Abstract
Low birth weight is linked to hypertension, chronic kidney disease and even end stage renal disease. We hypothesized that living kidney donors born with lower birth weight may be at increased risk of hypertension, albuminuria or reduced GFR beyond what is typical following uninephrectomy. 257 living kidney donors who donated at the University of Minnesota between 1967 and 2005 underwent iohexol GFR and urinary albumin excretion measurements. Predictors of iohexol GFR <60 ml/min/1.73m2, albuminuria and hypertension were examined using logistic regression. Predictors examined include age at GFR measurement, time since donation, BMI, gender, serum creatinine level (at donation and GFR measurement), systolic and diastolic blood pressure, race, and birth weight. The latter was obtained through self-report and verified through birth certificates and family members. Older age, higher BMI, and time from donation were associated with reduced GFR. Older age and higher BMI were also associated with hypertension. Birth weight was not associated with GFR <60 ml/min/1.73m2: OR=0.70, 95% CI (0.28, 1.74, p=0.45) or hypertension: OR=0.92, 95% CI (0.46, 1.84), p=0.82 but was associated with albuminuria: OR=0.37, 95% CI (0.15, 0.92), p=0.03. This data further strengthens the link between low birth weight and potential adverse renal outcomes.
Keywords: kidney, donor, outcomes, weight, albuminuria
Introduction
Several studies of living kidney donors revealed no decrease in life expectancy nor increased risk of developing end stage renal disease (ESRD) when compared to controls (1-10). Multiple studies have linked low birth weight (LBW) to late onset HTN and chronic kidney disease (CKD) (11-13). LBW, defined as ≤2.5 kg, is heavily embedded in epidemiological research as a surrogate marker for abnormalities in fetal programming (14). Low birth weight is an all-encompassing term for two sub categories: infants born prematurely (< 37 weeks gestation) but with a weight appropriate for gestational age (AGA) and infants born small for gestational age (termed intrauterine growth restriction (IUGR)) (12-14). Both animal models and human studies have shown that LBW is directly correlated with decreased nephron number, is inversely related to glomerular volume, and is associated with a greater risk for HTN, CKD, and ESRD (11-13, 15-27). These studies reinforce the Brenner hypothesis, which states that an early reduction in nephron number is followed by hyperfiltration of the remaining nephrons, accelerated renal decline and increased susceptibility to the development of HTN and ESRD (18). Whether the hyperfiltration engendered by the low number of nephrons coupled with the surgical reduction in renal mass conspire to promote the development of hypertension and reduced GFR in living kidney donors has not been studied. Considering the substantial link between LBW, HTN, and CKD, we hypothesized that living kidney donors with lower birth weight may be at increased risk of developing HTN, albuminuria, and reduced GFR beyond what is typically seen following uninephrectomy.
Methods
From June 1963 to December 2005, a total of 3,438 living donor nephrectomies were performed at the University of Minnesota. Our program requires potential donors to be free from diabetes, hypertension and have a GFR > 80 mL/min. Donors undergo a complete history, physical examination, visualization of the kidneys and vasculature and comprehensive laboratory assessment to rule out liver disease, active infections and systemic illnesses. No potential donor with any degree of albuminuria is accepted.
In December of 2003, we initiated a comprehensive effort to contact all those who donated since November 1963, consulting phone and internet directories and also asking their recipient. At the beginning of this effort, we generated donor lists of those known to be alive as of December 2003 and stratified them by gender and time from donation (in three year intervals). Within each stratum, 5% to 10% of donors were randomly selected to undergo GFR measurements. 80% of donors were successfully contacted and 257 underwent GFR measurement. If the selected donor refused participation, a new donor from the same strata was selected. We measured GFR using the plasma clearance of nonradioactive iohexol and urinary albumin excretion rate by first void urinary albumin creatinine ratio (UACR). Blood pressure was measured three times and their average was recorded. We defined hypertension by the donor's requirement for antihypertensive medications or by an average blood pressure > 140/90 mmHg in those not taking anti-hypertensives. Albuminuria is defined as > 30 mg/g Cr.
In the fall of 2012 all donors who previously underwent iohexol GFR measurement were contacted by telephone and asked to state their birth weight in pounds. Six months later a second survey was administered asking again for birth weight and gestational length (Appendix 1). Emphasis was placed on the importance of obtaining accurate information and donors were encouraged to consult birth records or family members to increase accuracy. Donors were also asked to document the source of the birth weight and gestational length to include one of three categories: validation from a birth record, verbal conveyance by a family member, or self-reported, i.e. without a source document or family verification. Of those who provided a birth weight in response to both surveys, the value from the more reliable source was used. Donors were encouraged to include a copy of their source document when returning the survey. All studies and procedures were approved by the institutional review board at the University of Minnesota and all participants provided written informed consent (UMN IRB #0301M39762).
Statistical Analysis
Continuous variables were compared using t-tests and categorical variables by chi-square or Fisher's tests. Logistic regression was performed to study the predictors of iohexol GFR <60 ml/min/1.73m2, albuminuria and hypertension. Predictors examined included age, systolic and diastolic blood pressure, serum creatinine (all at time of GFR measurement), iohexol GFR, years since donation, gender, and donor birth weight. Due to the low number of donors reporting a pre-term delivery, this variable was not incorporated into our set of predictors. Results are expressed as mean ± standard deviation (SD), unless otherwise specified. A p-value <0.05 was considered significant. All analyses were performed in SAS ver. 9.3 (Cary, NC).
Results
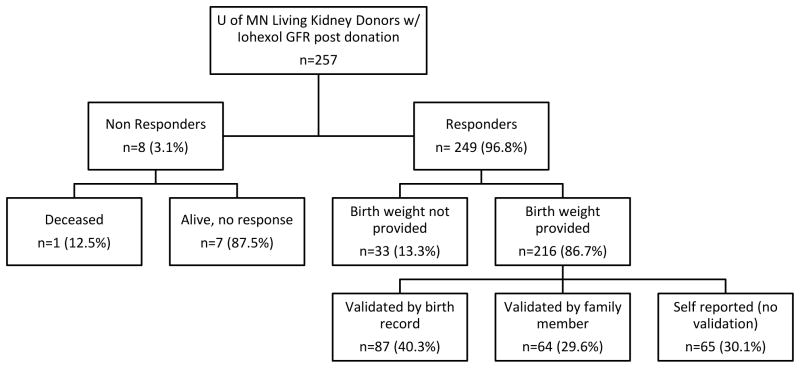
257 donors were studied in detail; 249 (97%) responded to the birth weight survey. Of the remaining eight, seven did not respond and one was deceased. Within this subset, we compared those who did (n=216) and did not (n=33) report a birth weight. Those reporting a birth weight were significantly younger at time of iohexol GFR measurement (52.2 (±9.6) vs. 57.8 (±10.0) years, p<0.01) (table 1). Otherwise, there was no difference between responders and non-responders.
Table 1. Donor characteristics.
| Birth Weight Reported | No Birth Weight Reported | P-value | |
|---|---|---|---|
| Demographics | |||
|
| |||
| n | 216 | 33 | |
| White | 213 (98.6%) | 32 (97.0%) | 0.44 |
| Female | 138 (63.9%) | 17 (51.5%) | 0.17 |
|
| |||
| At Donation | |||
|
| |||
| Age (years) | 40.6 (±11.2) | 43.6 (±10.2) | 0.14 |
| Serum creatinine (mg/dL) | 0.9 (±0.1) | 0.9 (±0.1) | 0.55 |
|
| |||
| At GFR Measurement | |||
|
| |||
| Age (years) | 52.2 (±9.6) | 57.8 (±10.0) | <0.01 |
| Time from donation (years) | 11.6 (±8.9) | 14.1 (±10.3) | 0.14 |
| BMI (kg/m2) | 28.0 (±4.9) | 28.1 (±4.8) | 0.86 |
| SBP (mmHg) | 121.2 (±14.0) | 126.0 (±17.1) | 0.08 |
| Hypertension | 50 (23.2%) | 11 (33.3%) | 0.21 |
| Diabetes mellitus | 7 (3.2%) | 1 (3.0%) | >0.99 |
| ACR (mg/g Cr) | 24 (±11.1) | 5 (±15.2) | 0.50 |
| Serum creatinine (mg/dL) | 1.0 (±0.2) | 0.9 (±0.2) | 0.73 |
| Iohexol GFR (ml/min/1.73m2) | 72.1 (±11.6) | 70.9 (±13.1) | 0.57 |
SBP: systolic blood pressure, DBP: diastolyic blood pressure, ACR: urine albumin to creatinine ratio
Of the 216 donors who reported birth weights, 15 described a low birth weight (≤2.5 kg). There were no differences between LBW and normal birth weight donors (table 2). Donors who reported birth weights on both surveys (n=168) provided similar values, with a mean of 3.4 (±0.6) kg at first survey and 3.4 (±0.5) kg at second survey. These two weights were also strongly correlated (r=0.89, p<0.01). In 84 cases the second birth weight was identical, 27 within 2 oz, and 57 were within 8 oz of the first. Of the 216 donors who provided a birth weight, 87 (40%) obtained this information from a birth record, 64 (30%) consulted a family member, and 65 (30%) reported it from memory without additional validation (figure 1). 229 (89%) donors responded to the gestational length survey, of which 186 provided an answer of either full term (n=177) or pre term (n=9) delivery. With respect to demographic variables, no significant differences existed between these two groups (table 3). Of those who provided a delivery term, 27 (15%) validated their answer on a birth record, 108 (58%) consulted a family member, and 51 (27%) reported it from memory.
Table 2. Characteristics of donors reporting birth weight.
| BW >2.5 Kg | BW ≤2.5 Kg | P-value | |
|---|---|---|---|
| Demographics | |||
|
| |||
| n | 201 | 15 | |
| White | 98.5% | 100.0% | >0.99 |
| Female | 62.7% | 80.0% | 0.18 |
|
| |||
| At Donation | |||
|
| |||
| Age (years) | 40.4 ± 11.3 | 42.9 ± 9.0 | 0.42 |
| Serum creatinine (mg/dL) | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.63 |
|
| |||
| At GFR Measurement | |||
|
| |||
| Age (years) | 52.2 ± 9.5 | 52.1 ± 10.6 | 0.97 |
| Time from donation (years) | 11.8 ± 8.9 | 9.2 ± 7.4 | 0.28 |
| BMI (kg/m2) | 28.0 ± 5.0 | 27.9 ± 3.8 | 0.99 |
| SBP (mmHg) | 121.6 ± 13.9 | 116.8 ± 14.6 | 0.20 |
| DBP (mmHg) | 72.9 ± 8.7 | 68.6 ± 8.1 | 0.07 |
| Hypertension | 22.9% | 26.7% | 0.75 |
| Diabetes mellitus | 3.5% | 0% | >0.99 |
| ACR (mg/g Cr) | 20 ± 10.0 | 4 ± 26.7 | 0.07 |
| Serum creatinine (mg/dL) | 1.0 ± 0.2 | 0.9 ± 0.1 | 0.38 |
| Iohexol GFR (ml/min/1.73m2) | 72.3 ± 11.6 | 69.6 ± 11.9 | 0.38 |
BW: birthweight, SBP: systolic blood pressure, DBP: diastolyic blood pressure, ACR: urine albumin to creatinine ratio
Figure 1. Birth weight survey response.
Table 3. Characteristics of donors reporting birth term.
| Full Term | Preterm | P-value | |
|---|---|---|---|
| Demographics | |||
|
| |||
| n | 177 | 9 | |
| White | 99.5% | 100.0% | >0.99 |
| Female | 64.4% | 77.8% | 0.41 |
|
| |||
| At Donation | |||
|
| |||
| Age (years) | 41.1 ± 11.3 | 36.2 ± 9.7 | 0.20 |
| Serum creatinine (mg/dL) | 0.9 ± 0.1 | 0.9 ± 0.2 | 0.46 |
|
| |||
| At GFR Measurement | |||
|
| |||
| Age (years) | 52.6 ± 9.4 | 51.3 ± 8.4 | 0.70 |
| Time from donation (years) | 11.4 ± 8.7 | 15.1 ± 12.8 | 0.23 |
| BMI (kg/m2) | 27.8 ± 4.7 | 28.9 ± 4.1 | 0.48 |
| SBP (mmHg) | 120.7 ± 14.0 | 119.3 ± 10.8 | 0.77 |
| DBP (mmHg) | 71.9 ± 8.1 | 72.3 ± 10.8 | 0.89 |
| Hypertension | 20.3% | 22.2% | >0.99 |
| Diabetes mellitus | 3.4% | 0% | >0.99 |
| ACR (mg/g Cr) | 161 ± 91.0 | 6 ± 66.7 | 0.051 |
| Serum creatinine (mg/dL) | 1.0 ± 0.2 | 0.9 ± 0.1 | 0.13 |
| Iohexol GFR (ml/min/1.73m2) | 71.3 ± 11.7 | 77.9 ± 13.2 | 0.10 |
SBP: systolic blood pressure, DBP: diastolyic blood pressure, ACR: urine albumin to creatinine ratio
Predictors of GFR <60 ml/min/1.73m2
Being older at time of GFR measurement: OR=1.15, 95% CI (1.08, 1.22), p<0.01, having an elevated BMI: OR=1.12, 95% CI (1.02, 1.23), p=0.02, and being female: OR=3.22, 95% CI (1.01, 10.30), p=0.049 were associated with an increased risk, while an increase in time from donation: OR=0.91, 95% CI (0.85, 0.97), p<0.01 was associated with a decreased risk. There was no significant effect of birth weight: OR=0.70, 95% CI (0.28, 1.74), p=0.45 (table 4).
Table 4. Risk of GFR<60, albuminuria and hypertension.
| Variable | GFR < 60 ml/min/1.73 m2 | Albuminuria | Hypertension | |||
|---|---|---|---|---|---|---|
|
| ||||||
| OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Age at GFR (years) | 1.15 (1.08, 1.22) | <0.01 | 1.00 (0.94, 1.07) | 0.99 | 1.08 (1.03, 1.13) | <0.01 |
| BMI (kg/m2) at GFR | 1.12 (1.02, 1.23) | 0.02 | 1.12 (1.02, 1.23) | 0.02 | 1.12 (1.04, 1.20) | <0.01 |
| Female | 3.22 (1.01, 10.30) | 0.049 | 0.48 (0.17, 1.39) | 0.18 | 1.20 (0.53, 2.75) | 0.66 |
| Birth Weight (kg) | 0.70 (0.28, 1.74) | 0.45 | 0.37 (0.15, 0.92) | 0.03 | 0.92 (0.46, 1.84) | 0.82 |
| Time from Donation (years) | 0.91 (0.85, 0.97) | <0.01 | 1.04 (0.99, 1.10) | 0.15 | 1.02 (0.98, 1.06) | 0.28 |
| Current Iohexol GFR (ml/min/1.73 m2) | NA | NA | 1.02 (0.97, 1.07) | 0.47 | 1.00 (0.97, 1.04) | 0.78 |
| SBP (mmHg) at GFR | 0.98 (0.94, 1.03) | 0.42 | 1.04 (0.99, 1.08) | 0.13 | 0.99 (0.96, 1.02) | 0.47 |
| DBP (mmHg) at GFR | 0.99 (0.93, 1.06) | 0.76 | 1.00 (0.93, 1.07) | 0.91 | 1.04 (0.98, 1.09) | 0.20 |
SBP: systolic blood pressure, DBP: diastolyic blood pressure, NA: Not applicable
Predictors of Albuminuria
An increase in BMI: OR=1.12, 95% CI (1.02, 1.23), p=0.02 was associated with an increased risk, while an increase in birth weight: OR=0.37, 95% CI (0.15, 0.92), p=0.03 was associated with a decreased risk (table 4).
Predictors of Hypertension
A higher age at GFR measurement: OR=1.08, 95% CI (1.03, 1.13), p<0.01 and increase in BMI: OR=1.12, 95% CI (1.04, 1.20), p<0.01 was associated with an increased risk. There was no significant effect of birth weight: OR=0.92, 95% CI (0.46, 1.84), p=0.82 (table 4).
Gestational Length and Outcomes
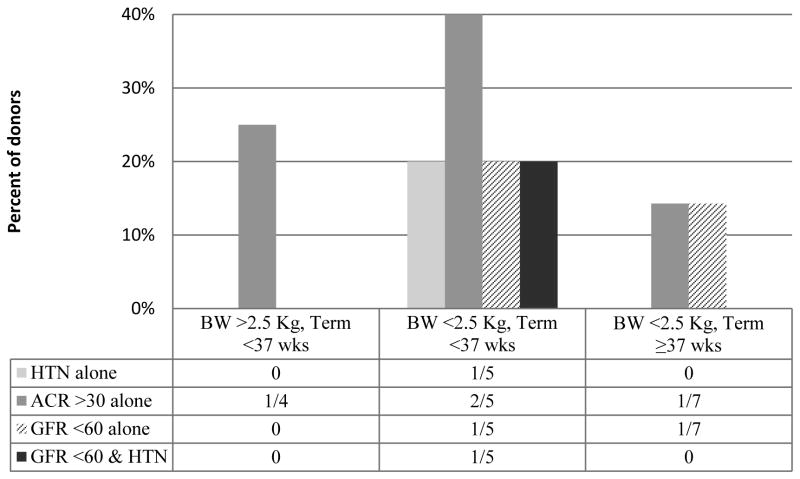
Due to the low number of donors meeting criteria for preterm and/ or low birth weight, further statistical analysis incorporating gestational length as a predictor for the risks of interest is not possible. Of the four donors reporting a birth weight > 2.5 kg and delivery at <37 weeks, one had albuminuria at time of GFR measurement and the remaining three neither had albuminuria, GFR <60, or HTN. Five donors were categorized as low birth weight and pre term: of these five, one met criteria for GFR<60, two had albuminuria, one had HTN, and one had both GFR<60 and HTN at the time of GFR measurement. Seven donors reported a low birth weight with full term delivery. Of this population, a single donor exhibited a GFR<60 without albuminuria or HTN and another had albuminuria alone (figure 2).
Figure 2. Donor outcome comparison of birth weight and gestational length.
Discussion
Overall, our results indicate an association between an individual's birth weight and risk of developing albuminuria following living kidney donation; with every kilogram increase in birth weight, the odds of developing albuminuria following donor nephrectomy decreased by 37%. However, birth weight was not a significant predictor of either GFR <60 ml/min/1.73m2 or HTN in our multivariate analysis. Not surprisingly, donors greater in age at the time of GFR measurement or who had an increase in BMI were at a higher risk for GFR <60 ml/min/1.73m2 and HTN. Women were at an increased risk of GFR <60 ml/min/1.73m2, though there was no significant association between gender and the outcomes of albuminuria or HTN. Interestingly, an increase in time since donation significantly decreased a donor's risk of having a GFR <60 ml/min/1.73m2 though there was no observed impact on albuminuria or HTN.
Previous studies in non-donor populations agree with our finding of low birth weight as a risk factor for albuminuria. However, these studies have also overwhelmingly shown an association between low birth weight, HTN, and CKD (11-13, 15-27). In 2012, Mu et al. performed meta-analysis on 27 studies reporting on the association between birth weight and blood pressure and revealed a 21% increased risk of developing HTN for individuals born <2.5 kg (19). Further meta-analysis by White et al. encompassed 31 studies involving >49,000 subjects from case controls and a single record linkage study of >2,000,000 individuals all documenting birth weight and kidney function at greater than 12 months of age. LBW individuals were at greater risk of developing CKD (OR=1.74), albuminuria (OR=1.81), ESRD (OR=1.58), and eGFR <60 mL/min/1.73 m2 (OR=1.79) (20). Birth weight may be a useful indicator of underlying errors in fetal programming and for each of these errors there is a vast array of phenotypic response. By nature of the extensive pre donation work-up, those accepted are overall incredibly healthy and in this study low birth weight donors were characteristically indiscernible from normal birth weight donors at time of donation. Thus, it is not surprising that our results do not match previous studies on non-donor low birth weight individuals.
In our study, we were unable to estimate prematurity associated risks, although all five donors born prematurely with low birth weight exhibited some combination of GFR<60, HTN, and/ or albuminuria, compared to 1/4 normal birth weight pre term donors, and 2/7 low birth weight full term donors. Overlap between IUGR, low birth weight, and prematurity make it difficult to surmise whether prematurity alone results in lasting negative renal deficits and disease. One such study lending credence to the impact of prematurity, employed ultrasound at 20 years of age to measure kidney length and volume of 51 individuals born <32 weeks gestation compared to full term controls. Findings included significantly smaller kidney size, particularly in women, regardless of IUGR, suggesting kidney growth inhibition as a consequence of preterm birth (28). Fitting to our results, other studies have argued for an amalgamation of IUGR and prematurity including long lasting reduction in kidney volume, increased risk of HTN, albuminuria, and decreased GFR (29-34).
Albuminuria >30 mg/g Cr is associated with an individual's ten year risk for ESRD and is further exacerbated in the context of a lower GFR (35). In this study, every increase of one kg in birth weight was associated with a decreased risk for albuminuria. Reassuringly, there was no association between low birth weight and risk for HTN or GFR <60 ml/min/1.73 m2. It should be noted the mean duration from donation to GFR measurement was 11.6 years and it is entirely possible the remaining kidney is able to compensate at this time. However, as indicated by the presence of albuminuria, hyperfiltration may be occurring which might ultimately lead to accelerated nephron loss and chronic kidney disease beyond what the individual would have experienced in the setting of two kidneys. This is far from proven and we intend to continue this study in the future to further monitor the outcomes of living kidney donors.
A potential limitation of this study is its retrospective nature and probability for recall bias. Previous studies ascertaining the validity of birth weight strongly cautioned against the self-reported birth weight in place of official records (36-39). In the absence of documentation, mothers are a crucial component in relaying birth information. One study assessing the accuracy of mothers' recall at one year post-delivery found 91% agreement with the weight listed on their child's birth certificate and a mean absolute difference < 1 oz (36, 38). To ascertain the accuracy of our data: donors were approached twice asking for their birth weight with an interlude > 6 months and those who responded to both surveys provided birth weights that were highly correlated. Emphasis was placed on consulting birth records followed by family members and 70% of the reported birth weights came from one of the aforementioned sources.
A second limitation is the overwhelming majority of white donors at our center; only 4/257 donors who previously underwent iohexol GFR measurement were non-white and 3/4 provided a birth weight. In the general population, African Americans are at greater risk for CKD and disease progression is more rapid among African Americans, Hispanics, and American Indians, furthered by low birth weight (26, 40). Decreased nephron number may be one cause for this disparity (26). African American women are also more likely to have a pre-term birth outcome (41). All of our non-white donors reported a birth weight >2.5 kg and none exhibited HTN, albuminuria, or GFR <60 at time of GFR measurement. While the apparent small size is an added limitation, the fact that these donors were randomly selected and are indeed representative of the larger group is somewhat reassuring.
In summary, our study highlights the possibility that birth weight may be associated with albuminuria and questioning donors about their birth weight, preferably from official records, should be considered as a potential modifier of long term risk.
Supplementary Material
Acknowledgments
Funding for this research provided by the National Institutes of Health as part of the PPG “Studies of Organ Transplantation in Animals and Men.”
Footnotes
Requests for offprints: Hassan N. Ibrahim, MD, Director, Division of Renal Diseases and Hypertension, University of Minnesota, 717 Delaware St. SE, Suite 353, MDC 1932, Minneapolis, MN 55414
Disclosure: The authors of this manuscript have no conflicts of interest to disclose.
Author Contributions: Berglund D: Data collection, drafting article, approval of article
MacDonald D: Data collection, drafting article, approval of article
Jackson S: Statistics, critical revision of article, approval of article
Spong R: Concept/ design, critical revision of article, approval of article
Issa N: Concept/ design, critical revision of article, approval of article
Kukla A: Concept/ design, critical revision of article, approval of article
Matas AJ: Concept/ design, critical revision of article, approval of article
Ibrahim HN: Concept/ design, critical revision of article, approval of article
References
- 1.Morgan BR, Ibrahim HN. Long-term outcomes of kidney donors. Curr Opin Nephrol Hypertens. 2011;20:605–609. doi: 10.1097/MNH.0b013e32834bd72b. [DOI] [PubMed] [Google Scholar]
- 2.Ibrahim H, Foley R, Tan L, Rogers T, Bailey RF, Guo H, et al. Long-term consequences of kidney donation. N Engl J Med. 2009;360:459–469. doi: 10.1056/NEJMoa0804883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Segev DL, Muzaale AD, Caffo BS, Mehta SH, Singer AL, Taranto SE, McBride MA, Montgomery RA. Perioperative mortality and long-term survival following live kidney donation. JAMA. 2010;303:959–966. doi: 10.1001/jama.2010.237. [DOI] [PubMed] [Google Scholar]
- 4.Najarian JS, Chavers BM, McHugh LE, Matas AJ. 20 years or more of follow-up of living kidney donors. Lancet. 1992;340:807–810. doi: 10.1016/0140-6736(92)92683-7. [DOI] [PubMed] [Google Scholar]
- 5.Kasiske BL, Ma JZ, Louis TA, Swan SK. Long-term effects of reduced renal mass in humans. Kidney Int. 1995;48:814–819. doi: 10.1038/ki.1995.355. [DOI] [PubMed] [Google Scholar]
- 6.Fehrman-Ekholm I, Elinder CG, Stenbeck M, Tyden G, Groth CG. Kidney donors live longer. Transplantation. 1997;64:976–978. doi: 10.1097/00007890-199710150-00007. [DOI] [PubMed] [Google Scholar]
- 7.Fehrman-Ekholm I, Duner F, Brink B, Tyden G, Elinder CG. No evidence of accelerated loss of kidney function in living kidney donors: results from a cross-sectional follow-up. Transplantation. 2001;72:444–449. doi: 10.1097/00007890-200108150-00015. [DOI] [PubMed] [Google Scholar]
- 8.Fehrman-Ekholm I, Norden G, Lennerling A, Rizell M, Mjornstedt L, Wramner L, Olausson M. Incidence of end-stage renal disease among live kidney donors. Transplantation. 2006;82:1646–1648. doi: 10.1097/01.tp.0000250728.73268.e3. [DOI] [PubMed] [Google Scholar]
- 9.Okamoto M, Akioka K, Nobori S, Ushigome H, Kozaki K, Kaihara S, Yoshimura N. Short- and long-term donor outcomes after kidney donation: Analysis of 601 cases over a 35-year period at Japanese single center. Transplantation. 2009;87:419–423. doi: 10.1097/TP.0b013e318192dc95. [DOI] [PubMed] [Google Scholar]
- 10.Fournier C, Pallet N, Cherqaoui Z, Pucheu S, Kreis H, Mejean A, Timsit MO, Landais P, Legendre C. Very long-term follow-up of living kidney donors. Transpl Int. 2012;25:385–390. doi: 10.1111/j.1432-2277.2012.01439.x. [DOI] [PubMed] [Google Scholar]
- 11.Luyckx V, Brenner B. Low birth weight, nephron number, and kidney disease. Kidney Int. 2005;68:S68–S77. doi: 10.1111/j.1523-1755.2005.09712.x. [DOI] [PubMed] [Google Scholar]
- 12.Chong E, Yosypiv I. Developmental Programming of Hypertension and Kidney Disease. International Journal of Nephrology. 2012 Nov 28; doi: 10.1155/2012/760580. Online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zohdi V, Sutherland MR, Lim K, Gubhaju L, Zimanyi M, Black MJ. Review Article: Low birth weight due to intrauterine growth restriction and/ or pre term birth: effects on nephron number and long-term renal health. International Journal of Nephrology. 2012 Aug 27; doi: 10.1155/2012/136942. Online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilcox AJ. On the importance-and the unimportance- of birthweight. Int J Epidemiol. 2001;30:1233–1241. doi: 10.1093/ije/30.6.1233. [DOI] [PubMed] [Google Scholar]
- 15.Manalich R, Reyes L, Herrera M, Melendi C, Fundora I. Relationship between weight at birth and the number and size of renal glomeruli in humans: a histomorphometric study. Kidney Int. 2000;58:770–773. doi: 10.1046/j.1523-1755.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- 16.Vehaskari V, Aviles D, Manning J. Prenatal programming of adult hypertension in the rate. Kidney Int. 2001;59:238–245. doi: 10.1046/j.1523-1755.2001.00484.x. [DOI] [PubMed] [Google Scholar]
- 17.Celsi G, Kistner A, Aizman R, Eklof AC, Ceccatelli S, de Santiago A, et al. Prenatal dexamethasone causes oligonephronia, sodium retention, and higher blood pressure in the offspring. Pediatr Res. 1998;44:317–322. doi: 10.1203/00006450-199809000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Brenner BM, Garcia DL, Anderson S. Glomeruli and blood pressure. Less of one, more the other? Am J Hypertens. 1988;1:335–347. doi: 10.1093/ajh/1.4.335. [DOI] [PubMed] [Google Scholar]
- 19.Mu M, Wang SF, Sheng J, Zhao Y, Li HZ, Hu CL, et al. Birth weight and subsequent blood pressure: a meta-analysis. Archive of Cardiovascular Disease. 2012;2:99–113. doi: 10.1016/j.acvd.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 20.White SI, Perkovic V, Cass A, Chang CL, Poulter NR, Spector T, et al. Is low birth weight an antecedent of CKD later in life? A systematic review of observational studies. Am J Kidney Dis. 2009;54:248–261. doi: 10.1053/j.ajkd.2008.12.042. [DOI] [PubMed] [Google Scholar]
- 21.Basioti M, Giapros V, Kostoula A, Cholevas V, Andronikou S. Growth restriction at birth and kidney function during childhood. Am J Kidney Dis. 2009;54:850–585. doi: 10.1053/j.ajkd.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 22.Hinchliffe SA, Lynch MRJ, Sargent PH, Howard CV, Van Velzen D. The effect of intrauterine growth retardation on the development of renal nephrons. Br J Obstet Gynaecol. 1992;99:296–301. doi: 10.1111/j.1471-0528.1992.tb13726.x. [DOI] [PubMed] [Google Scholar]
- 23.Leeson CPM, Kattenhorn M, Morley R, Lucas A, Deanfield JE. Impact of low birth weight and cardiovascular risk factors on endothelial function in early adult life. Circulation. 2001;103:1264–1268. doi: 10.1161/01.cir.103.9.1264. [DOI] [PubMed] [Google Scholar]
- 24.Goodfellow J, Bellamy MF, Gorman ST, Brownlee M, Ramsey MW, Lewis MJ, et al. Endothelial function is impaired in fit young adults of low birth weight. Cardiovasc Res. 1998;40:600–606. doi: 10.1016/s0008-6363(98)00197-7. [DOI] [PubMed] [Google Scholar]
- 25.Huxley RR, Shiell AW, Law CM. The role of size at birth and postnatal catch-up growth in determining systolic blood pressure: a systematic review of the literature. J Hypertens. 2000;18:815–831. doi: 10.1097/00004872-200018070-00002. [DOI] [PubMed] [Google Scholar]
- 26.Luyckx V, Brenner B. Nephron Endowment. In: Taal MW, Chertow GM, Marsden PA, Skorecki K, Yu ASL, Brenner B, editors. Brenner and Rector's The Kidney. Philadelphia, PA: Elsevier Saunders; 2012. pp. 782–803. [Google Scholar]
- 27.Viske BE, Irgens LM, Leivestad T, Hallan S, Iversen BM. Low birth weight increases risk for end-stage renal disease. J Am Soc Nephrol. 2008;19:151–157. doi: 10.1681/ASN.2007020252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keijzer-Veen MG, Devos AS, Meradji M, Dekker FW, Nauta J, van der Heijden BJ. Reduced renal length and volume 20 years after very preterm birth. Pediatr Nephrol. 2010;25:499–507. doi: 10.1007/s00467-009-1371-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drougia A, Giapros V, Hotoura E, Papadopoulou F, Argyropoulou M, Andronikou S. The effects of gestational age and growth restriction on compensatory kidney growth. Nephrol Dial Transplant. 2009;24:142–148. doi: 10.1093/ndt/gfn431. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt IM, Chellakooty M, Boisen KA, Damgaard IN, Mau Kai C, Olgaard K, et al. Impaired kidney growth in low-birth-weight children: distinct effects of maturity and weight for gestational age. Kidney Int. 2005;68:731–740. doi: 10.1111/j.1523-1755.2005.00451.x. [DOI] [PubMed] [Google Scholar]
- 31.Keijzer-Veen MG, Finken MJJ, Nauta J, Dekker FW, Hille ET, Frolich M, et al. Is blood pressure increased 19 years after intrauterine growth restriction and preterm birth? A prospective follow-up study in the Netherlands. Pediatrics. 2005;116:725–731. doi: 10.1542/peds.2005-0309. [DOI] [PubMed] [Google Scholar]
- 32.Leon DA, Johansson M, Rasmussen F. Gestational age and growth rate of fetal mass are inversely associated with systolic blood pressure in young adults: an epidemiologic study of 165,136 Swedish men aged 18 years”. Am J Epidemiol. 2000;152:597–604. doi: 10.1093/aje/152.7.597. [DOI] [PubMed] [Google Scholar]
- 33.Keijzer-Veen MG, Schrevel M, Finken MJJ, Dekker FW, Nauta J, Hille ET, et al. Microalbuminuria and lower glomerular filtration rate at young adult age in subjects born very premature and after intrauterine growth retardation. J Am Soc Nephrol. 2005;16:2762–2768. doi: 10.1681/ASN.2004090783. [DOI] [PubMed] [Google Scholar]
- 34.Bacchetta J, Harambat J, Dubourg L, Guy B, Liutkus A, Canterino I, et al. Both extrauterine and intrauterine growth restriction impair renal function in children born very preterm. Kidney Int. 2009;76:445–452. doi: 10.1038/ki.2009.201. [DOI] [PubMed] [Google Scholar]
- 35.Hallan SI, Ritz E, Lydersen S, Romundstad S, Kvenild K, Orth SR. Combining GFR and albuminuria to classify CKD improves prediction of ESRD. J Am Soc Nephrol. 2009;20:1069–1077. doi: 10.1681/ASN.2008070730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tehranifar P, Liao Y, Flom J, Terry MB. Validity of self-reported birth weight by adult women: sociodemographic influences and implications for life-course studies. Am J Epidemiol. 2009;170:910–917. doi: 10.1093/aje/kwp205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kemp M, Gunnell D, Maynard M, Smith GD, Frankel S. How accurate is self-reported birth weight among the elderly? J Epidemiol Community Health. 2000;54:639–640. doi: 10.1136/jech.54.8.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Little RE. Birthweight and gestational age: mothers' estimates compared with state and hospital records. Am J Public Health. 1986;76(11):1350–1351. doi: 10.2105/ajph.76.11.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andersson SW, Niklasson A, Lapidus L, Hallberg L, Bengtsson C, Hulthen Poor agreement between self-reported birth weight and birth weight from original records in adult women. Am J Epidemiol. 2000;152(7):609–616. doi: 10.1093/aje/152.7.609. [DOI] [PubMed] [Google Scholar]
- 40.Anderson AH, Berns JS, Bleicher MB, Feldman HI. Demographics of Kidney Disease. In: Taal MW, Chertow GM, Marsden PA, Skorecki K, Yu ASL, Brenner B, editors. Brenner and Rector's The Kidney. Philadelphia, PA: Elsevier Saunders; 2012. pp. 742–752. [Google Scholar]
- 41.Orr ST, Blackmore-Prince C, James SA, Griffin JM, Raghunathan T. Race, clinical factors and pre-term birth in a low-income urban setting. Ethn Dis. 2000;10(3):411–417. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.