Abstract
The present study was designed to understand the cigarette smoking-induced alterations in hormones and the resulting changes in platelet serotonin (5-hydroxytryptamine, 5-HT) and monoamine oxidase (MAO-B) activity in chronic smokers. Human male volunteers aged 35 ± 8 years, were divided into two groups, namely controls and smokers (12 ± 2 cigarettes per day for 7–10 years). Results showed that cigarette smoking significantly (p < 0.05) elevated plasma triiodothyronine (T3), cortisol and testosterone levels with significant (p < 0.05) reduction in plasma tryptophan and thyroxin (T4). Moreover, smokers showed reduced platelet 5-HT levels and MAO-B activity. In smokers, plasma cortisol was negatively correlated with tryptophan (r = −0.386), platelet MAO-B (r = −0.264), and 5-HT (r = −0.671), and positively correlated with testosterone (r = 0.428). However, testosterone was negatively correlated with platelet MAO-B (r = −0.315), and 5-HT (r = −.419) in smokers. Further, smokers plasma T3 levels were negatively correlated with platelet MAO-B (r = −0.398), and 5-HT (r = −0.541), whereas T4 levels were positively correlated with platelet MAO-B (r = 0.369), and 5-HT (r = 0.454). In conclusion, our study showed that altered testosterone and cortisol levels may aggravate behavior, mood disturbances and symptoms of depression by decreasing platelet 5-HT and MAO-B activity in smokers.
Keywords: Behaviour, Cigarette smoking, Hormones, 5-hydroxytryptamine, MAO-B activity
Background
Cigarette smoking is a reprehensible habit and is responsible for common diseases such as atherosclerosis and chronic obstructive pulmonary diseases [1]. The authentic stimulatory, euphoric, reinforcing and addictive properties of cigarette smoking make people not to quit the habit [2]. Earlier reports suggested that smoking could be protective against certain central nervous system disorders such as Parkinson’s, Alzheimer’s disease and dementia [3]. Besides, some other effects such as mood control, weight control and relief of tobacco withdrawal symptoms may slightly improve performance and health of people to cope with daily stress. [4, 5]. Further, unlike other drugs, anesthetics and alcohol, cigarette smoke does not impair performance in judgement, cognition and motor behavior [6]. Though the transient stimulatory, cognitive and reinforcing effects of cigarette smoking are specifically attributed to nicotine which activates the hypothalamic pituitary axis (HPA) [7], the effects of nicotine are not equivalent to cigarette smoke due to its heterogeneous composition.
Earlier studies revealed that smoking affects endocrine system chiefly, pituitary, thyroid, adrenal as well as testicular and ovarian functions [8]. However, its precise interactions and mechanisms on endocrine system are not clear. Blood platelets are peripheral model for central 5-hydroxytryptamine (5-HT) neurons due to similarities in the structure and function [9]. 5-HT receptors and 5-HT transporters, both expressed in platelets and neurons [10]. Monoamine oxidase isoenzyme B (MAO-B) in platelets has been suggested to have the same amino acid sequence as in brain [11]. Serotonergic neurotransmission is found to be linked with cigarette craving and smoking behavior [12]. The activity of MAO-B in platelets is one of the most extensively studied biological markers of personality and different forms of psychological disturbances [13–15]. Low platelet MAO-B activity has been associated with excessive risk taking, high impulsivity, anti-social behaviour and sensation seeking [16]. In healthy individuals there is a significant correlation between platelet MAO-B activity and monoamine metabolites, 5-hydroxyindoleacetic acid, homovanillic acid and 3-methoxy-4-hydroxyphenyl glycol, in cerebrospinal fluid [17]. The association of personality/behavior with platelet MAO-B and 5-HT is confirmed in different ways. Earlier reports revealed that cigarette smokers show depleted 5-HT levels and MAO-B activities [18]. However, the influence of hormones on 5-HT and MAO-B activities were not well understood in cigarette smokers.
Since, hormones are critical in cellular metabolism and coordination of physiological and behavioral responses such as growth, maturation and differentiation to biological stimuli, the present study was aimed to examine the changes induced by cigarette smoking on endocrine system and resulting influence on 5-HT and platelet MAO-B activity in cigarette smokers.
Methods
Experimental Details
Thirty human male volunteers (mean age 35 ± 8) in each group were chosen for the study. They were designated as non smokers or controls (n = 30) with no past or present history of smoking and other group was smokers (n = 30) with smoking at least 10 ± 2 cigarettes/day for the past 7–10 years. Subjects chosen for this study were with similar dietary habits, non alcoholics and free from chronic diseases, illness, use of any tranquillizers, drugs and anesthetics. All the volunteers were well informed about the experimentation and their written consent was obtained. The present study was approved by institutional ethical committee.
Isolation of Plasma and Platelets
Fasting blood samples were obtained from the subjects after 8 h of their last cigarette smoked. Blood was drawn into heparin-treated evacuated tubes and centrifuged immediately at 1,500×g for 10 min at 4 °C to separate plasma and red cells. Platelets were prepared by centrifuging at 200×g for 10 min at room temperature and the platelet-enriched plasma was carefully removed. The remaining pellet was washed three times with acid citrate dextrose buffer (36 mM citric acid, 5 mM KCl, 90 mM NaCl, 5 mM glucose, 10 mM EDTA, pH 6.8) and the washings were pooled with the original supernatant. The pooled suspension was centrifuged again at 200×g for 10 min to remove any residual erythrocytes. The platelets were then collected by centrifugation at 2,000×g for 20 min and the platelet pellet was washed three times with 10 ml of phosphate buffered saline (PBS, pH 6.8). The final pellet was resuspended in 700 μl of PBS for platelet analysis [19].
Determination of Plasma Tryptophan Content
Tryptophan content was estimated by adding 1.0 ml of Ehrilch’s reagent (3 % p-dimethylamino benzaldehyde in 2 N HCl) to 100 μl of plasma followed by 8.0 ml of 23.7 N H2SO4 and 0.1 ml of NaNO2. The absorbance was read at 540 nm against a blank. Standard solutions (20–100 μg) were run under similar conditions and the concentration of the sample was expressed as mg/dl [20].
Measuring of 5-HT Levels and MAO-B Activity
5-HT levels were estimated by suspending platelets in 3.5 ml of 0.02 N HCl and lysed by gentle agitation. One ml lysate was diluted to 2.0 ml with water. Platelet proteins were precipitated with 0.2 ml of 10 % ZnSO4 and 0.1 ml of 1 N NaOH and centrifuged to the supernatant 0.3 ml of 1 N HCl was added. Fluorescence is measured with an excitation at 295 nm and emission at 350 nm. Standard graph was prepared using serotonin creatinine sulfate [21].
The activity of the platelet monoamine oxidase enzyme was estimated spectrophotometrically as described previously [22]. Briefly, to 0.5 ml of assay medium (100 mM sodium phosphate buffer, pH 7.4; 1.0 mM tyramine; and 3.1 mM sodium azide) platelet sample was added and incubated for 10 min at 37 °C. The monoamine oxidase reaction was stopped by the addition of 0.5 ml H2O2 measuring solution (0.5 M phosphate and 5 U of horseradish peroxidase), which decreases the pH of the mixture to 4.5–4.7 followed by the addition of sodium dodecyl sulfate (final pH 2.1–2.3) and the colored product was measured at 414 nm. Specific activity was calculated using the molar extinction coefficient for the oxidized form of ABTS which was equal to 24,600 M−1 cm−1.
Measurement of TSH, T3, T4, Cortisol and Testosterone Levels
Levels of testosterone, cortisol, TSH, T3, and T4 in plasma samples of smokers and non-smokers were determined as described previously [23, 24]. The method is based on an automated immunoassay system (Advia® Centaur™, Bayer Health Care Diagnostika, Vienna, Austria) using a direct chemiluminiscence detection system according to the manufacturer’s instructions.
Statistical Analysis
Student’s ‘t’ test was performed for statistical significance between controls and smokers. Values were mean ± SD of 30 subjects in each group. Correlations between variables were assessed using Pearson’s correlation coefficients (r).
Results
General Characteristics of Controls and Cigarette Smokers
General characteristics of controls and smokers were summarized in Table 1. Smoker’s blood showed increased hemoglobin content and total WBC count with no significant difference in RBC, platelet counts and packed cell volume (PCV) when compared to controls.
Table 1.
General characteristics of controls and cigarette smokers
| Parameter | Controls (n = 30) | Smokers (n = 30) | |
|---|---|---|---|
| Age | 35 ± 8 | 35 ± 8 | |
| BMI | >23.5 | >24.2 | |
| Number of cigarettes/day | No | 10 ± 2 | |
| Smoking history | – | 7–10 years | |
| Alcohol consumption | No | No | |
| Chronic diseases | Not found | Not found | |
| RBC (m/cumm) | 4.32 ± 0.27 | 4.15 ± 0.18 | |
| WBC (cumm) | 7420 ± 137 | 8860 ± 145a | |
| Platelet count (×1,000/mm3) | 220 ± 60 | 232 ± 62 | |
| Hematocrit (PCV) | 48.7 ± 3.6 | 46.4 ± 3.1 |
Values are mean ± SD of each group. A p < 0.05 between groups
BMI body mass index; PCV packed cell volume
aIndicates significant difference from controls
Effect of Smoking on Plasma Tryptophan Levels
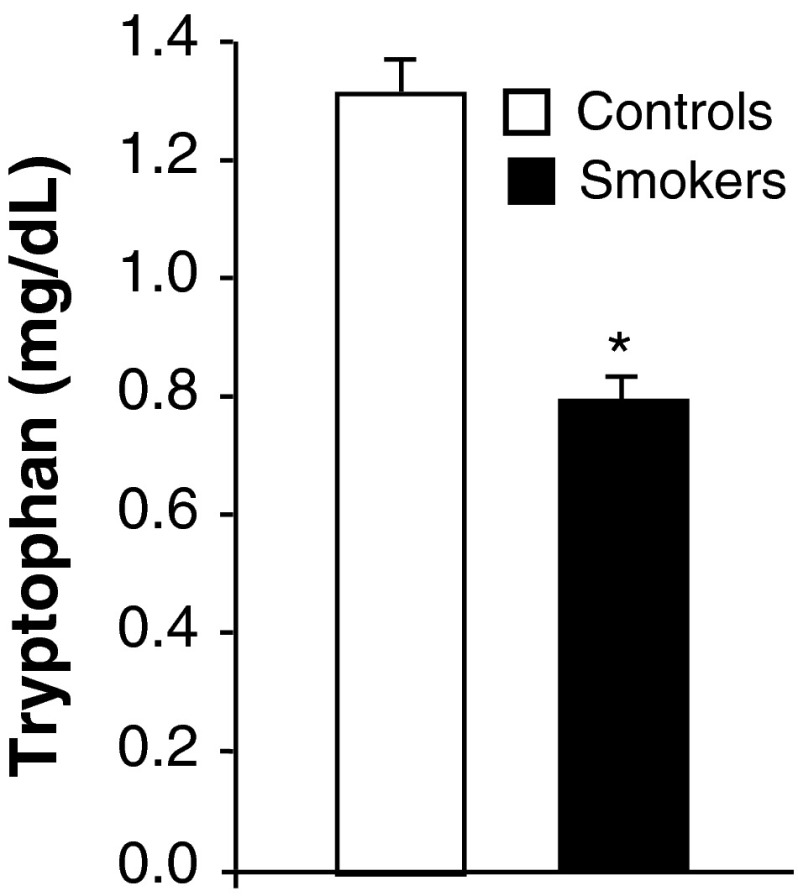
The influence of smoking on plasma tryptophan levels were analyzed and the data presented in Fig. 1. Smokers showed significantly lower tryptophan levels over the controls.
Fig. 1.
Effect of cigarette smoking on plasma tryptophan levels. Values are mean ± SD of each group. Tryptophan levels were expressed as mg/dl. *Indicates a p < 0.001 is statistically significant between groups
Changes in Serotonergic Markers of Smokers
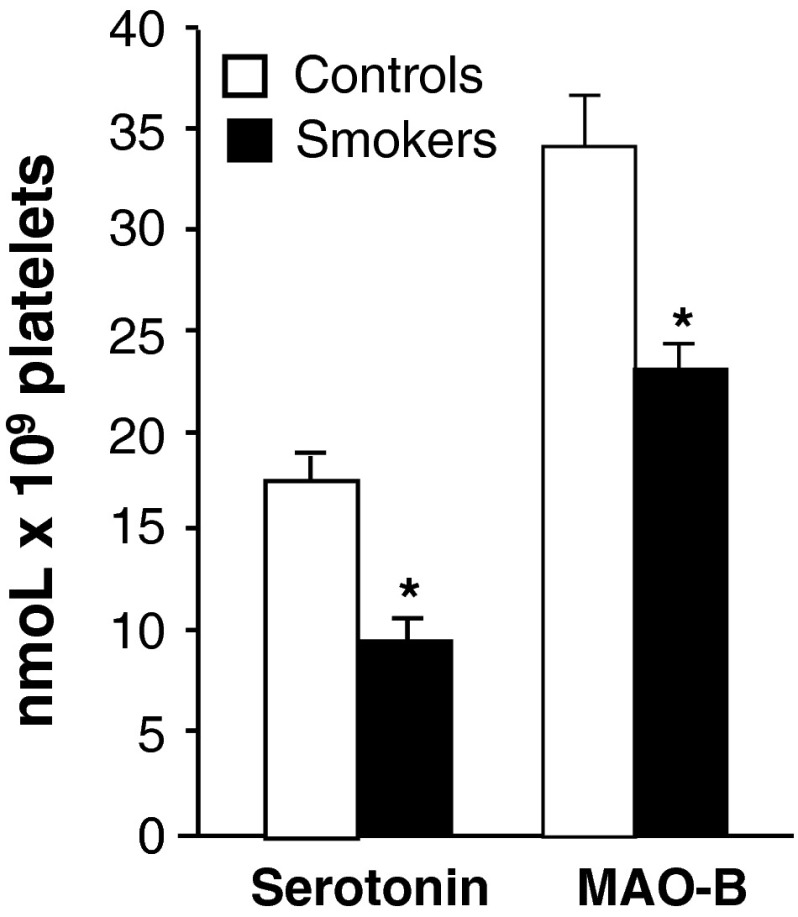
The serotonergic markers namely platelet 5-HT and MAO-B levels were determined in smokers and the data was presented in Fig. 2. Smokers showed significantly lowered platelet 5-HT levels as well as a significant reduction in the activity of MAO-B than to controls.
Fig. 2.
Effect of cigarette smoking on platelet serotonin and MAO-B activity. Values are mean ± SD of each group. Platelet serotonin levels and MAO-B activity were expressed as nmol × 109 platelets. *Indicates a p < 0.001 is statistically significant between groups
Influence of Smoking on Endocrine Status
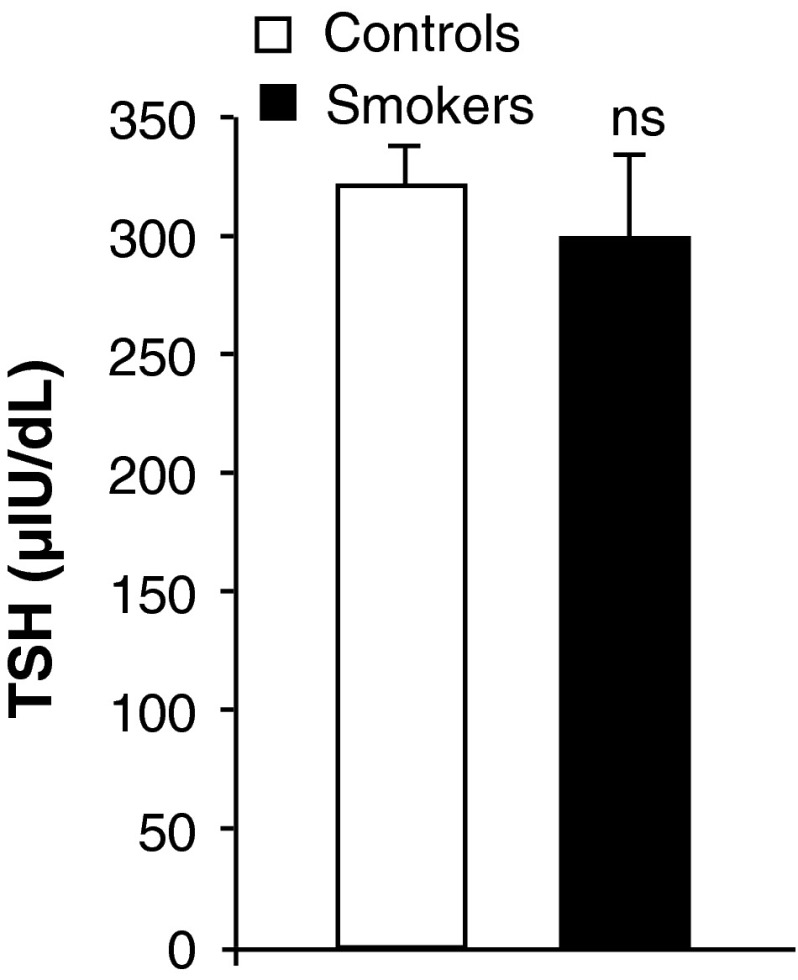
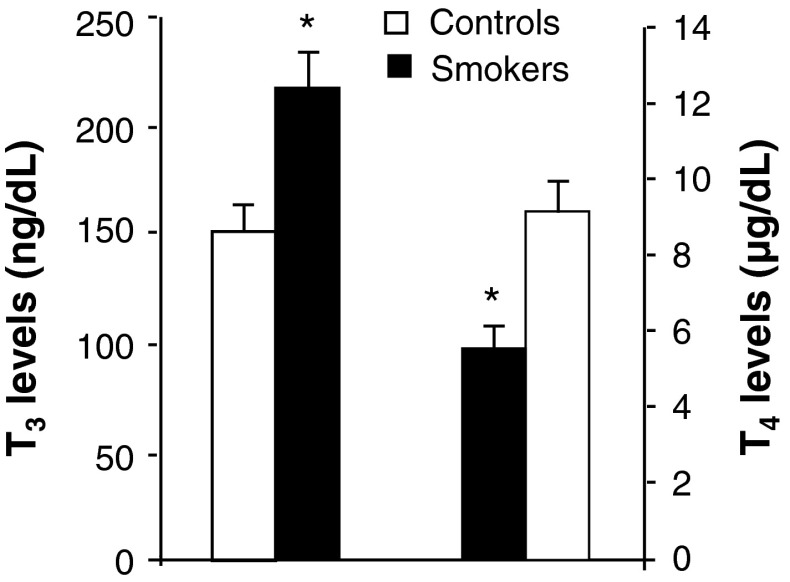
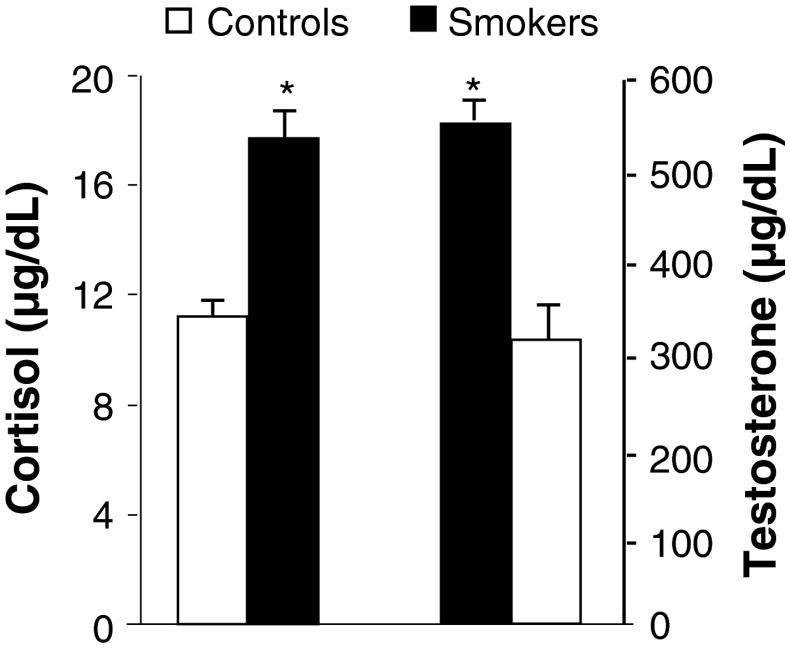
Impact of cigarette smoking on plasma endocrine status was assessed. The results indicated no detectable alterations in TSH levels (Fig. 3) in cigarette smokers compared to controls. However, increase in the levels of triiodothyronine was noticed with significant decrease in thyroxin (Fig. 4) levels in smokers than to controls. Smokers showed significant increase in cortisol and testosterone (Fig. 5) levels compared to controls.
Fig. 3.
Effect of cigarette smoking on plasma TSH levels. Values are mean ± SD of each group. TSH levels were expressed as μIU/dl. *Indicates a p < 0.001 is statistically significant between groups
Fig. 4.
Effect of cigarette smoking on plasma T3 and T4 levels. Values are mean ± SD of each group. T3 levels were expressed as ng/dl, T4 levels were expressed as μg/dl. *Indicates a p < 0.001 is statistically significant between groups
Fig. 5.
Effect of cigarette smoking on plasma cortisol and testosterone levels. Values are mean ± SD of each group. Cortisol and testosterone levels were expressed as μg/dl. *Indicates a p < 0.001 is statistically significant between groups
Association Between Endocrine System with Serotonin and MAO-B
Table 2 illustrates the correlations between variables in smokers. To ascertain the possible relation among behavioral markers such as 5-HT, MAO-B and endocrine status, Pearson correlation analysis was performed. In smokers, plasma cortisol was negatively correlated with tryptophan (r = −0.386), platelet MAO-B (r = −0.264), and 5-HT (r = −0.671), and positively correlated with testosterone (r = 0.428). However, testosterone was negatively correlated with platelet MAO-B (r = −0.315), and 5-HT (r = −0.419) in smokers. Further, smokers plasma T3 levels were negatively correlated with platelet MAO-B (r = −0.398), and serotonin (r = −0.541), whereas T4 levels were positively correlated with platelet MAO-B (r = 0.369), and serotonin (r = 0.454). No significant association was observed between TSH and behavioral markers, platelet 5-HT and MAO-B in smokers.
Table 2.
Correlation analysis between variables in cigarette smokers
| Parameter | Smokers | p value |
|---|---|---|
| Cortisol/tryptophan | r = −0.386 | p < 0.001 |
| Cortisol/MAO-B | r = −0.264 | p < 0.001 |
| Cortisol/testosterone | r = 0.428 | p < 0.001 |
| Cortisol/serotonin | r = −0.671 | p < 0.016 |
| Testosterone/MAO-B | r = −0.315 | p < 0.01 |
| Testosterone/serotonin | r = −0.419 | p < 0.001 |
| T3/serotonin | r = −0.541 | p < 0.017 |
| T3/MAO-B | r = −0.398 | p < 0.014 |
| T4/serotonin | r = 0.454 | p < 0.018 |
| T4/MAO-B | r = 0.369 | p < 0.011 |
Pearson correlation analysis was performed between variables. Student paired ‘t test’ was used to find out statistical significance
Discussion
Cigarette smoking is one of the most abused reinforcing agent and leading to morbidity and mortality [25]. In the present study, cigarette smokers showed decreased platelet MAO-B activity and 5-HT. Many studies have reported an association between tobacco smoking and reduced MAO-B activity in the brain and peripheral organs [26]. In vitro and in vivo studies are also confirmed the same, but the mechanism of this inhibition has not yet been elucidated. Studies revealed that chronic exposure of physiological concentrations of nicotine has no effect on the activity of MAO-A or MAO-B in rat brain suggesting that the inhibition of MAO activity is not nicotine related [27]. Nicotine shows some weak inhibition on MAO, at concentrations 2,000 times those found in chronic heavy smokers and is therefore unlikely to be physiologically relevant [28].
In the present study, cigarette smokers showed increase in testosterone, cortisol, T3 with decreased T4 levels in plasma of cigarette smokers. However, no significant change in TSH levels were noticed in smokers. In cigarette smokers testosterone and cortisol levels were negatively correlated with platelet MAO-B activity and serotonin. Testosterone and cortisol are end products of hypothalamic pituitary gonadal (HPG) axis and HPA. Testosterone inhibits HPA functioning at the hypothalamic level and cortisol has inhibitory effect on all levels of the HPG axis [29]. It has been shown that promoter of MAO-A contains three glucocorticoids/androgen response elements (GRE/ARE) and four binding sites for the SP 1 (specifity protein 1) and R1 (RAM2/CDCA7L) transcription factors of which androgens can interact directly with the third GRE/ARE and it also appears to act indirectly [30]. Since glucocorticoids and testosterone compete for the same sites this may actually mean that high testosterone levels may lead to lower transcription of MAO-A and lower MAO-A levels [31]. There is no study to confirm the same for MAO-B in cigarette smokers. Since MAO-A and MAO-B are highly conserved and they are coded by two different genes, and the regulation of MAO-B at transcription and translation level by testosterone cannot be ruled out in cigarette smokers suggesting lower MAO-B activity may not be the effect of nicotine but it could be the influence of altered testosterone levels.
In the present study, we noticed decreased platelet MAO-B activity and 5-HT in cigarette smokers. We found strong positive association between platelet MAO-B activity and 5-HT in smokers. 5-HT mediates its actions through a large family of receptors of which the 5-HT1A and HT2A receptors are the best characterized [32]. Thyroid hormone and cortisol exerts a powerful influence on 5-HT neurotransmission, metabolism and levels influence 5-HT1A and 5-HT2A receptor expression and regulating 5-HT-mediated neurotransmission [33, 34]. Thyroid hormones are known to play a significant role in adult brain function, but the precise mechanism(s) by which this occurs is not well understood [35, 36]. In cigarette smokers increased T3 and cortisol might be playing a role in 5-HT1A and 5-HT2A receptor function which would have lead to decreased serotonin concentration in platelets which needs further in-depth study. Brummett et al. [37] reported that single nucleotide polymorphism (SNP) in 5-HTR2C gene is associated with HPA axis activation. To date, there is no evidence for association of SNPs in 5-HTR2C in cigarette smokers. Triiodothyronine was reported to bind nuclear thyroid hormone receptors which are widely distributed throughout the brain, and in the central nervous system with the greatest densities in areas critical to mood and higher order cognition (e.g. hippocampus and frontal lobes) [35]. Thyroid hormone has neurogenic effects at the molecular level [38]. So far, only a very limited number of studies directly looked at the interaction between 5-HT and cortisol and thyroid responses in cigarette smokers. Several lines of evidence suggest that serotonergic neurotransmission, including the regulation of 5-HT1A receptors, is affected by nicotine [39]. Recent studies showed that some of nicotine’s psychotropic and endocrine effects are exerted by stimulation of 5-HT1A receptors on dorsal raphe neurons [40]. Increased cortisol secretion may play a role by inducing tryptophan 2,3-dioxygenase (tryptophan pyrrolase), the main metabolizing enzyme of tryptophan. Elevated cortisol levels could therefore explain lowered plasma tryptophan levels in cigarette smokers. The stimulating effect of smoking on cortisol secretion is likely to be related to the neuropharmacological actions of nicotine.
Our study shows that smokers might repeatedly stimulate their HPA axis during the day. In order to avoid hypercortisolism, counter-regulatory mechanisms through HPG axis are likely to be activated. Increased T3 levels also might be playing a role in the activation of HPA axis. In conclusion, altered testosterone and cortisol hormone levels may aggravate behavior, mood disturbances and symptoms of depression by decreasing platelet 5-HT and MAO-B activity in smokers. More mechanistic study is required to understand complex interplay of altered endocrine status and its role on behavior of cigarette smokers.
Acknowledgments
Authors thank University Grants Commission (UGC), New Delhi, India for providing Rajiv Gandhi National Senior Research Fellowship to P. Padmavathi.
Conflict of interest
None.
References
- 1.Padmavathi P, Reddy VD, Maturu P, Varadacharyulu NC. Smoking-induced alterations in platelet membrane fluidity and Na(+)/K(+)-ATPase activity in chronic cigarette smokers. J Atheroscler Thromb. 2010;17:619–627. doi: 10.5551/jat.2857. [DOI] [PubMed] [Google Scholar]
- 2.Baker TB, Brandon TH, Chassin L. Motivational influence on cigarette smoking. Ann Rev Psycol. 2004;55:463–491. doi: 10.1146/annurev.psych.55.090902.142054. [DOI] [PubMed] [Google Scholar]
- 3.Swan GE, Lessov-Schlaggar CN. The effect of tobacco smoke and nicotine on cognition and brain. Neuropsychol Rev. 2007;17:259–273. doi: 10.1007/s11065-007-9035-9. [DOI] [PubMed] [Google Scholar]
- 4.Jo YH, Talmage DA, Role LW. Nicotine receptor-mediated effects on appetite and food intake. J Neurobiol. 2002;53:618–632. doi: 10.1002/neu.10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parrott AC. Individual differences in stress and arousal during cigarette smoking. Psychopharmacology. 1994;115:389–396. doi: 10.1007/BF02245082. [DOI] [PubMed] [Google Scholar]
- 6.Prochaska JJ. Failure to treat tobacco use in mental health and addiction treatment settings: a form of harm reduction? Drug Alcohol Depend. 2010;110:177–182. doi: 10.1016/j.drugalcdep.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mello NK. Hormones, nicotine and cocaine: clinical studies. Horm Behav. 2010;58:57–71. doi: 10.1016/j.yhbeh.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berlin I. Endocrine and metabolic effects of smoking cessation. Curr Med Res Opin. 2009;25:527–534. doi: 10.1185/03007990802707626. [DOI] [PubMed] [Google Scholar]
- 9.Launay JM, Del Pino M, Chironi G, Callebert J, Peoch K, Megnien JL, Mallet J, Simon A, Rendu F. Smoking-induces long-lasting effects through a monoamine-oxidase epigenetic regulation. PLoS One. 2009;4:e7959–e7970. doi: 10.1371/journal.pone.0007959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cicin-Sian L, Matosic A, Mokrovic G, Balija M, Marusic S, Jernej B. Platelet monoamine oxidase kinetic, alcoholism subtypes and cigarette smoking. Neuropsychobiology. 2007;56:138–145. doi: 10.1159/000115780. [DOI] [PubMed] [Google Scholar]
- 11.Pombo S, Levy P, Bicho M, Ismail F, Cardoso JM. Neuropsychological function and platelet monoamine oxidase activity levels in type I alcoholic patients. Alcohol Alcohol. 2008;43:423–430. doi: 10.1093/alcalc/agn021. [DOI] [PubMed] [Google Scholar]
- 12.Hitsman B, Spring B, Pingitore R, Munafo M, Hedeker D. Effect of tryptophan depletion on the attentional salience of smoking cues. Psychopharmacology. 2007;192:317–324. doi: 10.1007/s00213-007-0722-2. [DOI] [PubMed] [Google Scholar]
- 13.Ruchkin VV, Koposov RA, afKlinteberg B, Oreland L, Grigorenko EL. Platelet MAO-B, personality, and psychopathology. J Abnorm Psychol. 2005;114:477–482. doi: 10.1037/0021-843X.114.3.477. [DOI] [PubMed] [Google Scholar]
- 14.Ertugrul A, Ucar G, Basar K, Memir B, Yabanoglu S, Ulug B. Influence of clozapine on platelet serotonin, monoamine oxidase and plasma serotonin levels. Psychiatry Res. 2007;149:49–57. doi: 10.1016/j.psychres.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 15.Paaver MN, Nordquist N, Parik J, Harro M, Oreland L, Harro L. Platelet MAO activity and the 5-HTT gene promoter polymorphism are associated with impulsivity and cognitive style in visual information processing. Psychopharmacol (Berl). 2007;194:545–554. doi: 10.1007/s00213-007-0867-z. [DOI] [PubMed] [Google Scholar]
- 16.Ensoo D, Paaver M, Pulver A, Harro M. Low platelet MAO activity assoicated with high dysfunctional impulsivity and antisocial behavior: evidence from drunk drivers. Psychopharmacology. 2004;72:356–358. doi: 10.1007/s00213-003-1664-y. [DOI] [PubMed] [Google Scholar]
- 17.Oreland L, Wiberg A, Asberg M, Traskman L, Sjostrand L, Thoren P, Bertilsson L, Tybring G. Platelet MAO activity and monoamine metabolites in cerebrospinal fluid in depressed and suicidal patients and in healthy controls. Psychiatry Res. 1981;4:21–29. doi: 10.1016/0165-1781(81)90004-4. [DOI] [PubMed] [Google Scholar]
- 18.Rendu F, Peoc’h K, Berlin I, Thomas D, Launay JM. Smoking related diseases: the central role of monoamine oxidase. Int J Environ Res Public Health. 2011;8:136–147. doi: 10.3390/ijerph8010136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Menashi S, Weintroub H, Crawford N. Characterization of human platelet surface and intra-cellular membranes isolated by free flow electrophoresis. J Biol Chem. 1981;256:4095–4101. [PubMed] [Google Scholar]
- 20.Spies JR. Determination of tryptophan in proteins. Anal Chem. 1967;39:1412–1416. doi: 10.1021/ac60256a004. [DOI] [PubMed] [Google Scholar]
- 21.Ansell GB, Beeson MF. A rapid and sensitive procedure for the combined assay of noradrenaline, dopamine, and serotonin in a single brain sample. Anal Biochem. 1968;23:196–206. doi: 10.1016/0003-2697(68)90351-5. [DOI] [PubMed] [Google Scholar]
- 22.Szutowicz A, Kobes RD, Orsulak PJ. Colorimetric assay for monoamine oxidase in tissues using peroxidase and 2,2′-azinodi(3-ethylbenzthiazoline-6-sulfonic acid) as chromogen. Anal Biochem. 1984;138:86–94. doi: 10.1016/0003-2697(84)90773-5. [DOI] [PubMed] [Google Scholar]
- 23.Regnier C, Bennet A, Malet T, Guez T, Plantavid M, Rochaix P, Monrozies X, Louvet JP, Caron P. Intraoperative testosterone assay for virilizing ovarian tumor topographic assessment: report of a leydig cell tumor of the ovary in a premenopausal woman with an adrenal incidentaloma. J Clin Endocrinol Metab. 2002;7:3074–3077. doi: 10.1210/jcem.87.7.8583. [DOI] [PubMed] [Google Scholar]
- 24.Kapelari K, Kirchlechner C, Hogler W, Schweitzer K, Virgolini I, Moncayo R. Pediatric reference intervals for thyroid hormone levels from birth to adulthood: a retrospective study. BMC Endocr Disord. 2008;8:15. doi: 10.1186/1472-6823-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fowler JS, Logan J, Wang GJ, Volkow ND, Telang F, Zhu W, Franceschi D, Pappas N, et al. Low monoamine oxidase B in peripheral organs in smokers. Proc Natl Acad Sci USA. 2003;100:11600–11605. doi: 10.1073/pnas.1833106100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castagnoli K, Steyn SJ, Magnin G, Van Der Schyf CJ, Fourie I, Khalil A, Castagnoli N., Jr Studies on the interaction of tobacco leaf and tobacco smoke constituents and monoamine oxidase. Neurotox Res. 2002;4:151–160. doi: 10.1080/10298420290015854. [DOI] [PubMed] [Google Scholar]
- 28.Lewis A, Miller JH, Lea RA. Monoamine oxidase and tobacco dependence. Neurotoxicology. 2007;28:182–195. doi: 10.1016/j.neuro.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 29.Viau V. Functional cross-talk between the hypothalamic-pituitary-gonadal and adrenal axes. J Neuroendocrinol. 2002;14:506–513. doi: 10.1046/j.1365-2826.2002.00798.x. [DOI] [PubMed] [Google Scholar]
- 30.Ou XM, Chen K, Shih JC. Glucocorticoid and androgen activator of monoamine oxidase A is regulated differently by R1 and Sp1. J Bio Chem. 2006;281:21512–21525. doi: 10.1074/jbc.M600250200. [DOI] [PubMed] [Google Scholar]
- 31.Sjoberg RL, Ducci F, Barr CS, Newman TK, Dellosso L, Virkkunen M, Goldman D. A non-additive interaction of a functional MAO-A VNTR and testosterone predicts antisocial behavior. Neuropsychopharmacology. 2008;33:425–430. doi: 10.1038/sj.npp.1301417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boess FG, Martin IL. Molecular biology of 5-HT receptors. Neuropharmacology. 1994;33:275–317. doi: 10.1016/0028-3908(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 33.Lanfumey L, Mongeau R, Cohen-Salmon C, Hamon M. Corticosteroid-serotonin interactions in the neurobiological mechanisms of stress-related disorders. Neurosci Biobehav Rev. 2008;32:1174–1184. doi: 10.1016/j.neubiorev.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 34.Stipcevic T, Kusacic-Kuna S, Dezelijin M, Dodig D, Korsic M, Pivac N, Muvk-Seler D. Platelet serotonin concentration and monoamine oxidase activity in hypothyroid patients. Horm Res. 2009;71:207–212. doi: 10.1159/000201109. [DOI] [PubMed] [Google Scholar]
- 35.Bauer M, Goetz T, Glenn T, Whybrow PC. The thyroid-brain interaction in thyroid disorders and mood disorder. J Neuroendocrinol. 2008;10:1101–1114. doi: 10.1111/j.1365-2826.2008.01774.x. [DOI] [PubMed] [Google Scholar]
- 36.Davis JD, Tremont G. Neuropsychiatric aspects of hypothyroidism and treatment reversibility. Minerva Endocrinol. 2007;32:49–65. [PubMed] [Google Scholar]
- 37.Brummett BH, Kuhn CM, Boyle SH, Babyak MA, Siegler IC, Williams RB. Cortisol responses to emotional stress in men: association with a functional polymorphism in the 5HTR2C gene. Biol Psychol. 2012;89:94–98. doi: 10.1016/j.biopsycho.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Despuza LA, Ladiwala U, Daniel SM, Agashe S, Vaidya RA, Vaidya VA. Thyroid hormone regulates hippocampal neurogenesis in the adult rat brain. Mol Cell Neurosci. 2005;29:414–426. doi: 10.1016/j.mcn.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 39.Kenny PJ, Markou A. Neurobiology of the nicotine withdrawal syndrome. Pharmacol Biochem Behav. 2001;70:531–549. doi: 10.1016/S0091-3057(01)00651-7. [DOI] [PubMed] [Google Scholar]
- 40.Broocks A, Bandelow B, Koch K, Bartmann U, Kinkelbur J, Schweiger U, Hohagen F, Hajak G. Smoking modulates neuroendocrine responses to ipsapirone in patients with panic disorder. Neuropsycopharmacology. 2002;27:270–278. doi: 10.1016/S0893-133X(02)00298-1. [DOI] [PubMed] [Google Scholar]