Abstract
Biological control of postharvest diseases, utilizing wild species and strains of antagonistic yeast species, is a research topic that has received considerable attention in the literature over the past 30 years. In principle, it represents a promising alternative to chemical fungicides for the management of postharvest decay of fruits, vegetables, and grains. A yeast-based biocontrol system is composed of a tritrophic interaction between a host (commodity), a pathogen, and a yeast species, all of which are affected by environmental factors such as temperature, pH, and UV light as well as osmotic and oxidative stresses. Additionally, during the production process, biocontrol agents encounter various severe abiotic stresses that also impact their viability. Therefore, understanding the ecological fitness of the potential yeast biocontrol agents and developing strategies to enhance their stress tolerance are essential to their efficacy and commercial application. The current review provides an overview of the responses of antagonistic yeast species to various environmental stresses, the methods that can be used to improve stress tolerance and efficacy, and the related mechanisms associated with improved stress tolerance.
INTRODUCTION
Postharvest decay of harvested crops caused by fungal pathogens results in significant losses of edible stored fruits, vegetables, and grains. Minimizing these losses is crucial as the worldwide demand for food increases (1). Environmental concerns and food safety issues have made the continued use of chemical fungicides a serious concern, and so several alternative management strategies have been explored. The utilization of wild species and strains of antagonistic yeast species is recognized as one of the promising alternatives. Since Wilson and Wisniewski proposed the first principles and concepts of postharvest biocontrol (2, 3), numerous reviews have been published (4–11). Research has focused on a wide variety of topics. However, the effects of environmental factors on biocontrol systems, especially the viability and efficacy of antagonists, still need to be thoroughly investigated.
Droby et al. (4) highlighted the importance of viewing the use of postharvest biocontrol agents as a tritrophic system involving a plant host, a biocontrol agent, and a pathogen (4). More recently, Liu et al. (8) further reiterated this viewpoint and emphasized that all three of these components are subject to environmental conditions (8). The majority of studies have been conducted on well-characterized strains of the model species such as Saccharomyces cerevisiae. Therefore, the information on stress responses derived from these studies may or may not have direct applicability to the wild species and strains of yeasts that have been identified and utilized in postharvest biocontrol research.
Ambient temperature is one of the major environmental stresses experienced by yeast. In S. cerevisiae (and likely other related yeasts), the heat shock transcription factor 1 (HSF1) protein family is the primary modulator of the heat shock response (HSR), while a second transcription factor, represented by Msn2 and Msn4 genes, also contributes substantially to heat shock gene expression. These transcription factors are responsible for the bulk, if not the entirety, of the HSR (12). Heat shock transcription factors Msn2 and Msn4 and the heat shock protein 12 (HSP12) molecular chaperone play an integral role in cold stress responses in yeast species. Importantly, the ability to quickly and efficiently translate genes associated with cold tolerance is a key adaptation in cold-adapted yeasts (13).
As another major environmental stress in biocontrol systems, oxidative stress causes yeast responses that include an increase in the level of a variety of antioxidants, mediated by complex transcriptional changes. These regulatory mechanisms are aimed at mitigating oxidative injury to cells, such as oxidative damage to DNA, proteins, and lipids. Yeast peroxiredoxin Tsa1 has been recently demonstrated to protect cells from protein-aggregate-induced oxidative stress (14). The adaptive response of yeast species to oxidative stress is largely regulated at the transcriptional level by transcription factors Yap1p, Skn7p, Snt2, Msn2p, and Msn4p. These transcription factors collectively coordinate cellular responses to different oxidative stressors by repressing or upregulating the transcription of specific genes, many of which are associated with antioxidant defenses (15, 16). The process of translation initiation is mediated by a set of highly conserved eukaryotic initiation factors (eIFs) and associated proteins. Translational regulation involves altered protein interactions, activity, and stability, as well as posttranslational modifications (17). More recently, the relocalization of mRNA to mRNA processing bodies (P-bodies) in response to environmental stress in S. cerevisiae has been reported to be biphasic. Some mRNAs are present early, whereas others are recruited much later, concomitant with recruitment of translation initiation factors, such as eIF4E (18).
The balance between energy-efficient growth and the ability to rapidly respond to fluctuating environments is a fundamental physiological challenge for all microorganisms, including yeasts. Cellular functions, such as metabolism, stress protection, growth, and proliferation, reflect cell responses to external factors that can be dynamically adjusted to both transient and long-term environmental changes (15, 19). Hershkovitz et al. (20) examined the transcriptomic response of the biocontrol yeast Metschnikowia fructicola to grapefruit peel and to the pathogen Penicillium digitatum (20). However, no comprehensive studies of the global changes in gene expression in postharvest yeast antagonists in response to abiotic stress have been conducted.
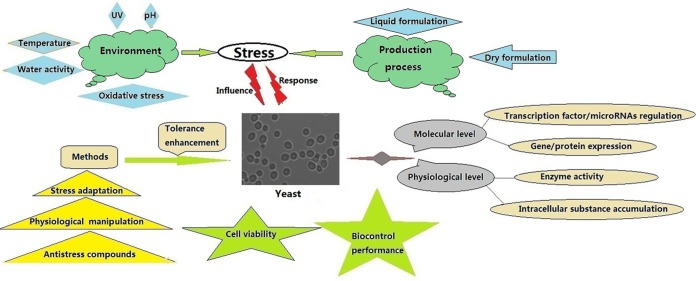
In order for the biological control of postharvest diseases to be successful, antagonists need to possess effective mechanisms to cope with the plethora of abiotic stresses to which they are exposed (5, 21, 22). In this regard, several methods, including physiological manipulation (23, 24), stress adaptation (25, 26), cross-protection (27, 28), and the use of exogenous antistress substances/protectants such as calcium (29), glycine betaine (30, 31), ascorbic acid (32, 33), sugars (33–36), and polyols (37), have been employed to enhance stress tolerance and improve biocontrol efficacy. The purpose of the current review is to provide an overview of research on the role of environmental stresses which affect the viability and performance of antagonistic yeasts. The review focuses on the physiological response of antagonists to stress conditions and on methods that can be used to improve stress tolerance (Fig. 1). Table 1 provides a list of representative studies of responses of antagonistic yeasts to different stresses.
FIG 1.
Diagram of responses of yeast antagonists to stresses and methods to improve their tolerance.
TABLE 1.
Representative studies of antagonistic yeasts in response to different stresses
| Stress | Yeast species | Reference |
|---|---|---|
| Temperature | ||
| Heat | Candida sake CPA-1 | 38 |
| Pichia anomala J121 | 21 | |
| Metschnikowia fructicola NRRL Y-27328 | 28 | |
| Candida oleophila I-182 | 27 | |
| Debaryomyces hansenii, Pichia membranaefaciens | 29 | |
| Pichia guilliermondii KW-103 | 36 | |
| Cold | Pichia anomala J121 | 21 |
| Candida sake CPA-1 | 38 | |
| Oxidative stress/low oxygen | ||
| ROS | Cryptococcus laurentii LS-28, Rhodotorula glutinis LS-11 | 39 |
| Metschnikowia fructicola NRRL Y-27328 | 28 | |
| Cystofilobasidium infirmominiatum PL1 | 30 | |
| Candida oleophila I-182 | 27 | |
| Pichia caribbica | 32 | |
| Oxygen limitation | Pichia anomala J121 | 40 |
| Cryptococcus laurentii, Trichosporon pullulans | 41 | |
| Rhodotorula glutinis, Trichosporon sp. | ||
| Water activity | Pichia anomala J121 | 21 |
| Candida sake CPA-1 | 38 | |
| Rhodosporidium paludigenum | 42 | |
| pH | Pichia anomala J121 | 21 |
| Candida sake CPA-1 | 38 | |
| Rhodosporidium paludigenum | 26 | |
| UV | Pichia anomala strain K | 43 |
ENVIRONMENTAL STRESSES
Temperature.
Once antagonistic yeasts have been applied to a plant surface, exposure to heat stress is inevitable. High temperatures can markedly reduce the viability of biocontrol agents, especially when preharvest applications are administered under field conditions. Some biocontrol yeasts can be administered as preharvest applications (44–46). Therefore, to develop recommendations for their mass production, application, and use, understanding their response to heat stress is essential. Without any protectant, the viability of Candida oleophila decreased with increasing temperature (39 to 41°C for 30 min) (27). In contrast, M. fructicola exhibited a high degree of thermotolerance, with viability dropping sharply only at temperatures above 43°C (28). More specifically, after a 30-min exposure, the viability of C. oleophila cells exposed to 41°C for 30 min dropped to 22%, while the viability of M. fructicola cells exposed to 43°C was 87%. Fredlund et al. (21) reported that Pichia anomala J121, the yeast used to preserve grains in storage, could grow at temperatures ranging from 3 to 37°C (21). This wide range of temperature tolerance greatly contributed to the competitive ability of this biocontrol yeast strain. An et al. (29) compared the levels of thermotolerance of two other biocontrol yeasts, Debaryomyces hansenii and Pichia membranaefaciens, and found that the survival of D. hansenii exposed to 40°C for 20 min was lower than 10% whereas the viability of P. membranaefaciens was approximately 40% after exposure to 50°C for 40 min (29). More recently, the viability of Pichia guilliermondii KW-103 was reported to decrease to as low as 4% when it was incubated at 45°C for 15 min (36). Although the induction of HSPs (47), a mitogen-activated protein kinase cascade (48), and other protein kinases (49) involved in heat response was previously reported, intracellular oxidative damage, resulting from the accumulation of reactive oxygen species (ROS) under heat stress conditions, was proposed to play a major role in the decrease of viability. In a study on the antagonistic yeast Candida sake, Teixidó et al. (38) did not directly investigate its survival in response to heat stress but provided growth profiles at temperatures ranging from 4 to 37°C in conjunction with low levels of water activity and unfavorable pH (38). Thus, different species and strains of yeast antagonists have various degrees of thermotolerance which make them more or less capable of surviving under different field or packing-house conditions. Different ranges of thermotolerance may also impact efficacy in a manner not directly related to cell viability, albeit this possibility has not yet been addressed in the literature. Vegetables, and especially fruits, are often put into cold storage and a controlled atmosphere in order to extend their commercial shelf life and availability. In the case of fruit crops, the time of storage can extend to up to 10 months. Therefore, the ability of a yeast species to colonize and develop on a host (commodity) at low temperatures is also an important feature. While this aspect of biocontrol systems has not been studied in detail, Vero et al. (50) have explored the use of yeast strains isolated from Antarctic soils as potential biocontrol agents for use under conditions of cold storage (50), and Sangorrín et al. recently reviewed the topic of cold-adapted biocontrol yeasts (51).
Oxidative stress and low oxygen.
ROS signaling has been proposed to be a component of the mode of action of yeast antagonists in biocontrol systems. The antagonistic yeasts may serve as an elicitor, triggering ROS signaling in host tissue and leading to the activation of host defenses (52–54). This premise is supported by studies analyzing gene/protein expression during yeast-fruit interactions (20, 55, 56). A proteomic analysis indicated that an antagonistic yeast, P. membranaefaciens, induced six antioxidant proteins in peach fruit, including catalase, glutathione peroxidase, and peroxiredoxin. These antioxidant proteins presumably contributed to ROS scavenging, as ROS levels became elevated as a result of pathogen invasion (55). Biocontrol agents must also be able to tolerate ROS-derived oxidative stress, which can affect their viability and efficacy. It has been reported that peroxidases and superoxide dismutase in grapefruit were induced by application of the yeast M. fructicola, based on transcriptome analysis (56). Castoria et al. (39) investigated the relationship between oxidative stress resistance and the fitness of the postharvest biocontrol yeasts Cryptococcus laurentii LS-28 and Rhodotorula glutinis LS-11 (39). C. laurentii LS-28, which had higher resistance to ROS-generated oxidative stress than R. glutinis LS-11, exhibited greater colonization and better biocontrol efficacy on apple fruit. It was suggested that oxidative stress resistance could be an important component of the fitness and suitability of a yeast species for use as a postharvest biocontrol agent. That report raised issues about the direct effects of oxidative stress on biocontrol yeasts. In this context, Liu et al. examined the response of M. fructicola, C. oleophila, and Cystofilobasidium infirmominiatum PL1 to oxidative stress (27, 28, 30). They found that C. infirmominiatum was the most sensitive to exogenous H2O2, while M. fructicola was the most tolerant (27, 28, 30). More specifically, survival of M. fructicola was 88% in 200 mM H2O2, that of C. oleophila was 28% in 100 mM H2O2, and that of C. infirmominiatum was 23% in 20 mM H2O2, after a 20-min incubation. Li et al. (32) reported that the survival of Pichia caribbica decreased significantly as the concentration of H2O2 increased from 5 to 20 mM (32). Almost all of the yeast cells died upon exposure to 20 mM H2O2 for 60 min. Thus, in similarity to their thermotolerance characteristics, different yeast species can differ dramatically in their levels of tolerance of oxidative stress.
In addition to oxidative stress, antagonistic yeasts may also have to deal with the low oxygen levels associated with controlled-atmosphere storage. This is especially true for the biocontrol agents used to manage mold in grain stored under airtight conditions (57). In this respect, P. anomala J121, a yeast strain used in the biocontrol of mold in grain during storage under conditions of low oxygen and high carbon dioxide, has been extensively studied. P. anomala exhibited strong biocontrol of grain mold pathogens such as Penicillium roqueforti and Aspergillus candidus in vitro (58) and also controlled P. roqueforti on a variety of grain hosts (59). Studies were conducted on high-moisture-content grains stored under airtight (anaerobic) conditions at both the laboratory scale (57) and the farm scale (60), and the results indicated that P. anomala J121 could act as an efficient biocontrol agent (61). Fredlund et al. (40) analyzed growth and metabolite production in P. anomala grown under two sets of oxygen-limited conditions: (i) initial aerobic conditions with restricted oxygen access during the growth period and (ii) initial microaerobic conditions followed by anaerobiosis (40). Biomass production was found to be higher under condition i, while the ethanol production rates during growth on glucose under conditions i and ii were similar, indicating that oxygen availability affected respiration but not the fermentation capacity of P. anomala. In addition, Tian et al. (41) reported that the concentrations of O2 and CO2 affected the viability of four antagonistic yeasts (Cryptococcus laurentii, Trichosporon pullulans, Rhodotorula glutinis, and Trichosporon sp.). All of these yeasts, except R. glutinis, could even grow well on nutrient yeast dextrose agar (NYDA) after 8 days of incubation at 25°C and at 20% CO2 (41). The survival of C. laurentii was also found to be about 80% after 15 days under controlled-atmosphere conditions (5% O2 and 5% CO2) (35).
Water activity.
Several attributes of yeast species make them suitable for use as biocontrol agents, including their ability to withstand osmotic stress (8). Many antagonistic yeast species can grow under conditions of low water activity (aw) (21). Once a yeast species has been applied to a commodity, aw depends on the environmental conditions within the host. For example, cereal grain is commonly harvested with a water content of 20% to 22%, corresponding to aw 0.92 to 0.95. When glycerol was used as an osmolyte, P. anomala was able to grow at aw 0.85 both in a liquid and on a solid substrate. In contrast, the yeast was unable to grow on a substrate containing NaCl below aw 0.92, which emphasizes the difference between a compatible solute and a salt solution (21). The growth of C. sake was also evaluated in nutrient yeast dextrose broth (NYDB) supplemented with an ionic solute, NaCl, and a nonionic solute, glycerol, in the aw range of 0.995 to 0.85. Results indicated that the minimum aw values for sustaining yeast growth were 0.92 with NaCl and 0.90 with glycerol. Both P. anomala and C. sake could grow at a lower aw when glycerol was used as an osmolyte rather than NaCl. Importantly, since the studies were performed in a nutrient-rich growth medium, the responses of a yeast species to low aw could be quite different in a minimal-growth medium or under the limited-nutrient conditions found on the intact surfaces of grains, fruits, and other plant surfaces.
As growth at low aw is advantageous for biocontrol activity, yeasts isolated from high-osmosis environments, such as the ocean, pickled juices, soy sauces, or honey, may be a good source of new antagonists (8). The ability of some yeasts to grow in these salt solutions led to the idea of combining yeast antagonists with various salts, such as CaCl2, MgCl2, and NaHCO3 (62, 63), which has now become a fairly common approach of enhancing biocontrol efficacy, as yeasts appear to be more tolerant of the low aw and the component salt ions. The effect of different solutes (NaCl, glycerol, and glucose) on the growth of Rhodosporidium paludigenum, an antagonistic yeast isolated from the south of East China Sea, was previously examined (42). Results indicated that low water activity (aw = 0.98, 0.97, 0.96, and 0.95) inhibited the growth of R. paludigenum in NYDB but that the yeast grew better in a medium supplemented with NaCl solute than with other nonionic solutes (glycerol and glucose). R. paludigenum grown in 6.6% NaCl-modified medium had higher viability (92.1%) under conditions of low water activity (aw = 0.95) than the control (81.1%) after a 48-h incubation. Salt-adapted R. paludigenum also showed higher tolerance of freeze stress and exhibited better biocontrol performance on pears and jujubes than the nonadapted culture. Overall, the ability of a yeast antagonist to grow at a low water potential may represent an important attribute regarding its use as a biocontrol agent and so should be considered when selecting and developing a potential biocontrol strain.
Other environmental factors.
When antagonists occupy a plant host (fruit, vegetable, or grain), the host pH either in the macroenvironment or in the microenvironment can have a direct impact on growth and establishment. C. sake is tolerant of a wide pH range (3–7), regardless of aw (38). Liu et al. (27) reported that oxidative-stress-adapted C. oleophila grew faster in liquid culture at pH 4.0 (the same pH value as that used to produce Golden Delicious apple flesh at commercial maturity) than non-stress-adapted yeast (27). The in vitro growth of the stress-adapted yeast at pH 4.0 correlated well with in vivo growth and resulted in greater efficacy in inhibiting apple rots caused by P. expansum than non-stress-adapted yeast. Wang et al. (26) assayed low pH tolerance in R. paludigenum and found that preexposure of the yeast to pH 4.0 or 5.5 for 36 h of incubation, adjusted with malic acid or lactic acid, improved the survival of the yeast when it was subsequently exposed to a lethal pH of 1.0 to 3.0 for 1 h (26). The preexposure to pH 4.0 or 5.5 also resulted in a higher growth rate on apple and greater biocontrol efficacy.
UV-B radiation (280 to 320 nm) is also a major environmental factor that can affect the viability of biocontrol agents when they are applied under field conditions. The effect of UV-B radiation on Pichia anomala strain K was evaluated in vitro and in vivo on apple fruit (43). To achieve lethal doses of 50% and 90%, respectively, UV-B at 0.89 and 1.6 Kj/m2 or at 3.2 and 5.76 Kj/m2 had to be applied in vitro or in vivo. Cosupplementation of P. anomala with several protectants, such as riboflavin, folic acid, gelatin, lignin, and tyrosine, was shown to be effective in reducing yeast mortality caused by UV-B radiation.
EXPOSURE TO STRESS DURING PRODUCTION
A commercial biocontrol product needs to have a formulation that provides adequate shelf life while retaining efficacy. Both dry and liquid formulations have been used for the production of commercial biocontrol products. During the production process, biocontrol agents are exposed to severe abiotic stresses that can have a profound effect on their viability and subsequent performance. Sugars, skim milk, and “antistress” substances are often utilized as protectants to ameliorate production-related stresses. In general, dry formulations, compared to wet formulations, offer the advantage of longer shelf life without the need for refrigeration as well as protection from contamination and greater convenience when the product is shipped and distributed (35, 64).
Dry formulation.
Freeze-drying is one of the most common methods used to obtain dry preparations of microorganisms (65, 66). It was reported that freeze-drying at −20°C using 10% skim milk as a protectant was the best method to preserve the viability of yeast (C. sake) cells (34). Freezing cells in liquid nitrogen resulted in the greatest injury to cells, reducing viability to <10%. The viability, efficacy, and stability of freeze-dried C. sake were further tested using different protective and rehydration media (67). The highest level of biocontrol activity was obtained when lactose and skim milk (10% lactose–10% skim milk) were used as protectants during the freeze-drying process and 1% peptone was added when cells were rehydrated prior to use. The use of nutrient protectants at those high concentrations, however, is not economically feasible for large-scale production. Melin et al. (64) compared different formulations (using freeze-drying, vacuum drying, fluidized bed drying, and liquid formulation) of P. anomala J121 and found that the best viability was obtained by freeze-drying the yeast (64). The initial viability after freeze-drying was as high as 80% when trehalose was used as a protectant during the freeze-drying process. Freeze-drying was also tested on Cryptococcus laurentii and Rhodotorula glutinis, and both the endogenous and exogenous levels of trehalose were found to play a critical role in enhancing cell viability (35, 68).
Freeze-drying is relatively expensive compared to other production methods and requires special equipment for batch drying. Therefore, many studies have explored alternative drying methods for producing biocontrol agents, including spray drying, fluidized bed drying, and vacuum drying. Abadias et al. (69) indicated that spray drying of C. sake resulted in a very high level of cell damage, thus greatly reducing viability (69). Although a mild heat treatment could induce thermotolerance, it did not improve the survival of yeast (C. sake) cells exposed to spray drying enough for the method to be considered a suitable approach to commercial production (70). Fluidized bed drying and vacuum drying, along with freeze-drying and liquid formulations, of the yeast antagonist P. anomala J121 have also been evaluated (71). A shelf life of a few months could be obtained, regardless of which production method was used. The use of fluidized bed drying required the addition of cottonseed flour as the main carrier ingredient during the drying process. This method of drying was also evaluated using the yeast-like fungus Aureobasidium pullulans (72). A. pullulans (Ach 1-1) was grown in a glucose-fed batch fermentor for 48 h. The cells were then dried in a fluidized bed dryer to obtain a viability level of 62%. Although viability declined with time, 28% of the initial viability was observed after 7 months when the material was stored at 4°C. Vacuum drying is similar to freeze-drying except that elevated drying temperatures and more-moderate vacuum levels are utilized. Cells are maintained in a nonfrozen state during the whole drying process and thus are metabolically active well into the drying process (73). The addition of trehalose, and the polymer polyvinylpyrrolidone, when vacuum drying the biocontrol agent, P. anomala J121, resulted in high levels of viability and a shelf life of at least 6 months at room temperature (64).
Liquid formulation.
The use of liquid media is another commonly employed method of producing biocontrol agents for commercial use. Liquid formulations have been extensively evaluated in the use of bacterial biocontrol agents such as Pseudomonas fluorescens (74) and Bacillus spp. (75) but not so extensively in the use of yeast-based biocontrol products. In the case of C. sake CPA-1, different media, temperatures, and levels of water activity have been evaluated in relation to a liquid formulation (76). Results indicated that a shelf life of approximately 7 months could be obtained without a significant loss in viability or efficacy when C. sake cells were grown in a sorbitol-modified medium and then stored in an isotonic solution of trehalose. In another pilot-scale study, production of a liquid formulation of Rhodotorula minuta was evaluated (77). The yeast was formulated at 109 CFU/ml in a phosphate buffer solution, with the addition of glycerol (20%) and xanthan (0.5%). The additives greatly inhibited contamination of the final product and prevented cell sedimentation. Using this formulation, a cell count of 107 CFU/ml could be maintained for up to 6 months when the formulated product was stored at 4°C. The maintenance of the viability of P. anomala in a liquid medium amended with lactose, starch, or trehalose was evaluated (78). Supplementing the storage medium with either lactose or trehalose allowed a high level of viability to be maintained over an 8-to-12-week period at all the temperatures tested (−20 to 30°C). Oxidative stress is one of the major factors leading to a decrease in the viability of yeast cells stored in a liquid formulation (77, 79). On the basis of this premise, L-ascorbic acid was added in combination with sugar protectants (trehalose and galactose) to enhance the viability and efficacy of Cryptococcus laurentii and Pichia membranaefaciens in a liquid formulation (33). The shelf life could be extended to 90 days at 4°C and to 15 days at 25°C. Table 2 provides a list of formulation studies conducted on antagonistic yeasts.
TABLE 2.
Representative formulation studies of antagonistic yeasts
| Formulation and yeast species | Reference(s) |
|---|---|
| Dry | |
| Freeze-drying | |
| Candida sake CPA-1 | 34 |
| Pichia anomala J121 | 64, 71 |
| Cryptococcus laurentii, Rhodotorula glutinis | 35, 68 |
| Spray drying | |
| Candida sake CPA-1 | 69 |
| Fluidized bed drying | |
| Pichia anomala J121 | 71 |
| Aureobasidium pullulans (yeast-like fungus) | 72 |
| Vacuum drying | |
| Pichia anomala J121 | 64, 71 |
| Liquid | |
| Candida sake CPA-1 | 76 |
| Rhodotorula minuta | 77 |
| Pichia anomala J121 | 71, 78 |
| Cryptococcus laurentii, Pichia membranaefaciens | 33 |
IMPROVING STRESS TOLERANCE AND BIOCONTROL EFFICACY
Methods for improving stress tolerance in antagonistic yeasts include preadaptation (acclimation) to a stress, physiological manipulation, and the addition of antistress compounds to either the growth medium or the storage medium. Few studies have utilized a transgenic approach to enhancement of stress tolerance due to regulatory and consumer concerns. Currently, the added value provided by transgenic approaches simply does not warrant the time or expense of pursuing this approach, except as an academic pursuit to better understand the molecular basis of stress tolerance.
Stress adaptation.
Stress adaptation, i.e., exposing an organism to a mild stress in order to increase its tolerance of a much stronger stress, has been reported to induce cross-protection against a variety of abiotic stresses in plants (80, 81) and against prokaryotic and eukaryotic microorganisms (82–84). Specifically regarding yeast antagonists, changes in gene expression during heat stress/oxidative stress adaptation and a subsequent improvement in stress tolerance and biocontrol efficacy have been reported (27, 28). A mild heat shock pretreatment (40°C, 30 min) improved the tolerance of M. fructicola to subsequent high-temperature stress (45°C, 20 to 30 min) and oxidative stress (0.4 mM H2O2, 20 to 60 min), at least partially due to the induction of trehalose-6-phosphate synthase 1 (TPS1) expression and the accumulation of trehalose (28). The role of TPS1 in abiotic stress tolerance has also been characterized in two model yeast species, S. cerevisiae (85) and Schizosaccharomyces pombe (86). Pretreatment of C. oleophila with 5 mM H2O2 for 30 min increased its tolerance of a subsequent severe oxidative stress (50 mM H2O2), high temperature (40°C), and low pH (pH 4) by activation of a cellular antioxidant system (27). In addition to heat stress and oxidative-stress adaptation, preexposure to a low pH has been recently reported to improve the viability of R. paludigenum exposed to a subsequent lethal pH (26). Pretreatment with mild concentrations of salt also improved the tolerance of freezing stress and the biocontrol efficacy of R. paludigenum (42). Similar results were obtained in bacterial species used for biocontrol, where preadaptation to osmotic stress was used to enhance the biocontrol efficacy of Bacillus subtilis and Brevibacillus sp. against Fusarium head blight in wheat (87).
Physiological manipulation.
Manipulation of the concentration of intracellular polyols and sugars may be a good approach for improving the ecological fitness of yeast antagonists under field conditions. In research conducted with C. sake, the culture medium (NYDB) was supplemented with glycerol, glucose, trehalose, NaCl, sorbitol, or proline to adjust the level of water activity (aw) and the intracellular concentration of polyols and sugars (23, 37). This resulted in improved ecological fitness and abiotic stress tolerance. Similarly, physiological manipulation was also successfully used on P. anomala to enhance its biocontrol efficacy and to reduce ochratoxin A contamination by Penicillium verrucosum (24). Yeast cells were grown in a liquid, molasses-based medium supplemented with proline to create different levels of aw (0.98 and 0.96), which resulted in a significant (up to 50%) increase in the concentrations of trehalose and arabitol.
Antistress compounds.
Sugars, polyols, and skim milk are commonly used in formulations of biocontrol agents to help maintain viability during the production process, including liquid culture (33, 78, 88), freeze-drying (34, 35, 67, 68), spray drying (69, 70), vacuum drying (64), and fluidized bed drying (71). Among sugar protectants, trehalose, a nonreducing disaccharide, is generally accepted to function as a protective metabolite. The protective function of trehalose is based on the water replacement hypothesis or the glass transition hypothesis (89, 90). Trehalose allows the intracellular solution to undergo a phase transition (becoming glass-like), to remain in disequlibrium with the external vapor pressure, in response to dehydration caused by drying or freezing. The glass-like structure of the cytoplasm prevents cellular injury resulting from the increased concentrations of salts and other potentially toxic compounds and also prevents membranes from adhering to each other. Thus, the plasma membrane is protected and lipid peroxidation is reduced (35, 91). In addition to trehalose, other disaccharides (sucrose and lactose), monosaccharides (glucose, fructose, and galactose), and trisaccharides (raffinose) have also been also evaluated for their protective effects (34). Glucose provided a highly significant (over 90%) protective effect on cell viability in Pichia guilliermondii exposed to 45°C (36). Ascorbic acid, as an antioxidant, has been applied alone (32) or in combination with various sugars (33) to enhance oxidative stress tolerance and biocontrol efficacy. Glycine betaine (N,N,N-trimethyl glycine), a compatible solute, not only acts as an osmoprotectant but also induces an antioxidant response in biocontrol yeasts at either the transcriptional level (Candida oleophila [31]) or the enzymatic level (Cystofilobasidium infirmominiatum [30]).
CONCLUSIONS AND FUTURE OUTLOOK
The commercial use of wild species or strains of yeasts as biocontrol agents presents unique challenges in terms of production and product stability. Most initial studies with biocontrol agents utilized high-cost nutrient media that are not economically feasible for large-scale production. Producing a product that has a long shelf life and maintains efficacy also represents a significant challenge. Although the effects of culture conditions on the physiology and stress tolerance of model yeasts have been extensively studied, much more research is needed to assess how much of the currently available information is applicable to the production of a wide array of natural, wild species and strains of yeasts. Such information may need to be generated on a case-by-case basis, especially for the use and production of “exotic” yeast species. The past 30 years of research have produced an exponential increase in the number of yeast species that have been identified as potential biocontrol agents for the management of postharvest diseases. The desire to circumvent the hurdles to commercialization of these yeasts is providing the impetus for increased research efforts to better understand how these novel yeast species respond to abiotic stress.
ACKNOWLEDGMENTS
This work was supported by National Natural Science Foundation of China (no. 31401794), a project from Department of Education in Anhui Province (no. KJ2014ZD25), and a collaborative project between Hefei University of Technology and USDA (no. 58-1931-4-008FN).
We thank the many scientists involved in the biocontrol yeast research for their efforts and thoughtful discussions on this topic and apologize to the investigators whose specific results could not be cited due to space limitations but whose work framed the issues and ideas that are discussed in this review.
REFERENCES
- 1.Wilson CL. 2013. Establishment of a world food preservation center. Agric Food Secur 2:1–4. doi: 10.1186/2048-7010-2-1. [DOI] [Google Scholar]
- 2.Wilson CL, Wisniewski ME. 1989. Biological control of postharvest diseases of fruits and vegetables: an emerging technology. Annu Rev Phytopathol 27:425–441. [Google Scholar]
- 3.Wisniewski ME, Wilson CL. 1992. Biological control of postharvest diseases of fruits and vegetables: recent advances. HortScience 27:94–98. [Google Scholar]
- 4.Droby S, Wisniewski M, Macarisin D, Wilson C. 2009. Twenty years of postharvest biocontrol research: is it time for a new paradigm? Postharvest Biol Technol 52:137–145. doi: 10.1016/j.postharvbio.2008.11.009. [DOI] [Google Scholar]
- 5.Ippolito A, Nigro F. 2000. Impact of preharvest application of biological control agents on postharvest diseases of fresh fruits and vegetables. Crop Prot 19:715–723. doi: 10.1016/S0261-2194(00)00095-8. [DOI] [Google Scholar]
- 6.Janisiewicz WJ, Korsten L. 2002. Biological control of postharvest diseases of fruits. Annu Rev Phytopathol 40:411–441. doi: 10.1146/annurev.phyto.40.120401.130158. [DOI] [PubMed] [Google Scholar]
- 7.Jijakli MH. 2011. Pichia anomala in biocontrol for apples: 20 years of fundamental research and practical applications. Antonie Van Leeuwenhoek 99:93–105. doi: 10.1007/s10482-010-9541-2. [DOI] [PubMed] [Google Scholar]
- 8.Liu J, Sui Y, Wisniewski M, Droby S, Liu Y. 2013. Review: utilization of antagonistic yeasts to manage postharvest fungal disease of fruit. Int J Food Microbiol 167:153–160. doi: 10.1016/j.ijfoodmicro.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Nunes CA. 2012. Biological control of postharvest diseases of fruit. Eur J Plant Pathol 133:181–196. doi: 10.1007/s10658-011-9919-7. [DOI] [Google Scholar]
- 10.Sharma RR, Singh D, Singh R. 2009. Biological control of postharvest diseases of fruits and vegetables by microbial antagonists: a review. Biol Control 50:205–221. doi: 10.1016/j.biocontrol.2009.05.001. [DOI] [Google Scholar]
- 11.Spadaro D, Gullino ML. 2004. State of the art and future prospects of the biological control of postharvest fruit diseases. Int J Food Microbiol 91:185–194. doi: 10.1016/S0168-1605(03)00380-5. [DOI] [PubMed] [Google Scholar]
- 12.Morano KA, Grant CM, Moye-Rowley WS. 2012. The response to heat shock and oxidative stress in Saccharomyces cerevisiae. Genetics 190:1157–1195. doi: 10.1534/genetics.111.128033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tronchoni J, Medina V, Guillamón JM, Querol A, Pérez-Torrado R. 2014. Transcriptomics of cryophilic Saccharomyces kudriavzevii reveals the key role of gene translation efficiency in cold stress adaptations. BMC Genomics 15:432. doi: 10.1186/1471-2164-15-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weids AJ, Grant CM. 2014. The yeast peroxiredoxin Tsa1 protects against protein-aggregate-induced oxidative stress. J Cell Sci 127:1327–1335. doi: 10.1242/jcs.144022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baker LA, Ueberheide BM, Dewell S, Chait BT, Zheng D, Allis CD. 2013. The yeast Snt2 protein coordinates the transcriptional response to hydrogen peroxide-mediated oxidative stress. Mol Cell Biol 33:3735–3748. doi: 10.1128/MCB.00025-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farrugia G, Balzan R. 2012. Oxidative stress and programmed cell death in yeast. Front Oncol 2:64. doi: 10.3389/fonc.2012.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simpson CE, Ashe MP. 2012. Adaptation to stress in yeast: to translate or not? Biochem Soc Trans 40:794–799. doi: 10.1042/BST20120078. [DOI] [PubMed] [Google Scholar]
- 18.Simpson CE, Lui J, Kershaw CJ, Sims PFG, Ashe MP. 2014. mRNA localization to P-bodies in yeast is bi-phasic with many mRNAs captured in a late Bfr1p-dependent wave. J Cell Sci 127:1254–1262. doi: 10.1242/jcs.139055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.López-Maury L, Marguerat S, Bähler J. 2008. Tuning gene expression to changing environments: from rapid responses to evolutionary adaptation. Nat Rev Genet 9:583–593. doi: 10.1038/nrg2398. [DOI] [PubMed] [Google Scholar]
- 20.Hershkovitz V, Sela N, Taha-Salaime L, Liu J, Rafael G, Kessler C, Aly R, Wisniewski M, Droby S. 2013. De-novo assembly and characterization of the transcriptome of Metschnikowia fructicola reveals differences in gene expression following interaction with Penicillium digitatum and grapefruit peel. BMC Genomics 14:168. doi: 10.1186/1471-2164-14-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fredlund E, Druvefors U, Boysen ME, Lingsten KJ, Schnürer J. 2002. Physiological characteristics of the biocontrol yeast Pichia anomala J121. FEMS Yeast Res 2:395–402. doi: 10.1111/j.1567-1364.2002.tb00109.x. [DOI] [PubMed] [Google Scholar]
- 22.Chen J, Li B, Qin G, Tian S. 2015. Mechanism of H2O2-induced oxidative stress regulating viability and biocontrol ability of Rhodotorula glutinis. Int J Food Microbiol 193:152–158. doi: 10.1016/j.ijfoodmicro.2014.10.025. [DOI] [PubMed] [Google Scholar]
- 23.Abadias M, Teixidó N, Usall J, Viñas I, Magan N. 2001. Improving water stress tolerance of the biocontrol yeast Candida sake grown in molasses-based media by physiological manipulation. Can J Microbiol 47:123–129. doi: 10.1139/w00-138. [DOI] [PubMed] [Google Scholar]
- 24.Mokiou S, Magan N. 2008. Physiological manipulation and formulation of the biocontrol yeast Pichia anomala for control of Penicillium verrucosum and ochratoxin A contamination of moist grain. Biocontrol Sci Technol 18:1063–1073. doi: 10.1080/09583150802585769. [DOI] [Google Scholar]
- 25.Teixidó N, Usall J, Torres R, Abadias Viñas MI. 2011. Improving the efficacy of postharvest biocontrol agents—production of environmental stress tolerant formulations. Acta Hort 905:221–226. [Google Scholar]
- 26.Wang Y, He S, Xia J, Yu T, Zheng X. 2014. Acid adaptation and biocontrol efficacy of antagonistic marine yeast Rhodosporidium paludigenum. Ann Microbiol 64:503–508. doi: 10.1007/s13213-013-0681-2. [DOI] [Google Scholar]
- 27.Liu J, Wisniewski M, Droby S, Norelli J, Hershkovitz V, Tian S, Farrell R. 2012. Increase in antioxidant gene transcripts, stress tolerance and biocontrol efficacy of Candida oleophila following sublethal oxidative stress exposure. FEMS Microbiol Ecol 80:578–590. doi: 10.1111/j.1574-6941.2012.01324.x. [DOI] [PubMed] [Google Scholar]
- 28.Liu J, Wisniewski M, Droby S, Tian S, Hershkovitz V, Tworkoski T. 2011. Effect of heat shock treatment on stress tolerance and biocontrol efficacy of Metschnikowia fructicola. FEMS Microbiol Ecol 76:145–155. doi: 10.1111/j.1574-6941.2010.01037.x. [DOI] [PubMed] [Google Scholar]
- 29.An B, Li B, Qin G, Tian S. 2012. Exogenous calcium improves viability of biocontrol yeasts under heat stress by reducing ROS accumulation and oxidative damage of cellular protein. Curr Microbiol 65:122–127. doi: 10.1007/s00284-012-0133-4. [DOI] [PubMed] [Google Scholar]
- 30.Liu J, Wisniewski M, Droby S, Vero S, Tian S, Hershkovitz V. 2011. Glycine betaine improves oxidative stress tolerance and biocontrol efficacy of the antagonistic yeast Cystofilobasidium infirmominiatum. Int J Food Microbiol 146:76–83. doi: 10.1016/j.ijfoodmicro.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 31.Sui Y, Liu J, Wisniewski M, Droby S, Norelli J, Hershkovitz V. 2012. Pretreatment of the yeast antagonist, Candida oleophila, with glycine betaine increases oxidative stress tolerance in the microenvironment of apple wounds. Int J Food Microbiol 157:45–51. doi: 10.1016/j.ijfoodmicro.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 32.Li C, Zhang H, Yang Q, Komla MG, Zhang X, Zhu S. 2014. Ascorbic acid enhances oxidative stress tolerance and biological control efficacy of Pichia caribbica against postharvest blue mold decay of apples. J Agric Food Chem 62:7612–7621. doi: 10.1021/jf501984n. [DOI] [PubMed] [Google Scholar]
- 33.Liu J, Tian SP, Li BQ, Qin GZ. 2009. Enhancing viability of two biocontrol yeasts in liquid formulation by applying sugar protectant combined with antioxidant. BioControl 54:817–824. doi: 10.1007/s10526-009-9229-6. [DOI] [Google Scholar]
- 34.Abadias M, Benabarre A, Teixidó N, Usall J, Viñas I. 2001. Effect of freeze drying and protectants on viability of the biocontrol yeast Candida sake. Int J Food Microbiol 65:173–182. doi: 10.1016/S0168-1605(00)00513-4. [DOI] [PubMed] [Google Scholar]
- 35.Li BQ, Tian SP. 2006. Effects of trehalose on stress tolerance and biocontrol efficacy of Cryptococcus laurentii. J Appl Microbiol 100:854–861. doi: 10.1111/j.1365-2672.2006.02852.x. [DOI] [PubMed] [Google Scholar]
- 36.Sui Y, Liu J. 2014. Effect of glucose on thermotolerance and biocontrol efficacy of the antagonistic yeast Pichia guilliermondii. Biol Control 74:59–64. doi: 10.1016/j.biocontrol.2014.04.003. [DOI] [Google Scholar]
- 37.Teixidó N, Viñas I, Usall J, Magan N. 1998. Improving ecological fitness and environmental stress tolerance of the biocontrol yeast Candida sake by manipulation of intracellular sugar alcohol and sugar content. Mycol Res 102:1409–1417. [Google Scholar]
- 38.Teixidó N, Viñas I, Usall J, Sanchis V, Magan N. 1998. Ecophysiological responses of the biocontrol yeast Candida sake to water, temperature and pH stress. J Appl Microbiol 84:192–200. [Google Scholar]
- 39.Castoria R, Caputo L, De Curtis F, De Cicco V. 2003. Resistance of postharvest biocontrol yeasts to oxidative stress: a possible new mechanism of action. Phytopathology 93:564–572. doi: 10.1094/PHYTO.2003.93.5.564. [DOI] [PubMed] [Google Scholar]
- 40.Fredlund E, Broberg A, Boysen ME, Kenne L, Schnürer J. 2004. Metabolite profiles of the biocontrol yeast Pichia anomala J121 grown under oxygen limitation. Appl Microbiol Biotechnol 64:403–409. doi: 10.1007/s00253-003-1464-7. [DOI] [PubMed] [Google Scholar]
- 41.Tian SP, Yao HJ, Qin GZ, Xu Y, Feng XY. 2004. Sensitivity of four yeasts to fungicides and CO2 concentrations and their antagonistic ability in combination with fungicide to pathogenic fungi in vitro. Agr Sci China 3:205–215. [Google Scholar]
- 42.Wang Y, Wang P, Xia J, Lou B, Wang J, Zhang XD. 2010. Effect of water activity on stress tolerance and biocontrol activity in antagonistic yeast Rhodosporidium paludigenum. Int J Food Microbiol 143:103–108. doi: 10.1016/j.ijfoodmicro.2010.07.035. [DOI] [PubMed] [Google Scholar]
- 43.Lahlali R, Brostaux Y, Jijakli MH. 2011. Control of apple blue mold by the antagonistic yeast Pichia anomala strain K: screening of UV protectants for preharvest application. Plant Dis 95:311–316. doi: 10.1094/PDIS-04-10-0265. [DOI] [PubMed] [Google Scholar]
- 44.El-Neshawy SM, El-Morsy FM. 2003. Control of gray mold of grape by pre-harvest application of Candida oleophila and its combination with a low dosage of Euparen. Acta Hort 600:95–102. [Google Scholar]
- 45.Karabulut OA, Tezcan H, Daus A, Cohen L, Wiess B, Droby S. 2004. Biological control of preharvest and postharvest rots in strawberries by Metschnikowia fructicola. Biocontrol Sci Technol 14:513–521. doi: 10.1080/09583150410001682287. [DOI] [Google Scholar]
- 46.Teixidó N, Usall J, Viñas I. 1999. Efficacy of preharvest and postharvest Candida sake biocontrol treatments to prevent blue mould on apples during cold storage. Int J Food Microbiol 50:203–210. doi: 10.1016/S0168-1605(99)00105-1. [DOI] [Google Scholar]
- 47.Hahn JS, Hu Z, Thiele DJ, Iyer VR. 2004. Genome-wide analysis of the biology of stress responses through heat shock transcription factor. Mol Cell Biol 24:5249–5256. doi: 10.1128/MCB.24.12.5249-5256.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen D, Toone WM, Mata J, Lyne R, Burns G, Kivinen K, Brazma A, Jones N, Bähler J. 2003. Global transcriptional responses of fission yeast to environmental stress. Mol Biol Cell 14:214–229. doi: 10.1091/mbc.E02-08-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fabrizio P, Pozza F, Pletcher SD, Gendron CM, Longo VD. 2001. Regulation of longevity and stress resistance by Sch9 in yeast. Science 292:288–290. doi: 10.1126/science.1059497. [DOI] [PubMed] [Google Scholar]
- 50.Vero S, Garmendia G, González MB, Bentancur O, Wisniewski M. 2013. Evaluation of yeasts obtained from Antarctic soil samples as biocontrol agents for the management of postharvest diseases of apple (Malus × domestica). FEMS Yeast Res 13:189–199. doi: 10.1111/1567-1364.12021. [DOI] [PubMed] [Google Scholar]
- 51.Sangorrín MP, Lopes CA, Vero S, Wisniewski M. 2014. Cold-adapted yeasts as biocontrol agents: biodiversity, adaptation strategies and biocontrol potential, p 441–464. In Buzzini P, Margesin R (ed), Cold-adapted yeasts. Springer, Berlin, Germany. [Google Scholar]
- 52.Macarisin D, Droby S, Bauchan G, Wisniewski M. 2010. Superoxide anion and hydrogen peroxide in the yeast antagonist–fruit interaction: a new role for reactive oxygen species in postharvest biocontrol? Postharvest Biol Technol 58:194–202. doi: 10.1016/j.postharvbio.2010.07.008. [DOI] [Google Scholar]
- 53.Chan Z, Tian S. 2006. Induction of H2O2-metabolizing enzymes and total protein synthesis by antagonistic yeast and salicylic acid in harvested sweet cherry fruit. Postharvest Biol Technol 39:314–320. doi: 10.1016/j.postharvbio.2005.10.009. [DOI] [Google Scholar]
- 54.Xu X, Qin G, Tian S. 2008. Effect of microbial biocontrol agents on alleviating oxidative damage of peach fruit subjected to fungal pathogen. Int J Food Microbiol 126:153–158. doi: 10.1016/j.ijfoodmicro.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 55.Chan Z, Qin G, Xu X, Li B, Tian S. 2007. Proteome approach to characterize proteins induced by antagonist yeast and salicylic acid in peach fruit. J Proteome Res 6:1677–1688. doi: 10.1021/pr060483r. [DOI] [PubMed] [Google Scholar]
- 56.Hershkovitz V, Ben-Dayan C, Raphael G, Pasmanik-Chor M, Liu J, Belausov E, Aly R, Wisniewski M, Droby S. 2012. Global changes in gene expression of grapefruit peel tissue in response to the yeast biocontrol agent Metschnikowia fructicola. Mol Plant Pathol 13:338–349. doi: 10.1111/j.1364-3703.2011.00750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Petersson S, Jonsson N, Schnürer J. 1999. Pichia anomala as a biocontrol agent during storage of high-moisture feed grain under airtight conditions. Postharvest Biol Technol 15:175–184. doi: 10.1016/S0925-5214(98)00081-7. [DOI] [Google Scholar]
- 58.Björnberg A, Schnürer J. 1993. Inhibition of the growth of grain-storage molds in vitro by the yeast Pichia anomala (Hansen) Kurtzman. Can J Microbiol 39:623–628. doi: 10.1139/m93-090. [DOI] [Google Scholar]
- 59.Petersson S, Schnürer J. 1998. Pichia anomala as a biocontrol agent of Penicillium roqueforti in high-moisture wheat, rye, barley, and oats stored under airtight conditions. Can J Microbiol 44:471–476. doi: 10.1139/w98-018. [DOI] [Google Scholar]
- 60.Olstorpe M, Borling J, Schnürer J, Passoth V. 2010. Pichia anomala yeast improves feed hygiene during storage of moist crimped barley grain under Swedish farm conditions. Anim Feed Sci Technol 156:47–56. doi: 10.1016/j.anifeedsci.2009.12.008. [DOI] [Google Scholar]
- 61.Schnürer J, Jonsson A. 2011. Pichia anomala J121: a 30-year overnight near success biopreservation story. Antonie Van Leeuwenhoek 99:5–12. doi: 10.1007/s10482-010-9509-2. [DOI] [PubMed] [Google Scholar]
- 62.Wisniewski M, Droby S, Chalutz E, Eilam Y. 1995. Effects of Ca2+ and Mg2+ on Botrytis cinerea and Penicillium expansum in vitro and on the biocontrol activity of Candida oleophila. Plant Pathol 44:1016–1024. doi: 10.1111/j.1365-3059.1995.tb02660.x. [DOI] [Google Scholar]
- 63.Yao H, Tian S, Wang Y. 2004. Sodium bicarbonate enhances biocontrol efficacy of yeasts on fungal spoilage of pears. Int J Food Microbiol 93:297–304. doi: 10.1016/j.ijfoodmicro.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 64.Melin P, Håkansson S, Schnürer J. 2007. Optimisation and comparison of liquid and dry formulations of the biocontrol yeast Pichia anomala J121. Appl Microbiol Biotechnol 73:1008–1016. doi: 10.1007/s00253-006-0552-x. [DOI] [PubMed] [Google Scholar]
- 65.Pembrey RS, Marshall KC, Schneider RP. 1999. Cell surface analysis techniques: what do cell preparation protocols do to cell surface properties? Appl Environ Microbiol 65:2877–2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Morgan CA, Herman N, White PA, Vesey G. 2006. Preservation of micro-organisms by drying; a review. J Microbiol Methods 66:183–193. doi: 10.1016/j.mimet.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 67.Abadias M, Teixidó N, Usall J, Benabarre A, Viñas I. 2001. Viability, efficacy, and storage stability of freeze-dried biocontrol agent Candida sake using different protective and rehydration media. J Food Prot 64:856–861. [DOI] [PubMed] [Google Scholar]
- 68.Li BQ, Zhou ZW, Tian SP. 2008. Combined effects of endo- and exogenous trehalose on stress tolerance and biocontrol efficacy of two antagonistic yeasts. Biol Control 46:187–193. doi: 10.1016/j.biocontrol.2008.04.011. [DOI] [Google Scholar]
- 69.Abadias M, Teixidó N, Usall J, Solsona C, Viñas I. 2005. Survival of the postharvest biocontrol yeast Candida sake CPA-1 after dehydration by spray-drying. Biocontrol Sci Technol 15:835–846. doi: 10.1080/09583150500187041. [DOI] [Google Scholar]
- 70.Cañamás TP, Viñas I, Usall J, Magan N, Solsona C, Teixidó N. 2008. Impact of mild heat treatments on induction of thermotolerance in the biocontrol yeast Candida sake CPA-1 and viability after spray-drying. J Appl Microbiol 104:767–775. doi: 10.1111/j.1365-2672.2007.03590.x. [DOI] [PubMed] [Google Scholar]
- 71.Melin P, Schnürer J, Håkansson S. 2011. Formulation and stabilisation of the biocontrol yeast Pichia anomala. Antonie Van Leeuwenhoek 99:107–112. doi: 10.1007/s10482-010-9522-5. [DOI] [PubMed] [Google Scholar]
- 72.Mounir R, Durieux A, Bodo E, Allard C, Simon JP, Achbani EH, El-Jaafari S, Jijakli MH. 2007. Production, formulation and antagonistic activity of the biocontrol like-yeast Aureobasidium pullulans against Penicillium expansum. Biotechnol Lett 29:553–559. doi: 10.1007/s10529-006-9269-2. [DOI] [PubMed] [Google Scholar]
- 73.Manzanera M, Vilchez S, Tunnacliffe A. 2004. High survival and stability rates of Escherichia coli dried in hydroxyectoine. FEMS Microbiol Lett 233:347–352. doi: 10.1111/j.1574-6968.2004.tb09502.x. [DOI] [PubMed] [Google Scholar]
- 74.Manikandan R, Saravanakumar D, Rajendran L, Raguchander T, Samiyappan R. 2010. Standardization of liquid formulation of Pseudomonas fluorescens Pf1 for its efficacy against Fusarium wilt of tomato. Biol Control 54:83–89. doi: 10.1016/j.biocontrol.2010.04.004. [DOI] [Google Scholar]
- 75.Schisler DA, Slininger PJ, Behle RW, Jackson MA. 2004. Formulation of Bacillus spp. for biological control of plant diseases. Phytopathology 94:1267–1271. doi: 10.1094/PHYTO.2004.94.11.1267. [DOI] [PubMed] [Google Scholar]
- 76.Abadias M, Usall J, Teixidó N, Viñas I. 2003. Liquid formulation of the postharvest biocontrol agent Candida sake CPA-1 in isotonic solutions. Phytopathology 93:436–442. doi: 10.1094/PHYTO.2003.93.4.436. [DOI] [PubMed] [Google Scholar]
- 77.Patiño-Vera M, Jiménez B, Balderas K, Ortiz M, Allende R, Carrillo A, Galindo E. 2005. Pilot-scale production and liquid formulation of Rhodotorula minuta, a potential biocontrol agent of mango anthracnose. J Appl Microbiol 99:540–550. doi: 10.1111/j.1365-2672.2005.02646.x. [DOI] [PubMed] [Google Scholar]
- 78.Melin P, Håkansson S, Eberhard TH, Schnürer J. 2006. Survival of the biocontrol yeast Pichia anomala after long-term storage in liquid formulations at different temperatures, assessed by flow cytometry. J Appl Microbiol 100:264–271. doi: 10.1111/j.1365-2672.2005.02778.x. [DOI] [PubMed] [Google Scholar]
- 79.Jakubowski W, Biliński T, Bartosz G. 2000. Oxidative stress during aging of stationary cultures of the yeast Saccharomyces cerevisiae. Free Radic Biol Med 28:659–664. doi: 10.1016/S0891-5849(99)00266-X. [DOI] [PubMed] [Google Scholar]
- 80.Bohnert HJ, Sheveleva E. 1998. Plant stress adaptations—making metabolism move. Curr Opin Plant Biol 1:267–274. doi: 10.1016/S1369-5266(98)80115-5. [DOI] [PubMed] [Google Scholar]
- 81.Wang W, Vinocur B, Altman A. 2003. Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta 218:1–14. doi: 10.1007/s00425-003-1105-5. [DOI] [PubMed] [Google Scholar]
- 82.Bergholz TM, Bowen B, Wiedmann M, Boor KJ. 2012. Listeria monocytogenes shows temperature-dependent and -independent responses to salt stress, including responses that induce cross-protection against other stresses. Appl Environ Microbiol 78:2602–2612. doi: 10.1128/AEM.07658-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Panadero J, Hernández-López MJ, Prieto JA, Randez-Gil F. 2007. Overexpression of the calcineurin target CRZ1 provides freeze tolerance and enhances the fermentative capacity of baker's yeast. Appl Environ Microbiol 73:4824–4831. doi: 10.1128/AEM.02651-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rangel DEN. 2011. Stress induced cross-protection against environmental challenges on prokaryotic and eukaryotic microbes. World J Microbiol Biotechnol 27:1281–1296. doi: 10.1007/s11274-010-0584-3. [DOI] [PubMed] [Google Scholar]
- 85.Rodriguez-Vargas S, Estruch F, Randez-Gil F. 2002. Gene expression analysis of cold and freeze stress in baker's yeast. Appl Environ Microbiol 68:3024–3030. doi: 10.1128/AEM.68.6.3024-3030.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Soto T, Fernández J, Vicente-Soler J, Cansado J, Gacto M. 1999. Accumulation of trehalose by overexpression of tps1, coding for trehalose-6-phosphate synthase, causes increased resistance to multiple stresses in the fission yeast Schizosaccharomyces pombe. Appl Environ Microbiol 65:2020–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Palazzini JM, Ramirez ML, Alberione EJ, Torres AM, Chulze SN. 2009. Osmotic stress adaptation, compatible solutes accumulation and biocontrol efficacy of two potential biocontrol agents on Fusarium head blight in wheat. Biol Control 51:370–376. doi: 10.1016/j.biocontrol.2009.07.008. [DOI] [Google Scholar]
- 88.Torres R, Usall J, Teixidó N, Abadias M, Viñas I. 2003. Liquid formulation of the biocontrol agent Candida sake by modifying water activity or adding protectants. J Appl Microbiol 94:330–339. doi: 10.1046/j.1365-2672.2003.01843.x. [DOI] [PubMed] [Google Scholar]
- 89.Cerrutti P, Segovia de Huergo M, Galvagno M, Schebor C, del Pilar Buera M. 2000. Commercial baker's yeast stability as affected by intracellular content of trehalose, dehydration procedure and the physical properties of external matrices. Appl Microbiol Biotechnol 54:575–580. doi: 10.1007/s002530000428. [DOI] [PubMed] [Google Scholar]
- 90.Cordone L, Cottone G, Giuffrida S. 2007. Role of residual water hydrogen bonding in sugar/water/biomolecule systems: a possible explanation for trehalose peculiarity. J Phys Condens Matter 19:205110. doi: 10.1088/0953-8984/19/20/205110. [DOI] [Google Scholar]
- 91.Herdeiro RS, Pereira MD, Panek AD, Eleutherio ECA. 2006. Trehalose protects Saccharomyces cerevisiae from lipid peroxidation during oxidative stress. BBA Gen Subjects 1760:340–346. doi: 10.1016/j.bbagen.2006.01.010. [DOI] [PubMed] [Google Scholar]