Abstract
Background:
Ejection fraction promotion in heart failure patients reduces mortalities and limitations.
Objectives:
This study was to investigate the effect of exercise on ejection fraction of chronic heart failure patients.
Patients and Methods:
The present study was conducted on patients with chronic heart failure. 66 patients were divided randomly into two experimental and control groups of 33 each. The subjects were male and female. The patients in experimental group followed an exercise program three sessions per week for 24 weeks. Echocardiography and quality of life questionnaire were used to gather data. The data were analyzed by SPSS 18 through pair and independent t-test.
Results:
The results indicated a significant difference in left ventricular diameter (LV-ESD, LV-EDD) and ejection fraction at the end of exercise program in experimental group and 24 weeks after in control group. There was a significant difference in quality of life in physical performance, activity limitation following physical problems, energy and fatigue, social performance, physical pain, and public health (P < 0.05 for all) between two groups.
Conclusions:
Exercise program increases ejection fraction and quality of life in chronic heart failure patients, associated with management of disease by health team.
Keywords: Heart Failure, Exercise, Quality of Life
1. Background
Heart disease is one of the most common chronic illnesses in elderly and middle-aged people. Patients with heart failure due to decreased left ventricular systolic function demonstrate large end-diastolic volumes and have little contractile reserve (1). The prevalence of congestive heart failure appears to have increased in the USA (2, 3). Nearly 15 million people in the world and more than 4.9 million people in the United States are suffering from heart failure (4).
One of the common characteristics of patients with heart failure is exercise intolerance, which may be due to mass loss of muscle power leading to reduced performance in individuals' daily affairs and ultimately loss of ejection fraction and quality of life (5). In addition, poor physical conditions, mental stress, and readmission to hospital could also decrease the quality of life in these patients (6, 7), ending with depression and psychological disorders in their social relationships. Congestive heart failure significantly compromises quality of life by contributing to physical, role, and social functioning impairment as well as increased psychological distress (8-11). During the past two decades, quality of life in chronic patients, as one of the indicators of effective care and treatment, has been one of the important issues for clinical research. Some studies are being done to investigate the effect of quality of life on the differences among patients, the prediction of disease, and evaluation of interventions (12-16).
An exercise program, developed based on the physical conditions of patients, can promote the quality of life in heart failure patients. Also, in patients with chronic but asymptomatic (Class II) heart failure, exercise training is recommended alongside pharmacological and non-pharmacological therapies (7, 17). Training induces a delay in the increase in blood lactate, the rates of creatine phosphate consumption and glycolysis are frequently high and blood free-fatty acids increase gradually during exercise(17, 18). Aerobic training has been shown to improve the physiological components of aerobic metabolism. In particular, studies have repeatedly demonstrated an improvement in VO2 peak. This improvement is usually cited as being between 15 - 25% though has been documented as high as 36% (19). Exercise may cause further increase in left ventricular end-diastolic volume in these patients that would increase wall tension and may be detrimental to left ventricular function Also, it could improve total peripheral vascular resistance (TPR) and peripheral perfusion (7, 19).
Aerobic training reverses left ventricular remodeling in asymptomatic individuals with chronic heart failure (20). Studies have shown that patients with stable conditions that have regular and moderate exercise have better quality of life and less mortality rates compared to the patients who do not exercise (8). Because of low cost, accessibility, and easy use, exercise training is an intervention that could be recommended for most patients with heart failure (17). These studies suggested that patients, who had physical problems and were dissatisfied with their status, experienced a considerable progress in their physical ability after an exercise period. The results showed that there was a significant relationship between exercise ability and the level of satisfaction with quality of life in these patients. Compared with usual care, exercise training reduces heart failure-related hospitalizations and results in clinical betterments in quality of life among heart failure patients (20, 21).
Lifestyle changes such as doing exercise, and managing stress and excitement by the health team in patients with heart failure can help to reduce signs and symptoms of disease progression and improve quality of life (22). These changes in lifestyle of cardiac patients require follow-up, care, education, and interventions such as physical activity under the supervision of health care teams especially nurses (23).
Due to the increased incidence of heart failure and importance of exercise and other supportive interventions, such as stress and excitement management, and the fact that heart failure patients suffer from several problems, utilizing moderate exercise programs and understanding the effect of other factors on ejection fraction and quality of life by medical staff especially nurses can help to improve these patients' health considerably.
2. Objectives
This study was aimed to investigate the effect of exercise training on the quality of life and echocardiography parameters of systolic function in patients with chronic heart failure in Iran.
3. Patients and Methods
3.1. Design and Sample
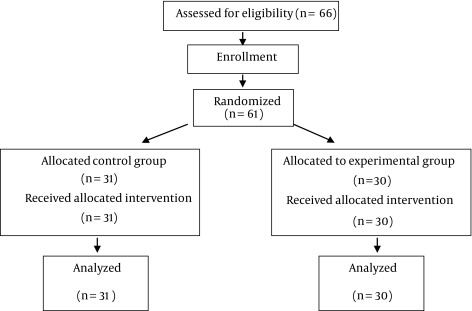
This is a quasi-experimental study in 2012. Statistician examined 66 patients for our study (33 patients each group with d = 0.13, p = 0.5 and q = 0.5). Subjects were randomly allocated (considering matching by gender and age) into two groups. The randomization code was developed with a computer random-number generator to select random permuted blocks. Then, these patients suffering from chronic heart failure were chosen. The patients were referred to the cardiology ward of Shahrekord University of Medical Sciences and were assessed for eligibility to participate in the study. Patients were randomized into control and experimental groups. Five patients were excluded from the study because of death, coronary bypass surgery, and refusal to receive treatment. Finally, 61 patients were participated in this study: control (including 31 patients) and experimental group (including 30 patients).
3.2. Inclusion Criteria
Inclusion criteria were male and female patients of 65 ± 15 years with chronic heart disease and consent to participate in the study. Other criteria for eligibility were diagnosis of heart failure, left ventricular ejection fraction of ≤ 40%, sinus rhythm, and being admitted by the Hospital. Chronic heart failure was based on clinical symptoms, echocardiographic evidence, and the physicians’ statements. In addition, the ability to perform the exercise program after the medication therapy, no difficulty with movement (such as rheumatoid arthritis, fractures, etc.), no heart transplant three months after exercise program, no advanced heart failure, and no traveling during the research were determined as other inclusion criteria.
3.3. Exclusion Criteria
Coronary bypass surgery during the research, neurological, orthopedic, peripheral vascular or severe pulmonary diseases probably intervening in successful completion of exercise tests, and being unwilling to cooperate were considered as exclusion criteria. Accordingly, in the experimental group three patients (one patient because of death and two patients for coronary bypass surgery) and in the control group, two patients (being unwilling to cooperate with the research team) were excluded from the study.
Procedure: The exercise program was performed three sessions per week for 24 weeks. The exercise program included 40 minutes, including 5-10 minutes for warm-up, 25 - 30 minutes of exercise (walking), and 5 minutes for cooling down) MET (Metabolic Equivalent) value of various common activities was light intensity activity: < 3MET). The patients, immediately after enrollment, underwent simple walking in a sport facility or gym in the hospital while they were supervised by a nurse or cardiologist. Exercise started until heart rate reached equal to 60% of heart rate reserve. After 6 sessions, the duration of the exercise (walking) was increased to 30 to 35 minutes, and heart rate to 70% of heart rate reserve. Each patient exercised based on his/her ability and resistance. Exercise was stopped when patients were physically tired or faced severe dyspnea, fatigue, dizziness or other problems that could jeopardize patient health based on Rhoten Fatigue Scale (RFS). To assess the reproducibility of gas exchange parameters, the cardiopulmonary test was repeated 3 to 5 days before starting the protocol for all patients and considered as baseline. The examination was repeated at the end of the study.
Patients in the control group received educational support but no exercise protocol; they were asked to continue their individually prescribed cardiovascular medications and their usual lifestyle but not to do any physical activity that caused breathlessness or fatigue and were supervised by their nurse and physicians.
Blood pressure and heart rate were measured before and after exercise. Drug treatment was not changed during the study in control and experimental groups. To further minimize the bias, we did not let the cardiologist who was informed of echocardiography results whether the participant was control or case (The physician who did perform echocardiography and the nurse who did data gathering were different from the physician and nurse that were monitoring the exercise).
3.4. Instruments
The required data were collected by MacNew Heart Disease Health-Related Quality of Life questionnaire (physical performance, limited activity following mental problems, limited activity following physical problems, energy and fatigue, physical problems, mental health, social performance and body pain). The self-administered MacNew questionnaire is designed to evaluate how daily activities and physical, emotional, and social functioning are influenced by heart disease, demographic characteristics, and echocardiography (2). Each of these 8 dimensions may be scored from 0 to 100. Scoring is performed according to criteria of SF-36 standards, with higher scores indicating better function (24, 25). The quality of life questionnaire was filled out and echocardiography was carried out on both groups by the researchers twice, before the exercise program at the hospital and 24 weeks after the exercise program (Figure 1).
Figure 1. Sampling flowchart.
3.5. Data Analysis
The obtained data were analyzed using SPSS software (version 11.5) through independent t-test. P < 0.05 was considered as the level of significance. For quantitative variables, mean and standard deviation and for qualitative variables, frequency and percent were used to report the data.
The ethical approval (with ethics code of 85-9-1) was obtained from Ethics Committee of the Shahrekord University of Medical Sciences and registration code of I.R.CT201306251376831 was issued by Iranian Registry of Clinical Trials for this study.
4. Results
The mean age of patients was 60 ± 4.25 years in the experimental group and 58 ± 4.22 in the control group. 60% in the experimental group and 74% in the control group were male. 83% of the patients in the experimental group and 81% in the control group had heart failure class III (Table 1).
Table 1. Baseline Characteristics in Patients with Congestive Heart Failure in Experimental and Control Groups a.
| Variables | Experimental Group b | Control Group b |
|---|---|---|
| Gender | ||
| Male | 18 (60) | 23 (74) |
| Female | 12 (40) | 8 (24) |
| Age, y | 60 ± 4.25 | 58 ± 4.22 |
| Disease diagnosis | ||
| Ischemic cardiomyopathy | 21 (70) | 20 (64.5) |
| Dilated cardiomyopathy | 2 (6.66) | 1 (3.22) |
| Hypertension | 7 (23.4) | 9 (32.27) |
| Peak oxygen uptake, mL.kg/1.min-1 | 17 ± 12 | 18 ± 15 |
| NYHA class | ||
| I | 0 | 0 |
| II | 6 (20) | 6 (19.33) |
| III | 25 (83) | 25 (81) |
| Ejection fraction | 32 ± 4 | 33 ± 5 |
| Medications t | ||
| Digitalis | 21 (70) | 22 (68) |
| ACE inhibitors | 24 (80) | 27 (87) |
| Diuretics | 28 (94) | 28 (90.3) |
| Warfarin | 12 (40) | 13 (42) |
a Data are presented as Mean ± SD and No. (%).
b No = 30
The results show that there was no significant difference in ejection fraction and demographic characteristics between the two groups before the study. The results also showed that after exercise training, ejection fraction in exercise group increased from 32 ± 4 to 37 ± 4, but in control group, it decreased from 33 ± 5 to 33 ± 4. (Table 2). Independent t-test indicated that the quality of life score in eight dimensions was not significantly different between the two groups at the beginning of the study (Table 3).
Table 2. Ejection Fraction between the Two Groups (Before and After the Study).
| Variables | Exercise training group | Control group | ||||
|---|---|---|---|---|---|---|
| Baseline | 6-MonthFollow-up | P value | Baseline | 6-MonthFollow-up | Р value | |
| LV-EDD, mL/m 2 | 65 ± 5 | 60 ± 5.2 | < 0.05 | 65 ± 4.7 | 66 ± 5.5 | > 0.05 |
| LV-ESD, mL/m 2 | 58 ± 5 | 54 ± 4.5 | < 0.05 | 57 ± 6 | 57 ± 5.7 | > 0.05 |
| LV-EDV, mL/m 2 | 224 ± 9 | 202 ± 8.5 | < 0.05 | 230 ± 7 | 228 ± 7.7 | > 0.05 |
| LV-ESV, mL/m 2 | 155 ± 6 | 127 ± 6.3 | < 0.05 | 157 ± 5 | 155 ± 7 | > 0.05 |
| LVEF, % | 32 ± 4 | 37 ± 5 | < 0.05 | 33 ± 5 | 31 ± 5 | < 0.05 |
Table 3. Different Dimensions of Life Quality in Patients with Congestive Heart Failure in Case and Control Groups at the Beginning of the Study a.
| Group | Control Group b | Experimental Group b | Р Value |
|---|---|---|---|
| Life quality (8 dimensions) | |||
| Physical performance | 55.08 ± 8.62 | 54.22 ± 10.87 | > 0.15 |
| Limited activity following mental problems | 56.84 ± 10.33 | 57.18 ± 12.39 | > 0.47 |
| Limited activity following physical problems | 55.14 ± 9.91 | 54.32 ± 12.41 | > 0.71 |
| Energy and fatigue | 55.43 ± 11.67 | 56.69 ± 13.62 | > 0.84 |
| Mental health | 62.9 ± 13.76 | 63.12 ± 16.83 | > 0.56 |
| Social performance | 67.82 ± 15.68 | 66.62 ± 19.71 | > 0.37 |
| Body pain | 72.8 ± 16.55 | 71.1 ± 17.47 | > 0.35 |
| Overall health | 64.85 ± 17 | 65.12 ± 15.93 | > 0.66 |
a Data are presented as Mean ± SD.
b No = 30
Independent t-test also showed that there was a significant difference in the physical performance, activity limitation following physical problem, energy and fatigue, social performance, physical pain, and public health at the end of the exercise program in the experimental group and 24 weeks after in the control group (Table 4). These results indicated that exercise program had a significant effect on promoting the patients’ quality of life score. The quality of life score was significantly different between before exercise program and at the end of the exercise program in the experimental group and 24 weeks after in the control group (Table 5).
Table 4. Different Dimensions of Life Quality in Patients with Congestive Heart Failure in Case and Control Groups After the Completion of Intervention a.
| Group | Control Group b | Experimental Group b | Р Value |
|---|---|---|---|
| Life quality (8 dimensions) | |||
| Physical performance | 46.12 ± 7.24 | 56.16 ± 12.19 | < 0.05 |
| Limited activity following mental problems | 66.43 ± 8.69 | 48.34 ± 12.27 | < 0.05 |
| Limited activity following physical problems | 59.32 ± 7.51 | 50.74 ± 11.65 | < 0.05 |
| Energy and fatigue | 52.12 ± 9.76 | 58.42 ± 13.06 | < 0.05 |
| Mental health | 59.76 ± 12.28 | 68.78 ± 16.29 | < 0.05 |
| Social performance | 59.9 ± 11.51 | 69.56 ± 18.74 | < 0.05 |
| Body pain | 74.43 ± 16.38 | 68.18 ± 17.96 | < 0.05 |
| Overall health | 57.92 ± 18.5 | 70.28 ± 19.33 | < 0.05 |
a Data are presented as Mean ± SD.
b No = 30
Table 5. Mean ± standard Deviation of Different Dimensions of Life Quality in Patients With Congestive Heart Failure in Case and Control Groups Prior to the Intervention and After the Completion of the Intervention a.
| Group | Control Group b | Experimental Group b | Р Value c |
|---|---|---|---|
| Prior to the intervention (at the beginning of the study) | 62.34 ± 11.25 | 61.01 ± 14.9 | > 0.61 |
| After completion of intervention | 58.43 ± 8.67 | 63.34 ± 12.69 | < 0.05 |
a Data are presented as Mean ± SD.
b No = 30
c Independent t-test indicates a statistically significant difference between case and control groups.
5. Discussion
The present study’s results showed that there was a significant change in left ventricular diameter and ejection fraction after exercise training in the experimental group. In a single-center study mortality decreased after exercise training (26) and some studies reported improved cardiac output response to exercise with no heightened pulmonary artery pressure (27, 28).
In a research, oxygen uptake increased by 26% at maximal exercise and 39% at the lactate threshold in the exercise group, whereas control values did not change (2). Also maximal oxygen uptake increased progressively after training. The increase in maximal oxygen uptake in the trained group paralleled an increase in maximal cardiac output but maximal cardiac output did not change in the control group (29).
In another study, the results showed that both resting and peak exercise pulmonary vascular resistance reduced after training therapy, indicating that the improvement in left ventricular systolic function may have led to a decreased preload and/or that an improvement of endothelial function may also have affected pulmonary resistance vessels (30).
Kitzman et al. found that an exercise program improved cardiac output but the response to the quality of life in individuals varied (31). Therefore, we require long term plans to determine the best response to increased ejection fraction. Why mild to moderate aerobic exercises lead to improved left ventricular ejection fraction and the general condition of patients with chronic heart failure, is not clearly known, but it could be argued that exercise leads to changes in lifestyle; increased mobility in patients and thus leads to higher peak VO2. On the other hand, exercise results in decrease in peripheral vascular resistance and thus increases blood flow in coronary vessels and reduces afterload.
In a study by van Tol et al. training sessions increased quality of life and ejection fraction (26). Exercise affects the endothelial membrane of peripheral vessels and therefore causes decreased resistance of peripheral vessels in relaxing and exercising. Therefore, it leads to decreased LV wall stress and afterload and increased cardiac output.
Also, in the present study the results showed a significant increase in quality of life especially in terms of physical performance, energy, social performance, and public health and a decrease in physical pain, activity limitation, physical problem, and fatigue at the end of the exercise program in the experimental group but not in the control group. Gary et al. obtained similar results, as well. They found that quality of life improved in the experimental group compared to the control group three months after the intervention but there was no significant difference in depression level at the end of the exercise program between the two groups (32).
In a study to determine the effect of a 12-week rehabilitation program on quality of life, aerobic ability, and the level of daily activity in the patients with heart failure, Collins et al. found a significant increase in physical performance, reduced sense of failure, and increased exercise tolerance in the experimental group. In addition, exercise had good outcome for the level of performance, ejection fraction, and the quality of life in these patients (33). Since exercise causes increased lung capacity, increased stroke volume, heart rate at rest and exercise, increased cardiac output, increased blood pressure and decreased diffusing capacity at rest and exercise, it leads to reduced anxiety, isolation, fatigue, and pain, enhances psychosocial function and improves overall health. Georgiou et al. found that exercise had a remarkable effect on reducing the cost of care and hospitalization, the referral to the physicians, and the length of survival (34). The study of Inglis et al. showed that exercise in patients with heart fibrillation decreased mortality rate, readmission, and days of hospitalization in the experimental group in comparison to the control group, but the level of anxiety and depression did not change significantly (35). Thus we can conclude that we need exercise programs, more care and intervention as well as specialized professionals in this field to enhance emotional, mental, physical, and social performance and public health and to decrease physical pain, activity limitation, and fatigue in heart patients. Exercise and lifestyle changes are the best tools for improving the ejection fraction and quality of life. We also must make use of the assistance of nurses for these patients’ better care.
Our findings show that quality of life is impaired in congestive heart failure patients. The results of this study indicate that exercise training in treating heart failure is important for increase in ejection fraction and improvement of the quality of life. Therefore, it is essential to address exercise, stress, excitement, and quality of life in these patients more seriously. In addition, dutiful and capable nurses can be helpful in this regard.
5.1. Limitations
Small sample size enrolled in this study limits the generalization of the study findings.
Acknowledgments
Hereby, we thank the Research and Technology Deputy of Shahrekord University of Medical Sciences.
Footnotes
Funding/Support:This study was supported by the Research and Technology Deputy of Shahrekord University of Medical Sciences.
References
- 1.Verdiani V, Ognibene A, Rutili MS, Lombardo C, Bacci F, Terreni A, et al. NT-ProBNP reduction percentage during hospital stay predicts long-term mortality and readmission in heart failure patients. J Cardiovasc Med (Hagerstown). 2008;9(7):694–9. doi: 10.2459/JCM.0b013e3282f447ae. [DOI] [PubMed] [Google Scholar]
- 2.Wisloff U, Stoylen A, Loennechen JP, Bruvold M, Rognmo O, Haram PM, et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation. 2007;115(24):3086–94. doi: 10.1161/CIRCULATIONAHA.106.675041. [DOI] [PubMed] [Google Scholar]
- 3.Hou N, Chui MA, Eckert GJ, Oldridge NB, Murray MD, Bennett SJ. Relationship of age and sex to health-related quality of life in patients with heart failure. Am J Crit Care. 2004;13(2):153–61. [PubMed] [Google Scholar]
- 4.van den Berg-Emons H, Bussmann J, Balk A, Keijzer-Oster D, Stam H. Level of activities associated with mobility during everyday life in patients with chronic congestive heart failure as measured with an "activity monitor". Phys Ther. 2001;81(9):1502–11. [PubMed] [Google Scholar]
- 5.O'Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301(14):1439–50. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carmona-Bernal C, Ruiz-Garcia A, Villa-Gil M, Sanchez-Armengol A, Quintana-Gallego E, Ortega-Ruiz F, et al. Quality of life in patients with congestive heart failure and central sleep apnea. Sleep Med. 2008;9(6):646–51. doi: 10.1016/j.sleep.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Lewis GD, Shah R, Shahzad K, Camuso JM, Pappagianopoulos PP, Hung J, et al. Sildenafil improves exercise capacity and quality of life in patients with systolic heart failure and secondary pulmonary hypertension. Circulation. 2007;116(14):1555–62. doi: 10.1161/CIRCULATIONAHA.107.716373. [DOI] [PubMed] [Google Scholar]
- 8.Juenger J, Schellberg D, Kraemer S, Haunstetter A, Zugck C, Herzog W, et al. Health related quality of life in patients with congestive heart failure: comparison with other chronic diseases and relation to functional variables. Heart. 2002;87(3):235–41. doi: 10.1136/heart.87.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carels RA. The association between disease severity, functional status, depression and daily quality of life in congestive heart failure patients. Qual Life Res. 2004;13(1):63–72. doi: 10.1023/B:QURE.0000015301.58054.51. [DOI] [PubMed] [Google Scholar]
- 10.Haworth JE, Moniz-Cook E, Clark AL, Wang M, Waddington R, Cleland JG. Prevalence and predictors of anxiety and depression in a sample of chronic heart failure patients with left ventricular systolic dysfunction. Eur J Heart Fail. 2005;7(5):803–8. doi: 10.1016/j.ejheart.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Edelmann F, Pieske B. [Exercise training in heart failure]. Herz. 2013;38(6):578–86. doi: 10.1007/s00059-013-3918-8. [DOI] [PubMed] [Google Scholar]
- 12.Dehkordi A, Heydarnejad MS, Fatehi D. Quality of Life in Cancer Patients undergoing Chemotherapy. Oman Med J. 2009;24(3):204–7. doi: 10.5001/omj.2009.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dehkordi AH, Heydarnejad MS. Factors-related to quality of life in post myocardial infarction patients. JHMU. 2009:7–10. [Google Scholar]
- 14.Heydarnejad S, Dehkordi AH. The effect of an exercise program on the health-quality of life in older adults. A randomized controlled trial. Dan Med Bull. 2010;57(1) [PubMed] [Google Scholar]
- 15.Hassanpour-Dehkordi A, Jivad N. Comparison of regular aerobic and yoga on the quality of life in patients with multiple sclerosis. Med J Islam Repub Iran. 2014;28:141. [PMC free article] [PubMed] [Google Scholar]
- 16.Heydarnejad MS, Hassanpour DA, Solati DK. Factors affecting quality of life in cancer patients undergoing chemotherapy. Afr Health Sci. 2011;11(2):266–70. [PMC free article] [PubMed] [Google Scholar]
- 17.Whellan DJ, O'Connor CM, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, et al. Heart failure and a controlled trial investigating outcomes of exercise training (HF-ACTION): design and rationale. Am Heart J. 2007;153(2):201–11. doi: 10.1016/j.ahj.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 18.Bangsbo J, Mohr M, Krustrup P. Physical and metabolic demands of training and match-play in the elite football player. J Sports Sci. 2006;24(7):665–74. doi: 10.1080/02640410500482529. [DOI] [PubMed] [Google Scholar]
- 19.Adsett J, Mullins R. Evidence Based Guidelines for Exercise and Chronic Heart Failure. Quenssland. 2010 [Google Scholar]
- 20.Haykowsky MJ, Liang Y, Pechter D, Jones LW, McAlister FA, Clark AM. A meta-analysis of the effect of exercise training on left ventricular remodeling in heart failure patients: the benefit depends on the type of training performed. J Am Coll Cardiol. 2007;49(24):2329–36. doi: 10.1016/j.jacc.2007.02.055. [DOI] [PubMed] [Google Scholar]
- 21.Davies EJ, Moxham T, Rees K, Singh S, Coats AJ, Ebrahim S, et al. Exercise training for systolic heart failure: Cochrane systematic review and meta-analysis. Eur J Heart Fail. 2010;12(7):706–15. doi: 10.1093/eurjhf/hfq056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dunlay SM, Gheorghiade M, Reid KJ, Allen LA, Chan PS, Hauptman PJ, et al. Critical elements of clinical follow-up after hospital discharge for heart failure: insights from the EVEREST trial. Eur J Heart Fail. 2010;12(4):367–74. doi: 10.1093/eurjhf/hfq019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hernandez AF, Greiner MA, Fonarow GC, Hammill BG, Heidenreich PA, Yancy CW, et al. Relationship between early physician follow-up and 30-day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303(17):1716–22. doi: 10.1001/jama.2010.533. [DOI] [PubMed] [Google Scholar]
- 24.Farzianpour F, Arab M, Hosseini SM, Pirozi B, Hosseini S. Evaluation of quality of life of the elderly population covered by healthcare centers of marivan and the influencing demographic and background factors in 2010. Iran Red Crescent Med J. 2012;14(11):695–6. doi: 10.5812/ircmj.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naderi N, Bakhshandeh H, Amin A, Taghavi S, Dadashi M, Maleki M. Development and Validation of the First Iranian Questionnaire to Assess Quality of Life in Patients With Heart Failure: IHF-QoL. Res Cardiovas Med. 2012;1(1):2–7. doi: 10.5812/cardiovascmed.4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Tol BA, Huijsmans RJ, Kroon DW, Schothorst M, Kwakkel G. Effects of exercise training on cardiac performance, exercise capacity and quality of life in patients with heart failure: a meta-analysis. Eur J Heart Fail. 2006;8(8):841–50. doi: 10.1016/j.ejheart.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 27.Giannuzzi P, Temporelli PL, Corra U, Tavazzi L, Elvd-Chf Study Group Antiremodeling effect of long-term exercise training in patients with stable chronic heart failure: results of the Exercise in Left Ventricular Dysfunction and Chronic Heart Failure (ELVD-CHF) Trial. Circulation. 2003;108(5):554–9. doi: 10.1161/01.CIR.0000081780.38477.FA. [DOI] [PubMed] [Google Scholar]
- 28.Hambrecht R, Niebauer J, Fiehn E, Kalberer B, Offner B, Hauer K, et al. Physical training in patients with stable chronic heart failure: effects on cardiorespiratory fitness and ultrastructural abnormalities of leg muscles. J Am Coll Cardiol. 1995;25(6):1239–49. doi: 10.1016/0735-1097(94)00568-B. [DOI] [PubMed] [Google Scholar]
- 29.Corvera-Tindel T, Doering LV, Woo MA, Khan S, Dracup K. Effects of a home walking exercise program on functional status and symptoms in heart failure. Am Heart J. 2004;147(2):339–46. doi: 10.1016/j.ahj.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Hambrecht R, Gielen S, Linke A, Fiehn E, Yu J, Walther C, et al. Effects of exercise training on left ventricular function and peripheral resistance in patients with chronic heart failure: A randomized trial. JAMA. 2000;283(23):3095–101. doi: 10.1001/jama.283.23.3095. [DOI] [PubMed] [Google Scholar]
- 31.Kitzman DW, Brubaker PH, Morgan TM, Stewart KP, Little WC. Exercise training in older patients with heart failure and preserved ejection fraction: a randomized, controlled, single-blind trial. Circ Heart Fail. 2010;3(6):659–67. doi: 10.1161/CIRCHEARTFAILURE.110.958785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gary RA, Sueta CA, Dougherty M, Rosenberg B, Cheek D, Preisser J, et al. Home-based exercise improves functional performance and quality of life in women with diastolic heart failure. Heart Lung. 2004;33(4):210–8. doi: 10.1016/j.hrtlng.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 33.Collins E, Langbein WE, Dilan-Koetje J, Bammert C, Hanson K, Reda D, et al. Effects of exercise training on aerobic capacity and quality of life in individuals with heart failure. Heart Lung. 2004;33(3):154–61. doi: 10.1016/j.hrtlng.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 34.Georgiou D, Chen Y, Appadoo S, Belardinelli R, Greene R, Parides MK, et al. Cost-effectiveness analysis of long-term moderate exercise training in chronic heart failure. Am J Cardiol. 2001;87(8):984–8. doi: 10.1016/s0002-9149(01)01434-5. [DOI] [PubMed] [Google Scholar]
- 35.Inglis S, McLennan S, Dawson A, Birchmore L, Horowitz JD, Wilkinson D, et al. A new solution for an old problem? Effects of a nurse-led, multidisciplinary, home-based intervention on readmission and mortality in patients with chronic atrial fibrillation. J Cardiovasc Nurs. 2004;19(2):118–27. doi: 10.1097/00005082-200403000-00006. [DOI] [PubMed] [Google Scholar]


