Abstract
L-carnitine is popular as a potential ergogenic aid because of its role in the conversion of fat into energy. The present study was undertaken to investigate the effect of short term supplementation of L-carnitine on metabolic markers and physical efficiency tests under short term calorie restriction. Male albino rats were divided into four groups (n = 12 in each)—control, calorie restricted (CR for 5 days, 25 % of basal food intake), L-carnitine supplemented (CAR, given orally for 5 days at a dose of 100 mg/kg), CR with L-carnitine supplementation (CR + CAR). Food intake and body weight of the rats were measured along with biochemical variables like blood glucose, tissue glycogen, plasma and muscle protein and enzymatic activities of CPT-1 (carnitine palmitoyl transferase-1) and AMP kinase. Results demonstrated that L-carnitine caused marked increase in muscle glycogen, plasma protein, CPT-1 activity and swim time of rats (P < 0.05) on short term supplementation. In addition to the substantive effects caused by CR alone, L-carnitine under CR significantly affected muscle glycogen, plasma protein, CPT-1 activity and AMP kinase (P < 0.05). Short term CR along with L-carnitine also resulted in increased swim time of rats than control, CR and L-carnitine treated rats (P < 0.05). The present study was an attempt towards developing an approach for better adherence to dietary restriction regimen, with the use of L-carnitine.
Keywords: L-carnitine, Calorie restriction, Forced swim test, Carnitine palmitoyl transferase-1, AMP kinase, Glycogen
Introduction
L-carnitine named from the Latin word, flesh, was discovered in muscle tissue 100 years ago and thereafter around 20 years later was identified as 3-hydroxy-4-N, N, N-trimethylaminobutyric acid, a water soluble quaternary amine [1]. This endogenous molecule synthesized in the human body remains a subject of much interest as it is essentially involved in energy production by increased fatty acid oxidation. It is a legal supplement that has gained attention over many years. The role of L-carnitine and its derivatives in carbohydrate and lipid metabolism continues to be subject of investigations [2]. Fatty acyl moieties inside the mitochondria are used for generating ATP by β-oxidation after being transported by carnitine. Moreover, carnitine decreases acetyl-CoA/CoA SH ratio regulating the PDC flux and thereby releases the inhibition on pyruvate dehydrogenase by acetyl-CoA. This step is necessary to generate ATP aerobically by TCA cycle [3]. This has directed research towards finding out any beneficial effect of L-CAR in aerobic exercise performance [1].
Energy metabolism is also affected due to the occurrence of calorie restriction (CR), which leads to modifications in specific enzymes and signalling pathways [4]. CR exhibits a variety of improvements related to overall health through involvement of multiple metabolic pathways. The beneficial effects on glucoregulatory functions, improved insulin sensitivity have been seen in rodents and rhesus monkeys [5, 6]. Studies have shown the effects of reduced caloric intake with a cut down on calories by 20–25 % of ad libitum fed subjects [7, 8]. A few studies have reduced the intake by a fixed number of calories rather than a percentage of the regular intake. CR is typically instituted for a period of 6–12 months and longer [9]; however some studies have also investigated short term CR lasting up to 10 days [10].
Calorie restriction has also emerged as an approach for combat situations, where there is a need for light- weight calorie dense, ready to eat ration which has minimum calories that can sustain metabolic functions during the emergency situations [11]. Within the last decade, substantial research has examined the effects of including exercise in CR regime. Holloszy et al. [12] have shown that male rats do not increase their caloric intake to compensate for their exercise induced caloric expenditure and therefore are often the choice for these kinds of studies. Some studies have displayed a positive effect of exercise in addition to those displayed by CR [13] while some haven’t [14]. Only a few studies have seen the effects of calorie restriction on performance capacity. No in-depth studies have been done. Inconsistency of the earlier results and lack of conclusive evidences for the effect of calorie restriction on performance capacity led us to study the supplementation of L-carnitine under CR. L-carnitine, being an ergogenic aid might help to overcome any initial fatigue associated with low calorie diets. The purpose of this study was to evaluate the effect of L-carnitine supplementation under short term CR regime on metabolic responses and exercise capacity.
Materials and Methods
Drug
L-carnitine tablets (Carnitor-500, under license from Sigma-Tau India Pvt. Ltd., manufactured by Modi-Mundipharma Pvt. Ltd.) were purchased and used in the study. The tablets were crushed and then dissolved in deionized water and given at a dose of 100 mg/kg body weight orally to the rats.
Experimental Animals and Treatment
Male Sprague–Dawley rats, weighing 200–250 g, bred and reared in the Experimental Animal Facility of the Defence Institute of Physiology and Allied Sciences, Delhi were used in the study. Animals were maintained at a temperature of 22 ± 1 °C and a humidity of 55–60 % in light-controlled room (lights on at 6:30 h, lights off at 18:30 h). Restricted day-time feeding regimen was employed in the study for monitoring of food intake, wherein the food was provided only for 6 h during the light phase of the day. Rats were habituated to this regimen 2 weeks prior to the experimentation. Food left-over was weighed on each day. Rats were provided commercial rodent diet supplied by M/S Golden feed Pvt Ltd., Delhi and water ad libitum. All procedures and protocols used in the present study were approved by the Animal Care and Use Committee of the Institute and followed the guidelines documented in the National Institute of Health’s Guide for the Care and Use of Laboratory Animals. The animals were randomly divided into four groups prior to the beginning of the experiments—Control, CR, CAR (L-carnitine supplemented) and CR + CAR (calorie restriction with L-carnitine supplementation) (n = 12 rats in each). The control and CAR rats were given food and water ad libitum. For calorie restriction, basal food intake of the rats was monitored over 7 days and thereafter 25 % restriction was made in the diets of both the experimental groups (CR and CR + CAR). L-carnitine was administered orally at a dose of 100 mg/kg body weight to the test animals for 5 consecutive days.
Sample Collection for Biochemical Analysis
After completion of the 5 days treatment, rats were fasted overnight, anaesthetized and killed. Glucose estimation was made in whole blood. Blood plasma was collected by centrifugation at 1,000×g for 10 min at 4 °C. The weight of the organs—liver, spleen, kidney, brain was recorded along with gastrocnemius muscle and epididymal fat tissue. For glycogen estimation the weighed portions of liver were dissolved in 30 % KOH immediately after removal, precipitated with 95 % ethanol in the presence of sodium sulphate. Ten percent liver and muscle homogenates (w/v) were prepared in 150 mm KCl using Polytron homogeniser and were centrifuged at 3,000×g for 15 min at 4 °C. The supernatants were divided into aliquots and frozen at −80 °C until assayed. Liver mitochondria were separated by differential centrifugation at 12,000×g for 30 min at 4 °C and stored at −80 °C for CPT-1 (Carnitine palmitoyl transferase-1) activity analysis.
Biochemical Estimations
Blood glucose was measured using glucose oxidase–peroxidase method. Tissue glycogen was estimated using the method of Montgomery [15]. CPT-1 activity was assayed using the method of Halperin and Pande [16]. Protein content was measured by the method of Lowry et al. [17]. AMP kinase in liver homogenates was measured using kit from Cusabio biotech Co., Ltd.
Physical Performance Measurement Tests
Forelimb Grip Strength
A Grip Strength Meter (dual/single channel, dunnett firmware version 2.3) from Linton Instrumentation, UK was used to measure forelimb grip strength. It is a determinant of muscular strength and an indicator of neuromuscular function. Method used has been described previously by Ta et al. [18]. The grip strength meter was positioned horizontally and rats held by the tail were allowed to grasp the smooth, metal pull bar (forelimbs only). They were then pulled backward in the horizontal plane. The force applied to the bar at the moment the grasp was released was recorded as the peak tension (g). The test commenced 2 h after the dose administration. It was repeated three consecutive times within the same session and the highest value recorded as the grip strength of that animal. Normalized values are expressed as g/kg body weight. Rats were trained prior to testing and each rat was tested (three trials equal one test session). The test was carried out before any treatment was given on day 0 (basal data) and on day 5 after 2 h of drug supplementation during the study period.
Forced Swim Test
Swimming, the exhaustive type of exercise developed by Porsolt et al. [19] has been selected in the present study as a model of physical exercise so that muscle trauma caused by other types of exercises like prolonged running on treadmill and exercise stimulated electric shock could be avoided [20]. In this test, the animal is released in a tank of water from which there is no escape and allowed to swim till the animal is unable to surface. The period of time that the animals swim till they are unable to rise to the surface within 10 s is recorded as swimming time. The temperature of the water is maintained at room temperature (30 ± 2 °C) until they sank. The animals were removed and allowed to recover for 5 min. before putting them back to their cages [21]. This test was conducted on a separate batch of rats. The animals were divided into groups of eight animals each; with a similar mean weight of each group. The swimming test was carried out before any treatment was given on day 0 (basal data) and on day 5 after 2 h of drug supplementation.
Statistical Analysis
All the data were presented as mean ± SD. Statistical analysis was performed using Graph Pad Prism version 5.0. One-way ANOVA followed by Newman-Keuls multiple comparison tests was used to evaluate the treatment effect with in different groups. Two-way ANOVA was used for statistical analysis in the forced swim test. Any change with P < 0.05 was considered significant.
Results
Effect on Food Intake
L-carnitine did not have an effect on the food intake over the 6 h period during the 5 days. The rats supplemented with L-carnitine ate normally with a mean food intake (1–5 days) of 16.6 g/rat. This was quite similar to the mean food intake of control rats 16 g/rat. No food was left after 6 h in case of CR groups.
Effect on Body Weight and Vital Organs Weight
The rate of body weight gain in the CAR group was not different than that of the control group over the study period (Control, 5.3 %; CAR, 5 %). Following the treatment, the CR group lost weight by 6.8 % and the rate of body weight decline in the CR + CAR group was 6.6 %. The body weight change between day 0 and day 5 was found to be significantly affected in CR and CR + CAR groups as compared to the control group (P < 0.05). No significant change in the organ weights was seen in any of the groups (data not shown).
Effect on Blood Glucose
The CR group had the lowest blood glucose levels in comparison with all the three groups. No significant change was observed in the blood glucose of CAR and CR + CAR rats vs control. However, CR + CAR had marginally higher blood glucose level than CR rats (Table 1).
Table 1.
Effect of L-carnitine supplementation alone (100 mg/kg body weight) and under calorie restriction (25 %) for 5 days on blood glucose, liver and muscle glycogen, plasma and muscle protein, enzyme activities of CPT-1 and AMP kinase
| Variables | Experimental groups | |||
|---|---|---|---|---|
| Control | CR | CAR | CR + CAR | |
| Blood glucose (mg/dl) | 60.18 ± 8.32 | 52 ± 14.05 | 66.55 ± 32.16 | 59 ± 15.76 |
| Liver glycogen (mg/g wet tissue) | 0.56 ± 0.75 | 0.24 ± 0.20 | 0.77 ± 0.52 | 0.36 ± 0.30 |
| Muscle glycogen (mg/g wet tissue) | 0.14 ± 0.02 | 0.05 ± 0.24 | 0.80 ± 0.33b,d | 0.30 ± 0.19c,e,f |
| Plasma protein (g/dl) | 5. 40 ± 1.2 | 8.6 ± 1.40a | 6.35 ± 1.57d | 7.28 ± 2.03c,e |
| Muscle protein (mg/ml) | 18.97 ± 4.09 | 18.12 ± 2.30 | 17.56 ± 2.52 | 15.98 ± 2.53 |
| CPT-1 activity (umol/min/mg protein) | 12.0 ± 4.0 | 15.73 ± 6.54 | 37 ± 14.68b,d | 40 ± 22.65c,e,f |
| AMP kinase (pg/mg protein) | 309 ± 53 | 303 ± 91 | 330 ± 104 | 130 ± 41c,e,f |
Expressed as mean ± SD (n = 12). Significance was set at P < 0.05
aIndicates comparison between control and CR
bIndicates comparison between control and CAR
cIndicates comparison between control and CR + CAR
dIndicates comparison between CR and CAR
eIndicates comparison between CR and CR + CAR
fIndicates comparison between CAR and CR + CAR
Effect on Liver and Muscle Glycogen
The CAR group had the highest liver glycogen concentration as compared to all the three groups. A decline was observed in the liver glycogen of CR + CAR rats as compared to the control and CAR rats but was slightly higher than that of CR rats (Table 2). A significant increase in the muscle glycogen content of CAR against the control, CR and CR + CAR rats (P < 0.05) was noted. The CR rats—CR had lowered glycogen content as compared to control. The glycogen concentration for CR + CAR rats and CR rats were significantly lesser than that of the CAR rats (P < 0.05) (Table 1).
Table 2.
Effect of L-carnitine supplementation alone (100 mg/kg body weight) and under calorie restriction (25 %) on forelimb grip strength (g/kg) of rats
| Days | Experimental groups | |||
|---|---|---|---|---|
| Control | CR | CAR | CR + CAR | |
| Day 0 | 1.21 ± 0.27 | 1.30 ± 0.17 | 1.20 ± 0.49 | 1.16 ± 0.22 |
| Day 5 | 1.36 ± 0.21 | 1.17 ± 0.28 | 1.32 ± 0.25 | 1.23 ± 0.24 |
Expressed as mean ± SD (n = 12)
Effect on Enzyme Activities
CPT-1—The carnitine supplemented groups—CAR and CR + CAR displayed significantly raised CPT-1 activity as compared to both the control and CR groups (P < 0.05). Also, the CR groups—CR and CR + CAR showed higher activities against control and CAR rats respectively (P < 0.05) (Table 1).
AMP kinase—AMPK activity was significantly lower in the CR + CAR rats when compared against control, CR and CAR groups. There was no significant change in any of the other groups (Table 1).
Effect on Plasma and Muscle Protein
Calorie restriction caused a significant increase in the plasma protein levels. The CR group had higher levels of plasma protein as compared to control, CAR and CR + CAR rats (P < 0.05). Likewise, the other CR group—CR + CAR had higher plasma protein concentration than that of control. No significant change was observed in muscle protein among the treated groups as compared to control (Table 1).
Effect on Forelimb Grip Strength
None of the treatments induced a change in the forelimb grip strength of rats as observed on day 5 of the treatment (Table 2).
Effect on Forced Swim Test
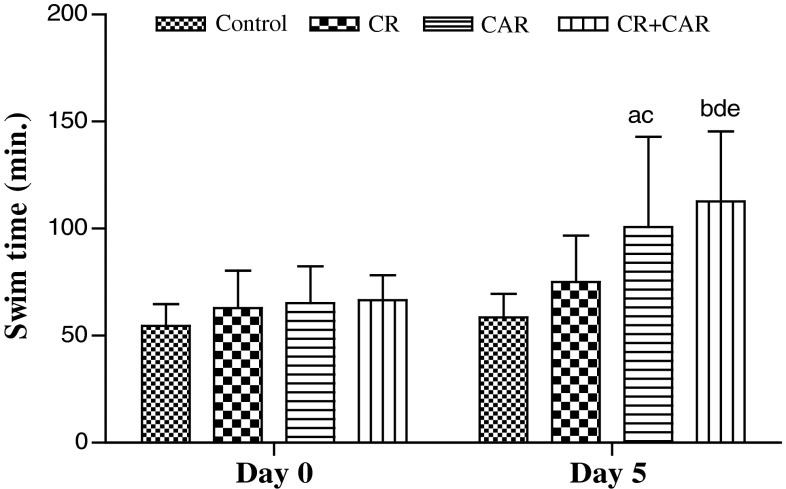
An increase in the swim time of the treated groups on day 5 was observed when compared to their basal data. Amongst the groups, there has been a significant improvement in physical performance of CAR (54 %) and CR + CAR (69 %) groups when compared to their basal swim time on day 0 (P < 0.05). It was observed that CR + CAR rats had relatively longer swim time than the rats on CR regime alone (P < 0.05) (Fig. 1).
Fig. 1.
Effect of L-carnitine supplementation (100 mg/kg body weight) and L-carnitine supplementation under calorie restriction on swim time (min) of rats on day 0 and day 5 of the study period. Expressed as mean ± SD (n = 8). ‘a’ indicates CAR group day 0 versus CAR group day 5, ‘b’ indicates CR + CAR group day 0 versus CR + CAR group day 5, ‘c’ indicates control group day 5 versus CAR group day 5, ‘d’ indicates control group day 5 versus CR + CAR day 5, ‘e’ indicates CAR group day 5 versus CR + CAR day 5. Significance was set at P < 0.05
Discussion
There are studies that suggest that L-carnitine supplementation may be beneficial in treating obesity, improving total energy expenditure and can affect body composition of animals [22]. Body weight regulation is controlled by numerous metabolic pathway intermediates and any major shift or imbalance in the energy homeostasis causes significant changes in body weight, as it is determined by both the energy intake and the expenditure [23]. Only a few studies report so far the effect of L-carnitine on weight reduction on an energy-reduced diet. As was expected on a calorie reduced diet, the rats lost a certain amount of weight. Brandsch and Eder [22] have shown no positive effect of L-carnitine supplementation on weight loss and body composition of rats fed an energy-deficient diet.
Early research suggests that although L-carnitine has a well-known action in fatty acid oxidation it is also central in carbohydrate metabolism [24]. It might lower the blood glucose levels by increasing its oxidation in cells. L-carnitine supplementation has shown improvement in glucose intolerance in conditions like obesity and diabetes [25]. However, the results are controversial as some researchers suggest it has no role in regulation of glucose oxidation [24], some say it decreases blood glucose [26, 27] while others suggest it increases [28, 29]. The lowered blood glucose levels in CR rats are inevitable as an early response to caloric restriction in mammals [30] as also noticed in the present study. Molfino et al. [31] has also suggested an increased intestinal uptake of carnitine during CR results in improved insulin resistance. In the present study, the blood glucose levels of carnitine supplemented rats were not affected significantly in normal as well as in CR state. This could be due to short study duration. Another study done by Bloomer et al. [32] with 8 weeks of supplementation did not show statistically significant improvement in fasting blood glucose.
According to Cederblad et al. [33] carnitine has a ‘glycogen sparing’ action and can act as an anti-catabolic agent to enhance energy production from fats thereby effectively reducing the need to burn glycogen. L-carnitine is an important factor in glycogen synthesis and ATP production [34] and its concentration in muscle is directly proportional to muscle glycogen stores [35]. This explains the rise in muscle and liver glycogen stores of the CAR treated groups in this study. A depletion of the glycogen reserves in the CR rats was observed as a result of energy reduced diet. L-carnitine feeding does not substantially leads to other beneficial effects except small increase in glucose levels, but does result in increased muscle and liver glycogen content. This increase is no longer observed in CR + CAR, thus suggesting the possible beneficial effects of CR + CAR.
Food intake immediately influences the nutritional status of the organism, as does the level of plasma proteins [36]. The plasma concentration of proteins is affected by the balance between their rates of synthesis and breakdown. Declining food intake does not correlate with low serum protein levels and vice versa [37]. Miller et al. [38] has demonstrated no effect of acute feeding (4 h) on protein synthesis. Decrease in plasma volume on a CR regime causes hypohydration, which thereby leads to an overall decrease in the blood volume. The negative water balance and decreased plasma volume is accompanied by an increase in plasma protein concentration [39]. This describes the upsurge in the plasma protein concentration of the energy restricted rats in the present study. L-carnitine supplementation does not seem to affect plasma protein levels as no change from the control was observed in the CAR group.
Increased fatty acid oxidation and reduced synthesis are hypothesised to occur during CR [40]. The hepatic lipid metabolism is controlled by AMPK in a large part through modulating acetyl CoA carboxylase and carnitine palmitoyl transferase pathway [41]. L-carnitine is an important regulator of lipid metabolism and its supplementation enhances CPT-1 activity [42]. The rise in the CPT-1 activity could have occurred as a result of calorie restriction and carnitine supplementation in the present study. The extent of the increase in CPT-1 activity may vary depending on the dose and duration of the study.
In conditions of low energy charge, AMP Kinase gets activated. AMPK detects shifts in AMP:ATP ratio and therefore when ATP is abundant AMPK has lower activity. Being a metabolic stress sensing protein kinase, it controls the energy balance and increases fatty acid oxidation [43]. It is also a glycogen sensor and may be inhibited by glycogen [44]. L-carnitine is responsible for generating ATP and glycogen [45]. In agreement with this, lowered AMPK activity of CR + CAR group of rats was observed.
The ATP generation on L-carnitine supplementation might have brought down the AMPK activity. This effect was however not seen on supplementation of L-carnitine alone in the present study. Both, AMPK activation and fatty acid oxidation are correlated, especially in rodents. Despite this association incongruity exists between them [43]. This might support the trend observed in CPT-1 and AMPK activity in this study. AMPK coordinates the interaction between peripheral and central energy regulation [46]. It has a vital role to play during exercise as well. Spiering et al. [47] showed that carnitine attenuates metabolic stress that occurs after exercise. Numerous studies suggest beneficial effects of increased carnitine intake in reducing physical fatigue and recovery from exercise stress [48, 49]. Conversely, some studies have shown no significant positive effects in exercise performance with carnitine [50, 51]. Calorie restriction has positive effects on physical performance [52]. In the present study, the longer swimming time of rats could be based on all of the above reasons. However, it was observed that both the short term feeding treatments—CAR and CR + CAR boost endurance more than CR (or no food restriction). The insignificant increase in the swim time exhibited by the control rats depicts the learning behavioral adaptation within the rats for the task.
The total muscle protein concentration remained unaltered in the present study. According to Fuster et al. [53] muscle protein synthesis is only significantly affected when there is complete omission of calories from the diet. Muscle function correlates closely with whole body protein, loss of weight or muscle mass [54]. Hand grip strength is an indicator of upper limb strength and reflects the maximum strength derived from combined contractions of extrinsic and intrinsic hand muscles. In the present study, no changes in the forelimb grip strength of the rats were observed. This might suggest that neither the CR regime employed nor the L-carnitine supplementation affected the muscle function. Increasing the dosage used in the study might lead to an enhanced effect as it is known from the past decade that L-carnitine has a metabolic role in skeletal muscle which if manipulated can have a significant impact on physiological function.
In conclusion, the present study has shown the biochemical changes and the metabolic responses induced along with the changes in physical performance to L-carnitine supplementation under short term calorie restriction. The short term administration of L-carnitine has shown performance enhancing properties even under calorie restriction. This implies that if given for a longer period it may exert a pronounced effect. The present study was an attempt towards developing an approach with the use of L-carnitine for better adherence to dietary restriction regimens like weight management, war like situations and in situations of food shortage.
Acknowledgment
Authors are thankful to DIPAS, Defence Research and Development Organisation (DRDO) for the financial support of the study.
References
- 1.Stephens FB, Constantin-Teodosiu D, Greenhaff PL. New insights concerning the role of carnitine in the regulation of fuel metabolism in skeletal muscle. J Physiol. 2007;581(2):431–444. doi: 10.1113/jphysiol.2006.125799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malaguarnera M. Carnitine derivatives: clinical usefulness. Curr Opin Gastroenterol. 2012;28(2):166–176. doi: 10.1097/MOG.0b013e3283505a3b. [DOI] [PubMed] [Google Scholar]
- 3.Hongu N, Sachan DS. Carnitine and choline supplementation with exercise alter carnitine profiles, biochemical markers of fat metabolism and serum leptin concentration in healthy women. J Nutr. 2003;133(1):84–89. doi: 10.1093/jn/133.1.84. [DOI] [PubMed] [Google Scholar]
- 4.Redman LM, Ravussin E. Caloric restriction in humans: impact on physiological, psychological and behavioural outcomes. Antioxid Redox Signal. 2011;14(2):275–287. doi: 10.1089/ars.2010.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Z, Masternak MM, Al-Regaiey KA, Bartke A. Adipocytokines and the regulation of lipid metabolism in growth hormone transgenic and calorie restricted mice. Endocrinology. 2007;148(6):2845–2853. doi: 10.1210/en.2006-1313. [DOI] [PubMed] [Google Scholar]
- 6.Bonkowski MS, Rocha JS, Masternak MM, Al Regaiey KA, Bartke A. Targeted disruption of growth hormone receptor interferes with the beneficial actions of calorie restriction. Proc Natl Acad Sci. USA. 2006;103:7901–5. [DOI] [PMC free article] [PubMed]
- 7.Civitarese AE, Carling S, Heilbronn LK, Hulver MH, Ukropcova B, Deutsch WA, Smith SR, Ravussin E, CALERIE Pennington Team Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med. 2007;4(3):e76. doi: 10.1371/journal.pmed.0040076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lefevre M, Redman LM, Heilbronn LK, Smith JV, Martin CK, Rood JC, Greenway FL, Williamson DA, Smith SR, Ravussin E, Pennington CALERIE team Caloric restriction alone and with exercise improves CVD risk in healthy non-obese individuals. Atherosclerosis. 2009;203(1):206–213. doi: 10.1016/j.atherosclerosis.2008.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Willcox BJ, Willcox DC, Todoriki H, Fujiyoshi A, Yano K, He Q, Curb JD, Suzuki M. Caloric restriction, the traditional Okinawan diet, and healthy aging: the diet of the world’s longest-lived people and its potential impact on morbidity and life span. Ann N Y Acad Sci. 2007;1114(1):434–455. doi: 10.1196/annals.1396.037. [DOI] [PubMed] [Google Scholar]
- 10.Jung KJ, Lee EK, Kim JY, Zou Y, Sung B, Heo HS, Kim MK, Lee J, Kim ND, Yu BP, Chung HY. Effect of short term calorie restriction on pro-inflammatory NF-Kb and AP-1 in aged rat kidney. Inflamm Res. 2009;58(3):143–150. doi: 10.1007/s00011-008-7227-2. [DOI] [PubMed] [Google Scholar]
- 11.Committee on Military Nutrition Research, Institute of Medicine, Not eating enough. Institute of Medicine: The National Academy Press; 1995. [Google Scholar]
- 12.Holloszy JO. Mortality rate and longevity of food restricted exercising male rats: a reevaluation. J Appl Physiol. 1997;82(2):03–399. doi: 10.1152/jappl.1997.82.2.399. [DOI] [PubMed] [Google Scholar]
- 13.Kim JH, Kwak HB, Leeuwenburgh C, Lawler JM. Lifelong exercise and mild (8%) calorie restriction attenuate age-induced alterations in plantaris muscle morphology, oxidative stress and IGF-1 in the Fischer-344 rat. Exp Gerantol. 2008;43:317–329. doi: 10.1016/j.exger.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huffman DM, Moellering DR, Grizzle WE, Stockyard CR, Johnson MS, Nagy TR. Effect of exercise and calorie restriction on biomarkers of aging in mice. Am J Physiol Regul Integr Comp Physiol. 2008;294(5):R1618–R1627. doi: 10.1152/ajpregu.00890.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montgomery R. Determination of glycogen. Arch Biochem Biophys. 1957;67(2):378–386. doi: 10.1016/0003-9861(57)90292-8. [DOI] [PubMed] [Google Scholar]
- 16.Halperin ML, Pande SV. Fatty acyl group transport into mitochondria: carnitine palmitoyltransferase EC 2.3.1.23 and carnitine–acylcarnitine translocase. Methods Enzymol. 1979;56:368–378. doi: 10.1016/0076-6879(79)56034-0. [DOI] [PubMed] [Google Scholar]
- 17.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193(1):265–275. [PubMed] [Google Scholar]
- 18.Ta LE, Low PA, Windebank AJ. Mice with cisplatin and oxaliplatin-induced painful neuropathy develop distinct early responses to thermal stimuli. Mol Pain. 2009;5(1):9. doi: 10.1186/1744-8069-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Porsolt RD, Bertin A, Jalfre M. Behavioural despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229(2):327–336. [PubMed] [Google Scholar]
- 20.Venditti P, Piro MC, Artiaco G, Di Meo S. Effect of exercise on tissue antioxidant capacity and heart electrical properties in male and female rats. Eur J Appl Physiol Occup Physiol. 1996;74(4):322–329. doi: 10.1007/BF02226928. [DOI] [PubMed] [Google Scholar]
- 21.Duraisami R, Mohite VA, Kasbe AJ. Antistress adaptogenic activity of standardized dried fruit extract of Aegle marmelos against diverse stressors. Asian J Pharm Clin Res. 2010;3(4):1–3. [Google Scholar]
- 22.Brandsch C, Eder K. Effect of L-carnitine on weight loss and body composition of rats fed a hypocaloric diet. Ann Nutr Metab. 2002;46(5):205–210. doi: 10.1159/000065408. [DOI] [PubMed] [Google Scholar]
- 23.Dokken BB and Tsao TS. The physiology of body weight regulation: are we too efficient for our own good? Diabetes Spectr. 2007;20:166–70.
- 24.De Gaetano A, Mingrone G, Castagneto M, Calvani M. Carnitine increases glucose disposal in humans. J Am Coll Nutr. 1999;18(4):289–295. doi: 10.1080/07315724.1999.10718866. [DOI] [PubMed] [Google Scholar]
- 25.Ringseis R, Keller J, Eder K. Role of carnitine in the regulation of glucose homeostasis and insulin sensitivity: evidence from in vivo and in vitro studies with carnitine supplementation and carnitine deficiency. Eur J Nutr. 2012;51(1):1–18. doi: 10.1007/s00394-011-0284-2. [DOI] [PubMed] [Google Scholar]
- 26.White TW, Gentry LR, Gentry GT, Fernandez JM, Chapa AM, Blouin DC. Effect of urea, fish meal and carnitine in liquid supplement on growth and metabolites of grazing calves. J Anim Sci. 1997;75:262. [Google Scholar]
- 27.Hadadinezhad S, Ghazaleh N, Razavi Z. Effects of l-carnitine on glycemic control and C-peptide levels in patients with type 2 diabetes mellitus. Turk Jem. 2008;12:1–3. [Google Scholar]
- 28.Chapa AM, Fernandez JM, White TW, Bunting LD, Gentry LR, Ward TL, Blum SA. Influence of intravenous l-carnitine administration in sheep preceding an oral urea drench. J Anim Sci. 1998;76:2930–2937. doi: 10.2527/1998.76112930x. [DOI] [PubMed] [Google Scholar]
- 29.Cetin M, Petek M, Polat U, Yalcin A. Effects of dietary carnitine supplementation on plasma carnitine and some serum biochemical parameters in lambs. Rev Méd Vét. 2003;154:195–198. [Google Scholar]
- 30.Mohamad-Shahi M, Karandish M, Haidari F, Omidian K, Fatemi-Tabatabayei SR, Rafiei H. Effect of daidzein-low-calorie diet on body weight, serum levels of glucose, resistin, and high sensitive C-reactive protein in high fat, high calorie diet induced rats. Saudi Med J. 2012;33:70–75. [PubMed] [Google Scholar]
- 31.Molfino A, Cascino A, Conte C, Ramaccini C, Rossi Fanelli F, Laviano A. Caloric restriction and l-carnitine administration improves insulin sensitivity in patients with impaired glucose metabolism. J Parenter Enter Nutr. 2010;34:295–299. doi: 10.1177/0148607109353440. [DOI] [PubMed] [Google Scholar]
- 32.Bloomer RJ, Fisher-Wellman KH, Tucker PS. Effect of oral acetyl l-carnitine arginate on resting and postprandial blood biomarkers in pre-diabetics. Nutr Metab (Lond). 2009;6:25. [DOI] [PMC free article] [PubMed]
- 33.Cederblad G, Bylund AC, Holm J, Scherstén T. Carnitine concentration in relation to enzyme activities and substrate utilization in human skeletal muscles. Scand J Clin Lab Invest. 1976;36:547–552. doi: 10.3109/00365517609054477. [DOI] [PubMed] [Google Scholar]
- 34.Nishida N, Sugimoto T, Takeuchi T, Kobayashi Y. Effect of l-carnitine on glycogen synthesis and ATP production in cultured hepatocytes of the newborn rat. J Nutr. 1989;119:1705–1708. doi: 10.1093/jn/119.11.1705. [DOI] [PubMed] [Google Scholar]
- 35.Brevetti G, Fanin M, De Amicis V, Carrozzo R, Di Lello F, Martone VD, Angelini C. Changes in skeletal muscle histology and metabolism in patients undergoing exercise deconditioning: effect of propionyl-l-carnitine. Muscle Nerve. 1997;20:1115–1120. doi: 10.1002/(SICI)1097-4598(199709)20:9<1115::AID-MUS4>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 36.Young VR, Marchini JS, Cortiella J. Assessment of protein nutritional status. J Nutr. 1990;120:1496–1502. doi: 10.1093/jn/120.suppl_11.1496. [DOI] [PubMed] [Google Scholar]
- 37.Fuhrman MP, Charney P, Mueller CM. Hepatic proteins and nutrition assessment. J Am Diet Assoc. 2004;104:1258–1264. doi: 10.1016/j.jada.2004.05.213. [DOI] [PubMed] [Google Scholar]
- 38.Miller BF, Robinson MM, Reuland DJ, Drake JC, Peelor FF, III, Bruss MD, Hamilton KL. Calorie restriction does not increase short-term or long-term protein synthesis. J Gerontol A. 2013;68:530–538. doi: 10.1093/gerona/gls219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Consolazio CF, Matoush LO, Johnson HL, Krzywicki HJ, Isaac GJ, Witt NF. Metabolic aspects of calorie restriction: hypohydration effects on body weight and blood parameters. Am J Clin Nutr. 1968;21:793–802. doi: 10.1093/ajcn/21.8.793. [DOI] [PubMed] [Google Scholar]
- 40.Bruss MD, Khambatta CF, Ruby MA, Aggarwal I, Hellerstein MK. Calorie restriction increases fatty acid synthesis and whole body fat oxidation rates. Am J Physiol Endocrinol Metab. 2010;298:E108–E116. doi: 10.1152/ajpendo.00524.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo H, Liu G, Zhong R, Wang Y, Wang D, Xia M. Cyanidin-3-O-β-glucoside regulates fatty acid metabolism via an AMP-activated protein kinase-dependent signaling pathway in human HepG2 cells. Lipids Health Dis. 2012;11:10. doi: 10.1186/1476-511X-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xi L, Brown K, Woodworth J, Shim K, Johnson B, Odle J. Maternal dietary L-carnitine supplementation influences fetal carnitine status and stimulates carnitine palmitoyltransferase and pyruvate dehydrogenase complex activities in swine. J Nutr. 2008;138:2356–2362. doi: 10.3945/jn.108.095638. [DOI] [PubMed] [Google Scholar]
- 43.Steinberg GR, Kemp BE. AMPK in health and disease. Physiol Rev. 2009;89:1025–1078. doi: 10.1152/physrev.00011.2008. [DOI] [PubMed] [Google Scholar]
- 44.McBride A, Ghilagaber S, Nikolaev A, Hardie DG. The glycogen-binding domain on the AMPK beta subunit allows the kinase to act as a glycogen sensor. Cell Metab. 2009;9:23–34. doi: 10.1016/j.cmet.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Owen L, Sunram-Lea SI. Metabolic agents that enhance ATP can improve cognitive functioning: a review of the evidencefor glucose, oxygen, pyruvate, creatine, and l-carnitine. Nutrients. 2011;3:735–755. doi: 10.3390/nu3080735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perrin C, Knauf C, Burcelin R. Intracerebroventricular infusion of glucose, insulin, and the adenosine monophosphate-activated kinase activator, 5-aminoimidazole-4-carboxamide-1-beta-d-ribofuranoside, controls muscle glycogen synthesis. Endocrinology. 2004;145:4025–4033. doi: 10.1210/en.2004-0270. [DOI] [PubMed] [Google Scholar]
- 47.Spiering BA, Kraemer WJ, Vingren JL, Hatfield DL, Fragala MS, Ho JY, Maresh CM, Anderson JM, Volek JS. Responses of criterion variables to different supplemental doses of l-carnitine l-tartrate. J Strength Cond Res. 2007;21:259–264. doi: 10.1519/00124278-200702000-00046. [DOI] [PubMed] [Google Scholar]
- 48.Kraemer WD, Volek JS, Spiering BA, Vingren JL. l-carnitine supplementation: a new paradigm for its role in exercise. Monatsh Chem. 2005;136:1383–1390. doi: 10.1007/s00706-005-0322-y. [DOI] [Google Scholar]
- 49.Spasov AA, Iezhitsa IN, Kravchenko MS, Pisarev VB, Snigur GL. Effects of l-, d-, and dl-carnitine on morphometric parameters of skeletal muscle and exercise performance of laboratory animals receiving carnitine-deficient diet. Bull Exp Biol Med. 2006;142:458–460. doi: 10.1007/s10517-006-0391-x. [DOI] [PubMed] [Google Scholar]
- 50.Brass EP. Carnitine and sports medicine: use or abuse? Ann N Y Acad Sci. 2004;1033:67–78. doi: 10.1196/annals.1320.006. [DOI] [PubMed] [Google Scholar]
- 51.Pekala J, Patkowska-Sokoła B, Bodkowski R, Jamroz D, Nowakowski P, Lochynski S, Librowski T. l-carnitine—metabolic functions and meaning in humans life. Curr Drug Metab. 2011;12:667–678. doi: 10.2174/138920011796504536. [DOI] [PubMed] [Google Scholar]
- 52.Gramignano G, Lusso MR, Madeddu C, Massa E, Serpe R, Deiana L, Lamonica G, Dessì M, Spiga C, Astara G, Macciò A, Mantovani G. Efficacy of l-carnitine administration on fatigue, nutritional status, oxidative stress, and related quality of life in 12 advanced cancer patients undergoing anticancer therapy. Nutr J. 2006;22:136–145. doi: 10.1016/j.nut.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 53.Fuster G, Busquets S, Almendro V, López-Soriano FJ, Argilés JM. Antiproteolytic effects of plasma from hibernating bears: a new approach for muscle wasting therapy? Clin Nutr. 2007;26:658–661. doi: 10.1016/j.clnu.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 54.Peng S. Plank LD, McCall JL, Gillanders LK, Mcllroy K, Gane EJ. Body composition, muscle function, and energy expenditure in patients with liver cirrhosis: a comprehensive study. Am J Clin Nutr. 2007;85:1257–1266. doi: 10.1093/ajcn/85.5.1257. [DOI] [PubMed] [Google Scholar]