Abstract
Bacterial glycerol ether lipids (alkylglycerols) have received increasing attention during the last decades, notably due to their potential role in cell resistance or adaptation to adverse environmental conditions. Major uncertainties remain, however, regarding the origin, biosynthesis, and modes of formation of these uncommon bacterial lipids. We report here the preponderance of monoalkyl- and dialkylglycerols (1-O-alkyl-, 2-O-alkyl-, and 1,2-O-dialkylglycerols) among the hydrolyzed lipids of the marine mesophilic sulfate-reducing proteobacterium Desulfatibacillum alkenivorans PF2803T grown on n-alkenes (pentadec-1-ene or hexadec-1-ene) as the sole carbon and energy source. Alkylglycerols account for one-third to two-thirds of the total cellular lipids (alkylglycerols plus acylglycerols), depending on the growth substrate, with dialkylglycerols contributing to one-fifth to two-fifths of the total ether lipids. The carbon chain distribution of the lipids of D. alkenivorans also depends on that of the substrate, but the chain length and methyl-branching patterns of fatty acids and monoalkyl- and dialkylglycerols are systematically congruent, supporting the idea of a biosynthetic link between the three classes of compounds. Vinyl ethers (1-alken-1′-yl-glycerols, known as plasmalogens) are not detected among the lipids of strain PF2803T. Cultures grown on different (per)deuterated n-alkene, n-alkanol, and n-fatty acid substrates further demonstrate that saturated alkylglycerols are not formed via the reduction of hypothetic alken-1′-yl intermediates. Our results support an unprecedented biosynthetic pathway to monoalkyl/monoacyl- and dialkylglycerols in anaerobic bacteria and suggest that n-alkyl compounds present in the environment can serve as the substrates for supplying the building blocks of ether phospholipids of heterotrophic bacteria.
INTRODUCTION
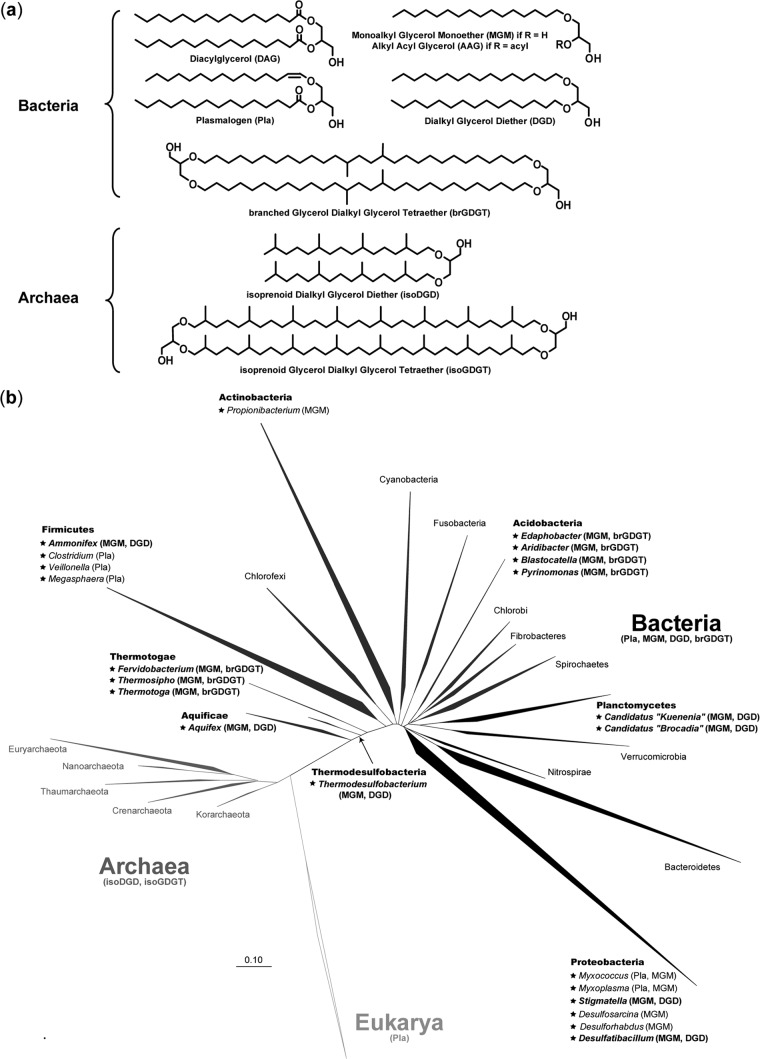
A major feature distinguishing between the two prokaryotic domains Archaea and Bacteria is the chemical composition of the cell wall (1, 2). As in eukaryotes, bacterial phospholipids generally consist of a glycerol moiety linked to fatty acids via ester bonds (diacylglycerols [DAG]). In contrast, the hydrophobic portion of archaeal membrane lipids consists of isoprenoid chains covalently bound to glycerol through ether bonds, forming isoprenoid di- and tetraethers of glycerol (isoprenoid glycerol dialkyl diether and glycerol dialkyl glycerol tetraether lipids, respectively) (Fig. 1a). Another major distinctive feature is the stereochemistry of the glycerol backbone, which in Archaea (which use 2,3-sn-glycerol) is the opposite of that in Bacteria and Eukarya (which utilize 1,2-sn-glycerol). These differences in lipid structures likely confer variations in the physical and physiological properties of the membranes of Bacteria and Archaea, with potential implications in terms of ecology and evolution (2–5). For instance, the glycerol ether lipids of archaeal membranes confer a lower degree of permeability to ions and a higher degree of stability than bacterial fatty acid membranes. The advantages of the archaeal membrane have been clearly shown in hyperthermophiles, halophiles, and acidophiles (see reference 2 and references therein), supporting the idea that ether-linked isoprenoid membrane lipids are well suited for life in extreme environments.
FIG 1.
Representative structures of the different types of bacterial and archaeal ether lipids (a) and schematic phylogenetic tree (small-subunit [SSU] rRNA; Greengenes database [http://greengenes.lbl.gov]) indicating the occurrence of ether lipids in the three domains of life (b). The occurrence of nonisoprenoidal ether lipids in specific genera within the different bacterial phyla is indicated. Bacterial genera indicated in bold are those capable of producing dialkyl glycerol diether (DGD) or tetraether (brGDGT) lipids. The scale bar corresponds to 0.1 substitutions per nucleotide.
Exceptions to the aforementioned chemical distinctions exist except for the stereochemistry of the glycerol backbone. Lipids with one ether-linked alkyl chain at position sn-1 of a glycerol moiety (1-O-alkylglycerols, monoalkyl glycerol monoethers [MGM]) (Fig. 1a) with, eventually, one ester-bound fatty acid at position sn-2 (1-alkyl-2-acylglycerols, AAG) (Fig. 1a) have been reported in aerobic and anaerobic bacteria (6–10) (Fig. 1b). Many strictly anaerobic and a few aerobic bacteria also contain so-called plasmalogens (Pla), which contain a vinyl-ether group at position sn-1 of the glycerol chain (1-alken-1′-yl-2-acylglycerols) (Fig. 1). These lipids likely play a role in cell resistance to adverse environmental conditions. Plasmalogens are also widespread in mammalian and human tissues, where they can have a number of important biological functions such as intracellular signaling (in response to stimuli) or a protective role during oxidant-induced stress (11, 12). There are indications that plasmalogens have distinct biosynthetic pathways in animal tissues and (anaerobic) bacteria, but mechanistic and evolutionary aspects remain unclear (12–14).
Even more remarkable is the existence of nonisoprenoid dialkyl glycerol diether lipids (DGD) and branched glycerol dialkyl glycerol tetraether lipids (brGDGT) (Fig. 1a). These lipids exhibit an intriguing combination of the structural characteristics of Bacteria and Archaea (15). To date, nonisoprenoid DGD have been reported only in (hyper)thermophilic bacteria (16–19) and in a few mesophilic members of the Planctomycetes (20) and aggregating myxobacteria (21, 22) (Fig. 1b). In addition to this restricted occurrence in isolated strains, little information is currently available regarding the biosynthesis and the physiological role of glycerol ether lipids in bacteria (15). Remarkably, the biosynthesis of ether lipids and their specific roles in membrane organization and stability in Archaea have received increasing attention in the last years (23–25), but there is still no bacterial corollary.
The limited information available about nonisoprenoid alkylglycerol biosynthesis and the rare occurrence of these lipids in bacterial isolates contrast with their widespread occurrence in the environment. During the last decade(s), various unprecedented structures of ether lipids have been identified in various recent and ancient ecosystems, suggesting a specific physiological role of these molecules in living cells and their potential usefulness as organic proxies (lipid biomarkers). Nonisoprenoidal MGM and DGD have been detected in hydrothermal environments (26–29) but are also omnipresent in many contemporary and ancient nonextreme marine ecosystems (30–33). MGM and DGD present at methane cold seeps have been systematically attributed to the sulfate-reducing bacteria (SRB) partners of Archaea involved in the anaerobic oxidation of methane (30, 34–37). Although sedimentary nonisoprenoidal ether lipids are regularly assigned to sulfate reducers and/or linked to the S cycle within marine/oceanic environments (30, 32), the presence of DGD in pure cultures of SRB has never been reported so far. Surprisingly, the investigation of ether lipids in biogeochemical studies is gaining popularity exponentially despite some major uncertainties regarding their origin, biosynthesis, physiological role, and modes of formation.
As part of an investigation of the physiology and the lipid composition of hydrocarbon-degrading sulfate-reducing Proteobacteria, we report here the predominance of monoalkyl- and dialkylglycerols (1-O-alkyl-, 2-O-alkyl-, and 1,2-O-dialkylglycerols) among the hydrolyzed lipids of Desulfatibacillum alkenivorans strain PF2803T. This marine mesophilic strain is shown to incorporate long-chain n-alkyl substrates (n-alkenes, n-alkanols, n-fatty acids), or their initial transformation products, directly into its cellular ether lipids. Substrate labeling allows proposing an unprecedented pathway to nonisoprenoidal MGM and DGD biosynthesis in anaerobic bacteria that does not involve the formation of plasmalogen-like intermediates. Overall, this work provides new insights into the possible biological origin and modes of formation of nonisoprenoid alkylglycerols in the environment.
MATERIALS AND METHODS
Chemicals.
Unlabeled alkenes (pentadec-1-ene and hexadec-1-ene, >99% pure) and 2,2-D2-hexadecanoic acid were purchased from Aldrich Chemical Co. 1-D2-pentadec-1-ene and perdeuterated hexadec-1-ene (D32-hexadec-1-ene) were synthesized in three steps from unlabeled pentadecanoic acid (Aldrich) and perdeuterated palmitic acid (C15D31COOH; Euriso-top), respectively, as described previously (38). 1,1,2,2-D4-hexadecanol was obtained by reduction of 2,2-D2-hexadecanoic acid with LiAlD4 (Aldrich) in diethyl ether and purified by column chromatography over silica gel using dichloromethane/ethyl acetate (9:1 [vol/vol]) as the eluent.
Source of bacterium and cultivation.
The sulfate-reducing bacterium D. alkenivorans strain PF2803T was isolated from marine sediment (Fos Harbor, Mediterranean Sea, France [39]). It belongs to the family Desulfobacteraceae in the Deltaproteobacteria (see Fig. S1 in the supplemental material). It is a hydrocarbonoclastic bacterium capable of utilizing n-alk-1-enes and their oxidized derivatives (n-alcohols, n-fatty acids) from C8 to C23 as a source of carbon and energy (39), and efficient growth is generally observed with midchain (C14 to C17) substrates. Strain PF2803T was grown at 30°C in 100 ml of defined anoxic sulfate-reducing medium with an unlabeled (pentadec-1-ene [C15:1] or hexadec-1-ene [C16:1]) or a labeled (1-D2-pentadec-1-ene, D32-hexadec-1-ene, 2,2-D2-hexadecanoic acid, or 1,1,2,2-D4-hexadecanol) n-alkyl compound as the sole carbon and energy source. Cultures with perdeuterated hexadecene were prepared by mixing equal volumes of unlabeled and labeled hexadecene (1 mM final concentrations) in order to facilitate growth. At the end of the exponential-growth phase (after ca. 2 to 3 weeks of incubation), cells were harvested by filtration through glass microfiber filters (GF/B; Whatman) and kept frozen before lipid analysis. By that time, ca. 70% to 90% of the substrate had been oxidized (39). All cultures were subcultured twice on the same substrate before lipid analysis.
Extraction and analysis of glycerol ethers and fatty acids.
Cells were directly hydrolyzed with 1 N KOH in methanol-water (1:1 [vol/vol]; reflux for 2 h), and unsaponifiable (neutral) and saponifiable (acid) lipids were extracted from the basic and the acidified solutions, respectively. Hydrolyzed neutral lipids were eventually purified by column chromatography over silica gel to get rid of the residual substrate. Following the addition of an internal standard (n-tricosanol), neutrals and acids were silylated by reaction with N,O-bis(trimethylsilyl [TMS])trifluoroacetanamide–pyridine (60°C, 1 h) before gas chromatography (GC) and GC-mass spectrometry (GC-MS) analyses were performed. Cells from a few cultures were submitted to acid hydrolysis instead of base hydrolysis using 1 N HCl–MeOH (reflux for 2 h). Following neutralization with 2 N KOH–MeOH and the addition of water, acid-hydrolyzed lipids were extracted from the methanolic phase and submitted to silylation before GC and GC-MS analyses were performed.
Alkylglycerols and fatty acids were identified and quantified using an HP 6890N gas chromatograph coupled to an HP 5975C mass spectrometer (both from Agilent). Compounds were injected on column and separated over an HP5-MS fused capillary column (30 m by 0.25 mm by 0.25 μm; Agilent). The temperature of the GC oven was programmed to increase from 60 to 130°C at 20°C min−1, from 130 to 250°C at 5°C min−1, and then at 3°C min−1 to 300°C and was held there for 30 min. The carrier gas (He) was maintained at 0.69 bar until the end of the temperature program, and then the pressure was programmed to increase from 0.69 bar to 1.49 bar at 0.04 bar min−1. Electron impact (EI) mass spectra were obtained at 70 eV (source temperature, 190°C; Quad temperature, 150°C; cycle time, 2 scans s−1).
The assignment of the methyl (Me) branch position in branched alkylglycerols and fatty acids was based on the structures of the hydrocarbons released by cleavage of the original ethers with hydroiodic acid (HI) (30) and on the formation of fatty acid pyrrolidide derivatives (38), respectively.
RESULTS
Alkylglycerol composition of D. alkenivorans grown on n-alk-1-ene.
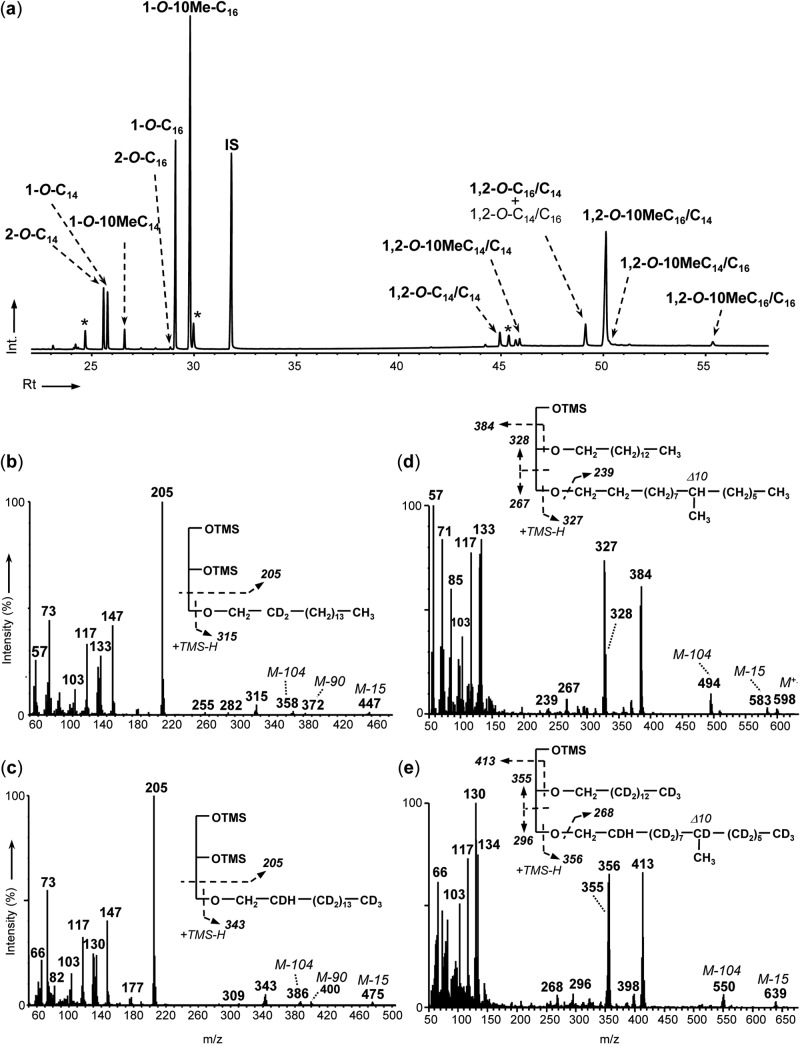
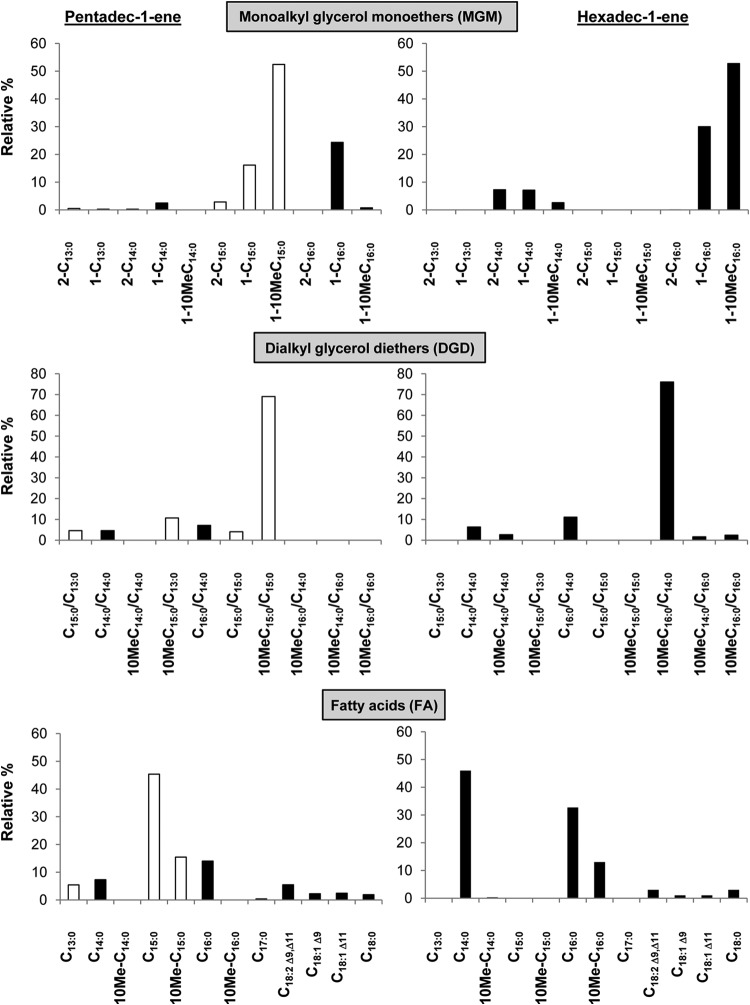
The neutral lipid fractions of strain PF2803T obtained after base hydrolysis of cells grown on C15:1 or C16:1 were dominated by the presence of series of MGM and DGD (Fig. 2a and 3). DGD were present in smaller amounts than MGM, accounting for one-fifth to two-fifths of the total ether lipids (Fig. 4). MGM essentially occurred as 1-O-monoalkylglycerols (1-O-MGM), with minor amounts of 2-O-monoalkylglycerols (2-O-MGM); both classes of MGM were identified based on their characteristic mass spectra exhibiting base peaks at m/z 205 and 218 for 1-O-MGM and 2-O-MGM, respectively (40). Specific fragment ions at M-15, M-90, M-104, and M-147 allowed the identification of the alkyl chain of each MGM (see, e.g., Fig. 2b and c). MGM had a carbon chain length directly dependent on that of the alkene substrate (Fig. 2a and 3). Growth on C16:1 yielded almost exclusively MGM with a C16 or C14 alkyl chain, whereas growth on C15:1 yielded a mixture of C-odd and C-even alkyl chains ranging from C13 to C16. For any substrate, the two main MGM produced had an alkyl chain length similar to that of the substrate, with or without an additional methyl branch at C-10. The presence of a methyl branch in the alkyl chain was inferred from the relative retention index (30) of the MGM. The exact position of the methyl branch within the alkyl chain was determined from the mass spectra of the alkanes released following HI cleavage of a purified glycerol ether fraction (data not shown). 1-Alken-1′-yl glycerols (i.e., the plasmalogen forms of MGM) were not observed in any neutral lipid fraction.
FIG 2.
Total-ion chromatogram (TIC) of neutral (saponified) lipids (as TMS derivatives) of D. alkenivorans PF2803T grown on unlabeled hexadec-1-ene (a) and mass spectra and structural characteristics of the TMS derivatives of 1-O-D2-hexadecylglycerol from a culture grown on either 1,1,2,2-D4-hexadecanol or 2,2-D2-hexadecanoic acid (b), 1-O-D30-hexadecylglycerol from a culture grown on D32-hexadec-1-ene (c), 1-O-methylhexadecanyl-2-O-tetradecanylglycerol from a culture grown on unlabeled hexadec-1-ene (d), and 1-O-D29-methylhexadecanyl-2-O-D27-tetradecanylglycerol from a culture grown on D32-hexadec-1-ene (e). *, contaminants; IS, internal standard; Int., intensity (%); Rt, retention time (min); OTMS, OSi(CH3)3.
FIG 3.
Relative abundances of monoalkyl glycerol monoethers (MGM), dialkyl glycerol diethers (DGD), and fatty acids (FA) released by hydrolysis of cells of D. alkenivorans PF2803T grown on n-alkene (optimal growth conditions, end of exponential-growth phase). Only those which comprised more than 0.5% are presented. Note that C15:0/C13:0 DGD includes a minor proportion of C13:0/C15:0 DGD and that C16:0/C14:0 DGD includes a minor proportion of C14:0/C16:0 DGD for growth on C16:1. Black bars represent C-even alkyl chains, and white bars represent C-odd alkyl chains.
FIG 4.
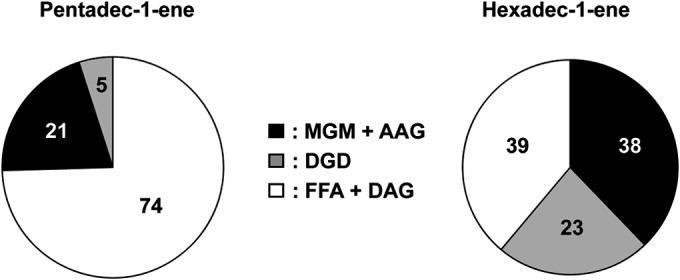
Proportions (%) of monoalkylglycerols (present in vivo as monoalkyl glycerol monoethers [MGM] and/or monoalkyl/monoacyl glycerols [AAG]), dialkylglycerols (DGD), and total fatty acids (present in vivo as free fatty acids [FFA] and/or diacyl glycerols [DAG]) in D. alkenivorans PF2803T grown on n-alkene (end of exponential-growth phase).
As seen with the MGM, the DGD were identified based on their mass spectra and their Kovats retention index values (30, 41). All spectra showed abundant fragment ions at m/z 130, 131, and 133 consistent with the fragmentation observed for 1,2-O-dialkylglycerols (41–43). Specific fragments ions at M-15 and M-104 were used to elucidate the molecular weight of DGD. Other diagnostic fragmentations corresponding to cleavage α to the O-alkyl groups and cleavage of the bond between the sn-1 and sn-2 carbon atoms allowed inferring the structure of both alkyl chains (Fig. 2d). Similarly to that of MGM, the chain length of DGD was directly dependent on that of the alkene substrate, with the C-even substrate yielding almost exclusively DGD with C-even alkyl chains and the C-odd substrate yielding preferentially C-odd alkyl chains (Fig. 3). The sn-1 alkyl chain of all DGD was similar to that of the corresponding MGM, whereas the sn-2 alkyl chain had the same chain length as or a shorter chain length than the sn-1 alkyl chain (Table 1 and Fig. 2a). The position of the methyl branch in branched DGD was assigned based on the corresponding branched MGM.
TABLE 1.
Labeled fatty acids and glycerol ether-linked alkyl chains formed by D. alkenivorans PF2803T grown on different (per)deuterated carbon substratesa
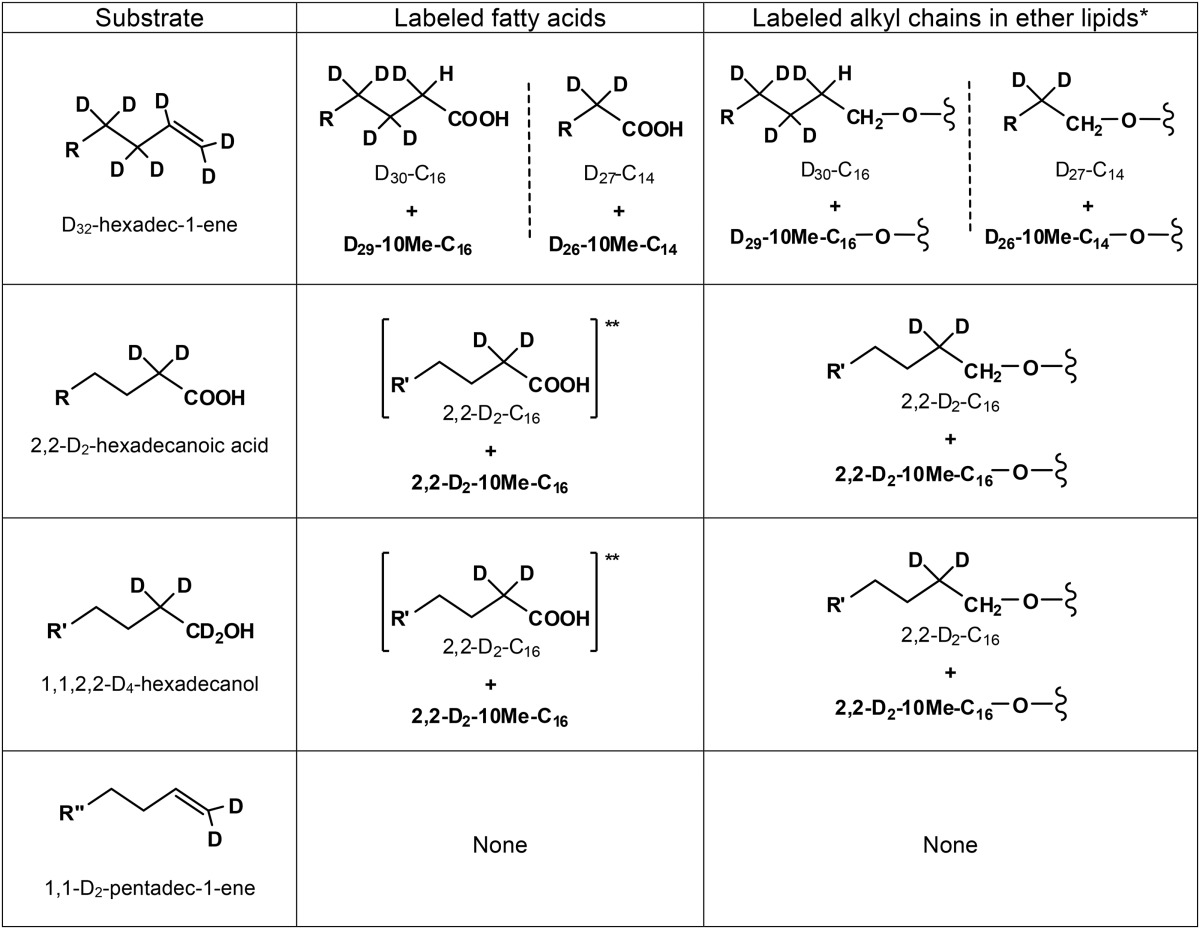
R = n-C12D25; R′ = n-C12H25; R″ = n-C11H23. *, see Fig. 3 for the position of the alkyl chains on the glycerol group; **, growth substrate.
Fatty acid composition of D. alkenivorans grown on n-alk-1-ene.
The hydrolysis of cells of D. alkenivorans grown on n-alkene also released significant proportions of fatty acids (FA) which were identified thanks to characteristic mass spectral fragmentation of silylated derivatives (38, 44). The identification of the methyl-branched fatty acids was based on their relative retention times (see Fig. S2a in the supplemental material) and on the interpretation of their mass spectra, which showed specific ions due to cleavage α to the branched carbon atoms (see Fig. S2d and e). The position of the methyl branch was further confirmed by comparison of the mass spectra with literature data or by the analysis of their pyrrolidide derivatives (38, 44).
As observed for glycerol ethers, the chain length distribution of the cellular FA of D. alkenivorans was strongly dependent on that of the alkene substrate (Fig. 3). Cells grown on a C-even alkene contained mainly FA with C-even carbon chains; similarly, FA with C-odd carbon chains predominated when cells were grown on a C-odd alkene. C-even FA formed from pentadec-1-ene were biosynthesized de novo from C2 units produced during substrate metabolism. With the exception of the presence of the saturated and unsaturated C18 FA commonly encountered in bacteria, the chain-length distributions as well as the methyl branching of the FA were remarkably congruent with those of the ether-bound alkyl moieties observed in MGM and DGD (Fig. 3).
The FA detected after cell hydrolysis likely came from the hydrolysis of AAG and DAG membrane lipids but also (presumably to a lesser extent) from the hydrolysis of FA coenzyme A thioesters constituting in vivo metabolites of the growth substrate (38). The amount of FA released from AAG is considered equal to the amount of MGM released by hydrolysis of cells. The difference between the total amount of FA and that released from AAG is assumed to represent the sum of FA originally present in the cells as free FA coenzyme A (CoA) (FFA) and DAG (Fig. 4).
Identification of (per)deuterated substrate-derived alkylglycerols and fatty acids.
In order to gain insights into alkylglycerol biosynthesis, D. alkenivorans was grown on different (per)deuterated n-alkene, n-alkanol, and n-fatty acid substrates (Table 1). The alkylglycerol and FA compositions of the cells grown on any labeled substrate were similar to those observed on unlabeled alkenes. Growth on D32-hexadecene, 2,2-D2-hexadecanoic acid, or 1,1,2,2-D4-hexadecanol yielded deuterated MGM, DGD, and FA, demonstrating that these cellular lipids were derived from the growth substrate (Table 1 and Fig. 2; see also Fig. S2 in the supplemental material). Alkylglycerols and FA produced from 1,1-D2-pentadecene showed exactly the same mass spectra in comparison to those formed from unlabeled pentadecene, indicating that the two deuterium atoms originally present at C-1 were lost during the biotransformation of the substrate by strain PF2803T (Table 1). This loss cannot have been due to the abiotic transformation of the substrate, since incubation of labeled pentadecene under sterile controls (with substrate but without cells) does not yield unlabeled C-odd FA (38).
Similar labeled alkylglycerols and FA were formed when strain PF2803T was grown on either 1,1,2,2-D4-hexadecanol or 2,2-D2-hexadecanoic acid (Table 1). MGM and FA with alkyl chains shorter than C16 as well as saturated and unsaturated C18 FA were not labeled. Specific fragmentation ions in the mass spectra of labeled MGM and FA were systematically shifted up by 2 atomic mass units (amu) compared with those in the spectra of their unlabeled analogues (see examples in Fig. 2b and reference 43 for MGM and in Fig. S2b and S2c in the supplemental material for FA). This indicates the presence of two deuterium atoms in the labeled compounds, since the TMS reagent used for derivatization was not deuterated. Figure 2b shows the mass spectrum of 1-O-D2-hexadecylglycerol. The O-alkyl fragment at m/z 315 indicates the presence of the two deuterium atoms in the alkyl chain. Since this MGM was formed from both 1,1,2,2-D4-hexadecanol and 2,2-D2-hexadecanoic acid substrates, the two deuterium atoms in the deuterated MGM were assumed to be at C-2 of the alkyl chain (Fig. 2b and Table 1). This location is in accordance with the loss of labeling in the same MGM biosynthesized from a 1,1-D2 substrate (Table 1) and with the position of the deuterium atoms in the D2-hexadecanoic acid formed during growth on 1,1,2,2-D4-hexadecanol (see Fig. S2b). The McLafferty fragment at m/z 134 in the mass spectrum of this fatty acid indeed indicates the presence of the two deuterium atoms at C-2 (see Fig. S2b). The addition of a methyl branching on either 1-O-D2-hexadecylglycerol or D2-hexadecanoic acid did not modify the location of the two deuterium atoms at C-2 of both alkyl chains (Table 1). Deuterated DGD were also biosynthesized from 1,1,2,2-D4-hexadecanol and 2,2-D2-hexadecanoic acid. They contained either 2 or 4 deuterium atoms, as attested by the shift of 2 to 4 amu observed in the characteristic fragment ions of their mass spectra (data not shown). Mass spectral analyses of labeled DGD allowed the identification of 1,2-O-D2-C16/C14, 1,2-O-D2-10MeC16/C14, and 1,2-O-D4-10MeC16/C16. DGD with both alkyl chains shorter than C16 were not labeled. Based on the aforementioned deuterated MGM, the deuterium atoms in labeled DGD are assumed to be systematically present at C-2 of (branched) C16 alkyl chains (Table 1).
Due to a higher degree of labeling, the growth of D. alkenivorans on D32-hexadecene yielded a higher number of deuterated alkylglycerols and FA than growth on other labeled substrates (Table 1). Although experiments were performed using a mixture of unlabeled and perdeuterated hexadecene, the labeled FA, MGM, and DGD had shorter chromatographic retention times than the unlabeled analogues and, thus, generally appeared completely separated during GC-MS analyses. Perdeuterated C14, 10Me-C14, C16, and 10Me-C16 fatty acids were detected. Their mass spectral characteristics are in agreement with those of FA formed during the biodegradation of deuterated acyclic hydrocarbons by other sulfate-reducing bacteria (38, 44, 45). The mass spectrum of the TMS derivative of the deuterated C16 FA showed a molecular ion at m/z 358, indicating the presence of 30 deuterium atoms in the molecule (m/z of 328 for the unlabeled C16:0 FA). The McLafferty ion (m/z 134) was upshifted by 2 amu compared to the corresponding ion in the unlabeled analogue (m/z 132), indicating the presence of two deuterium atoms in the labeled ion. Since one deuterium atom would come from the McLafferty rearrangement, we deduce the presence of one deuterium atom and one hydrogen atom at C-2 (Table 1). Similar analysis of the mass spectrum of the deuterated C14 FA indicates that it was fully deuterated (thus containing 27 deuterium atoms), which suggests that it was formed by β-oxidation (loss of a C-2 unit) of the D30-C16 FA. The mass spectra of the deuterated 10-methyl branched analogues indicate that they contained one deuterium atom less than the perdeuterated unbranched FA (Table 1; see also Fig. S2d and e in the supplemental material). These branched FA were thus likely formed by the exogenous addition of a methyl group at C-10 of the corresponding n-saturated fatty acid (D30-C16 and D27-C14) and not by the methylation of a monounsaturated analogue as often assumed for the biosynthesis of midchain methyl-branched fatty acids (38). Indeed, (i) desaturation and subsequent methylation at C-10 of the deuterated n-saturated fatty acids would have caused the loss of at least two deuterium atoms, and (ii) monounsaturated deuterated fatty acids that could have constituted potential intermediates in 10-Me-branched fatty acid biosynthesis were not detected. The present mechanism of methylation at C-10 remains unknown but seems to be common to many hydrocarbon-degrading sulfate-reducing bacteria (38, 44, 45).
As observed for unlabeled or partially deuterated substrates, the deuterated MGM biosynthesized by D. alkenivorans from D32-hexadecene exhibited the same labeled alkyl chains as that of the labeled FA. Several deuterated MGM were also biosynthesized by D. alkenivorans from D32-hexadecene (Table 1), namely, 1-O-D30-C16 and 1-O-D27-C14 and their 10-methyl-branched homologues (1-O-D29-10MeC16 and 1-O-D26-10MeC14, respectively) and 2-O-D27-C14. The number of deuterium atoms in these compounds was determined from the comparison of specific fragment ions in their mass spectra with those of unlabeled analogues. The mass spectrum of 1-O-D30-C16 is shown in Fig. 2c. The O-alkyl fragment at m/z 343 indicates the presence of the 30 deuterium atoms together with three hydrogen atoms in the alkyl chain. Although the exact positions of the hydrogen atoms cannot be deduced from the mass spectrum, the presence of two hydrogen atoms at C-1 and one hydrogen atom and one deuterium atom at C-2 can be inferred from (i) the systematic loss of labeling at C-1 in the same MGM biosynthesized from partially deuterated substrates (Table 1) and (ii) the presence of a hydrogen atom at C-2 of D30-C16 FA (Table 1). Likewise, 1-O-D29-10MeC16 is assumed to exhibit the same hydrogen pattern at C-1 and C-2 as 1-O-D30-C16, whereas all deuterated MGM with a C14 alkyl chain are assumed to contain two hydrogen atoms at C-1 (Table 1). Several deuterated DGD were also detected in hydrolyzed D. alkenivorans cells that grew on D32-hexadecene. The use of a mixture of unlabeled and perdeuterated hexadecene for these labeling experiments induced the formation of DGD with either one labeled and one unlabeled or two labeled alkyl chains (data not shown), but only the latter are considered here. DGD containing 53 to 59 deuterium atoms were identified based on the comparison of the molecular and/or M-15 ions in their mass spectra with those of mass spectra of unlabeled DGD (Fig. 2d and e). For each compound, the upshift of specific fragmentation ions allowed determination of the number of deuterium atoms in the sn-1 and sn-2 alkyl chains (Fig. 2d and e). Five deuterated DGD, namely, 1,2-O-D27-C14/D27-C14, 1,2-O-D26-10MeC14/D27-C14, 1,2-O-D30-C16/D27-C14, 1,2-O-D29-10MeC16/D27-C14, and 1,2-O-D29-10MeC16/D30-C16, were thus identified. The position of hydrogen atoms in each deuterated alkyl chains is considered similar to that inferred for deuterated MGM (see above and Table 1).
DISCUSSION
Dialkylglycerols in mesophilic bacteria.
The membrane lipids of the mesophilic sulfate reducer D. alkenivorans PF2803T are quite distinct from the diacyl glycerol membrane lipids that characterize most mesophilic bacteria, due to the presence of a relatively high (up to 60%) proportion of ether linkages (Fig. 4). In contrast to the Archaea, diether lipids are still uncommon in the bacterial domain, and have long been considered a specificity of thermophilic bacteria due to their original characterization in (hyper)thermophilic species from deep-branching phyla (16–18) (Fig. 1). Since then, diether or tetraether or mixed ether/ester lipids have been reported in an increasing number of bacterial species, including few mesophilic ones (10, 20, 46). Among SRB, diether lipids have thus far been reported only from thermophilic species (16, 19) whereas monoalkyl/monoacyl phospholipids have also been identified in the mesophilic species Desulfosarcina variabilis and Desulforhabdus amnigenus (7) (Fig. 1). Although the production of nonisoprenoidal dialkylglycerols (DGD) by mesophilic sulfate reducers has regularly been taken for granted, this is, as far as we know, the first report of such lipids in a pure strain of SRB. The widespread occurrence of MGM and DGD in different nonextreme environments and their biosynthesis by several types of mesophilic bacteria (Fig. 1) clearly indicate that glycerol ether lipids are not limited to extreme environments and/or extremophilic bacteria. The presence of ether lipids in phylogenetically very distinct lineages (Fig. 1b) further suggests that such lipids may be more prevalent among the bacteria than previously thought. Nevertheless, the reasons underlying the biosynthesis of ether lipids by mesophilic bacteria remain elusive. These compounds may constitute a relic from thermophilic ancestors, or vice versa since hyperthermophilic bacteria may not have emerged first (47, 48). The exact functional role of MGM and DGD is as yet unclear, but, like archaeal ether lipids, bacterial ether lipids likely provide the cell membrane with a higher degree of stability and/or impermeability. Thus, alkylglycerol biosynthesis by mesophilic bacteria may still constitute a way for these organisms to adapt to the environment in which they thrive, as recently hypothesized from the presence of unsaturated MGM in low-temperature oceanic waters (19). Alternatively, ether lipids might make cells more resistant to environmental stress and hydrolysis, as previously suggested for spore-forming myxobacteria (9).
Inferences on the structure of glycerol ether lipids in D. alkenivorans.
Like most membrane lipids, the MGM and DGD detected in hydrolyzed cells of D. alkenivorans strain PF2803T are likely present in vivo as AAG and DGD with attached polar head groups. As we did not analyze the polar lipids of D. alkenivorans in their intact form, the structure of these polar head groups remains unknown. However, bacterial lipids generally contain phosphatidyl head groups (phospholipids) whereas archaeal lipids can contain glycosidic (glycolipid) head groups and phosphatidyl (phospholipid) head groups (49). Moreover, base hydrolysis is sufficient for removing phospholipid head groups, whereas acid hydrolysis is needed to remove glycolipid head groups. Attempts to hydrolyze cells of D. alkenivorans strain PF2803T using acid instead of base catalysis did not yield different lipid profiles (data not shown). It may thus be conjectured that MGM and DGD were present in living cells of D. alkenivorans as monoalkyl/monoacyl- and dialkyl-glycerophospholipids, of which the exact nature of the polar head group remains to be characterized. 1-Alkyl-2-acyl-phosphatidylethanolamines, glycerols, and cholines (AAG-PE, AAG-PG, and AAG-PC, respectively) have been identified in the mesophilic sulfate-reducing Proteobacteria Desulfosarcina variabilis and Desulforhabdus amnigenus (7). AAG and DGD phospholipids are also commonly detected (essentially as PE and PG) in marine sediments hosting prokaryotic communities involved in the anaerobic oxidation of methane, where they are attributed to the SRB partners of methanotrophic Archaea (49). Nonisoprenoidal DGD with a glycosidic head group, however, do exist in nature, but their occurrence seems limited to very peculiar environments, such as the low-temperature alkaline Lost City Hydrothermal Field (29).
Also, the stereochemistry of the alkylglycerols produced by strain PF2803T was not determined, but it can reasonably be assumed that these compounds have a sn-1,2 stereochemistry. Indeed, sn-2,3 glycerol ether lipids have been shown to be specific to Archaea (3, 5), with no exception reported to date. This hypothesis on the stereochemistry of the glycerol backbone further relies on the sn-1,2 stereoconfiguration of the glycerol moieties of branched tetraether lipids (brGDGTs) (Fig. 1) present in soils and peats (15) and likely produced by Acidobacteria (10, 46).
Insights into bacterial (di)alkylglycerol biosynthesis.
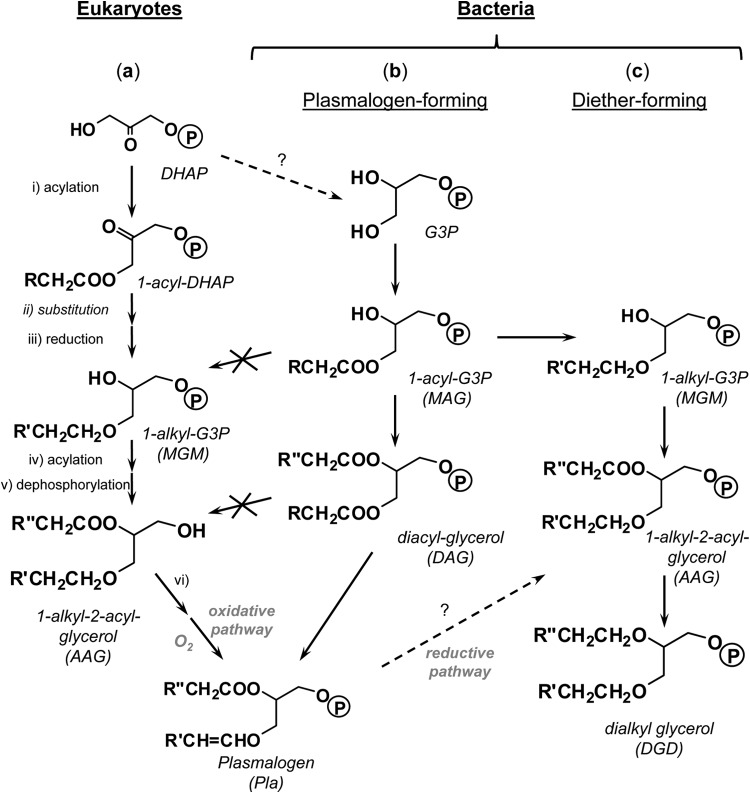
Most data currently available about nonisoprenoid ether lipid biosynthesis relate to 1-alkyl- and 1-alken-1′-yl glycerolipids found in animal and human tissues (see, e.g., references 50, 51, and 52). The biosyntheses of both compound classes in eukaryotes share common reaction steps, including reduction of fatty acids (as CoA derivatives) to the corresponding alcohols, acylation of dihydroxyacetone phosphate (DHAP) with acyl-CoA to form acyldihydroxyacetone phosphate (1-acyl-DHAP) (step i, Fig. 5a), substitution of the acyl group of 1-acyl-DHAP by a fatty alcohol to form 1-O-alkyl-DHAP (step ii, Fig. 5a), reduction of the carbonyl group of alkyl-DHAP to 1-O-alkyl-sn-glycero-3-phosphate (1-alkyl-G3P) (step iii, Fig. 5a), and acylation of the sn-2 position of 1-alkyl-G3P to yield 1-alkyl-2-acyl-glycero-3-phosphate (step iv, Fig. 5a), which is dephosphorylated to 1-alkyl-2-acyl-glycerol (AAG) (step v, Fig. 5a). AAG may then be converted to different types of monoether lipids, including plasmenyl lipids (Pla). In higher organisms, the latter compounds are formed by oxygen-dependent dehydrogenation at C-1 (step vi, Fig. 5a) of the 1-O-alkyl chain of a phosphorylethanolamine derivative of AAG. Plasmalogens are also widespread in anaerobes (e.g., 6, 51, 53), but the pathway and mechanism for anaerobic plasmalogen biosynthesis, which differ from that in higher (aerobic) organisms, remain incompletely understood (12, 13). Aside from the absence of oxygen, isotopic experiments have suggested G3P instead of DHAP as an intermediate in the anaerobic pathway to plasmalogen (51, 54, 55) (Fig. 5b). Striking evidence for a distinct route to plasmalogens in anaerobes compared to higher organisms comes from the demonstration that, in Clostridia, plasmalogens are formed from diacyl phospholipids (DAG derivatives) and not from AAG (13, 56) (Fig. 5b).
FIG 5.
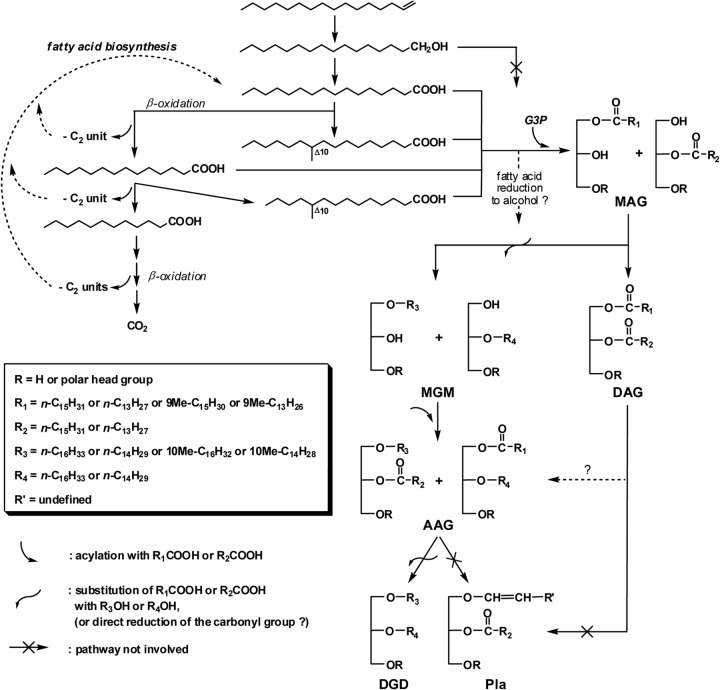
Simplified pathways to ether lipids in eukaryotes (a) and plasmalogen-forming (anaerobic) bacteria (b) and postulated pathway to mono- and dialkyl glycerol ether lipids in anaerobic bacteria (c). The latter pathway is hypothesized to involve intermediates with phosphorous polar head groups. The formation of DGD via the formation of 1-acyl-2-O-alkylglycerols is omitted to simplify the scheme. DHAP, dihydroxyacetone phosphate; G3P, sn-glycero-3-phosphate; 1-acyl-DHAP, acyldihydroxyacetone phosphate; 1-acyl-G3P, 1-acyl-sn-glycero-3-phosphate; MAG, monoacyl glycerol; 1-alkyl-G3P, 1-O-alkyl-sn-glycero-3-phosphate, MGM, monoalkyl glycerol monoether; Pla, plasmalogen (1-alken-1′-yl-2-acyl glycerol); DGD, 1,2-O-dialkyl glycerol diether.
Though the presence of DGD in (hyperthermophilic) bacteria has been known for more than 30 years (16, 17, 21), the pathways and mechanisms for their biosynthesis are not known. The lack of vinyl ethers (1-alken-1′-yl-glycerols) among the neutral lipids of D. alkenivorans strain PF2803T indicates that such lipid structures are not produced by the strain. Vinyl ethers are stable under the basic conditions used for hydrolysis of strain PF2803T cells and thus could not have been degraded during sample processing. The preservation of the labeling at C-2 in deuterated alkylglycerols formed from 2,2-D2-hexadecanoic acid and 1,1,2,2-D4-hexadecanol (Table 1) further demonstrates that saturated MGM and DGD are not formed via the reduction of the double bond(s) of hypothetic alken-1′-yl intermediates. Such a reductive pathway to alkylglycerols from alkenylglycerols (Fig. 5b) has been postulated to occur in fruiting-body-forming aerobic myxobacteria (9, 57) and is sometimes envisaged as the most accommodating pathway to ether lipids in bacteria, although elements of proof are still lacking (10, 14). The present results clearly support the idea of alternative biosynthetic pathways to mono- and dialkyl glycerol ether lipids in (anaerobic) bacteria (Fig. 5c and 6). Schematic biosynthetic routes to MGM and DGD in the mesophilic SRB D. alkenivorans PF2803T can be postulated (Fig. 6) based on the specific labeled alkylglycerols and fatty acids produced during growth on (per)deuterated n-alkyl substrates (Table 1). The first step involves the acylation at the sn-1 position (or, to a lesser extent, at the sn-2 position) of sn-glycerol-3-phosphate (G3P) with an acyl-CoA formed during the oxidation of the growth substrate to yield monoacylglycerols (MAG) (Fig. 6). Whether or not the initial step of this pathway involves the establishment of the G3P backbone from DHAP by G3P dehydrogenase (Fig. 5) is not known. The fatty ester side chains of MAG are then transformed into ether linked alkyl chains to form alkylglycerols (alkyl-G3P or MGM) (Fig. 6). This reaction step may involve either the substitution of the entire acyl chain by an alcohol (as seen in the biosynthesis of eukaryotic 1-O-alkylglycerols) (Fig. 5a) or the direct reduction of the ester group to the corresponding ether (albeit without the formation of an unsaturated intermediate); the exact mechanism remains to be fully determined. The connection of a second ether-linked alkyl chain to the glycerol backbone involves the same sequence of reactions as the formation of MGM, namely, acylation of MGM to form 1-alkyl-2-acylglycerols (or, to a lesser extent, 1-acyl-2-alkylglycerols [AAG]), followed by the replacement or reduction of the acyl chain of AAG to form 1,2-O-dialkylglycerols (DGD) (Fig. 6). Although our workup procedure did not allowed acylglycerols to be detected in their esterified form (i.e., as monoacyl-, monoalkyl/monoacyl-, or diacylglycerols), there are still several lines of evidence suggesting that monoacyl- and monoacyl/monoalkylglycerols constitute intermediates in monoalkyl- and dialkylglycerol biosynthesis, respectively. First, the similar chain-length, methyl-branching, and labeling patterns of FA, MGM, and DGD indicate a biosynthetic link between the three classes of compounds. Second, the loss of labeling at C-1 in alkylglycerols biosynthesized from 1,1,2,2-D4-hexadecanol (Table 1) indicates that a free alcohol substrate first needs to be oxidized to the corresponding FA before being incorporated into membrane phospholipids (Fig. 6). Then, if the transformation of MAG into MGM and AAG into DGD involves the substitution of the complete fatty ester side chain by an alkyl chain (as discussed above), FA need to be reduced back to the corresponding alcohols (Fig. 6) that then interact with the ether lipid-forming enzyme(s).
FIG 6.
Proposed pathway for the formation of glycerol ether lipids in mesophilic sulfate-reducing bacterium D. alkenivorans strain PF2803T during growth on medium- to long-chain n-alkyl substrates (R, R′, R″ > C12). This pathway is likely to involve intermediates with (as-yet-unknown) phosphorous polar head groups. G3P, sn-glycero-3-phosphate; MAG, monoacyl glycerol; MGM, monoalkyl glycerol monoether; AAG, alkyl acyl glycerol; DAG, diacyl glycerol; Pla, plasmalogen (1-alken-1′-yl-2-acyl glycerol); DGD, 1,2-O-dialkyl glycerol diether.
The genes and enzymes involved in MGM and DGD biosynthesis by (anaerobic) bacteria are not known (14) and definitely require investigation. Since the genome sequence of strain PF2803T is not yet available, the search for candidate genes involved in ether lipid biosynthesis in this strain could not been undertaken. It is feasible, however, that an as-yet-unknown acyltransferase(s) and an as-yet-unknown synthase(s) are responsible for the acylation and the eventual replacement of the acyl chains by alkyl chains, respectively. It might be envisaged that the mesophilic bacteria producing MGM and DGD acquired the enzymes carrying out the ether bond formation via horizontal gene transfer from thermophilic Bacteria or Archaea or vice versa, a process which has been shown to occur between thermophilic Archaea and Bacteria (58).
Interestingly, the labeled acyl and alkyl chains incorporated into phospholipids of strain PF2803T have conserved most of the deuterium labeling of the alkyl substrate used (Table 1), indicating that these carbon chains are derived from the first steps of oxidation of the substrate (Fig. 6). It can thus be envisaged that medium- to long-chain alkyl compounds (e.g., n-alkanes, n-alkenes, n-alkanols, and n-fatty acids) present in the environment can serve as the substrates for supplying the building blocks of ether phospholipids of marine heterotrophic bacteria. The prevalence of n-alkyl compounds in the marine environment may provide bacteria an interesting way for building their membranes by recycling sedimentary organic compounds. The recycling of fossil molecules by deep-sea benthic archaea has already been postulated (59) and could represent a crucial strategy for maintaining growth and maintenance under conditions of energy starvation in sedimentary environments (2).
Supplementary Material
ACKNOWLEDGMENT
This work was supported by grant ANR-12-BSV7-0003 (project BAGEL) from the French National Research Agency (ANR).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.03794-14.
REFERENCES
- 1.Woese CR, Kandler O, Wheelis ML. 1990. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci U S A 87:4576–4579. doi: 10.1073/pnas.87.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valentine DL. 2007. Adaptations to energy stress dictate the ecology and evolution of the archaea. Nat Rev Microbiol 5:316–323. doi: 10.1038/nrmicro1619. [DOI] [PubMed] [Google Scholar]
- 3.Koga Y, Kyuragi T, Nishihara M, Sone N. 1998. Did archaeal and bacterial cells arise independently from noncellular precursors? A hypothesis stating that the advent of membrane phospholipid with enantiomeric glycerophosphate backbones caused the separation of the two lines of descent. J Mol Evol 46:54–63. [DOI] [PubMed] [Google Scholar]
- 4.Peretó J, López-García P, Moreira D. 2004. Ancestral lipid biosynthesis and early membrane evolution. Trends Biochem Sci 29:469–477. doi: 10.1016/j.tibs.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Lombard J, López-García P, Moreira D. 2012. The early evolution of lipid membranes and the three domains of life. Nat Rev Microbiol 10:507–515. doi: 10.1038/nrmicro2815. [DOI] [PubMed] [Google Scholar]
- 6.Wagner F, Rottem S, Held H-D, Uhlig S, Zähringer U. 2000. Ether lipids in the cell membrane of Mycoplasma fermentans. Eur J Biochem 267:6276–6286. doi: 10.1046/j.1432-1327.2000.01709.x. [DOI] [PubMed] [Google Scholar]
- 7.Rütters H, Sass H, Cypionka H, Rullkotter J. 2001. Monoalkylether phospholipids in the sulfate-reducing bacteria Desulfosarcina variabilis and Desulforhabdus amnigenus. Arch Microbiol 176:435–442. doi: 10.1007/s002030100343. [DOI] [PubMed] [Google Scholar]
- 8.Paściak M, Holst O, Lindner B, Mordarska H, Gamian A. 2003. Novel bacterial polar lipids containing ether-linked alkyl chains, the structures and biological properties of the four major glycolipids from Propionibacterium propionicum PCM 2431 (ATCC 14157T). J Biol Chem 278:3948–3956. doi: 10.1074/jbc.M206013200. [DOI] [PubMed] [Google Scholar]
- 9.Ring MW, Schwär G, Thiel V, Dickschat JS, Kroppenstedt RM, Schulz S, Bode HB. 2006. Novel iso-branched ether lipids as specific markers of developmental sporulation in the myxobacterium Myxococcus xanthus. J Biol Chem 281:36691–36700. doi: 10.1074/jbc.M607616200. [DOI] [PubMed] [Google Scholar]
- 10.Sinninghe Damsté JS, Rijpstra WIC, Hopmans EC, Weijers JWH, Foesel BU, Overmann J, Dedysh SN. 2011. 13,16-Dimethyl octacosanedioic acid (iso-diabolic acid), a common membrane-spanning lipid of Acidobacteria subdivisions 1 and 3. Appl Environ Microbiol 77:4147–4154. doi: 10.1128/AEM.00466-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horrocks LA, Sharma M. 1982. Plasmalogens and O-alkyl glycerophospholipids, p 51–93. In Neuberger A, van Deenen LLM (ed), New comprehensive biochemistry (1. phospholipids), vol 4 Elsevier Biomedical Press, Amsterdam, The Netherlands. [Google Scholar]
- 12.Goldfine H. 2010. The appearance, disappearance and reappearance of plasmalogens in evolution. Prog Lipid Res 49:493–498. doi: 10.1016/j.plipres.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Guan Z, Johnston NC, Aygun-Sunar S, Daldal F, Raetz CRH, Goldfine H. 2011. Structural characterization of the polar lipids of Clostridium novyi NT. Further evidence for a novel anaerobic biosynthetic pathway to plasmalogens. Biochim Biophys Acta 1811:186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pearson A. 2014. Lipidomics for geochemistry, p 291–336. In Holland HD, Turekian KK (ed), Treatise on geochemistry, 2nd ed, vol 12 Elsevier, Oxford, United Kingdom. [Google Scholar]
- 15.Weijers JWH, Schouten S, Hopmans EC, Geenevasen JAJ, David ORP, Coleman JM, Pancost RD, Sinninghe Damsté JS. 2006. Membrane lipids of mesophilic anaerobic bacteria thriving in peats have typical archaeal traits. Environ Microbiol 8:648–657. doi: 10.1111/j.1462-2920.2005.00941.x. [DOI] [PubMed] [Google Scholar]
- 16.Langworthy TA, Holzer G, Zeikus JG, Tornabene TG. 1983. Iso- and anteiso-branched glycerol diethers of the thermophilic anaerobe Thermodesulfotobacterium commune. Syst Appl Microbiol 4:1–17. doi: 10.1016/S0723-2020(83)80029-0. [DOI] [PubMed] [Google Scholar]
- 17.Huber R, Wilharm T, Huber D, Trincone A, Burggraf S, Konig H, Rachel R, Rockinger I, Fricke H, Stetter KO. 1992. Aquifex pyrophilus gen. nov. sp. nov. represents a novel group of marine hyperthermophilic hydrogen-oxidizing bacteria. Syst Appl Microbiol 15:340–351. [Google Scholar]
- 18.Huber R, Rossnagel P, Woese CR, Rachel R, Langworthy TA, Stetter KO. 1996. Formation of ammonium from nitrate during chemolithoautotrophic growth of the extremely thermophilic bacterium Ammonifex degensii gen. nov. sp. nov. Syst Appl Microbiol 19:40–49. doi: 10.1016/S0723-2020(96)80007-5. [DOI] [PubMed] [Google Scholar]
- 19.Hamilton-Brehm SD, Gibson RA, Green SJ, Hopmans EC, Schouten S, Van der Meer MTJ, Shields JP, Sinninghe Damsté JS, Elkins JG. 2013. Thermodesulfobacterium geofontis sp. nov., a hyperthermophilic, sulfate-reducing bacterium isolated from Obsidian Pool, Yellowstone National Park. Extremophiles 17:251–263. doi: 10.1007/s00792-013-0512-1. [DOI] [PubMed] [Google Scholar]
- 20.Sinninghe Damsté JS, Rijpstra WIC, Geenevasen JAJ, Strous M, Jetten MSM. 2005. Structural identification of ladderane and other membrane lipids of planctomycetes capable of anaerobic ammonium oxidation (anammox): membrane lipids of anammox bacteria. FEBS J 272:4270–4283. doi: 10.1111/j.1742-4658.2005.04842.x. [DOI] [PubMed] [Google Scholar]
- 21.Caillon E, Lubochinsky B, Rigomier D. 1983. Occurrence of dialkyl ether phospholipids in Stigmatella aurantiaca DW4. J Bacteriol 153:1348–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asselineau C, Asselineau J. 1990. Analyse lipidique en taxonomie bactérienne: proposition d'une méthode standardisée. Biochem Cell Biol 68:379–386. doi: 10.1139/o90-053. [DOI] [PubMed] [Google Scholar]
- 23.Lombard J, Moreira D. 2011. Origins and early evolution of the mevalonate pathway of isoprenoid biosynthesis in the three domains of life. Mol Biol Evol 28:87–99. doi: 10.1093/molbev/msq177. [DOI] [PubMed] [Google Scholar]
- 24.Matsumi R, Atomi H, Driessen AJM, van der Oost J. 2011. Isoprenoid biosynthesis in Archaea—biochemical and evolutionary implications. Res Microbiol 162:39–52. doi: 10.1016/j.resmic.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Villanueva L, Sinninghe Damsté J, Schouten S. 2014. A re-evaluation of the archaeal membrane lipid biosynthetic pathway. Nat Rev Microbiol 12:438–448. doi: 10.1038/nrmicro3260. [DOI] [PubMed] [Google Scholar]
- 26.Zeng YB, Ward DM, Brassell SC, Eglinton G. 1992. Biogeochemistry of hot spring environments. 2. Lipid compositions of Yellowstone (Wyoming, USA) cyanobacterial and Chloroflexus mats. Chem Geol 95:327–345. [Google Scholar]
- 27.Jahnke LL, Eder W, Huber R, Hope JM, Hinrichs K-U, Hayes JM, Des Marais DJ, Cady SL, Summons RE. 2001. Signature lipids and stable carbon isotope analyses of Octopus Spring hyperthermophilic communities compared with those of Aquificales representatives. Appl Environ Microbiol 67:5179–5189. doi: 10.1128/AEM.67.11.5179-5189.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pancost RD, Pressley S, Coleman JM, Talbot HM, Kelly SP, Farrimond P, Schouten S, Benning L, Mountain BW. 2006. Composition and implications of diverse lipids in New Zealand geothermal sinters. Geobiology 4:71–92. doi: 10.1111/j.1472-4669.2006.00069.x. [DOI] [Google Scholar]
- 29.Bradley A, Fredricks H, Hinrichs K-U, Summons RE. 2009. Structural diversity of diether lipids in carbonate chimneys at the Lost City Hydrothermal Field. Org Geochem 40:1169–1178. doi: 10.1016/j.orggeochem.2009.09.004. [DOI] [Google Scholar]
- 30.Pancost RD, Bouloubassi I, Aloisi G, Sinninghe Damsté J. 2001. Three series of non-isoprenoidal dialkyl glycerol diethers in cold-seep carbonate crusts. Org Geochem 32:695–707. doi: 10.1016/S0146-6380(01)00015-8. [DOI] [Google Scholar]
- 31.Saito R, Oba M, Kaiho K, Maruo C, Fujibayashi M, Chen J, Chen Z-Q, Tong J. 2013. Ether lipids from the Lower and Middle Triassic at Qingyan, Guizhou Province, southern China. Org Geochem 58:27–42. doi: 10.1016/j.orggeochem.2013.02.002. [DOI] [Google Scholar]
- 32.Arning ET, Birgel D, Schulz-Vogt HN, Holmkvist L, Jørgensen BB, Larson A, Peckmann J. 2008. Lipid biomarker patterns of phosphogenic sediments from upwelling regions. Geomicrobiol J 25:69–82. doi: 10.1080/01490450801934854. [DOI] [Google Scholar]
- 33.Hernandez-Sanchez MT, Homoky WB, Pancost RD. 2014. Occurrence of 1-O-monoalkyl glycerol ether lipids in ocean waters and sediments. Org Geochem 66:1–13. doi: 10.1016/j.orggeochem.2013.10.003. [DOI] [Google Scholar]
- 34.Hinrichs K-U, Summons RE, Orphan V, Sylva SP, Hayes JM. 2000. Molecular and isotopic analysis of anaerobic methane-oxidizing communities in marine sediments. Org Geochem 31:1685–1701. doi: 10.1016/S0146-6380(00)00106-6. [DOI] [Google Scholar]
- 35.Teske A, Hinrichs KU, Edgcomb V, de Vera Gomez A, Kysela D, Sylva SP, Sogin ML, Jannasch HW. 2002. Microbial diversity of hydrothermal sediments in the Guaymas Basin: evidence for anaerobic methanotrophic communities. Appl Environ Microbiol 68:1994–2007. doi: 10.1128/AEM.68.4.1994-2007.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michaelis W, Seifert R, Nauhaus K, Treude T, Thiel V, Blumenberg M, Knittel K, Gieseke A, Peterknecht K, Pape T, Boetius A, Amann R, Jørgensen BB, Widdel F, Peckmann J, Pimenov NV, Gulin MB. 2002. Microbial reefs in the Black Sea fueled by anaerobic oxidation of methane. Science 297:1013–1015. doi: 10.1126/science.1072502. [DOI] [PubMed] [Google Scholar]
- 37.Pancost RD, Sinninghe Damsté JS. 2003. Carbon isotopic compositions of prokaryotic lipids as tracers of carbon cycling in diverse settings. Chem Geol 95:29–58. [Google Scholar]
- 38.Grossi V, Cravo-Laureau C, Méou A, Raphel D, Garzino F, Hirschler-Réa A. 2007. Anaerobic 1-alkene metabolism by the alkane- and alkene-degrading sulfate reducer Desulfatibacillum aliphaticivorans strain CV2803T. Appl Environ Microbiol 73:7882–7890. doi: 10.1128/AEM.01097-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cravo-Laureau C, Matheron R, Joulian C, Cayol J-L, Hirschler-Réa A. 2004. Desulfatibacillum alkenivorans sp. nov., a novel n-alkene-degrading, sulfate-reducing bacterium, and emended description of the genus Desulfatibacillum, Int J Syst Evol Microbiol 54:1639–1642. doi: 10.1099/ijs.0.63104-0. [DOI] [PubMed] [Google Scholar]
- 40.Myher JJ, Marai L, Kuksis A. 1974. Identification of monoacyl- and monoalkylglycerols by gas-liquid chromatography-mass spectrometry using polar siloxane liquid phase. J Lipid Res 15:586–592. [PubMed] [Google Scholar]
- 41.Teixidor P, Grimalt JO, Pueyo JJ, Rodriguez-Valera F. 1993. Isopranylglycerol diethers in non-alkaline evaporitic environments. Geochim Cosmochim Acta 57:4479–4489. doi: 10.1016/0016-7037(93)90497-K. [DOI] [Google Scholar]
- 42.Satouchi K, Saito K, Kates M. 1978. Studies on trimethylsilyl derivatives of 1,2-dialkylglycerols by gas-liquid chromatography mass spectrometry. Biomed Mass Spectrom 5:87–88. doi: 10.1002/bms.1200050117. [DOI] [PubMed] [Google Scholar]
- 43.Egge H. 1983. Mass spectrometry of ether lipids, p 17–47. In Mangold HK, Paltauf F (ed), Ether lipids. Biochemical and biomedical aspects. Academic Press, New York, NY. [Google Scholar]
- 44.Cravo-Laureau C, Grossi V, Raphel D, Matheron R, Hirschler-Réa A. 2005. Anaerobic n-alkane metabolism by a sulfate-reducing bacterium, Desulfatibacillum aliphaticivorans strain CV2803T. Appl Environ Microbiol 71:3458–3467. doi: 10.1128/AEM.71.7.3458-3467.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grossi V, Cravo-Laureau C, Rontani J-F, Cros M, Hirschler-Réa A. 2011. Anaerobic oxidation of n-alkenes by sulphate-reducing bacteria from the genus Desulfatiferula: n-ketones as potential metabolites. Res Microbiol 162:915–922. doi: 10.1016/j.resmic.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 46.Sinninghe Damsté JS, Rijpstra WIC, Hopmans EC, Foesel BU, Wust PK, Overmann J, Tank M, Bryant DA, Dunfield PF, Houghton K, Stott MB. 2014. Ether- and ester-bound iso-diabolic acid and other lipids in members of Acidobacteria subdivision 4. Appl Environ Microbiol 80:5207–5218. doi: 10.1128/AEM.01066-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brochier C, Philippe H. 2002. Phylogeny: a non-hyperthermophilic ancestor for Bacteria. Nature 417:244. doi: 10.1038/417244a. [DOI] [PubMed] [Google Scholar]
- 48.Whitfield J. 2004. Born in a watery commune. Nature 427:674–676. doi: 10.1038/427674a. [DOI] [PubMed] [Google Scholar]
- 49.Sturt HF, Summons RE, Smith K, Elvert M, Hinrichs K-U. 2004. Intact polar membrane lipids in prokaryotes and sediments deciphered by high-performance liquid chromatography/electrospray ionization multistage mass spectrometry—new biomarkers for biogeochemistry and microbial ecology. Rapid Commun Mass Spectrom 18:617–628. doi: 10.1002/rcm.1378. [DOI] [PubMed] [Google Scholar]
- 50.Hajra AK. 1983. Biosynthesis of O-alkylglycerol ether lipids, p 85–106. In Mangold HK, Paltauf F (ed), Ether lipids. Biochemical and biomedical aspects. Academic Press, New York, NY. [Google Scholar]
- 51.Paltauf F. 1983. Biosynthesis of 1-O-(1′alkenyl)glycerolipids (plasmalogens), p 107–128. In Mangold HK, Paltauf F (ed), Ether lipids. Biochemical and biomedical aspects. Academic Press, New York, NY. [Google Scholar]
- 52.Watschinger K, Werner ER. 2013. Orphan enzymes in ether lipid metabolism. Biochimie 95:59–65. doi: 10.1016/j.biochi.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goldfine H, Johnston NC. 2005. Membrane lipids of clostridia, p 297–310. In D̈urre P. (ed), Handbook on clostridia. CRC Press, Taylor & Francis Group, Boca Raton, FL. [Google Scholar]
- 54.Hill EE, Lands WEM. 1970. Formation of acyl and alkenyl glycerol derivatives in Clostridium butyricum. Biochim Biophys Acta 202:209–211. doi: 10.1016/0005-2760(70)90239-0. [DOI] [PubMed] [Google Scholar]
- 55.Prins RA, Van Golde LMG. 1976. Entrance of glycerol into plasmalogens of some strictly anaerobic bacteria and protozoa. FEBS Lett 63:107–111. doi: 10.1016/0014-5793(76)80204-9. [DOI] [PubMed] [Google Scholar]
- 56.Koga Y, Goldfine H. 1984. Biosynthesis of phospholipids in Clostridium butyricum: kinetics of synthesis of plasmalogens and the glycerol acetal of ethanolamine plasmalogen. J Bacteriol 159:597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lorenzen W, Ahrendt T, Bozhüyük KAJ, Bode HB. 2014. A multifunctional enzyme is involved in bacterial ether lipid biosynthesis. Nat Chem Biol 10:425–427. doi: 10.1038/nchembio.1526. [DOI] [PubMed] [Google Scholar]
- 58.Nelson KE, Clayton RA, Gill SR, Gwinn ML, Dodson RJ, Haft DH. 1999. Evidence for lateral gene transfer between Archaea and Bacteria from genome sequence of Thermotoga maritima. Nature 399:323–329. doi: 10.1038/20601. [DOI] [PubMed] [Google Scholar]
- 59.Takano Y, Chikaraishi Y, Ogawa NO, Nomaki H, Morono Y, Inagaki F, Kitazato H, Hinrichs K-U, Ohkouchi N. 2010. Sedimentary membrane lipids recycled by deep-sea benthic archaea. Nat Geosci 3:858–861. doi: 10.1038/ngeo983. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.