SUMMARY
The gastrointestinal (GI) tract contains much of the body’s serotonin (5-hydroxytryptamine, 5-HT), but mechanisms controlling the metabolism of gut-derived 5-HT remain unclear. Here we demonstrate that the microbiota plays a critical role in regulating host 5-HT. Indigenous spore-forming bacteria (Sp) from the mouse and human microbiota promote 5-HT biosynthesis from colonic enterochromaffin cells (ECs), which supply 5-HT to the mucosa, lumen and circulating platelets. Importantly, microbiota-dependent effects on gut 5-HT significantly impact host physiology, modulating GI motility and platelet function. We identify select fecal metabolites that are increased by Sp and that elevate 5-HT in chromaffin cell cultures, suggesting direct metabolic signaling of gut microbes to ECs. Furthermore, elevating luminal concentrations of particular microbial metabolites increases colonic and blood 5-HT in germ-free mice. Altogether, these findings demonstrate that Sp are important modulators of host 5-HT, and further highlight a key role for host-microbiota interactions in regulating fundamental 5-HT-related biological processes.
INTRODUCTION
In addition to its role as a brain neurotransmitter, the monoamine serotonin (5-hydroxytryptamine, 5-HT) is an important regulatory factor in the gastrointestinal (GI) tract and other organ systems. More than 90% of the body’s 5-HT is synthesized in the gut, where 5-HT activates as many as 14 different 5-HT receptor subtypes (Gershon and Tack, 2007) located on enterocytes (Hoffman et al., 2012), enteric neurons (Mawe and Hoffman, 2013) and immune cells (Baganz and Blakely, 2013). In addition, circulating platelets sequester 5-HT from the GI tract, releasing it to promote hemostasis and distributing it to various body sites (Amireault et al., 2013). As such, gut-derived 5-HT regulates diverse functions, including enteric motor and secretory reflexes (Gershon and Tack, 2007), platelet aggregation (Mercado et al., 2013), immune responses (Baganz and Blakely, 2013) and bone development (Chabbi-Achengli et al., 2012; Yadav et al., 2008) and cardiac function (Cote et al., 2003). Furthermore, dysregulation of peripheral 5-HT is implicated in the pathogenesis of several diseases, including irritable bowel syndrome (IBS) (Stasi et al., 2014), cardiovascular disease (Ramage and Villalon, 2008) and osteoporosis (Ducy and Karsenty, 2010).
The molecular mechanisms controlling the metabolism of gut 5-HT remain unclear. In the GI tract, 5-HT is synthesized by specialized endocrine cells, called enterochromaffin cells (ECs), as well as mucosal mast cells and myenteric neurons (Gershon and Tack, 2007), but the functions of these different pools of gut 5-HT are incompletely understood. In addition, two different isoenzymes of tryptophan hydroxylase (Tph), Tph1 and Tph2, mediate non-neuronal vs. neuronal 5-HT biosynthesis (Walther et al., 2003), but little is known regarding the endogenous signals that regulate Tph expression and activity.
Mammals are colonized by a vast and diverse collection of microbes that critically influences health and disease. Recent studies highlight a role for the microbiota in regulating blood 5-HT levels, wherein serum concentrations of 5-HT are substantially reduced in mice reared in the absence of microbial colonization (germ-free, GF), compared to conventionally-colonized (specific pathogen-free, SPF) controls (Sjogren et al., 2012; Wikoff et al., 2009). In addition, intestinal ECs are morphologically larger in GF vs. SPF rats (Uribe et al., 1994), which suggests that microbes could impact the development and/or function of 5-HT-producing cells. Interestingly, some species of bacteria grown in culture can produce 5-HT (Tsavkelova et al., 2006), raising the question of whether indigenous members of the microbiota contribute to host 5-HT levels through de novo synthesis. Based on this emerging link between the microbiota and serum 5-HT concentrations, we aimed to determine how pathways of 5-HT metabolism are affected by the gut microbiota, to identify specific microbial communities and factors involved in conferring serotonergic effects and to evaluate how microbial modulation of peripheral 5-HT impacts host physiology.
We show herein that the microbiota promotes 5-HT biosynthesis from colonic ECs in a postnatally inducible and reversible manner. Spore-forming microbes (Sp) from the healthy mouse and human microbiota sufficiently mediate microbial effects on serum, colon and fecal 5-HT levels. We further explore potential host-microbial interactions that regulate peripheral 5-HT by surveying microbial influences on the fecal metabolome. We find that particular microbial metabolites are elevated by Sp and likely signal directly to colonic ECs to promote 5-HT biosynthesis. Importantly, microbiota-mediated changes in colonic 5-HT regulate GI motility and hemostasis in the host, suggesting that targeting the microbiota can serve as a tractable approach for modulating peripheral 5-HT bioavailability and treating 5-HT-related disease symptoms.
RESULTS
The Gut Microbiota Modulates Host Peripheral Serotonin Levels
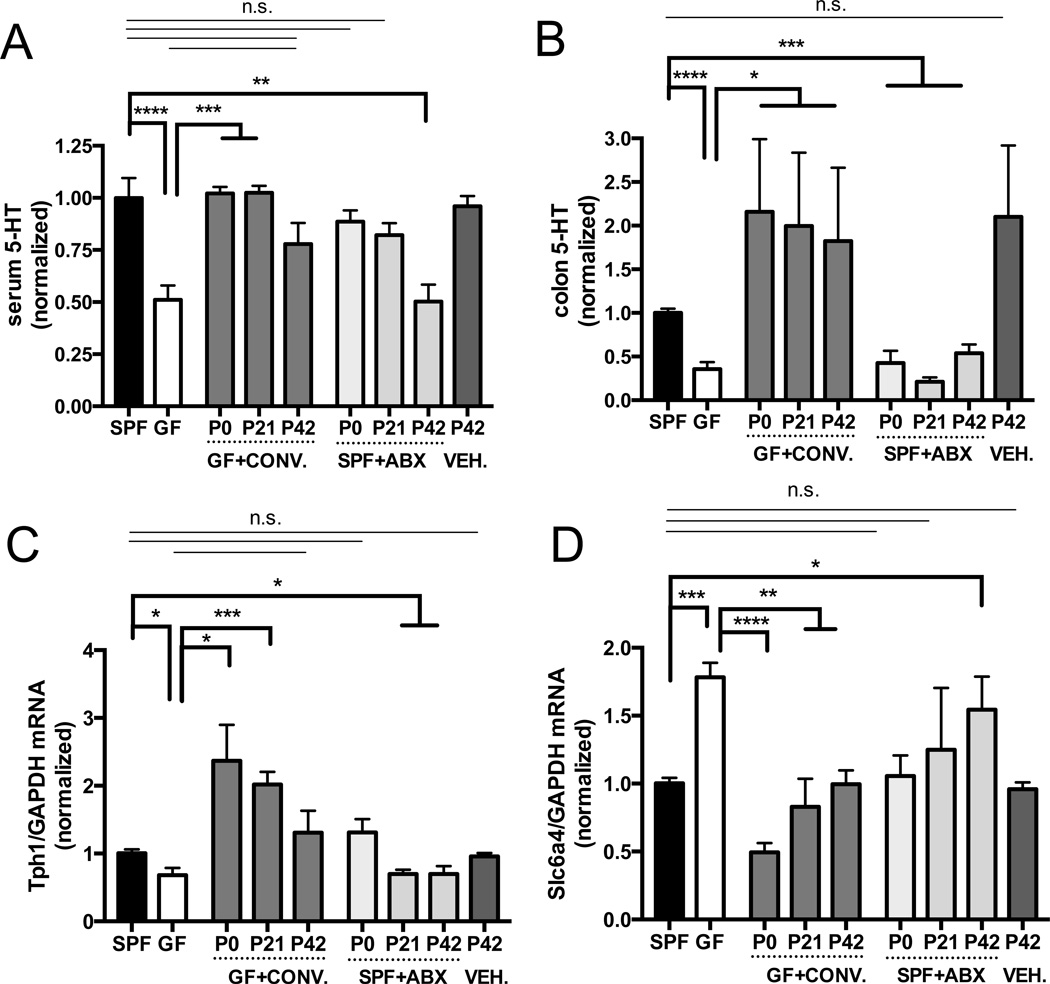
Adult GF mice display deficient serum (Sjogren et al., 2012; Wikoff et al., 2009) (Figure 1A) and plasma (Figure S1A) 5-HT concentrations compared to SPF controls, but the cellular sources of this disruption are undefined. Consistent with the understanding that much of the body’s 5-HT derives from the GI tract, we find that GF mice exhibit significantly decreased levels of colonic and fecal 5-HT compared to SPF controls (Figures 1B and S1A, and Table S1). This deficit in 5-HT is observed broadly across the distal, medial and proximal colon (Figure S1D), but not in the small intestine (Figures S1A, S2A and S2B), suggesting a specific role for the microbiota in regulating colonic 5-HT. Decreased levels of 5-HT are localized to colonic chromogranin A− positive (CgA+) enterochromaffin cells (ECs) (Figure 2), and not to small intestinal ECs (Figures S2A and S2B). Low 5-HT signal is seen in both GF and SPF colonic mast cells and enteric neurons (Figure 2A), which are minor producers of 5-HT (Gershon and Tack, 2007). There is no difference between adult GF and SPF mice in the abundance of CgA+ enteroendocrine cells (EECs) (Figure 2C), suggesting that decreases in colon 5-HT result from abnormal 5-HT metabolism rather than impaired development of EECs.
Figure 1. The Gut Microbiota Modulates Host Peripheral Serotonin Levels.
(A) Levels of serum 5-HT. Data are normalized to serum 5-HT in SPF mice. n=8–13.
(B) Levels of colon 5-HT relative to total protein. Data are normalized to colon 5-HT relative to total protein in SPF mice. n=8–13.
(C) Colonic expression of TPH1 relative to GAPDH. Data are normalized to expression levels in SPF mice. n=4.
(D) Colonic expression of SLC6A4 relative to GAPDH.
Data are normalized to expression levels in SPF mice. n=4. Data are presented as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, n.s.=not statistically significant. SPF=specific pathogen-free (conventionally-colonized), GF=germ-free, CONV.=SPF conventionalized, ABX=antibiotic-treated, VEH=vehicle (water)-treated. See also Figure S1.
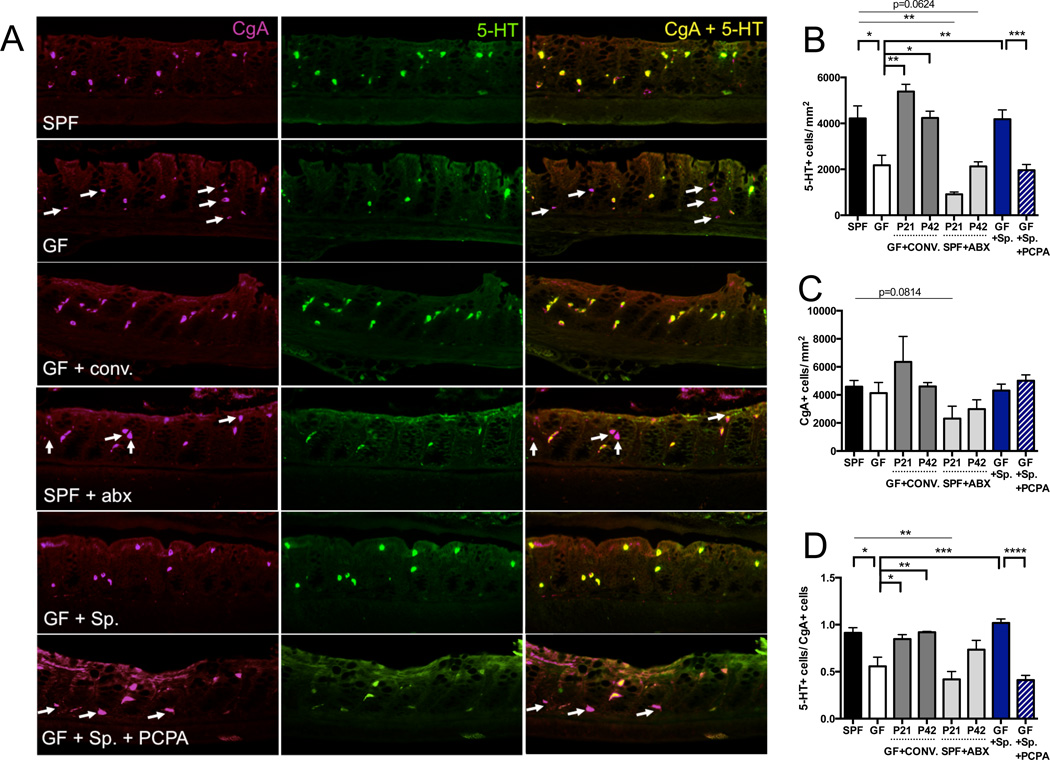
Figure 2. Indigenous Spore-forming Bacteria Increase 5-HT Levels in Colon Enterochromaffin Cells.
(A) Representative images of colons stained for chromagranin A (CgA) (left), 5-HT (center) and merged (right). Arrows indicate CgA-positive cells that lack 5-HT staining. n=3–7 mice/group.
(B) Quantitation of 5-HT+ cell number per area of colonic epithelial tissue. n=3–7 mice/group.
(C) Quantitation of CgA+ cell number per area of colonic epithelial tissue. n=3–7 mice/group.
(D) Ratio of 5-HT+ cells/CgA+ cells per area of colonic epithelial tissue. n=3–7 mice/group.
Data are presented as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. SPF=specific pathogen-free (conventionally-colonized), GF=germ-free, CONV.=SPF conventionalized, ABX=antibiotic-treated, Sp=spore-forming bacteria, PCPA=para-chlorophenylalanine. See also Figure S2.
To identify the specific steps of 5-HT metabolism that are affected by the microbiota, key intermediates of the 5-HT pathway were assessed in colons from GF vs. SPF mice. We find that GF colons exhibit decreased expression of TPH1 (Figures 1C and S1D; Sjogren et al., 2012), the rate-limiting enzyme for 5-HT biosynthesis in ECs, but no difference in expression of enzymes involved in 5-HT packaging, release and catabolism (Figure S1C). GF mice also display elevated colonic expression of the 5-HT transporter SLC6A4 (Figures 1D and S1E; Sjogren et al., 2012), synthesized broadly by enterocytes to enable 5-HT uptake (Wade et al., 1996). This could reflect a compensatory response to deficient 5-HT synthesis by host ECs, based on the finding that chemical Tph inhibition modulates SLC6A4 expression (Figures S2C and S2D). There is no difference between GF and SPF mice in colonic expression of neural-specific isoforms of 5-HT enzymes (Figure S1F), consistent with data showing no apparent difference in 5-HT-specific staining in enteric neurons (Figure 2). Despite deficient levels of colon, fecal and serum 5-HT (Figures 1A, 1B and S1A, and Table S1), GF mice exhibit significantly increased levels of the Tph substrate, tryptophan (Trp), in both feces (Table S1) and serum (Sjogren et al., 2012; Wikoff et al., 2009), suggesting that primary disruptions in host TPH1 expression result in Trp accumulation. Oral supplementation of GF mice with the Tph product, 5-hydroxytryptophan (5-HTP), sufficiently ameliorates deficits in colon and serum 5-HT, whereas supplementation with the Tph substrate Trp has no restorative effect (Figures S1G, S1H and S1I). Collectively, these data support the notion that the microbiota promotes 5-HT biosynthesis by elevating TPH1 expression in colonic ECs.
To confirm that deficient 5-HT levels in GF mice are microbiota-dependent, and further determine whether effects are age-dependent, GF mice were conventionalized with an SPF microbiota at birth (postnatal day (P) 0), weaning (P21), or early adulthood (P42) and then evaluated at P56 for levels of 5-HT and expression of 5-HT-related genes. GF mice conventionalized at each age with an SPF microbiota exhibit restored serum (Figure 1A) and colon (Figure 1B) 5-HT levels, with more pronounced effects seen at earlier ages of colonization. Colonic expression of TPH1 and SLC6A4 is similarly corrected by postnatal conventionalization of GF mice (Figures 1C and 1D), with more substantial changes from P0 conventionalization. Increases in 5-HT are localized to colonic ECs (Figure 2). These findings indicate that postnatal reconstitution of the gut microbiota can correct the 5-HT deficiency seen in GF mice and further suggest that gut microbes exert a continuous effect on 5-HT synthesis by modulating EC function. Overall, we demonstrate that microbiota-mediated elevation of host 5-HT is postnatally inducible, persistent from the time of conventionalization and not dependent on the timing of host development.
To assess the reversibility of microbial effects on host 5-HT metabolism, we depleted the gut microbiota in SPF mice via bi-daily antibiotic treatment beginning on P0, P21 or P42 and until P56. Treatment of P42 SPF mice with a cocktail of ampicillin, vancomycin, neomycin and metronidazole (Reikvam et al., 2011) sufficiently recapitulates GF-associated deficits in serum and colon 5-HT and alterations in host colonic TPH1 and SLC6A4 expression (Figures 1 and 2). Interestingly, P0 and P21 antibiotic treatment also induces GF-related deficits in colonic 5-HT, but the effects on serum 5-HT are more pronounced when administered at P42, compared to P0 and P21 (Figure 1), suggesting potential confounding effects of early life or prolonged antibiotic treatment on microbiota-mediated modulation of peripheral 5-HT. Antibiotics can elicit several direct effects on host cells (Shimizu et al., 2003; Westphal et al., 1994), which may underlie differences between P0 treatment and GF status. That P42 antibiotic treatment of SPF mice results in 5-HT phenotypes analogous to those seen in GF mice demonstrates that microbiota effects on host 5-HT can be abrogated postnatally and further supports the plasticity of 5-HT modulation by indigenous gut microbes. Altogether, these data indicate that the gut microbiota plays a key role in raising levels of colon and serum 5-HT, by promoting 5-HT in colonic ECs in an inducible and reversible manner.
Indigenous Spore-Forming Microbes Promote Host Serotonin Biosynthesis
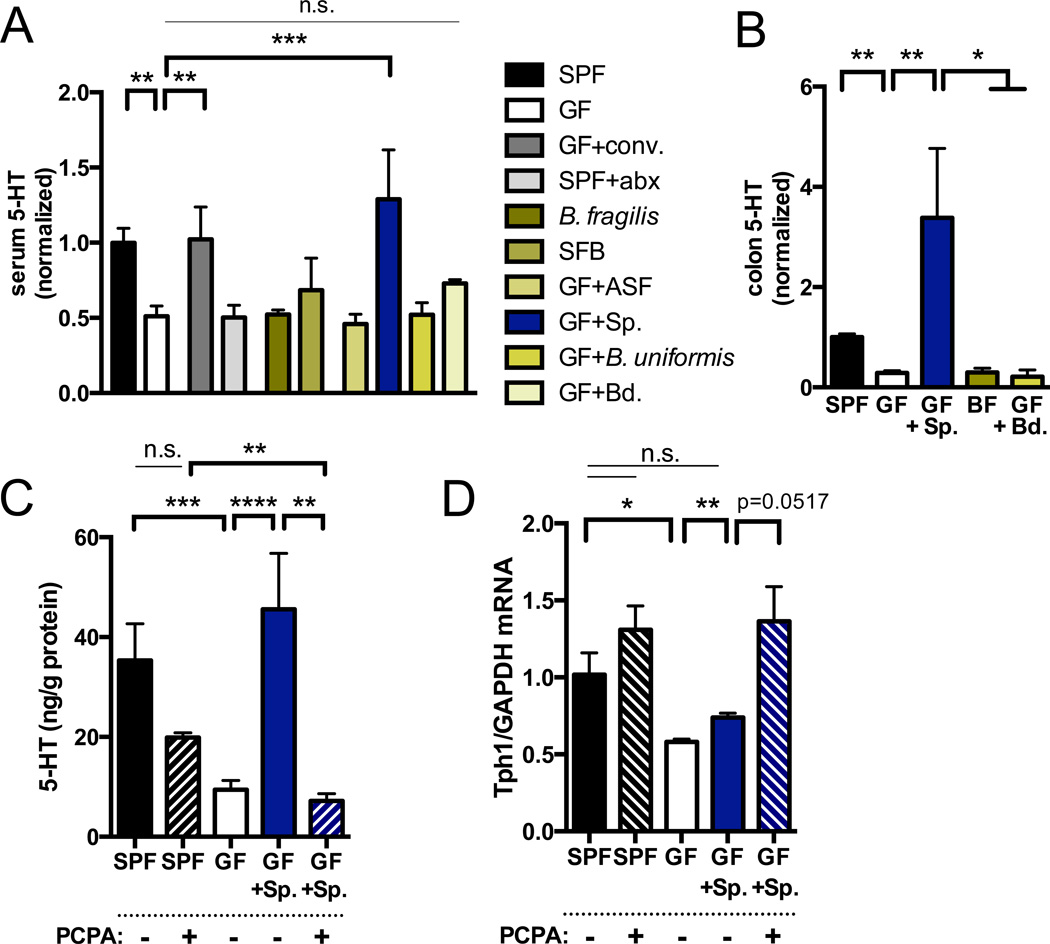
In light of our finding that 5-HT levels are decreased in colons but not small intestines of GF mice compared to SPF controls, we hypothesized that specific subsets of gut microbes are responsible for affecting host 5-HT pathways. Mice monocolonized with Bacteroides fragilis or Segmented Filamentous Bacteria (SFB) display deficits in serum 5-HT that are comparable to those seen in GF mice (Figure 3A). Moreover, postnatal colonization (P42) with Bacteroides uniformis, altered Schaedler flora (ASF), an eight-microbe consortium known to correct gross intestinal pathology in GF mice (Dewhirst et al., 1999), or with cultured Bacteroides spp. from the SPF mouse microbiota, has no significant effect on the 5-HT deficiency seen in GF mice (Figures 3A and 3B). Interestingly, however, GF mice colonized at P42 with indigenous spore-forming microbes from the mouse SPF microbiota (Sp), known to be dominated by Clostridial species (Atarashi et al., 2013; Stefka et al., 2014) (Figure S7 and Table S4), exhibit complete restoration of serum and colon 5-HT to levels observed in SPF mice (Figures 3A and 3B). Consistent with this, Sp colonization of GF mice increases 5-HT staining colocalized to CgA+ ECs (Figure 2), elevates host colonic TPH1 expression (Figure 3D) and decreases SLC6A4 expression (Figure 3E) toward levels seen in SPF mice. Improvements in serum 5-HT are observed within 2 days after inoculation of GF mice with Sp (Figure S2E), and do not correlate with amelioration of abnormal cecal weight (Figure S2F). Importantly, Sp also elevates colonic 5-HT in Rag1 knockout mice (Figure S2G), which lack adaptive immune cells, indicating that the effects of Sp on gut 5-HT are not dependent on Sp-mediated regulatory T cell induction (Stefka et al., 2014). Notably, the 5-HT-promoting effects of Sp are recapitulated by colonization of GF mice with spore-forming microbes from the healthy human colonic microbiota (hSp) (Figure S3), suggesting that the serotonergic function of this community is conserved across mice and humans.
Figure 3. Indigenous Spore-forming Bacteria Induce Colon 5-HT Biosynthesis and Systemic 5-HT Bioavailability.
(A) Levels of serum 5-HT. Data are normalized to serum 5-HT levels in SPF mice. SPF, n=13; GF, n=17; GF+conv.=P21 conventionalization, n=4; SPF+Abx= P42 antibiotic treatment, n=7; B. fragilis monoassociation (BF), n=6; SFB=Segmented Filamentous Bacteria monoassociation, n=4; ASF=Altered Schaedler Flora P21 colonization, n=4; Sp=spore-forming bacteria, P21 colonization, n=4; B. uniformis P21 colonization, n=4; Bd=Bacteroides consortium, n=3.
(B) Levels of colon 5-HT relative to total protein. Data are normalized to colon 5-HT relative to total protein in SPF mice. n=5–15.
(C) Levels of colon 5-HT relative to total protein after intrarectal treatment with the Tph inhibitor, PCPA, or vehicle. n=4.
(D) Colonic expression of TPH1 relative to GAPDH. Data are normalized to expression levels in SPF mice. n=3.
Data are presented as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, n.s.=not statistically significant. SPF=specific pathogen-free (conventionally-colonized), GF=germ-free, Sp=spore-forming bacteria, PCPA=para-chlorophenylalanine. See also Figure S3.
To determine whether the effects of Sp on host 5-HT depend on colonic Tph activity, we colonized GF mice with Sp on P42 and then administered the Tph inhibitor para-chlorophenylalanine (PCPA) intrarectally twice daily for 3 days prior to 5-HT assessments on P56 (Liu et al., 2008). Intrarectal injection of PCPA sufficiently blocks the ability of Sp to elevate colon and serum 5-HT levels (Figures 3C and S2C), as well as Sp-mediated increases in 5-HT staining in ECs (Figure 2). Similar effects of PCPA treatment on blocking increases in colon 5-HT, serum 5-HT and 5-HT staining in colonic ECs are seen in GF mice colonized with hSp (Figure S3). Interestingly, inhibiting Tph activity with PCPA results in a compensatory increase in colonic TPH1 and decrease in SLC6A4 (Figures 3D and S2D) expression in Sp-colonized mice, supporting the notion that microbiota-dependent changes in 5-HT transporter levels occur as a secondary response to Tph modulation.
To further evaluate whether changes in SLC6A4 expression are necessary for microbiota-mediated alterations in peripheral 5-HT, we tested the effects of microbiota manipulations on colon and serum 5-HT in SLC6A4 heterozygous (+/−) and complete (−/−) knockout (KO) mice. Depleting the microbiota via P42-P56 antibiotic treatment (Reikvam et al., 2011) of SPF SLC6A4 +/− and −/− mice effectively decreases colonic 5-HT levels (Figures S4A and S4B), indicating that the microbiota is required for promoting gut 5-HT in Slc6a4-deficient mice. Colonizing antibiotic-treated SLC6A4 +/− and −/− mice with Sp raises colon 5-HT to levels seen in SPF SLC6A4 +/− and −/− mice (Figure S4A), demonstrating that Slc6a4 is not required for conferring the effects of Sp on gut 5-HT. Antibiotic-induced decreases and Sp-induced increases in colon 5-HT levels can be attributed to modulation of 5-HT content in colonic ECs from SLC6A4 +/− and −/− mice (Figure S4C). Similar effects of antibiotic treatment and Sp colonization are seen for serum 5-HT in SLC6A4 +/− mice, whereas SLC6A4 −/− mice exhibit low to undetectable levels of serum 5-HT, highlighting the dependence of platelets on Slc6a4-mediated 5-HT uptake (Figure S4B). Taken together, these data support a role for Sp in promoting Tph1-mediated 5-HT biosynthesis by colonic ECs, regulating both colon and serum levels of 5-HT.
Microbiota-Mediated Regulation of Host Serotonin Modulates Gastrointestinal Motility
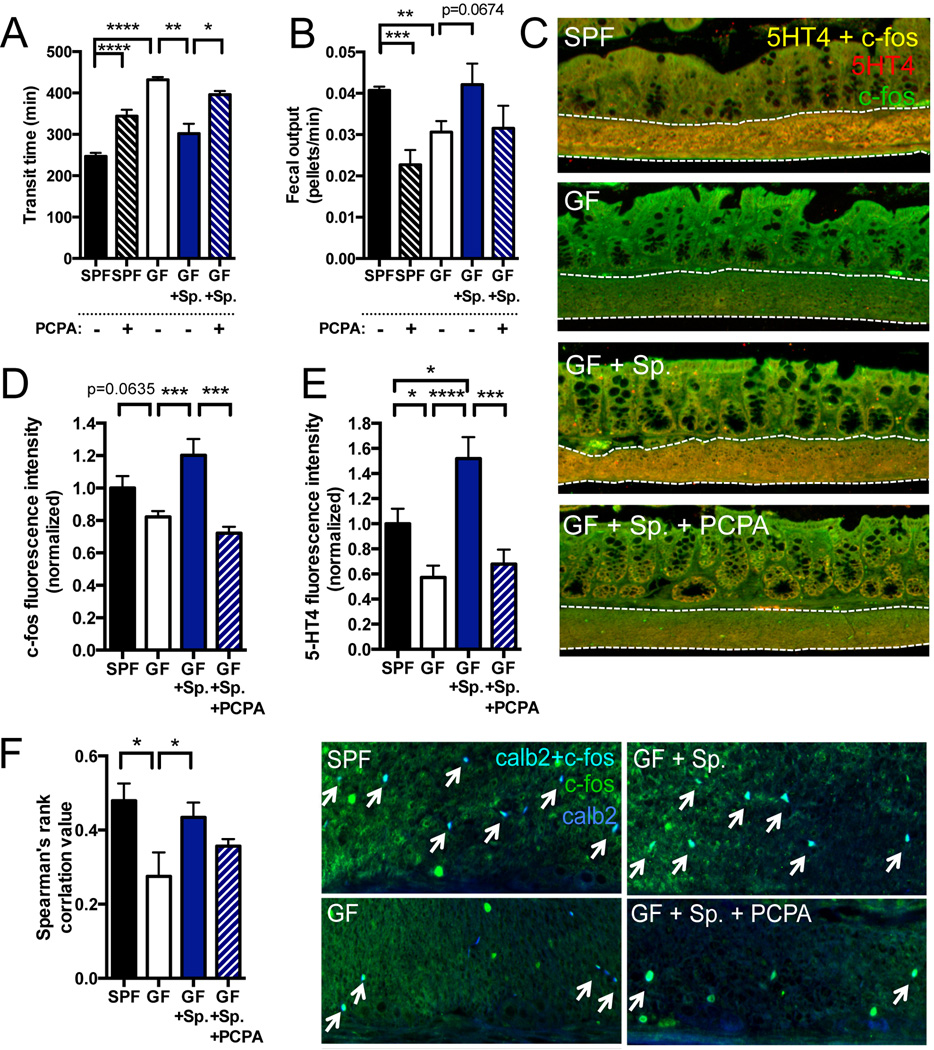
Intestinal 5-HT plays an important role in stimulating the enteric nervous system and GI function (Gershon and Tack, 2007). To determine whether microbiota-dependent modulation of colonic 5-HT impacts GI motility, we colonized P42 GF mice with Sp and then tested for GI transit and colonic neuronal activation at P56. Sp colonization ameliorates GF-associated abnormalities in GI motility, significantly decreasing total transit time and increasing the rate of fecal output in a Tph-dependent manner (Figures 4A and 4B). Similar effects are seen in SLC6A4 +/− and −/− mice, where Sp colonization of antibiotic-treated mice restores GI transit time toward levels seen in SPF SLC6A4 +/− and −/− controls (Figure S4E).
Figure 4. Microbiota-Mediated Regulation of Host Serotonin Modulates Gastrointestinal Motility.
(A) Total time for transit of orally administered carmine red solution through the GI tract. n=4–8.
(B) Defecation rate as measured by number of fecal pellets produced relative to total transit time. n=4–8.
(C) Representative images of c-fos and 5HT4 colocalization in the colonic submucosa and muscularis externa. n=4–5 mice/group.
(D) Quantitation of total c-fos fluorescence intensity in the colonic submucosa and muscularis externa. n=4–5 mice/group.
(E) Quantitation of total 5HT4 fluorescence intensity in the colonic submucosa and muscularis externa. n=4–5 mice/group.
(F) Quantitation and representative images of c-fos and calb2 (calretinin) colocalization in the colonic submucosa and muscularis externa. n=5–8 mice/group.
Data are presented as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. SPF=specific pathogen-free (conventionally-colonized), GF=germ-free, Sp=spore-forming bacteria, PCPA=para-chlorophenylalanine. See also Figure S4.
Consistent with deficits in GI motility, steady-state activation of 5-HT receptor subtype 4 (5HT4)-expressing neurons in the colonic submucosa and muscularis externa is decreased in GF mice compared to SPF controls, as measured by colocalized expression of 5HT4 with the immediate early gene, c-fos (Figure 4C, 4D and 4E). Colonization of GF mice with Sp increases 5HT4+ c-fos+ staining to levels seen in SPF mice, and this effect is dependent on colonic Tph activity (Figures 4C, 4D and 4E), which aligns well with the understanding that Sp-induced elevations in colonic 5-HT promote GI motility by activation of 5HT4+ enteric neurons (Mawe and Hoffman, 2013). In addition, colonic activation of intrinsic afferent primary neurons (IPANs) of the myenteric plexus is decreased in GF mice (McVey Neufeld et al., 2013) and improved by colonization with Sp, as measured by colocalization of c-fos and the IPAN marker, calretinin (Calb2) (Figure 4F). Inhibiting Tph activity with PCPA decreases IPAN activation in Sp-colonized mice, suggesting that some IPAN responses to Sp depend on host 5-HT synthesis (Figures 4F). Altogether, these findings indicate that Sp-mediated increases in colonic 5-HT biosynthesis are important for gut sensorimotor function.
Microbiota-Mediated Regulation of Host Serotonin Modulates Platelet Function
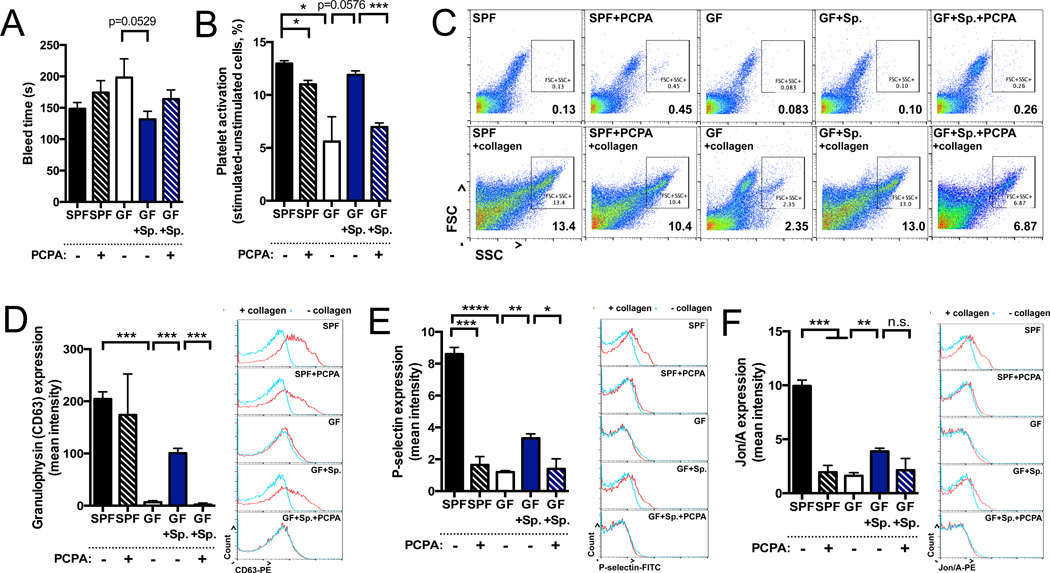
Platelets uptake gut-derived 5-HT and release it at sites of vessel injury to promote blood coagulation. To determine if microbiota-dependent modulation of colon (Figures 1 and 3) and plasma (Figure S1A) 5-HT impacts platelet function, we colonized P42 mice with Sp and then examined blood clotting, platelet activation and platelet aggregation at P56. In a tail bleed assay (Liu et al., 2012), GF mice exhibit trending increases in time to cessation of bleeding compared to SPF mice, suggesting impaired blood coagulation (Figure 5A). Colonization of GF mice with Sp ameliorates abnormalities in bleeding time to levels seen in SPF controls, and this effect is attenuated by intrarectal administration of PCPA (Figure 5A), indicating that Sp-mediated improvements in coagulation may be dependent on colonic Tph activity. Notably, the impact of acute colonic PCPA treatment on reducing 5-HT content and 5-HT-related functions in platelets may be tempered by the fact that mouse platelets have a lifespan of ~4 days (Odell and Mc, 1961). There were no significant differences between treatment groups in total platelet counts (Figure S5A).
Figure 5. Microbiota-Mediated Regulation of Host Serotonin Modulates Hemostasis.
(A) Time to cessation of bleeding in response to tail injury. n=7–16.
(B) Platelet activation, as measured by percentage of large, high granularity (FSChigh, SSChigh) events after collagen stimulation relative to unstimulated controls. n=3.
(C) Representative flow cytometry plots of large, high granularity (FSChigh, SSChigh) activated platelets after collagen stimulation (bottom), as compared to unstimulated controls (top). n=3.
(D)–(E) Geometric mean fluorescence intensity of granulophysin (CD63; D), P-selectin (E), JON/A (integrin αIIbβ3; F) expression in collagen-stimulated platelets (left). Representative histograms (right) of event count vs. fluorescence intensity (log scale) for platelets treated with collagen (red line) or vehicle (blue line). n=3.
Data for platelet assays are representative of three independent trials with at least three mice in each group. Data are presented as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, n.s.=not statistically significant. SPF=specific pathogen-free (conventionally-colonized), GF=germ-free, Sp=spore-forming bacteria, PCPA=para-chlorophenylalanine. See also Figure S5.
In light of inherent limitations of the tail bleed assay (Liu et al., 2012), we focused subsequent experiments particularly on platelet activity. Platelets isolated from GF mice display decreased activation in response to in vitro type I fibrillar collagen stimulation, as measured by reduced surface expression of the activation markers granulophysin (CD63), P-selectin and JON/A (integrin αIIbβ3) (Figures 5D, 5E and 5F) (Ziu et al., 2012). Sp colonization of GF mice leads to partial restoration in the expression of platelet activation markers, and this effect depends on colonic Tph activity (Figures 5D, 5E and 5F). Moreover, platelets isolated from GF mice exhibit impaired aggregation in response to in vitro collagen stimulation, as measured by decreased levels of high granularity, high mass aggregates detected by both flow cytometry (De Cuyper et al., 2013; Nieswandt et al., 2004) (Figures 5B 5C, S5C an S5D) and imaging (Figure S5B). Colonization of GF mice with Sp restores levels of platelet aggregation to those seen in SPF mice. These effects of Sp on correcting impaired platelet aggregation are attenuated by colonic PCPA injection, indicating dependence on Tph activity. Overall, these findings suggest that Sp-mediated elevations in colonic 5-HT, and thus platelet 5-HT, promote platelet activation and aggregation relevant to hemostasis.
Microbial Metabolites Mediate Effects of the Microbiota on Host Serotonin
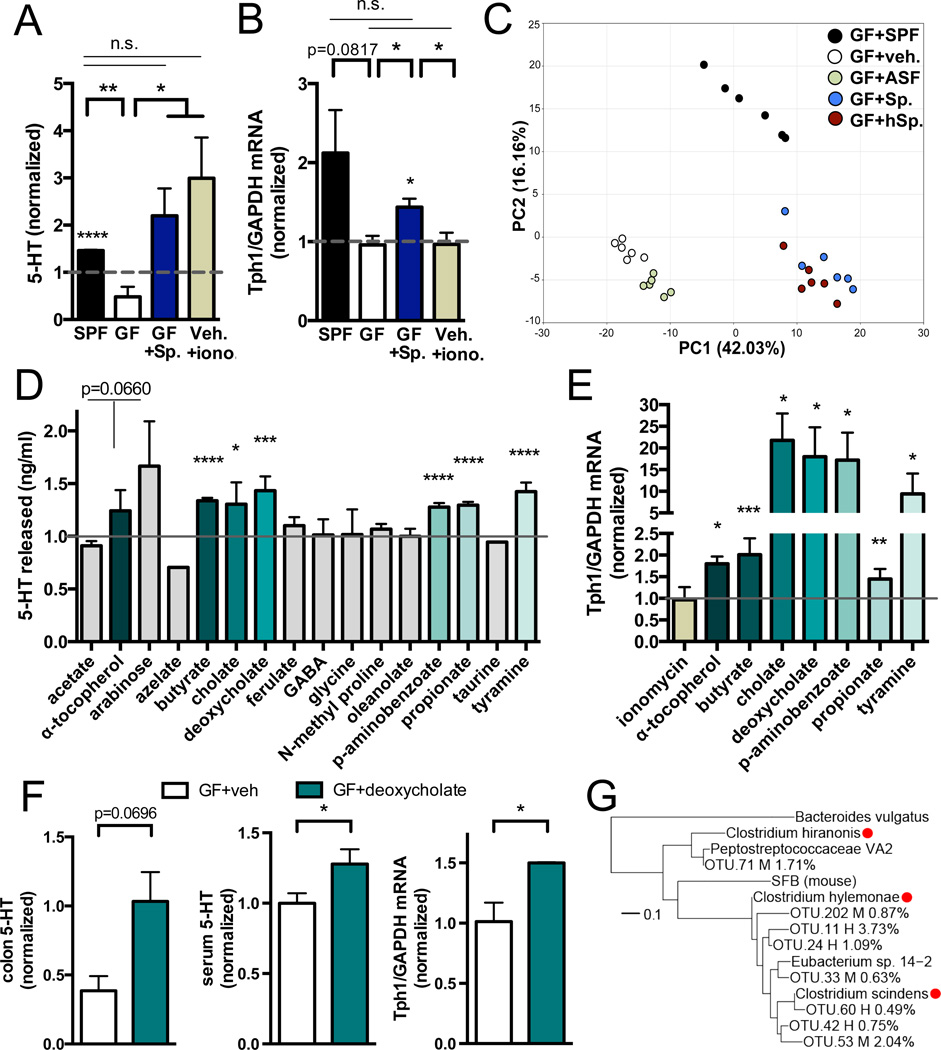
In light of the important role for Sp in regulating 5-HT-related intestinal and platelet function, we aimed to identify specific microbial factors responsible for conferring the serotonergic effects of Sp. Based on our finding that Sp elevates 5-HT particularly in colonic ECs (Figure 2), we hypothesized that Sp promotes levels of a soluble factor that signals directly to ECs to modulate TPH1 expression and 5-HT biosynthesis. To test this, we prepared filtrates of total colonic luminal contents from Sp-colonized mice and controls, and evaluated their effects on levels of 5-HT in RIN14B chromaffin cell cultures (Nozawa et al., 2009). Relative to vehicle-treated controls, there is no significant effect of filtered colonic luminal contents from GF mice on levels of 5-HT released or TPH1 expressed from RIN14B cells (Figures 6A and 6B). Filtered colonic luminal contents from SPF and Sp-colonized mice sufficiently induce 5-HT from RIN14B cells (Figure 6A), to levels comparable to those elicited by the calcium ionophore, ionomycin, as a positive control. TPH1 expression is also elevated in chromaffin cells exposed to SPF and Sp luminal filtrates, suggesting increased 5-HT synthesis. This is in contrast to ionomycin, which stimulates 5-HT release, but has no effect on TPH1 expression, from RIN14B cells. Importantly, these findings suggest that microbiota-mediated increases in gut 5-HT are conferred via direct signaling of a soluble, Sp-modulated factor to colonic ECs.
Figure 6. Microbial Metabolites Mediate Effects of the Microbiota on Host Serotonin.
(A) Levels of 5-HT released from RIN14B cells after exposure to colonic luminal filtrate from SPF, GF and Sp-colonized mice, or to ionomycin (iono). Data are normalized to 5-HT levels in vehicle-treated controls (hatched gray line at 1). Asterisks directly above bars indicate significance compared to controls; asterisks at the top of the graph denote significance between experimental groups. n=3.
(B) Expression of TPH1 relative to GAPDH in RIN14B cells after exposure to colon luminal filtrate from SPF, GF and Sp-colonized mice, or to ionomycin (iono). Data are normalized to gene expression in vehicle-treated controls (hatched gray line at 1). Asterisks directly above bars indicate significance compared to controls, whereas asterisks at the top of the graph denote significance between experimental groups. n=4.
(C) Principal components analysis of the fecal metabolome from GF mice colonized with SPF, ASF, Sp, or hSp. n=6.
(D) Levels of 5-HT released from RIN14B cells after exposure to metabolites: acetate (1 mM), α-tocopherol (8 uM), arabinose (50 uM), azelate (50 uM), butyrate (100 uM), cholate (75 uM), deoxycholate (25 uM), ferulate (25 uM), GABA (25 uM), glycine (50 uM), N-methyl proline (0.5 uM), oleanolate (50 uM), p-aminobenzoate (1 uM), propionate (100 uM), taurine (50 uM), tyramine (100 uM). Data are normalized to 5-HT levels in vehicle-treated controls (gray line at 1). n=5–19.
(E) Expression of TPH1 relative to GAPDH in RIN14B cells after metabolite exposure. Data are normalized to expression in vehicle-treated controls (gray line at 1). n=3–4.
(F) Levels of 5-HT in colons (left) and serum (center) of GF mice at 30 min after intrarectal injection of deoxycholate (125 mg/kg) or vehicle. Expression of TPH1 relative to GAPDH (right) at 1 hr post injection. n=3–8.
(G) Phylogenetic tree displaying key Sp. (M) and hSp. (H) operational taxonomic units (OTUs) relative to reference Clostridium species with reported 7α-dehydroxylation activity (red circles). Relative abundances are indicated in parentheses. n=3.
Data are presented as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, n.s.=not statistically significant. SPF=specific pathogen-free (conventionally-colonized), GF=germ-free, Sp=spore-forming bacteria, iono=15uM ionomycin, ASF=Altered Schaedler Flora, hSp=humanderived spore-forming bacteria. See also Figures S6 and S7.
We utilized metabolomic profiling to identify candidate Sp-dependent, 5-HT-inducing molecules in feces from adult mice. Sp colonization of GF mice leads to statistically significant alterations in 75% of the 416 metabolites detected, of which 76% are elevated and 24% are reduced, relative to vehicle-treated GF controls (Tables S1 and S2). Similar changes are seen with hSp colonization, leading to co-clustering of Sp and hSp samples by principal components analysis (PCA) (Figure 6C). ASF colonization has a mild effect, significantly modulating 50% of metabolites detected (66% increased, 36% decreased) (Table S2), and forming a distinct but proximal cluster to GF controls by PCA (Figure 6C). Postnatal conventionalization of GF mice with an SPF microbiota alters 66% of all metabolites detected (59% increased, 41% decreased) (Table S2), and produces substantial changes in the metabolome that are distinguishable from the effects of Sp, hSp and ASF along PC2 (Figure 6C). Notably, Sp, hSp and SPF colonization results in similar shifts along PC1, compared to vehicle and ASF-treated controls, suggesting common metabolic alterations among communities that similarly elevate peripheral 5-HT levels. Metabolomics profiling confirms that fecal 5-HT is commonly upregulated in the Sp, hSp and SPF fecal metabolome, and comparatively low in ASF and GF samples (Table S1). Simple linear regression reveals 83 metabolites that co-vary with 5-HT (r2 ≥ 0.25), 47 of which correlate positively and 36 of which correlate negatively with 5-HT levels (Table S3 and Figure S6A).
To determine whether specific metabolites mediate the effects of Sp on 5-HT, we tested a subset of biochemicals that were commonly upregulated by Sp, hSp and SPF, and that positively correlated with 5-HT levels (Table S3 and Figure S6A), for their ability to induce 5-HT in vitro and in vivo. We also tested the short chain fatty acids, acetate, butyrate and propionate, which were previously shown to be produced by Sp (Atarashi et al., 2013) and to stimulate 5-HT release from ECs (Fukumoto et al., 2003). Of 16 metabolites examined, α-tocopherol, butyrate, cholate, deoxycholate, p-aminobenzoate (PABA), propionate and tyramine elevate 5-HT in RIN14B chromaffin cell cultures (Figure 6D). Elevations in 5-HT correspond to increases in TPH1 expression from RIN14B cells (Figure 6E), suggesting that particular metabolites induced by Sp enhance 5-HT biosynthesis by ECs. We further tested for sufficiency to induce 5-HT in vivo. Notably, raising luminal concentrations of deoxycholate in colons of GF mice to levels seen in SPF mice (Sayin et al., 2013) sufficiently increases colon and serum 5-HT compared to vehicle-injected controls (Figures 6F and S6B). This restoration of peripheral 5-HT correlates with elevations in colonic TPH1 expression (Figure 6F). Increases in colon and serum 5-HT are also seen with injection of α-tocopherol, PABA and tyramine into colons of GF mice (Figures S6B and S6C). Consistent with in vitro RIN14B data, oleanolate has no statistically significant effect on elevating colon or serum 5-HT in GF mice (Figures S6B and S6C). Importantly, the effects of a single rectal injection of deoxycholate or α-tocopherol on raising colon 5-HT levels in GF mice are weak and transient, peaking within 1 hour of injection (Figure S6C). Consistent with this, there is no significant effect of acute colonic metabolite injection on GI transit time (Figure S6D), and there is only a trending improvement on platelet activation (Figure S6E). Our finding that Sp colonization leads to lasting increases in colon and blood 5-HT levels (Figure 3), and long-term changes in the fecal metabolome (Figure 6C and Tables S1 and S2), suggests that Sp colonization results in persistent elevations of 5-HT-modulating luminal metabolites. Future studies on whether chronic, colon-restricted increases in Sp-regulated metabolites sufficiently correct GI motility and platelet function in GF mice, and whether this occurs in a 5-HT-dependent manner, are warranted. In addition, we demonstrate that select concentrations of Sp-associated metabolites sufficiently promote 5-HT in vitro and in vivo, but whether the metabolites are necessary for mediating the serotonergic effects of Sp is unclear. Overall, these data reveal that indigenous spore-forming microbes promote 5-HT biosynthesis from colonic ECs, modulating 5-HT concentrations in both colon and blood. Furthermore, we identify select microbial metabolites that confer the serotonergic effects of indigenous spore-forming microbes, likely by signaling directly to colonic ECs to promote Tph1 expression and 5-HT biosynthesis.
DISCUSSION
The GI tract is an important site for 5-HT biosynthesis, but the regulatory mechanisms underlying the metabolism of gut-derived 5-HT are incompletely understood. Here we demonstrate that the gut microbiota plays a key role in promoting levels of colon and blood 5-HT, largely by elevating synthesis by host ECs. This host-microbiota interaction contributes to a growing appreciation that the microbiota regulates many aspects of GI physiology by signaling to host cells. Whether particular members of the microbiota contribute 5-HT by de novo synthesis remains unclear. Some bacteria, including Corynebacterium spp., Streptococcus spp. and Escherichia coli, are reported to synthesize 5-HT in culture (Roshchina, 2010), but this is believed to occur independently of Tph, by decarboxylation of tryptophan to tryptamine (Williams et al., 2014), as seen in plants (Oleskin et al., 1998). Our finding that colonic PCPA administration blocks the ability of the microbiota to promote colonic and blood 5-HT (Figures 3C and 3D) suggests that gut microbes require host Tph activity to upregulate peripheral 5-HT. Furthermore, SPF Tph1 KO mice lack >90% of intestinal and blood 5-HT levels (Savelieva et al., 2008), indicating that <10% of peripheral 5-HT is contributed directly by microbial synthesis or by Tph2-mediated biosynthesis in these mice. We find that the microbiota regulates relatively high levels of peripheral 5-HT, 64% of colonic (Figure 1) and 49% of serum concentrations (Figure 1; Sjogren et al., 2012; Wikoff et al., 2009), further supporting the notion that the microbiota modulates 5-HT metabolism primarily by affecting host colonic ECs. Consistent with the understanding that ECs secrete low levels of 5-HT into the lumen, fecal concentrations of 5-HT are also significantly increased by the microbiota. Interestingly, 5-HT is reported to stimulate the growth of Enterococcus faecalis, E. coli and Rhodospirillum rubrum in culture (Oleskin et al., 1998; Tsavkelova et al., 2006). In addition, 5-HT is a structural analogue of auxins found in E. faecalis, R. rubrum and Staphylococcus aureus, among other bacteria. Whether particular members of the microbiota alter host 5-HT biosynthesis to, in turn, support colonization, growth or resilience of particular gut microbes is an interesting question for future study.
We demonstrate that indigenous spore-forming microbes from colons of SPF mice (Sp) and from a healthy human colon (hSp) sufficiently mediate microbiota effects on colonic and blood 5-HT. While we show that B. fragilis, B. uniformis, SFB, ASF and a consortium of Bacteroides species cultured from mice, including B. thetaiotaomicron, B. acidifaciens and B. vulgatus, have no effect on host peripheral 5-HT (Figure 3), whether other non-Sp microbial species or communities are capable of modulating colonic and serum 5-HT remains unclear. Interestingly, Sp and hSp are known to promote regulatory T cell levels in the colons, but not small intestines, of GF and SPF mice (Atarashi et al., 2013). This regional specificity is also seen with microbiota-induced 5-HT biosynthesis, which occurs in colonic, but not small intestinal, ECs (Figures S1A, S2A and S2B). We find that Sp elevates colon 5-HT levels even in Rag1 KO mice (Figure S2G), indicating that the serotonergic effects of Sp are not dependent on T and B cells. Whether 5-HT modulation contributes to the immunosuppressive effects of Sp, however, is unclear. In light of increasing evidence that innate and adaptive immune cells express a variety of 5-HT receptors (Baganz and Blakely, 2013), future studies examining whether Sp-mediated increases in peripheral 5-HT levels impact cellular immune responses will be of interest.
Consistent with our finding that the microbiota modulates colon and serum 5-HT via interactions with host colonic ECs, we find that particular fecal metabolites are similarly elevated by SPF, Sp and hSp microbiota, and sufficiently promote 5-HT in chromaffin cell cultures and in vivo (Figure 6 and Table S3). Deoxycholate is a secondary bile acid, produced by microbial biotransformation of cholate. Notably, deoxycholate is reported to promote GI motility by activating TGR5 G-protein-coupled receptors on ECs (Alemi et al., 2013), which is consistent with our finding that Sp-induced metabolites raise 5-HT levels in ECs and that Sp colonization improves GI motility. Particular Clostridium species are known to possess high 7α-dehydroxylation activity required for the production of deoxycholate from cholate (Kitahara et al., 2001; Narushima et al., 2006), which is in line with our finding that Sp microbes, comprised largely of Clostridia, increase deoxycholate levels. Deoxycholate concentrations are substantially higher in the colon versus small intestine (Sayin et al., 2013), which, coupled to the finding that bacterial load and diversity is greater in the colon versus small intestine (Sekirov et al., 2010), could contribute to the regional specificity of microbiota-mediated increases in 5-HT synthesis to colonic ECs. Phylogenetic analysis of 16S rDNA sequences reveals that a subset of microbes recovered from Sp-colonized mice cluster taxonomically with known 7α- dehydroxylating Clostridia (Figures 6G and S7). Notably, there are striking phylogenetic commonalities between taxa identified in Sp- and hSp-colonized mice (Figure S7), consistent with their very similar luminal metabolomic profiles (Figure 6C) and ability to promote 5-HT synthesis from colonic ECs (Figure S3).
We also reveal that the metabolites α-tocopherol, tyramine and PABA are elevated in feces by Sp. hSp or SPF colonization, co-vary with fecal 5-HT levels, and sufficiently induce 5-HT in vitro and in vivo (Figures 6 and S6 and Table S1). α-tocopherol is a naturally abundant form of vitamin E, with reported therapeutic effects for several diseases (Brigelius-Flohe and Traber, 1999). Interestingly, patients with depression exhibit decreased plasma α-tocopherol (Maes et al., 2000; Owen et al., 2005), and treatment with α-tocopherol reduces depressive-like behavior in pre-clinical models (Lobato et al., 2010), suggesting a link between α-tocopherol and 5-HT-related disease. Tyramine is a trace amine that acts as a neurotransmitter and catecholamine-releasing agent. Particular bacteria can produce tyramine by decarboxylation of tyrosine in the gut, where tyramine is reported to stimulate fast ileal contractions and neuropeptide Y release (Marcobal et al., 2012). PABA is an intermediate of folic acid synthesis and essential nutrient for some bacteria. Particular species can generate PABA from chorismate (de Crecy-Lagard et al., 2007), but physiological roles for PABA in the GI tract are unclear. Subsets of microbes from Sp- and hSp-colonized mice relate phylogenetically to Clostridia with putative genes for α-tocopherol and tyrosine metabolism (Figures 6G and S7). Screening Sp microbes for target metabolic functions could serve as a tractable approach for further parsing the Sp consortium into the minimal species required for increasing 5-HT biosynthesis by ECs.
While there is increasing evidence for a bi-directional relationship between the gut microbiota and gut sensorimotor function, the particular microbes and mechanisms involved are unclear. The microbiota is required for normal IPAN excitability (McVey Neufeld et al., 2013), and recent studies reveal that changes in the microbiota can alter levels of neuroactive molecules, such as nitric oxide, substance P and endocannabinoids, which have the potential to influence gut motor activity (Quigley, 2011). Mucosal immune responses (Collins, 1996), including key interactions between macrophages and enteric neurons (Muller et al., 2014), also modulate GI motility via the gut microbiota. It will be interesting to determine whether 5-HT-mediated effects on immunity (Baganz and Blakely, 2013) contribute to its effects on GI motility. Notably, deconjugated bile salts are reported to alter gut sensorimotor activity (Appleby and Walters, 2014), which supports our hypothesis that Sp-induced increases in deoxycholate, among other metabolites, contribute to its ability to elevate colonic 5-HT and decrease intestinal transit time.
While we demonstrate that Sp-mediated induction of colonic and blood 5-HT regulates GI motility and platelet function in mice, further research is needed to explore additional implications of microbially induced 5-HT on host health and disease (O'Mahony et al., 2014). Peripheral 5-HT modulates several cellular processes, including osteoblast differentiation, erythropoiesis and immunity. Moreover, gross abnormalities in brain structure are observed in Tph1+/− embryos from Tph1−/− mothers (Cote et al., 2007), indicating that maternal peripheral 5-HT is important for offspring neurodevelopment. Placentally-derived 5-HT also influences neurodevelopment, influencing thalamocortical axon guidance (Bonnin et al., 2011). Interestingly, the indigenous microbiota also modulates hippocampal levels of 5-HT (Clarke et al., 2013), revealing a role for the microbiota in regulating the brain serotonergic system. Overall, our findings provide a mechanism by which select microbes and their metabolic products can be used to promote endogenous, localized 5-HT biosynthesis and further alter host physiology.
EXPERIMENTAL PROCEDURES
See Supplemental information for additional details and references
PCPA Treatment
At 2 weeks post-bacterial treatment, mice were anesthetized with isoflurane, and PCPA (90 mg/kg) (Liu et al., 2008) was administered intrarectally every 12 hours for 3 days using a sterile 3.5 Fr silicone catheter inserted 4 cm into the rectum. Mice were suspended by tail for 30 s before return to the home cage. For mock treatment, mice were anesthetized and intrarectally injected with sterile water as vehicle.
Serotonin Measurements
Serotonin levels were detected in sera and supernatant of tissue homogenates by ELISA according to the manufacturer’s instructions (Eagle Biosciences). Readings from tissue samples were normalized to total protein content as detected by BCA assay (Thermo Pierce). Data compiled across multiple experiments are expressed as 5-HT concentrations normalized to SPF controls within each experiment.
RIN14B In Vitro Culture Experiments
RIN14B cells (ATCC) were seeded at 105 cells/cm2 and cultured according to methods described in (Nozawa et al., 2009). Total colonic luminal contents were collected from adult SPF, GF and GF mice colonized with spore-forming bacteria, suspended at 120 ul/mg in HBSS supplemented with 0.1% BSA and 2 uM fluoxetine, and centrifuged at 12,000 ×g for 10 min. Supernatants were passed through 0.2 um pore syringe filters. Cultured RIN14B cells were incubated with colonic luminal filtrate for 1 hr at 37°C.
GI Transit Assay
Mice were orally gavaged with 200 ul sterile solution of 6% carmine red (Sigma Aldrich) and 0.5% methylcellulose (Sigma Aldrich) in water, and placed in a new cage with no bedding (Li et al., 2011). Starting at 120 minutes post-gavage, mice were monitored every 10 minutes for production of a red fecal pellet. GI transit time was recorded as the total number of minutes elapsed (rounded to the nearest 10 minutes) before production of a red fecal pellet. For mice treated intrarectally with PCPA or metabolites, GI transit assay was conducted 1 hour after the third injection.
Platelet Activation and Aggregation Assays
Blood samples were collected by cardiac puncture, diluted with a 2× volume of HEPES medium and centrifuged through PST lithium hepararin vacutainers (Becton Dickinson). Expression of platelet activation markers was measured by flow cytometry (Nieswandt et al., 2004; Ziu et al., 2012). Platelet aggregation assays were conducted according to methods described in (De Cuyper et al., 2013). Remaining unstained PRP was used to generate PRP smears. Slides were stained with Wright Stain (Camco) according to standard procedures.
16S rRNA Gene Sequencing and Analysis
Fecal samples were collected at two weeks after orally gavaging GF mice with Sp or hSp. Bacterial genomic DNA was extracted from mouse fecal pellets using the QIAamp DNA Stool Mini Kit (Qiagen). The library was generated according to methods from (Caporaso et al., 2011). The V4 regions of the 16S rRNA gene were PCR amplified, purified and then sequenced using the Illumina MiSeq platform. Operational taxonomic units (OTUs) were chosen de novo with UPARSE pipeline (Edgar, 2013). Taxonomy assignment and rarefaction were performed using QIIME1.8.0 (Caporaso et al., 2010). Phylogenetic trees were built using PhyML (Guindon et al., 2010) and visualized using iTOL (Letunic and Bork, 2007).
Supplementary Material
ACKNOWLEDGMENTS
The authors acknowledge the assistance of Taren Thron, Sara McBride and Alyssa Maskell for caring for the animals, Andrew Stefka and Taylor Feehley (University of Chicago) for contributing pilot serum and fecal samples, Dr. Nathan Dalleska and Jesse Allen (Caltech) for conducting pilot LC/MS experiments, Said Bogatyrev (Caltech) for helpful advice, Natasha Shelby (Caltech) for editing the manuscript and the late Dr. Paul H. Patterson for his valuable support. This work was supported by the NIH Director’s Early Independence Award (5DP5OD017924; to E.Y.H.), Caltech Center for Environmental Microbial Interactions Award (to E.Y.H.), NSF Emerging Frontiers in Research and Innovation Award (EFRI-1137089; to R.F.I. and S.K.M.), NHGRI grant (R01HG005826; to R.F.I.), NIDDK grant (DK078938; to S.K.M.) and NIMH grant (MH100556; to S.K.M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHORS CONTRIBUTIONS
J.M.Y., K.Y., G.P.D., G.G.S., P.A., L.M. and E.Y.H. performed the experiments and analyzed the data, J.M.Y. and E.Y.H. designed the study, C.R.N., R.F.I and S.K.M. provided novel reagents, R.F.I. and S.K.M. provided valuable support and contributed equally, J.M.Y. and E.Y.H. wrote the manuscript. All authors discussed the results and commented on the manuscript.
REFERENCES
- Alemi F, Poole DP, Chiu J, Schoonjans K, Cattaruzza F, Grider JR, Bunnett NW, Corvera CU. The receptor TGR5 mediates the prokinetic actions of intestinal bile acids and is required for normal defecation in mice. Gastroenterology. 2013;144:145–154. doi: 10.1053/j.gastro.2012.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amireault P, Sibon D, Cote F. Life without peripheral serotonin: insights from tryptophan hydroxylase 1 knockout mice reveal the existence of paracrine/autocrine serotonergic networks. ACS chemical neuroscience. 2013;4:64–71. doi: 10.1021/cn300154j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleby RN, Walters JR. The role of bile acids in functional GI disorders. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2014;26:1057–1069. doi: 10.1111/nmo.12370. [DOI] [PubMed] [Google Scholar]
- Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232–236. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- Baganz NL, Blakely RD. A dialogue between the immune system and brain, spoken in the language of serotonin. ACS chemical neuroscience. 2013;4:48–63. doi: 10.1021/cn300186b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnin A, Goeden N, Chen K, Wilson ML, King J, Shih JC, Blakely RD, Deneris ES, Levitt P. A transient placental source of serotonin for the fetal forebrain. Nature. 2011;472:347–350. doi: 10.1038/nature09972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigelius-Flohe R, Traber MG. Vitamin E: function and metabolism. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1999;13:1145–1155. [PubMed] [Google Scholar]
- Chabbi-Achengli Y, Coudert AE, Callebert J, Geoffroy V, Cote F, Collet C, de Vernejoul MC. Decreased osteoclastogenesis in serotonin-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:2567–2572. doi: 10.1073/pnas.1117792109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, Dinan TG, Cryan JF. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Molecular psychiatry. 2013;18:666–673. doi: 10.1038/mp.2012.77. [DOI] [PubMed] [Google Scholar]
- Collins SM. The immunomodulation of enteric neuromuscular function: implications for motility and inflammatory disorders. Gastroenterology. 1996;111:1683–1699. doi: 10.1016/s0016-5085(96)70034-3. [DOI] [PubMed] [Google Scholar]
- Cote F, Fligny C, Bayard E, Launay JM, Gershon MD, Mallet J, Vodjdani G. Maternal serotonin is crucial for murine embryonic development. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:329–334. doi: 10.1073/pnas.0606722104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote F, Thevenot E, Fligny C, Fromes Y, Darmon M, Ripoche MA, Bayard E, Hanoun N, Saurini F, Lechat P, et al. Disruption of the nonneuronal tph1 gene demonstrates the importance of peripheral serotonin in cardiac function. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:13525–13530. doi: 10.1073/pnas.2233056100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Crecy-Lagard V, El Yacoubi B, de la Garza RD, Noiriel A, Hanson AD. Comparative genomics of bacterial and plant folate synthesis and salvage: predictions and validations. BMC genomics. 2007;8:245. doi: 10.1186/1471-2164-8-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewhirst FE, Chien CC, Paster BJ, Ericson RL, Orcutt RP, Schauer DB, Fox JG. Phylogeny of the defined murine microbiota: altered Schaedler flora. Applied and environmental microbiology. 1999;65:3287–3292. doi: 10.1128/aem.65.8.3287-3292.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducy P, Karsenty G. The two faces of serotonin in bone biology. The Journal of cell biology. 2010;191:7–13. doi: 10.1083/jcb.201006123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto S, Tatewaki M, Yamada T, Fujimiya M, Mantyh C, Voss M, Eubanks S, Harris M, Pappas TN, Takahashi T. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. American journal of physiology Regulatory, integrative and comparative physiology. 2003;284:R1269–R1276. doi: 10.1152/ajpregu.00442.2002. [DOI] [PubMed] [Google Scholar]
- Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132:397–414. doi: 10.1053/j.gastro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Hoffman JM, Tyler K, MacEachern SJ, Balemba OB, Johnson AC, Brooks EM, Zhao H, Swain GM, Moses PL, Galligan JJ, et al. Activation of colonic mucosal 5-HT(4) receptors accelerates propulsive motility and inhibits visceral hypersensitivity. Gastroenterology. 2012;142:844–854. e844. doi: 10.1053/j.gastro.2011.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Chalazonitis A, Huang YY, Mann JJ, Margolis KG, Yang QM, Kim DO, Cote F, Mallet J, Gershon MD. Essential roles of enteric neuronal serotonin in gastrointestinal motility and the development/survival of enteric dopaminergic neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:8998–9009. doi: 10.1523/JNEUROSCI.6684-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobato KR, Cardoso CC, Binfare RW, Budni J, Wagner CL, Brocardo PS, de Souza LF, Brocardo C, Flesch S, Freitas AE, et al. alpha-Tocopherol administration produces an antidepressant-like effect in predictive animal models of depression. Behavioural brain research. 2010;209:249–259. doi: 10.1016/j.bbr.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Maes M, De Vos N, Pioli R, Demedts P, Wauters A, Neels H, Christophe A. Lower serum vitamin E concentrations in major depression. Another marker of lowered antioxidant defenses in that illness. Journal of affective disorders. 2000;58:241–246. doi: 10.1016/s0165-0327(99)00121-4. [DOI] [PubMed] [Google Scholar]
- Marcobal A, De las Rivas B, Landete JM, Tabera L, Munoz R. Tyramine and phenylethylamine biosynthesis by food bacteria. Critical reviews in food science and nutrition. 2012;52:448–467. doi: 10.1080/10408398.2010.500545. [DOI] [PubMed] [Google Scholar]
- Mawe GM, Hoffman JM. Serotonin signalling in the gut--functions, dysfunctions and therapeutic targets. Nature reviews Gastroenterology & hepatology. 2013;10:473–486. doi: 10.1038/nrgastro.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVey Neufeld KA, Mao YK, Bienenstock J, Foster JA, Kunze WA. The microbiome is essential for normal gut intrinsic primary afferent neuron excitability in the mouse. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2013;25:e183–e188. doi: 10.1111/nmo.12049. [DOI] [PubMed] [Google Scholar]
- Mercado CP, Quintero MV, Li Y, Singh P, Byrd AK, Talabnin K, Ishihara M, Azadi P, Rusch NJ, Kuberan B, et al. A serotonin-induced N-glycan switch regulates platelet aggregation. Scientific reports. 2013;3:2795. doi: 10.1038/srep02795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller PA, Koscso B, Rajani GM, Stevanovic K, Berres ML, Hashimoto D, Mortha A, Leboeuf M, Li XM, Mucida D, et al. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell. 2014;158:300–313. doi: 10.1016/j.cell.2014.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narushima S, Itoha K, Miyamoto Y, Park SH, Nagata K, Kuruma K, Uchida K. Deoxycholic acid formation in gnotobiotic mice associated with human intestinal bacteria. Lipids. 2006;41:835–843. doi: 10.1007/s11745-006-5038-1. [DOI] [PubMed] [Google Scholar]
- O'Mahony SM, Clarke G, Borre YE, Dinan TG, Cryan JF. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behavioural brain research. 2014 doi: 10.1016/j.bbr.2014.07.027. [DOI] [PubMed] [Google Scholar]
- Odell TT, Jr, Mc DT. Life span of mouse blood platelets. Proc Soc Exp Biol Med. 1961;106:107–108. doi: 10.3181/00379727-106-26252. [DOI] [PubMed] [Google Scholar]
- Oleskin AV, Kirovskaia TA, Botvinko IV, Lysak LV. Effect of serotonin (5-hydroxytryptamine) on the growth and differentiation of microorganisms. Mikrobiologiia. 1998;67:305–312. [PubMed] [Google Scholar]
- Owen AJ, Batterham MJ, Probst YC, Grenyer BF, Tapsell LC. Low plasma vitamin E levels in major depression: diet or disease? European journal of clinical nutrition. 2005;59:304–306. doi: 10.1038/sj.ejcn.1602072. [DOI] [PubMed] [Google Scholar]
- Quigley EM. Microflora modulation of motility. Journal of neurogastroenterology and motility. 2011;17:140–147. doi: 10.5056/jnm.2011.17.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramage AG, Villalon CM. 5-hydroxytryptamine and cardiovascular regulation. Trends in pharmacological sciences. 2008;29:472–481. doi: 10.1016/j.tips.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Roshchina VV. Evolutionary Considerations of Neurotransmitters in Microbial, Plant, and Animal Cells. Microbial Endocrinology. 2010:17–52. [Google Scholar]
- Savelieva KV, Zhao S, Pogorelov VM, Rajan I, Yang Q, Cullinan E, Lanthorn TH. Genetic disruption of both tryptophan hydroxylase genes dramatically reduces serotonin and affects behavior in models sensitive to antidepressants. PLoS One. 2008;3:e3301. doi: 10.1371/journal.pone.0003301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayin SI, Wahlstrom A, Felin J, Jantti S, Marschall HU, Bamberg K, Angelin B, Hyotylainen T, Oresic M, Backhed F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell metabolism. 2013;17:225–235. doi: 10.1016/j.cmet.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Shimizu S, Hattori R, Gabazza EC, Majima Y. In vivo and in vitro effects of macrolide antibiotics on mucus secretion in airway epithelial cells. American journal of respiratory and critical care medicine. 2003;168:581–587. doi: 10.1164/rccm.200212-1437OC. [DOI] [PubMed] [Google Scholar]
- Sjogren K, Engdahl C, Henning P, Lerner UH, Tremaroli V, Lagerquist MK, Backhed F, Ohlsson C. The gut microbiota regulates bone mass in mice. J Bone Miner Res. 2012;27:1357–1367. doi: 10.1002/jbmr.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasi C, Bellini M, Bassotti G, Blandizzi C, Milani S. Serotonin receptors and their role in the pathophysiology and therapy of irritable bowel syndrome. Techniques in coloproctology. 2014 doi: 10.1007/s10151-013-1106-8. [DOI] [PubMed] [Google Scholar]
- Stefka AT, Feehley T, Tripathi P, Qiu J, McCoy K, Mazmanian SK, Tjota MY, Seo G, Cao S, Theriault BR, et al. Commensal bacteria protect against food allergen sensitization. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:13145–13150. doi: 10.1073/pnas.1412008111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsavkelova EA, Klimova S, Cherdyntseva TA, Netrusov AI. Hormones and hormone-like substances of microorganisms: a review. Prikladnaia biokhimiia i mikrobiologiia. 2006;42:261–268. [PubMed] [Google Scholar]
- Uribe A, Alam M, Johansson O, Midtvedt T, Theodorsson E. Microflora modulates endocrine cells in the gastrointestinal mucosa of the rat. Gastroenterology. 1994;107:1259–1269. doi: 10.1016/0016-5085(94)90526-6. [DOI] [PubMed] [Google Scholar]
- Wade PR, Chen J, Jaffe B, Kassem IS, Blakely RD, Gershon MD. Localization and function of a 5-HT transporter in crypt epithelia of the gastrointestinal tract. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1996;16:2352–2364. doi: 10.1523/JNEUROSCI.16-07-02352.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther DJ, Peter JU, Bashammakh S, Hortnagl H, Voits M, Fink H, Bader M. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003;299:76. doi: 10.1126/science.1078197. [DOI] [PubMed] [Google Scholar]
- Westphal JF, Vetter D, Brogard JM. Hepatic side-effects of antibiotics. The Journal of antimicrobial chemotherapy. 1994;33:387–401. doi: 10.1093/jac/33.3.387. [DOI] [PubMed] [Google Scholar]
- Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, Siuzdak G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci U S A. 2009;106:3698–3703. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BB, Van Benschoten AH, Cimermancic P, Donia MS, Zimmermann M, Taketani M, Ishihara A, Kashyap PC, Fraser JS, Fischbach MA. Discovery and Characterization of Gut Microbiota Decarboxylases that Can Produce the Neurotransmitter Tryptamine. Cell host & microbe. 2014;16:495–503. doi: 10.1016/j.chom.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav VK, Ryu JH, Suda N, Tanaka KF, Gingrich JA, Schutz G, Glorieux FH, Chiang CY, Zajac JD, Insogna KL, et al. Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell. 2008;135:825–837. doi: 10.1016/j.cell.2008.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziu E, Mercado CP, Li Y, Singh P, Ahmed BA, Freyaldenhoven S, Lensing S, Ware J, Kilic F. Down-regulation of the serotonin transporter in hyperreactive platelets counteracts the pro-thrombotic effect of serotonin. Journal of molecular and cellular cardiology. 2012;52:1112–1121. doi: 10.1016/j.yjmcc.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.