Abstract
Low back pain is very disabling and dispiriting because of the physical impediment it causes and its psychological effects. Innumerable factors have been implicated in its etiology. In spite of improvements in diagnostic modalities, a considerable number of such cases fall in the ambiguous zone of unknown etiology or ‘idiopathic.’Early diagnosis of low back pain will allow effective prevention and treatment to be offered. This study was conducted to assess the contribution of vitamin D levels and other biochemical factors to chronic low back pain in such cases. All patients attending the orthopedics OPD for low back pain in whom a precise anatomical cause could not be localized, were prospectively enrolled in this study. We measured serum levels of glucose, calcium, phosphorus, uric acid, rheumatoid factor, C reactive protein, alkaline phosphatase, total protein, albumin and 25 (OH) D concentrations in 200 cases and 200 control samples. The patients showed significantly lower vitamin D levels compared to controls with p value < 0.0001. The maximum number of low back pain patients were in the age group of 31–50 years (42 %).The average BMI was 23.27 ± 5.17 kg/sq m, 73 % of total patient population were females and 27 % were known case of type 2 diabetes mellitus. Calcium, alkaline phosphatase, was positively correlated with vitamin D and glucose showed a negative correlation with vitamin D in the patient population. The problem of low back pain provides a challenge to health care providers. The problem in developing countries is compounded by ignorance to report for early treatment and occupational compulsions in rural areas and sedentary lifestyle in urban youth. The authors strongly recommend early frequent screening for vitamin D along with glucose, protein, albumin, calcium, phosphorus, CRP as part of general health checkup for non-specific body pain, especially low back pain.
Keywords: Low back pain, Vitamin D. glucose, CRP, RA factor, Biochemical markers
Introduction
Low back pain, a leading cause of disability, interferes with quality of life and work performance and is the most common reason for medical consultations [1]. It is a very commonly attributed to the upright posture and many hours spent sitting, at work. Difficult to diagnose and even more difficult to treat medically, it could result in an enormous socio-economic burden. In up to 80 % of patients with low back pain, a precise anatomic cause cannot be localized [2] Back pain is often self-treated with over the counter nonprescription medications or alternative therapies, a further impediment for effective diagnosis and management.
Vitamin D, sunshine vitamin is one of the most intensely investigated nutrients of the 21st century. It plays a crucial role in the development and maintenance of a healthy skeleton throughout life. Experts largely agree that this fat-soluble pro-hormone is vital to bone health.
Due to the important role it plays in calcium homeostasis and bone mineralization. A number of studies have been done to assess the vitamin D status in subjects with nonspecific back pain where no organic cause could be ascertained. The results have largely been contradictory. A meta-analysis of 22 studies found no statistically significant link between vitamin D levels and chronic pain syndromes. However some studies hint towards a cause effect relationship between vitamin D levels and pain [3, 4].
It has been reported by various researchers that Indians have a very precarious vitamin D balance in spite of ample sunlight exposure due to dietary imbalances and aesthetic reasons. Hence evaluation of vitamin D status among Indians with back pain may reveal interesting trends. So, this study was designed to assess specifically the levels of vitamin D and some other biochemical factors in patients presenting with idiopathic low back pain.
Materials and Methods
Place of Study
This was a single center cross-sectional prospective study of patients presenting consecutively to the department of orthopaedics with back pain. The study was conducted at XXX over a period of 2 years.
Study Population
Inclusion criteria for recruitment as subjects were symptomatic men and women, age more than 18 years. The presenting symptoms taken for inclusion were difficulty in squatting/getting up from squatting position/climbing stairs, difficulty in using oriental toilets, difficulty in attending prayers in the sitting position, and altered gait. A number of conditions amounted to exclusion from the study. Patients with hepatic, renal, dermatological disorders, alcoholics and pregnant women were excluded from the study. Also, patients with clinico-radiological evidence of rheumatoid arthritis, fracture dislocation, tuberculosis, tumor of spine, prolapsed intervertebral disc, lordo-scoliosis, were excluded from the study. History of surgery, hospitalization, or major medical illness within the past one year and history of hormone replacement therapy, intake of glucocorticoids, biophosphonates, teriparatide, and other drugs affecting bone metabolism also amounted to exclusion from the study. Intake of conventional calcium or vitamin D supplements was not considered an exclusion criterion. The controls were chosen from the volunteers in the departments of biochemistry and orthopaedics. Sunlight exposure and dietary intake of calcium or vitamin D were not measured due to managerial and administrative constraints.
Methodology
All subjects were enrolled after obtaining a written informed voluntary consent. They were subjected to a complete history and physical examination geared towards assessing bone and mineral status. The data was recorded according to a prepared Proforma which included anthropometric data.
Biochemical Analysis
A 12–16 h fasting sample of blood collected from median cubital vein and stored at −20 degree C till further analysis. The tests performed included fasting plasma glucose (FPG), calcium, phosphorus, alkaline phosphatase, uric acid, 25-hydroxy vitamin D, C reactive protein (CRP) and Rheumatoid Factor (R.A. Factor). PTH could not be assayed because of resource limitation.
Assay Methods
Blood sugar, calcium, phosphorus, alkaline phosphatase and uric acid levels were measured on fully automated biochemistry analyser (AU 480) using commercially available kits. 25 OH vitamin D was measured by ELISA using commercially available kit supplied by DRG International (USA) while R.A. Factor was assayed by ELISA kit provided by Biolegend. CRP was estimated by commercially available immune-turbidimetric kit by Randox on AU 480 autoanalyser.
Statistical Analysis
Data are presented as mean ± standard deviation. Student's t test was used to compare the data. Pearson’s coefficient was calculated for the correlation. p < 0.05 was considered significant.
Observations
In our study, a total of 200 low back pain patients’ data were evaluated. The patients were divided into four groups according to age so as to ascertain the age group in which back pain is more prevalent. This is important keeping in mind incapacitation caused by back pain and its consequent economic repercussions. Our study revealed that 42 % of the patients were in the age group of 31–50 years followed by 39 % cases in 51–70 year age group, 15 % cases were ≤30 years and 4 % were ≥70 years. Table 1 shows the biochemical and anthropometrical data of the low back pain patients [n = 200] attending the orthopedics OPD. The low vitamin D level in the subjects with back pain is evident from the table. CRP is a non-specific marker of inflammation and its levels decrease with therapy. The high levels in our study could an indication of low grade chronic inflammation in our subjects. RA factor ia an antibody level that rises in rheumatoid arthritis. However a positive R.A. Factor test does not always reinforce RA as there are several other conditions that can also give positive R.A. Factor results. Healthy people without RA can also test positive for R.A. Factor, particularly older people [5]. The sole complaint of low back pain was seen in 55 % patients and associated knee joint pain in 35 % and associated shoulder pain in 10 % cases. Fifty four patients were known cases of diabetes mellitus, 45 had thyroid disorder and 36 were known to have high blood pressure (Table 2). Table 3 shows the level of 25 OH vitamin D in cases and controls. It was significantly lower in cases compared to controls (p < 0.0001). The CRP and RA factor levels were higher in the cases as compared to the controls. The non-specific nature of CRP and the elevation of RA factor in conditions other than rheumatoid arthritis could be the possible reasons behind this finding.
Table 1.
Mean, SD and range of various parameters in low back pain patients (n = 200)
| Parameters | Mean ± SD | Range |
|---|---|---|
| Age (years) | 46.19 ± 15.69 | 13–75 |
| Sex (females:males) | 146:54 | – |
| Weight (kg) | 58.16 ± 13.22 | 34–80 |
| Height (m) | 1.58 ± 0.10 | 1.4–1.82 |
| Body mass index (kg/m2) | 23.27 ± 5.17 | 16.63–31.47 |
| 25 OHVit. D (ng/ml) | 35.26 ± 20.98 | 6.05–115.30 |
| Alkaline phosphatase (U/L) | 145.27 ± 80.63 | 44–311 |
| Calcium (mg/dl) | 8.39 ± 0.91 | 7.0–10.5 |
| Phosphorous (mg/dl) | 3.56 ± 1.20 | 2.0–9.2 |
| Uric acid (mg/dl) | 4.58 ± 1.55 | 2.6–7.4 |
| Fasting plasma glucose (mg/dl) | 107.54 ± 35.37 | 64.57–197 |
| Rheumatoid factor (IU/ml) | 9.23 ± 7.85 | 6.7–16.7 |
| Total leukocyte count | 7553.15 ± 3135.77 | 4,400–1,4000 |
| Erythrocyte sedimentation rate | 35.74 ± 24.51 | 8–113 |
| C reactive protein (mg/dl) | 17.23 ± 16.02 | 1.01–33.25 |
Table 2.
Chief complaints and past history in patients of low back pain (n = 200)
| Chief complaints (n = 200) | Past history (n = 200) |
|---|---|
| Low back pain (n = 109) | Type II diabetes mellitus (n = 54) |
| With associated knee joint pain (n = 71) | Thyroid disorder (n = 45) |
| With associated shoulder pain (n = 20) | High blood pressure (n = 36) |
Table 3.
Vitamin D levels in cases & controls
| Parameter | Cases (n = 200) | Controls (n = 200) | p value |
|---|---|---|---|
| 25 OH vitamin D (mean ± SEM) | 35.26 ± 2.000 | 43.89 ± 0.4783 | p < 0.0001 |
| C reactive protein (mean ± SD) | 17.23 ± 16.02 | 1.02 ± 0.57 | p < 0.0001 |
| R A factor (mean ± SD) | 9.23 ± 7.85 | 3.47 ± 0.98 | <0.001 |
We then compared individual parameters among the different age groups as well as ascertained the prevalence of the various biochemical risk factors among the subjects. Approximately 6 % of patients had calcium less than 8.5 mg/dl. Vitamin D levels were insufficient (less than 30 ng/ml) in 50 % of the subjects with back pain. FPG was >126 mg/dl in 20.7 % cases. This refers to the arthritis associated with diabetes mellitus. BMI was <18.5 kg/m2 in 24.3 % cases, between 18.5–25 in 37.8 % cases and >30 kg/m2 in 5.4 % cases. Alkaline phosphatase levels were elevated over 280 U/L in 9 % cases of low back pain. Raised ESR or CRP, normocytic normochromic anaemia, thrombocytosis, low albumin, raised alkaline phosphatase are indicators of inflammatory arthritis.
We then divided all the patients’ age wise into four age groups, ≤30 years, 31–45 years, 46–60 years and ≥61 years, calculated the means of all parameters (Table 4) and performed ANOVA to see if the differences were statistically significant between the different age groups. CRP showed a significant difference in the four age groups. Vitamin D levels demonstrated a decreasing trend with increasing age with deficient levels in subjects aged more than 60 years. A similar trend was observed for serum calcium levels denoting the positive effect vitamin D levels have on calcium levels.
Table 4.
Mean ± standard deviation of biochemical parameters in different age groups (n = 200)
| Age group (years) | ≤30 | 31–45 | 46–60 | ≥61 |
|---|---|---|---|---|
| Number of cases (n) | 30 | 67 | 64 | 39 |
| M:F | 8:22 | 7:60 | 12:52 | 11:28 |
| Age (yrs) | 18.29 ± 5.87 | 42.28 ± 461 | 59.45 ± 6.64 | 73.25 ± 2.06 |
| Weight (kg) | 49.82 ± 15.92 | 59.09 ± 13.06 | 60.07 ± 11.11 | 75 ± 5.57 |
| Height (m) | 1.53 ± 0.13 | 1.59 ± 0.09 | 1.59 ± 0.09 | 1.57 ± 0.13 |
| BMI (kg/m2) | 20.90 ± 5.08 | 23.19 ± 4.95 | 24.02 ± 5.37 | 31.22 ± 5.04 |
| Vitamin D (ng/ml) | 43.62 ± 32.26 | 36.40 ± 18.95 | 34.69 ± 18.62 | 24.86 ± 6.60 |
| ALP (U/L) | 161.76 ± 105.06 | 149.96 ± 83.90 | 130.88 ± 67.37 | 144 ± 41.63 |
| Calcium (mg/dl) | 8.55 ± 0.78 | 8.38 ± 0.93 | 8.35 ± 0.97 | 8.13 ± 0.85 |
| Phosphorus (mg/dl) | 3.93 ± 0.74 | 3.39 ± 1.10 | 3.44 ± 1.18 | 4.78 ± 2.92 |
| Uric acid (mg/dl) | 4.16 ± 1.3 | 4.26 ± 1.14 | 5.10 ± 1.87 | 5.10 ± 1.60 |
| Plasma glucose (mg/dl) | 98.76 ± 28.53 | 106.74 ± 30.91 | 109.78 ± 41.44 | 122.50 ± 40.75 |
| Protein (g/dl) | 7.64 ± 0.58 | 7.80 ± 0.51 | 7.66 ± 0.66 | 7.45 ± 1.16 |
| Rheumatoid factor (IU/ml) | 8.40 ± 1.5 | 9.28 ± 4.33 | 9.35 ± 2.19 | 20.12 ± 27.46 |
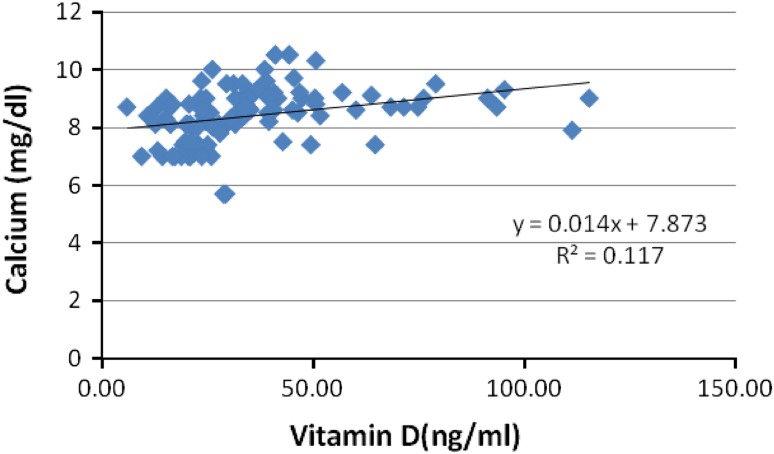
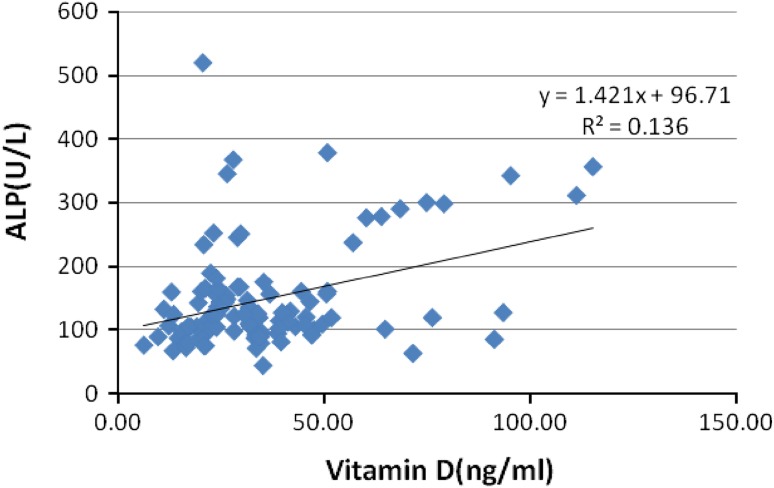
Calcium (Fig. 1), alkaline phosphatase (Fig. 2), were positively correlated with vitamin D and glucose (Fig. 3) showed a negative correlation with vitamin D in the patient population. The positive correlation between calcium, vitamin D and ALP levels is self-explanatory owing to the interplay between these factors in bone metabolism. This negative correlation between vitamin D and glucose levels can be explained by the role of vitamin D in insulin signaling and its role in maintenance of glucose tolerance.
Fig. 1.
Correlation of calcium with vitamin D in low back pain patients [n = 200]
Fig. 2.
Correlation of vitamin D with alkaline phosphatase in low back pain patients [n = 200]
Fig. 3.
Correlation of vitamin D (ng/ml) with glucose levels (mg/dl) in low back pain [n = 200]
Discussion
Low back pain (LBP) is defined as pain localized in the area below the costal margins and above the inferior gluteal folds. [6] It is a disorder afflicting a large chunk of the population both in the young as well as the older age groups owing to a multitude of aggravating factors. The sedentary lifestyle and improper posture are the main culprits. In a sizeable number of such subjects, no anatomic defect can be discerned making management of these idiopathic cases difficult.
Chronic pain is more prevalent in older women as compared to men [7]. In our study there were 146 females and 54 males in patient group. This can be attributed to differential work profiles as well as precarious nutritional status of women owing to social and economic factors. The back pain in post-menopausal women can be attributed to decreased bone mass, sarcopenia, vertebral fractures, and inflammation. All these findings have been associated with vitamin D deficiency [8].
The higher rates in urban populations as compared with rates in rural populations and the sharply higher rates among workers in enclosed workshops of low-income countries suggest a disturbing trend: LBP prevalence may be on the rise among populations owing to incessant urbanization and rapid industrialization. Therefore it is pertinent to identify the causes and plan subsequent individualized therapy for back pain.
In our study we have attempted to evaluate the role of hypovitaminosis D as a probable etiological factor in idiopathic chronic back pain. The vitamin D status is assessed by serum 25 hydroxy vitamin D owing to its longer half-life and stability. Serum 25 (OH) D is a potent indicator of total body vitamin D, the main regulator hormone in calcium and vitamin D metabolism. [9] Vitamin D deficiency may be without florid signs like bone tenderness in around 63 % patients, [10] an important finding, as without bony tenderness and without proper awareness on the part of physicians, vitamin D deficiency can easily be overlooked. Severe vitamin D deficiency causes a reversible myopathy characterized by muscle weakness, wasting, and instability of gait. The etiology of this myopathy is multifactorial and attributed to secondary hyperparathyroidism, hypocalcemia, hypophosphatemia, and calcitriol deficiency itself [11]. The direct association between vitamin D deficiency and myopathy was underscored with the finding of vitamin D receptors (VDR) in skeletal muscle.
Bischoff-Ferrari et al. reported from their study on 4,100 individuals that 25 (OH) D concentrations between 40 and 94 nmol/L were associated with better musculoskeletal function in the lower extremities than are concentrations <40 nmol/L In both active and inactive ambulatory persons aged > or = 60 y [12]. Furthermore, vitamin D improves postural and dynamic balance, both of which are independent and significant fall predictors. A 2004 meta-analysis found that vitamin D supplementation as compared with calcium alone or placebo reduced the risk of falls significantly (22 %) in both ambulatory and institutionalized elderly subjects. A potential role of vitamin D in initiating protein synthesis in musculature was suggested [13].
A number of mechanisms have been proposed to explain the pathogenesis of back pain in vitamin D deficiency. Continuous regeneration of nerve cells runs smoothly only when adequate vitamin D is present. Studies have shown that vitamin D supplements helped those with nerve pain. Vitamin D deficiency promotes skeletal muscle hypersensitivity and sensory hyper-innervation [14, 15] Inflammation exacerbates pain, impairs healing, and causes further structural damage to the back. Vitamin D deficient people have much higher levels of inflammatory markers than those that get enough vitamin D. Adequate vitamin D decreases this inflammation and has been shown to alleviate lower back pain. Up-regulation of MKP-1 by vitamin D is the novel pathway by which vitamin D inhibits LPS-induced p38 activation and cytokine production in monocytes/macrophages [16]. RANK-RANKL osteoprotegerin system, modulated by vitamin D, directly influences the release of pro inflammatory cytokines. It is now known that vitamin D deficiency makes muscles weaker and that vitamin D repletion boosts muscle strength [17].
Schreuder et al. conducted a study on the effect of vitamin D supplementation for nonspecific musculoskeletal pain in Non-Western immigrants in Netherlands. They reported a positive effect of vitamin D supplementation in such subjects [18]. A number of theories were put forward to explain this phenomenon. Noteworthy among them included the rapid nongenomic influence of vitamin D on the metabolism of muscle cells [19], growth of muscle fibers by a slow genomic effect on muscle cells, and a nonspecific effect on the central or peripheral nervous system pointing towards a anti depressive influence of vitamin D thereby affecting food intake [20]. Kalra et al. reported that vitamin D levels were less than 10 ng/mL in 55.55 % cases and between 10 and 30 ng/mL in 38.46 % of patients with back pain. Thus, the prevalence of vitamin D deficiency and insufficiency was 220/234 (94.01 %) in his cohort of North Indian patients with musculoskeletal complaints [21]. Our findings are in accordance with Kalra et al.
We observed a negative correlation between vitamin D and glucose levels in the patient population. According to a recent Indian study by Bachali et al., the mean 25 (OH) D level was significantly lower (20.09 ng/ml) in type 2 diabetes compared to controls (23.89 ng/ml) and 25 (OH) D levels negatively correlated with homeostasis model assessment—Insulin Resistance (HOMA-IR) in total subjects. They concluded that vitamin D has a decisive role in glucose metabolism and its deficiency can result in insulin resistance and diabetes. [22]. We also demonstrated a similar trend among our cohort of patients with back pain. Stümer et al. [23] and Gebhardt et al. [24] observed a strong association between pain severity and CRP levels in patients with sciatic pain but not in those with chronic LBP. CRP—an acute phase reactant is an indicator of low grade inflammation. The higher level in our cases denotes the probable role of inflammation in back pain [25].
Our findings provide a plausible explanation as well as justification for advocating dietary supplementation as well as therapeutic medication to achieve euvitaminosis D in musculoskeletal pain patients. It is also of utmost importance to initiate population based screening for vitamin D status especially in at risk populations. A holistic approach inclusive of appropriate sunlight exposure, vitamin D & calcium supplementation and appropriate physical exercise is necessary to mitigate the morbidity induced by the incapacitations caused by abnormal vitamin D homeostasis.
References
- 1.Ehrlich GE. Low back pain. Bull World Health Organ. 2003;81:671–676. [PMC free article] [PubMed] [Google Scholar]
- 2.Deyo RA, Rainville J, Kent DL. What cam the history and physical examination tell us about low back pain. JAMA. 1992;268:760–765. doi: 10.1001/jama.1992.03490060092030. [DOI] [PubMed] [Google Scholar]
- 3.Straube S, Andrew Moore R, McQuay HJ. Vitamin D and chronic pain. Pain. 2009;141:10–13. doi: 10.1016/j.pain.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 4.Kulie T, Groff A, Redmer J, Hounshell J, Schrager S. Vitamin D: an evidence-based review. J Am Board Fam Med. 2009;22:698–706. doi: 10.3122/jabfm.2009.06.090037. [DOI] [PubMed] [Google Scholar]
- 5.Suresh E. Diagnosis of early rheumatoid arthritis: what the non-specialist needs to know. J R Soc Med. 2004;97(9):421–424. doi: 10.1258/jrsm.97.9.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johansen JV, Manniche C, Kjaer P. Vitamin D levels appear to be normal in Danish patients attending secondary care for low back pain and a weak positive correlation between serum level vitamin D and modic changes was demonstrated: a cross-sectional cohort study of consecutive patients with non-specific low back pain. BMC Musculoskelet Disord. 2013;14:78. doi: 10.1186/1471-2474-14-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leveille SG, Zhang Y, McMullen W, Kelly- Hayes M, Felson DT. Sex differences in musculoskeletal pain in older adults. Pain. 2005;116:332–338. doi: 10.1016/j.pain.2005.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Souza e Silva AV, Lacativa PGS, Russo LAT, de Gregório LH, Pinheiro RAC, Marinhei LPF. Association of back pain with hypovitaminosis D in postmenopausal women with low bone mass. BMC Musculoskelet Disord. 2013;14:184. doi: 10.1186/1471-2474-14-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallagher JC, Sai A. Dose response to vitamin D supplementation in postmenopausal women. Ann Intern Med. 2012;157(5):384–385. doi: 10.7326/0003-4819-157-5-201209040-00015. [DOI] [PubMed] [Google Scholar]
- 10.Kanekar A, Sharma M, Joshi VR. Vitamin D Deficiency—A Clinical Spectrum:Is There a Symptomatic Nonosteomalacic State?. Int J Endocrinol. 2009;2010, Article ID 521457, 6 pages. [DOI] [PMC free article] [PubMed]
- 11.Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81:353–373. doi: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- 12.Bischoff-Ferrari HA, Dietrich T, Orav EJ, Hu FB, Zhang Y, Karlson EW, et al. Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged > or = 60 y. Am J Clin Nutr. 2004;80:752–758. doi: 10.1093/ajcn/80.3.752. [DOI] [PubMed] [Google Scholar]
- 13.Bischoff-Ferrari HA, Dawson-Hughes B, Willett WC, Staehelin HB, Bazemore MG, Zee RY, et al. Effect of vitamin D on falls: a metaanalysis. JAMA. 2004;291:1999–2006. doi: 10.1001/jama.291.16.1999. [DOI] [PubMed] [Google Scholar]
- 14.Tague SE, Clarke GL, Winter MK, McCarson KE, Wright DE, Smith PG. Vitamin D deficiency promotes skeletal muscle hypersensitivity and sensory hyperinnervation. J Neurosci. 2011;31(39):13728–13738. doi: 10.1523/JNEUROSCI.3637-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Boer IH. Serum 25-hydroxyvitamin D concentration and risk for major clinical disease events in a community-based population of older adults: a cohort study. Ann Intern Med. 2012;156(9):627–634. doi: 10.7326/0003-4819-156-9-201205010-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Leung DY, Richers BN, Liu Y, Remigio LK, Riches DW, et al. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase. J Immunol. 2012;188(5):2127–2135. doi: 10.4049/jimmunol.1102412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lacativa PGS, Farias ML. Osteoporosis and inflammation. Arq Bras Endocrinol Metab. 2010;54(2):123–132. doi: 10.1590/S0004-27302010000200007. [DOI] [PubMed] [Google Scholar]
- 18.Schreuder F, Bernsen R, van der Wouden JC. Vitamin D supplementation for nonspecific musculoskeletal pain in non-western immi- grants: a randomized controlled trial. Ann Fam Med. 2012;10:547–555. doi: 10.1370/afm.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bischoff HA, Borchers M, Gudat F, et al. In situ detection of 1,25-dihydroxyvitamin D receptor in human skeletal muscle tissue. Histochem J. 2001;33(1):19–24. doi: 10.1023/A:1017535728844. [DOI] [PubMed] [Google Scholar]
- 20.Jorde R, Sneve M, Figenschau Y, Svartberg J, Waterloo K. Effects of vitamin D supplementation on symptoms of depression in over- weight and obese subjects: randomized double blind trial. J Intern Med. 2008;264(6):599–609. doi: 10.1111/j.1365-2796.2008.02008.x. [DOI] [PubMed] [Google Scholar]
- 21.Kalra S, Kalra B, Khandelwal SK. Vitamin D status in patients with musculoskeletal symptoms in Haryana. India J Med Nutr Nutraceut. 2012;1:50–53. doi: 10.4103/2278-019X.94631. [DOI] [Google Scholar]
- 22.Bachali S, Dasu K, Ramalingam K, Naidu JN. Vitamin D deficiency and insulin resistance in normal and type 2 diabetes subjects. Indian J Clin Biochem. 2012 doi: 10.1007/s12291-012-0239-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stürmer T, Raum E, Buchner M, Gebhardt K, Schiltenwolf M, Richter W, et al. Pain and high sensitivity C reactive protein in patients with chronic low back pain and sciatic pain. Ann Rheum Dis. 2005;64:921–925. doi: 10.1136/ard.2004.027045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gebhardt K, Brenner H, Stürmer T, Raum E, Richter W, Schiltenwolf M, et al. The course of high-sensitive C reactive protein in correlation with pain and clinical function in patients with lumbosciatic pain and chronic low back pain - a 6 months prospective longitudinal study. Eur J Pain. 2006;10:711–719. doi: 10.1016/j.ejpain.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Park CH, Lee SH. Investigation of high-sensitivity C-reactive protein and erythrocyte sedimentation rate in low back pain patients. Korean J Pain. 2010;23(2):147–150. doi: 10.3344/kjp.2010.23.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]