Abstract
Background
The American Fistula First Breakthrough Initiative currently aims for a 66% arterio-venous fistula (AVF) rate, while in the UK, best practice tariffs target AVF and arterio-venous graft (AVG) rates of 85%. The present study aims to assess whether these goals can be achieved.
Methods
We conducted a retrospective cohort study on patients who initiated haemodialysis from 1995 to 2006. Outcomes were the final failure-free survival of the first permanent access and the type of second access created. Prevalent use rates for the access types were calculated on the 1st January of each year for the second half of the study period.
Results
Two hundred and eleven out of 246 patients (86%) received an AVF, 16 (6%) an AVG and 19 (8%) a permanent catheter (PC) as the first permanent access. Eighty-six (35%) patients had final failure of the primary access. One- and 3-year final failure-free survival rates were 73 and 65% for AVF compared with 40 and 20% for AVG and 62 and 0% for PC, respectively. In patients with primary AVF, female sex {hazard ratio (HR) 2.20 [confidence interval (CI) 1.29–3.73]} and vascular disease [HR 2.24 (CI 1.26–3.97)] were associated with a poorer outcome. A similar trend was observed for autoimmune disease [HR 2.14 (CI 0.99–4.65)]. As second accesses AVF, AVG and PC were created in 47% (n = 40), 38% (n = 33) and 15% (n = 13). The median prevalent use rate was 80.5% for AVF, 14% for AVG and 5.5% for PC.
Conclusions
The vascular access targets set by initiatives from the USA and UK are feasible in unselected haemodialysis patients. High primary AVF rates, the superior survival rates of AVFs even in patient groups at higher risk of access failure and the high rate of creation of secondary AVFs contributed to these promising results.
Keywords: arterio-venous fistula, arterio-venous graft, chronic haemodialysis, incidence and prevalence, vascular access
Introduction
Almost 300 000 patients in Europe and 1.5 million patients worldwide rely on a vascular access for chronic haemodialysis [1, 2]. The ideal haemodialysis access should fulfil three criteria. It should be long lasting, provide an adequate blood flow and have a low rate of associated complications [3]. Currently, three types of vascular access are commonly used: native arterio-venous fistulas (AVFs), synthetic arterio-venous grafts (AVGs) and permanent tunnelled catheters (PCs).
While no access form perfectly fulfils all quality criteria, the use of an AVF is unanimously recommended by international guidelines [4] because of higher patency rates and fewer access-associated interventions. Despite these guidelines, various studies have repeatedly found striking differences in the types of vascular access used in different regions and countries. In 2002, the ‘Dialysis Outcomes and Practice Patterns Study’ (DOPPS) found 80% of the dialysis patients in Europe to have undergone chronic haemodialysis via an AVF, while in the USA, the majority (58%) of the patients were dialysed via an AVG and only 24% were dialysed via an AVF [5]. The continuous efforts of the American fistula first initiative have since led to an increase in the AVF rate to 60% in the USA [6]. Interestingly, wide variations in vascular access use were even found within Europe, with AVF percentages reaching from 67% (UK) to 90% (Italy) [5].
To improve AVF rates, the American Fistula First Breakthrough Initiative currently aims for a 66% AVF rate in haemodialysis patients [7], while the clinical practice guidelines for vascular access for haemodialysis of the UK Renal Association (5th edition, 2008–11) state that 85% of all prevalent patients on haemodialysis should receive dialysis via an arterio-venous fistula [8], and best practice tariffs (BPT) are being implemented to target definitive vascular access rates (the sum of AVF and AVG) of 85% by 2013/2014 [9]. The feasibility of these performance goals has been controversially discussed [10]. We therefore assessed whether these ambitious goals can be achieved and maintained in an unselected cohort of incident haemodialysis patients.
Materials and methods
Study design and population
We conducted a retrospective cohort study which encompassed all patients receiving a first permanent vascular access and initiating chronic haemodialysis (HD) at the University Hospital Basel between January 1995 and the end of June 2006. Overall, 269 patients entered the chronic haemodialysis programme during the study period. Patients transferring from another dialysis centre (n = 20) and patients for whom incomplete data sets were available (n = 3) were excluded. A total of 246 patients were eligible for the analysis. All access-related surgical procedures were performed or supervised by a dedicated board-certified vascular surgeon. Patients were routinely dialysed in a haemodiafiltration post-dilution modus with a blood flow of ≥300 mL/min for AVF or AVG. Shunts were punctured with 16 G needles by experienced nurses. A rope ladder rotation technique was applied and anticoagulation was performed with low molecular heparins. Haemostasis after needle removal was performed by manual compression and not by clamps in most cases.
Data source
Since the beginning of the dialysis programme at the University Hospital Basel, all clinical data have been prospectively and continuously collected in standardized flow sheets and in medical records.
The diagnosis of glomerulonephritis or interstitial nephropathy as the cause of end-stage renal disease was histology-based (kidney biopsy or nephrectomy). In cases of clinically suspected glomerulonephritis or interstitial nephropathy but missing biopsy proof, the underlying kidney pathology was grouped as ‘unknown’. Comorbid conditions were confirmed by the abstractors based on medical history, current medication and clinical testing at the time of entry to chronic haemodialysis as described previously [11]. In short, vascular disease was defined as the presence of at least one of the following diagnoses: coronary artery disease (CAD), peripheral arterial disease (PAD) or cerebrovascular disease (CVD). The diagnosis of active malignancy was based on a histological diagnosis. The diagnosis of autoimmune disease (AID) was based on the judgement of the ‘Interdisciplinary Vasculitis Board’ of the University Hospital Basel.
Patients were grouped by their first permanent vascular access type. Access types were classified as follows: AVF, AVG and PC. The date of creation of the first permanent vascular access, the start of haemodialysis, the date of any first complication which needed intervention and the date of complete loss of the access site for dialysis requiring creation of a new access were noted. Additionally, the type of the second permanent vascular access was recorded. Vascular access-related complications included thrombosis (occlusion), stenosis, aneurysm, steal syndrome, infection and other reason for dysfunction, e.g. insufficient access maturation or difficult anatomic situation to ensure appropriate puncture. The cases of inappropriately recorded complications were classified as ‘unknown’. As a consequence of the study design which was exclusively based on patients initiating haemodialysis, prevalent use rates of the different access types were recorded for the second half of the study period, ensuring a stable and representative population of prevalent patients. Concretely, the prevalent use rates of AVF, AVG and PC, calculated by dividing the number of AVF, AVG and PC in use by the number of patients reported, were recorded on the 1st January each year from 2001 to 2006.
The study protocol was approved by the Institutional Review Board and was carried out according to the principles of the Declaration of Helsinki.
Endpoints and outcome
Main outcome measures were the final failure-free survival of the first permanent access, the type of the second vascular access created and the prevalent use rates of the different access types. To avoid overestimation of access survival by including unutilized accesses created prior to haemodialysis, follow-up started with creation of the primary permanent vascular access or the start of chronic haemodialysis, whatever came last. Final failure-free survival was defined as the time from the start of follow-up to complete loss of the primary permanent access site for dialysis (i.e. need for creation of a new access). Additionally, complication-free survival, defined as the time from the start of follow-up to the occurrence of the first access-related complication requiring intervention, and the type of the complication leading to the intervention were assessed. Included were all events, regardless of whether the event occurred after or prior to the start of haemodialysis or within or after the access maturation time. In patients who received an access prior to haemodialysis and had an event prior to the start of haemodialysis follow-up time to failure was defined as zero to avoid the generation of negative follow-up time (i.e. from the start of haemodialysis backwards to the occurrence of the event during the pre-haemodialysis period). In survival analyses, reasons for censoring were switch from haemodialysis to peritoneal dialysis or kidney transplantation, loss to follow-up, death or the end of the observational period.
Statistical analysis
The statistical analyses were performed using SPSS/PC (IBM SPSS Statistics 19). A P-value of <0.05 was considered statistically significant, significance levels are two-tailed, and confidence intervals (CI) are 95% CI.
Discrete variables were expressed as counts (%). In the case of normal distribution, continuous variables were expressed as mean ± standard deviation. In the case of not normally distributed variables, they were expressed as median (range). The group comparisons were performed with the Fisher exact test for categorical variables and t-test for continuous variables if normally distributed or the Mann-Whitney test if not normally distributed. Access survival was analysed by the Cox regression analysis. Cumulative survival was calculated using the Kaplan–Meier analysis and differences were evaluated by log-rank statistics. To address the limitations of the data due to a decreasing number of patients in long-term follow-up, the Cox regression and survival analyses were modelled with a follow-up period of 4 years.
Results
Patient characteristics
Baseline characteristics of the population are summarized in Table 1. The patients' age ranged from 15 to 90 years (median 65.3). Overall, body mass index (BMI) was significantly higher in male than female patients (P = 0.004). While significantly more women (14%, n = 15) than men (4%, n = 5) suffered from underweight (BMI < 18.5) (P = 0.004), there was no significant difference in gender regarding overweight (P = 0.06) and obesity (P = 0.4). Fifty per cent (n = 122) of the patients suffered either from CAD, PAD and/or CVD. Diabetes mellitus type 2 was more common in men than in women (P = 0.04). While the distribution of diabetic nephropathy did not differ significantly (P = 0.4), analgesic nephropathy was more common in women than in men (P = 0.003). No differences in the primary vascular access type existed between men and women (Table 1). There were no significant differences in patient characteristics when stratified by the type of access (data not shown).
Table 1.
Patient characteristics and type of primary permanent vascular accessa
| Overall | Male | Female | P-value | |
|---|---|---|---|---|
| Number | 246 | 138 (56) | 108 (44) | |
| Age (years) | 65.3 [15.2–89.6] | 63.6 [15.2–88.1] | 65.9 [20.9–89.6] | 0.7b |
| BMI | 23.5 [11.8–50.9] | 24.1 [16.7–50.9] | 22.3 [11.8–37.0] | 0.004b |
| Diabetes mellitus | 89 (36) | 56 (41) | 33 (31) | 0.1c |
| Type 1 | 10 (4) | 4 (3) | 6 (6) | 0.3c |
| Type 2 | 79 (32) | 52 (38) | 27 (25) | 0.04c |
| Vascular disease | 122 (50) | 70 (51) | 52 (48) | 0.7c |
| CAD | 69 (28) | 44 (32) | 25 (23) | 0.2c |
| PAD | 70 (29) | 39 (29) | 31 (29) | 1.0c |
| CVD | 39 (16) | 22 (16) | 17 (16) | 1.0c |
| Malignancies | 65 (27) | 35 (26) | 30 (28) | 0.8c |
| AID | 19 (8) | 8 (6) | 11 (10) | 0.2c |
| Underlying kidney disease | ||||
| Diabetes mellitus | 45 (18) | 28 (20) | 17 (16) | 0.4c |
| ADPKD | 23 (9) | 11 (8) | 12 (11) | 0.5c |
| Analgesic nephropathy | 16 (7) | 3 (2) | 13 (12) | 0.003c |
| Glomerulonephritis | 29 (12) | 18 (13) | 11 (10) | 0.6c |
| Interstitial nephropathy | 3 (1) | 2 (2) | 1 (1) | 1.0c |
| Vascular nephropathy | 38 (15) | 23 (17) | 15 (14) | 0.6c |
| Otherd | 63 (26) | 40 (29) | 23 (21) | 0.2c |
| Unknown | 29 (12) | 13 (9) | 16 (15) | 0.2c |
| Type of vascular access | ||||
| AVF | 211 (86) | 123 (89) | 88 (82) | 0.1c |
| AVG | 16 (6) | 6 (4) | 10 (9) | 0.2c |
| PC | 19 (8) | 9 (7) | 10 (9) | 0.5c |
aData are displayed as counts (%) or median [range]. Vascular disease: including all patients suffering from at least one subgroup of CAD, PAD or CVD. CAD, coronary artery disease; PAD, peripheral arterial disease; CVD, cerebrovascular disease; AID, autoimmune disease; ADPKD, autosomal dominant polycystic kidney disease; AVF, native arterio-venous fistula; AVG, arterio-venous graft; PC, permanent catheter.
bMann–Whitney test.
cFisher's exact test.
dAmong others primary focal segmental glomerulosclerosis (n = 13), nephrectomy due to renal cell carcinoma (n = 7), atheroembolic renal disease (n = 4), amyloidosis (n = 4), multiple myeloma (n = 4) and reflux nephropathy (n = 4).
Follow-up and patients outcome
During the observational period, the median time on HD was 1.7 years (range 0.0–10.5 years). At the end of the observational period, 85 (35%) of 246 patients were still on haemodialysis, 23 (9%) received a living and 39 (16%) a deceased donor kidney transplantation. Three patients (1%) were transferred to peritoneal dialysis, 2 (1%) regained kidney function, 87 (35%) patients died and 7 (3%) were lost to follow-up.
Incidence of the primary permanent vascular access
In 211 patients (86%), the first permanent vascular access was a native AVF, in 16 patients (6%) an AVG and in 19 patients (8%) a PC (Table 2). In nine patients (seven males and two females; 48%), PC was created as a bridging vascular access to living donor kidney transplantation and in one female patient (5%) to peritoneal dialysis. Two male and two female patients (21%) received a PC due to a short expected survival and five patients (all female; 26%) due to a difficult vascular situation.
Table 2.
Type of primary and secondary permanent vascular access
| Type of vascular access | Primary access, n = 246 (%) | Needing second access, 86/246 (35%) | Type of second access |
|---|---|---|---|
| Native arterio-venous fistula (AVF) | 211 (86) | 67/211 (32) | AVF: 36/67(54) |
| AVG: 24/67 (36) | |||
| PC: 7/67 (10) | |||
| Arterio-venous graft (AVG) | 16 (6) | 12/16 (75) | AVF: 2/12 (17) |
| AVG: 8/12 (66) | |||
| PC: 2/12 (17) | |||
| Permanent catheter (PC) | 19 (8) | 7/19 (37) | AVF: 2/7 (29) |
| AVG: 1/7 (14) | |||
| PC: 4/7 (57) |
Data are displayed as counts (%).
Final failure of the primary permanent vascular access
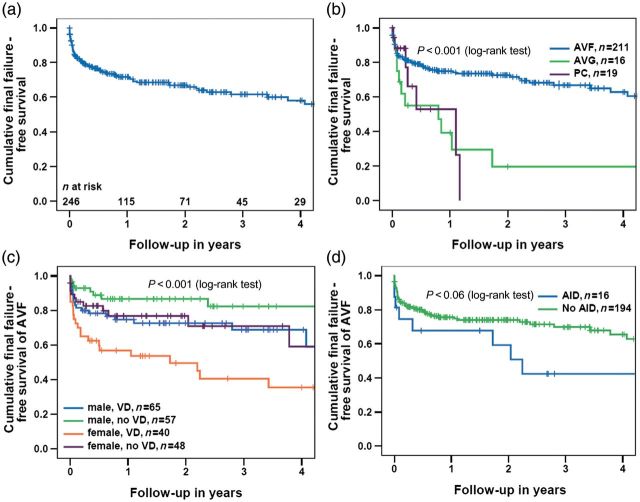
Overall, 86 (35%) patients experienced final failure of the first permanent access. In eight patients (all AVF), final failure occurred before the start of haemodialysis. One- and 3-year final failure-free survival rates were 72 and 62%, respectively (Figure 1a). Stratified by the access type, survival differed significantly between the groups (P < 0.001). One- and 3-year final failure-free survival rates were 75 and 67% for AVF compared with 39 and 20% for AVG and 53 and 0% for PC, respectively (Figure 1b). In multivariable Cox's regression analysis, female gender [hazard ratio (HR) 1.98 (CI 1.23–3.19), P = 0.005], a history of vascular disease [HR 2.32 (CI 1.39–3.89), P = 0.001] and the use of AVG [HR 2.52 (CI 1.29–4.92), P = 0.007] were independent risk factors for final access failure (Table 3). In the subgroup of patients with a primary AVF, female sex [HR 2.20 (CI 1.29–3.73), P = 0.004] and vascular disease [HR 2.24 (CI 1.26–3.97), P = 0.006] were associated with a poorer outcome; a similar trend was seen for AID [HR 2.14 (CI 0.99–4.65), P = 0.06] (Table 3). Figure 1c shows the final failure-free access survival in patients with AVF. Stratified by gender and vascular disease, final failure-free survival differed significantly between the groups (P < 0.001). One- and 3-year final failure-free survival rates were 75 and 69% versus 87 and 82% for men with versus without vascular disease, and 57 and 40 versus 77 and 71% for women with and without vascular disease (Figure 1c). Figure 1d shows the final failure in patients with AVF stratified by AID (P = 0.06).
Fig. 1.
(a) Overall final failure-free survival of the first permanent vascular access over time, (b) overall final failure-free survival, stratified by the type of vascular access, (c) final failure-free survival of AVF, stratified by gender and vascular disease and (d) final failure-free survival of AVF, stratified by AID. AVF, native arterio-venous fistulas; AVG, arterio-venous graft; PC, permanent catheter; VD, vascular disease; AID, autoimmune disease. P-values (log-rank test) between subgroups in (b): AVF versus AVG: P < 0.001; AVF versus PC: P = 0.055; AVG versus PC: P = 0.8; AVF versus AVG and PC: P < 0.001.
Table 3.
Cox's regression of time to final failure of the primary permanent vascular access
| Overall (n = 246) univariate |
Overall (n = 246) multivariate |
AVF only (n = 211) univariate |
AVF only (n = 211) multivariate |
|||||
|---|---|---|---|---|---|---|---|---|
| HR (CI) | P-value | HR (CI) | P-value | HR (CI) | P-value | HR (CI) | P-value | |
| Female | 2.13 (1.36–3.35) | 0.001 | 1.98 (1.23–3.19) | 0.005 | 2.08 (1.25–3.46) | 0.005 | 2.20 (1.29–3.73) | 0.004 |
| Age | 1.01 (0.99–1.03) | 0.3 | 1.00 (0.98–1.02) | 0.8 | 1.00 (0.98–1.02) | 0.8 | 0.99 (0.97–1.01) | 0.3 |
| BMI | 0.99 (0.94–1.04) | 0.6 | 1.00 (0.95–1.05) | 0.9 | 0.99 (0.93–1.04) | 0.6 | 0.99 (0.93–1.04) | 0.6 |
| DM | 1.31 (0.84–2.05) | 0.2 | 1.37 (0.83–2.25) | 0.2 | 1.52 (0.91–2.52) | 0.1 | 1.50 (0.85–2.66) | 0.2 |
| Vascular disease | 2.22 (1.38–3.57) | 0.001 | 2.32 (1.39–3.89) | 0.001 | 2.07 (1.21–3.54) | 0.008 | 2.24 (1.26–3.97) | 0.006 |
| AID | 1.76 (0.90–3.41) | 0.1 | 1.93 (0.95–3.92) | 0.07 | 2.01 (0.95–4.23) | 0.07 | 2.14 (0.99–4.65) | 0.06 |
| Malignoma | 1.67 (1.04–2.66) | 0.032 | 1.54 (0.93–2.57) | 0.09 | 1.40 (0.80–2.43) | 0.2 | 1.65 (0.92–2.96) | 0.09 |
| AVF | 1.00 | 1.00 | ||||||
| AVG | 3.14 (1.64–6.01) | 0.001 | 2.52 (1.29–4.92) | 0.007 | ||||
| PC | 2.22 (1.00–4.91) | 0.050 | 1.95 (0.85–4.45) | 0.1 | ||||
Follow-up cut at 4 years, events overall: 78, events AVF: 60. BMI, body mass index; DM, diabetes mellitus; AID, autoimmune disease; AVF, native arterio-venous fistula; AVG, arterio-venous graft; PC, permanent catheter.
First complication of the primary permanent vascular access
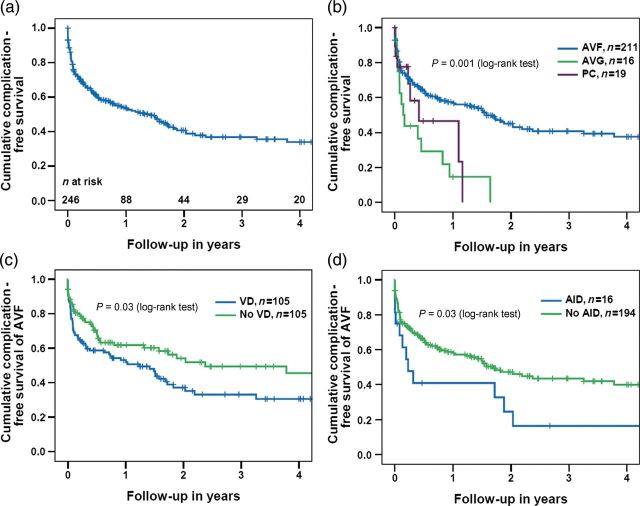
Overall, 133 patients (54%) experienced at least one vascular access-related complication during the observational period. Thrombosis (n = 50, 38%) and stenosis (n = 43, 32%) were the most common primary complications (Table 4). In 13 patients (AVF in 12 patients and PC in 1 patient), a complication occurred before the start of haemodialysis. One- and 3-year complication-free survival rates were 54 and 37%, respectively (Figure 2a). Stratified by the access type, the median complication-free survival differed significantly between the groups (P = 0.001). One- and 3-year complication-free survival rates were 57 and 41% for AVF compared with 15 and 0% for AVG and 46 and 0% for PC, respectively (Figure 2b). In Cox's regression analysis, female gender [HR 1.45 (CI 1.00–2.08), P = 0.048], a history of vascular disease [HR 1.55 (CI 1.05–2.30), P = 0.03], AID [HR 1.81 (CI 1.02–3.23), P = 0.04] and the use of an AVG [HR 2.37 (CI 1.33–4.22), P = 0.003] were all associated with an increased risk of access-related complications (Table 5). When restricting the analysis to patients receiving an AVF as the primary access, vascular disease [HR 1.69 (CI 1.10–2.60), P = 0.02] and AID [HR 2.00 (CI 1.06–3.75), P = 0.03] remained independently associated with a poor prognosis. One- and 3-year complication-free survival rates were 53 and 33% versus 62 and 49% with and without vascular disease (Figure 2c), and 41 and 16 versus 59 and 44% with and without AID (Figure 2d), respectively.
Table 4.
First complication of the primary permanent vascular access
| Complication (n) | 133 (100%) |
|---|---|
| Thrombosis | 50 (38) |
| Stenosis | 43 (32) |
| Aneurysm | 2 (2) |
| Infection | 2 (2) |
| Othersa | 18 (13) |
| Unknown | 18 (13) |
aAmong others steal syndrome and insufficient access maturation.
Fig. 2.
(a) Overall complication-free survival of the first permanent vascular access over time, (b) Overall complication-free survival, stratified by the type of vascular access, (c) complication-free survival of AVF, stratified by vascular disease and (d) complication-free survival of AVF, stratified by AID. AVF, native arterio-venous fistula; AVG, arterio-venous graft; PC, permanent catheter; VD, vascular disease; AID, autoimmune disease. P-values (log-rank test) between subgroups in (b): AVF versus AVG: P < 0.001; AVF versus PC: P = 0.1; AVG versus PC: P = 0.4; AVF versus AVG and PC: P = 0.001.
Table 5.
Cox's regression of time to first complication of primary permanent vascular access
| Overall (n=246) univariate |
Overall (n=246) multivariate |
AVF only (n=211) univariate |
AVF only (n=211) multivariate |
|||||
|---|---|---|---|---|---|---|---|---|
| HR (CI) | P-value | HR (CI) | P-value | HR (CI) | P-value | HR (CI) | P-value | |
| Female | 1.57 (1.11–2.22) | 0.01 | 1.45 (1.00–2.08) | 0.048 | 1.40 (0.95–2.05) | 0.09 | 1.37 (0.92–2.05) | 0.1 |
| Age | 1.00 (0.99–1.01) | 0.9 | 0.99 (0.98–1.01) | 0.3 | 1.00 (0.98–1.01) | 0.7 | 0.99 (0.97–1.00) | 0.1 |
| BMI | 1.01 (0.97–1.04) | 0.7 | 1.01 (0.98–1.05) | 0.6 | 1.01 (0.97–1.05) | 0.6 | 1.01 (0.97–1.05) | 0.8 |
| DM | 1.14 (0.80–1.63) | 0.5 | 1.16 (0.79–1.70) | 0.5 | 1.21 (0.82–1.78) | 0.3 | 1.15 (0.75–1.77) | 0.5 |
| Vascular disease | 1.49 (1.05–2.11) | 0.03 | 1.55 (1.05–2.30) | 0.03 | 1.54 (1.04–2.27) | 0.03 | 1.69 (1.10–2.60) | 0.02 |
| AID | 1.80 (1.05–3.08) | 0.03 | 1.81 (1.02–3.23) | 0.04 | 1.90 (1.04–3.48) | 0.04 | 2.00 (1.06–3.75) | 0.03 |
| Malignoma | 1.40 (0.95–2.04) | 0.1 | 1.40 (0.93–2.11) | 0.1 | 1.32 (0.86–2.02) | 0.2 | 1.48 (0.94–2.33) | 0.1 |
| AVF | 1.00 | 1.00 | ||||||
| AVG | 2.68 (1.52–4.73) | 0.001 | 2.37 (1.33–4.22) | 0.003 | ||||
| PC | 1.69 (0.85–3.37) | 0.1 | 1.49 (0.73–3.03) | 0.3 | ||||
Follow-up cut at 4 years; overall: 128 events, AVF: 105 events. BMI, body mass index; DM, diabetes mellitus; AID, autoimmune disease; AVF, native arterio-venous fistula; AVG, arterio-venous graft; PC, permanent catheter.
Type of the second permanent vascular access
The rate of patients needing a second vascular access was 32% for patients primarily dialysed by AVF, 75% by AVG and 37% by PC (Table 2). After the exclusion of patients waiting for a living donor kidney transplant or to the start of peritoneal dialysis, the need for second access was 66% in patients treated primarily by PC. Overall, as second access, AVF, AVG and PC were created in 47% (n = 40), 38% (n = 33) and 15% (n = 13). An AVF as second access was created in 54% (n = 36) and 17% (n = 2) of the patients primarily dialysed by AVF and AVG, respectively (Table 2). Two (29%) of the patients primarily dialysed by PC received an AVF as second access, one female with advanced cancer disease and unexpected long survival and one male with advanced cancer disease and catheter sepsis (Table 2).
Prevalent use rates of AVF, AVG and PC
The prevalent use rates for AVF, AVG and PC are summarized in Table 6. At the reference day, a median of 67 patients (range 49–84) were on haemodialysis and the median time on haemodialysis was 1.92 years (range 1.51–2.15). Except at the reference day in 2004, the median time on haemodialysis did not significantly differ between patients treated by AVF, AVG or PC and was overall in the range of 1.39–2.10 years. The median prevalent use rates over 6 years were 80.5% (range 75–86%) for AVF, 14% (range 10–19%) for AVG and 5.5% (range 2–8%) for PC (Table 6). There was a trend towards a lower AVF prevalent use rate in women compared with men (range 66–74 versus 79–93%). Consequently, AVG and PC rates tended to be higher in female patients. These differences were consistent over time but did not reach the pre-defined 5% significance level (Table 7).
Table 6.
Prevalent use rates of permanent vascular access types over 6 yearsa
| Reference date | Overall |
AVF |
AVG |
PC |
P-valueb | ||||
|---|---|---|---|---|---|---|---|---|---|
| n | Time on HD | n (%) | Time on HD | n (%) | Time on HD | n (%) | Time on HD | ||
| 1.1.2001 | 49 | 1.60 [0.03–5.67] | 42 (86) | 1.28 [0.05–5.67] | 5 (10) | 1.91 [0.03–4.90] | 2 (4) | 2.42 [1.62–3.21] | 0.5 |
| 1.1.2002 | 61 | 1.51 [0.10–6.04] | 50 (82) | 1.42 [0.10–6.04] | 8 (13) | 2.28 [0.79–5.90] | 3 (5) | 0.66 [0.16–4.21] | 0.3 |
| 1.1.2003 | 63 | 1.91c [0.05–7.04] | 50 (79) | 1.92c [0.05–7.04] | 12 (19) | 0.85 [0.08–6.90] | 1 (2) | 5.21 [5.21–5.21] | 0.2 |
| 1.1.2004 | 71 | 1.92c [0.04–8.67] | 53 (75) | 2.44c [0.04–8.67] | 13 (18) | 1.57 [0.14–7.90] | 5 (7) | 0.35 [0.04–0.87] | 0.01 |
| 1.1.2005 | 78 | 2.15c [0.11–9.67] | 60 (77) | 2.07c [0.11–9.67] | 12 (15) | 3.39c [0.24–8.90] | 6 (8) | 2.12 [1.14–5.61] | 0.7 |
| 1.1.2006 | 84 | 2.02c [0.03–9.84] | 69 (82) | 2.02c [0.03–9.84] | 10 (12) | 3.65c [0.13–7.55] | 5 (6) | 0.51 [0.04–3.36] | 0.1 |
| 2001–06 | 67 [49–84] | 1.92 [1.51–2.15] | 80.5% [75–86] | 1.97 [1.28–2.44] | 14% [10–19] | 2.09 [0.85–3.65] | 5.5% [2–8] | 1.39 [0.35–5.21] | |
aData are displayed as counts (%) or median [range].
bP-value of difference in median time on dialysis between groups (Kruskal–Wallis test). Reference date: prevalent use rates were recorded at each 1 January from 2001 to 2006 for the second half of the study period.
cPatients with >1 year interruption of HD due to transplantation were excluded from the analysis of time on HD: 2003: 1 AVF; 2004: 1 AVF; 2005: 2 AVF, 2 AVG; 2006: 3 AVF, 1 AVG. AVF, native arterio-venous fistula; AVG, arterio-venous graft; PC, permanent catheter.
Table 7.
Prevalent use rates of permanent vascular access types over 6 years by gendera
| Reference date | Overall |
AVF |
AVG |
PC |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Male (n) | Female (n) | Male, n (%) | Female, n (%) | Male, n (%) | Female, n (%) | Male, n (%) | Female, n (%) | P-value | |
| 1.1.2001 | 30 | 19 | 28 (93) | 14 (74) | 2 (7) | 3 (16) | 0 | 2 (10) | 0.1 |
| 1.1.2002 | 35 | 26 | 32 (91) | 18 (69) | 2 (6) | 6 (23) | 1 (3) | 2 (8) | 0.06 |
| 1.1.2003 | 36 | 27 | 32 (89) | 18 (67) | 4 (11) | 8 (29) | 0 | 1 (4) | 0.07 |
| 1.1.2004 | 38 | 33 | 29 (76) | 24 (73) | 6 (16) | 7 (21) | 3 (8) | 2 (6) | 0.9 |
| 1.1.2005 | 43 | 35 | 37 (86) | 23 (66) | 5 (12) | 7 (20) | 1 (2) | 5 (14) | 0.07 |
| 1.1.2006 | 51 | 33 | 45 (88) | 24 (73) | 5 (10) | 5 (15) | 1 (2) | 4 (12) | 0.1 |
| 2001–2006 | 37 [30–51] | 30 [19–35] | 88.5% [76–93] | 71% [66–74] | 10.5% [6–16] | 20.5% [15–29] | 2% [0–8] | 9% [4–14] | |
aData are displayed as counts (%) and median [range], P-value of difference between male and female (Fisher's exact test). Reference date: prevalent use rates were recorded at each 1st January from 2001 to 2006 for the second half of the study period. AVF, native arterio-venous fistula; AVG, arterio-venous graft; PC, permanent catheter.
Discussion
This study investigated the feasibility of the ambitious AVF targets proposed by the Fistula First Breakthrough Initiative and the UK BPT. We found a primary AVF rate of 86% and an average AVF long-term prevalent use rate of over 80%. The final failure-free survival rates for AVF were significantly higher than for AVG and PC. The rationale to promote AVF use in haemodialysis patients is compelling. AVFs have been shown to allow safe and adequate dialysis, to require fewer salvage interventions, provide better patency rates and to be associated with lower mortality than AVGs or PCs [12–14]. Recently, the creation of an AVF has even been suggested to be associated with beneficial cardiovascular effects, by decreasing arterial stiffness and blood pressure and increasing left ventricular ejection fraction [15]. Furthermore, these medical benefits come with significant economic advantages: The US Renal Data System showed the 1-year use of an AVF to be almost 20 000 US dollars cheaper than the 1 year use of a PC [16]. However, various reports have suggested demographic factors such as old age [17], female gender [18] and obesity [19, 20] to be associated with decreased AVF survival and have called for a re-appraisal of the AVF targets in these patient groups.
Our findings extend these previous studies by establishing vascular disease, AID and female gender as well as the use of a non-AVF access as the most important independent risk factors for primary vascular access complications and final failure. The risk factors for AVF complications and final failure were similar to those observed in the overall study population. The prognostic importance of vascular disease and female gender is at least partially triggered by their effects on vessel and lumen size. The importance of vessel size for adequate AVF maturation and adequacy was highlighted in a study by Wong et al. [21] that showed that fistulas constructed from veins or arteries <1.6 mm consistently failed to mature. Consequently, Silva et al. [22] were able to reach the primary AVF patency rates of 83% by increasing minimum vessel sizes to 2.0 mm for the artery and 2.5 mm for the vein. In agreement with these observations, AVFs placed on the lower arm without pre-surgical vascular mapping were shown to carry an especially bad prognosis in females, while upper arm AVFs faired equally well in both genders [23]. Therefore, pre-surgical vascular mapping to optimize vessel selection for fistula formation appears to be especially important in women. However, recent results by Miller et al. [18] suggest that despite pre-surgical vascular mapping and equal vascular diameters in adequate and failing fistulas, female patients were more likely to suffer from technical failure and early AVF thrombosis. The mechanisms behind the gender difference in prognosis not attributable to vessel size are currently not fully understood. The increased risk of final vascular access failure in women translated into a trend towards a lower long-term prevalent AVF use rate in female patients. However, the AVF survival rates for women and patients with known vascular diseases in this study were still considerably longer than the survival period observed for AVGs and PCs.
The importance of AIDs for vascular access complications and final failure observed in this study is most likely caused by a hypercoagulability state induced by these diseases. For example, thrombotic events are recognized as an important complication of Wegener's granulomatosis and patients with systemic lupus erythematodes are more likely to suffer vascular access thromboses [24]. Again, AVF survival rates in patients with AID were still considerably higher than survival rates observed for AVGs and PCs.
There are conflicting data in the literature concerning the effect of age [17, 25] and obesity [19, 26, 27] on vascular access outcomes. We found neither factor to negatively impact outcome in this non-selected patient cohort [5].
In our cohort, primary AVF creation in association with a high secondary AVF rate led to prevalent AVF and non-PC use rates persistently over those targeted by the Fistula First Breakthrough Initiative and the UK BPT [7, 8]. However, the range of prevalent AVF use rates of 75–86% indicates that the AVF percentage of 85% targeted by the UK clinical practice guidelines probably represents the upper limit of what is achievable overall and might not be achievable in this subgroup of female patients.
Our study is limited by its retrospective, single-centre design and the relatively small number of patients enrolled. However, all data were collected prospectively at the time of dialysis, and baseline characteristics and mortality rates were similar to those observed in other dialysis studies as well as the European Renal Association Annual Report. Further, we did not analyse surrogate markers with respect to haemodialysis quality in relation to the access type.
In conclusion, the vascular access targets set by the Fistula First Breakthrough Initiative and the UK BPT are feasible and sustainable in unselected haemodialysis patients. In this cohort, high primary AVF rates, the superior survival rates of AVFs even in patient groups at higher risk for access complications and failure (e.g. women, history of vascular or AID) and the high rate of creation of secondary AVFs contributed to these promising results.
Acknowledgements
The authors thank Catherine Haenlin, patient administrator of our dialysis unit, for her help during the data collection process. Further, a particular debt of gratitude is owed to our nursing team for their enthusiasm and daily work to achieve the best care for our patients.
Conflict of interest statement. A part of the study was presented as abstract and poster at the Kidney Week of the American Society of Nephrology 2011 in Philadelphia. The authors of this manuscript have no conflicts of interest to disclose.
References
- 1.JASN Editorial Board. 2002 Albert Lasker Award for Clinical Medical Research. J Am Soc Nephrol. 2002;13:3027–3030. doi: 10.1097/01.asn.0000041965.26075.84. doi:10.1097/01.ASN.0000041965.26075.84. [DOI] [PubMed] [Google Scholar]
- 2.European Renal Association EDaTA. 2007. Annual Report.
- 3.Hayashi R, Huang E, Nissenson AR. Vascular access for hemodialysis. Nat Clin Pract Nephrol. 2006;2:504–513. doi: 10.1038/ncpneph0239. [DOI] [PubMed] [Google Scholar]
- 4.Vascular Access 2006 Work Group. Clinical practice guidelines for vascular access. Am J Kidney Dis. 2006;48(Suppl 1)):S176–S247. doi: 10.1053/j.ajkd.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 5.Pisoni RL, Young EW, Dykstra DM, et al. Vascular access use in Europe and the United States: results from the DOPPS. Kidney Int. 2002;61:305–316. doi: 10.1046/j.1523-1755.2002.00117.x. doi:10.1046/j.1523-1755.2002.00117.x. [DOI] [PubMed] [Google Scholar]
- 6. Fistula First. http://www.fistulafirst.org/ (2 February 2012, date last accessed)
- 7.Lynch JR, Wasse H, Armistead NC, McClellan WM. Achieving the goal of the Fistula First breakthrough initiative for prevalent maintenance hemodialysis patients. Am J Kidney Dis. 2011;57:78–89. doi: 10.1053/j.ajkd.2010.08.028. doi:10.1053/j.ajkd.2010.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. The Renal Association: Vascular Access for Haemodialysis. http://www.renal.org/Clinical/GuidelinesSection/VascularAccess.aspx. (16 February 2012, date last accessed)
- 9.Department of Health. Payment by Results (PbR) Road Testing for 2011–12. 2010. http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/documents/digitalasset/dh_123079.pdf. (29 August 2011, date last accessed)
- 10.Fluck R, Raftery M. The motion to be debated: ‘Reduced payments for chronic haemodialysis patients dialysing on a tunnelled catheter are justified. British Renal Society/Renal Association Conference 2011; 2011; Birmingham,. [Google Scholar]
- 11.Breidthardt T, Moser-Bucher CN, Praehauser C, et al. Morbidity and mortality on chronic haemodialysis: a 10-year Swiss single centre analysis. Swiss Med Wkly. 2011;141:w13150. doi: 10.4414/smw.2011.13150. [DOI] [PubMed] [Google Scholar]
- 12.Tonelli M, Muirhead N. Access type as a predictor of dialysis adequacy in chronic hemodialysis patients. ASAIO J. 2000;46:279–282. doi: 10.1097/00002480-200005000-00007. doi:10.1097/00002480-200005000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Allon M. Current management of vascular access. Clin J Am Soc Nephrol. 2007;2:786–800. doi: 10.2215/CJN.00860207. doi:10.2215/CJN.00860207. [DOI] [PubMed] [Google Scholar]
- 14.Polkinghorne KR, McDonald SP, Atkins RC, Kerr PG. Vascular access and all-cause mortality: a propensity score analysis. J Am Soc Nephrol. 2004;15:477–486. doi: 10.1097/01.asn.0000109668.05157.05. doi:10.1097/01.ASN.0000109668.05157.05. [DOI] [PubMed] [Google Scholar]
- 15.Korsheed S, Eldehni MT, John SG, Fluck RJ, McIntyre CW. Effects of arteriovenous fistula formation on arterial stiffness and cardiovascular performance and function. Nephrol Dial Transplant. 2011;26:3296–3302. doi: 10.1093/ndt/gfq851. [DOI] [PubMed] [Google Scholar]
- 16.National Institute of Health NIoDaDaKD. Bethesda, MD: 2008. US Renal Data System: USRDS 2008 Annual Data Report. [Google Scholar]
- 17.Vachharajani TJ, Moossavi S, Jordan JR, et al. Re-evaluating the Fistula First Initiative in Octogenarians on Hemodialysis. Clin J Am Soc Nephrol. 2011;6:1663–1667. doi: 10.2215/CJN.05830710. doi:10.2215/CJN.05830710. [DOI] [PubMed] [Google Scholar]
- 18.Miller CD, Robbin ML, Allon M. Gender differences in outcomes of arteriovenous fistulas in hemodialysis patients. Kidney Int. 2003;63:346–352. doi: 10.1046/j.1523-1755.2003.00740.x. doi:10.1046/j.1523-1755.2003.00740.x. [DOI] [PubMed] [Google Scholar]
- 19.Kats M, Hawxby AM, Barker J, et al. Impact of obesity on arteriovenous fistula outcomes in dialysis patients. Kidney Int. 2007;71:39–43. doi: 10.1038/sj.ki.5001904. doi:10.1038/sj.ki.5001904. [DOI] [PubMed] [Google Scholar]
- 20.Plumb TJ, Adelson AB, Groggel GC, et al. Obesity and hemodialysis vascular access failure. Am J Kidney Dis. 2007;50:450–454. doi: 10.1053/j.ajkd.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 21.Wong V, Ward R, Taylor J, Selvakumar S, How TV, Bakran A. Factors associated with early failure of arteriovenous fistulae for haemodialysis access. Eur J Vasc Endovasc Surg. 1996;12:207–213. doi: 10.1016/s1078-5884(96)80108-0. doi:10.1016/S1078-5884(96)80108-0. [DOI] [PubMed] [Google Scholar]
- 22.Silva MB, Jr, Hobson RW, 2nd, Pappas PJ, et al. A strategy for increasing use of autogenous hemodialysis access procedures: impact of preoperative noninvasive evaluation. J Vasc Surg. 1998;27:302–307. doi: 10.1016/s0741-5214(98)70360-x. discussion 307–308 doi:10.1016/S0741-5214(98)70360-X. [DOI] [PubMed] [Google Scholar]
- 23.Miller PE, Tolwani A, Luscy CP, et al. Predictors of adequacy of arteriovenous fistulas in hemodialysis patients. Kidney Int. 1999;56:275–280. doi: 10.1046/j.1523-1755.1999.00515.x. doi:10.1046/j.1523-1755.1999.00515.x. [DOI] [PubMed] [Google Scholar]
- 24.Shafi ST, Gupta M. Risk of vascular access thrombosis in patients with systemic lupus erythematosus on hemodialysis. J Vasc Access. 2007;8:103–108. [PubMed] [Google Scholar]
- 25.Lok CE, Oliver MJ, Su J, Bhola C, Hannigan N, Jassal SV. Arteriovenous fistula outcomes in the era of the elderly dialysis population. Kidney Int. 2005;67:2462–2469. doi: 10.1111/j.1523-1755.2005.00355.x. doi:10.1111/j.1523-1755.2005.00355.x. [DOI] [PubMed] [Google Scholar]
- 26.Chan MR, Young HN, Becker YT, Yevzlin AS. Obesity as a predictor of vascular access outcomes: analysis of the USRDS DMMS Wave II study. Semin Dial. 2008;21:274–279. doi: 10.1111/j.1525-139X.2008.00434.x. doi:10.1111/j.1525-139X.2008.00434.x. [DOI] [PubMed] [Google Scholar]
- 27.Allon M. Fistula first: recent progress and ongoing challenges. Am J Kidney Dis. 2011;57:3–6. doi: 10.1053/j.ajkd.2010.11.002. doi:10.1053/j.ajkd.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]