Abstract
Cellular extrusion is a mechanism that removes dying cells from epithelial tissues to prevent compromising their barrier function. Extrusion occurs in all observed epithelia in vivo and can be modeled in vitro by inducing apoptosis in cultured epithelial monolayers. We established that actin and myosin form a ring that contracts in the surrounding cells that drives cellular extrusion. It is not clear, however, if all apoptotic pathways lead to extrusion and how apoptosis and extrusion are molecularly linked. Here, we find that both intrinsic and extrinsic apoptotic pathways activate cellular extrusion. The contraction force that drives cellular extrusion requires caspase activity. Further, necrosis does not trigger the cellular extrusion response, but instead necrotic cells are removed from epithelia by a passive, stochastic movement of epithelial cells.
Keywords: Extrusion, Apoptosis, Contraction ring, Actomyosin dynamics, Caspases
Introduction
During development and throughout adulthood, cells forming epithelial tissues undergo extensive turnover via cell division and death [1]. Aside from homeostatic death, epithelia are frequently exposed to toxins, inflammatory cytokines, pathogens or other forms of stress that lead to increased apoptosis or necrosis [2, 3] that could damage the epithelium. This damage could compromise the protective barrier that epithelia provide the organs and tissues they encase. Poor epithelial barriers could lead to malformations in developing embryos or edema, tissue damage, chronic inflammation and infections in adults. However, even when large numbers of epithelia cells become apoptotic, the barrier is still maintained using a process termed ‘cellular extrusion’. During extrusion, an apoptotic cell signals its neighbors to form an actin and myosin contractile ring that simultaneously squeezes the dying cell out of the monolayer replacing the space the apoptotic cell leaves, thereby preventing any gaps from forming within the epithelium [4].
Several studies have linked cellular extrusion and apoptosis in vivo and in vitro [4–9]. Two models proposed that apoptosis could drive cellular extrusion, yet neither model has been examined experimentally. In one model, Mills et al. [10] suggested that extrusion results from the contractile forces that drive apoptotic cell blebbing. In the other, Peralta-Soler et al. [6] suggested the apoptotic cell would signal reorganization of actin filaments and cell junctions at the interface between the dying cell and its neighbors to orchestrate cell extrusion. Later, Rosenblatt et al. [4], through a cell addition assay, demonstrated that early apoptotic cells induce the formation of actin cables on cells in a monolayer, suggesting that the apoptotic cell signals the neighboring cells to induce the actomyosin ring to form and contract. This was an important step for understanding how apoptotic cells might activate extrusion, yet several questions remain. How does apoptosis trigger extrusion and which apoptotic signals are important for activating extrusion? Is extrusion activated in response to both intrinsic and extrinsic apoptotic stimuli? Is apoptosis required to initiate extrusion or can extrusion occur independently of apoptosis? Can other forms of cell death such necrosis activate cell extrusion?
Here, we find that extrusion can be triggered by either intrinsic or extrinsic apoptotic stimuli. By investigating different steps in these apoptotic pathways, we found that completion of extrusion requires caspase activation. Although necrotic cells resulting from caspase inhibition do not extrude, they are removed from epithelia by stochastic movement of epithelial cells.
Materials and methods
Cell culture
MDCK II cells (gift from K. Matlin, University of Chicago, Chicago, IL) were cultured in Dulbecco’s Modified Eagle Medium (DMEM) high glucose (Invitrogen 11965-092) with 5% FBS, 2mM L-glutamine, 50 U/ml penicillin, 50 μg/ml streptomycin (all from Invitrogen) at 5% CO2, 37°C. 16-HBE-14o (gift from D. Gruenert, California Pacific Medical Center, San Francisco, CA) were cultured in Minimum Essential Media (MEM) low glucose (Invitrogen 11095-080) with 5% FBS, 2mM L-glutamine, 50 U/ml penicillin, 50 μg/ml streptomycin in a flask coated with a solution of human fibronectin type I (BD Biosciences), bovine collagen I (Purecol; Inamed biomaterials), and BSA (Invitrogen) at 5% CO2, 37°C.
Induction of cell death
To induce apoptosis, MDCK monolayers or HBE bilayers were treated with 120 mJ/cm2 short-wave (UV-C) light, using a Spectrolinker (Spectroline) and incubated for 2 h after irradiation or by treating with 500 μM etoposide (Sigma-Aldrich) for 5h or 100 ng/ml superKiller TRAIL (Enzo Life Sciences) along with 100 ng/ml Cyclohexamide (Calbiochem) for 5h. In some experiments, MDCK cells were pre-treated with 50μM z-VAD-fmk (Promega) or 0.1 % DMSO (Sigma) before inducing apoptosis.
Cell staining
Cells were fixed with 4% formaldehyde in PBS for 20 min, permeabilized for 5 min with 0.5% Triton in PBS, rinsed 3 times with 0.1% Triton in PBS, and blocked with AbDil (PBS with 0.1% Triton X-100 and 2% BSA) for 20 min before incubating with primary antibodies. Cells were then incubated with the following primary antibodies (diluted in AbDil) for 1 h: 1:100 mouse monoclonal anti-Bax clone 6A7 (Sigma), 1:200 rabbit monoclonal anti active caspase-3 (BD pharmigen), 1:100 rabbit polyclonal anti cytochrome c (Santa Cruz), mouse monoclonal anti cytochrome c (Abcam), 1:50 monoclonal mouse anti HMGB1 (Sigma) and 1:100 polyclonal rabbit anti AIF (Cell Signaling). After washing coverslips 3 times in 0.1% Triton X-100, coverslips were incubated in secondary antibodies (all diluted 1:100 in AbDil): Alexa Fluor® 488 goat anti-mouse, Alexa Fluor® 568 goat anti-mouse, Alexa Fluor® 488 goat anti-rabbit, Alexa Fluor® 568 goat anti-rabbit and Alexa Fluor® 647 goat anti-rabbit (all from Molecular probes, Invitrogen). Along with secondary antibodies, we incubated the cells with 1 μg/ml Hoescht 33342 (Sigma-Aldrich) and 0.25 μg/ml Alexa Fluor® 568 phalloidin or 0.25 μg/ml Alexa Fluor® 647 phalloidin (Molecular Probes, Invitrogen). After incubation with secondary antibody for 45 min, the coverslips were rinsed once with 0.1% Triton in PBS and then mounted on a micro slide (Gold Seal Products) using ProLong Gold antifade reagent (Invitrogen).
Microscopy
Fluorescence micrographs cells were obtained using a Leica DM 6000B microscope captured with a Micromax charge-coupled device camera (Roper Scientific). IP Lab Software was used to control the camera and to process images. All images were processed further using Photoshop (Adobe) and Illustrator (Adobe) software. Movies were made with an Olympus IX81 spinning disk microscope with a Weather Station incubation chamber, prior motorized stage, ZDC-laser focus, and motorized objectives for Z-sectioning. Slidebook TM 5.0 software (3i intelligent Imaging Innovations) was used to control the camera and to process images. Images were later processed using ImageReady software (Adobe)
Immunoblot analysis
Antibodies used for immunoblot analysis included polyclonal rabbit anti-Bax (Santa Cruz), monoclonal rabbit anti-Bak (Abcam), monoclonal mouse anti-Bcl-2 (Abcam), polyclonal rabbit anti-GFP (Invitrogen) and Monoclonal mouse anti-alpha tubulin (Sigma) and diluted to 1:10,000 in PBST (0.05% Tween 20 in PBS) with 5% milk.
DNA constructs
Bcl-2 alpha isoform ORF (Open Biosystems) was PCR cloned and recombined into the donor vector pDONR221 (Invitrogen) following company’s instructions. The entry vector, pDONR-Bcl2-221, was then recombined with the retroviral vector pMIG [11] modified to function as a destination vector (Gift from Dr. Alana Welm [12]). The expression vector pMIG-Bcl2 and the empty pMIG vector were used to produce retroviral particles. We synthesized Bax and Bak shRNA oligos: GCUCUGAGCAGAUCAUGAA and CCCAUUCACUACAGGUGAA, respectively [13] and cloned them into the lentiviral vector pLL5.0 (Gift from Dr. James Bear [14]). The generated vectors pLL5.0 Bax shRNA and pLL5.0 Bak shRNA along with the control vector pLL5.0 N.S. shRNA (Also a gift from Dr. James Bear) were used to make lentiviral particles. The gene fusion rat HMGB1-EGFP construct (Gift from Dr. Marco Bianchi [15]) was PCR cloned into the retroviral vector pMSCVhyg (Clontech).
Statistical analysis
The statistical analysis of data collected from three independent experiments was performed using the parametric unpaired two-tailed t test. In each graph, p values are shown and error bars are Standard Error of the Mean (SEM), unless indicated differently.
Results
Both intrinsic and extrinsic apoptotic stimuli elicit cellular extrusion in epithelial monolayers
To determine if different apoptotic pathways could trigger cell extrusion and to establish where in the apoptotic pathway extrusion is activated, we investigated whether a variety of intrinsic and extrinsic apoptotic stimuli activate extrusion in Madin-Darby Canine Kidney (MDCK) cell monolayers. Although previous studies have found that both extrinsic and intrinsic pathways can activate extrusion, these studies were done in different cell types and with only one type of stimulus [4, 6, 7]. We treated MDCK monolayers with either etoposide, a topoisomerase II inhibitor that causes double-stranded DNA breaks and activates the intrinsic death pathway, or TRAIL (Tumor Necrosis Factor (TNF)-related apoptosis-inducing ligand) to activate the extrinsic pathway. We then assayed for the number of cells undergoing apoptotic cellular extrusion by counting the number of cells with a contractile actin ring that stained positively for the apoptosis marker, active caspase-3 (Fig. 1a–c). Both treatments increased the percentage of cells undergoing apoptotic cellular extrusion compared to controls (Fig. 1b, c). To determine the treatment at which apoptotic cellular extrusion is maximized, we used increasing doses of apoptotic stimuli. Doses higher than 1mM etoposide or 500μg/ml TRAIL destroyed the monolayer integrity, which impaired the ability of cells to extrude (Fig. S1 and S2). Therefore, compared to apoptosis studies in single cells, apoptotic cell extrusion can only be assessed when between 2–8% of the monolayer undergoes apoptosis. Because of this, all of our extrusion assays are conducted within this range of percentage of apoptotic cell extrusion.
Figure 1. Both intrinsic and extrinsic apoptotic stimuli elicit cellular extrusion in epithelial monolayers.

MDCK monolayers treated with DMSO, etoposide, cycloheximide (CHX) or TRAIL plus CHX were fixed and stained for DNA, actin and active caspase-3 (a). Insets show a magnified view of the contraction ring, the apoptotic extruding cell and the neighbor cells distributed in a rosette pattern, characteristic of extrusion. Apoptotic extruding cells (a) were quantified (b and c) from three independent experiments. Cells treated with TRAIL or CHX were stained for DNA, actin, active caspase-3, and active Bax (d) or cytochrome c (e). Cartoons in (d) illustrate the microscope focal planes; the bottom view focuses on the contraction ring of the extruding cell and the top view focuses on the cell extruded out of the layer. Insets in (e) show the cytochrome c distribution of a live neighbor cell (bottom view) and of an extruding cell (top view). Scale bars, 10 μm. The number of cells counted for statistical analysis in (b) and (c) was as followed: DMSO (n = 1046), Etoposide (n = 1449), +CHX/−TRAIL- (n = 1425), and +CHX/+TRAIL(n = 1306).
Additionally, treatment with camptothecin, a topoisomerase I inhibitor, or Fas ligand (Fas-L), which activate the intrinsic or extrinsic pathways of cell death respectively, produced similar results to those of etoposide and TRAIL (data not shown). The same treatments in another epithelial cell line, human bronchial (HBE), gave similar results (Fig. 2e and 3d) and all cells undergoing apoptosis also undergo extrusion. Together, these results suggest that multiple types of epithelia can trigger extrusion in response to a variety of intrinsic or extrinsic apoptotic stimuli. These results also suggest that the extrusion pathway should initiate downstream of where the extrinsic and intrinsic apoptosis pathways converge.
Figure 2. Over-expression of Bcl-2 or knockdown of Bax and Bak blocks cell extrusion induced by intrinsic apoptosis stimuli.
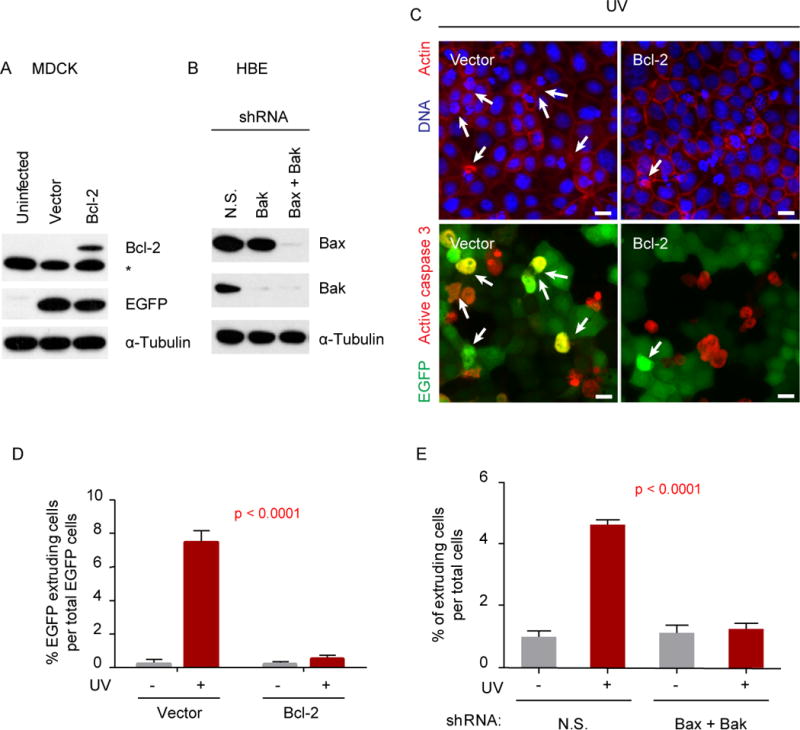
Immunoblot analysis of Bcl-2 in MDCK (a) or Bax and Bak in HBE (b) cells transduced with the indicated constructs. In (a), *denotes non-specific immunogen; and in (b), N.S. stand for non-specific shRNA. Extrusion in MDCK monolayers with over-expression of Bcl-2 block cell death and extrusion, where arrows indicate apoptotic extruding EGFP-positive cells (c). The EGFP-negative cells in the vector control and Bcl-2 monolayers (c) are internal control cells. Note that internal control cells are extruding in both Vector and Bcl-2 monolayers. Scale bars, 10 μm. The number of apoptotic extruding cells was quantified (d and e) from three independent experiments. The p values in (d) and (e) correspond to t-test analyses of the experiments indicated with red bars. The number of cells counted for statistical analysis in (d) and (e) were as followed: Vector −UV=790, Vector +UV=619, Bcl2 −UV=1319, Bcl2 +UV=1050, NS −UV=1907, NS +UV=2078, Bax/Bak −UV=2047, and Bax/Bak +UV=2356.
Figure 3. Neither over-expression of Bcl-2 nor Bax/Bak knockdown blocks cell extrusion induced by extrinsic apoptosis stimuli.

Extrusion in MDCK and HBE monolayers transduced with Bcl-2 or Bax/Bak shRNAs were treated with TRAIL and CHX (a and b, respectively). Arrows in (a) and (b) show apoptotic extruding cells. In (a), the arrows in upper panels point to closed actin rings with condensed and fragmented DNA and those in lower panels show EGFP-positive cells with caspase-3 activity. The EGFP-negative cells in (a) are internal control cells. The number of apoptotic extruding cells was quantified (c and d) from three independent experiments. The p values in (c) and (d) correspond to t-test analyses of the experiments indicated with red bars. Monolayers treated with TRAIL and CHX were stained for DNA, actin, and either active Bax or cytochrome c (e). Bottom views show actin rings of extruding cells while insets of top views show the apoptotic extruding cell features. The second of the Bcl-2 panels shows two adjacent extruding cells positive for active Bax: one EGFP-positive and the other EGFP-negative (internal control). Scale bars, 10 μm. The number of cells counted for statistical analysis in (c) and (d) were as followed: Vector +CHX/−TRAIL=905, Vector +CHX/+TRAIL=681, Bcl2 +CHX/−TRAIL=1288, Bcl2 +CHX/+TRAIL=1146, NS +CHX/−TRAIL=924, NS +CHX/+TRAIL=850, Bax/Bak +CHX/−TRAIL=1157, and Bax/Bak +CHX/+TRAIL=1004.
Both intrinsic and extrinsic apoptotic pathways target the mitochondrial pathway during apoptotic cellular extrusion
The extrinsic apoptotic pathway can directly activate executor caspases such as caspase 3 through the initiator caspases 8 and 10, skipping the mitochondrial pathway [16–18]. However, the extrinsic pathway does have the ability to induce mitochondrial outer membrane permeabilization (MOMP) for release of mitochondrial apoptotic factors such as cytochrome c and Smac/Diablo that will facilitate the activation of caspases and enhance the execution of apoptosis. This process occurs through the proteolytic activation of the BH3-only protein Bid by caspase 8, which in turn activates the multidomain pro-apoptotic protein Bax. Activated Bax translocates to the mitochondria where it oligomerizes to form pores and permeabilize the mitochondrial outer membrane [16, 19]. Therefore, since both intrinsic and extrinsic apoptotic pathways have the ability to target the mitochondria during apoptosis, it is possible that the mitochondria, through the release of a sequestered factor, could play a role in the activation of the extrusion machinery.
To test this possibility, we investigated if extrinsic and intrinsic stimuli target the mitochondria during apoptotic cell extrusion by immunostaining MDCK monolayers for cytochrome c and the activated form of Bax after inducing apoptosis with either etoposide or TRAIL (Fig. 1d, e and S3). All apoptotic extruding cells, triggered by either etoposide or TRAIL treatment, show strong staining for the active form of Bax (as represented in Fig. 1d, S3a). Cytochrome c staining is also diffuse in all apoptotic extruding cells analyzed, characteristic of cytoplasmic cytochrome c release after mitochondrial permeabilization, when compared to the punctate, mitochondrial staining seen in live cells (seen in bottom view of Fid 1e and S3b). These results show that both the extrinsic and intrinsic pathways activate mitochondrial permeabilization during apoptotic cell extrusion and suggested that mitochondria might sequester factors required for activation of the extrusion pathway during apoptosis.
Either overexpression of Bcl-2 or knockdown of Bax and Bak block cell extrusion via the intrinsic but not extrinsic apoptotic pathway
To determine the potential role of mitochondria in the activation of cellular extrusion, we blocked its permeabilization, and evaluated if cellular extrusion was blocked when the intrinsic pathway of apoptosis was stimulated. To block MOMP, and therefore the release of mitochondrial factors during apoptosis, we use two approaches: overexpression of the anti-apoptotic protein Bcl-2 or knockdown the pro-apoptotic genes Bax and Bak. To do this, we transduced MDCK cells with a vector containing a Bcl-2-IRES-EGFP cassette or sequentially transduced HBE cells with vectors containing a Bax shRNA and a Bak shRNA sequence (Fig. 2a, b). For the respective controls, we transduced MDCK cells with an empty vector (IRES-EGFP) or HBE cells with non-specific shRNA sequence (Fig 2a, b). Epithelial monolayers overexpressing Bcl-2 or Bax/Bak knockdown were treated with UV-C irradiation or etoposide and scored for their abilities to undergo apoptosis and extrusion. In addition to apoptosis, overexpression of Bcl-2 or downregulation of both Bax and Bak inhibited cell extrusion (Fig 2c–e and S4), suggesting that mitochondria permeabilization is required for the activation of the extrusion pathway. We obtained similar results when we over-expressed Bcl-2 in HBE cells (data not shown). Therefore, the signal that initiates extrusion could either be sequestered in the mitochondria or activated downstream mitochondrial permeabilization (i.e. caspase activation).
Although our data show TRAIL targets the mitochondria during apoptotic cell extrusion, it might not require MOMP to induce apoptosis, as it can directly activate executioner caspases via caspase 8 and 10. If the extrusion-initiating signal is sequestered in the mitochondria, then blocking MOMP after initiating the extrinsic pathway should not allow cells to extrude. However, if the extrusion initiating signal is activated downstream of caspase activation, then TRAIL-dependent activation of the extrinsic death pathway should cause both apoptosis and extrusion even when MOMP is blocked. To test between these models, we treated MDCK and HBE monolayers with TRAIL and evaluated cell death and extrusion when MOMP is blocked by Bcl-2 overexpression or Bax/Bak knockdown (Fig. 3a–d). Neither overexpression of Bcl-2 nor Bax/Bak knockdown blocked apoptosis induced by TRAIL treatment, as expected from other studies [16, 18]. Importantly, cell extrusion also occurred in these lines, favoring the hypothesis that the extrusion-initiating signal is activated downstream caspase activation.
To verify that TRAIL-induced apoptotic cell extrusion occurred independently of mitochondrial permeabilization, we immunostained TRAIL-treated monolayers for active Bax and cytochrome c (Fig 3e). Apoptotic extruding cells from monolayers where Bcl-2 was overexpressed still showed release of cytochrome c; however, cytochrome c staining appeared punctate when Bax and Bak were knocked down (Fig 3e, right panel insets). Although some mitochondrial permeability may occur through other mechanisms (i.e. VDAC) [20, 21], lack of complete diffuse cytochrome c staining suggested that Bax/Bak knockdown blocked most of MOMP. Correspondingly, activated Bax was absent in apoptotic extruding cell where Bax and Bak were knocked down, yet Bcl-2 overexpression still resulted in activated Bax staining (Fig 3e, left panel insets). Failure of Bcl-2 overexpression to block TRAIL-induced Bax activation and cytochrome c release suggests that other anti-apoptotic Bcl-2 family members, rather than Bcl-2, could efficiently inhibit Bax activation and cytochrome c release triggered by TRAIL (i.e. Bcl-xL, Mcl-1, Bfl-1 and Bcl-w) [22–24]. However, because knockdown of Bak/Bax blocked both Bax activation and significant cytochrome c release but still allowed apoptotic extrusion to occur; we reasoned that the caspase cascade may be important for cellular extrusion.
Epithelial cellular extrusion requires caspase activity for the proper formation and contraction of the actomyosin ring
To test the role of caspase activity for activating apoptotic cell extrusion, we inhibited caspase activation using the pan-caspase inhibitor benzyloxycarbonyl-Val-Ala-Asp (OMe)-fluoromethyl-ketone (z-VAD-fmk) after UV irradiation or etoposide-treatment. As expected, we observed an almost complete inhibition of apoptotic cell death, as evaluated by caspase activation and DNA morphology (Fig 4a, c). Since either pharmacological or genetic inhibition of caspase following apoptotic stimuli could lead to other cell death mechanisms such as necrosis [25–29] or caspase-independent cell death [30–33], we evaluated the High Motility Group Box 1 (HMGB1) marker localization to explore the viability of the z-VAD-fmk–treated UV-irradiated cells. In living cells, HMGB1 is localized in the nucleus of interphase cells. During apoptosis, HMGB1 remains in the fragmented nucleus or is released into the cytoplasm. During necrosis or other forms of non-apoptotic cell death, HMGB1 is released extracellularly and its staining is lost [15, 34, 35].
Figure 4. Epithelial cellular extrusion requires caspase activity for proper formation and contraction of the actomyosin ring.

MDCK monolayers incubated with 50 μM zVAD-fmk (caspase inhibitor) and irradiated with UV-C were immuno-stained for DNA and HMGB1 as well as active caspase-3, cytochrome c, AIF or actin (a and b). Apoptotic (with active caspase-3) and necrotic cells (negative for both active caspase-3 and HMGB1) as well as extruding and uncontracted cells were quantified in monolayers subjected to treatments indicated in (c) and (d). Results shown represent mean, where error bars are ± SEM from three independent experiments. The number of cells counted for statistical analysis in (c) and (d) were as followed: DMSO −UV=1832, DMSO +UV=1952, zVAD −UV=1899, and zVAD +UV=1875.MDCK monolayers stably expressing the fusion HMGB1-EGFP were treated with UV-C irradiation with or without zVAD (e) and time-lapsed every 10 minutes for 12 hours. Propidium iodide (PI) was added to monitor permeability of the dying cells. Arrows in the top panels show an apoptotic extruding cell, where asterisks in the second panel highlight rosette pattern formed around the dying cell, which is characteristic of cell extrusion. Arrows in the third panel show a necrotic, uncontracted cell, which loses its HMGB1 (bottom panel) and becomes PI-positive and bursts. Arrowheads in the third panel point to another cell undergoing necrosis at a faster rate than the cell with arrows. In the bottom panel, numbers indicate four cells changing positions to illustrate the stochastic movement of cells in the epithelium while yellow arrows indicate the direction the cell moves. Scale bars, 10 μm.
To evaluate cell death in the presence of the pan-caspase inhibitor, MDCK monolayers incubated with z-VAD-fmk and treated with either UV-C or etoposide were immunostained for HMGB1 in combination with DNA, active caspase-3, cytochrome C or AIF (Fig. 4a). Very few cells (1.5%) within these monolayers were apoptotic, as characterized by positive caspase-3 and HMGB1 immunostaining, and chromatin condensation and fragmentation (Fig. 4a, c). A higher percentage (5.3%) of cells with z-VAD-fmk showed neither HMGB1 nor caspase-3 staining (Fig. 4a, c). The sum of both percentages corresponds to the percentage of cells that would normally die through apoptosis in the absence of z-VAD-fmk (Fig 4c). Further, the mitochondria in the HMGB1 negative cells appear to be permeabilized, as cytochrome c was absent from mitochondria (Fig 4a), confirming that these cells were indeed dying, although through a cell death mechanism different from apoptosis. To determine whether these cells were dying through caspase-independent cell death or necrosis, we first evaluated apoptosis inducing factor (AIF) localization. During caspase-independent cell death, AIF is released from the mitochondrial outer membrane space and translocates to the nucleus to mediate chromatin condensation and large-scale DNA fragmentation [30, 36, 37]. Although AIF is absent from mitochondria, it does not translocate into the nucleus, suggesting that these cells were probably dying through necrosis, rather than caspase-independent cell death (Fig. 4a). Their DNA appearance, the absence of HMGB1 and caspase staining as well as their positive staining for propidium iodide (PI), strongly suggest that the z-VAD-fmk-treated cells were dying through necrosis (Fig 4a, e).
When evaluating whether cells extrude from monolayers treated with z-VAD-fmk, we found that the small population of apoptotic cells with caspase-3 activity undergo cell extrusion, while the HMGB1 negative cells do not (Fig. 4d). Instead, HMGB1 negative cells show a slight accumulation of actin at the cell cortex where they contact their neighboring cells, resembling a non-continuous actin ring (Fig 4b). Although the necrotic cells appear to form a reduced actin extruding ring, they remain uncontracted in the monolayer compared to control cells at two hours post-irradiation (Fig. 4b, d).To determine whether these cells eventually extrude, we filmed these cells by time-lapse microscopy by treating MDCK cells stably expressing a HMGB1-EGFP protein fusion with z-VAD-fmk and UV-C (Fig. 4e). To monitor cell permeabilization, we added PI to the culture medium. As previously observed, control monolayers treated with the DMSO and UV irradiation undergo apoptotic cell extrusion one to two hours post-irradiation, as characterized by contracting, blebbing cells surrounded by their neighbor cells in a rosette pattern (Fig. 4e, asterisks). These apoptotic cells retain HMGB1 in their nuclei and are negative for PI staining throughout the extrusion process until the extruding ring has been closed at 70–80 minutes after extrusion initiates (Fig. 4e, movie 1). By contrast, when caspases are blocked, cells undergo necrosis, lose HMGB1 and become PI-permeant, while no extrusion occurs (Fig. 4b, e, Note lack of rosette pattern). In this case, necrotic cells have impaired plasma membrane integrity and should compromise the epithelium protective barrier, compared to monolayers where apoptotic cells extrude. Nevertheless, although necrotic cells are unable to trigger the extrusion machinery, they are eventually removed from the epithelium by a passive cell movement. The movement by constant cell rearrangements in the epithelium acts to shove the necrotic cells out of the way as their integrity declines (Fig. 4e, movies 2 and 3). These movies are representative of other movies, where apoptotic extrusion occurs within 90 minutes, whereas necrotic cell removal can take up to 5 hours (Fig. S5). This wide variation is likely due to variance in the stochastic movements of cells.
Discussion
Although several studies both in vivo and in vitro show a connection between cellular extrusion and apoptosis [4–9], little was previously known about how the pathways of apoptosis and extrusion are linked. In this study, we found that several intrinsic (UV irradiation, etoposide, camptothecin and serum deprivation) and extrinsic (Fas-L and TRAIL cytokines) apoptotic stimuli induce cells to extrude from monolayers of two different cell lines (MDCK and HBE). We also found that caspase activity is required for cellular extrusion. When caspases are inhibited, cells become necrotic and are removed from epithelia by random epithelial cell movement. Taken together, our results lead to a model whereby all known apoptotic signals feed into a conserved step in the pathway important for apoptotic extrusion—caspase activation (Fig. 5).
Figure 5. A model for apoptotic regulation of cellular extrusion.

This model illustrates pathways that can link apoptosis with extrusion. Here, caspases may orchestrate the removal of the apoptotic cells by activating several signals. Question mark suggests that other non-apoptotic signals may also contribute to formation of actin extruding ring, which partially forms in the presence of caspase inhibitors.
Caspases proteolytically inactivate or activate a variety of targets, leading to the controlled pathway of morphological changes and break down of a cell during apoptosis. Caspases also regulate several non-apoptotic processes including activation and proliferation of T and B-lymphocytes, cell differentiation during keratinization of skin and spermatogenesis, cell motility and cell shaping [39–41]. In addition, caspase-8 activity has been suggested to play a role in cell survival [42–43]. In this work, we find that caspase activity is required to initiate the removal of dying cells from epithelia. Our findings reveal a new role for caspases in a non-apoptotic event—extrusion.
Two models were previously proposed to explain the regulation of cellular extrusion. One model proposes that the force driving cellular extrusion comes from the dying cell itself and thus it is a cell autonomous mechanism [10, 44]. The second one, a non-cell autonomous model, proposes that the contraction of an actomyosin ring formed in the neighboring cells provides the main force for the removal of the dying cell [4]. However, because extrusion is a mechanism that requires multiple coordinated caspase-dependent events, a combination of these two models may occur (Fig. 5). During apoptosis, caspases target important actin and myosin regulators such as Gas2, LIM-kinase 1 and ROCK, which activate contractile forces required for apoptotic cell blebbing [45–49]. These forces, as initially proposed by Mills et al. [10], could be an important component of actomyosin contractions occurring during extrusion. Therefore, even though contraction of the actomyosin ring in the surrounding cells is required for extrusion, contractions in the dying cell may also be required to ensure efficient extrusion (Fig. 5).
While contractions to remove the dying cell occur, caspases could also control the reorganization of adherens and tight junctions, which are required for extrusion. This reorganization plays a crucial role during extrusion not only for the release of the dying cell from the epithelium, but also for the maintenance of the epithelial protective barrier. As shown by Kessler and Müller [50, 51], caspases trigger a progressive and orchestrated disassembly of adherens junctions. Early in apoptosis, cleavage of β-catenin promotes the removal of E-cadherin from the membrane. Later in apoptosis, all adherens junction components are removed from the membrane. At the same time, while the apoptotic cell is released, the neighboring cells form new adherens junctions, closing the gap the apoptotic cell would leave. In addition, the detachment of the apoptotic cell from the extracellular matrix is also regulated by caspases [10].
Although caspase activity is required for cellular extrusion, other upstream signals may be required to initiate early steps in extrusion activation. Indeed, we find that even when caspases are inhibited with zVAD, a noncontinuous ring of actin still forms around the dying cell; however, it is unclear whether this ring forms in the live or dying cells. Therefore, other upstream signals could partially participate in the formation of the actomyosin ring, but without caspase activation, the extruding ring would not fully form or contract (Fig. 5).
In our experiments when blocking caspases inhibited apoptosis, cells instead become necrotic and did not extrude, but were eventually removed by random movements of surrounding cells. It is important to note, however, that other studies find that necrosis of single cells due to laser ablation heal by a purse-string mechanism that mimics extrusion [38, 52]. Although this mechanism might be different from extrusion, these cells may still contain extrusion-inducing signals that the necrotic cells in our study would have lost over time. Therefore, how an epithelial cell dies within the epithelium could have significant effects on the barrier function of the epithelium. If a cell dies by apoptosis or sudden necrosis, such as a wound, the barrier will be preserved, whereas if it dies by necrosis following impaired apoptosis, the barrier of the epithelium could become compromised.
The fact that apoptosis connects with the extrusion pathway at a late point in the apoptotic pathway ensures that any apoptotic stimulus will feed into the extrusion pathway. The requirement of caspase activity also suggests that caspase activity functions as an important checkpoint in the removal of cells from the monolayer only after a cell has passed the point of no return in the apoptosis pathway. Early events in the apoptosis pathway, therefore, might not trigger extrusion as a way to give cells another chance to fix any potential threat before committing to apoptosis, such as DNA damage, protein agglomeration, ER stress, or damaged mitochondria.
Our findings establish the connection between apoptosis and cellular extrusion and bring new insights in the molecular basis of cellular extrusion. Our work also reveals another non-apoptotic role for caspases, regulation of cellular extrusion. However, further investigations will need to determine which caspases participate in the activation of extrusion and which factors targeted by caspases play decisive roles during this event.
Supplementary Material
Figure S1. Etoposide-induced apoptotic dose response curve.
MDCK monolayers were treated with increasing concentrations of etoposide (a and b) to determine the concentration at which the percentage of extrusion is maximized without compromising the monolayer integrity. Circles show holes in the monolayers (a) due to the excessive loss via apoptosis, thereby preventing further extrusion. Although, maximal extrusion occurs at 1000 μM etoposide (b), we chose 500 μM etoposide, which was less stressful for the monolayer (a). Scale bars, 10 μm.
Figure S2. TRAIL-induced apoptotic dose response curve.
MDCK monolayers were treated with 100 ng/ml CHX and increasing concentrations of TRAIL (a and b) to determine the concentration at which the percentage of extrusion is maximized without compromising the monolayer integrity. For these analyses, cells were stained for DNA, actin and active caspase-3. The white circle shows a hole in the monolayers (a) due to the excessive loss via apoptosis, preventing extrusion. Although, maximal extrusion occurs at 500 ng/ml TRAIL (b), we chose 100 ng/ml TRAIL, which was less stressful for the monolayer (a). Scale bars, 10 μm.
Figure S3. Etoposide-induced apoptotic cell extrusion targets the mitochondrial pathway of apoptosis.
MDCK monolayers treated with etoposide were stained for DNA, actin, active caspase-3, and active Bax (a) or cytochrome c (b). The bottom views focus on the actin contractile ring and the top views focus on the cell extruded out of the layer. Arrows point to the apoptotic extruding cells. Scale bars, 10 μm.
Figure S4. Downregulation of Bax and Bak blocks cell extrusion induced by an intrinsic apoptosis stimulus.
HBE monolayers transduced with either Bax and Bak or non-specific (N.S.) shRNA constructs were treated with UV-C irradiation, and immunostained for DNA, actin, and active caspase-3. Arrows in the top panels show apoptotic extruding cells while arrows in lower panel show the actin contractile rings. Scale bars, 10 μm.
Figure S5. Time of cell removal.
MDCK monolayers stably expressing the fusion HMGB1-EGFP incubated with either DMSO or the caspase inhibitor zVAD and exposed to UV-C irradiation were time-lapsed every 10 minutes for 12 hours. Propidium iodide (PI) was added to monitor permeability of the dying cells. The graph shows time ranges required for removal of apoptotic and necrotic cell. Error bars represent standard deviation.
Supplementary Movie 1. Extrusion of an apoptotic cell.
MDCK monolayers stably expressing the fusion HMGB1-EGFP incubated with DMSO and exposed to UV-C irradiation were timelapsed every 10 minutes for 12 hours. Propidium iodide (red) labels permeable dying cells.
Supplementary Movie 2. Slow burst of a necrotic cell.
MDCK monolayers stably expressing the fusion HMGB1-EGFP incubated with the caspase inhibitor zVAD and exposed to UV-C irradiation were time-lapsed every 10 minutes for 12 hours. Propidium iodide (red) labels permeable dying cells.
Supplementary Movie 3. Rapid burst of another necrotic cell.
MDCK monolayers incubated with the caspase inhibitor zVAD and exposed to UV-C irradiation were time-lapsed every 10 minutes for 12 hours. Propidium iodide (red) labels permeable dying cells.
Acknowledgments
We thank Dr. Bryan Welm for use of his microscope, and Drs. Alana Welm, Marco Bianchi, and James Bear for providing the lentiviral and other constructs, and Drs. Karl Matlin and Gruenert for cell lines. We would also like to thank Anna Roth, James Laird and Tony Trinh for their participation and support in this work. This work was supported by a National Institute of Health Innovator Award No. DP2 OD002056-01 to J. Rosenblatt and P30 CA042014 awarded to The Huntsman Cancer Institute for core facilities.
References
- 1.Merritt AJ, Potten CS, Kemp CJ, Hickman JA, Balmain A, Lane DP, Hall PA. The role of p53 in spontaneous and radiation-induced apoptosis in the gastrointestinal tract of normal and p53-deficient mice. Cancer Res. 1994;54:614–617. [PubMed] [Google Scholar]
- 2.Gobe GC, Buttyan R, Wyburn KRL, Etheridge MR, Smith PJ. Clusterin expression and apoptosis in tissue remodeling associated with renal regeneration. Kidney Int. 1995;47:411–420. doi: 10.1038/ki.1995.54. [DOI] [PubMed] [Google Scholar]
- 3.Villar CC, Zhao XR. Candida albicans induces early apoptosis followed by secondary necrosis in oral epithelial cells. Mol Oral Microbiol. 2010 Jun;25(3):215–25. doi: 10.1111/j.2041-1014.2010.00577.x. [DOI] [PubMed] [Google Scholar]
- 4.Rosenblatt J, Raff MC, Cramer LP. An epithelial cell destined for apoptosis signals its neighbors to extrude it by an actin- and myosin-dependent mechanism. Curr Biol. 2001;11(23):1847–57. doi: 10.1016/s0960-9822(01)00587-5. [DOI] [PubMed] [Google Scholar]
- 5.Nagai H, Kalnins VI. Normally occurring loss of single cells and repair of resulting defects in retinal pigment epithelium in situ. Exp Eye Res. 1996;62(1):55–61. doi: 10.1006/exer.1996.0007. [DOI] [PubMed] [Google Scholar]
- 6.Peralta Soler A, Mullin JM, Knudsen KA, Marano CW. Tissue remodeling during tumor necrosis factor-induced apoptosis in LLC-PK1 renal epithelial cells. Am J Physiol. 1996;270(5 Pt 2):F869–79. doi: 10.1152/ajprenal.1996.270.5.F869. [DOI] [PubMed] [Google Scholar]
- 7.Beeman NE, Baumgartner HK, Webb PG, Schaack JB, Neville MC. Disruption of occludin function in polarized epithelial cells activates the extrinsic pathway of apoptosis leading to cell extrusion without loss of transepithelial resistance. BMC Cell Biol. 2009 Dec 9;10:85. doi: 10.1186/1471-2121-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pentecost M, Otto G, Theriot JA, Amieva MR. Listeria monocytogenes invades the epithelial junctions at sites of cell extrusion. PLoSPathog. 2007;2(1):e3. doi: 10.1371/journal.ppat.0020003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ninov N, Chiarelli DA, Martín-Blanco E. Extrinsic and intrinsic mechanisms directing epithelial cell sheet replacement during Drosophila metamorphosis. Development. 2007;134:367–379. doi: 10.1242/dev.02728. [DOI] [PubMed] [Google Scholar]
- 10.Mills JC, Stone NL, Pittman RN. Extranuclear apoptosis. The role of the cytoplasm in the execution phase. J Cell Biol. 1999;146(4):703–8. doi: 10.1083/jcb.146.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Parijs L, Refaeli Y, Lord JD, Nelson BH, Abbas AK, Baltimore D. Uncoupling IL-2 signals that regulate T cell proliferation, survival, and Fas-mediated activation-induced cell death. Immunity. 1999;11(3):281–8. doi: 10.1016/s1074-7613(00)80103-x. [DOI] [PubMed] [Google Scholar]
- 12.Welm AL, Sneddon JB, Taylor C, Nuyten DS, van de Vijver MJ, Hasegawa BH, Bishop JM. The macrophage-stimulating protein pathway promotes metastasis in a mouse model for breast cancer and predicts poor prognosis in humans. Proc Natl Acad Sci U S A. 2007;104(18):7570–5. doi: 10.1073/pnas.0702095104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kepp O, Rajalingam K, Kimmig S, Rudel T. Bak and Bax are non-redundant during infection-and DNA damage-induced apoptosis. EMBO J. 2007;26(3):825–34. doi: 10.1038/sj.emboj.7601533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cai L, Marshall TW, Uetrecht AC, Schafer DA, Bear JE. Coronin 1B coordinates Arp2/3 complex and cofilin activities at the leading edge. Cell. 2007;128(5):915–29. doi: 10.1016/j.cell.2007.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 16.Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, Debatin KM, Krammer PH, Peter ME. Two CD95 (APO-1/Fas) signaling pathways. Embo J. 1998;17(6):1675–87. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walczak H, Krammer PH. The CD95 (APO-1/Fas) and the TRAIL (APO-2L) apoptosis systems. Exp Cell Res. 2000;256(1):58–66. doi: 10.1006/excr.2000.4840. Review. [DOI] [PubMed] [Google Scholar]
- 18.Debatin KM, Krammer PH. Death receptors in chemotherapy and cancer. Oncogene. 2004;23:2950–2966. doi: 10.1038/sj.onc.1207558. [DOI] [PubMed] [Google Scholar]
- 19.Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94(4):481–90. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- 20.Shimizu S, Narita M, Tsujimoto Y. Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature. 1999;399(6735):483–7. doi: 10.1038/20959. Erratum in: Nature 407(6805):767. [DOI] [PubMed] [Google Scholar]
- 21.Tsujimoto Y, Shimizu S. VDAC regulation by the Bcl-2 family of proteins. Cell Death Differ. 2000;7:1174–1181. doi: 10.1038/sj.cdd.4400780. [DOI] [PubMed] [Google Scholar]
- 22.Borner C. The Bcl-2 protein family: sensors and checkpoints for life-or-death decisions. MolImmunol. 2003;39(11):615–47. doi: 10.1016/s0161-5890(02)00252-3. [DOI] [PubMed] [Google Scholar]
- 23.Deng J, Carlson N, Takeyama K, Dal Cin P, Shipp M, Letai A. BH3 profiling identifies three distinct classes of apoptotic blocks to predict response to ABT-737 and conventional chemotherapeutic agents. Cancer Cell. 2007 Aug;12(2):171–85. doi: 10.1016/j.ccr.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Chonghaile TN, Letai A. Mimicking the BH3 domain to kill cancer cells. Oncogene. 2008;27(Suppl 1):S149–57. doi: 10.1038/onc.2009.52. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vercammen D, Brouckaert G, Denecker G, Van de Craen M, Declercq W, Fiers W, Vandenabeele P. Dual signaling of the Fas receptor: initiation of both apoptotic and necrotic cell death pathways. J Exp Med. 1998;188(5):919–30. doi: 10.1084/jem.188.5.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawahara A, Ohsawa Y, Matsumura H, Uchiyama Y, Nagata S. Caspase-independent cell killing by Fas-associated protein with death domain. J Cell Biol. 1998;143(5):1353–60. doi: 10.1083/jcb.143.5.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chautan M, Chazal G, Cecconi F, Gruss P, Golstein P. Interdigital cell death can occur through a necrotic and caspase-independent pathway. Curr Biol. 1999;9(17):967–70. doi: 10.1016/s0960-9822(99)80425-4. [DOI] [PubMed] [Google Scholar]
- 28.Matsumura H, Shimizu Y, Ohsawa Y, Kawahara A, Uchiyama Y, Nagata S. Necrotic death pathway in Fas receptor signaling. J Cell Biol. 2000;151(6):1247–56. doi: 10.1083/jcb.151.6.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edinger AL, Thompson CB. Death by design: apoptosis, necrosis and autophagy. Curr Opin Cell Biol. 2004;16(6):663–9. doi: 10.1016/j.ceb.2004.09.011. Review. [DOI] [PubMed] [Google Scholar]
- 30.Daugas E, Susin SA, Zamzami N, Ferri KF, Irinopoulou T, Larochette N, Prevost MC, Leber B, Andrews D, Penninger J, Kroemer G. Mitochondrio-nuclear translocation of AIF in apoptosis and necrosis. FASEB J. 2000;14:729–739. [PubMed] [Google Scholar]
- 31.Susin SA, Daugas E, Ravagnan L, et al. Two distinct pathways leading to nuclear apoptosis. J Exp Med. 2000;192:571–80. doi: 10.1084/jem.192.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loeffler M, Daugas E, Susin SA, et al. Dominant cell death induction by extramitochondrially targeted apoptosis-inducing factor. FASEBJ. 2001;15:758–67. doi: 10.1096/fj.00-0388com. [DOI] [PubMed] [Google Scholar]
- 33.Bröker LE, Kruyt FA, Giaccone G. Cell death independent of caspases: a review. Clin Cancer Res. 2005;11(9):3155–62. doi: 10.1158/1078-0432.CCR-04-2223. Review. [DOI] [PubMed] [Google Scholar]
- 34.Rovere-Querini P, Capobianco A, Scaffidi P, Valentinis B, Catalnotti F, Giazzon M, Dumitiriu IE, Muller S, Iannacone M, Traversari C, Bianchi M, Manfredi AA. HMGB1 is an endogenous immune adjuvant released by necrotic cells. EMBO Rep. 2004;5:825–830. doi: 10.1038/sj.embor.7400205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thorburn J, Frankel AE, Thorburn A. Regulation of HMGB1 release by autophagy. Autophagy. 2009;5(2):247–9. doi: 10.4161/auto.5.2.7552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, Larochette N, Goodlett DR, Aebersold R, Siderovski DP, Penninger JM, Kroemer G. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397:441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- 37.Candé C, Cecconi F, Dessen P, Kroemer G. Apoptosis-inducing factor (AIF): key to the conserved caspase-independent pathways of cell death? J Cell Sci. 2002;115(Pt 24):4727–34. doi: 10.1242/jcs.00210. Review. [DOI] [PubMed] [Google Scholar]
- 38.Tamada M, Perez TD, Nelson WJ, Sheetz MP. Two distinct modes of myosin assembly and dynamics during epithelial wound closure. J Cell Biol. 2007;176(1):27–33. doi: 10.1083/jcb.200609116. Erratum in: J Cell Biol. 2007 Feb 12;176(4):545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kennedy NJ, Kataoka T, Tschopp J, Budd RC. Caspase activation is required for T cell proliferation. J Exp Med. 1999;190:1891. doi: 10.1084/jem.190.12.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chun HJ, Zheng L, Ahmad M, Wang J, Speirs CK, Siegel RM, Dale JK, Puck J, Davis J, Hall CG, Skoda-Smith S, Atkinson TP, Straus SE, Lenardo MJ. Pleiotropic defects in lymphocyte activation caused by caspase-8 mutations lead to human immunodeficiency. Nature. 2002;419(6905):395–9. doi: 10.1038/nature01063. [DOI] [PubMed] [Google Scholar]
- 41.Kuranaga E, Miura M. Nonapoptotic functions of caspases: caspases as regulatory molecules for immunity and cell-fate determination. Trends Cell Biol. 2007;17(3):135–44. doi: 10.1016/j.tcb.2007.01.001. Review. [DOI] [PubMed] [Google Scholar]
- 42.Kang TB, Ben-Moshe T, Varfolomeev EE, Pewzner-Jung Y, Yogev N, Jurewicz A, Waisman A, Brenner O, Haffner R, Gustafsson E, Ramakrishnan P, Lapidot T, Wallach D. Caspase-8 serves both apoptotic and nonapoptotic roles. J Immunol. 2004;173(5):2976–84. doi: 10.4049/jimmunol.173.5.2976. [DOI] [PubMed] [Google Scholar]
- 43.Yu L, Alva A, Su H, Dutt P, Freundt E, Welsh S, Baehrecke EH, Lenardo MJ. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science. 2004;304(5676):1500–2. doi: 10.1126/science.1096645. [DOI] [PubMed] [Google Scholar]
- 44.Wang F, Wang F, Zou Z, Liu D, Wang J, Su Y. Active deformation of apoptotic intestinal epithelial cells with adhesion-restricted polarity contributes to apoptotic clearance. Lab Invest. 2010 Nov 1; doi: 10.1038/labinvest.2010.182. [DOI] [PubMed] [Google Scholar]
- 45.Brancolini C, Benedetti M, Schneider C. Microfilament reorganization during apoptosis: the role of Gas2, a possible substrate for ICE-like proteases. EMBO J. 1995;14(21):5179–90. doi: 10.1002/j.1460-2075.1995.tb00202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sgorbissa A, Benetti R, Marzinotto S, Schneider C, Brancolini C. Caspase-3 and caspase-7 but not caspase-6 cleave Gas2 in vitro: implications for microfilament reorganization during apoptosis. J Cell Sci. 1999;112(Pt 23):4475–82. doi: 10.1242/jcs.112.23.4475. [DOI] [PubMed] [Google Scholar]
- 47.Sebbagh M, Renvoize C, Hamelin J, Riche N, Bertoglio J, Breard J. Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing. Nat Cell Biol. 2001;3:346–352. doi: 10.1038/35070019. [DOI] [PubMed] [Google Scholar]
- 48.Coleman ML, Sahai EA, Yeo M, Bosch M, Dewar A, Olson MF. Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat Cell Biol. 2001;3:339–345. doi: 10.1038/35070009. [DOI] [PubMed] [Google Scholar]
- 49.Tomiyoshi G, Horita Y, Nishita M, Ohashi K, Mizuno K. Caspase-mediated cleavage and activation of LIM-kinase 1 and its role in apoptotic membrane blebbing. Genes Cells. 2004;9(6):591–600. doi: 10.1111/j.1356-9597.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- 50.Kessler T, Müller HA. Cleavage of Armadillo/beta-catenin by the caspase DrICE in Drosophila apoptotic epithelial cells. BMC Dev Biol. 2009 Feb 20;9:15. doi: 10.1186/1471-213X-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suzanne M, Steller H. Letting go: modification of cell adhesion during apoptosis. J Biol. 2009;8(5):49. doi: 10.1186/jbiol152. Epub 2009 May 28. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bement WM, Forscher P, Mooseker MS. A novel cytoskeletal structure involved in purse string wound closure and cell polarity maintenance. J Cell Biol. 1993;121(3):565–78. doi: 10.1083/jcb.121.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Etoposide-induced apoptotic dose response curve.
MDCK monolayers were treated with increasing concentrations of etoposide (a and b) to determine the concentration at which the percentage of extrusion is maximized without compromising the monolayer integrity. Circles show holes in the monolayers (a) due to the excessive loss via apoptosis, thereby preventing further extrusion. Although, maximal extrusion occurs at 1000 μM etoposide (b), we chose 500 μM etoposide, which was less stressful for the monolayer (a). Scale bars, 10 μm.
Figure S2. TRAIL-induced apoptotic dose response curve.
MDCK monolayers were treated with 100 ng/ml CHX and increasing concentrations of TRAIL (a and b) to determine the concentration at which the percentage of extrusion is maximized without compromising the monolayer integrity. For these analyses, cells were stained for DNA, actin and active caspase-3. The white circle shows a hole in the monolayers (a) due to the excessive loss via apoptosis, preventing extrusion. Although, maximal extrusion occurs at 500 ng/ml TRAIL (b), we chose 100 ng/ml TRAIL, which was less stressful for the monolayer (a). Scale bars, 10 μm.
Figure S3. Etoposide-induced apoptotic cell extrusion targets the mitochondrial pathway of apoptosis.
MDCK monolayers treated with etoposide were stained for DNA, actin, active caspase-3, and active Bax (a) or cytochrome c (b). The bottom views focus on the actin contractile ring and the top views focus on the cell extruded out of the layer. Arrows point to the apoptotic extruding cells. Scale bars, 10 μm.
Figure S4. Downregulation of Bax and Bak blocks cell extrusion induced by an intrinsic apoptosis stimulus.
HBE monolayers transduced with either Bax and Bak or non-specific (N.S.) shRNA constructs were treated with UV-C irradiation, and immunostained for DNA, actin, and active caspase-3. Arrows in the top panels show apoptotic extruding cells while arrows in lower panel show the actin contractile rings. Scale bars, 10 μm.
Figure S5. Time of cell removal.
MDCK monolayers stably expressing the fusion HMGB1-EGFP incubated with either DMSO or the caspase inhibitor zVAD and exposed to UV-C irradiation were time-lapsed every 10 minutes for 12 hours. Propidium iodide (PI) was added to monitor permeability of the dying cells. The graph shows time ranges required for removal of apoptotic and necrotic cell. Error bars represent standard deviation.
Supplementary Movie 1. Extrusion of an apoptotic cell.
MDCK monolayers stably expressing the fusion HMGB1-EGFP incubated with DMSO and exposed to UV-C irradiation were timelapsed every 10 minutes for 12 hours. Propidium iodide (red) labels permeable dying cells.
Supplementary Movie 2. Slow burst of a necrotic cell.
MDCK monolayers stably expressing the fusion HMGB1-EGFP incubated with the caspase inhibitor zVAD and exposed to UV-C irradiation were time-lapsed every 10 minutes for 12 hours. Propidium iodide (red) labels permeable dying cells.
Supplementary Movie 3. Rapid burst of another necrotic cell.
MDCK monolayers incubated with the caspase inhibitor zVAD and exposed to UV-C irradiation were time-lapsed every 10 minutes for 12 hours. Propidium iodide (red) labels permeable dying cells.


