Abstract
Human urine gives evidence of the metabolism in the body and contains numerous organic acids and other compounds at a variety of concentration. The concentration of organic acids in urine varies from population to population due to genotype, food habits and other epigenetic and environmental influences. Knowledge of the reference values for urinary organic acids in a healthy pediatric population is very important for critical evaluation. This study was designed to quantify 16 organic acids in a healthy north Indian pediatric population. Early morning urine samples from healthy pediatric subjects of age 1 day to 16 years who did not have symptoms of any disease were analyzed for organic acid content. The children were not on any supplemental vitamins or drugs and were on a free and unrestricted diet. The creatinine concentration of each sample was determined before organic acid analysis. Organic acids were extracted from urine with ethyl acetate, extracted residue was air dried, converted into trimethylsilyl derivatives and analysed by gas chromatography mass spectrometry. Here we reported the age wise mean values and standard deviations for each compound, adjusted for creatinine content (mmol/mol of creatinine). We found the concentration of most of the metabolites are higher in our population in comparison to other populations. Such data may help to provide a basis for diagnosing metabolic abnormalities in patients in a specific ethnicity.
Keywords: Urinary organic acids, Gas chromatography mass spectroscopy, Healthy pediatric population
Introduction
Genetic disorders are the major cause of newborn deaths. Inborn errors of metabolism (IEM) are a group of genetic diseases in which the body is unable to metabolize proteins, fats or carbohydrates and as a result organic compounds are accumulated in the body fluids such as urine, blood and CSF. IEM are caused primarily by mutation of a single gene which results in deficiency of a specific enzyme activity that leads to disturbance of biochemical reactions. If these diseases are not diagnosed and treated prior to onset or soon after onset, these patients may die in the newborn period or may be left with neurological deficits like mental-motor retardation. It is therefore essential to detect the affected children rapidly in order to institute the treatments that are available for some of these disorders. Also detection of these disorders can facilitate prenatal diagnosis in subsequent pregnancies as most of them are inherited in an autosomal recessive manner with an incidence of <1 in 1,000 [1]. However the actual rate may be higher, because a newborn may die before the condition is ever diagnosed.
Human urine gives evidence of the metabolism in the body and contains numerous organic acids and other chemical compounds at a variety of concentrations. Gas chromatography mass spectrometry (GC–MS) was one of the latest modern biochemical techniques for analysis of IEM. This method was first applied to diagnose IEM in 1966 by Tanaka et al. [2]. Many IEM in which organic acids accumulate in urine, have been discovered using GC–MS. GC–MS is indispensable for both qualitative and quantitative analysis of urinary metabolites.
For making a diagnosis and interpreting test results, reference ranges for urinary organics in a healthy pediatric population are required. For diagnosing IEM it is very important to determine the normal concentration of organic acids in the urine of healthy pediatric populations. The concentration of organic acids in urine varies from population to population due to genetic factors, food habits and other influences. There are very few reports in the literature concerning reference ranges in healthy newborns [3–5], children [5–8] and adults [9, 10] and none exist for Indian newborns and children. In the Indian context no reference range has been established for urinary organic acids. We in our lab have established and now report the means and standard deviations of 16 organic acids that are present in the urine of a healthy pediatric population.
Materials and Methods
This study was carried out at Maulana Azad Medical College and associated Lok Nayak Hospital, New Delhi, India. The study subjects were drawn from a healthy pediatric population of age 1 day to 16 years who did not have any symptoms of disease, were not on any supplemental vitamins or drugs and on a free and unrestricted diet. The children were clinically in good health. The ethical clearance certificate for the study was obtained from the Institutional ethical committee. The study participants completed questionnaires on their general background like medical and family histories during their baseline visit. These participants were also evaluated for the presence of various risk factors like behavioral problems using a standard questionnaire. The questionnaire consisted of socio-demographic factors such as name, gender and address, contact number, antenatal history, prenatal history, history of metabolic disorders, family history, socioeconomic history and questions concerning urinary symptoms.
10 ml morning urine was collected into urine culture bottles or into clean glass containers for urinary profiling. These specimens were stored frozen at −20 °C until use. The creatinine concentration of each sample was determined before organic acid analysis. The creatinine was determined by Jaffe’s method.
The organic acids were quantitated as earlier described [11] with slight modifications: In a glass tube, urine equivalent to 0.25 mg of creatinine, 40 μl of internal standard (100 μmol/l of tropic acid in methanol) was taken and the pH was adjusted to 14 with 7.5 mol/l NaOH. After that 500 μl of 50 g/l aqueous hydroxylamine hydrochloride solution was mixed for the oximation of keto groups. The solution was incubated at 60 °C for 30 min in an oven. The cooled solution was adjusted to pH 1 with 6 mol/l HCl, saturated with 1 g of NaCl, vortex mixed for 2 min and extracted with 6 ml of ethyl acetate. The organic phase was transferred into a second glass tube containing sodium sulphate and further transferred into a PFTA glass vail and evaporated to dryness at 60 °C. Derivatization of the organic acids was accomplished by 100 μl of BSTFA + TMCS: pyridine (1:1 by vol.) solution.The reaction mixture was kept at 80 °C for 10 min. One micro liter of the derivatized organic acid extract was injected into the GC–MS manually.
GC–MS
The analyses were performed on a gas chromatograph (Agilent Technologies, 7890 GC system) coupled to a mass selective detector (Agilent Technologies, 5975C inert XL EI/CI MSD with Triple-Axis Detector) and ChemStation software (E.02.00.493, Agilent Technologies). A HP-5MS 5 % phenyl methyl silox (30 m × 250 μM × 0.25 μM) column was used as stationary phase. Helium was used as carrier gas at a linear velocity of 36.445 cm/s and the injector split ratio was set to 1:10. The scan range was m/z 30–500, which allowed 3.09 scans/s in TIC mode. The oven program was started at 50 °C with initial holding for 3 min and was increased at the rate of 10 °C/min to 140 °C with a hold for 1 min, and then it was increased at the rate of 20 °C/min to 280 °C, with a final hold for 3 min. The total run time was 23 min. The temperatures of the injector port and transfer line were both 250 °C.
Standard Curve for Method Comparison
For quantitation we prepared a standard curve for 14 organic acids normally present in urine, for this we prepared stock solutions of 20 mM concentrations of each compound. The calibrators were purchased from Sigma Aldrich. Then a master mix of all 14 compounds at a final concentration of 200 μM was prepared by adding 100 μl of each solution and final volume was made up to 10 ml with distilled water. Dilutions of different concentrations (25, 50, 75 and 100 μM/l) were prepared from this master mix and extracted from an aqueous calibration mixture through the procedure described for use with urine samples. Internal standard tropic acid was also added to all calibration mixtures. The signal from the MSD and the whole mass spectra in the mass range 30–500 amu were recorded simultaneously [12].
The identities of the peaks with reference spectra were confirmed by computerized comparison of the mass spectra underlying the peaks with the reference spectra of the NIST mass spectra library. The correct retention time and, for data analysis, one product ion mass were determined for each of the marker compounds and the presence of a peak with the expected retention time was confirmed. Quantification of the organic acids was based on the specific ion masses (Table 1) those of internal standards versus m/z 118 or 280 of tropic acid, according to the ion being quantified. The ratio of response factor of the parent ion and qualifier ion for a compound was used to quantify analytes whose pure standards were available to us. The analytes for which we did not have pure standards were assigned a relative response factor that corresponded to the response factor for the internal standard. The urinary concentrations of organic acids in relation to creatinine (mmol/mol creatinine) were calculated from peak area ratios of the unknown versus the internal standard.
Table 1.
Organic acids with their respective retention time and specific ion masses used for their identification and quantification by GC–MS
| Organic acid | Precursor ion | Product ion | Retention time (in min) |
|---|---|---|---|
| Lactic acid | 219 | 191 | 9.128 |
| Oxalic acid | 219 | 190 | 10.295 |
| Pyruvic acid | 247 | 232 | 10.592 |
| Methylmalonic acid | 247 | 218 | 11.554 |
| Ethylmalonic acid | 261 | 217 | 12.531 |
| 2-Ketocaproic acid | 274 | 247 | 13.362 |
| Fumaric acid | 245 | 217 | 13.558 |
| Lactic acid dimer | 291 | 262 | 14.211 |
| Glutaric acid | 276 | 261 | 14.325 |
| 3-Methylglutaric acid | 275 | 247 | 14.573 |
| Succinylacetone | 212 | 182 | 14.880 |
| Adipic acid | 275 | 217 | 15.405 |
| Suberic acid | 303 | 217 | 16.981 |
| Azelaic acid | 317 | 217 | 17.544 |
| Sebacic acid | 331 | 215 | 18.095 |
| Orotic acid | 326 | 311 | 17.28 |
We analysed 100 normal urine samples from a healthy pediatric population of age group 1 day to 16 years. Out of these 100 pediatric subjects, 59 were male and 41 were female. The samples we analyzed were divided into four groups according to the age: 1–45 days (n = 10, breast feeding and toddlers), 45 days to 1 year (n = 6), 3–6 years (n = 15, children of preschool age), 6–12 years (n = 48, children of school age) and 12–16 years (n = 12, teenagers). After eliminating outlier values from the data, the Kolmogorov–Smirnov test was used for the normality of the distribution. Most of the results were acceptable Gaussian distributions. The results that were not in Gaussian distributions were transformed to Gaussian distributions after logarithmic transformation. The results are presented as mean ± 2SD in the units of Gaussian distribution. The normal range was calculated as the average of ratios of the area of the analyte over the area of internal standard derived from normal urines, corrected by the response factors, plus two standard deviations for each of the target compounds. We used tropic acid as internal standard and also attempted to compare the recovery of organic acids from aqueous mixture and matrix based calibrators. Six duplicate sets of calibration urine and aqueous calibration solution were analyzed in the same daily runs. Interday as well as intra-day precision was estimated for aqueous calibrator and urine calibrator over 2 months and this experiment is repeated after every 6 months. Both calibration urine and aqueous calibration solution contained the same concentrations of organic acids, after the endogenous urinary concentration were subtracted. Inter day variation and intraday variation in the recovery of organic acid was also estimated for 2 months and the variation was <10 %. For purposes of internal quality control some of these samples were run at St. Louis University USA for validation.
Results
The means and standard deviations of 16 urinary organic acids for each age group in healthy north Indian pediatric populations are listed in Table 2. Many metabolites show an irregular pattern of excretion: lactate, oxalate, pyruvate, ethylmalonic acid, 3 phenylbutyric acid, and succinylacetone were in this group. Adipic acid, suberic acid and sebacic acid show a decreased excretion with age in the initial two age groups and an increase in the 3rd age group and again a decrease in older age group. Azelaic acid, 3-methylglutaric acid and glutaric acid show a similar pattern of excretion. The excretion of fumaric acid, lactic acid and orotic acid decreased with age. Methylmalonic acid showed a bimodal excretion pattern. A typical chromatogram of a normal urine sample is shown in Fig. 1. We have identified the retention time of 79 compounds which we found in normal urine sample (Table 3), 75 % of the compounds were present in all urine samples and the rest, 25 %, were present in only some samples. The retention time of 53 compounds were identified by the computerized comparisons of mass spectra lying within the peak with the NIST mass spectra library. The library match that showed a qualitative match of more than 80 % were considered for the identification of retention times and the rest were ignored. Table 3 shows the trimethylsilyl ester form of the compounds identified in the NIST mass spectrum library match, the respective retention time of the compounds and their qualifier ions. Some compounds eluted out at the same retention times, like aconitic acid and orotic acid. We observed that the peak area of lactic acid dimer was directly proportional to the peak area of lactic acid in most of the urine sample. Hippuric acid was detected in all urine samples but the peak height varied greatly. Phosphate was extracted along with the organic acids in most of the urine samples but it did not interfere with the analysis. Figure 2 shows a chromatogram of a urine sample spiked with 16 organic acids. Peaks corresponding to the acids being quantitated are numbered in the order of increasing retention time. Table 4 depicts the relative recovery and precision of organic acids added in aqueous calibrator and urine calibrator.
Table 2.
Mean and 2SD values obtained for 16 organic acids quantitated by GC–MS in four age groups of healthy north Indian pediatric population, concentration of organic acids is given in mmol/mol of creatinine
| Analyte | 1–45 days | 45 days to 1 year | 1–3 years | 3–6 years | 6–12 years | 12–16 years |
|---|---|---|---|---|---|---|
| n = 10 | n = 6 | n = 9 | n = 15 | n = 48 | n = 12 | |
| Mean ± 2SD (range) | Mean ± 2SD (range) | Mean ± 2SD (range) | Mean ± 2SD (range) | Mean ± 2SD (range) | Mean ± 2SD (range) | |
| Lactate | 34.9 ± 22.8 (12.1–57.7) | 21.8 ± 13.4 (8.4–35.2) | 22.5 ± 18 (4.5–40.5) | 9.8 ± 3.2 (3.6–13.0) | 14.8 ± 19.4 (0.0–34.2) | 6.9 ± 7.8 (0.0–14.7) |
| Oxalic acid | 96.3 ± 85.8 (10.5–182.1) | 160.7 ± 10.8 (149.9–171.5) | 85.0 ± 87.4 (0.0–172.4) | 38.4 ± 55.6 (0.0–94.0) | 149.8 ± 180.8 (0.0–330.6) | 69.5 ± 123.2 (0.0–192.2) |
| Pyruvate | 23.1 ± 18.8 (4.3–41.9) | 12.3 ± 1.4 (10.9–13.7) | 20.1 ± 25.0 (0.0–45.1) | 12.9 ± 12.4 (0.5–25.3) | 27.9 ± 40.4 (0.0–68.3) | 17.2 ± 23.6 (0.0–40.8) |
| Methylmalonic acid | 23.7 ± 13.2 (10.5–36.9) | 38.0 ± 27.2 (10.8–65.2) | 29.1 ± 27.8 (1.3–56.9) | 42.5 ± 67.0 (0.0–109.5) | 21.4 ± 13.2 (8.2–34.6) | 21.2 ± 28.4 (0.0–49.6) |
| Ethylmalonic acid | 31.8 ± 23.2 (8.6–55.0) | 24.9 ± 17.4 (7.5–42.3) | 48.1 ± 30.4 (17.7–78.5) | 18.5 ± 21.0 (0.0–39.5) | 27.9 ± 35.0 (0.0–62.9) | 8.6 ± 11.6 (0.0–20.2) |
| Fumaric acid | 19.2 ± 16.8 (2.4–36.0) | 23.3 ± 17.4 (5.9–40.7) | 11.4 ± 8.8 (2.6–20.2) | 10.0 ± 10.6 (0.0–20.6) | 12.7 ± 19.0 (0.0–31.7) | 5.6 ± 6.4 (0.0–12.0) |
| Lactic acid dimer | 15.6 ± 6.4 (9.2–22.0) | 19.4 ± 10.6 (8.4–30.0) | 16.2 ± 7.6 (8.6–23.8) | 15.4 ± 18.2 (0.0–33.6) | 13.1 ± 16.0 (0.0–29.1) | 7.6 ± 9.4 (0.0–17.0) |
| Glutaric acid | 21.2 ± 8.6 (12.6–29.8) | 14.4 ± 20.4 (0.0–34.8) | 15.7 ± 13.8 (1.9–29.5) | 5.3 ± 7.4 (0.0–12.7) | 11.3 ± 15.8 (0.0–27.1) | 6.1 ± 8.8 (0.0–14.9) |
| 3-Methylglutaric acid | 18.5 ± 9.0 (9.5–27.5) | 35.8 ± 22.2 (13.6–58.0) | 26.1 ± 18.8 (7.3–44.9) | 23.7 ± 28.8 (0.0–52.5) | 19.2 ± 21.6 (0.0–30.6) | 10.9 ± 13.6 (0.0–24.5) |
| 3-Phenylbutyric acid | 19.9 ± 7.0 (12.9–26.7) | 37.4 ± 19.8 (17.6–57.2) | 21.8 ± 15.6 (6.2–37.4) | 19.0 ± 21.6 (0.0–40.6) | 21.5 ± 26.8 (0.0–48.3) | 10.2 ± 12.8 (0.0–23.0) |
| Succinylacetone | 12.9 ± 6.0 (6.9–18.9) | 10.6 ± 4.0 (6.6–14.6) | 10.8 ± 4.2 (6.6–15.0) | 12.7 ± 4.8 (7.9–17.5) | 14.9 ± 13.2 (1.7–28.1) | 11.8 ± 15.0 (0.0–26.8) |
| Adipic acid | 20.4 ± 29.4 (0.0–49.8) | 29.0 ± 26.8 (2.2–55.8) | 19.2 ± 21.2 (0.0–40.4) | 11.6 ± 16.6 (0.0–28.2) | 28.1 ± 43.4 (0.0–71.5) | 11.6 ± 17.4 (0.0–29.0) |
| Suberic acid | 19.5 ± 15.6 (3.9–35.1) | 12.9 ± 4.6 (8.3–17.5) | 12.6 ± 19.8 (0.0–32.4) | 10.6 ± 16.0 (0.0–26.6) | 24 ± 37.2 (0.0–61.2) | 15.4 ± 27.0 (0.0–42.4) |
| Azelaic acid | 5.1 ± 4.6 (0.5–9.7) | 19.9 ± 20.0 (0.0–39.9) | 18.0 ± 12.4 (5.6–30.4) | 4.9 ± 10.8 (0.0–15.7) | 18 ± 27.6 (0.0–45.6) | 7.5 ± 13.6 (0.0–21.1) |
| Sebacic acid | 12.9 ± 13.4 (0.0–26.3) | 21.4 ± 13.6 (7.8–35.0) | 8.0 ± 2.9 (2.2–13.8) | 4.6 ± 3.4 (1.2–8.0) | 10.7 ± 16.2 (0.0–26.9) | 4.5 ± 4.8 (0.0–9.3) |
| Orotic acid | 2.1 ± 1.2 (0.9–3.3) | 2.1 ± 2.0 (0.1–4.1) | 3.2 ± 4.2 (0.0–7.4) | 1.3 ± 1.4 (0.0–2.7) | 3 ± 4.8 (0.0–7.8) | 2.0 ± 5.8 (0.0–7.8) |
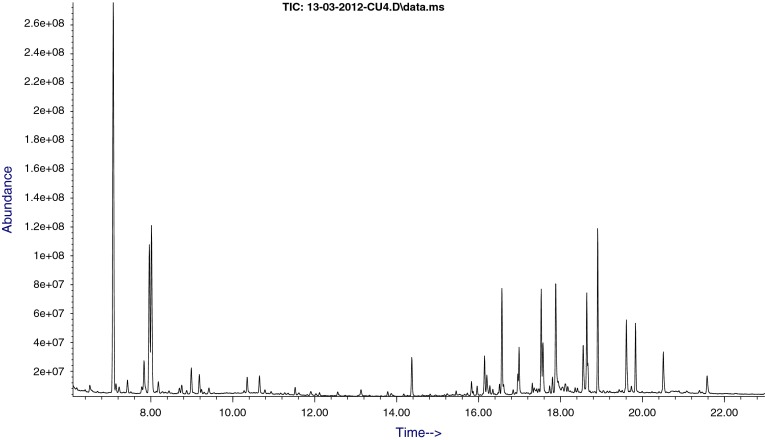
Fig. 1.
Chromatogram of a normal urine sample
Table 3.
Respective retention time of compounds identified in 100 normal urine sample
| S. No | Analyte | Trimethylsilyl ester of the compound identified in NIST Library | Retention times (min) | Precursor ion | Daughter ion |
|---|---|---|---|---|---|
| 1 | Propionic acid | Silanol trimethyl propionate | 4.521 | 146 | 131 |
| 2 | Butyric acid | Butanoic acid trimethylsilyl ester | 6.254 | 159 | 144 |
| 3 | Isovaleric acid | Butanoic acid, 3-methyl, trimethylsilyl ester | 6.892 | 159 | 132 |
| 4 | Tiglic acid | 3-Butenoic acid, 3-methyl, trimethylsilyl ester | 8.156 | 172 | 157 |
| 5 | Lactic acid | Propanoic acid, 2-trimethylsilyl-oxy-trimethylsilyl ester | 9.128 | 219 | 191 |
| 6 | Hexanoic acid | Hexanoic acid trimethylsilyl ester | 9.296 | 173 | 132 |
| 7 | Glycolic acid | Acetic acid, [(trimethylsilyl)oxy]-, trimethylsilyl ester | 9.371 | 205 | 177 |
| 8 | Glyoxylic acid | Glyoxylic oxime acid, bis(trimethylsilyl)ester | 10.225 | 233 | 218 |
| 9 | Oxalic acid | Ethanedioic acid bis(trimethylsilyl) ester | 10.295 | 219 | 190 |
| 10 | Pyruvic acid | Pyruvic acid oxime, bis(trimethylsilyl)-deriv. | 10.592 | 247 | 232 |
| 11 | 3Hydroxy isovaleric acid | Butanoic acid, 3-methyl-3(trimethylsilyl)oxy(trimethylsilyl) ester | 11.457 | 247 | 231 |
| 12 | Methylmalonic acid | Propanedioic acid, methyl, bis(trimethylsilyl) ester | 11.554 | 247 | 218 |
| 13 | 2Ethyl,3hydroxypropionic acid | 2Ethyl, 3hydroxypropionic acid, di-TMS | 11.754 | 247 | 233 |
| 14 | Urea | Urea, N,N′-bis(trimethylsilyl)ester | 11.867 | 204 | 189 |
| 15 | Benzoic acid | Benzoic acid trimethylsilyl ester | 11.970 | 194 | 179 |
| 16 | Phosphoric acid | Silanol trimethyl phosphate (3:1) | 12.477 | 314 | 299 |
| 17 | Ethylmalonic acid | Propanedioic acid, ethyl bis(trimethylsilyl) ester | 12.531 | 261 | 217 |
| 18 | Glycerol | Trimethylsilyl ether of glycerol | 12.558 | 293 | 205 |
| 19 | 2Ketoisocaproic acid | 2Ketoisocaproic acid oxime, bis(trimethylsilyl)-deriv. | 12.661 | 274 | 247 |
| 20 | Succinic acid | Butanedioic acid, bis(trimethylsilyl) ester | 13.055 | 262 | 247 |
| 21 | 3-Methylsuccinic acid | Butanedioic acid, methyl, bis(trimethylsilyl) ester | 13.266 | 261 | 186 |
| 22 | Glyceric acid | Propanoic acid, 2,3,bis(trimethylsilyl)oxy-trimethylsilyl ester | 13.434 | 322 | 292 |
| 23 | Fumaric acid | 2-Butenedioic acid(E)-bis(trimethylsilyl) ester | 13.558 | 245 | 217 |
| 24 | 2,3-Dihydroxybutyric acid | (R*S*)-2,3-Dihydroxybutanoic acid, tris(trimethylsilyl) derive. | 13.655 | 321 | 292 |
| 25 | Lactic acid dimer | Lactic acid dimer, bis(trimethylsilyl)- | 14.211 | 291 | 262 |
| 26 | Glutaric acid | pentanedioic acid, bis(trimethylsilyl) ester | 14.325 | 276 | 261 |
| 27 | 3-Methylglutaric acid | Pentanedioic acid, 3-methyl, bis(trimethylsilyl) ester | 14.573 | 275 | 247 |
| 28 | 3-Methyl glutaconic acid | 2-Pentanedioic acid, 3-methyl, bis(trimethylsilyl) ester, (E) | 14.849 | 288 | 273 |
| 29 | Succinylacetone | 5-Methyl-3-isoxazolepropionic acid trimethylsilyl ester | 14.880 | 212 | 182 |
| 30 | 3Phenylbutyric acid | 3Phenylbutyric acid bis trimethylsilyl ester | 14.908 | 236 | 221 |
| 31 | Adipic acid | Hexanedioic acid, bis(trimethylsilyl) ester | 15.405 | 275 | 217 |
| 32 | 3-Methyladipic acid | Hexanedioic acid, 3-methyl, bis(trimethylsilyl) ester | 15.675 | 289 | 186 |
| 33 | Pimelic acid | Heptanedioic acid, bis(trimethylsilyl) ester | 16.226 | 289 | 245 |
| 34 | 4-Hydroxyphenyl acetic acid | Benzeneacetic acid, 4-[(trimethylsily)oxy]-trimethylsilyl ester | 16.529 | 296 | 281 |
| 35 | Suberic acid | Octanedioic acid bis trimethylsilyl ester | 16.981 | 303 | 217 |
| 36 | Orotic acid | 4-Pyrimidine carboxylic acid,2,6-bis(trimethylsily)oxy-trimethylsilyl ester | 17.280 | 372 | 357 |
| 37 | Cis-Aconitic acid | 1-Propene-1,2,3-tricarboxylic acid, tris (trimethylsilyl) ester, [E]- | 17.280 | 375 | 346 |
| 38 | Azelaic acid | Azelaic acid bis trimethylsilyl ester | 17.544 | 317 | 217 |
| 39 | Sebacic acid | Decanedioic acid bis trimethylsilyl ester | 18.095 | 331 | 215 |
| 40 | Hippuric acid | m-Trimethylsilyloxy(trimethylsilyl) hippurate | 19.414 | 399 | 324 |
| 41 | Tropic acid (ISTD)a | Tropic acid trimethylsilyl ester | 16.178 | 280 | 118 |
| 42 | Phenyllactic acid | Benzenepropanoic acid, alpha-[(trimethylsily)oxy]-, trimethylsilyl ester | 16.145 | 295 | 267 |
| 43 | Stearic acid | Octadecanoic acid, trimethylsilyl ester | 19.829 | 356 | 341 |
| 44 | Palmitic acid | Hexadecanoic acid, trimethylsilyl ester | 18.906 | 328 | 313 |
| 45 | Indole acetic acid | 1H-Indole-1-acetic acid,trimethylsilyl ester | 18.349 | 247 | 232 |
| 46 | Phthalic acid | 1,2-Benzenedicarboxylic acid, bis(trimethylsilyl) ester | 16.983 | 310 | 295 |
| 47 | 2,5-Furandicarboxylic acid | 2,5-Furandicarboxylic acid, bis(trimethylsilyl) ester | 16.599 | 300 | 285 |
| 48 | 4-Hydroxyphenylacetic acid | Benzeneacetic acid, 4-[(trimethylsilyl)oxy]-trimethylsilyl ester | 16.567 | 296 | 281 |
| 49 | 2-Furoic acid, 5hydroxymethyl | 2-Furancarboxylic acid,5-[[(trimethylsilyl)oxy)methyl]-, trimethylsily ester | 15.859 | 286 | 271 |
| 50 | Hydroquinone | Silane, [1,4-phenylenebis(oxy)]bis[trimethyl- | 14.368 | 254 | 239 |
| 51 | 2-Keto, 3-methylvaleric acid | 2-Keto-3-methylvalerate oxime,bis[trimethylsilyl]-deriv. | 12.483 | 289 | 274 |
| 52 | Oleic acid | Oleic acid, trimethylsilyl ester | 19.732 | 354 | 339 |
| 53 | Vanillylpropionic acid | Benzenepropionic acid, 3-methoxy-4[(trimethylsilyl)oxy]-, trimethylsilyl | 18.193 | 340 | 325 |
| 54 | Citric acid or isocitric acid | 1,2,3-Propanetricarboxylic acid, 2-[(trimethylsilyl)oxy]-, tris(trimethylsilyl) | 17.804 | 480 | 465 |
| 55 | m-Anisic acid or vanillic acid | Benzoic acid, 3-methoxy-4-[(trimethylsilyl)oxy]-, trimethylsilyl ester | 17.426 | 312 | 297 |
| 56 | Homovanillic acid | Trimethylsilyl[3-methoxy-4-(trimethylsilyloxy)phenyl]acetate | 17.474 | 326 | 311 |
| 57 | Malonic acid | Propanedioic acid, bis(trimethylsilyl) ester | 12.607 | 248 | 233 |
| 58 | 4-Hydroxybutyric acid | Butanoic acid, 4-[(trimethylsilyl)oxy]-, trimethylsilyl ester | 10.787 | 233 | 204 |
| 59 | p-Cresol | Silane, trimethyl(4-methylphenoxy) | 10.581 | 180 | 165 |
| 60 | Ethylene glycol | 1,2-Bis(trimethylsilyloxy)ethane | 7.832 | 206 | 191 |
| 61 | 4-Hydroxyhippuric acid | Glycine, N-[4-(trimethylsilyl)oxy]benzoyl]-,trimethylsilyl ester | 19.819 | 339 | 324 |
| 62 | 2-Hydroxyhippuric acid | Glycine, N-[2-(trimethylsilyl)oxy]benzoyl]-, trimethylsilyl ester | 19.122 | 339 | 324 |
| 63 | 3-Hydroxysebacic acid | 3-Trimethylsiloxysebacic acid, bis(trimethylsilyl)-ester | 19.041 | 419 | 377 |
| 64 | Linolenic acid | Linolenic acid, trimethylsilyl ester | 18.279 | 350 | 335 |
| 65 | 3,5-Dihydroxybenzoic acid | 3,5-Bis(trimethylsiloxy)benzoic acid, trimethylsilyl ester | 17.739 | 370 | 355 |
| 66 | Furoylglycine | Furolglycine, trimethylsilyl ester | 16.696 | 241 | 226 |
| 67 | Pyroglutamic acid | l-Proline,5-oxo-1(trimethylsilyl)-, trimethylsilyl ester; N,O-Bis-(trimethylsilyl)-2-pyrrolidone carboxylic acid | 15.654 | 258 | 230 |
| 68 | 5-Hydroxyhydantoin | Hydantoin, 5-hydroxy-tris-O-(trimethylsilyl)- | 15.621 | 332 | 317 |
| 69 | 2Methyl-3hydroxybutyric acid | Butanoic acid, 2-methyl-3-[(trimethylsilyl)oxy], trimethylsilyl ester | 14.427 | 247 | 218 |
| 70 | Threonolactone | 2(3H)-Furanone,dihydro-3,4-bis[(trimethylsilyl)oxy]-,trans | 14.076 | 262 | 247 |
| 71 | 3-Hydroxyvaleric acid | 5-Trimethylsilyloxy-n-valeric acid, trimethylsilyl ester | 11.181 | 247 | 231 |
| 72 | 4-Hydroxybutyric acid | Butanoic acid, 4-[(trimethylsilyl)oxy], trimethylsilyl ester | 10.786 | 233 | 204 |
| 73 | Acetyltyrosine | N-Acetyltyrosine, di-TMS | 19.397 | 352 | 308 |
| 74 | Ferulic acid | Trimethylsilyl 3-methoxy-4-(trimethylsilyloxy)cinnamate | 19.197 | 338 | 323 |
| 75 | Tartaric acid | Tartaric acid,bis-O-(trimethylsilyl)-,bis(trimethylsilyl) ester | 16.653 | 438 | 423 |
| 76 | Anthranilic acid | Benzoic acid,2-[(trimethylsilyl)amino]-, trimethylsilyl ester | 16.399 | 281 | 266 |
| 77 | 3Hydroxy-3-methylglutaric acid | Pentanedioic acid, 3-methyl-3-[(trimethylsilyl)oxy]-, bis(trimethylsilyl)ester | 16.329 | 363 | 273 |
| 78 | 3-Methylglutaconic acid | 2-Pentenedioic acid, 3-methyl-, bis(trimethylsilyl)ester, (E) | 15.232 | 288 | 273 |
| 79 | 4-Hydroxyphenyllactic acid | Benzenepropionic acid,alpha,4-bis[(trimethylsilyl)oxy]-, trimethylsilyl ester | 18.247 | 398 | 383 |
aInternal standard, not found in biological samples
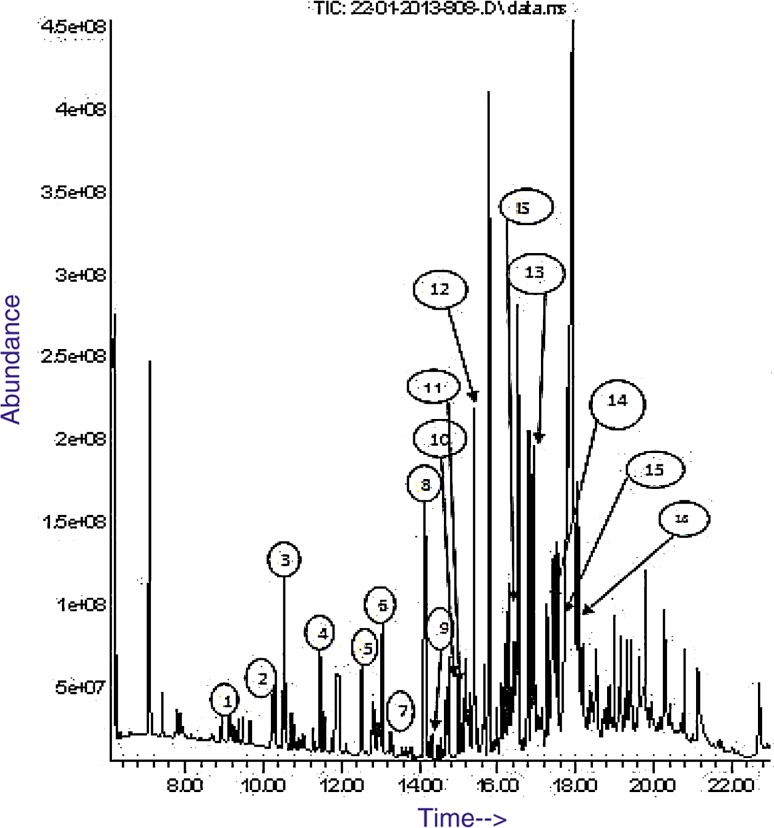
Fig. 2.
Chromatogram of a normal urine sample spiked with 16 organic acids. 1 Lactic acid, 2 oxalic acid, 3 pyruvic acid, 4 methylmalonic acid, 5 ethylmalonic acid, 6 2-ketocaproic acid, 7 fumaric acid, 8 lactic acid dimer, 9 glutaric acid, 10 3-methylglutaric acid, 11 succinylacetone, 12 adipic acid, 13 suberic acid, 14 orotic acid, 15 azelaic acid, 16 sebacic acid
Table 4.
Relative recovery and precision of organic acids added in aqueous calibrator and urine calibrator
| Organic acid | Relative recovery (%) | Intraday imprecision (n = 24) | Interday imprecision (n = 24) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Aqueous calibrator | Urine calibrator | Aqueous calibrator mean conc. (mmol/l) | CV (%) | Urine calibrator mean conc. (mmol/l) | CV (%) | Aqueous calibrator mean conc. (mmol/l) | CV (%) | Urine calibrator mean conc. (mmol/l) | CV (%) | |
| Lactate | 72.5 | 72.75 | 9.9 | 1.2 | 9.7 | 0.01 | 9.2 | 0.9 | 9.7 | 3.0 |
| Oxalic acid | 104.75 | 113.25 | 13.9 | 0.8 | 15.1 | 4.6 | 14.1 | 1.7 | 15.1 | 4.6 |
| Pyruvate | 91.75 | 81.75 | 12.4 | 1.6 | 11 | 4.2 | 11.9 | 3.4 | 10.7 | 7.8 |
| Methylmalonic acid | 97.5 | 99 | 12.8 | 0.04 | 13.5 | 9.1 | 13.4 | 1.2 | 5.6 | 1.6 |
| Ethylmalonic acid | 83.25 | 83 | 9.9 | 1.2 | 10.4 | 1.4 | 13.5 | 0.9 | 6.4 | 2.9 |
| Fumaric acid | 74 | 75.25 | 9.9 | 0.7 | 10.7 | 3.9 | 9.8 | 0.06 | 7.7 | 1.4 |
| Lactic acid dimer | 104.5 | 98.5 | 14 | 0.05 | 12.8 | 1.2 | 13.8 | 0.01 | 3.8 | 1.0 |
| Glutaric acid | 119.5 | 118.5 | 15.9 | 1.0 | 15.8 | 1.6 | 16 | 1.0 | 5.8 | 2.5 |
| 3Methylglutaric acid | 82.5 | 127.5 | 10.8 | 0.5 | 17 | 0.1 | 11.4 | 1.0 | 27.0 | 0.4 |
| 3Phenylbutyric acid | 71.75 | 84.75 | 9.9 | 0.1 | 11.3 | 2.7 | 8.9 | 3.8 | 4.3 | 3.0 |
| Succinylacetone | 77 | 83.25 | 10.5 | 0.07 | 11.1 | 4.1 | 9.8 | 6.3 | 11.1 | 2.9 |
| Adipic acid | 109 | 105.75 | 14.5 | 1.3 | 14.1 | 0.9 | 14.6 | 2.7 | 14.1 | 2.3 |
| Suberic acid | 134.75 | 133.5 | 18 | 1.0 | 17.8 | 0.5 | 17.9 | 1.2 | 17.8 | 0.3 |
| Azelaic acid | 88.75 | 98.25 | 10.9 | 1.9 | 13.4 | 1.4 | 13.7 | 1.2 | 6.5 | 1.4 |
| Sebacic acid | 56.25 | 56 | 7.5 | 2.6 | 7.3 | 6.9 | 7.5 | 9.8 | 7.8 | 1.9 |
| Orotic acid | 66.75 | 67.75 | 8.9 | 5.1 | 9.6 | 5.5 | 8.9 | 4.8 | 4.0 | 6.8 |
Discussion
Major biochemical changes take place in the human body between birth and adulthood, and many of these are reflected by metabolites excreted in body fluids (blood, CSF and urine). Concentrations of metabolites that are abnormal for pediatric age group may be considered normal in adults. The most pronounced changes are seen in the newborn period and during puberty. Population specific reference ranges had been established for other populations [3, 5–8, 13]. Most pediatric reference values have been established for Caucasian population from samples collected from hospitalized infants and children who did not have primary organic aciduria. The diversity of ethnic groups needs to be included into pediatrics reference values so that it can fully reflect levels in healthy multicultural population like ours. It is clinically inappropriate to apply the reference values specific for an ethnic group to other ethnic population [14]. The concentrations of organic acids varies from population to population due to genotype, food habits and other epigenetic and environmental influences. Reference ranges for urinary organic acids in a healthy pediatric population are indispensible for critical evaluation. Quantitative data for 16 organic acids in four age groups of healthy north Indian pediatrics population have been presented in Table 2. The concentration of urinary organic acids were given in a ratio to urinary creatinine concentration because the creatinine excretion rate is relatively constant for metabolic body size. The age groups were selected on the basis of changes in feeding and physical activity. Due to food preferences and metabolic differences significant variation in acid excretion is apparent in each age group. The excretion of metabolites were higher in younger age groups decreased at an intermediate age and then increased in older age groups. There was a significant difference found in the excretion of methylmalonic acid in our study in comparison with other studies concerned [5, 13]. The higher reference range of this metabolite may be due to dietary deficiency; moreover amino acid breakdown may play a role, protein intake being insufficient in our pediatric population [15]. Also we found higher concentrations of urinary succinylacetone is higher in our study as compared to other studies, The possible reason for this could be the extraction procedure that we follow. The oximation is done on urine samples at 60 °C for 30 min. At high temperature and low pH (<3) in the presence of hydroxylamine hydrochloric acid, succinylacetoacetic acid get converted into succinylacetone thus increasing its concentration. Another reason could be the presence of 3-methyl-4-propyl-5-isoxazol methyl trimethylsilyl ester whose m/z ratio (ions-212,170,142) are same as succinylacetone and its retention time is also near to succinylacetone thus increasing the concentration in a fallacious manner. A GC–MS/MS is likely to identify better these co-eluting peaks. This is probably a limitation of our procedure.
We found that the concentration of organic acids quantitated were different in a North Indian pediatric population compared to other populations [5–7, 9, 13]. In our case almost all values were higher: this may be due to a low creatinine concentration in our population [16, 17]. A significant gap exists between the urinary organic acid concentrations in developed and developing countries. Area specific reference values are needed to improve the health care of the pediatric population. Such data may help to produce a basis for diagnosing metabolic abnormalities in patients in a specific ethnicity.
Acknowledements
We acknowledge the financial support given by two government funding agencies: the Council of Scientific and Industrial Research (CSIR) and the Indian Council of Medical Research (ICMR) to conduct this study. Thanks to ICMR. ICMR Grant No. FNo. 54/10/2011-BMS.
Conflict of interests
None.
References
- 1.Saudubray J-M, Charpentier C. Clinical phenotype: diagnosis/algorithms. In: Scriver CBA, Sly W, Valle D, editors. The metabolic and molecular bases of inherited disease. New York: McGraw Hill; 2001. pp. 1327–1402. [Google Scholar]
- 2.Tanaka K, Budd MA, Efron ML, Isselbacher KJ. Isovaleric acidemia: a new genetic defect of leucine metabolism. Proc Natl Acad Sci USA. 1966;56(1):236–242. doi: 10.1073/pnas.56.1.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bjorkman L, McLean C, Steen G. Organic acids in urine from human newborns. Clin Chem. 1976;22(1):49–52. [PubMed] [Google Scholar]
- 4.Mills GA, Walker V. Urinary excretion of cyclohexanediol, a metabolite of the solvent cyclohexanone, by infants in a special care unit. Clin Chem. 1990;36(6):870–874. [PubMed] [Google Scholar]
- 5.Guneral F, Bachmann C. Age-related reference values for urinary organic acids in a healthy Turkish pediatric population. Clin Chem. 1994;40(6):862–866. [PubMed] [Google Scholar]
- 6.Chalmers RA, Healy MJ, Lawson AM, Hart JT, Watts RW. Urinary organic acids in man. III. Quantitative ranges and patterns of excretion in a normal population. Clin Chem. 1976;22(8):1292–1298. [PubMed] [Google Scholar]
- 7.Thompson JA, Miles BS, Fennessey PV. Urinary organic acids quantitated by age groups in a healthy pediatric population. Clin Chem. 1977;23(9):1734–1738. [PubMed] [Google Scholar]
- 8.Aksu A, Morrow G, 3rd, Barness LA. Urinary excretion of non-nitrogenous organic acids by healthy infants and children. Clin Chem. 1974;20(5):603–605. [PubMed] [Google Scholar]
- 9.Lawson AM, Chalmers RA, Watts RW. Urinary organic acids in man. I. Normal patterns. Clin Chem. 1976;22(8):1283–1287. [PubMed] [Google Scholar]
- 10.Liebich HM, Forst C. Basic profiles of organic acids in urine. J Chromatogr. 1990;525(1):1–14. doi: 10.1016/S0378-4347(00)83375-7. [DOI] [PubMed] [Google Scholar]
- 11.Mardens Y, Kumps A, Planchon C, Wurth C. Comparison of two extraction procedures for urinary organic acids prior to gas chromatography–mass spectrometry. J Chromatogr. 1992;577(2):341–346. doi: 10.1016/0378-4347(92)80256-P. [DOI] [PubMed] [Google Scholar]
- 12.Greter J, Jacobson CE. Urinary organic acids: isolation and quantification for routine metabolic screening. Clin Chem. 1987;33(4):473–480. [PubMed] [Google Scholar]
- 13.Boulat O, Gradwohl M, Matos V, Guignard JP, Bachmann C. Organic acids in the second morning urine in a healthy Swiss paediatric population. Clin chem lab med: CCLM/FESCC. 2003;41(12):1642–1658. doi: 10.1515/CCLM.2003.248. [DOI] [PubMed] [Google Scholar]
- 14.Pediatric reference intervals: critical gap analysis and establishment of a national initiative. Clin Biochem. 2006;39(6):559–60. [DOI] [PubMed]
- 15.Kanjilal B, Mazumdar PG, Mukherjee M, Rahman MH. Nutritional status of children in India: household socio-economic condition as the contextual determinant. Int J Equity Health. 2010;9(1):19. doi: 10.1186/1475-9276-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bailey PK, Tomson CR, Kinra S, Ebrahim S, Radhakrishna KV, Kuper H, et al. Differences in estimation of creatinine generation between renal function estimating equations in an Indian population: cross-sectional data from the Hyderabad arm of the Indian migration study. BMC Nephrol. 2013;14:30. doi: 10.1186/1471-2369-14-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallagher D, Visser M, De Meersman RE, Sepulveda D, Baumgartner RN, Pierson RN, et al. Appendicular skeletal muscle mass: effects of age, gender, and ethnicity. J Appl Physiol. 1997;83(1):229–239. doi: 10.1152/jappl.1997.83.1.229. [DOI] [PubMed] [Google Scholar]