Abstract
Advances in chelation therapy and noninvasive monitoring of iron overload have resulted in substantial improvements in the survival of transfusion dependent patients with thalassemia major. Myocardial decompensation and sepsis remain the major causes of death. While endocrine abnormalities are a well-recognized problem in these iron-overloaded patients, adrenal insufficiency and its consequences are under-appreciated by the hematology community. The aims of this study were to determine the prevalence of adrenal insufficiency in thalassemia major subjects, to identify risk factors for adrenal insufficiency, and to localize the origin of the adrenal insufficiency within the hypothalamic-pituitary-adrenal axis. Eighteen subjects with thalassemia major (18.9 ± 9.3 years old, 7 female) were tested for adrenal insufficiency using a glucagon stimulation test (GST). Those found to have adrenal insufficiency (stimulated cortisol < 18 μg/dL) subsequently underwent an ovine corticotrophin-releasing hormone (oCRH) stimulation test to define the physiological basis for the adrenal insufficiency. The prevalence of adrenal insufficiency was 61%, with an increased prevalence in males over females (92% vs. 29%, p=0.049). Ten of 11 subjects who failed the GST subsequently demonstrated normal ACTH and cortisol responses to oCRH, indicating a possible hypothalamic origin to their adrenal insufficiency.
Keywords: thalassemia, iron overload, adrenal insufficiency
Introduction
Thalassemia is one of the most common genetic blood disorders worldwide. With recent improvements in medical therapy, patients with thalassemia major are living longer and, therefore, more likely to exhibit complications of chronic iron overload, including various endocrinopathies. Adrenal insufficiency, in particular, is important to identify because it is a potentially life-threatening co-morbidity for which preventative treatment is readily available. While severe day-to-day adrenal insufficiency is uncommon, adrenal insufficiency can play an important role in the event of acute cardiac decompensation, stress, or sepsis. Furthermore, screening for adrenal insufficiency is commonly overlooked by physicians who manage patients with thalassemia major.
Adrenal insufficiency reportedly affects between 13% and 46% of patients with thalassemia major 1-5. The prevalence appears to be increased in patients with greater transfusion burden, poor linear growth, and wasting 1,3. Adrenal insufficiency may also complicate the management of heart failure in thalassemia major patients who often require glucocorticoid support for hypotension 6. Thus, patients with thalassemia major and undiagnosed adrenal insufficiency may be at increased risk of significant morbidity or even mortality.
The pathophysiological basis of adrenal insufficiency in thalassemia major has not been well-defined, and there are currently no clear guidelines on how to diagnose adrenal insufficiency in patients with thalassemia major. Chronic transfusions produce iron overload in several organs 7. Deposition of iron in the pituitary gland leads to hypogonadotropic hypogonadism 8 and other manifestations of hypopituitarism, including growth hormone (GH) deficiency and central hypothyroidism 9. It would make sense, then, that pituitary iron deposition might reduce adrenocorticotropin hormone (ACTH) secretion producing secondary adrenal insufficiency.
However, the adrenal glands might also be directly affected by iron toxicity. Patients with thalassemia major have been shown to have higher baseline ACTH levels than do controls, suggesting primary impairment of adrenocortical function and decreased adrenocortical reserve at baseline 5,10. Other studies have shown relative preservation of the hypothalamic-pituitary-adrenal (HPA) axis in patients with thalassemia major, but evidence of decreased adrenal androgen production consistent with direct adrenal iron toxicity 11.
The administration of glucagon acutely stimulates ACTH and cortisol secretion in normal individuals 12, and therefore is a useful screening test to assess the integrity of the HPA axis. Once adrenal insufficiency has been established, the ovine CRH (oCRH) stimulation test is another endocrine function test that directly stimulates ACTH secretion from the pituitary gland, and subsequently cortisol secretion. A normal response does not rule out partial tertiary adrenal insufficiency, but an abnormal response can help to localize the defect 13,14.
Thus, the primary purpose of this study was to examine the function of the HPA axis in patients with thalassemia major by conducting dynamic testing to enable us to pin-point the origin of the dysfunction, leading to a better understanding of the pathophysiology of adrenal insufficiency in this population. Our secondary purpose was to identify risk factors that predict adrenal insufficiency in our population of patients with thalassemia major.
Materials and Methods
Patients
This prospective study was conducted at Children's Hospital Los Angeles, Los Angeles, California, USA between September 2010 and January 2012. The study was approved by the Institutional Review Board at Children's Hospital Los Angeles (CCI#: 10-00070). Informed consent was obtained from subjects ≥ 18 years of age or parents/legal guardians of subjects < 18 years of age. Assent was obtained from subjects < 18 years of age.
Nineteen subjects with thalassemia major (74% β0/β0; 16% E/β0; 10% HbH Constant Spring) were enrolled in the study, including one who withdrew prior to completing any study visits. All subjects required chronic transfusion therapy of 10-15 mL/kg every 2-4 weeks to maintain a pre-transfusion hemoglobin > 9.5 g/dL. All subjects were on appropriate chelation therapy as directed by their primary hematologist. Sixteen subjects were on deferasirox monotherapy (31.9 ± 13 mg/kg), and the remaining two were on combination therapy with deferasirox (36 ± 5.6 mg/kg), desferoxamine (64 ± 22.6 mg/kg) and deferiprone (68 mg/kg). Subjects were excluded if they were < 7 years of age, if they had been diagnosed with diabetes requiring treatment with insulin, or if they had been taking glucocorticoids on a daily basis or within one month of the study visits.
Data Collection
Demographic data included age, gender, height, weight, ethnicity, and number of years transfused. The five most recent ferritin levels (all drawn within 6 months of the study date) and MRI values for pituitary, liver, pancreatic, and cardiac iron were also recorded.
Glucagon Stimulation Test (GST)
All subjects underwent a GST as the initial test for adrenal insufficiency. Subjects were required to fast overnight for at least 8 hours. Baseline morning (8-9 AM) fasting levels of glucose, ACTH, cortisol, insulin, GH, plasma renin activity (PRA), dehydroepiandrosterone sulfate (DHEA-S), and aldosterone were measured, followed by injection of glucagon [GlucaGen, glucagon (rDNA origin), Bedford Laboratories, Bedford, OH, USA] at a dose of 0.1 mg/kg (maximum dose of 1 mg) subcutaneously at T=0 minutes. This was followed by blood sampling for measurements of glucose, ACTH, cortisol, and GH, at T = +90, +120, +180, and +240 minutes. A failed GST, suggesting adrenal insufficiency, was defined as all cortisol levels < 18 μg/dL. Previous studies have cited cut-offs ranging from < 14.5 μg/dL to < 21 μg/dL 15-18. We selected a cut-off of 18 μg/dL to allow for greater sensitivity.
Ovine corticotropin-releasing hormone (oCRH) Stimulation Test
Subjects who failed the GST underwent a second, confirmatory test for adrenal insufficiency, the oCRH stimulation test. This test was performed within 35 ± 27 days following the GST. Subjects were required to fast for at least 4 hours. For this test, baseline morning (8-9 AM) fasting levels of ACTH and cortisol were drawn, followed by injection of oCRH (Acthrel, corticorelin ovine triflutate, Ferring Pharmaceuticals, Inc., Suffern, NY, USA) at a dose of 1 μg/kg intravenously at T=0 minutes. This was followed by blood sampling for measurements of ACTH and cortisol at T = +15, +30, +45, +60, +75, and +90 minutes. A failed oCRH stimulation test (suggesting adrenal insufficiency) was defined as a stimulated cortisol level < 16.3 μg/dL 17.
MRI Methods
All MRI studies were performed on 1.5T General Electric CVi and Philips Achieva scanners. MRI methods for the liver, heart, and pancreas have been previously described 19-21. Organ iron burden was quantified from MRI scans done on the day of the GST (2/18 subjects), using linear interpolation of values from annual MRI scans done before and after the stimulation test (12/18 subjects), or from the most recent MRI study (246 ± 190 days prior to the study; 4/18 subjects). MRI methods for the pituitary have also been previously described 8. Pituitary iron measurements were unavailable for 2/18 patients. Pituitary iron burden was quantified from MRI scans using linear interpolation of values from MRI scans done before and after the stimulation test (7/18 subjects), or from the most recent MRI study (294 ± 230 days prior to the study; 9/18 subjects).
Laboratory Assays
All assays were performed at Quest Diagnostics Nichols Institute, San Juan Capistrano, CA, USA. Plasma glucose was measured by spectrophotometry (hexokinase). Plasma ACTH was measured by immunochemiluminometric assay (ICMA). Serum cortisol was measured by liquid chromatography-tandem mass spectrometry (LC/MS/MS). Serum insulin was measured following acid treatment and PEG precipitation by radioimmunoassay (RIA). Serum GH was measured by ICMA. PRA was measured by Angiotensin I generation RIA. DHEA-S was measured by ICMA. Serum aldosterone was measured by LC/MS/MS.
Statistical Analysis
All normally distributed results are expressed as mean ± SD. All non-normally distributed results are expressed as median; 5th and 95th percentiles. Comparisons between continuous variables of two normally distributed groups of data were addressed by linear regression. Comparisons between groups (adrenal insufficiency vs. no adrenal insufficiency) and continuous normally distributed variables (age, duration of chelation, ferritin levels, and MRI iron levels) were addressed by unpaired two-tailed T test. Fisher's exact test was used to compare adrenal insufficiency vs. no adrenal insufficiency across gender. Comparisons between groups (adrenal insufficiency vs. no adrenal insufficiency) and continuous non-normally distributed variables (GH) were addressed by non-parametric Wilcoxon test. Statistical analyses were performed using JMP statistical software version 5.1.2.
Results
Eighteen subjects completed the study. Subjects ranged from 7 to 43 years of age and the majority of patients were male and of Asian ethnicity (Table I). The initial study day was 13.8 ± 7.9 days following the most recent blood transfusion.
Table I. Baseline participant characteristics.
| n | 18 |
| Age (yrs) | 18.9 ± 9.3 |
| Female Sex, n (%) | 7 (39) |
| BMI SDS | -0.11 ± 1.11 |
| Years transfused | 16.4 ± 9.3 |
| Ethnicity | |
| Cambodian, n (%) | 1 (5.5) |
| Chinese, n (%) | 7 (39) |
| Filipino, n (%) | 2 (11) |
| Greek, n (%) | 1 (5.5) |
| Hispanic, n (%) | 1 (5.5) |
| Indian, n (%) | 3.5 (19) |
| Thai, n (%) | 1 (5.5) |
| Vietnamese, n (%) | 1.5 (8) |
| 8:00 AM cortisol (μg/dL) | 8.6 ± 3.6 |
| Ferritin (ng/mL) | 1409 ± 1408 |
Data are mean ± SD for normally distributed continuous variables or n (%) for categorical variables
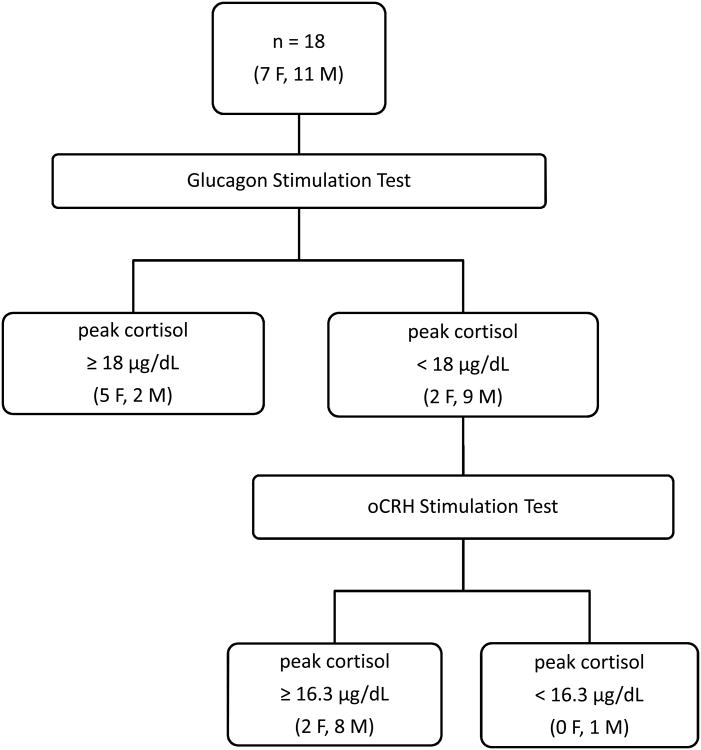
The mean basal morning cortisol level of this cohort was 8.6 ± 3.6 μg/dL (reference range: 5-21 μg/dL). Eleven subjects (61%) failed the GST, defined as a peak stimulated cortisol level < 18 μg/dL (Fig. 1). There were no significant correlations between peak stimulated cortisol level and basal morning cortisol level, age, peak GH level, or number of years transfused. There was no significant difference between subjects who passed and who failed the GST with regard to age, number of years transfused, peak GH level, or basal morning cortisol level. There was a trend toward lower ferritin levels in the group of subjects who failed the GST (p = 0.078), although this was not statistically significant (Table II). Analysis of MRI characteristics can be found in Table III. Patients who failed the GST had somewhat lower liver iron content (LIC) and smaller pituitary volume (p = 0.08 and 0.11, respectively) than those who passed.
Figure 1.
Diagram of 18 thalassemia major patients who underwent glucagon stimulation testing and 11 thalassemia major patients who underwent ovine Corticotrophin Releasing Hormone stimulation testing.
Table II. Glucagon stimulation test results of 18 subjects with thalassemia major.
| Adrenal Sufficiency | Adrenal Insufficiency | p | |
|---|---|---|---|
| n | 7 | 11 | n/a |
| Age (yrs) | 18.8 ± 7.8 | 19 ± 10.5 | 0.96 |
| Female Sex, n (%) | 5 (71) | 2 (18) | 0.049 |
| Transfusion history (yrs) | 15.1 ± 7.3 | 17.3 ± 10.6 | 0.6 |
| 8:00 AM cortisol (mcg/dL) | 9.6 ± 5.3 | 8.0 ± 2.0 | 0.48 |
| Peak cortisol (mcg/dL) | 22.1 ± 3.7 | 13.3 ± 2.6 | n/a |
| 8:00 AM ACTH (pg/mL) | 22 ± 11 | 25 ± 20 | 0.72 |
| Peak ACTH (pg/mL) | 97 ± 66 | 47 ± 50 | 0.12 |
| Peak GH (ng/mL) | 9.6 (5.5-34) | 7.8 (2.4-32) | 0.47 |
| Ferritin (ng/mL) | 2401 ± 1979 | 802 ± 500 | 0.078 |
| Days since last transfusion | 14 ± 10 | 13.6 ± 6.8 | 0.93 |
Data are mean ± SD for normally distributed continuous variables; median (10th and 90th percentiles) for non-normally distributed variables; or n (%) for categorical variables
Table III. MRI characteristics of 18 subjects who underwent GST.
| Adrenal Sufficiency | Adrenal Insufficiency | p | |
|---|---|---|---|
| n | 7 | 11 | n/a |
| Heart R2* | 46.2 ± 42.2 | 46.5 ± 29.0 | 0.98 |
| Pancreas R2* | 190 ± 186 | 109 ± 106 | 0.32 |
| LIC | 13 ± 11.9 | 3.6 ± 3.7 | 0.08 |
| Pituitary R2* Sagittal Z | 4.6 ± 3.1 | 3.8 ± 2.5 | 0.6 |
| Pituitary R2* Coronal Z | 4.9 ± 3 | 4 ± 3.7 | 0.59 |
| Pituitary Volume Z | 0.6 ± 1.5 | - 0.6 ± 1.1 | 0.11 |
Data are mean ± SD for normally distributed continuous variables, LIC = Liver iron content measured by MRI
There was a marked sex difference in peak cortisol levels following glucagon stimulation with males having a blunted response (14.5 ± 4.9 μg/dL in males vs. 20.1 ± 4.4 μg/dL in females, p = 0.02, T test). Nine of 11 (92%) male subjects demonstrated adrenal insufficiency compared with two of 7 (29%) female subjects (p = 0.049, Fisher exact test). Males had somewhat lower basal morning cortisol level (7.5 ± 1.9 μg/dL) than did females (10.4 ± 4.9 μg/dL), but this was not statistically significant (p = 0.18, T test). Males also had significantly lower body mass index standard deviation score (SDS) than did females (-0.57 ± 0.29 vs. 0.60 ± 0.37, p = 0.02, T test). There were no significant differences between males and females with regard to age, number of years transfused, peak GH level, ferritin level, or MRI iron measurements.
Adrenal mineralocorticoid and androgen levels were compared between the group with adrenal insufficiency and the group without adrenal insufficiency, but no significant differences were found. Age was used as an independent variable and compared to basal and stimulated cortisol levels, GH level, ferritin, and MRI iron measurements, but there were no significant correlations.
Of the 11 subjects who underwent subsequent oCRH stimulation testing, only one demonstrated an inadequate response to oCRH with a stimulated cortisol level < 16.3 μg/dL (Figure 1, Table IV). This subject (#8) had an elevated basal morning ACTH level of 62 pg/mL (reference range: 7-50 pg/mL), a peak cortisol level of 13.6 μg/dL, and an elevated peak ACTH level of 144 pg/mL, all suggesting primary adrenal insufficiency.
Table IV. Biochemical Profiles of Subjects who underwent oCRH testing.
| Subject | Sex | Age (yrs) | 8:00 AM Cortisol (μg/dL) | Peak oCRH Cortisol (μg/dL) | 8:00 AM ACTH (pg/mL) | Peak oCRH ACTH (pg/mL) |
|---|---|---|---|---|---|---|
| 1 | F | 43.7 | 11.6 | 20.2 | 23 | 85 |
| 2 | M | 9.4 | 12.2 | 21.2 | 17 | 46 |
| 3 | M | 12.3 | 12 | 22.5 | 38 | 115 |
| 4 | M | 10.4 | 7 | 20.7 | 13 | 76 |
| 5 | M | 21 | 10.5 | 21.3 | 28 | 94 |
| 6 | M | 29.1 | 14.2 | 23.1 | 15 | 39 |
| 7 | M | 16.8 | 9.4 | 22.5 | 33 | 128 |
| 8 | M | 15.1 | 8.9 | 13.6 | 62 | 144 |
| 9 | F | 18.9 | 3.5 | 20.4 | 11 | 164 |
| 10 | M | 8.6 | 3.7 | 27 | 12 | 106 |
| 11 | M | 25 | 3.5 | 24.2 | 15 | 116 |
Of the remaining 10 subjects who failed the GST and yet passed the oCRH stimulation test, the basal morning cortisol level was 8.7 ± 4.0 μg/dL and peak oCRH-stimulated cortisol level was 22.3 ± 2.1 μg/dL. The basal morning ACTH level was 20.5 ± 9.5 pg/mL and peak ACTH level was 96.9 ± 37.7 pg/mL. Three of the 10 subjects had markedly decreased basal morning cortisol levels < 4 μg/dL with correspondingly low-normal ACTH levels of ≤ 15 pg/mL.
Discussion
In our study cohort, the prevalence of adrenal insufficiency, defined by a failed GST, was 61%. This is relatively high compared with other studies 1,2,4,5,22, but may be due to a relatively conservative peak cortisol cut-off used (18 μg/dL), as well as a somewhat small study cohort. There have been various proposed cut-off levels to define adrenal insufficiency using the GST in non-thalassemia major populations. One study compared the GST, ITT, and CRH stimulation tests in children with short stature and found no intra-individual differences between peak cortisol levels for all three tests. A peak cortisol level of 16.3 μg/dL yielded 88.5% sensitivity and 86.8% specificity for the diagnosis of adrenal insufficiency 17. Another study compared the GST to the ITT in children with GH deficiency and also found similar peak cortisol levels in both tests. ROC analysis determined a cut-off of 14.6 μg/dL to have 66.67% sensitivity and 100% specificity for adrenal insufficiency 15. Yet another study compared the GST to ITT in 49 adults, who had undergone resection of hypothalamic or pituitary tumors, and found that a peak cortisol cut-off of 21.7 μg/dL following glucagon stimulation yielded 100% sensitivity and 32% specificity for the diagnosis of adrenal insufficiency 16. In our study, we selected a cut-off of 18 μg/dL to allow for greater sensitivity, since we were planning a confirmatory test for those who failed. In spite of this, if a lower cut-off (peak cortisol of 14.6 μg/dL) were used to define adrenal insufficiency, the prevalence of adrenal insufficiency in our population was still 44%.
Adrenal insufficiency was far more common in males; this has not been previously described. However, the literature has suggested that females with thalassemia major may experience a lower frequency of complications. Females with thalassemia major have better overall survival, which is not related to compliance 23. Females also have a lower incidence of heart failure despite similar levels of cardiac and liver iron compared to males 24. Males have been diagnosed with osteoporosis/osteopenia more frequently and more severely than females, even when correcting for their degree of hypogonadism 25. Proposed hypotheses for sex differences in thalassemia major include a protective role of estrogens, less oxidative damage in females, and greater resistance of females to the damaging effects of iron 24, though none of these hypotheses has been studied in thalassemia major patients.
We postulated that both hepatic and extrahepatic iron burdens would correlate with the risk of hormonal insufficiency, based on our observations in both the pituitary gland and pancreas 8,26. Previous studies have found greater transfusion burden and serum ferritin levels to be a risk factor for primary adrenal insufficiency 1. This was not the case in our study; rather, the mean ferritin and LIC in the subjects with adrenal insufficiency trended lower than those of the subjects without adrenal insufficiency. However, prior reports of adrenal insufficiency have come from cohorts with much poorer access to iron chelation than our study population, suggesting that chronic, severe iron overload is required to develop primary adrenal insufficiency.
Another possible explanation for our apparently discrepant observations is that adrenal insufficiency is multifactorial and can occur from derangements at multiple levels in the HPA axis. For example, the single patient we found with primary adrenal insufficiency had survived a failed allogeneic bone marrow transplantation 2.5 years prior to his study date; heavy corticosteroid use is a known risk factor for adrenal insufficiency27. Although pituitary iron was detectable in some of our patients, oCRH stimulation produced a satisfactory ACTH response in 10/11 subjects evaluated, suggesting that pituitary function was intact. Thus, the adrenal insufficiency described here represents a failure of glucagon to provoke its normal physiologic response. The mechanism by which glucagon stimulates the HPA axis has not been fully elucidated, but it is known that glucagon does not act directly on the pituitary corticotrophs to produce ACTH. Indirect mechanisms that have been proposed include promoting secretion of GH secretagogues that have ACTH-releasing activity 28, stimulating endogenous CRH and arginine vasopressin to mediate ACTH release 29, and releasing ACTH through peptides produced by glucagon degradation or enhanced catecholamine production 30. If, indeed, glucagon acts through CRH, then hypothalamic undersecretion of CRH (tertiary adrenal insufficiency) appears to be the dominant mechanism of adrenal insufficiency in our study population.
Since the hypothalamus is predominantly protected from iron overload by the blood-brain barrier 31, a diagnosis of tertiary adrenal insufficiency would explain why we observed no relationship between adrenal insufficiency and increased iron overload. Hypothalamic dysfunction has been proposed as the etiology of short stature in patients with thalassemia major 32,33, who were found to have decreased GH reserve and decreased nocturnal GH secretion with normal IGF-I generation by the liver. Support for hypothalamic dysfunction in our population was also evident by MRI. Subjects with adrenal insufficiency had pituitary volumes that were one standard deviation smaller than those of subjects without adrenal insufficiency, despite having similar pituitary iron levels. Chronically decreased secretion of releasing factors from the hypothalamus could lead to atrophy of the hormone-producing cells in the pituitary contributing to a smaller overall pituitary volume. Indeed, patients with pituitary stalk transection have been found to have pituitary gland hypoplasia on MRI 34. There is no clear explanation as to why these subjects would have impaired synthesis or secretion of hypothalamic hormones.
Individuals with thalassemia major have a number of chronic stressors that could potentially impair hypothalamic function. Chronic anemia forces them to maintain higher cardiac output to preserve oxygen delivery, increasing their basal metabolic rate 35; this could potentially impact the HPA axis similar to derangements in gonadal function observed in female endurance athletes 36. Despite regular transfusion therapy, patients with thalassemia major develop mild tissue hypoxia during the latter third of their transfusion cycle, and fluctuations in tissue oxygenation may potentially modulate hypothalamic function. Thalassemia major patients also experience chronic oxidative vascular stress from circulating labile iron species. Indeed, the pattern of iron chelation (hours per day that chelator is present) is very important to protect vascular endothelia from accelerated aging. Lastly, we cannot exclude the possibility of an iron chelator effect, especially since iron burdens trended lower in the patients with adrenal insufficiency. All of these patients were taking deferasirox, which has not been reported to cross the blood-brain barrier. Although adrenal insufficiency has never been described with iron chelators before, this question has never been systematically studied, either. Carefully designed prospective studies will be required to test any of these potential mechanisms.
Based on the results of this study, given the relatively high prevalence of biochemical adrenal insufficiency in our population, we would recommend annual screening of patients for adrenal insufficiency. With heart failure and infection as leading causes of morbidity and mortality in patients with thalassemia major 37, we would further consider administration of stress doses of glucocorticoids peri-operatively and during acute stressful situations with low or borderline low adrenal reserve. It remains to be seen whether this type and degree of adrenal insufficiency is clinically significant. It is possible that these patients exhibit an impaired HPA axis response to glucagon, but may be capable of mounting an appropriate response to physiological stress. Patients with thalassemia major have actually been found in prior studies to have normal cortisol responses to surgical stress when compared with controls 10. Further research is warranted to explore the underlying mechanisms and consequences before maintenance therapy can be recommended.
In conclusion, we found a prevalence of adrenal insufficiency of 61% in our population of patients with thalassemia major, with male patients predominantly affected. All but one of these patients had intact HPA function following administration of oCRH, implying a possible hypothalamic origin to their adrenal insufficiency. These findings are novel to the field, and further studies are necessary to explore these possibilities and to characterize HPA axis function in thalassemia major patients.
Acknowledgments
This research was supported in part by the NIH NCRR GCRC Grant M01 RR-46 and was performed at the GCRC at Children's Hospital Los Angeles, Los Angeles, CA, USA.
Funding/Support: NIH NCRR GCRC Grant M01 RR-46
Footnotes
Institution where study was conducted: Children's Hospital Los Angeles, 4650 Sunset Blvd, Los Angeles, CA, 90027
Disclosures: Thomas D. Coates has received Speakers Honoraria from Novartis and has served on Medical Advisory Boards for Apotex and Shire. Michell E. Geffner receives research funding from Pfizer, Eli Lilly, Novo Nordisk, Ipsen, and Versartis; is a member of the Steering Committee for a research study conducted by Daiichi Sankyo; receives consulting fees from Pfizer and Ipsen; and is the pediatric endocrinology editor for UpToDate. John C. Wood serves as a consultant for Shire and Biomed-Informatics and receives research funding from Shire. He also has served on Medical Advisory Boards for Apotex.The remaining authors have no conflicts of interest or funding to disclose.
References
- 1.Elsedfy HH, El KM, Tarif R, et al. Adrenal function in thalassemia major adolescents. Pediatr Endocrinol Rev. 2011;8(Suppl 2):295–299. [PubMed] [Google Scholar]
- 2.Jaruratanasirikul S, Tanchotikul S, Wongcharnchailert M, et al. A low dose adrenocorticotropin test (1 microg ACTH) for the evaluation of adrenal function in children with beta-thalassemia receiving hypertransfusion with suboptimal iron-chelating therapy. J Pediatr Endocrinol Metab. 2007;20:1183–1188. doi: 10.1515/jpem.2007.20.11.1183. [DOI] [PubMed] [Google Scholar]
- 3.Srivatsa A, Marwaha RK, Muraldharan R, Trehan A. Assessment of adrenal endocrine function in Asian thalassemics. Indian Pediatr. 2005;42:31–35. [PubMed] [Google Scholar]
- 4.Gulati R, Bhatia V, Agarwal SS. Early onset of endocrine abnormalities in beta-thalassemia major in a developing country. J Pediatr Endocrinol Metab. 2000;13:651–656. doi: 10.1515/jpem.2000.13.6.651. [DOI] [PubMed] [Google Scholar]
- 5.Scacchi M, Danesi L, Cattaneo A, et al. The pituitary-adrenal axis in adult thalassaemic patients. Eur J Endocrinol. 2010;162:43–48. doi: 10.1530/EJE-09-0646. [DOI] [PubMed] [Google Scholar]
- 6.Wood JC. Cardiac complications in thalassemia major. Hemoglobin. 2009;33(Suppl 1):S81–S86. doi: 10.3109/03630260903347526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Telfer P. Update on survival in thalassemia major. Hemoglobin. 2009;33(Suppl 1):S76–S80. doi: 10.3109/03630260903347336. [DOI] [PubMed] [Google Scholar]
- 8.Noetzli LJ, Panigrahy A, Mittelman SD, et al. Pituitary iron and volume predict hypogonadism in transfusional iron overload. Am J Hematol. 2011;87:167–171. doi: 10.1002/ajh.22247. [DOI] [PubMed] [Google Scholar]
- 9.Geffner ME, Karlsson H. Use of recombinant human growth hormone in children with thalassemia. Horm Res. 2009;71(Suppl 1):46–50. doi: 10.1159/000178037. [DOI] [PubMed] [Google Scholar]
- 10.Banani SA, Omrani GH. Cortisol and adrenocorticotropic hormone response to surgical stress (splenectomy) in thalassemic patients. Pediatr Surg Int. 2000;16:400–403. doi: 10.1007/s003830000401. [DOI] [PubMed] [Google Scholar]
- 11.Sklar CA, Lew LQ, Yoon DJ, David R. Adrenal function in thalassemia major following long-term treatment with multiple transfusions and chelation therapy. Evidence for dissociation of cortisol and adrenal androgen secretion. Am J Dis Child. 1987;141:327–330. doi: 10.1001/archpedi.1987.04460030105036. [DOI] [PubMed] [Google Scholar]
- 12.Rao RH, Spathis GS. Intramuscular glucagon as a provocative stimulus for the assessment of pituitary function: growth hormone and cortisol responses. Metabolism. 1987;36:658–663. doi: 10.1016/0026-0495(87)90150-8. [DOI] [PubMed] [Google Scholar]
- 13.Lytras N, Grossman A, Perry L, et al. Corticotrophin releasing factor: responses in normal subjects and patients with disorders of the hypothalamus and pituitary. Clin Endocrinol (Oxf) 1984;20:71–84. doi: 10.1111/j.1365-2265.1984.tb00061.x. [DOI] [PubMed] [Google Scholar]
- 14.Orth DN, Jackson RV, DeCherney GS, et al. Effect of synthetic ovine corticotropin-releasing factor. Dose response of plasma adrenocorticotropin and cortisol. J Clin Invest. 1983;71:587–595. doi: 10.1172/JCI110804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Iorgi N, Napoli F, Allegri A, et al. The accuracy of the glucagon test compared to the insulin tolerance test in the diagnosis of adrenal insufficiency in young children with growth hormone deficiency. J Clin Endocrinol Metab. 2010;95:2132–2139. doi: 10.1210/jc.2009-2697. [DOI] [PubMed] [Google Scholar]
- 16.Berg C, Meinel T, Lahner H, et al. Diagnostic utility of the glucagon stimulation test in comparison to the insulin tolerance test in patients following pituitary surgery. Eur J Endocrinol. 2010;162:477–482. doi: 10.1530/EJE-09-0824. [DOI] [PubMed] [Google Scholar]
- 17.Bottner A, Kratzsch J, Liebermann S, et al. Comparison of adrenal function tests in children--the glucagon stimulation test allows the simultaneous assessment of adrenal function and growth hormone response in children. J Pediatr Endocrinol Metab. 2005;18:433–442. doi: 10.1515/jpem.2005.18.5.433. [DOI] [PubMed] [Google Scholar]
- 18.De Sanctis V, Skordis N, Galati MC, et al. Growth hormone and adrenal response to intramuscular glucagon test and its relationship to IGF-1 production and left ventricular ejection fraction in adult B-thalassemia major patients. Pediatr Endocrinol Rev. 2011;8(Suppl 2):290–294. [PubMed] [Google Scholar]
- 19.Noetzli LJ, Papudesi J, Coates TD, Wood JC. Pancreatic iron loading predicts cardiac iron loading in thalassemia major. Blood. 2009;114:4021–4026. doi: 10.1182/blood-2009-06-225615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wood JC, Noetzli L. Cardiovascular MRI in thalassemia major. Ann N Y Acad Sci. 2010;1202:173–179. doi: 10.1111/j.1749-6632.2010.05571.x. [DOI] [PubMed] [Google Scholar]
- 21.Wood JC, Enriquez C, Ghugre N, et al. MRI R2 and R2* mapping accurately estimates hepatic iron concentration in transfusion-dependent thalassemia and sickle cell disease patients. Blood. 2005;106:1460–1465. doi: 10.1182/blood-2004-10-3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poomthavorn P, Isaradisaikul B, Chuansumrit A, et al. High prevalence of “biochemical” adrenal insufficiency in thalassemics: is it a matter of different testings or decreased cortisol binding globulin? J Clin Endocrinol Metab. 2010;95:4609–4615. doi: 10.1210/jc.2010-0205. [DOI] [PubMed] [Google Scholar]
- 23.Borgna-Pignatti C, Rugolotto S, De SP, et al. Survival and complications in patients with thalassemia major treated with transfusion and deferoxamine. Haematologica. 2004;89:1187–1193. [PubMed] [Google Scholar]
- 24.Marsella M, Pepe A, Borgna-Pignatti C. Better survival and less cardiac morbidity in female patients with thalassemia major: a review of the literature. Ann N Y Acad Sci. 2010;1202:129–133. doi: 10.1111/j.1749-6632.2010.05588.x. [DOI] [PubMed] [Google Scholar]
- 25.Kyriakou A, Savva SC, Savvides I, et al. Gender differences in the prevalence and severity of bone disease in thalassaemia. Pediatr Endocrinol Rev. 2008;6(Suppl 1):116–122. [PubMed] [Google Scholar]
- 26.Noetzli LJ, Mittelman SD, Watanabe RM, et al. Pancreatic iron and glucose dysregulation in thalassemia major. Am J Hematol. 2011;87:155–160. doi: 10.1002/ajh.22223. [DOI] [PubMed] [Google Scholar]
- 27.Tauchmanova L, Selleri C, Rosa GD, et al. High prevalence of endocrine dysfunction in long-term survivors after allogeneic bone marrow transplantation for hematologic diseases. Cancer. 2002;95:1076–1084. doi: 10.1002/cncr.10773. [DOI] [PubMed] [Google Scholar]
- 28.Arvat E, Maccagno B, Ramunni J, et al. Interaction between glucagon and hexarelin, a peptidyl GH secretagogue, on somatotroph and corticotroph secretion in humans. Eur J Endocrinol. 2000;143:601–606. doi: 10.1530/eje.0.1430601. [DOI] [PubMed] [Google Scholar]
- 29.Arvat E, Maccagno B, Ramunni J, et al. Interaction between glucagon and human corticotropin-releasing hormone or vasopressin on ACTH and cortisol secretion in humans. Eur J Radiol. 2000;143:99–104. doi: 10.1530/eje.0.1430099. [DOI] [PubMed] [Google Scholar]
- 30.Broglio F, Prodam F, Gottero C, et al. Ghrelin does not mediate the somatotroph and corticotroph responses to the stimulatory effect of glucagon or insulin-induced hypoglycaemia in humans. Clin Endocrinol (Oxf) 2004;60:699–704. doi: 10.1111/j.1365-2265.2004.02038.x. [DOI] [PubMed] [Google Scholar]
- 31.Moos T, Rosengren NT, Skjorringe T, Morgan EH. Iron trafficking inside the brain. J Neurochem. 2007;103:1730–1740. doi: 10.1111/j.1471-4159.2007.04976.x. [DOI] [PubMed] [Google Scholar]
- 32.Roth C, Pekrun A, Bartz M, et al. Short stature and failure of pubertal development in thalassaemia major: evidence for hypothalamic neurosecretory dysfunction of growth hormone secretion and defective pituitary gonadotropin secretion. Eur J Pediatr. 1997;156:777–783. doi: 10.1007/s004310050711. [DOI] [PubMed] [Google Scholar]
- 33.Cavallo L, Gurrado R, Gallo F, et al. Growth deficiency in polytransfused beta-thalassaemia patients is not growth hormone dependent. Clin Endocrinol (Oxf) 1997;46:701–706. doi: 10.1046/j.1365-2265.1997.1951005.x. [DOI] [PubMed] [Google Scholar]
- 34.Christophe C, Van VG, Dooms G, et al. Panhypopituitarism without diabetes insipidus: magnetic resonance imaging of pituitary stalk transection. Eur J Pediatr. 1990;149:235–236. doi: 10.1007/BF02106279. [DOI] [PubMed] [Google Scholar]
- 35.Vaisman N, Akivis A, Sthoeger D, et al. Resting energy expenditure in patients with thalassemia major. Am J Clin Nutr. 1995;61:582–584. doi: 10.1093/ajcn/61.3.582. [DOI] [PubMed] [Google Scholar]
- 36.Loucks AB, Mortola JF, Girton L, Yen SS. Alterations in the hypothalamic-pituitary-ovarian and the hypothalamic-pituitary-adrenal axes in athletic women. J Clin Endocrinol Metab. 1989;68:402–411. doi: 10.1210/jcem-68-2-402. [DOI] [PubMed] [Google Scholar]
- 37.Borgna-Pignatti MD, Cappellini MD, De SP, et al. Survival and complications in thalassemia. Ann N Y Acad Sci. 2005;1054:40–47. doi: 10.1196/annals.1345.006. [DOI] [PubMed] [Google Scholar]