Abstract
Background
Acute kidney injury (AKI) requiring renal replacement therapy (RRT) is associated with high in-hospital morbidity and mortality in critically ill patients. Long-term outcomes have received little attention.
Methods
The aim of this study was to characterize AKI–chronic kidney disease (CKD) nexus in critically ill patients with AKI (RIFLE class F). We performed a single-centre prospective observational study of 425 consecutive critically ill patients with AKI requiring RRT. None of these patients had pre-existing kidney disease. Primary outcomes were vital status and renal function at hospital discharge and at 5 and 10 years of follow-up.
Results
The overall in-hospital mortality of the study cohort was 47%, the mortality rates at 1, 5 and 10 years were 65, 75 and 80%, respectively. At hospital discharge, recovery of renal function was complete in 56% of survivors. None of these patients developed CKD during follow-up. Ninety percent of the 100 survivors with partial recovery of renal function had ongoing CKD during long-term follow-up. CKD progressed to end-stage renal disease (ESRD) in 12 patients (3% of the cohort or 5% of survivors). The patients with post-AKICKD had a higher prevalence of hypertension, a higher rate of fatal cardiac diseases and a higher all-cause death rate.
Conclusion
Long-term survival of critically ill patients with AKI requiring RRT is poor and determined by the development of de novo CKD. There is a need for close follow-up of patients surviving AKI to prevent progressive CKD and to reduce associated lethal cardiac events.
Keywords: acute kidney injury, long-term mortality, risk of chronic kidney disease, survival
Introduction
Acute kidney injury (AKI) occurring in the setting of intensive care medicine (ICU) complicates the clinical course of approximately 40% of ICU patients [1, 2]. Its incidence is rising due to more aggressive diagnostic and therapeutic interventions in an ageing population with multiple comorbid diseases [3]. On average, 5% of ICU patients with severe AKI require renal replacement therapy (RRT) [4, 5].
AKI rarely develops as isolated organ dysfunction, but presents with a broad spectrum of severity, and has heterogeneous unpredictable clinical outcomes [6]. Undoubtedly, this patient population has a poor short-term outcome with excessive in-hospital mortality rates (40–60%), prolonged ICU and hospital stay as well as reduced health-related quality of life [7].
Prior to the era of chronic kidney disease (CKD) staging, it was generally accepted that patients who survive an episode of AKI had a ‘good’ renal outcome as assessed by a rapid return of renal function towards baseline values in most patients and by a low incidence of end-stage renal disease (ESRD) [8]. Traditionally it was thought that AKI was reversible and, as a consequence, survivors of AKI were not followed up. As recently as 2005, an epidemiologic surveillance study reported that ‘although the majority of patients with severe AKI will die, most survivors will become independent from RRT within one year’, but it did not mention the level of kidney function regained [9].
The systematic review and meta-analysis reported by Schmitt et al. [10] found that there is impaired recovery of kidney function after AKI in the aged (65 years and older). The recognition of age-related functional and structural alterations of human kidneys is of clinical importance, as the prevalence of elderly individuals admitted to hospital continues to increase over time, as does the incidence of AKI in these patients.
More recently, however, a number of studies assessing large-scale database cohorts of patients demonstrated that patients who survive AKI have a significant risk for the development of advanced stages (4/5) of CKD. In one of the first analyses linking AKI to progressive CKD, Ishani et al. [11] assessed a random sample of Medicare beneficiaries. They found that the adjusted hazard ratio for developing ESRD was 41 for patients with AKI on CKD relative to those patients without either acute or CKD. It was 13 for patients with AKI and without previous CKD, and 8.4 for CKD without AKI. Because this study utilized diagnostic codes, it is difficult to determine the proportions of patients who suffered AKI and then progressed directly to ESRD when compared with patients who suffered AKI, recovered renal function and then progressed to ESRD.
Lo et al. [12], utilizing a database from the health insurer Kaiser Permanente, evaluated retrospectively the risk of progressive CKD in a cohort of patients with baseline estimated glomerular filtration rate (eGFR) of at least 45 mL/min/1.73 m2. In this study, the investigators found that an episode of dialysis-requiring AKI was associated with a 28-fold increase of developing advanced CKD and a 2-fold increase in mortality compared with patients who did not suffer dialysis-requiring AKI.
Extending these lines of investigations, Bucaloiu et al. [13] retrospectively analysed the impact of AKI on long-term mortality and progressive kidney disease using a hospital database. The authors limited their analyses to patients with no evidence of pre-existing kidney disease who manifested complete recovery of kidney function after AKI. A total of 1610 discharged patients with reversible AKI (mostly AKIN stage I) were successfully matched with 3652 control patients who had not experienced AKI. Reversible AKI was associated with significant risk of de novo CKD and death. The authors identified numerous predictors of de novo CKD, including increasing age, lower baseline eGFR, burden of comorbidities and severity of AKI. However, it is important to recognize that retrospective database studies use codes for AKI and do not give the causes of AKI. They often lack actual baseline eGFR (within two months prior to renal injury), do not differentiate between CKD stages and underestimate the burden of comorbid disease and its effects on renal function. The recent meta-analysis published by Coca et al. [14] identified AKI as an independent risk factor for CKD, ESRD, long-term non-renal morbidity and death.
Palevsky [15] suggested two alternative models for the relationship among patient risk factors, development of AKI, and de novo CKD. According to the first model, risk factors predispose to the development of AKI. The development of AKI mediates the development of subsequent CKD, which increases mortality risk. The alternative model suggests that risk factors for the development of AKI are also risk factors for the subsequent development of CKD and mortality. In the second model, AKI may be either a direct mediator for the development of CKD and mortality or only a marker of risk. Whether or not AKI is a marker or mediator, there is a need for prospective follow-up for patients who sustain an episode of AKI.
The aims of our investigations were to quantify long-term mortality and incidence of de novo CKD in critically ill patients with AKI necessitating RRT. The analyses were based on the 10-year follow-up summary data from our prospective cohort study, the interim analyses at 1 and 5 years have been published [16, 17].
Materials and methods
Study design
This prospective single-centre 10-year follow-up observational study was carried out according to the principles of the Declaration of Helsinki. The nature of the study was explained in detail to the patients or their next of kin; they all consented to participate in the investigations. Great attention was paid to the use of non-identifiable data exclusively. The internal ethical board approved the study protocol [16].
Study cohort
Inclusion criteria, renal characteristics of patients, indication for initiation of RRT as well as the performance of RRT have been previously described in detail [16]. Briefly, 425 critically ill patients with severe AKI necessitating RRT at the dialysis unit of the University Hospital Munich-Innenstadt (1990–2001) formed the cohort. None of the patients had radiological signs of CKD or persisting functional abnormalities (urine microscopy, urine dipstick proteinuria, microalbuminuria defined as more than 30 mg/L at two evaluations within 3 months prior) or decreased glomerular filtration rate (GFR <90 mL/min/1.73 m2). The GFR values were measured by creatinine clearance from 24-h urine or calculated by the Cockroft-Gault formula. The values were obtained from measurements performed within 3 months prior to initiation of RRT.
Only patients with a clinical diagnosis of acute tubular necrosis (ATN) participated in the cohort study. Presumed ATN was differentiated from other causes of AKI by detailed and accurate medical history, thorough physical examination, laboratory tests and imaging studies. A fractional excretion of sodium of more than 2% and the presence of granular casts on microscopic examinations of the urine were considered typical for ATN. None of the study patients underwent a kidney biopsy. A total of 143 patients with pre-existing CKD, or with AKI due to other causes than ATN, were excluded from the investigations. The severity of AKI at initiation of RRT was defined by the RIFLE classification scheme. All patients had either an acute increase in serum creatinine three times above baseline or anuria for 12 h (RIFLE Class F).
The indications for the initiation of RRT were volume overload with pulmonary oedema inadequately controlled with diuretics; hyperkalaemia refractory to conservative measures; the need for hyperalimentation in the presence of insufficient urinary output; uraemic signs or symptoms for which uraemia could not be ruled out as a precipitating cause; and/or a blood urea nitrogen concentration >100 mg/dL [15].
Outcome parameters
The primary outcome variables of the study were specified in advance as (i) in-hospital all-cause mortality, (ii) long-term survival, (iii) recovery of renal function at hospital discharge and (iv) development of de novo CKD.
Clinical data during hospital stay were recorded at the day of initiation of RRT. In-hospital outcome (death, survival) and recovery of renal function at discharge were taken from the patient's medical record. Information related to long-term outcome (survival, eGFR) was obtained from the family doctor annually for a follow-up period of 10 years.
The follow-up of patients was performed by (a) annual calls to the patients' family and (b) by an annual questionnaire sent to their treating physicians.
All patients were informed at initiation of the study period about the purpose and the necessary data collection for follow-up. The large majority of the survivors (226 patients) lived in the area in and around Munich. The death of the patient was either confirmed by the family or doctor or taken from the death register. Around 50% took up the offer to have their initial follow-ups performed in the out-patient department of our university. Almost all patients felt that the frequent follow-up contacts functioned for them as continued specialized care. Very few patients moved from Munich to another area in Germany. Their addresses were given to the follow-up centre. The follow-up at 10 years was mostly performed by the treating physicians (general practice, internal medicine practice, nephrologists in those with kidney dysfunction) of the patients.
Definition of recovery of renal function from AKI
Recovery of renal function was defined as complete if the decreased glomerular filtration rate returned to the pre-AKI baseline level of eGFR. Partial recovery of renal function was defined as persistent change of serum creatinine concentrations to more than 0.3 mg/dL above baseline, but without continued requirement for RRT. CKD was defined and staged according to the National Kidney Foundation (NKF) Clinical Practice Guidelines for CKD [18]. ESRD was defined as stage 5 of CKD maintained on regular dialysis.
Statistics
Statistical analyses were performed with SPSS for Windows (Version 15, SPSS, Chicago, IL, USA). Continuous variables were reported as mean ± SD. Categorical data were expressed as proportions (percentage). Fisher's exact test was used to analyse the categorical data. Multivariate logistic regression analysis was performed to identify independent determinants of long-term mortality of hospital survivors. A two-sided P-value of <0.05 was considered statistically significant.
Results
Characteristics of the cohort at initiation of RRT
The cohort consisted of 425 medical and surgical ICU patients with AKI secondary to clinically diagnosed ATN and a need for RRT (RIFLE Class F). The study population was characterized by a high mean age, heavy burden of comorbid diseases (cardiovascular, metabolic, pulmonary) and severe underlying illness (Table 1). None of the patients had pre-existing kidney disease. They all had normal GFR. Clinically diagnosed ATN arose in the presence of ischaemia (cardiovascular surgery, septic or cardiogenic shock), sepsis or nephrotoxins or often combined insults. However, no patient had more than one episode of AKI during the hospital stay. AKI presented in all patients as part of a multiple organ failure syndrome. In total, 75% of the patients required mechanical ventilation, 58% needed vasopressor support and 48% of the patients had oliguria at initiation of RRT. The main mode of RRT was intermittent haemodialysis (Table 1). RRT was initiated when one of the classical indications was present. Its frequency was prescribed according to the need of the patient (either alternate days or daily). The prescribed doses were a single-pool Kt/V urea of 1.3 ± 0.3 (delivered dose sp Kt/V 1.1 ± 0.3) for intermittent haemodialysis (IHD) and an effluent volume rate of 24.0 ± 2.4 mL/kg/h (delivered 20.5 ± 3.5 mL/kg/h) for continuous veno-venous haemodialysis (CRRT).
Table 1.
Patient characteristics at initiation of RRTa
| Number of patients | 425 |
| Age (years) | 65 ± 12 |
| Gender (M) | 66% |
| Comorbid conditions | |
| Hypertension | 48% |
| Diabetes mellitus | 22% |
| COPD | 14% |
| Chronic heart failure | 18% |
| Setting of AKI | |
| Surgery | 41% |
| Medicine | 59% |
| Severity of illness | |
| APACHE III | 88 ± 29 |
| Number of failed organs | 2.4 ± 0.2 |
| Renal data | |
| GFR (mL/min/1.73 m2)b | 113 ± 12 |
| Presumed cause of ATN | |
| Ischaemia | 60% |
| Sepsis | 33% |
| Nephrotoxins | 7% |
| Markers of AKI | |
| Serum creatinine (mg/dL) | 4.3 ± 1.1 |
| Oliguria | 48% |
| RIFLE Class F | 100% |
| Mode of RRT | |
| IHD | 68% |
| CRRT | 13% |
| IHD/CRRT | 19% |
aResults are given as mean ± SD or percentage. AKI, acute kidney injury; ATN, acute tubular necrosis; CKD, chronic kidney disease; GFR, glomerular filtration rate; RRT, renal replacement therapy; IHD, intermittent haemodialysis; CRRT, continuous veno-venous haemodialysis.
bMeasured by creatinine clearance from 24-h urine or calculated by the Crockroft–Gault formula.
Completeness of follow-up
None of the study patients was lost for follow-up.
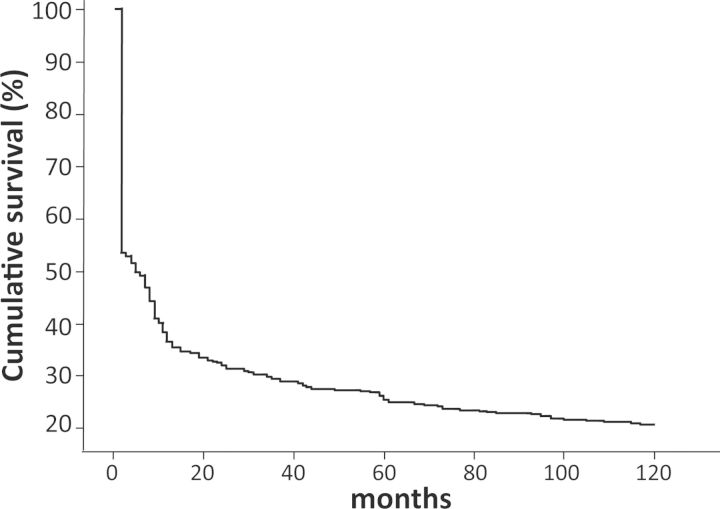
Mortality of critically ill patients during in-hospital stay and long-term follow-up (Figure 1).
Fig. 1.
Outcome of the study cohort. ICU, intensive care unit; CRR, complete renal recovery; PRR, partial renal recovery; NRF, normal renal recovery; CKD, chronic kidney disease.
The overall in-hospital mortality was 47%; the 1-, 5- and 10-year mortality rates were 65, 75 and 80%, respectively (Table 2 and Figure 2). The corresponding mortality rates of survivors were 34, 53 and 62% (Table 2). Major clinical causes of deaths in survivors were congestive heart failure, acute myocardial infarction and sudden death (65%).
Table 2.
Long-term outcomes of critically ill patients surviving AKI requiring RRT
| 1 year (%) | 5 years (%) | 10 years (%) | |
|---|---|---|---|
| Mortality rate | |||
| Cohort of AKI patients | 65 | 75 | 80 |
| Survivors of AKI | 34 | 53 | 62 |
| Renal recovery | |||
| Normal eGFR | 74 | 87 | 86 |
| CKD 2 | 11 | 3 | 0 |
| CKD 3 | 11 | 3 | 3 |
| CKD 4 | 3 | 4 | 3 |
| CKD 5 | 1 | 3 | 8 |
AKI, acute kidney injury; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; RRT, renal replacement therapy.
Fig. 2.
Survival curve of the cohort of critically ill patients with AKI requiring RRT.
Long-term renal function in survivors of an AKI episode requiring RRT
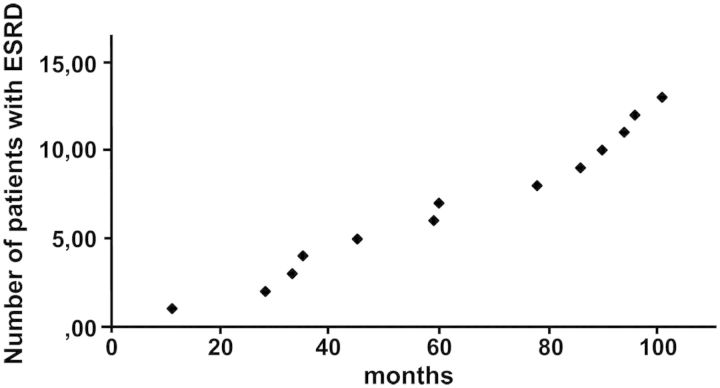
At hospital discharge, recovery of renal function was complete in 56%, i.e. 126 of the 226 survivors (assessed by eGFR). None of these 126 patients developed CKD during the 10 year follow-up period. Partial recovery of renal function was documented in 100 of the patients discharged from hospital (44%). During the first year of follow-up of these patients, low eGFR improved in 26 patients, normalized in 10 patients, showed no change in 56 patients and progressed in 8 patients. Thereafter, impaired eGFR remained stable or showed progression. In the subgroup of 90 patients with initial partial recovery of renal function from AKI, in 12 patients ESRD occurred within the follow-up period (Figure 3). None of these patients had suffered a second episode of AKI.
Fig. 3.
Number of survivors from AKI (RIFLE class F) developing end-stage renal disease during a 10-year follow-up period. ESRD, end-stage renal disease.
There were significant differences in the prevalence of chronic arterial hypertension among patients with normal renal function and those with CKD (45 vs. 76%, P < 0.001). At the end of the 10-year follow-up, 85 patients of the 226 survivors were still alive. The majority of long-term survivors had normal renal function, five patients had CKD stages 2–4 and eight patients underwent maintenance dialysis.
The mortality rate of survivors regaining normal eGFR after AKI was significantly better than that of survivors with CKD (46 vs. 83%, P < 0.001). Multivariate logistic regression analysis incorporating the variables age, gender, APACHE II score, comorbid disease index, mode of RRT, setting of AKI showed that independent predictors of long-term mortality were de novo CKD, extra-renal comorbid disease and surgical setting of AKI (Table 3). It should be noted that the subgroup of patients with sepsis was relatively small (35%) and the majority of these patients died during the hospital stay. Thus these survivors were too small a cohort to reach significant conclusions.
Table 3.
Independent predictors of long-term mortality of survivors of ICU AKI
| Variable | Odds ratio (95 % confidence Interval) | P-value |
|---|---|---|
| De novo CKD | 4.3 (2.9–6.2) | 0.001 |
| Comorbidity | 2.9 (1.9–4.5) | 0.001 |
| Surgery | 1.5 (1.1–2.2) | 0.01 |
CKD, chronic kidney disease.
Discussion
Our prospective single-centre cohort study of 425 consecutive critically ill patients without evidence for pre-existing CKD demonstrates that AKI requiring RRT has significant long-term negative implications. AKI per se drives the excess in-hospital mortality of these patients and also increases the risk for long-term death. There is no doubt that AKI is causally associated with long-term outcomes and not simply a marker for unmeasured risk factors in a sicker patient population. The analysis of renal recovery from AKI in ICU patients without prior kidney disease clearly showed that partial recovery of renal function at hospital discharge was followed by progression to CKD.
AKI requiring RRT is associated with poor survival in ICU patients. The present report is the first dealing prospectively with the association between long-term mortality after critical illness and AKI requiring RRT. We found fatality rates in our ICU patients of 75% at 5 years and 80% at 10 years after initiation of RRT. These results are in line with published survival rates of 16–47% at 5 years [19–22] or of 17% at 10 years [23]. Variations in the long-term survival among studies utilizing different research methods are likely caused by the combined effects of differences in case mixture and demographic variables (severity of illness and burden of comorbidity). Furthermore, published survival analyses varied in the extent of loss to follow-up, restriction to homologous groups (cardiac surgery, >65 years), and the use of administrative codes for AKI, acute illness or pre-existing comorbidity [24]. Our cohort consisted only of critically ill patients with defined extra-renal pre-existing diseases, a clear classification of AKI (RIFLE Class F) necessitating RRT, and a complete follow-up.
The survival analysis of our ICU cohort suggested a three-phase pattern of death after AKI requiring RRT. Phase 1, during the index ICU and hospital stay, was associated with a dismally high case-fatality rate. In phase 2, from hospital discharge and lasting several years, there is an excess of deaths in survivors compared with comparable ICU patients without AKI [22]. The duration of this phase differs among studies depending on the cause of critical illness. In phase 3, up to 10 years observation, the survival curves of our ICU patients flattened. Major determinants of long-term mortality of survivors of an AKI episode were de novo CKD, comorbidities and setting of AKI in our study population and in retrospective analyses [8, 19]. Although in our patients who died, no autopsy was performed, a significant proportion of the deaths are believed to have been caused by cardiac diseases. This observation is in accordance with findings reported by other authors that myocardial infarction and heart failure are more frequent in this population [22, 25].
Traditionally, AKI was considered a reversible condition, as GFR recovered in most of the survivors discharged from hospital. However, recovery of renal function is directly related to the cause of AKI [26, 27] and to a large extent to pre-existing CKD [28]. The frequency of ESRD in survivors of AKI due to ATN has been shown to be low, if pre-insult glomerular filtration had been low in the normal range [27, 29]. None of our patients had pre-existing CKD; they all had clinically diagnosed ATN as a cause of AKI. At hospital discharge, recovery of renal function was complete in 56% of surviving patients as assessed by serial determinations of eGFR. None of the patients discharged with normal eGFR showed any deterioration in renal function during their follow-up. ESRD secondary to progressive CKD occurred in 12 patients (3%) of the total cohort or 5% of all survivors. Serial measurements up to 10 years documented that in progressive CKD, eGFR declined continuously rather than abruptly.
There is broad experimental support for a causal link between AKI and post-AKI–CKD. Numerous studies demonstrate that AKI can induce permanent damage to the microvasculature and subsequent abnormalities in kidney structure and function. Through inflammation and fibrosis, the residual kidney damage can lead to progressive structural kidney damage, which may then predispose to faster decline of GFR by worsening hypertension [30, 31] which are well-known risk factors for cardiovascular disease.
The prospective design, long-term follow-up and completeness of data are the strength of our analysis. The data set may be of value for treatment decisions in the ICU or the discussions with patients or their relatives about the prognosis of AKI, at least in patients with normal GFR prior to the renal insult(s).
However, our investigations have some limitations. By nature, observational studies cannot prove that AKI plays a causal role in long-term outcomes of critically ill patients. However, the data obtained by our cohort study provide several lines of evidence, that AKI is not a short-term illness, but a renal syndrome with truly linked long-lasting effects on patient outcomes. Secondly, as this was a long-standing single-centre study, results may not be replicable in other settings. However, our cohort of aged surgical and medically critically ill patients with a burden of comorbid diseases reflects well the characteristics of current patient populations treated in mixed ICUs showing similar short-term outcomes [26, 32]. Randomized studies are needed to provide definitive proof for the AKI–CKD nexus.
There is an unmet need for strategies to prevent the development of AKI, to hasten the recovery of renal function and to minimize the adverse outcomes following AKI. Survivors of an episode of AKI should be closely followed-up after discharge to detect early development of CKD and its detrimental cardiovascular effects on long-term survival [33].
Conflict of interest statement. None declared.
References
- 1.Ostermann M, Chang RW. Challenges of defining acute kidney injury. QJM. 2011;104:237–243. doi: 10.1093/qjmed/hcq185. [DOI] [PubMed] [Google Scholar]
- 2.Hoste EA, Clermont G, Kersten A, et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care. 2006;10:R73. doi: 10.1186/cc4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagshaw SM, George C, Bellomo R. Changes in the incidence and outcome for early acute kidney injury in a cohort of Australian intensive care units. Crit Care. 2007;11:R68. doi: 10.1186/cc5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 5.Cruz DN, Bolgan I, Perazella MA, et al. North East Italian Prospective Hospital Renal Outcome Survey on Acute Kidney Injury (NEiPHROS-AKI): targeting the problem with the RIFLE Criteria. Clin J Am Soc Nephrol. 2007;2:418–425. doi: 10.2215/CJN.03361006. [DOI] [PubMed] [Google Scholar]
- 6.Star RA. Treatment of acute renal failure. Kidney Int. 1998;54:1817–1831. doi: 10.1046/j.1523-1755.1998.00210.x. [DOI] [PubMed] [Google Scholar]
- 7.Dennen P, Douglas IS, Anderson R. Acute kidney injury in the intensive care unit: an update and primer for the intensivist. Crit Care Med. 2010;38:261–275. doi: 10.1097/CCM.0b013e3181bfb0b5. [DOI] [PubMed] [Google Scholar]
- 8.Liano F, Felipe C, Tenorio MT, et al. Long-term outcome of acute tubular necrosis: a contribution to its natural history. Kidney Int. 2007;71:679–686. doi: 10.1038/sj.ki.5002086. [DOI] [PubMed] [Google Scholar]
- 9.Bagshaw SM, Laupland KB, Doig CJ, et al. Prognosis for long-term survival and renal recovery in critically ill patients with severe acute renal failure: a population-based study. Crit Care. 2005;9:R700–R709. doi: 10.1186/cc3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmitt R, Coca S, Kanbay M, et al. Recovery of kidney function after acute kidney injury in the elderly: a systematic review and meta-analysis. Am J Kidney Dis. 2008;52:262–271. doi: 10.1053/j.ajkd.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Ishani A, Xue JL, Himmelfarb J, et al. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol. 2009;20:223–228. doi: 10.1681/ASN.2007080837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lo LJ, Go AS, Chertow GM, et al. Dialysis-requiring acute renal failure increases the risk of progressive chronic kidney disease. Kidney Int. 2009;76:893–899. doi: 10.1038/ki.2009.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bucaloiu ID, Kirchner HL, Norfolk ER, et al. Increased risk of death and de novo chronic kidney disease following reversible acute kidney injury. Kidney Int. 2012;81:477–485. doi: 10.1038/ki.2011.405. [DOI] [PubMed] [Google Scholar]
- 14.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81:442–448. doi: 10.1038/ki.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palevsky PM. Chronic-on-acute kidney injury. Kidney Int. 2012;81:430–431. doi: 10.1038/ki.2011.435. [DOI] [PubMed] [Google Scholar]
- 16.Schiffl H. Renal recovery from acute tubular necrosis requiring renal replacement therapy: a prospective study in critically ill patients. Nephrol Dial Transplant. 2006;21:1248–1252. doi: 10.1093/ndt/gfk069. [DOI] [PubMed] [Google Scholar]
- 17.Schiffl H, Fischer R. Five-year outcomes of severe acute kidney injury requiring renal replacement therapy. Nephrol Dial Transplant. 2008;23:2235–2241. doi: 10.1093/ndt/gfn182. [DOI] [PubMed] [Google Scholar]
- 18.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 19.Morgera S, Kraft AK, Siebert G, et al. Long-term outcomes in acute renal failure patients treated with continuous renal replacement therapies. Am J Kidney Dis. 2002;40:275–279. doi: 10.1053/ajkd.2002.34505. [DOI] [PubMed] [Google Scholar]
- 20.Ahlstrom A, Tallgren M, Peltonen S, et al. Survival and quality of life of patients requiring acute renal replacement therapy. Intensive Care Med. 2005;31:1222–1228. doi: 10.1007/s00134-005-2681-6. [DOI] [PubMed] [Google Scholar]
- 21.Frost L, Pedersen RS, Bentzen S, et al. Short and long term outcome in a consecutive series of 419 patients with acute dialysis-requiring renal failure. Scand J Urol Nephrol. 1993;27:453–462. doi: 10.3109/00365599309182277. [DOI] [PubMed] [Google Scholar]
- 22.Hobson CE, Yavas S, Segal MS, et al. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation. 2009;119:2444–2453. doi: 10.1161/CIRCULATIONAHA.108.800011. [DOI] [PubMed] [Google Scholar]
- 23.Fertmann J, Wolf H, Kuchenhoff H, et al. Prognostic factors in critically ill surgical patients requiring continuous renal replacement therapy. J Nephrol. 2008;21:909–918. [PubMed] [Google Scholar]
- 24.Rimes-Stigare C, Awad A, Martensson J, et al. Long-term outcome after acute renal replacement therapy: a narrative review. Acta Anaesthesiol Scand. 2011;56:138–146. doi: 10.1111/j.1399-6576.2011.02567.x. [DOI] [PubMed] [Google Scholar]
- 25.Rihal CS, Textor SC, Grill DE, et al. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation. 2002;105:2259–2264. doi: 10.1161/01.cir.0000016043.87291.33. [DOI] [PubMed] [Google Scholar]
- 26.Ng KP, Chanouzas D, Fallouh B, et al. Short and long-term outcome of patients with severe acute kidney injury requiring renal replacement therapy. QJM. 2012;105:33–39. doi: 10.1093/qjmed/hcr133. [DOI] [PubMed] [Google Scholar]
- 27.Bhandari S, Turney JH. Survivors of acute renal failure who do not recover renal function. QJM. 1996;89:415–421. doi: 10.1093/qjmed/89.6.415. [DOI] [PubMed] [Google Scholar]
- 28.Wu VC, Huang TM, Lai CF, et al. Acute-on-chronic kidney injury at hospital discharge is associated with long-term dialysis and mortality. Ki.dney Int. 2011;80:1222–1230. doi: 10.1038/ki.2011.259. [DOI] [PubMed] [Google Scholar]
- 29.Ponte B, Felipe C, Muriel A, et al. Long-term functional evolution after an acute kidney injury: a 10-year study. Nephrol Dial Transplant. 2008;23:3859–3866. doi: 10.1093/ndt/gfn398. [DOI] [PubMed] [Google Scholar]
- 30.Spurgeon-Pechman KR, Donohoe DL, Mattson DL, et al. Recovery from acute renal failure predisposes hypertension and secondary renal disease in response to elevated sodium. Am J Physiol Renal Physiol. 2007;293:F269–F278. doi: 10.1152/ajprenal.00279.2006. [DOI] [PubMed] [Google Scholar]
- 31.Venkatachalam MA, Griffin KA, Lan R, et al. Acute kidney injury: a springboard for progression in chronic kidney disease. Am J Physiol Renal Physiol. 2010;298:F1078–F1094. doi: 10.1152/ajprenal.00017.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Faulhaber-Walter R, Hafer C, Jahr N, et al. The Hannover Dialysis Outcome study: comparison of standard versus intensified extended dialysis for treatment of patients with acute kidney injury in the intensive care unit. Nephrol Dial Transplant. 2009;24:2179–2186. doi: 10.1093/ndt/gfp035. [DOI] [PubMed] [Google Scholar]
- 33.Siew ED, Peterson JF, Eden SK, et al. Outpatient Nephrology Referral Rates after Acute Kidney Injury. J Am Soc Nephrol. 2012;23:305–312. doi: 10.1681/ASN.2011030315. [DOI] [PMC free article] [PubMed] [Google Scholar]