Abstract
Lactobacillus plantarum is the lactic acid bacterial species most frequently found in the fermentation of food products of plant origin on which phenolic compounds are abundant. L. plantarum strains showed great flexibility in their ability to adapt to different environments and growth substrates. Of 28 L. plantarum strains analyzed, only cultures from 7 strains were able to hydrolyze hydroxycinnamic esters, such as methyl ferulate or methyl caffeate. As revealed by PCR, only these seven strains possessed the est_1092 gene. When the est_1092 gene was introduced into L. plantarum WCFS1 or L. lactis MG1363, their cultures acquired the ability to degrade hydroxycinnamic esters. These results support the suggestion that Est_1092 is the enzyme responsible for the degradation of hydroxycinnamic esters on the L. plantarum strains analyzed. The Est_1092 protein was recombinantly produced and biochemically characterized. Surprisingly, Est_1092 was able to hydrolyze not only hydroxycinnamic esters, since all the phenolic esters assayed were hydrolyzed. Quantitative PCR experiments revealed that the expression of est_1092 was induced in the presence of methyl ferulate, an hydroxycinnamic ester, but was inhibited on methyl gallate, an hydroxybenzoic ester. As Est_1092 is an enzyme active on a broad range of phenolic esters, simultaneously possessing feruloyl esterase and tannase activities, its presence on some L. plantarum strains provides them with additional advantages to survive and grow on plant environments.
INTRODUCTION
Lactobacillus plantarum is a highly versatile lactic acid bacterial species found in many different ecological niches, such as vegetables, meat, fish, and dairy products, as well as in the gastrointestinal tract (1). The genome of L. plantarum strain WCFS1 was the first to be fully sequenced, and it was, in fact, the first of any of the Lactobacillus genomes to be published (2). When the genome diversity of L. plantarum on a full genome scale was analyzed, it was revealed that L. plantarum strains are predicted to lack 9 to 20% of the genes of the L. plantarum WCFS1 reference genome, and about 50 genes appeared to be specific to strain WCFS1, as they were not found in any other strain (1). This variability confirms the flexibility of L. plantarum in its ability to adapt to different environments and growth substrates.
Phenolic compounds are important constituents of food products of plant origin, as they are related to the sensory characteristics of the food and are beneficial to consumer health (3). Therefore, it is interesting to know the metabolic pathways of biosynthesis or degradation of these compounds in bacteria. L. plantarum is the lactic acid bacterium that is the most frequently found in the fermentation of food products of plant origin, being the bacterial model for the study of phenolic compound metabolism (4). Among these compounds, the metabolism of phenolic esters is greatly relevant, as they are widely spread throughout the plant kingdom (3). Esters of phenolic acids mainly belong to two distinguishing constitutive carbon frameworks: the hydroxycinnamic and the hydroxybenzoic structures (3) (see Fig. S1 in the supplemental material). In relation to hydroxybenzoic esters, two esterase enzymes able to hydrolyze them have been described in L. plantarum. The L. plantarum TanA (TanALp) and L. plantarum TanB (TanBLp) esterases, also known as tannases, hydrolyze the ester bonds of gallic and protocatechuic acids (5, 6). TanBLp is an inducible enzyme present in all L. plantarum strains, whereas TanALp is not inducible by methyl gallate and is rarely present in strains of this species (6). Subsequently, the hydroxybenzoic acids formed by tannase action are decarboxylated by a decarboxylase enzyme recently described (7).
In relation to the metabolism of hydroxycinnamic esters, the decarboxylase enzyme involved in their metabolism (phenolic acid decarboxylase) has been characterized (8, 9); however, knowledge about the esterases (feruloyl esterases) implicated is still limited. Feruloyl esterases are the enzymes involved in the release of phenolic compounds from plant cell walls and constitute an interesting group of enzymes with a potentially broad range of applications in the food, fuel, pharmaceutical, and paper pulp industries (10–13). The potential for feruloyl esterases to be able to open up the plant cell wall is significant for the design of processes for improved biomass utilization (13). Ferulic acid released from the plant cell wall is an effective industrial component by virtue of its antioxidant and photoprotectant properties (11). In human and herbivore digestion, feruloyl esterases are important for the deesterification of dietary fiber, releasing hydroxycinnamates and derivatives, which have been shown to have positive effects, such as antioxidant, anti-inflammatory, and antimicrobial activities (13).
The availability of the L. plantarum WCFS1 genome allows the application of bioinformatics tools to predict the functions of the genes and to reconstruct metabolic pathways and regulatory networks. However, understanding protein function is always a major goal in biology. Most of the genes in sequenced genomes are annotated on the basis of sequence similarity to other proteins that have already been characterized (14). However, the definite approach to assigning a molecular function to a predicted open reading frame is to isolate and biochemically characterize the corresponding protein (14). In this regard, a wide study to dissect the complex array of esterase activities in L. plantarum WCFS1 cells was designed by our group (15–23). From the esterases assayed, only Lp_0796 was able to hydrolyze hydroxycinnamic acids and was therefore considered a feruloyl esterase. Given the industrial significance of feruloyl esterases and taking into account the great variability present on the L. plantarum pangenome, in this work the metabolism of esters from hydroxycinnamic acids was studied in several L. plantarum strains and the enzyme involved on this metabolism was genetically and biochemically characterized.
MATERIALS AND METHODS
Strains and growth conditions.
In this study, 29 L. plantarum strains were analyzed. L. plantarum strains WCFS1, NC8, and LPT 57/1 were kindly provided by M. Kleerebenzem (NIZO Food Research, The Netherlands), L. Axelsson (Norwegian Institute of Food, Fisheries and Aquaculture Research, Norway), and J. L. Ruíz-Barba (Instituto de la Grasa, CSIC, Spain), respectively. Eight strains were purchased from the Spanish Type Culture Collection (CECT): L. plantarum CECT 220 (ATCC 8014), CECT 221 (ATCC 14431), CECT 223, CECT 224, CECT 749 (ATCC 10241), CECT 4185, CECT 4645 (NCFB 1193), and the type strain L. plantarum subsp. plantarum CECT 748 (ATCC 14917, DSMZ 20174). Seven strains were purchased from the German Collection of Microorganisms and Cell Cultures (DSMZ): L. plantarum DSM 1055, DSM 2648, DSM 10492, DSM 12028, DSM 13273, DSM 20246, and the type strain L. plantarum subsp. argentoratensis DSM 16365. Eleven strains were isolated from must grape or wine of different wine-producing areas of Spain over the period from 1998 to 2001: L. plantarum RM28, RM31, RM34, RM35, RM38, RM39, RM40, RM41, RM71, RM72, and RM73 (24). In addition, two Lactobacillus paraplantarum strains purchased from the DSMZ, DSM 10641 (ATCC 10776) and DSM 10667T, were also analyzed. Among these strains, the complete genome sequences of the WCFS1 (GenBank assembly number GCA_000203855.3), NC8 (GenBank assembly number GCA_000247735.2), and ATCC 14917T (GenBank assembly number GCA_000143745.1) strains are available.
The L. plantarum strains were routinely grown in MRS medium adjusted to pH 6.5 and incubated at 30°C. For the degradation assays, the L. plantarum strains were cultivated in a modified basal and defined medium for L. plantarum described previously (18, 25).
Lactococcus lactis MG1363 was used as a host for heterologous gene expression in the pNZ:Tu plasmid (26). Escherichia coli DH10B was used for DNA manipulations. E. coli BL21(DE3) was used for expression in the pURI3-Cter vector (27). The pGro7 vector (TaKaRa) overexpressing GroES/GroEL E. coli chaperones was also used. E. coli strains were cultured in Luria-Bertani (LB) medium at 37°C and 140 rpm. When required, ampicillin and chloramphenicol were added to the medium at concentrations of 100 and 20 μg/ml, respectively.
Hydrolysis of esters of phenolic acids by L. plantarum cultures.
The sterilized modified basal medium was supplemented with the filter-sterilized esters of phenolic acids at a 1 mM final concentration. L. plantarum WCFS1 transformed with the pNZ:Tu plasmid harboring the est_1092 gene was grown in RPM (a modified basal and defined medium for L. plantarum described previously [18, 25]) containing chloramphenicol (5 μg/ml). L. lactis was cultivated in M17 medium supplemented with glucose (final concentration, 0.5%), and L. lactis strains harboring pNZ:Tu derivatives were grown in medium to which chloramphenicol (5 μg/ml) was added. The L. plantarum- or L. lactis-inoculated media were incubated in the darkness without shaking at 30°C for 7 days. Incubated media with cells and without phenolic compound and incubated media without cells and with phenolic compounds were used as controls. The reaction products were extracted twice with one-third of the reaction volume of ethyl acetate (Lab-Scan, Ireland). The solvent fractions were filtered through a 0.45-μm-pore-size polyvinylidene difluoride (PVDF) filter (Teknokroma, Spain) and analyzed by high-pressure liquid chromatography (HPLC).
PCR detection of est_1092 gene.
Bacterial chromosomal DNA was isolated from overnight cultures as described previously (28). The est_1092 gene (GenBank accession number CP001617.1) was amplified by PCR using 10 ng of chromosomal DNA. PCRs were performed in 0.2-ml centrifuge tubes in a total volume of 25 μl containing 1 μl of template DNA (approximately 10 ng), 20 mM Tris-HCl (pH 8.0), 50 mM KCl, 2.5 mM MgCl2, 200 μM each deoxynucleoside triphosphate, 1 U of AmpliTaq Gold DNA polymerase, and 1 μM each primer. The reactions were performed using oligonucleotides 1230 and 1231 to amplify the est_1092 gene in a personal Eppendorf thermocycler using the following cycling parameters: an initial 10 min at 98°C for enzyme activation, denaturation at 94°C for 1 min, annealing at 50°C for 30 s, and extension at 72°C for 1 min. The expected size of the amplicon was 0.9 kb. PCR fragments were resolved on a 0.7% agarose gel.
Heterologous expression of est_1092 in L. lactis.
The est_1092 gene from L. plantarum DSM 1055 was amplified by PCR using the F-1092 (5′-CCATGGTATCAAAAGAATTGAGTCGGTCAATAATTG) and R-1092 (5′-TCTAGATCATCAGGCCATATGTTCCTGCAA) oligonucleotides (the underlined sequences represent NcoI and XbaI sites, respectively). The F-1092 forward primer introduced an NcoI site at about the initiation codon of the est_1092 gene, and the R-1092 reverse primer introduced an XbaI site downstream of the stop codon. The PCR product was digested with the two restriction enzymes and ligated into the corresponding restriction sites of vector pNZ:Tu (26). The ligation mixture was transformed into L. lactis MG1363 by electroporation (29), and the transformants containing the recombinant pNZ:Tu-1092 plasmid were checked by restriction mapping and sequencing of the inserted fragment.
Production and purification of Est_1092 from L. plantarum DSM 1055.
The est_1092 gene from L. plantarum DSM 1055 was PCR amplified by the use of PrimeStar HS DNA polymerase (TaKaRa) and primers 1230 (5′-TAACTTTAAGAAGGAGATATACATatgatatcaaaagaattgagtcggt) and 1231 (5′-GCTATTAATGATGATGATGATGATGggccatatgttcctgcaaaaagcg) (the nucleotides pairing the expression vector sequence are indicated in italics, and the nucleotides pairing the lp_1092 gene sequence are indicated in lowercase letters). The 0.9-kb purified PCR product was inserted into the pURI3-Cter vector using a restriction enzyme- and ligation-free cloning strategy (27). The vectors produce recombinant proteins having a six-histidine affinity tag in their C termini. E. coli DH10B cells were transformed; recombinant plasmids were isolated; and plasmids containing the correct insert were identified by restriction enzyme analysis, verified by DNA sequencing, and then transformed into E. coli BL21(DE3) cells for expression.
E. coli BL21(DE3) was cotransformed with the recombinant plasmid pURI3-Cter-1092 and the pGro7 plasmid (TaKaRa), a vector overexpressing GroES/GroEL chaperones. E. coli cells were grown in LB medium containing 100 μg/ml ampicillin, 20 μg/ml chloramphenicol, and 2 mg/ml arabinose until an optical density at 600 nm (OD600) of 0.4 was reached and then induced by adding isopropyl-β-d-thiogalactopyranoside (IPTG) at a final concentration of 0.4 mM. Following induction, the cells were grown at 22°C for 20 h and collected by centrifugation (8,000 × g, 15 min, 4°C). The cells were disrupted, and the Est_1092 protein (GenBank accession number ACT61979.1) was purified by affinity chromatography as described previously (18), except that the bound enzyme was eluted using 150 mM McIlvaine buffer (pH 5.0) (30).
Spectrophotometric assays for esterase activity and substrate specificity.
Esterase activity was determined by a spectrophotometric method previously described, but p-nitrophenyl butyrate (Sigma-Aldrich) was used as the substrate (18).
The substrate specificity of Est_1092 was determined by using different p-nitrophenyl esters of various chain lengths (Sigma-Aldrich), i.e., p-nitrophenyl acetate (C2), p-nitrophenyl butyrate (C4), p-nitrophenyl caprylate (C8), p-nitrophenyl laurate (C12), p-nitrophenyl myristate (C14), and p-nitrophenyl palmitate (C16), as the substrates as described previously (18, 31) but using 50 mM McIlvaine buffer, pH 5.0.
The enzymatic substrate profile of purified protein was determined by using an ester library described previously (18, 32), but two additional esters of hydroxybenzoic acids, ethyl protocatechuate (ethyl 3,4-dihydroxybenzoate) and methyl gallate, were included. p-Nitrophenol was used as a pH indicator to monitor ester hydrolysis colorimetrically (18). Assays with blanks without enzyme were carried out for each substrate, data were collected in triplicate, and the average activities were quantified. Results are shown as means ± standard deviations.
Esterase activity on gallate esters (tannase activity) was determined using a rhodanine assay specific for gallic acid (33). The amount of gallic acid in the reaction mixture was determined as described previously (5). One unit of tannase activity was defined as the amount of enzyme required to release 1 μmol of gallic acid per minute under standard reaction conditions.
HPLC analysis of Est_1092 activity on phenolic esters.
The activity of Est_1092 against 20 potential substrates was analyzed by HPLC. The substrates assayed were esters derived from benzoic and cinnamic acids. Among the benzoic acids, gallic esters (methyl gallate, ethyl gallate, propyl gallate, and lauryl gallate), benzoic esters (methyl benzoate, ethyl benzoate, and vinyl benzoate), hydroxybenzoic esters (methyl 4-hydroxybenzoate and ethyl 4-hydroxybenzoate), vanillic ester (methyl vanillate), and dihydroxybenzoic esters (methyl 2,4-dihydroxybenzoate, methyl 2,5-dihydroxybenzoate, ethyl 3,4-dihydroxybenzoate or ethyl protocatechuate, and ethyl 3,5-dihydroxybenzoate) were analyzed. In relation to hydroxycinnamic acids, ferulic esters (methyl ferulate and ethyl ferulate), caffeic ester (methyl caffeate), p-coumaric ester (methyl p-coumarate), and sinapic ester (methyl sinapinate) were analyzed. In addition, epicatechin gallate was also assayed as a potential substrate. Est_1092 (100 μg) was incubated in McIlvaine buffer (50 mM), pH 5.0, at 30°C in the presence of the substrate (1 mM). As controls, McIlvaine buffer containing the reagents but not the enzyme were incubated under the same conditions. The reaction products were extracted twice with ethyl acetate; the solvent fractions were filtered through a 0.45-μm-pore-size PVDF filter and analyzed by HPLC with photodiode array detection (DAD). A Thermo chromatograph (Thermo Electron Corporation, Waltham, MA, USA) equipped with a P4000 SpectraSystem pump and AS3000 autosampler and a UV6000LP photodiode array detector were used. A gradient of solvent A (water and acetic acid, 98:2, vol/vol) and solvent B (water, acetonitrile, and acetic acid, 78:20:2, vol/vol/vol) was applied to a reversed-phase Nova-Pak C18 cartridge (25 cm by 4.0 mm [inside diameter]; particle size, 4.6 μm) at room temperature as described previously (18). The identification of degradation compounds was carried out by comparing the retention times and spectral data for each peak with those of standards from commercial suppliers or by liquid chromatography-DAD/electrospray ionization-mass spectrometry.
Biochemical characterization of Est_1092.
The effects of pH and temperature on the esterase activity of Est_1092 were studied by using buffers of different pHs ranging from 3 to 9. The buffers (100 mM) used were acetic acid-sodium acetate (pH 3 to 5), sodium phosphate (pH 6 to 7), Tris-HCl (pH 8), and glycine-NaOH (pH 9). The optimal temperature was assayed by incubating purified Est_1092 esterase in 50 mM McIlvaine buffer (pH 5.0) at different temperatures (5, 20, 30, 37, 40, 45, 55, and 65°C). For temperature stability measurements, the recombinant esterase was incubated in 50 mM McIlvaine buffer, pH 5.0, at 20, 30, 37, 45, 55, and 65°C for 5, 15, and 30 min and 1, 2, 4, 6, and 20 h. Aliquots were withdrawn at these incubation times to test the remaining activity under standard conditions. The nonheated enzyme was considered a control, and the activity obtained with the control was given a value of 100%. The analyses were performed in triplicate.
To study the effect of metals and ions on Est_1092 activity, the enzyme was incubated in the presence of the different additives at a final concentration of 1 mM for 5 min at room temperature. Then, the substrate was added and the reaction mixture was incubated at 30°C. The residual esterase activity was measured after the incubation of the purified enzyme with each additive. The additives analyzed were MgCl2, KCl, CaCl2, HgCl2, ZnCl2, CuCl2, NiCl2, MnCl2, Triton X-100, Tween 20, Tween 80, SDS, urea, EDTA, dimethyl sulfoxide, cysteine, dithiothreitol, phenylmethylsulfonyl fluoride, diethyl pyrocarbonate, and β-mercaptoethanol. The esterase activity measured in the absence of any additive was taken as a control and was given a value of 100%. The experiments were done in triplicate.
RNA isolation, reverse transcription-PCR, and quantitative PCR.
For RNA isolation, L. plantarum MRS cultures were grown to an OD600 of 0.8 to 0.9 and then supplemented with methyl ferulate or methyl gallate at a 30 mM final concentration. After 10 min of incubation, the cultures were immediately processed for RNA extraction as previously described (34). After DNase I treatment, the DNA-free RNA was retrotranscribed using a High-Capacity cDNA reverse transcription kit (Applied Biosystems) according to the manufacturer's instructions. From the DNA obtained, quantitative gene expression was analyzed in an ABI Prism 7500 Fast real-time PCR system (Applied Biosystems). The SYBR green method was used, and each assay was performed in triplicate using a SYBR green real-time PCR master mix (Applied Biosystems). Amplification was initiated at 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. Control PCRs were included to confirm the absence of primer dimer formation (no-template control) and to verify that there was no DNA contamination (without reverse transcriptase enzyme as a negative control). Specific primer pairs were designed with the Primer Express (version 3.0) program to amplify internal regions of the lp_0796 and est_1092 esterase genes. Oligonucleotides 977 (5′-GCCAACATGCCGTCATTTTA) and 978 (5′-CCGCACATCATTGGCACTT) were used to amplify 56 bp of lp_0796, and primers 1031 (5′-TCCTCGCGGGCATGTT) and 1032 (5′-CCGTCGCTTGTTGTGCTAATT) were used to amplify a 59-bp fragment of est_1092. The level of expression of the endogenous control gene (16S rRNA) was assayed with primers 597 (5′-GGGTAATCGGCCACATTGG) and 598 (5′-CTGCTGCCTCCCGTAGGA). Amplifications were performed in triplicate. All real-time PCR assays amplified a single product, as determined by melting curve analysis and by electrophoresis. A standard curve was plotted with cycle threshold (CT) values obtained from amplification of known quantities of cDNA and used to determine the efficiency (E) of amplification as 10−1/slope. The levels of expression of the target genes were normalized. BestKeeper analysis (35, 36) was applied, and the geometric mean of the most stably expressed housekeeping gene (16S rRNA) was used as a normalization factor. Results were analyzed using the comparative CT method (also named the double delta-delta CT [2ΔΔCT] method). Relative expression levels were calculated with 7500 Fast system relative quantification software using the L. plantarum 16S rRNA gene as the endogenous gene and the growth in the absence of compound as the growth condition calibrator. In order to measure L. plantarum gene expression, amplification of the endogenous control gene was performed simultaneously, and its expression was compared with that of the target gene.
Statistical analysis.
The two-tailed Student's t test, performed using GraphPad InStat (version 3.0) software (GraphPad Software, San Diego, CA), was used to determine the differences between means. The data are representative means from at least three independent experiments.
RESULTS AND DISCUSSION
Metabolism of esters from hydroxycinnamic acids by L. plantarum strains.
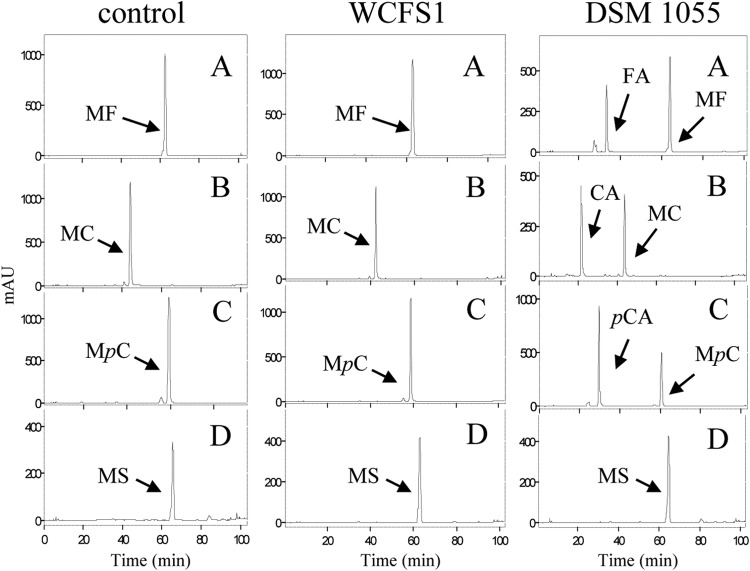
Previously, it has been described that L. plantarum WCFS1 cultures are unable to hydrolyze any of the four esters from hydroxycinnamic acids assayed (methyl ferulate, methyl caffeate, methyl p-coumarate, and methyl sinapinate), even though this strain possesses Lp_0796, an enzyme exhibiting feruloyl esterase activity (18). In order to know if this is a general behavior among L. plantarum strains, 27 additional strains were assayed. L. plantarum cultures were grown for 7 days in the presence of the four model substrates for feruloyl esterases at a 1 mM final concentration. After various incubation times, the phenolic compounds present in the supernatants were analyzed by HPLC. Similar to the findings for L. plantarum WCFS1, most of the strains analyzed (20 strains) were unable to hydrolyze the esters assayed. However, seven strains (L. plantarum DSM 1055, CECT 220, CECT 221, CECT 223, CECT 224, RM35, and RM73) partially hydrolyzed methyl ferulate, methyl caffeate, and methyl p-coumarate (Fig. 1) but were unable to hydrolyze methyl sinapinate.
FIG 1.
HPLC analysis of hydroxycinnamic ester degradation by L. plantarum cultures. Modified basal medium containing 1 mM methyl ferulate (A), methyl caffeate (B), methyl p-coumarate (C), or methyl sinapinate (D) was inoculated with L. plantarum WCFS1 or DSM 1055 and incubated at 30°C for 7 days. A noninoculated control medium was incubated under the same conditions. Detection was performed at 280 nm. The peaks detected for methyl ferulate (MF), methyl caffeate (MC), methyl p-coumarate (MpC), methyl sinapinate (MS), ferulic acid (FA), caffeic acid (CA), and p-coumaric acid (pCA) are indicated. The chromatograms were recorded at 280 nm. mAU, milli-arbitrary units.
Currently, the complete genomes of almost 20 L. plantarum strains are available. Among the strains analyzed in this study, the genomes of three strains which were unable to hydrolyze the esters assayed (L. plantarum strains WCFS1, ATCC 14917T, and NC8) are available. Initially, it was thought that it could be possible that the gene encoding the esterase involved in the metabolism of these esters from hydroxycinnamic acids is absent from these three genomes. Therefore, the L. plantarum genomes sequenced were studied for the presence or absence of proteins annotated as an esterase. Only one protein annotated as an esterase/lipase was absent from the L. plantarum WCFS1, ATCC 14917T, and NC8 genomes and present in the genome of L. plantarum strains JDM1 (JDM1_1092 protein, GenBank accession number YP_003062676.1), ZJ316 (zj316_1310 protein, GenBank accession number YP_007413995.1), 2025 (N876_10330 protein, GenBank accession number ERJ63142), EGD-AQ4 (N692_11190 protein, GenBank accession number EQM54850.1), and WHE92 (O209_09595 protein, GenBank accession number EYR71161.1). This is a 295-amino-acid-residue protein with an expected molecular mass of 33.5 kDa which exhibited the conserved motif Gly142-X-Ser-X-Gly146 typical of serine hydrolases. Two conserved domains were found in Est_1092, pfam00135 of the carboxyl esterase family and pfam07859 alpha/beta hydrolase fold, a catalytic domain found in a very wide range of enzymes, including esterases. Even though the ability of the sequenced L. plantarum strains possessing this protein to hydrolyze esters from hydroxycinnamic acids is unknown, this protein could be a potential candidate for the wanted esterase. In order to corroborate this hypothesis, the strains assayed previously in this study were analyzed for the presence of this protein (denominated Est_1092 from here on). DNA from these strains was used to amplify the 0.9-kb DNA fragment containing the est_1092 gene. A clear association between the presence of the est_1092 gene and the ability to degrade hydroxycinnamic esters by the L. plantarum cultures was observed. The est_1092 gene was present only in strains CECT 220, CECT 221, CECT 223, CECT 224, RM35, RM73, and DSM 1055, the same strains which possess hydroxycinnamic esterase activity in cultures (see Fig. S2 in the supplemental material).
Other lactobacillus species presented on their genomes genes for proteins similar to Est_1092. Degrees of identity higher than 50% were found between Est_1092 and proteins annotated as an esterase/lipase in L. pentosus IG1 (the G0M163 protein, 82.4% identity), a triacylglycerol lipase in L. gasseri CECT 5714 (the J2Z3C4 protein, 59.8% identity), an esterase/lipase in L. johnsonii ATCC 33200 (the C2E7B7 protein, 57.8%), a lipase in L. acidophilus ATCC 700396 (the Q5FJE2 protein, 56.3%), a lipase in L. crispatus strain ST1 (the D5GYE3 protein, 55.8%), a triacylglycerol lipase in L. helveticus DSM 20075 (the C9LZH1 protein, 54.8%), and a hydrolase in L. jensenii JV_V16 (the D6S2C7 protein, 53.4% identity). However, the biochemical activity of these proteins remains unknown.
Identification of Est_1092 as an enzyme possessing cinnamoyl esterase activity.
An experimental procedure in which this gene was introduced into strains devoid of this activity was performed to ascertain the involvement of Est_1092 in the hydroxycinnamic esterase activity observed. As est_1092 is absent in L. plantarum WCFS1, this strain could be a host adequate for the study of Est_1092 enzymatic activity. In order to demonstrate the activity of Est_1092, the gene encoding this protein was cloned into replicative plasmid pNZ:Tu, resulting in pNZ:Tu-1092. Subsequently, the pNZ:Tu-1092 plasmid was introduced into L. plantarum WCFS1 competent cells. Contrary to the findings for L. plantarum WCFS1, L. plantarum WCFS1(pNZ:Tu-1092) cultures grown in the presence of the model substrates for feruloyl esterases partially hydrolyzed the substrates assayed, with the exception of methyl sinapinate (see Fig. S3 in the supplemental material). As the pNZ:Tu plasmid also replicates on L. lactis, its derivative pNZ:Tu-1092 plasmid was also introduced into L. lactis cells. Cultures of L. lactis MG1363 cells grown in the presence of the four feruloyl esterase substrates were not able to hydrolyze them, although methyl p-coumarate was minimally degraded (see Fig. S3 in the supplemental material). The presence of Est_1092 on L. lactis cells confers to them the ability to hydrolyze partially the four model substrates assayed. It could be observed that in both host bacteria, the substrate most degraded was methyl ferulate, followed by methyl p-coumarate. As only the minor hydrolysis of methyl sinapinate was observed on L. lactis cells, it is possible that Est_1092 could be expressed more efficiently on this bacterium and, therefore, the activity on L. plantarum cells was not detected. These results confirmed that Est_1092 is an esterase able to hydrolyze hydroxycinnamic esters. Apart from the feruloyl esterase Lp_0796 described previously in L. plantarum strains (18), among lactic acid bacteria, only feruloyl esterases from L. johnsonii (12) and L. acidophilus (37) had been previously identified.
Est_1092 is an esterase active on a broad range of esters from phenolic acids.
Given the industrial significance of feruloyl esterases and the difficulty of distinguishing these enzymes on the basis of sequence comparisons alone (38), the function of these proteins needs to be confirmed through the biochemical characterization of the expressed protein. Once the feruloyl esterase activity of Est_1092 was confirmed, it was biochemically characterized. The est_1092 gene from the L. plantarum DSM 1055 strain was cloned into the pURI3-Cter expression vector (27) and transformed into E. coli BL21(DE3). SDS-PAGE analysis of cell extracts showed that there was one major protein band, of approximately 35 kDa, present as inclusion bodies in the insoluble fraction (data not shown). To obtain Est_1092 in a soluble form, plasmid pGro7, producing GroES/GroEL chaperones, was used. When pURI3-Cter-1092 and pGro7 plasmids were used simultaneously, Est_1092 appeared in the intracellular soluble fraction of the cells (Fig. 2). Est_1092 was purified by immobilized metal affinity chromatography, although some overproduced GroEL proteins were retained in the resin and eluted along with Est_1092.
FIG 2.
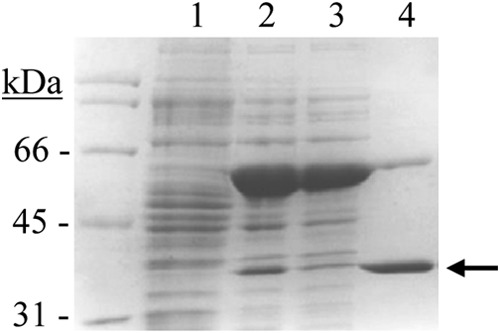
Purification of Est_1092 from L. plantarum DSM 1055. SDS-PAGE analysis of the expression and purification of Est_1092. Soluble cell extracts of IPTG-induced E. coli BL21(DE3)(pURI3-Cter) (lane 1) or E. coli BL21(DE3)(pURI3-Cter-1092) (pGro7) (lane 2), flowthrough from the affinity resin (lane 3), and protein eluted after His affinity resin (lane 4) were analyzed. Arrow, the overproduced and purified protein. The 12.5% gel was stained with Coomassie blue. Molecular mass markers are located in the left lane (SDS-PAGE standards; Bio-Rad).
Esterase activity on pure Est_1092 protein was confirmed using p-nitrophenyl esters possessing different acyl chain lengths from C2 to C16. Est_1092 was active on all the substrates assayed, exhibiting a clear preference for p-nitrophenyl butyrate (Fig. 3A), which was selected as the substrate used to determine the biochemical properties of Est_1092 (see Fig. S4 in the supplemental material). The optimal pH for Est_1092 activity was found to be 5.0, although more than 80% of the maximal activity was observed at pH 6.5 (see Fig. S4A in the supplemental material). In relation to the optimal temperature, Est_1092 exhibited maximum activity at 30°C, although over the range from 5 to 30°C it also presented 90% of its maximal activity (see Fig. S4B in the supplemental material). This activity at a low temperature could be related to the role of L. plantarum in the fermentation of food substrates which is carried out at low temperatures. Interestingly, Est_1092 also showed high thermostability, since it retained up to 70% of its activity after incubation for 20 h at 37°C or 40% of its activity after incubation for 20 h at 45 to 65°C (see Fig. S4C in the supplemental material). Regarding the effects of several ions and additives, the activity of Est_1092 was slightly increased by Tween 80 but highly inhibited by SDS (see Fig. S4D in the supplemental material).
FIG 3.
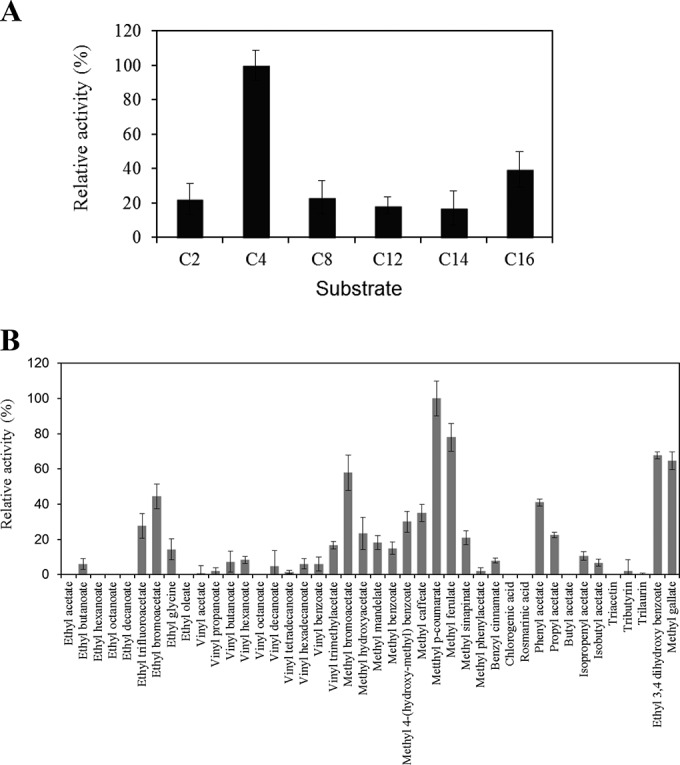
Substrate profile of Est_1092 toward chromogenic substrates (p-nitrophenyl esters) with different acyl chain lengths (C2, acetate; C4, butyrate; C8, caprylate; C12, laurate; C14, myristate; C16, palmitate) (A) or a general ester library (B). The relative specificities obtained toward different substrates are displayed, and the lines on the top of each bar represent the standard deviation estimated from three independent assays. The maximum activity observed was defined as 100%.
The substrates in a library previously used to determine the substrate profile of L. plantarum esterases were assayed (18, 21, 23). This library included two additional esters from hydroxybenzoic acids, ethyl protocatechuate (ethyl 3,4-dihydroxybenzoate) and methyl gallate, used to check tannase activity. As expected, the ester library confirmed that the four model substrates for feruloyl esterases were efficiently hydrolyzed by Est_1092 (Fig. 3B). Surprisingly, Est_1092 also exhibited activity against the two model hydroxybenzoic esters hydrolyzed by bacterial tannases (tannin acyl hydrolases), gallate and protocatechuate esters (5, 6, 39, 40). The specific activity of Est_1092 on methyl gallate hydrolysis was compared to the activity reported for the two tannase enzymes (TanALp and TanBLp) previously described on L. plantarum (6). By using the rhodanine assay, Est_1092 showed a specific activity of 25 U/mg, which was similar to the activity of TanALp (39 U/mg) but significantly lower than the specific activity observed for TanBLp (404 U/mg) (6). This result could indicate that, among the enzymes possessing tannase activity on L. plantarum, gallate esters seem to be hydrolyzed mainly by the action of TanBLp, the only one of these enzymes which is present in all L. plantarum strains.
Hydrolytic activity on esters from hydroxybenzoic acids is not a common activity on feruloyl esterases, such as L. plantarum Lp_0796 (data not shown) (41). To our knowledge, an enzyme (Tan410) possessing feruloyl esterase and tannase activity has been reported only from a cotton soil metagenomic library (42). The ability of an enzyme such as Est_1092 or Tan410 to hydrolyze both acid types makes it an interesting enzyme for biotechnological applications.
In relation to tannase substrates, it has been described that only the esters derived from gallic and protocatechuic acids were hydrolyzed (5, 6, 39, 40, 43). It seems that other hydroxybenzoic acids without hydroxyl groups and with substituents other than —H or —OH at position 2 are not metabolized by bacterial tannases. Therefore, the hydrolysis of nine additional benzoic esters, including benzoic esters (methyl benzoate, ethyl benzoate, and vinyl benzoate), hydroxybenzoic esters (methyl 4-hydroxybenzoate and ethyl 4-hydroxybenzoate), vanillic ester (methyl vanillate), and dihydroxybenzoic esters (methyl 2,4-dihydroxybenzoate, methyl 2,5-dihydroxybenzoate, and ethyl 3,5-dihydroxybenzoate), was analyzed by HPLC. Unexpectedly and contrary to the findings for bacterial tannases, all the benzoic esters assayed were hydrolyzed (see Fig. S5 in the supplemental material). Therefore, the Est_1092 esterase from L. plantarum is not only a feruloyl esterase which hydrolyzes esters from hydroxycinnamic acids and a tannase which hydrolyzes esters from hydroxybenzoic acids but also is an esterase active on a broad range of esters from phenolic acids.
Expression of est_1092 in the presence of phenolic esters.
Esters from phenolic acids are common in foods, as phenolic acids account for almost one-third of the dietary phenols (44). L. plantarum strains could be found on environmental niches on which phenolic acids are abundant. In order to gain insights into the specific physiological role of the esterases hydrolyzing hydroxycinnamic acids on L. plantarum DSM 1055, Lp_0796 and Est_1092, the relative expression of both esterase-encoding genes upon exposure to a hydroxybenzoic ester (methyl gallate) or a hydroxycinnamic ester (methyl ferulate) was studied. L. plantarum DSM 1055 cultures were induced for 10 min by the presence of 30 mM methyl gallate or methyl ferulate, which were used as potential esterase substrates. The gene expression levels obtained were substantially different between the two esterase-encoding genes, indicating the presence of two different patterns of expression of these proteins (see Fig. S6 in the supplemental material). The est_1092 gene, present in only a few L plantarum strains, showed an expression level affected by the presence of both potential substrates. Upon methyl gallate exposure, the level of est_1092 transcription was reduced 85-fold. From this strong reduction it could be hypothesized that Est_1092 plays an active role in the synthesis of methyl gallate but not in its hydrolysis. The repression of est_1092 and the low specific tannase activity shown by Est_1092 support the hypothesis that Est_1092 does not play a relevant role during tannin degradation. On the contrary, the hydroxycinnamic ester (methyl ferulate) induces an approximately 2.5-fold increase in the level of expression of the est_1092 gene. This expression behavior allows the assumption that est_1092 encodes an inducible feruloyl esterase in L. plantarum. This expression pattern differs from that observed on the feruloyl esterase Lp_0976. In L. plantarum DSM 1055, the expression of the lp_0796 gene was reduced 1.4-fold in the presence of its substrate, methyl ferulate. Similarly, the expression of lp_0796 in L. plantarum WCFS1 under methyl ferulate exposure was also reduced (5-fold) (data not shown). The repression observed in lp_0796 could explain the degradation pattern presented by L. plantarum WCFS1 cultures in the presence of esters from hydroxycinnamic acids. Even though L. plantarum WCFS1 possesses at least one enzyme exhibiting feruloyl esterase activity (Lp_0796), cultures from this strain were unable to hydrolyze the esters from hydroxycinnamic acids presented on the culture media, probably due to the repression of the lp_0796 gene.
The presence on L. plantarum strains of enzymes able to metabolize phenolic compounds confers on them a selective advantage for life in environments where compounds of plant origin are abundant. In addition, the flexibility of the L. plantarum genome that allows it to adapt to different environments and growth substrates has led to the presence in some L. plantarum strains of an enzyme able to hydrolyze a broad range of esters from phenolic acids. This new esterase will be induced by esters from hydroxycinnamic acids, providing L. plantarum strains an additional advantage to survive and grow on plant environments where these compounds are abundant.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants AGL2011-22745 (MINECO) and RM2012-00004 (INIA). M. Esteban-Torres is the recipient of a JAE predoctoral fellowship from CSIC. J. M. Landete is the recipient of a Ramón y Cajal contract, and L. Santamaría is the recipient of an FPI fellowship from MINECO.
We are grateful to J. M. Barcenilla and M. V. Santamaría for their assistance.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.00323-15.
REFERENCES
- 1.Siezen RJ, van Hylckama Vlieg JET. 2011. Genomic diversity of Lactobacillus plantarum, a natural metabolic engineer. Microb Cell Fact 10(Suppl 1):S3. doi: 10.1186/1475-2859-10-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kleerebezem M, Boekhorst J, van Kranenburg R, Molenaar D, Kipers OP, Leer R, Tarchini R, Peters SA, Sandbrink HM, Fiers MWEJ, Stiekema W, Lankhorst RMK, Bron PA, Hoffer SM, Groot MNN, Kerkhoven R, de Vries M, Ursing B, de vos WM, Siezen RJ. 2003. Complete genome sequence of Lactobacillus plantarum WCFS1. Proc Natl Acad Sci U S A 100:1990–1995. doi: 10.1073/pnas.0337704100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shahidi F, Naczk M. 2003. Phenolics in food and nutraceuticals. CRC Press, London, United Kingdom. [Google Scholar]
- 4.Rodríguez H, Curiel JA, Landete JM, de las Rivas B, López de Felipe F, Gómez-Cordovés C, Mancheño JM, Muñoz R. 2009. Food phenolics and lactic acid bacteria. Int J Food Microbiol 132:78–90. doi: 10.1016/j.ijfoodmicro.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 5.Curiel JA, Rodríguez H, Acebrón I, Mancheño JM, de las Rivas B, Muñoz R. 2009. Production and physicochemical properties of recombinant Lactobacillus plantarum tannase. J Agric Food Chem 57:6224–6230. doi: 10.1021/jf901045s. [DOI] [PubMed] [Google Scholar]
- 6.Jiménez N, Esteban-Torres M, Mancheño JM, de las Rivas B, Muñoz R. 2014. Tannin degradation by a novel tannase enzyme present in some Lactobacillus plantarum strains. Appl Environ Microbiol 80:2991–2997. doi: 10.1128/AEM.00324-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiménez N, Curiel JA, Reverón I, de las Rivas B, Muñoz R. 2013. Uncovering the Lactobacillus plantarum WCFS1 gallate decarboxylase involved in tannin degradation. Appl Environ Microbiol 79:4253–4263. doi: 10.1128/AEM.00840-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cavin JF, Barthelmebs L, Diviès C. 1997. Molecular characterization of an inducible p-coumaric acid decarboxylase from Lactobacillus plantarum: gene cloning, transcriptional analysis, overexpression in Escherichia coli, purification, and characterization. Appl Environ Microbiol 63:1939–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodríguez H, Landete JM, Curiel JA, de las Rivas B, Mancheño JM, Muñoz R. 2008. Characterization of the p-coumaric acid decarboxylase from Lactobacillus plantarum CECT 748T. J Agric Food Chem 56:3068–3072. doi: 10.1021/jf703779s. [DOI] [PubMed] [Google Scholar]
- 10.Benoit I, Navarro D, Marnet N, Rakotomanomana N, Lesage-Meessen L, Sigoillot JC, Asther M, Asther M. 2006. Feruloyl esterases as a tool for the release of phenolic compounds from agro-industrial by-products. Carbohydr Res 341:1820–1827. doi: 10.1016/j.carres.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 11.Koseki T, Fushinobu S, Ardiansyah Shirakawa H, Komai M. 2009. Occurrence, properties, and applications of feruloyl esterases. Appl Microbiol Biotechnol 84:803–810. doi: 10.1007/s00253-009-2148-8. [DOI] [PubMed] [Google Scholar]
- 12.Lai KL, Lorca GL, González CF. 2009. Biochemical properties of two cinnamoyl esterases purified from a Lactobacillus johnsonii strain isolated from stool samples of diabetes-resistant rats. Appl Environ Microbiol 75:5018–5024. doi: 10.1128/AEM.02837-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faulds CB. 2010. What can feruloyl esterases do for us? Phytochem Rev 9:121–132. doi: 10.1007/s11101-009-9156-2. [DOI] [Google Scholar]
- 14.Kuznetsova E, Proudfoot M, Sanders SA, Reinking J, Savchenko A, Arrowsmith CH, Edwards AM, Yakunin AF. 2005. Enzyme genomics: application of general enzymatic screens to discover new enzymes. FEMS Microbiol Rev 29:263–279. doi: 10.1016/j.femsre.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 15.Álvarez Y, Esteban-Torres M, Acebrón I, de las Rivas B, Muñoz R, Martínez-Ripoll M, Mancheño JM. 2011. Preliminary X-ray analysis of twinned crystals of the Q88Y25_Lacpl esterase from Lactobacillus plantarum WCFS1. Acta Crystallogr Sect F Struct Biol Cryst Commun 67:1436–1439. doi: 10.1107/S1744309111036682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Álvarez Y, Esteban-Torres M, Cortés-Cabrera A, Gago F, Acebrón I, Benavente R, Mardo K, de las Rivas B, Muñoz R, Mancheño JM. 2014. Esterase LpEst1 from Lactobacillus plantarum: a novel and atypical member of the αβ hydrolase superfamily of enzymes. PLoS One 9:e92257. doi: 10.1371/journal.pone.0092257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benavente R, Esteban-Torres M, Acebrón I, de las Rivas B, Muñoz R, Alvarez Y, Mancheño JM. 2013. Structure, biochemical characterization and analysis of the pleomorphism of carboxylesterase Cest-2923 from Lactobacillus plantarum WCFS1. FEBS J 280:6658–6671. doi: 10.1111/febs.12569. [DOI] [PubMed] [Google Scholar]
- 18.Esteban-Torres M, Reverón I, Mancheño JM, de las Rivas B, Muñoz R. 2013. Characterization of a feruloyl esterase from Lactobacillus plantarum. Appl Environ Microbiol 17:5130–5136. doi: 10.1128/AEM.01523-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Esteban-Torres M, Barcenilla JM, Mancheño JM, de las Rivas B, Muñoz R. 2014. Characterization of a versatile arylesterase from Lactobacillus plantarum active on wine esters. J Agric Food Chem 62:5118–5125. doi: 10.1021/jf500991m. [DOI] [PubMed] [Google Scholar]
- 20.Esteban-Torres M, Mancheño JM, de las Rivas B, Muñoz R. 2014. Production and characterization of a tributyrin esterase from Lactobacillus plantarum suitable for cheese lipolysis. J Dairy Sci 97:6737–6744. doi: 10.3168/jds.2014-8234. [DOI] [PubMed] [Google Scholar]
- 21.Esteban-Torres M, Mancheño JM, de las Rivas B, Muñoz R. 2014. Characterization of a cold-active esterase from Lactobacillus plantarum suitable for food fermentations. J Agric Food Chem 62:5128–5132. doi: 10.1021/jf501493z. [DOI] [PubMed] [Google Scholar]
- 22.Esteban-Torres M, Santamaría L, de las Rivas B, Muñoz R. 2014. Characterization of a cold-active and salt-tolerant esterase from Lactobacillus plantarum with potential application during cheese ripening. Int Dairy J 39:312–315. doi: 10.1016/j.idairyj.2014.08.004. [DOI] [Google Scholar]
- 23.Esteban-Torres M, Mancheño JM, de las Rivas B, Muñoz R. 2015. Characterization of a halotolerant lipase from the lactic acid bacteria Lactobacillus plantarum useful in food fermentations. LWT Food Sci Technol 60:246–252. doi: 10.1016/j.lwt.2014.05.063. [DOI] [Google Scholar]
- 24.Moreno-Arribas MV, Polo MC, Jorganes F, Muñoz R. 2003. Screening of biogenic amine production by lactic acid bacteria isolated from grape must and wine. Int J Food Microbiol 84:117–123. doi: 10.1016/S0168-1605(02)00391-4. [DOI] [PubMed] [Google Scholar]
- 25.Rozès N, Peres C. 1998. Effects of phenolic compounds on the growth and the fatty acid composition of Lactobacillus plantarum. Appl Microbiol Biotechnol 49:108–111. doi: 10.1007/s002530051145. [DOI] [Google Scholar]
- 26.Landete JM, Peirotén A, Rodríguez E, Margolles A, Medina M, Arques JL. 2014. Anaerobic green fluorescent protein as a marker of Bifidobacterium strains. Int J Food Microbiol 175:6–13. doi: 10.1016/j.ijfoodmicro.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 27.Curiel JA, de las Rivas B, Mancheño JM, Muñoz R. 2011. The pURI family of expression vectors: a versatile set of ligation independent cloning plasmids for producing recombinant His-fusion proteins. Protein Expr Purif 76:44–53. doi: 10.1016/j.pep.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 28.Vaquero I, Marcobal A, Muñoz R. 2004. Tannase activity by lactic acid bacteria isolated from grape must and wine. Int J Food Microbiol 96:199–204. doi: 10.1016/j.ijfoodmicro.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Holo H, Nes IF. 1989. High-frequency transformation, by electroporation, of Lactococcus lactis subsp. cremoris grown with glycine in osmotically stabilized media. Appl Environ Microbiol 55:3119–3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sumby KM, Matthews AH, Grbin PR, Jiranek V. 2009. Cloning and characterization of an intracellular esterase from the wine-associated lactic acid bacterium Oenococcus oeni. Appl Environ Microbiol 75:6729–6735. doi: 10.1128/AEM.01563-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brod FCA, Vernal J, Bertoldo JB, Terenzi H, Arisi AC. 2010. Cloning, expression, purification, and characterization of a novel esterase from Lactobacillus plantarum. Mol Biotechnol 44:242–249. doi: 10.1007/s12033-009-9232-2. [DOI] [PubMed] [Google Scholar]
- 32.Liu AMF, Somers NA, Kazlauskas RJ, Brush TS, Zocher TS, Enzelberger MM, Bornscheuer UT, Horsman GP, Mezzetti A, Schmidt-Dannert C, Schmid RD. 2001. Mapping the substrate selectivity of new hydrolases using colorimetric screening: lipases from Bacillus thermocatenulatus and Ophiostoma piliferum, esterases from Pseudomonas fluorescens and Streptomyces diastatochromogenes. Tetrahedron Asym 12:545–556. doi: 10.1016/S0957-4166(01)00072-6. [DOI] [Google Scholar]
- 33.Inoue KH, Hagerman AE. 1988. Determination of gallotannins with rhodanine. Anal Biochem 169:363–369. doi: 10.1016/0003-2697(88)90296-5. [DOI] [PubMed] [Google Scholar]
- 34.Saulnier DM, Molenaar D, de Vos WM, Gibson GR, Kolida S. 2007. Identification of prebiotic fructooligosaccharide metabolism in Lactobacillus plantarum WCFS1 trough microarrays. Appl Environ Microbiol 73:1753–1765. doi: 10.1128/AEM.01151-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP. 2004. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper—Excel-based tool using pair-wise correlations. Biotechnol Lett 26:509–515. doi: 10.1023/B:BILE.0000019559.84305.47. [DOI] [PubMed] [Google Scholar]
- 36.Smyth GK, Speed T. 2003. Normalization of cDNA microarray data. Methods 31:265–273. doi: 10.1016/S1046-2023(03)00155-5. [DOI] [PubMed] [Google Scholar]
- 37.Wang X, Geng X, Egashira Y, Sanada H. 2004. Purification and characterization of a feruloyl esterase from the intestinal bacterium Lactobacillus acidophilus. Appl Environ Microbiol 70:2367–2372. doi: 10.1128/AEM.70.4.2367-2372.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fojan P, Jonson PH, Petersen MTN, Petersen SB. 2000. What distinguishes an esterase from a lipase: a novel structural approach. Biochimie 82:1033–1041. doi: 10.1016/S0300-9084(00)01188-3. [DOI] [PubMed] [Google Scholar]
- 39.Jiménez N, Barcenilla JM, López de Felipe F, de las Rivas B, Muñoz R. 2014. Characterization of a bacterial tannase from Streptococcus gallolyticus UCN34 suitable for tannin degradation. Appl Microbiol Biotechnol 98:6329–6337. doi: 10.1007/s00253-014-5603-0. [DOI] [PubMed] [Google Scholar]
- 40.Jiménez N, Reverón I, Esteban-Torres M, López de Felipe F, de las Rivas B, Muñoz R. 2014. Genetic and biochemical approaches towards unravelling the degradation of gallotannins by Streptococcus gallolyticus. Microb Cell Fact 13:154. doi: 10.1186/s12934-014-0154-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benoit I, Danchin EGJ, Bleichrodt RJ, de Vries RP. 2008. Biotechnological applications and potential of fungal feruloyl esterases based on prevalence, classification and biochemical diversity. Biotechnol Lett 30:387–396. doi: 10.1007/s10529-007-9564-6. [DOI] [PubMed] [Google Scholar]
- 42.Yao J, Chen QL, Shen AX, Cao W, Liu YH. 2013. A novel feruloyl esterase from a soil metagenomic library with tannase activity. J Mol Catalysis B Enzymatic 95:55–61. doi: 10.1016/j.molcatb.2013.05.026. [DOI] [Google Scholar]
- 43.Chávez-González M, Rodríguez-Durán LV, Balagurusamy N, Pardo-Barragán A, Rodríguez R, Contreras JC, Aguilar CN. 2012. Biotechnological advances and challenges of tannase: an overview. Food Bioprocess Technol 5:445–459. doi: 10.1007/s11947-011-0608-5. [DOI] [Google Scholar]
- 44.Haminiuk CWI, Maciel GM, Plata-Oviedo MSV, Peralta RM. 2012. Phenolic compounds in fruits—an overview. Int J Food Sci Technol 47:2013–2044. doi: 10.1111/j.1365-2621.2012.03067.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.