Abstract
Objective
To determine visceral adiposity (VAT), subcutaneous adiposity (SAT) and regional body adipose differences between HIV-infected and non-HIV-infected subjects in relation to body-mass index (BMI) and World Health Organization (WHO) BMI categories.
Design, Setting, and Participants
Analysis of 306 HIV-infected and 107 community-derived, HIV-negative subjects evaluated for metabolic studies between 1999–2006. Analyses were stratified by gender. Additional analyses were performed stratifying subjects by metabolic syndrome status.
Results
HIV-infected men and women demonstrated decreased total extremity fat by 1.1 kg and 0.85 kg, respectively, relative to non-HIV-infected control subjects. VAT was increased among HIV-infected men and women in the normal (18.5 – 24.9 kg/m2) and overweight categories (25.0–29.9 kg/m2) relative to control subjects, but not among those in the obese category (≥ 30.0 kg/m2). In contrast, abdominal SAT was reduced among HIV-infected men in normal and overweight categories, but similar among HIV-infected women and control subjects in these categories. Abdominal SAT was increased among HIV-infected women in the obese category relative to control subjects. Similar results were obtained limiting the analysis to HIV (n=204) and control subjects (n=89) without the metabolic syndrome.
Conclusions
Peripheral lipoatrophy is a consistent finding among HIV-infected men and women with metabolic abnormalities. Relative increases in VAT are most pronounced among male and female HIV-infected subjects in the normal-weight and overweight categories. Gender differences in abdominal SAT accumulation are observed, with preservation of SAT among HIV-infected women relative to control subjects.
Keywords: BMI, VAT, body composition, HIV
INTRODUCTION
The use of highly active antiretroviral therapy (HAART) has been associated with the development of metabolic complications including dyslipidemia, insulin resistance, and altered body fat distribution.1–6 Changes in adipose distribution include peripheral lipoatrophy of face and limbs and/or central lipohypertrophy in the dorsocervical, breast, and abdominal regions.7–10 More importantly, since visceral fat accumulation has been linked to the development of cardiovascular disease and type II diabetes in the non-HIV-infected population, visceral adipose gain associated with HAART has raised concerns regarding long-term risk for cardiovascular disease in the HIV-infected population.11–13
Several studies have investigated changes in body composition that occur in HIV-infected individuals. The FRAM (Fat Redistribution and Metabolic Change in HIV Infection) Study compared fat distribution between HIV-infected individuals and HIV-negative controls and revealed a greater degree of fat loss in peripheral and most central depots among HIV-infected men and women when compared to controls.14, 15 Importantly, FRAM along with several recent studies have shown that peripheral lipoatrophy is not linked to central lipohypertrophy in the majority of HIV-infected individuals.6, 14–16 However, weight itself may influence the amount of adipose tissue present, and there may be differences in VAT (visceral adipose tissue), SAT (subcutaneous adipose tissue), and regional body adipose measurements within specific anthropometric categories. To our knowledge, differences in body composition between HIV-infected individuals and HIV-negative controls stratified by standard anthropometric cut-points for body-mass index (BMI) have not been studied. We therefore sought to examine the relationship between VAT, SAT or regional body adipose measurements and BMI among HIV-infected and non-HIV-infected subjects and determine how these relationships differed between these groups of subjects within the World Health Organization (WHO) body-mass index (BMI) categories of normal-weight, overweight, and obese.17
METHODS
PATIENTS AND CONTROLS
Data on body composition parameters were prospectively collected from 1999–2006 in 306 HIV-infected subjects participating in metabolic studies at the Massachusetts General Hospital (MGH) 18–27 and 107 HIV-negative subjects simultaneously recruited from the community as controls for the HIV studies 20–22, 26, 27 and as participants for metabolic studies in non-HIV-infected individuals 28, 29. HIV-infected subjects in some 18–23, 25, 27 but not all studies were recruited based on the presence of lipodystrophy. Among the HIV-infected patients, 70% were characterized as having lipodystrophy based on previously used definition 19. Among studies in which HIV-infected and non-HIV-infected subjects were simultaneously recruited, subjects of similar weight were recruited. Dietary data on a subset were recently published. 30 HIV-infected subjects with known wasting or evaluated for studies of AIDS wasting were not included in the analysis. HIV-infected subjects aged 18 – 60 years were recruited from newspaper advertisement, community and referral-based practices. For subjects receiving antiretroviral (ARV) therapy, a stable regimen for a minimum of 6 weeks prior to evaluation was required. Subjects in both groups were excluded if they had a history of diabetes mellitus; were receiving concurrent therapy with insulin, antidiabetic agents, glucocorticoids, growth hormone or growth hormone releasing analogues, supraphysiologic testosterone replacement, or anabolic steroids; were current substance abusers; had a major opportunistic infection within the 6 weeks prior to the study; or were pregnant or breast-feeding within the past year. The HIV-negative controls recruited through hospital and local advertisements. Other criteria, including age, medication use and reproductive status were similar between the HIV and non-HIV groups. For both HIV-infected and control groups, baseline data were obtained before any intervention. If subjects participated in more than one study, only data from the initial study were used. Collection of all data was approved by the Institutional Review Board at MGH as well as at MIT (Massachusetts Institute of Technology), and all participants provided informed consent.
PROTOCOL
All subjects were studied after an overnight fast of 12 hours. Each individual had a complete medical history (including documentation of current antiretroviral use) and a physical examination, which included measurement of height by stadiometer and weight by digital scale.
Subjects underwent total-body dual-energy x-ray absorptiometry (Hologic QDR-4500A, Hologic Inc., Waltham, MA) to determine regional fat and lean mass.31 Cross-sectional abdominal computed tomography (CT) scans were performed as described by Borkan et al to assess distribution of subcutaneous and visceral abdominal fat (SAT and VAT, respectively). A lateral scout image was obtained to identify the level of the L4 pedicle, which served as the landmark for the 1cm single-slice image. 32 Subjects also received a standard 75-g oral glucose tolerance test (OGTT), with glucose and insulin determinations at 30, 60, 90, and 120 minutes following the OGTT. CD4 count, HIV viral load, and concentrations of glucose, insulin, cholesterol, HDL and triglycerides were determined by methods described elsewhere.19
STATISTICAL ANALYSIS
We performed an analysis to determine the impact of BMI as a continuous variable on various body composition parameters (VAT, SAT, VAT/SAT, trunk fat, total extremity fat, and trunk:total extremity fat) in both HIV-infected and non-HIV-infected subjects, stratified by gender, using linear fit modeling. Slopes were tested between the HIV and non-HIV groups by ANCOVA, testing for an interaction between the groups. If the slopes were parallel (i.e. not statically significantly different), ANCOVA was subsequently performed to determine the difference in Y-intercepts. In a subanalysis, HIV-infected patients and controls were characterized based on NCEP/ATP III criteria for the metabolic syndrome.33 We used ANCOVA analysis in these subgroups without metabolic syndrome to determine the relationship of BMI to body composition parameters among subjects meeting an identical criterion for analysis.
Subjects were also stratified by gender into 3 categories based on WHO BMI class (normal weight = BMI of 18.5 – 24.9 kg/m2; overweight = BMI 25.0 – 29.9 kg/m2; obese = BMI ≥ 30.0 kg/m2) 17 to determine differences in body composition between HIV-infected and non-HIV-infected subjects within each category. Data are expressed as mean ± standard deviation except where indicated. P values were derived from the ANOVA test to determine differences between HIV and control subjects, and a p value <0.05 was considered significant. All statistical analyses were performed using SAS JMP software, version 5.0.1 (SAS Institute).
RESULTS
Demographics
Three hundred and six HIV-infected subjects (168 males, 138 females) and 107 non-HIV-infected controls (68 males, 39 females) were evaluated. There were no statistically significant differences in age, race, or BMI between the HIV-infected and control subjects, stratified by gender. HIV-infected males demonstrated higher total cholesterol and triglyceride levels, lower HDL levels, higher fasting insulin and insulin AUC (area-under-the-curve), as well as higher fasting glucose and glucose AUC compared to male controls. HIV-infected females also demonstrated lower HDL, higher fasting insulin, and higher insulin AUC levels compared to female controls (Table 1). Demographics were also similar in the subanalysis limited to HIV and control subjects without the metabolic syndrome (Table 2).
Table 1.
Demographics for HIV-Infected and HIV-Negative Control Subjects
| MALES | P Value† | FEMALES | P Value† | |||
|---|---|---|---|---|---|---|
| HIV+ (N=168) | Controls (N=68) | HIV+ (N=138) | Controls (N=39) | |||
| Demographics | ||||||
|
| ||||||
| Age (Yr) | 43±7 | 41±8 | 0.09 | 41±7 | 41±10 | 0.75 |
|
| ||||||
| Race (%) | 0.17 | 0.81 | ||||
|
| ||||||
| Caucasian | 66.1 | 79.4 | - | 34.8 | 41.0 | - |
|
| ||||||
| African American | 20.2 | 14.7 | - | 46.4 | 41.0 | - |
|
| ||||||
| Hispanic | 9.5 | 2.9 | - | 10.9 | 12.8 | - |
|
| ||||||
| Other | 4.2 | 2.9 | - | 8.0 | 5.1 | - |
|
| ||||||
| BMI (kg/m2) | 26.1±3.9 | 27.1±3.9 | 0.08 | 27.9±5.8 | 27.4±5.1 | 0.60 |
|
| ||||||
| HIV Parameters | ||||||
|
| ||||||
| CD4 (#/mm3) ‡ | 442±250 | 899±383 | <0.0001 | 452±253 | 950±316 | <0.0001 |
|
| ||||||
| Viral Load (copies/mL)¶ ‡ | 50 (50,12150) | - | - | 116 (50,5471) | - | - |
|
| ||||||
| Duration HIV (Yr) ‡ | 8.7±5.0 | - | - | 8.3±4.4 | - | - |
|
| ||||||
| Currently Taking PI (%) | 67.3 | - | - | 44.9 | - | - |
|
| ||||||
| Currently Taking NRTI (%) | 85.7 | - | - | 79.0 | - | - |
|
| ||||||
| Currently Taking NNRTI (%) | 38.0 | - | - | 31.1 | - | - |
|
| ||||||
| Not Taking Antiretrovirals (%) | 9.5 | - | - | 18.8 | - | - |
|
| ||||||
| % Categorized with Lipodystrophy | 66.7 | - | - | 73.9 | - | - |
|
| ||||||
| Metabolic Parameters | ||||||
|
| ||||||
| Total cholesterol (mg/dL)*‡ | 198±50 | 181±37 | 0.01 | 185±44 | 177±34 | 0.29 |
|
| ||||||
| HDL (mg/dL)*‡ | 38±11 | 45±11 | <0.0001 | 46±13 | 56±15 | <0.0001 |
|
| ||||||
| Triglyceride (mg/dL)*‡ | 259±231 | 141±147 | 0.0001 | 158±154 | 113±165 | 0.12 |
|
| ||||||
| Fasting Insulin (μIU/mL)*‡ | 16±15 | 11±8 | 0.04 | 11±8 | 8±6 | 0.047 |
|
| ||||||
| Insulin AUC (μIU/mL × 120 min)*‡ | 9216±5348 | 6431±4430 | 0.02 | 8683±4953 | 5500±2781 | 0.009 |
|
| ||||||
| Fasting Glucose (mg/dL)*‡ | 93±13 | 90±13 | 0.049 | 86±10 | 86±15 | 0.87 |
|
| ||||||
| Glucose AUC (mg/dL × 120 min)*‡ | 17496±3809 | 15094±3207 | 0.0004 | 15797±3431 | 15925±4515 | 0.89 |
Results expressed as mean ± standard deviation
p values derived from ANOVA test
Results expressed as median (interquartile range)
Data for CD4 available in 19 HIV− males and 128 HIV+/18 HIV− females; for viral load in 48 HIV+ males and 103 HIV+ females; for duration of HIV in 167 HIV+ males; for total cholesterol in 166 HIV+/67 HIV− males and 130 HIV+ females; for triglyceride in 67 HIV− males and 130 HIV+ females; for HDL in 67 HIV− males and 130 HIV+ females; for fasting insulin in 116 HIV+/48 HIV− males and 125 HIV+/37 HIV− females; for insulin AUC in 96 HIV+/25 HIV− males and 110 HIV+/18 HIV− females; for fasting glucose in 67 HIV− males and 130 HIV+ females; for glucose AUC in 147 HIV+/39 HIV− males and 116 HIV+/19 HIV− females
To convert mg/dL to mmol/L for total cholesterol and HDL, multiply by 0.0259; for triglycerides, multiply by 0.0113; for glucose, multiply by 0.0555; and to convert μIU/mL to pmol/L for insulin, multiply by 6.945
Abbreviations: BMI: Body Mass Index, SAT: Abdominal Subcutaneous Adipose Tissue, VAT: Visceral Adipose Tissue, HDL: High-Density Lipoprotein, AUC: Area-Under-the-Curve
Table 2.
Demographics for HIV-Infected and HIV-Negative Control Subjects Without Metabolic Syndrome
| MALES | P Value† | FEMALES | P Value† | |||
|---|---|---|---|---|---|---|
| HIV+ (N=115) | Controls (N=56) | HIV+ (N=89) | Controls (N=33) | |||
| Demographics | ||||||
|
| ||||||
| Age (Yr) | 43±7 | 41±8 | 0.17 | 40±7 | 40±10 | 0.96 |
|
| ||||||
| Race (%) | 0.17 | 0.70 | ||||
|
| ||||||
| Caucasian | 60.9 | 76.8 | - | 31.5 | 39.4 | - |
|
| ||||||
| African American | 22.6 | 16.1 | - | 48.3 | 39.4 | - |
|
| ||||||
| Hispanic | 11.3 | 3.6 | - | 11.2 | 15.2 | - |
|
| ||||||
| Other | 5.2 | 3.6 | - | 9.0 | 6.1 | - |
|
| ||||||
| BMI (kg/m2) | 25.0±3.4 | 26.3±3.6 | 0.029 | 26.9±6.2 | 26.5±4.6 | 0.71 |
|
| ||||||
| HIV Parameters | ||||||
|
| ||||||
| CD4 (#/mm3) ‡ | 395±227 | 930±408 | <0.0001 | 443±241 | 944±325 | <0.0001 |
|
| ||||||
| Viral Load (copies/mL)¶ ‡ | 171 (50,16475) | - | - | 158 (50, 5881) | - | - |
|
| ||||||
| Duration HIV (Yr) | 8.6±5.3 | - | - | 7.5±4.4 | - | - |
|
| ||||||
| Currently Taking PI (%) | 68.7 | - | - | 40.4 | - | - |
|
| ||||||
| Currently Taking NRTI (%) | 80.9 | - | - | 83.1 | - | - |
|
| ||||||
| Currently Taking NNRTI (%) | 35.7 | - | - | 36.0 | - | - |
|
| ||||||
| Not Taking Antiretrovirals (%) | 13.0 | - | - | 16.9 | - | - |
|
| ||||||
| % Categorized with Lipodystrophy | 55.7 | - | - | 71.9 | - | - |
|
| ||||||
| Metabolic Parameters | ||||||
|
| ||||||
| Total cholesterol (mg/dL)*‡ | 193±48 | 179±37 | 0.048 | 178±44 | 172±30 | 0.50 |
|
| ||||||
| HDL (mg/dL)* | 40±12 | 47±11 | 0.0001 | 48±15 | 59±14 | 0.0004 |
|
| ||||||
| Triglyceride (mg/dL)* | 222±196 | 103±64 | <0.0001 | 124±68 | 74±35 | <0.0001 |
|
| ||||||
| Fasting Insulin (μIU/mL)*‡ | 12±9 | 9±4 | 0.03 | 11±9 | 7±4 | 0.018 |
|
| ||||||
| Insulin AUC (μIU/mL × 120 min)*‡ | 7744±3952 | 5879±3392 | 0.05 | 8073±4560 | 5603±2831 | 0.04 |
|
| ||||||
| Fasting Glucose (mg/dL)* | 91±11 | 87±9 | 0.02 | 85±10 | 83±8 | 0.25 |
|
| ||||||
| Glucose AUC (mg/dL × 120 min)*‡ | 17127±3271 | 14726±2639 | 0.0001 | 15714±3677 | 15089±2743 | 0.50 |
Results expressed as mean ± standard deviation
p values derived from ANOVA test
Results expressed as median (interquartile range)
Data for CD4 available in 16 HIV− males and 88 HIV+/17 HIV− females; for viral load in 34 HIV+ males and 72 HIV+ females; for total cholesterol in 113 HIV+ males; for fasting insulin in 76 HIV+/38 HIV− males and 84 HIV+/31 HIV− females; for insulin AUC in 57 HIV+/22 HIV− males and 73 HIV+/17 HIV− females; for glucose AUC in 94 HIV+/36 HIV− males and 79 HIV+/18 HIV− females
To convert mg/dL to mmol/L for total cholesterol and HDL, multiply by 0.0259; for triglycerides, multiply by 0.0113; for glucose, multiply by 0.0555; and to convert μIU/mL to pmol/L for insulin, multiply by 6.945
Abbreviations: BMI: Body Mass Index, SAT: Abdominal Subcutaneous Adipose Tissue, VAT: Visceral Adipose Tissue, HDL: High-Density Lipoprotein, AUC: Area-Under-the-Curve
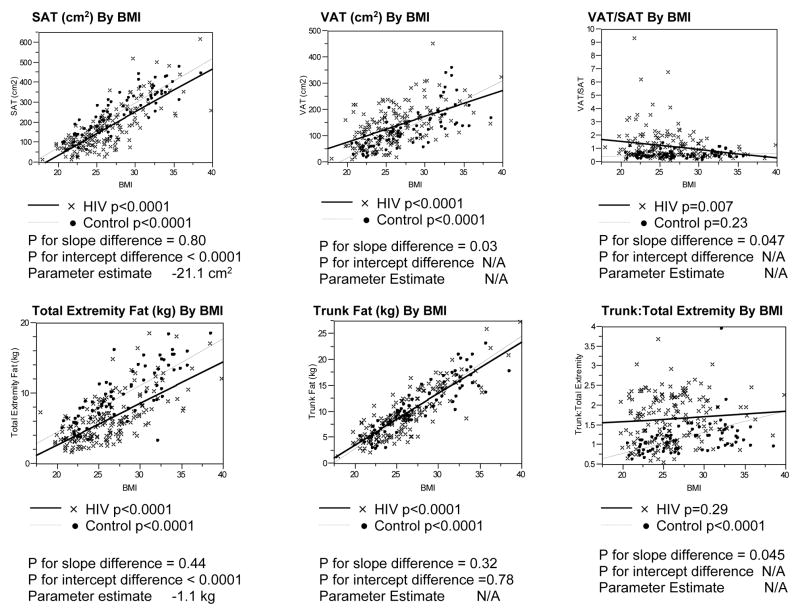
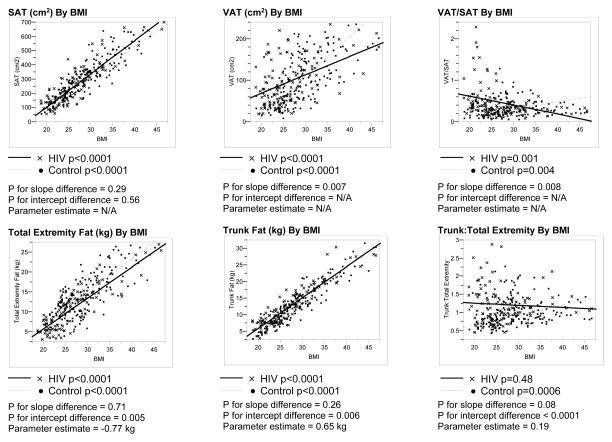
MEN
Significant associations between BMI and SAT, VAT, trunk fat, and total extremity fat were observed for both HIV-infected and non-HIV-infected men (Figure 1). Regression lines were parallel, and intercepts differed significantly in the comparison of the relationship of SAT to BMI between the HIV-infected and non-HIV-infected groups. SAT was decreased by approximately 21 cm2 for a given BMI among the male HIV-infected compared to non-HIV-infected subjects. In contrast, the slopes of the regression lines relating VAT or VAT/SAT to BMI differed significantly between HIV-infected and non-HIV-infected groups.
Figure 1. Linear Regression Analyses of Body Composition Parameters vs. BMI Among HIV-Infected and Control Males.
P values for differences in slopes between the HIV-infected and control groups determined using ANCOVA, testing for an interaction between the groups.
P values for differences in Y-intercepts determined using ANCOVA if the p values for the slopes were not statistically significantly different.
Parameter estimate derived from ANCOVA assessing the relationship of each body composition parameter to BMI and HIV status.
Abbreviations: SAT, Subcutaneous Adipose Tissue; BMI, Body Mass Index; VAT, Visceral Adipose Tissue
Trunk fat was not increased for a given BMI in male HIV-infected vs. non-HIV-infected, but total extremity fat was lower by 1.1 kg across the range of BMI for male HIV-infected compared to non HIV-infected subjects. Trunk:total extremity fat was increased for male HIV-infected vs. non-HIV-infected but differences between HIV and non-HIV-infected men decreased with increasing BMI. Trunk:total extremity fat did not increase with BMI among HIV-infected men but did increase with BMI among non-HIV-infected men. (Figure 1)
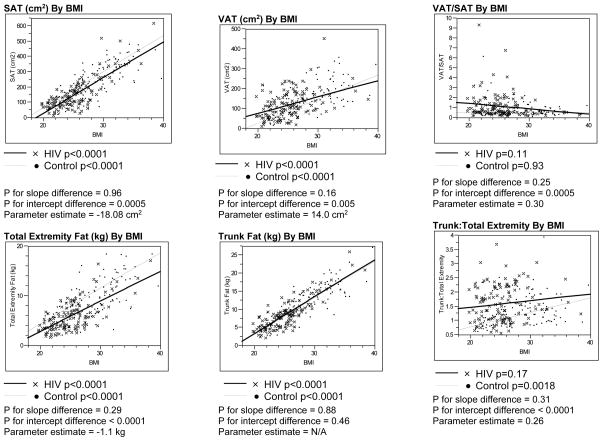
Analyses of HIV-infected patients and controls without metabolic syndrome as defined by NCEP/ATP III guidelines revealed similar results to the primary findings among all the subjects (Figure 2).
Figure 2. Linear Regression Analyses of Body Composition Parameters vs. BMI Among HIV-Infected and Control Males Without Metabolic Syndrome.
P values for differences in slopes between the HIV-infected and control groups without NCEP/ATP III defined metabolic syndrome were determined using ANCOVA, testing for an interaction between the groups.
P values for differences in Y-intercepts were determined using ANCOVA if the p values for the slopes were not statistically significantly different.
Parameter estimate derived from ANCOVA assessing the relationship of each body composition parameter to BMI and HIV status.
Abbreviations: SAT, Subcutaneous Adipose Tissue; BMI, Body Mass Index; VAT, Visceral Adipose Tissue
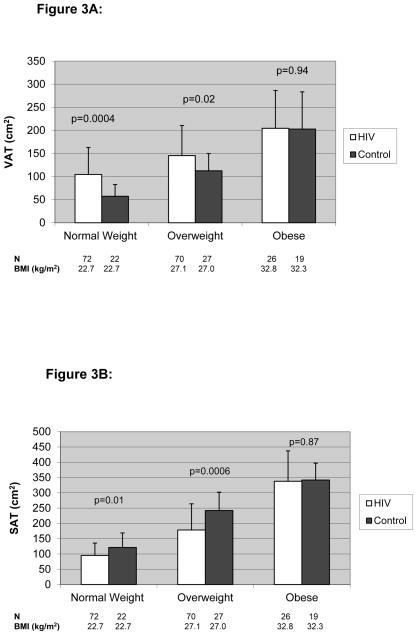
Seventy-two (43%) HIV-infected and 22 (32%) male control subjects were normal weight, 70 (42%) HIV-infected and 27 (40%) male control subjects were overweight, and 26 (15%) HIV-infected and 19 (28%) male control subjects were obese using WHO criteria. VAT was significantly higher and SAT was significantly lower among HIV-infected compared to non-HIV-infected men in the normal-weight and overweight categories, but not in the obese category. The difference in VAT was most significant among those in the normal-weight category (104.6±58.5 vs. 57.3±25.5 cm2, p=0.0004; HIV-infected vs. controls), whereas the difference in SAT was most significant among those in the overweight category (178.5±85.6 vs. 242.2±60.0 cm2, p=0.0006, HIV-infected vs. controls). (Figure 3A and B)
Figure 3.
Figure 3A. VAT Based on BMI Class Among HIV-Infected and Control Males
Results are expressed as mean±standard deviation.
The white bar denotes HIV-infected subjects while the black bar denotes non-HIV-infected controls.
- Normal Weight = BMI 18.5 – 24.9
- Overweight = BMI 25.0 – 29.9
- Obese = BMI ≥ 30.0).
P values derived from ANOVA test to determine differences between HIV-infected and non-HIV-infected subjects
Abbreviations: VAT, Visceral Adipose Tissue; BMI, Body Mass Index
Figure 3B. SAT Based on BMI Class Among HIV-Infected and Control Males
Results are expressed as mean±standard deviation.
The white bar denotes HIV-infected subjects while the black bar denotes non-HIV-infected controls.
- Normal Weight = BMI 18.5 – 24.9
- Overweight = BMI 25.0 – 29.9
- Obese = BMI ≥ 30.0).
P values derived from ANOVA test to determine differences between HIV-infected and non-HIV-infected subjects
Abbreviations: SAT, Subcutaneous Adipose Tissue; BMI, Body Mass Index
Consistent with linear regression modeling, trunk fat was not increased among HIV vs. non HIV-infected men for any BMI category, whereas total extremity fat was decreased in the normal and overweight categories and tended to be decreased in the obese category. Trunk:total extremity fat was therefore significantly increased in the normal and overweight categories, driven primarily by lower total extremity fat values. (Table 3A)
Table 3A.
Differences in DEXA Regional Body Composition Between HIV-Infected and Non-HIV-Infected Males
| Normal BMI | P value† | Overweight BMI | P value† | Obese BMI | P value† | ||||
|---|---|---|---|---|---|---|---|---|---|
| HIV+(N=72) | Control (N=22) | HIV+ (N=70) | Control (N=27) | HIV+ (N=26) | Control (N=19) | ||||
| BMI (kg/m2) | 22.7±1.5 | 22.7±1.0 | 0.92 | 27.1±1.4 | 27.0±1.5 | 0.67 | 32.8±2.6 | 32.3±1.0 | 0.39 |
|
| |||||||||
| Trunk Fat (kg) | 6.3±2.3 | 5.5±1.9 | 0.15 | 10.3±3.2 | 10.6±2.3 | 0.66 | 16.7±4.1 | 16.1±2.9 | 0.58 |
|
| |||||||||
| Total Extremity Fat (kg) | 4.3±1.8 | 5.9±1.6 | 0.0003 | 6.8±2.9 | 9.6±2.4 | <0.0001 | 10.5±3.5 | 12.4±3.4 | 0.09 |
|
| |||||||||
| Trunk:Total Extremity Fat | 1.63±0.65 | 0.92±0.18 | <0.0001 | 1.68±0.58 | 1.14±0.26 | <0.0001 | 1.69±0.53 | 1.45±0.69 | 0.19 |
|
| |||||||||
| Total Fat (kg) | 11.7±3.4 | 12.5±3.3 | 0.32 | 18.2±5.3 | 21.3±3.9 | 0.006 | 28.7±6.0 | 29.9±4.5 | 0.46 |
Results expressed as mean ± standard deviation
p values derived from ANOVA test
NRTI and NNRTI usage did not differ among HIV-infected men within the three BMI categories. The percentage of HIV-infected men receiving PIs was lower in the obese category compared to the other two BMI categories (48.0% PI use in obese vs. 74.2% in normal and 78.8% in overweight, p=0.01). However, VAT (151.4±70.4 vs. 136.2±72.0 cm2, p= 0.23, no PI vs. PI use) and SAT (188.4±119.0 vs. 162.1±108.6 cm2, p=0.19, no PI vs. PI use) did not differ by PI use, nor did PI use influence VAT or SAT in regression modeling accounting for BMI category (p=0.74 for VAT and p=0.52 for SAT).
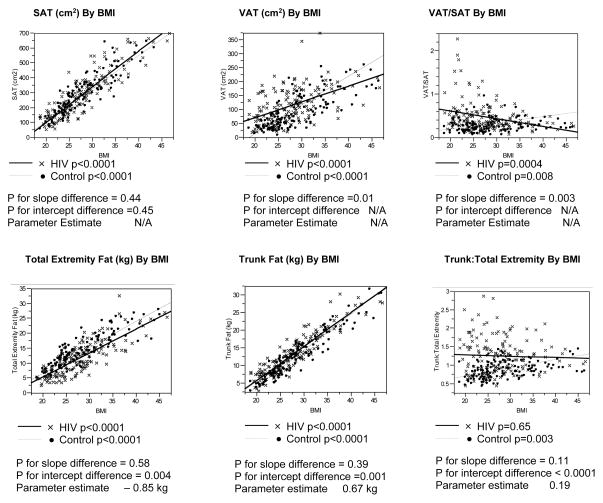
WOMEN
Significant associations were observed between BMI and SAT, VAT, trunk fat, and total extremity fat for both HIV-infected and non-HIV-infected women (Figure 3). The relationship of SAT to BMI was not significantly different between the HIV-infected and non-HIV-infected women. In contrast, the relationships of VAT and VAT/SAT to BMI differed significantly between HIV-infected and non-HIV-infected women.
Regression lines were parallel and intercepts differed significantly in the comparisons of trunk fat and total extremity fat to BMI between HIV-infected and non-HIV-infected women. For a given BMI, female HIV-infected subjects demonstrated increased trunk fat and decreased total extremity fat by differences of approximately 0.67 kg and 0.85 kg, respectively, compared to non-HIV-infected subjects. Trunk:total extremity fat did not increase with BMI among HIV-infected women in contrast to non-HIV-infected women. (Figure 4)
Figure 4. Linear Regression Analyses of Body Composition Parameters vs. BMI Among HIV-Infected and Control Females.
P values for differences in slopes between the HIV-infected and control groups determined using ANCOVA, testing for an interaction between the groups.
P values for differences in Y-intercepts were determined using ANCOVA if the p values for the slopes were not statistically significantly different.
Parameter estimate derived from ANCOVA assessing the relationship of each body composition parameter to BMI and HIV status.
Abbreviations: SAT, Subcutaneous Adipose Tissue; BMI, Body Mass Index; VAT, Visceral Adipose Tissue
Analyses of HIV-infected patients and controls without metabolic syndrome as defined by NCEP/ATP III guidelines revealed similar results to the primary findings among all the subjects (Figure 5).
Figure 5. Linear Regression Analyses of Body Composition Parameters vs. BMI Among HIV-Infected and Control Females Without Metabolic Syndrome.
P values for differences in slopes between the HIV-infected and control groups without NCEP/ATP III defined metabolic syndrome were determined using ANCOVA, testing for an interaction between the groups.
P values for differences in Y-intercepts were determined using ANCOVA if the p values for the slopes were not statistically significantly different.
Parameter estimate derived from ANCOVA assessing the relationship of each body composition parameter to BMI and HIV status.
Abbreviations: SAT, Subcutaneous Adipose Tissue; BMI, Body Mass Index; VAT, Visceral Adipose Tissue
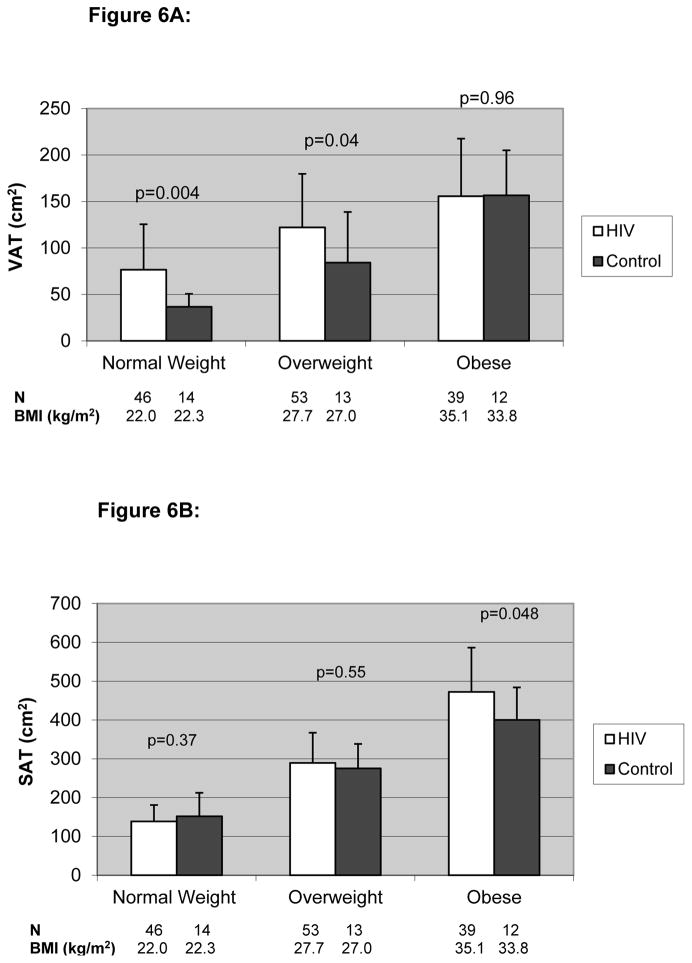
Forty-six (33%) HIV-infected and 14 (36%) female control subjects were normal weight; 53 (39%) HIV-infected and 13 (33%) female control subjects were overweight and 39 (28%) HIV-infected and 12 (31%) female control subjects were obese using WHO criteria. VAT was significantly higher among HIV-infected compared to non-HIV-infected women in the normal-weight and overweight categories, but not in the obese category. The difference in VAT was most significant among those in the normal-weight category (76.6±48.9 vs. 36.7±13.9 cm2, p=0.004; HIV-infected vs. controls). In contrast, SAT was significantly higher among HIV-infected compared to non-HIV-infected women in the obese category (472.4±113.9 vs. 400.1±83.7 cm2, p=0.048). (Figure 6A and B)
Figure 6.
Figure 6A. VAT Based on BMI Class Among HIV-Infected and Control Females
Results are expressed as mean±standard deviation.
The white bar denotes HIV-infected subjects while the black bar denotes non-HIV-infected controls.
- Normal Weight = BMI 18.5 – 24.9
- Overweight = BMI 25.0 – 29.9
- Obese = BMI ≥ 30.0).
P values derived from ANOVA test to determine differences between HIV-infected and non-HIV-infected subjects
Abbreviations: VAT, Visceral Adipose Tissue; BMI, Body Mass Index
Figure 6B. SAT Based on BMI Class Among HIV-Infected and Control Females
Results are expressed as mean±standard deviation.
The white bar denotes HIV-infected subjects while the black bar denotes non-HIV-infected controls.
- Normal Weight = BMI 18.5 – 24.9
- Overweight = BMI 25.0 – 29.9
- Obese = BMI ≥ 30.0).
P values derived from ANOVA test to determine differences between HIV-infected and non-HIV-infected subjects
Abbreviations: SAT, Subcutaneous Adipose Tissue; BMI, Body Mass Index
Trunk fat values were significantly higher in the HIV-infected women in the normal and overweight categories and tended to be higher in the obese category (Table 3B). HIV-infected women demonstrated higher trunk fat:total extremity fat across all BMI categories.
Table 3B.
Differences in DEXA Regional Body Composition Between HIV-Infected and Non-HIV-Infected Females
| Normal BMI | P value† | Overweight BMI | P value† | Obese BMI | P value† | ||||
|---|---|---|---|---|---|---|---|---|---|
| HIV+ (N=46) | Control (N=14) | HIV+ (N=53) | Control (N=13) | HIV+ (N=39) | Control (N=12) | ||||
| BMI (kg/m2) | 22.0±1.8 | 22.3±1.8 | 0.70 | 27.7±1.4 | 27.0±1.5 | 0.08 | 35.1±4.4 | 33.8±2.0 | 0.33 |
|
| |||||||||
| Trunk Fat (kg) | 7.4±1.8 | 6.3±2.0 | 0.04 | 13.3±2.7 | 11.3±3.1 | 0.02 | 20.6±4.6 | 18.1±3.1 | 0.07 |
|
| |||||||||
| Total Extremity Fat (kg) | 6.8±2.8 | 8.5±2.1 | 0.048 | 11.5±3.5 | 12.9±2.7 | 0.19 | 17.9±5.1 | 18.4±3.2 | 0.73 |
|
| |||||||||
| Trunk:Total Extremity Fat | 1.28±0.59 | 0.74±0.17 | 0.002 | 1.27±0.47 | 0.91±0.31 | 0.01 | 1.22±0.37 | 0.99±0.15 | 0.04 |
|
| |||||||||
| Total Fat (kg) | 15.1±3.5 | 15.8±3.8 | 0.55 | 25.8±4.7 | 25.1±4.2 | 0.63 | 39.6±7.9 | 37.4±5.6 | 0.38 |
Results expressed as mean ± standard deviation
p values derived from ANOVA test
PI, NRTI, and NNRTI usage was similar among the HIV-infected women across the three BMI categories. VAT (118.1±64.0 vs. 120.4±66.9 cm2, p= 0.85, no PI vs. PI use) and SAT (300.4±181.0 vs. 278.2±139.1cm2, p=0.46, no PI vs. PI use) did not differ by PI use, nor did PI use influence VAT or SAT in regression modeling accounting for BMI category (p=0.76 for VAT and p=0.53 for SAT).
DISCUSSION
Although BMI has been used as a simple anthropometric predictor of type 2 diabetes, hypertension, dyslipidemia, and cardiovascular disease among non-HIV-infected individuals,34–37 few studies have evaluated body composition between HIV-infected and non-HIV-infected control subjects in relation to BMI. We therefore examined the relationship between body composition and BMI for HIV-infected compared to non-HIV-infected subjects in linear regression modeling and used the WHO BMI criteria to determine fat distribution differences between HIV-infected and non-HIV-infected subjects within these anthropometric subgroups.17
Our study demonstrated the significant presence of peripheral lipoatrophy among HIV-infected compared to non-HIV-infected subjects, supporting the findings of FRAM and others.14–16, 38 For any given BMI, HIV-infected males demonstrated 1.1 kg less total extremity fat and HIV-infected females 0.85 kg less total extremity fat compared to their respective non-HIV-infected controls. To our knowledge, this is the first study to quantify the degree of fat loss in relation to BMI between HIV-infected and non-HIV-infected subjects, stratified by gender. We have also shown that in addition to the presence of peripheral lipoatrophy among HIV-infected individuals, significant alterations in VAT, SAT, and truncal fat occur in the context of BMI within gender categories.
Among HIV-infected men, abdominal SAT was significantly lower compared to non-HIV-infected males by an average difference of 21 cm2, and the largest differences were seen among normal and overweight HIV-infected men compared to control subjects. In contrast, VAT was increased among HIV-infected men in the normal and overweight category compared to control subjects. Simultaneous increases in VAT and decreases in SAT may help to explain the observation that trunk fat per se was not different between HIV and non-HIV-infected men.
Among HIV-infected women, trunk fat was increased by approximately 0.67 kg compared to non-HIV-infected female control subjects. Similar to the observation in men, VAT was increased most among HIV-infected women in the normal and overweight categories relative to controls. In contrast to the findings among men, SAT was not different among normal weight and overweight subjects (HIV vs. control), and thus the increased trunk fat in these categories was primarily due to increased VAT, whereas in the obese category, the increased trunk fat was due to increased SAT. Women have more total body fat than men and tend to preserve gluteal and femoral fat stores39, which may help to explain observed differences between HIV-infected men and women in this and other studies.14, 15
HIV-infected and control subjects (both males and females) also demonstrated increasing VAT and SAT deposition with increasing BMI, supporting the results of other studies of non-HIV-infected individuals.40 Thus although absolutes levels of VAT increase with increasing BMI, relative differences compared to control subjects are greatest for normal and overweight HIV-infected patients (both males and females). These findings suggest that: 1) obesity alone does not predict an increased visceral adiposity among HIV-infected subjects relative to non-HIV-infected subjects; and 2) HIV-infected subjects at relatively lower BMIs may have increased risk of metabolic complications given the degree of visceral adiposity observed.
In the FRAM study 14, 15, patients were categorized based on the presence of lipoatrophy. Among men, a trend toward more VAT and significantly more trunk fat was demonstrated in HIV-infected patients without lipoatrophy compared to those with lipoatrophy. In contrast, VAT was lower compared to non-HIV-infected controls in those with lipoatrophy. Among women, more VAT and trunk fat were seen among HIV-infected subjects without lipoatrophy compared both to HIV-infected with lipoatrophy and to non-HIV-infected controls. Subjects were not compared in relation to BMI or within BMI categories in FRAM, but rather, adipose tissue volume for each subject was divided by height-squared and then multiplied by 1.752 to correspond to a typical height. In contrast, HIV-infected patients and controls were compared in relation to BMI and within BMI categories in our study, suggesting relatively more VAT deposition among HIV-infected patients compared to controls at lower BMIs, particularly in the normal and overweight BMI categories, for both genders. Thus our data extend those of FRAM, demonstrating relative differences in fat accumulation and fat loss by BMI category between genders.
This study has a number of limitations. We assessed body composition among HIV-infected individuals with a high proportion of metabolic abnormalities. Our results therefore cannot be generalized to all HIV-infected individuals or to HIV-infected individuals with wasting. However, similar results were obtained when body composition parameters were compared between HIV-infected and non-HIV-infected patients using an identical criterion of absence of NCEP/ATP III defined metabolic syndrome. These data suggest that the changes in body composition among the HIV-infected patients relative to controls in this study were not significantly biased by selection of patients with a high proportion of metabolic abnormalities. We did not follow patients longitudinally to determine changes in adipose distribution over time. Finally, we were unable to analyze the respective contributions from deep and superficial subcutaneous compartments, which may help to define the specific adipose changes that are occurring among HIV-infected individuals. Despite these limitations, these data provide new information on the relationship of body composition to BMI among HIV-infected patients.
In conclusion, we have demonstrated differences in central and peripheral fat depots in relation to BMI as well as by WHO BMI category in the comparisons of male and female HIV-infected vs. control subjects. Loss of extremity fat was the most consistent finding, but increased VAT was also observed relative to control subjects both among HIV-infected men and women. The differences in VAT were most obvious among normal and overweight subjects. Gender differences in abdominal SAT accumulation were observed, with preservation of SAT among HIV-infected women relative to control subjects.
Acknowledgments
Funding/Support: This work was funded in part by NIH DKRO1-49302 (SG), NIH DK-02844 (CH), NIH T32HD-052961 (JL), University of Western Ontario (London, Ontario, Canada) Research Fellowship Fund (TJ), NIH MO1-RR01066 and the Mary Fisher SARE Fund (SG). The funding sources had no role in the choice of methods, the contents or form of this work, or the decision to submit the results for publication.
We are grateful to the nursing staff of the MGH and MIT General Clinical Research Centers for their dedicated patient care and to Matt Kron for his help with the analysis.
Footnotes
Financial Disclosures: None related to this project
References
- 1.Carr A, Samaras K, Burton S, et al. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS. 1998;12(7):F51–58. doi: 10.1097/00002030-199807000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Morse CG, Kovacs JA. Metabolic and skeletal complications of HIV infection: the price of success. JAMA. 2006 Aug 16;296(7):844–854. doi: 10.1001/jama.296.7.844. [DOI] [PubMed] [Google Scholar]
- 3.Walli R, Herfort O, Michl GM, et al. Treatment with protease inhibitors associated with peripheral insulin resistance and impaired oral glucose tolerance in HIV-1-infected patients. AIDS. 1998;12(15):F167–173. doi: 10.1097/00002030-199815000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Periard D, Telenti A, Sudre P, et al. Atherogenic dyslipidemia in HIV-infected individuals treated with protease inhibitors. The Swiss HIV Cohort Study. Circulation. 1999;100(7):700–705. doi: 10.1161/01.cir.100.7.700. [DOI] [PubMed] [Google Scholar]
- 5.Hadigan C, Meigs JB, Corcoran C, et al. Metabolic abnormalities and cardiovascular disease risk factors in adults with human immunodeficiency virus infection and lipodystrophy. Clin Infect Dis. 2001;32(1):130–139. doi: 10.1086/317541. [DOI] [PubMed] [Google Scholar]
- 6.Mulligan K, Parker RA, Komarow L, et al. Mixed patterns of changes in central and peripheral fat following initiation of antiretroviral therapy in a randomized trial. J Acquir Immune Defic Syndr. 2006 Apr 15;41(5):590–597. doi: 10.1097/01.qai.0000214811.72916.67. [DOI] [PubMed] [Google Scholar]
- 7.Lo JC, Mulligan K, Tai VW, Algren H, Schambelan M. “Buffalo hump” in men with HIV-I infection. Lancet. 1998;351:871–875. doi: 10.1016/S0140-6736(97)11443-X. [DOI] [PubMed] [Google Scholar]
- 8.Striker R, Conlin D, Marx M, Wiviott L. Localized adipose tissue hypertrophy in patients receiving human immunodeficiency virus protease inhibitors. Clin Infect Dis. 1998;27(1):218–220. doi: 10.1086/517682. [DOI] [PubMed] [Google Scholar]
- 9.Saint-Marc T, Partisani M, Poizot-Martin I, et al. A syndrome of peripheral fat wasting (lipodystrophy) in patients receiving long-term nucleoside analogue therapy. AIDS. 1999;13(13):1659–1667. doi: 10.1097/00002030-199909100-00009. [DOI] [PubMed] [Google Scholar]
- 10.Gervasoni C, Ridolfo AL, Trifiro G, et al. Redistribution of body fat in HIV-infected women undergoing combined antiretroviral therapy. AIDS. 1999;13(4):465–471. doi: 10.1097/00002030-199903110-00004. [DOI] [PubMed] [Google Scholar]
- 11.Pouliot MC, Despres JP, Nadeau A. Visceral Obesity in Men: Associations with Glucose Tolerance, Plasma Insulin and Lipoprotein Levels. Diabetes. 1992;41:826–834. doi: 10.2337/diab.41.7.826. [DOI] [PubMed] [Google Scholar]
- 12.Miller KD, Jones E, Yanovski JA, Shankar R, Feuerstein I, Falloon J. Visceral abdominal-fat accumulation associated with use of indinavir. Lancet. 1998;351(9106):871–875. doi: 10.1016/S0140-6736(97)11518-5. [DOI] [PubMed] [Google Scholar]
- 13.Rexrode KM, Carey VJ, Hennekens CH, et al. Abdominal obesity and coronary heart disease in women. JAMA. 1998;280:1843–1848. doi: 10.1001/jama.280.21.1843. [DOI] [PubMed] [Google Scholar]
- 14.Fat distribution in women with HIV infection. J Acquir Immune Defic Syndr. 2006 Aug 15;42(5):562–571. doi: 10.1097/01.qai.0000229996.75116.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bacchetti P, Gripshover B, Grunfeld C, et al. Fat distribution in men with HIV infection. J Acquir Immune Defic Syndr. 2005;40(2):121–131. doi: 10.1097/01.qai.0000182230.47819.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tien PC, Cole SR, Williams CM, et al. Incidence of lipoatrophy and lipohypertrophy in the women’s interagency HIV study. J Acquir Immune Defic Syndr. 2003 Dec 15;34(5):461–466. doi: 10.1097/00126334-200312150-00003. [DOI] [PubMed] [Google Scholar]
- 17.WHO. Global Database on Body Mass Index: BMI Classification. Available at: http://www.who.int/bmi/index.
- 18.Dolan SE, Frontera W, Librizzi J, et al. The effects of a supervised home-based aerobic and progressive resistance training regimen in HIV-infected women: A randomized trial. Archives of Internal Medicine. 2006;166:1225–1231. doi: 10.1001/archinte.166.11.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hadigan C, Corcoran C, Basgoz N, Davis B, Sax P, Grinspoon S. Metformin in the treatment of HIV lipodystrophy syndrome: A randomized controlled trial. JAMA. 2000;284(4):472–477. doi: 10.1001/jama.284.4.472. [DOI] [PubMed] [Google Scholar]
- 20.Koutkia P, Canavan B, Breu J, Grinspoon S. Growth hormone (GH) responses to GH-releasing hormone-arginine testing in human immunodeficiency virus lipodystrophy. J Clin Endocrinol Metab. 2005 Jan;90(1):32–38. doi: 10.1210/jc.2004-1342. [DOI] [PubMed] [Google Scholar]
- 21.Rietschel P, Hadigan C, Corcoran C, et al. Assessment of Growth Hormone Dynamics in Human Immunodeficiency Virus- Related Lipodystrophy. J Clin Endocrinol Metab. 2001;86(2):504–510. doi: 10.1210/jcem.86.2.7175. [DOI] [PubMed] [Google Scholar]
- 22.Hadigan C, Kamin D, Liebau J, et al. Depot-specific regulation of glucose uptake and insulin sensitivity in HIV-lipodystrophy. Am J Physiol Endocrinol Metab. 2006 Feb;290(2):E289–298. doi: 10.1152/ajpendo.00273.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hadigan C, Yawetz S, Thomas A, Havers F, Sax PE, Grinspoon S. Metabolic effects of rosiglitazone in HIV lipodystrophy: A randomized controlled trial. Ann Intern Med. 2004;140(10):786–794. doi: 10.7326/0003-4819-140-10-200405180-00008. [DOI] [PubMed] [Google Scholar]
- 24.Fitch KV, Anderson EJ, Hubbard JL, et al. Effects of a lifestyle modification program in HIV-infected patients with the metabolic syndrome. AIDS. 2006 Sep 11;20(14):1843–1850. doi: 10.1097/01.aids.0000244203.95758.db. [DOI] [PubMed] [Google Scholar]
- 25.Driscoll SD, Meininger GE, Lareau MT, et al. Effects of exercise training and metformin on body composition and cardiovascular indices in HIV infected patients. AIDS. 2004;18(3):465–473. doi: 10.1097/00002030-200402200-00013. [DOI] [PubMed] [Google Scholar]
- 26.Dolan SE, Huang JS, Killilea KM, Sullivan MP, Aliabadi N, Grinspoon S. Reduced bone density in HIV-infected women. AIDS. 2004 Feb 20;18(3):475–483. doi: 10.1097/00002030-200402200-00014. [DOI] [PubMed] [Google Scholar]
- 27.Hadigan C, Borgonha S, Rabe J, Young V, Grinspoon S. Increased rates of lipolysis among HIV-infected men receiving highly active antiretroviral therapy. Metabolism. 2002;51:1143–1147. doi: 10.1053/meta.2002.34704. [DOI] [PubMed] [Google Scholar]
- 28.Fleischman A, Johnsen S, Systrom M, et al. Effects of a Nucleoside Reverse Transcriptase Inhibitor, Stavudine, on Insulin Sensitivity and Mitochondrial Function in Muscle of Healthy Adults. AJP: Endocrinology and Metabolism. 2007 Feb 6;2006 doi: 10.1152/ajpendo.00550.2006. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bernstein LE, Berry J, Kim S, Canavan B, Grinspoon SK. Effects of etanercept in patients with the metabolic syndrome. Arch Intern Med. 2006 Apr 24;166(8):902–908. doi: 10.1001/archinte.166.8.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joy T, Keough HM, Hadigan C, et al. Dietary Fat Intake and Relationship tp Serum Lipid Levels Among HIV-infected Subjects with Metabolic Abnormalities in the Era of HAART. AIDS. 2007 doi: 10.1097/QAD.0b013e32823644ff. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mazess RB, Barden HS, Bisek JP, Hanson J. Dual-energy x-ray absorptiometry for total-body and regional bone-mineral and soft-tissue composition. Am J Clin Nutr. 1990 Jun;51(6):1106–1112. doi: 10.1093/ajcn/51.6.1106. [DOI] [PubMed] [Google Scholar]
- 32.Borkan GA, Gerzof SG, Robbins AH, Hults DE, Silbert CK, Silbert JE. Assessment of abdominal fat content by computed tomography. Am J Clin Nutr. 1982 Jul;36(1):172–177. doi: 10.1093/ajcn/36.1.172. [DOI] [PubMed] [Google Scholar]
- 33.Executive Summary of The Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 34.Suwaidi JA, Wright RS, Grill JP, et al. Obesity is associated with premature occurrence of acute myocardial infarction. Clin Cardiol. 2001 Aug;24(8):542–547. doi: 10.1002/clc.4960240804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y, Rimm EB, Stampfer MJ, Willett WC, Hu FB. Comparison of abdominal adiposity and overall obesity in predicting risk of type 2 diabetes among men. Am J Clin Nutr. 2005 Mar;81(3):555–563. doi: 10.1093/ajcn/81.3.555. [DOI] [PubMed] [Google Scholar]
- 36.Mora S, Lee IM, Buring JE, Ridker PM. Association of physical activity and body mass index with novel and traditional cardiovascular biomarkers in women. JAMA. 2006 Mar 22;295(12):1412–1419. doi: 10.1001/jama.295.12.1412. [DOI] [PubMed] [Google Scholar]
- 37.Gelber RP, Gaziano JM, Manson JE, Buring JE, Sesso HD. A Prospective Study of Body Mass Index and the Risk of Developing Hypertension in Men. Am J Hypertens. 2007 Apr;20(4):370–377. doi: 10.1016/j.amjhyper.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mulligan K, Anastos K, Justman J, et al. Fat distribution in HIV-infected women in the United States: DEXA substudy in the Women’s Interagency HIV Study. J Acquir Immune Defic Syndr. 2005 Jan 1;38(1):18–22. doi: 10.1097/00126334-200501010-00004. [DOI] [PubMed] [Google Scholar]
- 39.Blaak E. Gender differences in fat metabolism. Curr Opin Clin Nutr Metab Care. 2001 Nov;4(6):499–502. doi: 10.1097/00075197-200111000-00006. [DOI] [PubMed] [Google Scholar]
- 40.Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000 Dec;21(6):697–738. doi: 10.1210/edrv.21.6.0415. [DOI] [PubMed] [Google Scholar]