Abstract
The key enzymes and pathways involved in polyhydroxyalkanoate (PHA) biosynthesis in haloarchaea have been identified in recent years, but the haloarchaeal enzymes for PHA degradation remain unknown. In this study, a patatin-like PHA depolymerase, PhaZh1, was determined to be located on the PHA granules in the haloarchaeon Haloferax mediterranei. PhaZh1 hydrolyzed the native PHA (nPHA) [including native polyhydroxybutyrate (nPHB) and native poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (nPHBV) in this study] granules in vitro with 3-hydroxybutyrate (3HB) monomer as the primary product. The site-directed mutagenesis of PhaZh1 indicated that Gly16, Ser47 (in a classical lipase box, G-X-S47-X-G), and Asp195 of this depolymerase were essential for its activity in nPHA granule hydrolysis. Notably, phaZh1 and bdhA (encoding putative 3HB dehydrogenase) form a gene cluster (HFX_6463 to _6464) in H. mediterranei. The 3HB monomer generated from nPHA degradation by PhaZh1 could be further converted into acetoacetate by BdhA, indicating that PhaZh1-BdhA may constitute the first part of a PHA degradation pathway in vivo. Interestingly, although PhaZh1 showed efficient activity and was most likely the key enzyme in nPHA granule hydrolysis in vitro, the knockout of phaZh1 had no significant effect on the intracellular PHA mobilization, implying the existence of an alternative PHA mobilization pathway(s) that functions effectively within the cells of H. mediterranei. Therefore, identification of this patatin-like depolymerase of haloarchaea may provide a new strategy for producing the high-value-added chiral compound (R)-3HB and may also shed light on the PHA mobilization in haloarchaea.
INTRODUCTION
Polyhydroxyalkanoate (PHA) is accumulated in the form of granules and serves as storage compound of carbon and energy in bacteria (1) and archaea (2) during growth in the presence of excess carbon sources. Several proteins, which are known as PHA granule-associated proteins (PGAPs), are embedded on or attached to the PHA granules. These include PHA synthases, phasins, regulatory proteins, and depolymerases (3). A PHA-accumulating host may utilize the accumulated PHA for growth and survival under conditions of carbon starvation (4). PHA depolymerase (PhaZ) is the key enzyme that functions in PHA mobilization.
In bacteria, PHA depolymerases are grouped into two classes: intracellular (catalyzing the degradation of endogenous PHA) (iPhaZ) and extracellular (catalyzing the degradation of exogenous PHA) (ePhaZ) PHA depolymerases (5). The extracellular PHA depolymerase degradation process is well known, but the mechanism underlying the metabolic pathway and regulation of PHA degradation in vivo remains poorly understood (5). Native polyhydroxybutyrate (nPHB) is degraded by iPhaZs in vitro to 3-hydroxybutyrate (3HB) (6, 7) or 3-hydroxybutyryl-coenzyme A (3HB-CoA) in the presence of CoA (8, 9). The PHB degradation product, 3HB-CoA, is the precursor of PHB synthesis; hence, the simultaneous synthesis and mobilization of PHB (10, 11) have been observed, which may be controlled by the [acetyl-CoA]/[CoA] and [NADH]/[NAD+] ratios (8). Notably, the PHA degradation process is not simply along the reverse pathway of PHA synthesis. Crotonyl-CoA and (S)-3HB-CoA (one stereoisomer of 3HB-CoA) were recently found to be involved in nPHB degradation, which indicated that PHB degradation is separate from PHB synthesis and is connected to the β-oxidation cycle in Ralstonia eutropha (9).
As far as we know, nine intracellular PHB depolymerases or PHB oligomer hydrolases have been detected in the bacterium R. eutropha H16 (6), and these are classified as PhaZa, PhaZb, PhaZc, and PhaZd based on their degradation substrates, active sites, and molecular masses. PhaZa1 degrades nPHB to 3HB-CoA in the presence of CoA (8, 9), whereas PhaZb and PhaZc mainly hydrolyze 3HB oligomer to 3HB (12, 13). PhaZd degrades artificial PHB (aPHB) and nPHB granules and releases mainly 3HB oligomer with only a small amount of 3HB monomer (6, 7). A genetic analysis showed the PHB depolymerase redundancy in R. eutropha H16. PhaZa1 is particularly important for PHB degradation (14), while the deletion of phaZa2, phaZa3, phaZb, phaZc, phaZd1, or phaZd2 alone has no significant effect on PHB mobilization (6, 7, 12, 13, 15).
Due to its clear genetic background (16) and the well-established genetic manipulation tools (17), Haloferax mediterranei has been developed as an excellent model system for investigation of the PHA metabolism in haloarchaea. During growth in medium supplemented with glucose as the carbon source, H. mediterranei accumulates a considerable amount of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) containing a high ratio (∼10 mol%) of 3-hydroxyvalerate (3HV) monomer (18). The PHA synthase (PhaEC) (18), acetoacetyl-CoA reductases (PhaB1 and PhaB2) (19), and haloarchaeal-type β-ketothiolases (PhaA and BktB) (20), which are indispensable for PHBV biosynthesis in H. mediterranei, have been identified. The PHA granule-associated phasin protein (PhaP) (21) and regulatory protein (PhaR) (22), which are important for PHA accumulation and granule formation, have also been characterized. However, the key enzymes of PHA degradation have not been identified yet for any haloarchaeal species.
In this study, we identified a novel PHA depolymerase (PhaZh1, a patatin-like protein) on the PHA granules in H. mediterranei. PhaZh1 (HFX_6464) had no similarity or identity to any known PHA depolymerase of bacteria. The function and enzyme activity of PhaZh1 were investigated through gene knockout and complementation, protein activity assays, and site-directed mutagenesis. We showed that the primary product of the native PHBV (nPHBV) granule hydrolysis by PhaZh1 was the 3HB monomer, which could be further converted into acetoacetate by BdhA (HFX_6463), a putative 3HB dehydrogenase in H. mediterranei. As PhaZh1 showed efficient activity in nPHA hydrolysis in vitro and the main products are hydroxyalkanoate monomers, it may provide a novel strategy for producing the chiral compound (R)-3HB from PHB accumulated by haloarchaea.
MATERIALS AND METHODS
Strains, culture conditions, and plasmids.
The strains and plasmids used in this study are shown in Table 1. The primers are listed in Table 2. Escherichia coli strains were grown in lysogeny broth (LB) medium at 37°C, with ampicillin at a final concentration of 100 μg ml−1 when necessary. E. coli JM109 (23) was used as a host for plasmid construction. The plasmids were shuttled into and isolated from E. coli JM110 (24) before transforming H. mediterranei strain EPS, in which a gene cluster involved in exopolysaccharide (EPS) synthesis has been deleted (25). H. mediterranei EPS was grown in AS-168 medium in the presence of 50 μg ml−1 uracil, and the recombinant H. mediterranei EPS strains harboring the derivatives of either pWL502 (21) or pHFX (17) were grown in AS-168SY medium (AS-168 medium without yeast extract) at 37°C (17). Haloferax volcanii H1424 was grown in Hv-YPC medium (26) at 45°C in the presence of 50 μg ml−1 uracil and 40 μg ml−1 thymidine, and recombinant strains harboring plasmids of the pTA1228 (27) derivatives were grown in Hv-YPC medium. For PHA accumulation research, the H. mediterranei strains were grown in AS-168 or AS-168SY medium for 2 days and then transferred (1:25 [vol/vol] inoculation) into PA medium (PHA production medium [25] with 20 g liter−1 starch replaced by 10 g liter−1 glucose) for 3 days. For PHA degradation research, PHA-rich cells of H. mediterranei strains were resuspended and incubated in PD medium (PA medium without carbon) for 5 days.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| Strains | ||
| Escherichia coli JM109 | recA1 supE44 endA1 hsdR17 gyrA96 relA1 thi | 23 |
| Escherichia coli JM110 | dam dcm mutant of E. coli JM109 | 24 |
| Haloferax mediterranei CGMCC 1.2087 (ATCC 33500) | CGMCC | |
| Haloferax mediterranei EPS | pyrF and eps deletion mutant of H. mediterranei | 25 |
| Haloferax mediterranei EPSΔphaZh1 | phaZh1 deletion mutant of H. mediterranei EPS | This study |
| Haloferax volcanii H1424 | ΔpyrE2 ΔhdrB pitANph Δmrr cdc48d, C-terminal truncation | 26 |
| Plasmids | ||
| pHFX | 4.0 kb, vector for gene knockout; Ampr | 17 |
| pDZh1 | 5.1 kb, integration vector of pHFX for phaZh1 knockout | This study |
| pTA1228 | 8.3 kb, shuttle vector with pyrE2-marker, P.tnaA tryptophanase promoter, T.syn terminator, and N-terminal His6 tag; Ampr | 27 |
| pTA01 | 8.0 kb, P.tnaA of pTA1228 replaced with P.phaR | This study |
| pTA02 | 8.0 kb, pTA01 without KpnI restriction site | This study |
| pTA03 | 8.0 kb, pTA02 with C-terminal His6 tag | This study |
| pTA03-phaZh1 | 9.0 kb, expression plasmid for PhaZh1 | This study |
| pTA03-phaZh1-G16A | 9.0 kb, expression plasmid for PhaZh1G16A | This study |
| pTA03-phaZh1-S47A | 9.0 kb, expression plasmid for PhaZh1S47A | This study |
| pTA03-phaZh1-D195A | 9.0 kb, expression plasmid for PhaZh1D195A | This study |
| pTA05 | 8.0 kb, pTA02 with N-terminal His6 tag | This study |
| pTA05-bdhA | 8.9 kb, expression plasmid for BdhA | This study |
| pWL502 | 7.9 kb, shuttle vector with pyrF marker; Ampr | 21 |
| pWLZh1 | 9.0 kb, expression plasmid for PhaZh1 with its promoter region | This study |
TABLE 2.
Primers used in this study
Sequences representing restriction sites are underlined.
PHA analysis and isolation of nPHA granules.
The PHA content and concentration in the cells were analyzed via gas chromatography (GC) (GC-6820; Agilent, USA) as described previously (28). Native PHA granules were isolated by sucrose density gradient (1.0 M, 1.3 M, 1.6 M, and 2.0 M sucrose in 100 mM Tris-HCl buffer, pH 7.15) centrifugation (210,000 × g at 4°C for 2 h) as previously described (21).
The molecular weight distribution of PHA was analyzed via gel permeation chromatography (GPC) (1260; Agilent, USA). Lyophilized cells with 15 mg of PHA were treated with chloroform for 2 to 3 days at room temperature. After centrifugation (3,000 × g for 30 min) to remove debris, the sample was filtered through a disassembled syringe filter. The PHA dissolved in chloroform was then purified by ethanol precipitation (29). The samples used for GPC analysis were dissolved in chloroform at a concentration of 6 mg ml−1 and filtered through a 0.45-μm syringe filter. The conditions for the GPC analysis were as follows: two PLgel 5-μm 10E5A columns (300 mm by 7.5 mm, Agilent Technologies); column and refractive index detector temperature, 35°C; mobile phase, chloroform; flow rate, 1 ml min−1; and injection volume, 50 μl. Polymer polystyrene (Agilent polymer standard kit) was used as a standard.
SDS-PAGE and MALDI-TOF/TOF MS analysis.
The isolated nPHA granules were resuspended in SDS-PAGE loading buffer (50 mM Tris-HCl, 2% SDS, 0.1% bromophenol blue, and 10% glycerol, pH 6.8). Coomassie brilliant blue R-250 was used for protein staining. The protein of interest was identified via matrix-assisted laser desorption ionization–tandem time of flight mass spectrometry (MALDI-TOF/TOF MS) as described by Shevchenko et al. (30).
Gene deletion, complementation, and mutation.
For construction of the phaZh1-knockout plasmid, a 497-bp DNA fragment located immediately upstream and a 644-bp DNA fragment located immediately downstream of the phaZh1 open reading frame (ORF) were amplified with the primer pairs phaZh1-DF1/DR1 and phaZh1-DF2/DR2, respectively. These two DNA fragments were used as templates for overlapping extension PCR with the primer pair phaZh1-DF1/DR2. The overlapping PCR product was digested with BamHI plus KpnI and then inserted into the pyrF-based integration plasmid pHFX (17), which resulted in pDZh1. After pDZh1 was introduced into H. mediterranei EPS, the ΔphaZh1 mutant strain was screened and identified as previously described (17).
For construction of the phaZh1 complementation plasmid, the ORF of phaZh1 with its promoter region (i.e., the intergenic region between bdhA and phaZh1) was amplified with the primer pair phaZh1-WL-F/R. The PCR product was digested with NcoI plus XbaI and then inserted into the pWL502 plasmid (21) to yield pWLZh1.
Through overlapping extension PCR, phaZh1 mutations were amplified with the primer pairs listed in Table 2. The PCR products were digested with EcoRI plus KpnI and then inserted into the plasmid pTA03, resulting in derivatives of pTA03 with mutated phaZh1.
Expression and purification of His-tagged proteins in H. volcanii.
The plasmids and primers used in this study are listed in Tables 1 and 2. For gene cloning and protein expression in H. volcanii H1424, several new expression vectors were developed, at first based on the plasmid pTA1228 (27), in which the tryptophanase promoter P.tnaA was replaced by the strong promoter P.phaR (22). Briefly, the P.phaR promoter was amplified with the primer pair P.phaR-F/R and was then digested with BamHI plus KpnI and inserted into pTA1228 to yield pTA01. After digestion with KpnI, the 3′ overhang of the linear pTA01 was removed with T4 DNA polymerase and then self-ligated to generate pTA02. After digestion with EcoRI plus BamHI, pTA02 was ligated with the fragment of the annealing primer pair TA03-F/R or TA05-F/R, resulting in pTA03 or pTA05, respectively.
For construction of the PhaZh1- or BdhA-expressing plasmid, the ORF of phaZh1 or bdhA was amplified with the primer pair phaZh1-TA-F/R or bdhA-TA-F/R, respectively. These PCR products were digested with EcoRI plus KpnI or KpnI plus BamHI and then inserted into the plasmids pTA03 and pTA05, resulting in pTA03-phaZh1 and pTA05-bdhA, respectively. The H. volcanii H1424 transformant harboring the expressing plasmid was cultivated in 2-liter flasks with 500 ml (1:100 [vol/vol] inoculation) of Hv-YPC medium at 45°C for 36 h. After the collected cells were ultrasonicated at 4°C (the following operations were all at the same temperature), the crude extracts were separated from the debris by centrifugation (10,000 × g for 15 min). His-tagged protein was purified with Ni-agarose columns. Briefly, the column was activated with 10 ml of binding buffer (2 M NaCl and 20 mM Tris-HCl, pH 8.0). The crude extracts were then loaded onto the column, and the proteins with nonspecific adsorption were eluted with 50 ml of wash buffer (2 M NaCl, 20 mM Tris-HCl, and 20 mM imidazole, pH 8.0). Afterwards, the His-tagged proteins were eluted with 10 ml of elution buffer (2 M NaCl, 20 mM Tris-HCl, and 500 mM imidazole, pH 8.0). Finally, the imidazole in the solution was diluted with binding buffer, and the protein was concentrated via ultrafiltration. Using the bicinchoninic acid method (31), the concentration of purified protein was measured spectrophotometrically (DU800; Beckman Coulter, USA) at 562 nm.
Enzyme assay.
The nPHA depolymerase activity was analyzed by measuring the decrease in nPHA granule turbidity at 650 nm (32). Briefly, the assay mixture contained 2 M KCl, 100 mM Tris-HCl (pH 7.5), 5 mM MgCl2, and the purified nPHA granules (initial optical density at 650 nm [OD650], ∼1). The reaction was started by adding the purified PhaZh1 (100 μg ml−1) at 45°C.
The 3HB dehydrogenase activity assay was performed at room temperature in buffer (500 μl) containing 2 M KCl, 100 mM Tris-HCl (pH 7.5), 5 mM MgCl2, 4 mM sodium 3-hydroxybutyrate (Sigma), 5 mM NAD+, and 25 μg of purified BdhA. After the protein was added, the NADH produced in the solution was monitored spectrophotometrically (DU800; Beckman Coulter, USA) at 340 nm (the extinction coefficient of NADH [εNADH] at 340 nm is 6.22 × 103 liters mol−1 cm−1). Sodium 3-hydroxybutyrate can be replaced with the nPHA granule degradation solution with the pH adjusted to 7.0.
PHA degradation product and glucose concentration analysis.
The nPHA granule degradation product was identified via high-pressure liquid chromatography (HPLC) (HPLC-1220; Agilent, USA) as described previously (33) using sodium 3-hydroxybutyrate (Sigma) as the standard.
The glucose concentration in the medium was measured with the SBA-40D sensor (Institute of Biology, Shandong Academy of Sciences, China). After centrifugation (10,000 × g for 5 min), the supernatant was diluted with double-distilled water (ddH2O) (1:20, vol/vol), and 25 μl of the dilution was subjected to the glucose concentration assay, with 1 g liter−1 glucose as the standard.
Protein sequence analysis and gene cluster search.
The protein domain and active site were analyzed using NCBI BLASTP with the conserved-domain database (34, 35). The GeneDoc program (http://www.nrbsc.org/gfx/genedoc/) was used to analyze the homology of protein sequences. The conserved gene cluster was analyzed through a Sequence Similarity Database (SSDB) gene cluster search (KEGG) (http://www.kegg.jp/kegg/ssdb/).
RESULTS
Mobilization of PHA in H. mediterranei.
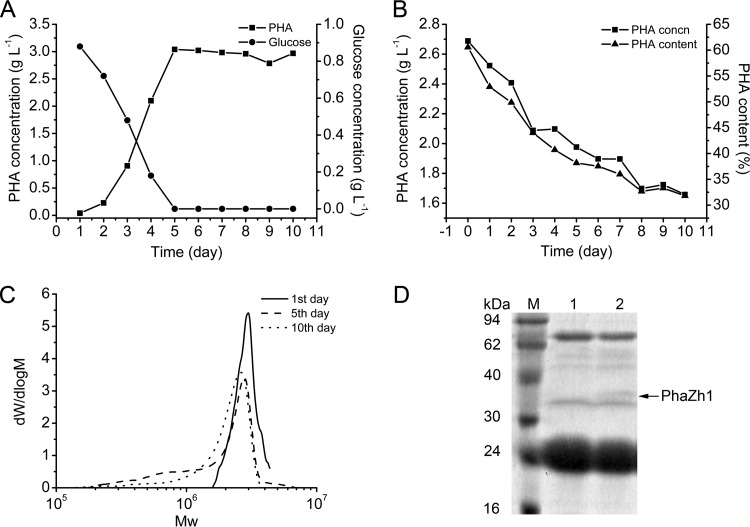
PHA is a carbon and energy storage compound accumulated within the bacterial cells, it is usually mobilized when the exogenous carbon is inadequate; e.g., R. eutropha can use the PHB degradation products for growth and survival under conditions of carbon starvation (4). Here we investigated PHA degradation in H. mediterranei in carbon-rich medium (Fig. 1A) and carbon-free medium (Fig. 1B). The concentration and content of PHA accumulated in the cells were analyzed via GC assay to determine whether the accumulated PHA was mobilized. The concentration of glucose, which was the sole carbon source in the medium, was measured as described in Materials and Methods. Unlike the case for bacteria (15), which mobilize the accumulated PHA when the exogenous carbon source in the medium is exhausted, the accumulated PHA in H. mediterranei was not obviously mobilized after the glucose in the medium was used up even for a period of 5 days (Fig. 1A).
FIG 1.
PHA accumulation and degradation in the haloarchaeon H. mediterranei. (A) PHA accumulation in H. mediterranei grown in medium with glucose as the sole carbon source. (B) PHA degradation in H. mediterranei incubated in fresh medium without carbon. (C) GPC analysis of the molecular weight (Mw) distribution of PHA extracted on days 1, 5, and 10 as indicated in panel B. (D) SDS-PAGE analysis of proteins located on the PHA granules. Lanes 1 and 2, PHA granule isolated on days 0 and 5 as shown in panel B, respectively. The arrow indicates PhaZh1. Lane M, protein markers.
Considering that other nutrients (such as N or P) in the medium may be exhausted or any unknown metabolites may be accumulated and thus inhibit the PHA mobilization, we then transferred the PHA-rich cells into a fresh carbon-free medium (Fig. 1B). Notably, when PHA-rich cells were incubated in this freshly prepared carbon-free medium, the previously accumulated PHA was degraded gradually in H. mediterranei (Fig. 1B). Over a period of 5 days, the PHA content in the cells decreased from 61% (of dry cell weight) to 38%, and 0.71 g liter−1 PHA was degraded. After 10 days, approximately 47% of the accumulated PHA was mobilized. The molecular weight distribution of PHA in the degradation stage was analyzed via GPC assay as described in Materials and Methods (Fig. 1C). With an increase in the incubation time, the molecular weight of PHA decreased. This finding demonstrated that PHA was degraded in PHA-rich cells incubated in carbon-free medium. Because the PHA content decreased slowly from the 5th day to the 10th day (Fig. 1B and C), incubation of PHA-rich cells in carbon-free medium for 5 days was chosen for the following PHA degradation research.
PhaZh1 is a novel PGAP in H. mediterranei.
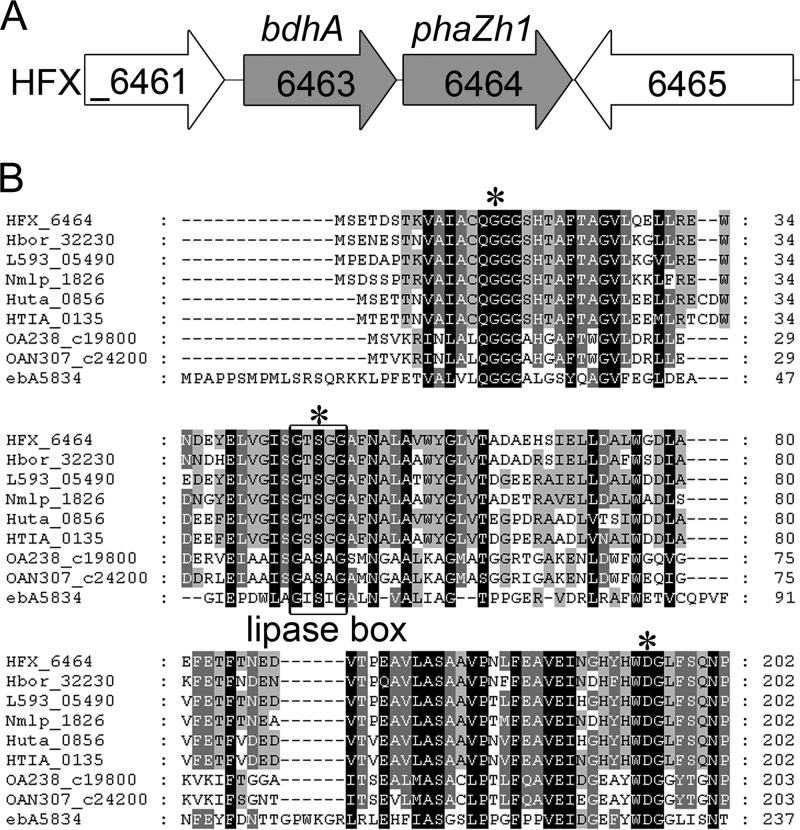
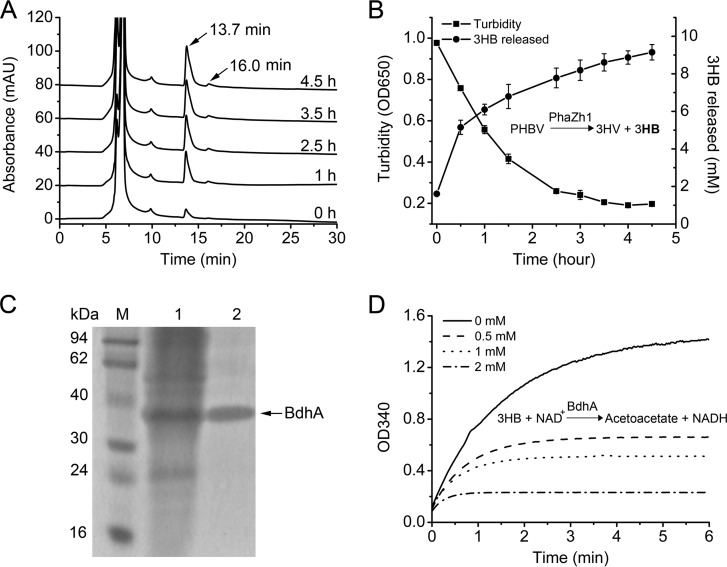
Depolymerase is the key enzyme involved in PHA degradation. To isolate the potential PHA depolymerase from H. mediterranei, we collected and purified nPHA granules from cells in which PHA was accumulated and degraded, as described in Materials and Methods. The proteins located on the granules were analyzed by SDS-PAGE (Fig. 1D). A new protein located on the granule was purified from the PHA degradation cells that were incubated in carbon-free medium for 5 days (Fig. 1B). The new protein was identified by MALDI-TOF/TOF MS as HFX_6464 (Fig. 2A). Interestingly, HFX_6464 was a patatin-like protein (36) (belonging to the Pfam01734 family). Patatin is a major storage protein found in potatoes that also has lipid acyl hydrolase and acyl transferase activities (37). A classical lipase box (G-T-S47-G-G) was found in the sequence of HFX_6464 (Fig. 2B), and such a box is also found in several bacterial PhaZs and is very important for the hydrolysis activity of these PHA depolymerases in bacteria (6, 38, 39).
FIG 2.
Genetic organization of bdhA-phaZh1 cluster and alignment of partial sequences around conserved residues of PhaZh1 and its homologous proteins. (A) Overview of the genetic organization of the bdhA-phaZh1 cluster (HFX_6463 to _6464) in the genome of the haloarchaeon H. mediterranei. (B) Alignment of amino acid sequences of PhaZh1 (HFX_6464, YP_006351567)-homologous proteins from five archaea (Halogeometricum borinquense [Hbor_32230, YP_004044416], Natronomonas moolapensis [Nmlp_1826, YP_007486766], Salinarchaeum sp. strain Harcht-Bsk1 [L593_05490, YP_008053845], Halorhabdus tiamatea [HTIA_0135, YP_008375597], and Halorhabdus utahensis [Huta_0856, YP_003129772]) and three bacteria (Octadecabacter antarcticus [OA238_c19800, YP_007699542], Octadecabacter arcticus [OAN307_c24200, YP_007704668], and Aromatoleum aromaticum [ebA5834, YP_160340]) with a gene cluster similar to bdhA-phaZh1 in their genomes. The lipase box (G-X-S-X-G) is boxed. The putative catalytic sites are shown with asterisks. Identical residues are shaded in black, and similar residues are shaded in gray. The numbers on the right indicate the positions of the amino acids of the respective proteins.
Notably, HFX_6463 (bdhA), which encodes a putative NAD+-dependent 3HB dehydrogenase (40), and HFX_6464 formed a gene cluster in the megaplasmid pHM500 of H. mediterranei (Fig. 2A). Moreover, through an SSDB gene cluster search (see Materials and Methods), we found five other archaeal and three bacterial strains that had the same genetic organization in their genomes (Fig. 2B). Among the eight strains, the gene encoding PHA synthase (phaC) can be found in the genomes of the five archaeal strains and the bacterium Aromatoleum aromaticum, which indicated that these six strains may also accumulate PHA in their cells.
All of these facts indicated that HFX_6464 may act as a PHA depolymerase in H. mediterranei. HFX_6464 was previously annotated as RssA, and we here designated it PhaZh1, the potential haloarchaeal PHA depolymerase.
Effect of PhaZh1 on PHA degradation in H. mediterranei.
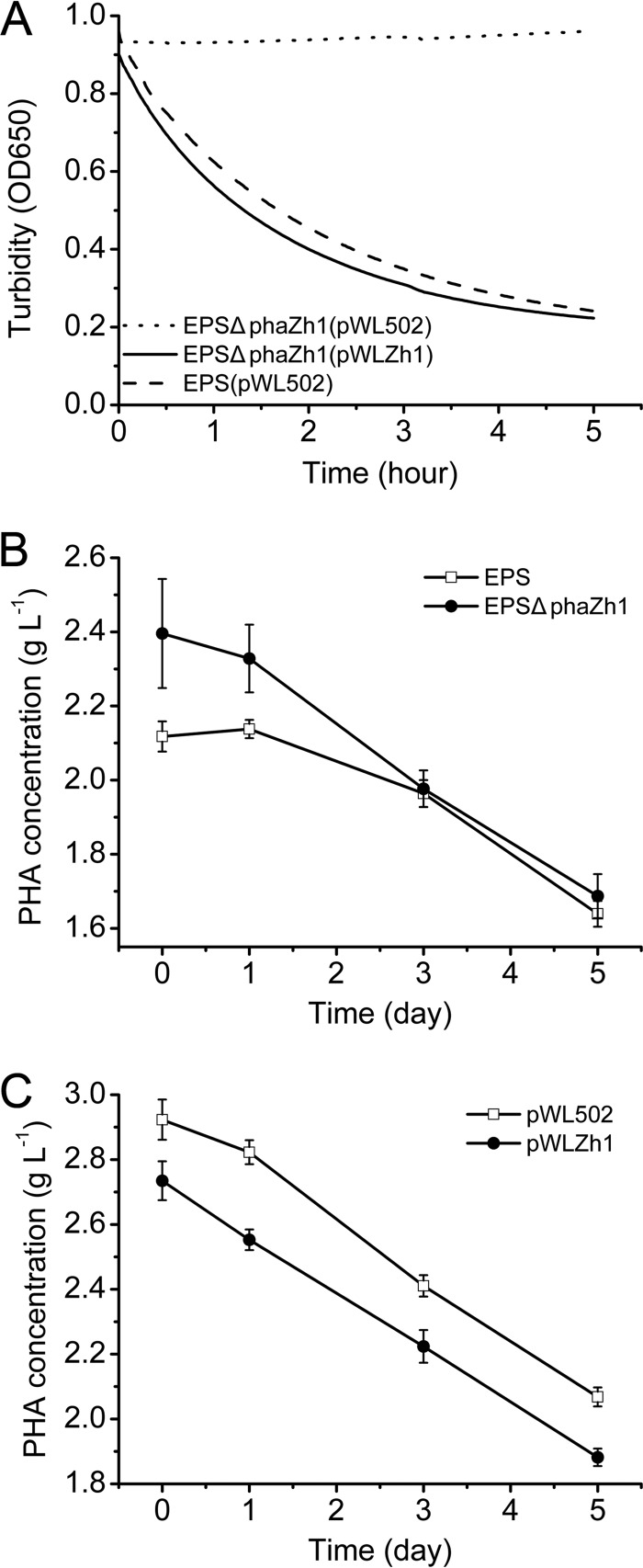
To determine the function of PhaZh1 in nPHA granule degradation, we knocked out the phaZh1 gene from H. mediterranei EPS (Table 1). The nPHA granules isolated from the strain H. mediterranei EPS harboring pWL502 (an empty plasmid) were gradually degraded in vitro (Fig. 3A). After 5 h, the turbidity of granule suspension at 650 nm was decreased from 0.96 to 0.24. However, for the nPHA granules isolated from the phaZh1 mutant strain H. mediterranei EPSΔphaZh1 harboring the pWL502 plasmid, the granule degradation was not detectable in vitro (Fig. 3A). In contrast, the nPHA granules isolated from the phaZh1 complementation strain H. mediterranei EPSΔphaZh1(pWLZh1) recovered the ability of autodegradation (Fig. 3A), which was similar to that of the wild-type strain. This result indicated that PhaZh1 is involved in the nPHA granule hydrolysis in vitro.
FIG 3.
Effect of phaZh1 deletion or complementation on PHA degradation in the haloarchaeon H. mediterranei. (A) Degradation of nPHA granules isolated from the H. mediterranei EPS strain with the pWL502 plasmid, the H. mediterranei EPSΔphaZh1 strain with the pWL502 plasmid, and the H. mediterranei EPSΔphaZh1 strain with the pWLZh1 plasmid. The nPHA granules were isolated from cells in the PHA mobilization phase. The turbidity of the granules was monitored (DU800; Beckman Coulter) at 650 nm. (B) Effect of phaZh1 deletion on intracellular PHA degradation in H. mediterranei EPS. EPS, wild-type strain; EPSΔphaZh1, ΔphaZh1 mutant strain. (C) Effect of phaZh1 complementation on intracellular PHA degradation in H. mediterranei EPSΔphaZh1. pWL502, empty plasmid; pWLZh1, expression plasmid for PhaZh1 with its promoter region.
To determine the in vivo function of PhaZh1 in H. mediterranei, the effect of phaZh1 deletion on intracellular PHA degradation in H. mediterranei was investigated. The PHA concentrations in the H. mediterranei EPSΔphaZh1 and H. mediterranei EPS strains were determined via GC assay (Fig. 3B). The H. mediterranei EPSΔphaZh1 strain accumulated slightly more PHA (2.40 ± 0.15 g liter−1) than the H. mediterranei EPS strain (2.12 ± 0.04 g liter−1) on day 0. After 5 days, however, the PHA concentration in the H. mediterranei EPSΔphaZh1 strain gradually decreased to 1.69 ± 0.06 g liter−1, which was equal to that found in the H. mediterranei EPS strain (1.63 ± 0.03 g liter−1). Hence, PhaZh1 mutation (ΔphaZh1) had a slight effect on PHA accumulation but had no significant effect on intracellular PHA mobilization in H. mediterranei EPS.
The effect of phaZh1 complementation on PHA degradation in the ΔphaZh1 mutant strain was investigated. The expression of PhaZh1 (with plasmid pWLZh1) in the mutant strain H. mediterranei EPSΔphaZh1 resulted in less PHA accumulation (2.73 ± 0.06 g liter−1) than that found in the control strain (with empty plasmid pWL502) (2.92 ± 0.06 g liter−1) on day 0 in another parallel experimental assay (Fig. 3C). However, the PHA concentration in the complementation strain was also decreased to a level equal to that observed in the control strain over a period of 5 days. These results indicated that although PhaZh1 showed efficient activity and was most likely the key enzyme in nPHA granule hydrolysis in vitro (Fig. 3A), either deletion or complementation of the phaZh1 gene had no significant effect on PHA mobilization in vivo (Fig. 3B and C).
Analysis of the nPHA granule hydrolysis product.
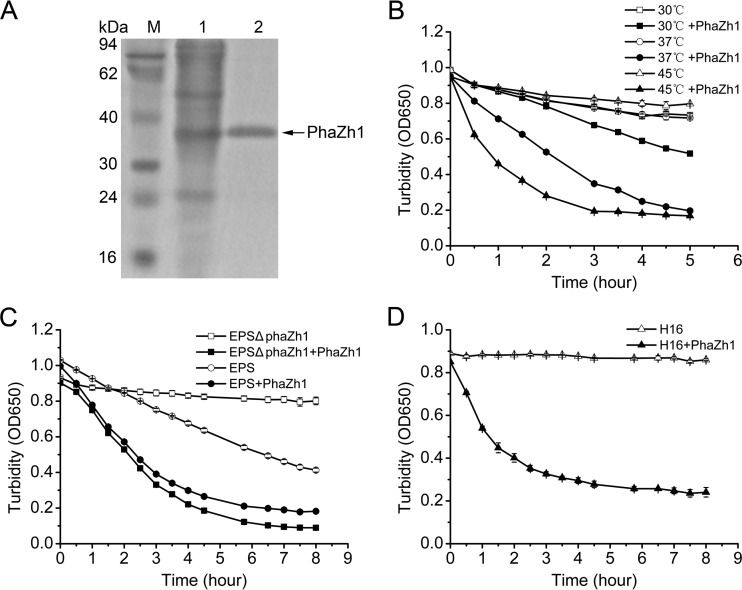
To further biochemically determine the hydrolysis activity of PhaZh1 with the nPHA granules, we expressed PhaZh1 with a C-terminal His6 tag (His6-PhaZh1) in the haloarchaeon H. volcanii H1424 (26). The promoter of the expression plasmid pTA1228 (27) used in this study was replaced with a strong constitutive promoter of P.phaR in which its negative cis element has been deleted (22). The PHA depolymerase activity of His6-PhaZh1 (Fig. 4A) was higher at 45°C than that at 37°C or 30°C (Fig. 4B), which was consistent with the optimal growth temperature (45°C) of H. mediterranei (41). The purified His6-PhaZh1 hydrolyzed the nPHA granules isolated from the haloarchaeon strain H. mediterranei EPSΔphaZh1 (Fig. 4B and C) and even the nPHB (which did not autodegrade in the hypersaline buffer) obtained from the bacterial strain R. eutropha H16 (Fig. 4D). The PHA degradation rate for nPHA granules isolated from the strain H. mediterranei EPS was significantly raised when the purified His6-PhaZh1 was added (Fig. 4C). These results clearly indicated that PhaZh1 is the key enzyme for nPHA granule hydrolysis in vitro.
FIG 4.
Hydrolysis of nPHA granules by purified PhaZh1. (A) SDS-PAGE analysis of purified PhaZh1 from the haloarchaeon H. volcanii H1424 harboring the pTA03-phaZh1 plasmid. Lane 1, soluble crude extract; lane 2, His6-tagged PhaZh1 purified with a Ni-agarose column; lane M, protein markers. (B) PHA depolymerase activities of purified PhaZh1 at different temperature (30°C, 37°C, and 45°C). The nPHA granule was isolated from the haloarchaeon H. mediterranei EPSΔphaZh1. (C) Hydrolysis of nPHA granules isolated from the H. mediterranei EPS and H. mediterranei EPSΔphaZh1 strains by purified PhaZh1. (D) Hydrolysis of nPHA granules isolated from the bacterium R. eutropha H16 by purified PhaZh1.
The nPHA granule hydrolysis products were identified via HPLC (33) using sodium 3-hydroxybutyrate (Sigma) as the standard. We found that the 3HB monomer (the typical retention time was 13.7 min) but not a mixture of the 3HB monomer and dimer (6) was detected in the hydrolysis reaction mixture (Fig. 5A). When the turbidity (OD650) of the reaction solution was decreased to 0.197 ± 0.01, the amount of the 3HB monomer released from the nPHA granules was increased to 9.15 ± 0.41 mM (Fig. 5B), and the final pH of the solution was 5.0 to 6.0, in contrast to the initial pH of 7.0. Changes in pH indicated that some acidic materials were produced in the solution. The analysis of the peak spectrum of the HPLC assay results revealed a small peak at 16 min (Fig. 5A), which may represent the 3HV monomer (33). This result confirmed that PhaZh1 hydrolyzed the nPHA granules isolated from H. mediterranei and that the main product was 3HB monomer.
FIG 5.
Analysis of products of nPHA granule hydrolysis and the enzyme activity of BdhA. (A) HPLC analysis of the product of nPHA granule hydrolysis by purified PhaZh1 after different reaction times. The curves from 1 to 4.5 h were translated to the vertical direction for convenient observation. The retention time of the 3-hydroxybutyrate (3HB) monomer was 13.7 min. (B) Turbidimetric assay (OD650) of nPHA granule (isolated from the haloarchaeon H. mediterranei EPSΔphaZh1) hydrolysis by purified PhaZh1 (◼). The product (3HB) (●) released in the solution was identified via HPLC assay (A) using sodium 3-hydroxybutyrate (Sigma) as a standard. (C) SDS-PAGE analysis of BdhA purified from the haloarchaeon H. volcanii H1424 harboring the pTA05-bdhA plasmid. Lane 1, soluble crude extract; lane 2, His6-tagged BdhA purified with a Ni-agarose column; lane M, protein markers. (D) Properties of BdhA with different molarities of acetoacetate (0 to 2 mM) added to the reaction solution. The absorption at 340 nm of NADH produced in the solution was monitored spectrophotometrically.
Expression, purification, and functional analysis of BdhA.
Interestingly, the bdhA gene located in the bdhA-phaZh1 (HFX_6463 to _6464) gene cluster encodes a putative 3HB dehydrogenase. It is well known that the 3HB monomer can be converted to acetoacetate by NAD+-dependent 3HB dehydrogenase, generating NADH that can be easily monitored (13, 40, 42). To test the function of the BdhA of H. mediterranei, we expressed the BdhA with an N-terminal His6 tag in H. volcanii H1424 and purified it with Ni-agarose columns (Fig. 5C). Notably, when the purified His6-tagged BdhA was added to the solution containing sodium 3-hydroxybutyrate and NAD+ in a hypersaline buffer, the absorption at 340 nm (for detection of NADH production) increased rapidly. Because the final solution of the nPHA granule hydrolysis by PhaZh1 contains 3HB and the pH is about 5.0 to 6.0, it was adjusted to pH 7.0 before being used to replace the sodium 3-hydroxybutyrate for the BdhA activity assay. The results clearly indicated that BdhA is a 3HB dehydrogenase in haloarchaea (Fig. 5D). Interestingly, when the molarity of the acetoacetate added to the solution increased, the NADH produced in the enzymatic reaction gradually decreased (Fig. 5D). The addition of 2 mM acetoacetate to the solution strongly inhibited the enzyme activity of BdhA within 0.5 min, indicating that the reaction product acetoacetate clearly inhibited the enzyme activity of BdhA. These results implied that BdhA could participate in nPHA granule hydrolysis, and PhaZh1 and BdhA may constitute the first part of a PHA degradation pathway in vivo.
Analysis of the putative active sites of PhaZh1.
The product, 3HB and likely the 3HV monomer (Fig. 5A), of nPHA granule hydrolysis by PhaZh1 was different from the mixture of 3HB monomer and dimer found in bacteria (6). As mentioned above, PhaZh1 is a patatin-like protein (36) that has not been reported to hydrolyze nPHA granules previously. Through BLASTP (protein-protein BLAST) analysis using the NCBI (34, 35), we analyzed the putative active sites of PhaZh1 based on the crystal structure of an isozyme of patatin, Pat17 (PDB id 1OXW) (36), which indicated that Gly16 of PhaZh1 may form the putative oxyanion hole and that Ser47 (in the lipase box G-T-S47-G-G) and Asp195 (in the motif W-D195-G) may constitute the putative catalytic dyad (Fig. 2B).
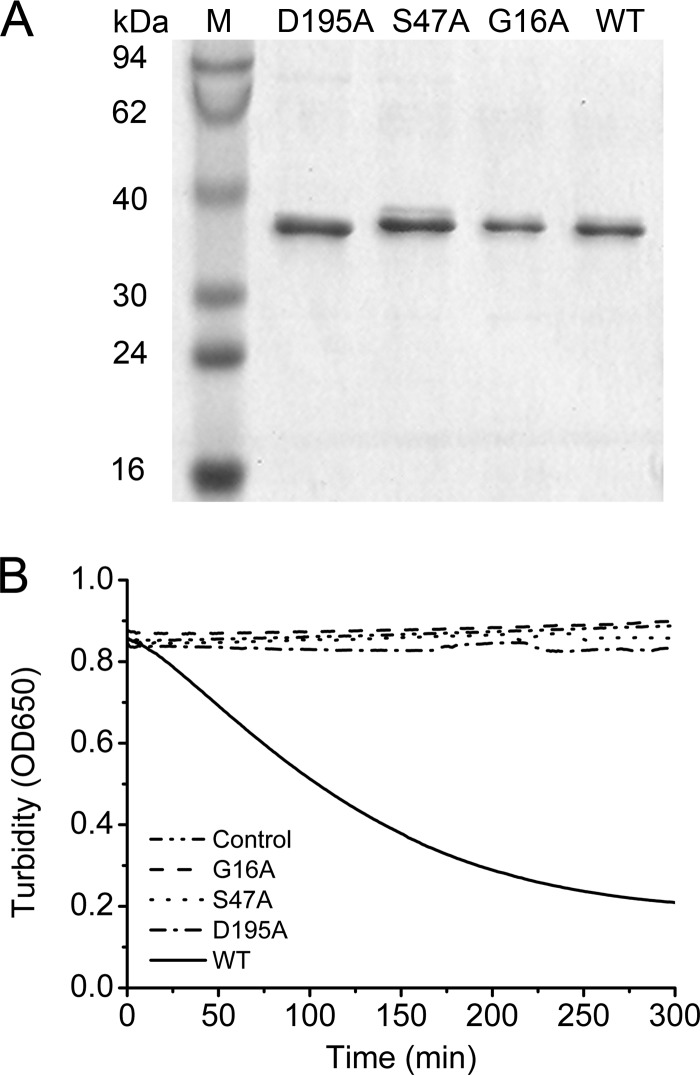
We replaced the three amino acid residues with alanine to verify whether these putative active sites were indeed important for the hydrolysis activity of PhaZh1. Three PhaZh1 mutants (G16A, S47A, and D195A) were constructed as described in Materials and Methods. All three PhaZh1 mutants (Fig. 6A) lost the ability to hydrolyze the nPHA granules (Fig. 6B). This result confirmed that Gly16, Ser47, and Asp195 are indeed essential for nPHA granule hydrolysis. Ser47 in the classical lipase box (G-X-S-X-G) may be the active-site serine (6, 38, 39), and Asp195 may form the charge relay dyad with Ser47 in PhaZh1. Gly16 of PhaZh1 may act as an oxyanion hole liganded to the Ser47-Asp195 catalytic dyad, similar to Lys17 of patatin B2 (43). Taken together, these mutagenesis studies implied that Gly16, Ser47, and Asp195 are the essential residues, among which Ser47-Asp195 is likely the catalytic dyad of PhaZh1.
FIG 6.
Site-directed mutagenesis of PhaZh1. (A) SDS-PAGE analysis of PhaZh1 muteins purified from the haloarchaeon H. volcanii H1424 harboring pTA03 plasmid derivatives of phaZh1 mutants. Lanes D195A, S47A, and G16A, Asp195, Ser47, and Gly16 in the amino acid sequence of PhaZh1 were replaced with alanine; lane WT, wild-type PhaZh1; lane M, protein markers. (B) Turbidimetric assay (OD650) of the hydrolysis of nPHA granules (isolated from the haloarchaeon H. mediterranei EPSΔphaZh1) by purified PhaZh1 and its muteins. Control, without purified protein.
DISCUSSION
Although many intracellular PHA depolymerases have been found in bacteria, such proteins were not clearly recognized by homology searches in the genome of H. mediterranei. Using the two-dimensional electrophoresis (2-DE) method, five PGAPs (HFX_5217 to HFX_5221; MaoC, PhaR, PhaP, PhaE, and PhaC) have been isolated from the nPHA granules accumulated in H. mediterranei cells at the stationary phase of growth (21, 22). Interestingly, these five PGAPs are encoded by a gene cluster (pha cluster; HFX_5217 to _5221) in the genome. The functions of these PGAPs (except MaoC) in PHA biosynthesis or granule formation have been characterized recently (18, 21, 22). However, no putative PHA depolymerase was found in this PHA granule proteome. In this study, when we transferred the PHA-rich cells of H. mediterranei into a fresh medium without a carbon resource, a novel PGAP was observed at the nPHA granules and was identified as the intracellular PHA depolymerase PhaZh1(Fig. 1D). This indicated that PhaZh1 might be induced under the condition of carbon starvation.
Through BLASTP analysis (34, 35), PhaZh1 was found to be a patatin-like protein (36), and proteins with a patatin catalytic domain are widely found in the three domains of life (44–46). Modification of putative conserved residues of serine, aspartate, and histidine of patatin B2 demonstrated that Ser54 and Asp192 constitute the active site (43), which was further revealed by the crystal structure of a patatin isozyme, Pat17 (36). Patatin is a serine hydrolase with a Ser-Asp catalytic dyad (43), which contains a nucleophile serine that is embedded in the lipase box (G-X-S-X-G) and an aspartate that is often found in an Asp-Gly-Gly motif (36). An anion-binding element, Gly-Gly-X-Arg, is also important for the enzyme activity of patatin (36). The patatin-related phospholipase A found in plants (pPLA) and animals (cPLA2) cleaves phospholipids at the sn1 or sn2 position of glycerophospholipids to yield free fatty acids (47). However, a patatin-like protein that hydrolyzes the PHA granules has not yet been reported. Based on a partial sequence around the conserved residue alignment of PhaZh1 with Pat17 (36), we proposed that Gly16, Ser47, and Asp195 of PhaZh1 may be the essential residues (Fig. 2B). The site-directed mutagenesis of PhaZh1 indicated that Gly16, Ser47, and Asp195 are indeed essential for nPHA granule hydrolysis. The putative catalytic dyad of PhaZh1 is Ser47-Asp195, and that is different from the case for all known bacterial PHA depolymerases, which have the putative catalytic triad Ser-Asp-His (7, 42). The difference in catalytic domain between PhaZh1 and its bacterial counterparts may explain why the products of nPHA hydrolysis by PhaZh1 are hydroxyalkanoate monomers, which are different from that obtained by bacterial depolymerase PhaZd1 or PhaZd2 (6). The latter is a mixture of hydroxyalkanoate monomer and dimer. Interestingly, a patatin-like phospholipase protein (H16_A0225) was recently identified as a new PGAP in R. eutropha (48). Because of their same locations on the PHA granules and their conserved patatin-like catalytic domains between PhaZh1 and H16_A0225, we speculate that H16_A0225 may be also involved in PHA degradation in R. eutropha.
PhaZh1 catalyzed the hydrolysis of nPHA granules purified from the haloarchaeon H. mediterranei and even nPHB granules purified from the bacterium R. eutropha H16 in vitro (Fig. 4). The nPHB granule isolated from R. eutropha H16 degraded without depolymerase addition in 50 mM potassium phosphate buffer (8) but showed no significant degradation in 2 M KCl buffer (Fig. 4D), which indicated that depolymerase PhaZa1 located on the nPHB granule did not work in hypersaline buffer. Thus, PhaZh1 was the key enzyme for nPHB granule hydrolysis in hypersaline buffer (Fig. 4D). BdhA (a putative 3HB dehydrogenase) catalyzed the redox reaction (40) in the presence of NAD+ in the solution of nPHA granule hydrolysis by PhaZh1, which indicated that BdhA is a 3HB dehydrogenase in haloarchaea. PhaZh1 with BdhA degraded the nPHA granule to acetoacetate in vitro, indicating that they may constitute the first part of a PHA degradation pathway in vivo. The main product of nPHA granule hydrolysis by PhaZh1 was found to be the 3HB monomer (Fig. 5A). It is known that the monomers of PHA degradation by PhaZ are typically in the R configuration (49), and the ΔbktB mutant of H. mediterranei accumulated a considerable amount of PHB instead of PHBV in the cells (20). Thus, PhaZh1 has the potential to produce the chiral compound (R)-3HB, which is a high-value-added product with industrial and medical applications (49).
Although PhaZh1 was the key enzyme of nPHA granule hydrolysis in vitro, deletion of the phaZh1 gene had no significant effect on PHA mobilization in vivo in H. mediterranei (Fig. 3B and C). Similarly to the PHB depolymerase redundancy in the bacterium R. eutropha H16 (6, 7, 15), other PHA depolymerases should exist and have significant effects on the in vivo PHA degradation in H. mediterranei. These candidates for PHA depolymerase may include several predicted hydrolases, as well as the PhaCs that may contribute to both PHA synthesis and mobilization. Interestingly, among the nine intracellular PHB depolymerases or PHB oligomer hydrolases in R. eutropha H16, PhaZa1 is the only PHB depolymerase located on the PHB granules (8, 48). The other PHA depolymerases that may exist in H. mediterranei may not be located on the PHA granules (similarly to PhaZh1) or not function in vitro. Therefore, PhaZh1 may not play a significant role in PHA mobilization within the cell, but it is the key enzyme for nPHA degradation in vitro (Fig. 4).
Taking the results together, the patatin-like protein PhaZh1, which is located on the PHA granules in H. mediterranei, was identified as a novel PHA depolymerase. PhaZh1 is distinct from its bacterial counterparts in terms of its catalytic domain and hydrolysis product. Notably, hydroxyalkanoate monomers were the product of nPHA granule hydrolysis by PhaZh1. These specific features may provide a new strategy for producing the high-value-added chiral compound (R)-3HB. In addition, this study implies that other PHA degradation pathways may also exist in H. mediterranei. Identification of these pathways may provide more insights into PhaZh1 function in vivo, as well as the mechanisms of PHA mobilization in haloarchaea.
ACKNOWLEDGMENTS
This work was supported by grants from the National Natural Science Foundation of China (grant no. 31330001 and 31370096) and the Hundred Talents Program of the Chinese Academy of Sciences (to H.X.).
We sincerely acknowledge Weiqi Weng and Jiuyuan Ding, who provided R. eutropha H16 cells with accumulated PHB and helped with the HPLC analysis.
REFERENCES
- 1.Grage K, Jahns AC, Parlane N, Palanisamy R, Rasiah IA, Atwood JA, Rehm BHA. 2009. Bacterial polyhydroxyalkanoate granules: biogenesis, structure, and potential use as nano-/micro-beads in biotechnological and biomedical applications. Biomacromolecules 10:660–669. doi: 10.1021/bm801394s. [DOI] [PubMed] [Google Scholar]
- 2.Fernandez-Castillo R, Rodriguezvalera F, Gonzalezramos J, Ruizberraquero F. 1986. Accumulation of poly(beta-hydroxybutyrate) by halobacteria. Appl Environ Microbiol 51:214–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jendrossek D, Pfeiffer D. 2014. New insights in the formation of polyhydroxyalkanoate granules (carbonosomes) and novel functions of poly(3-hydroxybutyrate). Environ Microbiol 16:2357–2373. doi: 10.1111/1462-2920.12356. [DOI] [PubMed] [Google Scholar]
- 4.Handrick R, Reinhardt S, Jendrossek D. 2000. Mobilization of poly(3-hydroxybutyrate) in Ralstonia eutropha. J Bacteriol 182:5916–5918. doi: 10.1128/JB.182.20.5916-5918.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jendrossek D, Handrick R. 2002. Microbial degradation of polyhydroxyalkanoates. Annu Rev Microbiol 56:403–432. doi: 10.1146/annurev.micro.56.012302.160838. [DOI] [PubMed] [Google Scholar]
- 6.Sznajder A, Jendrossek D. 2014. To be or not to be a poly(3-hydroxybutyrate) (PHB) depolymerase: PhaZd1 (PhaZ6) and PhaZd2 (PhaZ7) of Ralstonia eutropha, highly active PHB depolymerases with no detectable role in mobilization of accumulated PHB. Appl Environ Microbiol 80:4936–4946. doi: 10.1128/AEM.01056-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abe T, Kobayashi T, Saito T. 2005. Properties of a novel intracellular poly(3-hydroxybutyrate) depolymerase with high specific activity (PhaZd) in Wautersia eutropha H16. J Bacteriol 187:6982–6990. doi: 10.1128/JB.187.20.6982-6990.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uchino K, Saito T, Gebauer B, Jendrossek D. 2007. Isolated poly(3-hydroxybutyrate) (PHB) granules are complex bacterial organelles catalyzing formation of PHB from acetyl coenzyme A (CoA) and degradation of PHB to acetyl-CoA. J Bacteriol 189:8250–8256. doi: 10.1128/JB.00752-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eggers J, Steinbüchel A. 2013. Poly(3-hydroxybutyrate) degradation in Ralstonia eutropha H16 is mediated stereoselectively to (S)-3-hydroxybutyryl coenzyme A (CoA) via crotonyl-CoA. J Bacteriol 195:3213–3223. doi: 10.1128/JB.00358-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doi Y, Segawa A, Kawaguchi Y, Kunioka M. 1990. Cyclic nature of poly(3-hydroxyalkanoate) metabolism in Alcaligenes eutrophus. FEMS Microbiol Lett 55:165–169. [DOI] [PubMed] [Google Scholar]
- 11.Taidi B, Mansfield DA, Anderson AJ. 1995. Turnover of poly(3-hydroxybutyrate) (Phb) and its influence on the molecular-mass of the polymer accumulated by Alcaligenes eutrophus during batch culture. FEMS Microbiol Lett 129:201–205. doi: 10.1016/0378-1097(95)00158-2, . [DOI] [Google Scholar]
- 12.Kobayashi T, Shiraki M, Abe T, Sugiyama A, Saito T. 2003. Purification and properties of an intracellular 3-hydroxybutyrate-oligomer hydrolase (PhaZ2) in Ralstonia eutropha H16 and its identification as a novel intracellular poly(3-hydroxybutyrate) depolymerase. J Bacteriol 185:3485–3490. doi: 10.1128/JB.185.12.3485-3490.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kobayashi T, Uchino K, Abe T, Yamazaki Y, Saito T. 2005. Novel intracellular 3-hydroxybutyrate-oligomer hydrolase in Wautersia eutropha H16. J Bacteriol 187:5129–5135. doi: 10.1128/JB.187.15.5129-5135.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uchino K, Saito T, Jendrossek D. 2008. Poly(3-hydroxybutyrate) (PHB) depolymerase PhaZa1 is involved in mobilization of accumulated PHB in Ralstonia eutropha H16. Appl Environ Microbiol 74:1058–1063. doi: 10.1128/AEM.02342-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.York GM, Lupberger J, Tian JM, Lawrence AG, Stubbe J, Sinskey AJ. 2003. Ralstonia eutropha H16 encodes two and possibly three intracellular poly[d-(−)-3-hydroxybutyrate] depolymerase genes. J Bacteriol 185:3788–3794. doi: 10.1128/JB.185.13.3788-3794.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han J, Zhang F, Hou J, Liu XQ, Li M, Liu HL, Cai L, Zhang B, Chen YP, Zhou J, Hu SN, Xiang H. 2012. Complete genome sequence of the metabolically versatile halophilic archaeon Haloferax mediterranei, a poly(3-hydroxybutyrate-co-3-hydroxyvalerate) producer. J Bacteriol 194:4463–4464. doi: 10.1128/JB.00880-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu H, Han J, Liu X, Zhou J, Xiang H. 2011. Development of pyrF-based gene knockout systems for genome-wide manipulation of the archaea Haloferax mediterranei and Haloarcula hispanica. J Genet Genomics 38:261–269. doi: 10.1016/j.jgg.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 18.Lu QH, Han J, Zhou LG, Zhou J, Xiang H. 2008. Genetic and biochemical characterization of the poly(3-hydroxybutyrate-co-3-hydroxyvalerate) synthase in Haloferax mediterranei. J Bacteriol 190:4173–4180. doi: 10.1128/JB.00134-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng B, Cai S, Han J, Liu H, Zhou J, Xiang H. 2010. Identification of the phaB genes and analysis of the PHBV precursor supplying pathway in Haloferax mediterranei. Acta Microbiol Sin 50:1305–1312. doi: 10.13343/j.cnki.wsxb.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Hou J, Feng B, Han J, Liu HL, Zhao DH, Zhou J, Xiang H. 2013. Haloarchaeal-type beta-ketothiolases involved in poly(3-hydroxybutyrate-co-3-hydroxyvalerate) synthesis in Haloferax mediterranei. Appl Environ Microbiol 79:5104–5111. doi: 10.1128/AEM.01370-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cai SF, Cai L, Liu HL, Liu XQ, Han J, Zhou J, Xiang H. 2012. Identification of the haloarchaeal phasin (PhaP) that functions in polyhydroxyalkanoate accumulation and granule formation in Haloferax mediterranei. Appl Environ Microbiol 78:1946–1952. doi: 10.1128/AEM.07114-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cai SF, Cai L, Zhao DH, Liu GM, Han J, Zhou J, Xiang H. 2015. A novel DNA-binding protein, PhaR, plays a central role in the regulation of polyhydroxyalkanoate accumulation and granule formation in the haloarchaeon Haloferax mediterranei. Appl Environ Microbiol 81:373–385. doi: 10.1128/AEM.02878-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual, 3rd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 24.Palmer BR, Marinus MG. 1994. The dam and dcm strains of Escherichia coli—a review. Gene 143:1–12. doi: 10.1016/0378-1119(94)90597-5. [DOI] [PubMed] [Google Scholar]
- 25.Zhao DH, Cai L, Wu JH, Li M, Liu HL, Han J, Zhou J, Xiang H. 2013. Improving polyhydroxyalkanoate production by knocking out the genes involved in exopolysaccharide biosynthesis in Haloferax mediterranei. Appl Microbiol Biotechnol 97:3027–3036. doi: 10.1007/s00253-012-4415-3. [DOI] [PubMed] [Google Scholar]
- 26.Stroud A, Liddell S, Allers T. 2012. Genetic and biochemical identification of a novel single-stranded DNA-binding complex in Haloferax volcanii. Front Microbiol 3:224. doi: 10.3389/fmicb.2012.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brendel J, Stoll B, Lange SJ, Sharma K, Lenz C, Stachler AE, Maier LK, Richter H, Nickel L, Schmitz RA, Randau L, Allers T, Urlaub H, Backofen R, Marchfelder A. 2014. A complex of Cas proteins 5, 6, and 7 is required for the biogenesis and stability of clustered regularly interspaced short palindromic repeats (CRISPR)-derived RNAs (crRNAs) in Haloferax volcanii. J Biol Chem 289:7164–7177. doi: 10.1074/jbc.M113.508184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han J, Lu Q, Zhou L, Zhou J, Xiang H. 2007. Molecular characterization of the phaECHm genes, required for biosynthesis of poly(3-hydroxybutyrate) in the extremely halophilic archaeon Haloarcula marismortui. Appl Environ Microbiol 73:6058–6065. doi: 10.1128/AEM.00953-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han J, Li M, Hou J, Wu L, Zhou J, Xiang H. 2010. Comparison of four phaC genes from Haloferax mediterranei and their function in different PHBV copolymer biosyntheses in Haloarcula hispanica. Saline Syst 6:9. doi: 10.1186/1746-1448-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shevchenko A, Wilm M, Vorm O, Mann M. 1996. Mass spectrometric sequencing of proteins from silver stained polyacrylamide gels. Anal Chem 68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- 31.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. 1985. Measurement of protein using bicinchoninic acid. Anal Biochem 150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 32.Handrick R, Reinhardt S, Schultheiss D, Reichart T, Schüler D, Jendrossek V, Jendrossek D. 2004. Unraveling the function of the Rhodospirillum rubrum activator of polyhydroxybutyrate (PHB) degradation: the activator is a PHB-granule-bound protein (phasin). J Bacteriol 186:2466–2475. doi: 10.1128/JB.186.8.2466-2475.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu J, Takahashi A, Ueda S. 2014. 3-Hydroxybutyrate oligomer hydrolase and 3-hydroxybutyrate dehydrogenase participate in intracellular polyhydroxybutyrate and polyhydroxyvalerate degradation in Paracoccus denitrificans. Appl Environ Microbiol 80:986–993. doi: 10.1128/AEM.03396-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marchler-Bauer A, Lu SN, Anderson JB, Chitsaz F, Derbyshire MK, DeWeese-Scott C, Fong JH, Geer LY, Geer RC, Gonzales NR, Gwadz M, Hurwitz DI, Jackson JD, Ke ZX, Lanczycki CJ, Lu F, Marchler GH, Mullokandov M, Omelchenko MV, Robertson CL, Song JS, Thanki N, Yamashita RA, Zhang DC, Zhang NG, Zheng CJ, Bryant SH. 2011. CDD: a conserved domain database for the functional annotation of proteins. Nucleic Acids Res 39:D225–D229. doi: 10.1093/nar/gkq1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Altschul SF, Wootton JC, Gertz EM, Agarwala R, Morgulis A, Schäffer AA, Yu YK. 2005. Protein database searches using compositionally adjusted substitution matrices. FEBS J 272:5101–5109. doi: 10.1111/j.1742-4658.2005.04945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rydel TJ, Williams JM, Krieger E, Moshiri F, Stallings WC, Brown SM, Pershing JC, Purcell JP, Alibhai MF. 2003. The crystal structure, mutagenesis, and activity studies reveal that patatin is a lipid acyl hydrolase with a Ser-Asp catalytic dyad. Biochemistry 42:6696–6708. doi: 10.1021/bi027156r. [DOI] [PubMed] [Google Scholar]
- 37.Andrews DL, Beames B, Summers MD, Park WD. 1988. Characterization of the lipid acyl hydrolase activity of the major potato (Solanum tuberosum) tuber protein, patatin, by cloning and abundant expression in a baculovirus vector. Biochem J 252:199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jendrossek D, Hermawan S, Subedi B, Papageorgiou AC. 2013. Biochemical analysis and structure determination of Paucimonas lemoignei poly(3-hydroxybutyrate) (PHB) depolymerase PhaZ7 muteins reveal the PHB binding site and details of substrate-enzyme interactions. Mol Microbiol 90:649–664. doi: 10.1111/mmi.12391. [DOI] [PubMed] [Google Scholar]
- 39.Tseng CL, Chen HJ, Shaw GC. 2006. Identification and characterization of the Bacillus thuringiensis phaZ gene, encoding new intracellular poly-3-hydroxybutyrate depolymerase. J Bacteriol 188:7592–7599. doi: 10.1128/JB.00729-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williamson DH, Krebs HA, Mellanby J. 1962. Enzymic determination of d(−)-beta-hydroxybutyric acid and acetoacetic acid in blood. Biochem J 82:90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oren A, Hallsworth JE. 2014. Microbial weeds in hypersaline habitats: the enigma of the weed-like Haloferax mediterranei. FEMS Microbiol Lett 359:134–142. doi: 10.1111/1574-6968.12571. [DOI] [PubMed] [Google Scholar]
- 42.Chen HJ, Pan SC, Shaw GC. 2009. Identification and characterization of a novel intracellular poly(3-hydroxybutyrate) depolymerase from Bacillus megaterium. Appl Environ Microbiol 75:5290–5299. doi: 10.1128/AEM.00621-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hirschberg HJHB, Simons JWFA, Dekker N, Egmond MR. 2001. Cloning, expression, purification and characterization of patatin, a novel phospholipase A. Eur J Biochem 268:5037–5044. doi: 10.1046/j.0014-2956.2001.02411.x. [DOI] [PubMed] [Google Scholar]
- 44.Kienesberger PC, Oberer M, Lass A, Zechner R. 2009. Mammalian patatin domain containing proteins: a family with diverse lipolytic activities involved in multiple biological functions. J Lipid Res 50:S63–S68. doi: 10.1194/jlr.R800082-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tirawongsaroj P, Sriprang R, Harnpichamchai P, Thongaram T, Champreda V, Tanapongpipat S, Pootanakit K, Eurwilaichitr L. 2008. Novel thermophilic and thermostable lipolytic enzymes from a Thailand hot spring metagenomic library. J Biotechnol 133:42–49. doi: 10.1016/j.jbiotec.2007.08.046. [DOI] [PubMed] [Google Scholar]
- 46.Banerji S, Flieger A. 2004. Patatin-like proteins: a new family of lipolytic enzymes present in bacteria? Microbiology 150:522–525. doi: 10.1099/mic.0.26957-0. [DOI] [PubMed] [Google Scholar]
- 47.Scherer GFE, Ryu SB, Wang XM, Matos AR, Heitz T. 2010. Patatin-related phospholipase A: nomenclature, subfamilies and functions in plants. Trends Plant Sci 15:693–700. doi: 10.1016/j.tplants.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 48.Sznajder A, Pfeiffer D, Jendrossek D. 2015. Comparative proteome analysis reveals four novel polyhydroxybutyrate (PHB) granule-associated proteins in Ralstonia eutropha H16. Appl Environ Microbiol 81:1847–1858. doi: 10.1128/AEM.03791-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ren Q, Ruth K, Thöny-Meyer L, Zinn M. 2010. Enatiomerically pure hydroxycarboxylic acids: current approaches and future perspectives. Appl Microbiol Biotechnol 87:41–52. doi: 10.1007/s00253-010-2530-6. [DOI] [PMC free article] [PubMed] [Google Scholar]