Abstract
Clozapine can cause severe adverse effects yet it is associated with reduced mortality risk. We test the hypothesis this association is due to increased clinical monitoring and investigate risk of premature mortality from natural causes. We identified 14 754 individuals (879 deaths) with serious mental illness (SMI) including schizophrenia, schizoaffective and bipolar disorders aged ≥ 15 years in a large specialist mental healthcare case register linked to national mortality tracing. In this cohort study we modeled the effect of clozapine on mortality over a 5-year period (2007–2011) using Cox regression. Individuals prescribed clozapine had more severe psychopathology and poorer functional status. Many of the exposures associated with clozapine use were themselves risk factors for increased mortality. However, we identified a strong association between being prescribed clozapine and lower mortality which persisted after controlling for a broad range of potential confounders including clinical monitoring and markers of disease severity (adjusted hazard ratio 0.4; 95% CI 0.2–0.7; p = .001). This association remained after restricting the sample to those with a diagnosis of schizophrenia or those taking antipsychotics and after using propensity scores to reduce the impact of confounding by indication. Among individuals with SMI, those prescribed clozapine had a reduced risk of mortality due to both natural and unnatural causes. We found no evidence to indicate that lower mortality associated with clozapine in SMI was due to increased clinical monitoring or confounding factors. This is the first study to report an association between clozapine and reduced risk of mortality from natural causes.
Key words: clozapine, mortality, clinician contact, schizophrenia, schizoaffective disorder, bipolar affective disorder
Introduction
Individuals with serious mental illness (SMI), including schizophrenia, schizoaffective and bipolar disorder have 1.5–2.5 times the mortality risk of the general population1–3 and a 10–20 year reduction in life expectancy4 with the majority of the excess deaths being due to natural causes.1 Difficulty performing activities of daily living, substance misuse, adverse lifestyle choices, psychopathology and medications appear to contribute to the elevated mortality rate.5–7
Antipsychotic medications, the mainstay of SMI treatment, are associated with increased risk of physical morbidity and premature death in individuals with SMI.8–10 Importantly, mortality risk to these individuals may vary between the antipsychotics they are prescribed.11 A number of investigations, including several large scale cohort studies, have reported that clozapine has the lowest risk of all-cause mortality and suicide specifically compared to other antipsychotics.12–15 Clozapine is highly effective; however, its use is restricted owing to safety concerns regarding the risk of agranulocytosis, seizures, myocarditis and other adverse cardiovascular/respiratory effects in patients prescribed this antipsychotic.16,17 Consequently, clozapine can only be prescribed to individuals who receive at least monthly blood monitoring (with no signs of agranulocytosis) and is restricted to individuals with treatment-resistant SMI.
Despite treatment guidelines, substantial delays to clozapine initiation remain and antipsychotic polypharmacy and high doses are commonly used prior to clozapine.18 There is evidence that clozapine is under used, with calls for restrictions on clozapine to be reevaluated.15,18 However, clozapine is the only antipsychotic where routine specialist clinical contact is mandated. Frequent mental health assessments may be protective against mortality in patients with schizophrenia19 so it is possible that monitoring rather than clozapine per se reduces premature mortality risk in these patients. Few studies investigating antipsychotic use and all-cause mortality have controlled for clinical contact; those that do have failed to differentiate between clozapine and other antipsychotics,19 or else have only controlled for hospitalizations rather than all face-to-face clinical contact.15 In addition, there is a prevailing view that clozapine confers protection on overall mortality through preventing suicide in individuals with SMI,15,20 yet no adequately powered studies have examined clozapine’s associations with natural causes of mortality in this patient group. In this investigation we draw on data from a large electronic case register to test the hypotheses that the protective effect of clozapine on mortality in individuals with SMI is due to more frequent clinical monitoring and that clozapine is associated with lower risk of mortality from natural as well as unnatural causes in these patients.
Methods
Setting
This study used data from the South London and Maudsley NHS Foundation Trust (SLAM) Case Register; a large, anonymized, electronic mental health records database (described elsewhere)21 which has been used extensively in previous research.5,22,23 In the United Kingdom, mental health services are provided according to defined geographic catchment areas under the National Health Service (NHS). SLAM delivers all aspects of specialist mental healthcare to approximately 1.2 million residents of 4 London boroughs (Lambeth, Southwark, Lewisham, and Croydon). Since 2006, fully electronic clinical records have been maintained by all SLAM services. The Clinical Record Interactive Search (CRIS) system allows researchers to search on structured data and free-text fields for the 200 000 plus individuals represented in the system.
Ethics statement
CRIS was approved as an anonymised data resource for secondary analysis by Oxfordshire Research Ethics Committee C (08/H0606/71) and governance is provided for all projects and dissemination through a patient-led oversight committee. A linkage with death certification was further approved by the same committee.
Inclusion criteria
The cohort comprised individuals who had received an SMI diagnosis (WHO ICD-10 codes: F20, F25, F31)24 during an observation period from January 1, 2007 to December 31, 2011 inclusive, and who were 15 years or older at the time of their first SMI diagnosis in this period. Those individuals who had been prescribed clozapine prior to entering the observation period were excluded. Consequently, the analyses compared individuals who were newly prescribed clozapine against those with no evidence of this agent being prescribed. As mentioned above, electronic clinical records have been maintained by all SLAM services since 2006. Consequently we were able to search over a minimum of 12 months prior to the start of the observation period for clozapine prescriptions. However, we also searched earlier records where these were represented in the SLAM electronic health record database. Diagnosis and medication data within free-text fields in the SLAM Case Register were extracted using natural language processing applications (described below) which supplemented information in structured fields.
Data extraction from free-text fields
Applications for extracting information from free-text fields were built using Generalized Architecture for Text Engineering (GATE). GATE is a widely used program which provides a suite of tools to assist with natural language processing tasks such as information extraction from clinical notes. These applications were designed to extract data from the free text taking into account the linguistic context and validated (against human raters). These applications go well beyond a basic key word search. For example, natural language processing makes it possible to differentiate between a current prescription of clozapine and instances where the word clozapine is used in other contexts. We tested precision (positive predictive value) of the application for extracting/coding medications data on randomly selected instances where the application coded the patient as being prescribed clozapine (n = 279). We then determined if this was correct by manually searching through the underlying document. To determine recall (sensitivity) we extracted a random set of documents (n = 200) that contained the word clozapine, read these documents to ascertain whether the patient was actually prescribed clozapine, then determined if this was in agreement with the coding performed by the natural language processing application.
Main outcome measure
Mortality (all-cause/cause-specific) during the observation period (2007–2011, inclusive) was determined through routine nationwide mortality tracing linked to the electronic health record.2 In the United Kingdom, all death certifications are linked to NHS number (a unique identifier for UK NHS medical records). Healthcare providers are required by law to keep these records up to date. NHS numbers for all previous and current SLAM contacts are checked monthly against the national mortality database and deaths electronically flagged. Moreover, a linkage to additional data derived from death certificates allowed us to distinguish natural from unnatural causes of mortality where ICD-10 codes S00-T98 (injury, poisoning and certain other consequences of external causes), V01-Y98 (external causes of morbidity and mortality) and U509 (death from injury or poisoning event awaiting determination) were classified as unnatural mortality with the remaining codes classified as natural mortality.
Exposure variables
People who took clozapine at any stage during their follow-up period were designated as the exposed group. The follow-up period commenced from the first diagnosis of SMI during the observation period through to the censor date (last day of the observation period–December 31, 2011) or the patient’s death, whichever occurred earliest. In addition, those who had been prescribed any type of antipsychotic during follow-up were identified. Prescribing data were obtained from the SLAM pharmacy dispensing database and structured medications fields in the source electronic health record, as well as from free text using GATE as previously described. Using these data we calculated the duration over which patients were prescribed clozapine during the observation period.
We defined clinical monitoring as the proportion of days on which each individual with SMI received face to face clinical contact during follow-up. Any day on which a service user was an inpatient (for some or all of that day) or had engaged in face to face clinical interaction with a mental health professional was counted as a one clinically monitored day. The clinical monitoring was recorded as a percentage (a continuous variable and in tertiles) rather than the absolute number of days to account for varying follow-up periods. In addition we adjusted for inpatient and outpatient contact as separate covariates.
Since the introduction of Mental Health Act 2007, patients compulsorily detained in hospital in England and Wales for treatment may, on discharge, be placed on a supervised community treatment order (CTO), requiring them to comply with certain conditions related to their mental health treatment. One of the main indicators for CTOs are to enhance adherence to medications.25 Similarly, delivering medications as long acting injectable (LAI) or depot is indicated when individuals have difficulty adhering to oral medication regimes. We defined CTOs or having received any medication via LAI (prior to or during follow-up) as a marker of potential nonadherence with the view that those with poor adherence in the past may be more likely to be nonadherent during follow-up.
Covariates were also defined from the Health of the Nation Outcome Scale (HoNOS) instrument, taking the administration closest to the diagnosis date. HoNOS is a standard measure of patient wellbeing in UK mental health services, completed by clinicians after routine assessments,26,27 and whose validity has been assessed in a number of previous studies.26,28–30 HoNOS subscales measuring syndrome severity, other mental and physical health problems and functional status were included in this analysis. In addition, recorded diagnoses of opiate or alcohol use disorders (diagnosed before or during the observation period) were also taken from structured fields and extracted from free text using the GATE application described above. Diagnostic categories were based on the first SMI diagnosis received during the observation period. Further covariates were drawn from routinely completed fields in the source records including ethnic group and being married or cohabiting. Age was calculated on the diagnosis date.
Socioeconomic status was measured using area-level index of multiple deprivation which was calculated at the level of lower super output area for the residence: a UK address-grouping construct which contains an average of 1500 residents per area unit. The index of multiple deprivation incorporates several area-level domains defined from the (2001) national UK census (employment, income, education, health, barriers to housing and services, crime, living environment) with each domain given a weighting to reflect its importance. The address recorded closest to the time the individual entered the study cohort was used, with a separate category assigned to those who were homeless.31
Statistical analysis
Cohort members who were prescribed clozapine during the follow-up period were compared to never-exposed counterparts with respect to demographic and other risk factors for premature mortality. Kaplan–Meier curves with a log-rank test were used to compare those who were and were not prescribed clozapine. Having checked proportional hazards assumptions, Cox regression procedures were used to model associations between clozapine and risk of all-cause mortality. Several different models were constructed where we controlled for sets of related covariates with the final model adjusting for all covariates examined. The following sensitivity analyses were carried out: (1) excluding patients treated in one of the 4 London boroughs (Lewisham) where data on clozapine exposure were less complete (unavailability of pharmacy dispensing data); (2) exclusion of those who were not taking any antipsychotics during the observation period and individuals with potentially lower adherence to treatment regimes (those who had received medication as LAI at any time in SLAM services or had been placed on a CTO); (3) restricting the sample to those with ICD-10 F20 schizophrenia diagnosis; and (4) restricting the comparator group to individuals who were newly prescribed olanzapine during the observation period (the clozapine group included 189 individuals who were newly prescribed both olanzapine and clozapine during the observation period); (5) excluding those who came from outside the catchment (SLaM provides some national specialist services) in case these individuals had substantial contact with non-SLaM specialist care services which would not be represented on CRIS; (6) excluding those who were prescribed clozapine for less than 30 days. We also investigated whether including inpatient and outpatient contact as separate covariates or as a combined variable made any difference to results.
We used standard propensity score methods for reducing the effects of confounding by indication in observational studies.32 The propensity score was defined as the probability of being treated with clozapine during the observation period based on a regression model which included factors relating to demographic and socioeconomic status; diagnosis and severity of symptoms; mental and physical health problems; substance use disorders and clinical monitoring (listed in table 1). We included the propensity score in place of these covariates in a Cox model. In addition we constructed a fully adjusted Cox model where we only included those at risk of being both treated and untreated by clozapine based on their propensity scores. Finally, Cox regression models were repeated for natural and unnatural causes of death as separate outcomes.
Table 1.
Sample Characteristics and Percentage of Deaths
| Risk factors |
N individuals (N deaths) |
% deaths |
|---|---|---|
| Total | 14 754 (879) | 6.0 |
| Taking clozapine during follow-up period | ||
| No | 14 006 (864) | 6.2 |
| Yes | 748 (15) | 2.0 |
| Taking any antipsychotic during follow-up period | ||
| No | 3860 (265) | 6.9 |
| Yes | 10 894 (614) | 5.6 |
| Demographic and socioeconomic factors | ||
| Age (mean 43.2, SD 16.1, range 15–96 years) | ||
| 15 to < 35 years | 5071 (67) | 1.3 |
| 35 to < 45 years | 6461 (229) | 3.5 |
| 55 years and over | 3222 (583) | 18.1 |
| Gender | ||
| Female | 6769 (416) | 6.2 |
| Male | 7985 (463) | 5.8 |
| Ethnicity | ||
| White British | 6106 (488) | 8.0 |
| Other white background | 1534 (100) | 6.5 |
| South Asian | 405 (16) | 4.0 |
| East Asian | 450 (22) | 4.9 |
| Caribbean | 1550 (105) | 6.8 |
| Other Black background | 3236 (97) | 3.0 |
| Mixed, other or unknown | 1473 (51) | 3.5 |
| Married or cohabiting | ||
| No | 12 728 (757) | 6.0 |
| Yes | 2026 (122) | 6.0 |
| Deprivation level in area of residence (tertiles) | ||
| Low levels of deprivation | 4495 (294) | 6.5 |
| Medium levels of deprivation | 4498 (264) | 5.9 |
| High levels of deprivation | 4512(261) | 5.8 |
| Homeless | 304 (10) | 3.3 |
| Diagnosis and severity of symptoms | ||
| SMI Diagnosis | ||
| Schizophrenia (ICD10 code - F20) | 9437 (609) | 6.5 |
| Schizoaffective disorder (ICD10 code - F25) | 805 (43) | 5.3 |
| Bipolar affective disorder (ICD10 code - F31) | 4512 (227) | 5.0 |
| Overactive, aggressive behavior | ||
| Not a problem | 6745 (388) | 5.8 |
| Minor problems only | 2636 (176) | 6.7 |
| Significant problem | 2558 (175) | 6.8 |
| Hallucinations and delusions | ||
| Not a problem | 5379 (321) | 6.0 |
| Minor problems only | 1821 (118) | 6.5 |
| Significant problem | 4675 (292) | 6.3 |
| Depressed mood | ||
| Not a problem | 5268 (363) | 6.9 |
| Minor problems only | 3355 (226) | 6.7 |
| Significant problem | 3274 (144) | 4.4 |
| Additional mental and physical health problems | ||
| Nonaccidental self-injury | ||
| Not a problem | 10 259 (670) | 6.5 |
| Minor problem only | 900 (41) | 4.6 |
| Significant problem | 756 (25) | 3.3 |
| Problem-drinking or drug taking | ||
| Not a problem | 8798 (593) | 6.7 |
| Minor problems only | 1130 (48) | 4.3 |
| Significant problem | 1907 (95) | 5.0 |
| Physical illness or disability problems | ||
| Not a problem | 7205 (174) | 2.4 |
| Minor problems only | 2003 (168) | 8.4 |
| Significant problem | 2701 (397) | 14.7 |
| Functional status | ||
| Activities of daily living (ADLs) | ||
| Not a problem | 5513 (188) | 3.4 |
| Minor problems only | 2780 (164) | 5.9 |
| Significant problem | 3574 (385) | 10.8 |
| Standard of living conditions | ||
| Not a problem | 6966 (433) | 6.2 |
| Minor problems only | 2260 (146) | 6.5 |
| Significant problem | 2381 (146) | 6.1 |
| Occupational and recreational activities | ||
| Not a problem | 5245 (283) | 5.4 |
| Minor problems only | 2768 (183) | 6.6 |
| Significant problem | 3609 (248) | 6.9 |
| Social relationships | ||
| Not a problem | 4461 (287) | 6.4 |
| Minor problems only | 3138 (193) | 6.2 |
| Significant problem | 4249 (251) | 5.9 |
| Substance use disorders | ||
| Ever diagnosed with alcohol use disorder | ||
| No | 13 492 (807) | 6.0 |
| Yes | 1262 (72) | 5.7 |
| Ever diagnoses with opioid use disorder | ||
| No | 14 441 (868) | 6.0 |
| Yes | 313 (11) | 3.5 |
| Clinical monitoring (percentage of days in face-to-face contact with SLAM services during observation period, in tertiles) | ||
| Low level of contact | 4918 (260) | 5.3 |
| Medium level of contact | 4918 (293) | 6.0 |
| High level of contact | 4918 (326) | 6.6 |
Results
Over the 5 year observation period we identified 14 754 individuals (879 deaths) with a diagnosis of schizophrenia, schizoaffective disorder or bipolar disorder who met the inclusion criteria. The mean (SD) follow-up period was 1105 (571) days. Overall, 8.9% of follow-up days included face to face contact between patient and clinician. The GATE application that was used to extract data on clozapine from electronic patient records was validated against manual record review resulting in precision (positive predictive value) and recall (sensitivity) for clozapine annotations of 96% and 92%, respectively, for “current use” (with a margin for error of 3 months with respect to the exact date the prescriptions was issued) and 99% and 92% respectively for historical use (ie, having ever been prescribed clozapine). Among those prescribed clozapine the mean time over which they were prescribed this antipsychotic was 521.7 days SD 567.8. For 503 people who were prescribed clozapine for at least 30 days the mean duration over which clozapine was prescribed was 774.4 days SD 533.4. It should be noted that some patients will have continued on clozapine after the observation period ended. Consequently, the time on clozapine reported in this analysis does not represent the full length of the time a patient may be placed on clozapine.
Table 1 provides numbers of cases and deaths by diagnosis, levels of symptom severity, and other cohort characteristics. A quarter of service users included in the study were not prescribed any antipsychotics during follow-up (3860 individuals, 265 deaths). The majority of patients were prescribed some form of antipsychotic during follow-up (10 894 individuals, 614 deaths) with 748 individuals commencing clozapine during their follow-up period, 15 of whom died. Among those diagnosed with schizophrenia, 6.5% were newly prescribed clozapine during follow-up compared to 6.5% for schizoaffective disorder and 1.8% for bipolar disorder. Notably low numbers of deaths were recorded among those who were homeless (3.3%) and those who had ever been diagnosed with an opiate use disorder (3.5%); however these groups were younger than the remainder of the cohort and associations with reduced risk of mortality were not significant after adjusting for age.
The characteristics of those individuals with or without clozapine exposure are compared in table 2. Those prescribed clozapine had higher levels of clinical contact, were more likely to be male, to be from a non-Caribbean black background, to have received schizophrenia as a first SMI diagnosis, and were younger [mean age (SD): 36.7(12.7) vs 43.5(16.1) years]; they were significantly less likely to be in a relationship; to be from non-British white background and to have received a first SMI diagnosis of bipolar disorder. In addition, those prescribed clozapine were significantly more likely to have poor functional status on the HoNOS scale (including problems with ADL impairment, occupational and recreational activities, social relationships, living conditions) and to have worse psychopathology (increased likelihood of having problems with overactive aggressive behavior, problems with hallucinations and delusions, subclinical depressed mood, minor problems with drinking or drug taking), or to have been diagnosed with an alcohol use disorder. Nine of the 15 patient characteristics associated with being prescribed clozapine were also significantly associated with an increased risk of mortality in age and gender adjusted models (ie, being male, unmarried, having increased clinical contact, overactive aggressive behavior, minor depression, problems with drinking or drug taking, living conditions, ADLs and occupational or recreational activities). The only characteristic associated with clozapine that was protective against mortality was younger age.
Table 2.
Characteristics of Those Individuals Who Were and Were Not Prescribed Clozapine During Follow-Up
| N (%) of individuals | ||
|---|---|---|
| Risk factors | Not prescribed clozapine | Prescribed clozapine |
| Total | 14 006 (100%) | 748 (100%) |
| Demographic and socio-economic factors | ||
| Age * mean(SD): 43.5(16.1)NC vs 36.7(12.7)C | ||
| 15 to < 35 years | 4705 (33.6%) | 366 (48.9%) |
| 35 to < 45 years | 6150 (43.9%) | 311 (41.6%) |
| 55 years and over | 3151 (22.5%) | 71 (9.5%) |
| Gender* | ||
| Female | 6508 (46.5%) | 261 (34.9%) |
| Male | 7498 (53.5%) | 487 (65.1%) |
| Ethnicity* | ||
| White British | 5813 (41.5%) | 293 (39.2%) |
| Other white background | 1482 (10.6%) | 52 (7.0%) |
| South Asian | 386 (2.8%) | 19 (2.5%) |
| East Asian | 428 (3.1%) | 22 (2.9%) |
| Caribbean | 1474 (10.5%) | 76 (10.2%) |
| Other Black background | 3012 (21.5%) | 224 (30.0%) |
| Mixed, other or unknown | 1411 (10.1%) | 62 (8.3%) |
| Married or cohabiting* | ||
| No | 12 033 (85.9%) | 695 (92.9%) |
| Yes | 1973 (14.1%) | 53 (7.1%) |
| Deprivation level in area of residence (tertiles) | ||
| Low levels of deprivation | 4268 (32.5%) | 228 (33.0%) |
| Medium levels of deprivation | 4266 (32.5%) | 232 (33.6%) |
| High levels of deprivation | 4301 (32.8%) | 211 (30.5%) |
| Homeless | 283 (2.2%) | 21 (3.0%) |
| Diagnosis and severity of symptoms | ||
| SMI Diagnosis* | ||
| Schizophrenia (ICD10 code - F20) | 8820 (63.0%) | 617 (82.5%) |
| Schizoaffective disorder (ICD10 code - F25) | 753 (5.4%) | 52 (7.0%) |
| Bipolar affective disorder (ICD10 code - F31) | 4433 (31.7%) | 79 (10.6%) |
| Overactive, aggressive behavior* | ||
| Not a problem | 6442 (57.2%) | 303 (45.3%) |
| Minor problems only | 2454 (21.8%) | 182 (27.2%) |
| Significant problem | 2374 (21.1%) | 184 (27.5%) |
| Hallucinations and delusions * | ||
| Not a problem | 5225 (46.6%) | 154 (23.1%) |
| Minor problems only | 1716 (15.3%) | 105 (15.7%) |
| Significant problem | 4267 (38.1%) | 408 (61.2%) |
| Depressed mood* | ||
| Not a problem | 4981(44.4%) | 287 (43.0%) |
| Minor problems only | 3136 (27.9%) | 219 (32.8%) |
| Significant problem | 3112 (27.7%) | 162 (24.3%) |
| Additional mental and physical health problems | ||
| Nonaccidental self-injury | ||
| Not a problem | 9688 (86.1%) | 571 (85.7%) |
| Minor problem only | 847 (7.5%) | 53 (8.0%) |
| Significant problem | 714 (6.4%) | 42 (6.3%) |
| Problem-drinking or drug taking* | ||
| Not a problem | 8335 (74.6%) | 463 (70.0%) |
| Minor problems only | 1052 (9.4%) | 78 (11.8%) |
| Significant problem | 1786 (16.0%) | 121 (18.3%) |
| Physical illness or disability problems | ||
| Not a problem | 6791 (60.4%) | 414 (62.2%) |
| Minor problems only | 1880 (16.7%) | 123 (18.5%) |
| Significant problem | 2572 (22.9%) | 129 (19.4%) |
| Functional status | ||
| Activities of daily living (ADLs)* | ||
| Not a problem | 5265 (47.0%) | 248 (37.4%) |
| Minor problems only | 2618 (23.4%) | 162 (24.4%) |
| Significant problem | 3321 (29.6%) | 253 (38.2%) |
| Standard of living conditions* | ||
| Not a problem | 6610 (60.3%) | 356 (55.4%) |
| Minor problems only | 2137 (19.5%) | 123 (19.1%) |
| Significant problem | 2217 (20.2%) | 164 (25.5%) |
| Occupational and recreational activities* | ||
| Not a problem | 4995 (45.5%) | 250 (38.8%) |
| Minor problems only | 2610 (23.8%) | 158 (24.5%) |
| Significant problem | 3372 (30.7%) | 237 (36.7%) |
| Social relationships* | ||
| Not a problem | 4256 (38.1%) | 205 (30.7%) |
| Minor problems only | 2968 (26.6%) | 170 (25.5%) |
| Significant problem | 3957 (35.4%) | 292 (43.8%) |
| Substance use disorders | ||
| Ever diagnosed with alcohol use disorder* | ||
| No | 12 825 (91.6%) | 667 (89.2%) |
| Yes | 1181 (8.4%) | 81 (10.8%) |
| Ever diagnoses with opiate use disorder | ||
| No | 13 703 (97.8%) | 738 (98.7%) |
| Yes | 303 (2.2%) | 10 (1.3%) |
| Clinical monitoring (percentage of days in face-to-face contact with SLAM services during observation period, in tertiles)* | ||
| Low level of contact | 4847 (34.6%) | 71 (9.5%) |
| Medium level of contact | 4835 (34.5%) | 83 (11.1%) |
| High level of contact | 4324 (30.9%) | 594 (79.4%) |
Notes: *P value < .05 for comparison between those who were and were not prescribed clozapine.
NC not prescribed clozapine during follow-up.
C prescribed clozapine during follow-up.
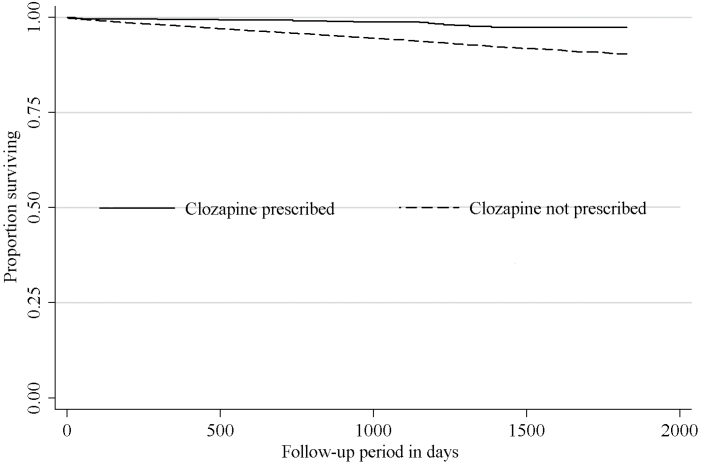
Figure 1 displays Kaplan–Meier curves comparing survival over time of those who were and were not prescribed clozapine. Those prescribed clozapine displayed significantly better survival (p < .001). Moreover, Cox regression models displayed in table 3 indicated a strong association between clozapine exposure and reduced risk of all-cause mortality which remained significant and not substantially reduced in strength after adjusting for a range of potential confounders, including clinical monitoring. Adjustment for propensity scores, inclusion of inpatient and outpatient contact as separate variables or combined, and the previously specified sensitivity analyses made little difference to this finding. In fully adjusted models for cause-specific mortality as an outcome (table 4), the association remained significant and strong for reduced risk of mortality both from natural and unnatural causes.
Fig. 1.
Kaplan–Meier curves displaying the survival status of people with serious mental illness comparing those who were prescribed clozapine with those who were not (N = 14 754).
Table 3.
Multivariate Cox Regression Analyses of Association Between Receiving Clozapine Treatment and All-Cause Mortality in Individuals With Serious Mental Illness.14 754 Cases (879 Deaths)
| Prescribed clozapine during follow-up perioda | Hazard ratio (95% CI) | P values |
|---|---|---|
| Crude | 0.3 (0.2–0.4) | <.001 |
| Adjusted for contact with SLAM servicesb | 0.2 (0.1–0.3) | <.001 |
| Adjusted for contact with SLAM services,b
non-clozapine antipsychoticsc socio-demographic factorsd |
0.4 (0.2–0.7) | .001 |
| Adjusted for contact with SLAM services,b
non-clozapine antipsychoticsc socio-demographic factors,d SMI diagnosis and severitye other mental & physical healthf |
0.4 (0.2–0.7) | .001 |
| Adjusted for contact with SLAM services,b
non-clozapine antipsychoticsc socio-demographic factors,d SMI diagnosis and severitye other mental and physical healthf functional statusg |
0.4 (0.2–0.7) | .001 |
| Fully adjustedh | 0.4 (0.2–0.7) | .001 |
| Fully adjustedh
restricting to those taking any antipsychotics |
0.4 (0.2–0.7) | .001 |
| Fully adjustedh
restricting to those with ICD-10 F20 Schizophrenia diagnosis |
0.5 (0.3–0.8) | .012 |
| Fully adjustedh
excluding potentially noncompliant individuals (those who had received medication as depot or had placed on a supervised community treatment order at any time in SLAM services |
0.3 (0.2–0.6) | .001 |
| Fully adjustedh
comparing to those who were newly prescribed olanzapine during follow-up |
0.4 (0.2–0.8) | .008 |
| Fully adjustedh
excluding those referred in from outside the 4 boroughs of the SLaM catchment |
0.4(0.2–0.7) | .002 |
| Fully adjustedh
adjusting for inpatient and outpatient contact as separate covariates |
0.4(0.2–0.7) | .002 |
| Fully adjustedh
including only those who were at risk of being both treated or untreated with clozapine (based on propensity scores) |
0.4(0.2–0.7) | .001 |
| Adjusted by using propensity score as a covariate | 0.3(0.2–0.5) | <.001 |
| Adjusted by using propensity score as a covariate, excluding those who had been prescribed clozapine for less than 30 days |
0.2(0.1–0.5) | <.001 |
Notes: aFollow-up period begins at first diagnosis during observation window (from January 1, 2007 to December 31, 2011, inclusive) and end with death or end of observation window (whichever is sooner).
bPercentage of time during observation period where patient had face to face contact with SLAM services (measured as a continuous variable).
cUse of 1 or more antipsychotic other than clozapine.
dAge, gender, ethnicity, married or cohabiting, deprivation level in area of residence.
eSMI diagnosis, overactive aggressive behaviour, hallucinations and delusions, depressed mood.
fNonaccidental self-injury, problem drinking/drug taking, physical illness, or disability problems.
gImpairment in activities of daily living, standard of living conditions, occupational and recreational activities, social relationships.
hAll of above plus ever having had alcohol or opioid use disorder diagnoses.
Table 4.
Multivariate Cox Regression Analyses of Association Between Receiving Clozapine Treatment and Cause-Specific Mortality in Individuals With Serious Mental Illness
| Hazard ratio (95% CI), p value | ||
|---|---|---|
| Prescribed clozapine during follow-up perioda | Natural causes of mortality Deaths = 713 |
Unnatural causes of mortality Deaths = 91 |
| Crude | 0.3 (0.1–0.5), p <.001 | 0.3 (0.1–1.4), p = .140 |
| Fully adjustedb | 0.5 (0.2–0.9), p = .022 | 0.2 (0.05–0.9), p = .039 |
Notes: aFollow-up period begins at first diagnosis during observation window (from January 1, 2007 to December 31, 2011, inclusive) and ends with death or end of observation window (whichever is sooner).
bAdjusted for percentage of time during observation period where patient had face to face contact with SLAM services (measured as a continuous variable); use of 1 or more antipsychotic other than clozapine; age; gender; ethnicity; married or cohabiting; deprivation level in area of residence; SMI diagnosis; overactive aggressive behaviour; hallucinations and delusions; depressed mood; non accidental self-injury; problem drinking/drug taking; physical illness or disability problems; impairment in activities of daily living; standard of living conditions; occupational and recreational activities; social relationships; ever having had alcohol or opioid use disorder diagnoses.
Discussion
This is the first investigation to test the hypothesis that lower mortality in clozapine users is a result of intensive clinical monitoring, and to describe the association of clozapine with both natural and unnatural mortality. Using electronic health record data from a comprehensive specialist mental health care service within a geographic catchment, we found no evidence that the reduced risk of mortality associated with clozapine was accounted for by clinical monitoring or other covariates. Individuals with SMI who were prescribed clozapine had more severe psychopathology (hallucinations, delusions, aggression, subclinical depression, addiction) and poorer functional status (problems with ADL impairment, occupational and recreational activities, social relationships, living conditions). Nine of the 15 patient characteristics associated with being prescribed clozapine were themselves significantly associated with an increased risk of mortality in age and gender adjusted models. However despite this increased level of adversity those prescribed clozapine had substantially reduced risk of natural—and unnatural—mortality. These associations persisted after controlling for a broad range of potential confounders including contact with specialist mental health services, markers of disease severity, use of other antipsychotics, other aspects of mental and physical health and use of alcohol or other drugs. It was also robust to propensity score adjustment/sensitively analysis a method to reduce the effects of confounding by indication.32
In addition, excluding those who had been prescribed clozapine for less than 1 month increased the strength of association between clozapine and reduced risk of mortality in individuals with SMI.
Our findings are consistent with other large epidemiological cohort studies which have reported an association between clozapine and lower all-cause mortality. Amongst 67 000 clozapine-treated patients in the United States (1989 to 1996), all-cause mortality was lower during the period of clozapine use than nonuse (HR 0.46).13 Similarly, a more recent study conducted in Finland using record-based data on medication prescription (66 881 SMI cases), found that clozapine was associated with the lowest mortality risk of all major antipsychotics.15 However, in the above studies, a potential protective effect of the clinical monitoring required for clozapine treatment could not be excluded as a reason for lower mortality. Our own findings suggest that increased clinical monitoring is not the reason for the lower mortality among those prescribed clozapine.
The association between clozapine and lower all-cause mortality was not purely attributable to lower suicide risk, as previously hypothesized.13,20 We discriminated between unnatural and natural cause mortality in our cohort of individuals with SMI and found, even after adjustment for a broad range of potential confounders, that clozapine was associated with a lower risk of both natural and unnatural deaths. Our results contrast with findings of associations with increased risk of sudden cardiac death,9 respiratory related death,33 and potentially fatal metabolic34,35 and hematological disturbances.20 However, other studies indicate that clozapine may not confer additional cardiovascular risk or even be protective against cardiovascular related mortality compared to other antipsychotics.36,37 Our results do not exclude the possibly that clozapine may be associated with a higher risk of specific causes of death through individual pathways but do suggest that associations with lower risk of other mortality may outweigh these.
This investigation has a number of strengths. The sample included all patients with SMI in contact with mental health services within a defined area over a 5-year period. In the United Kingdom, healthcare providers are legally required to keep death records up to date. Mortality tracing in the source records system (updated monthly) is based on national certification so that only deaths occurring outside the United Kingdom are likely to have been missed. We would expect our data to be representative of patients with SMI living in urban and suburban areas since SLAM is a near-monopoly provider of specialist mental healthcare for its geographic catchment. We drew on complete electronic clinical records for close to fifteen thousand cases, providing the statistical power to control for a range of potential confounders. The findings were also robust to a series of sensitivity analyses.
Limitations include the possibility of residual confounding, particularly medication use prior to the observation period.38 We only examined cases newly prescribed clozapine during the observation period and did not investigate mortality associations beyond 5 years. Despite our strategy of using propensity scores to perform sensitivity analysis and adjustment sensitivity, confounding by indication is an important consideration which cannot be ruled out entirely in any observational study. However, we found no evidence to suggest that clinicians were reserving clozapine for patients who were healthier (apart from being younger). Instead, those individuals who were prescribed clozapine had more severe psychopathology and poorer functional status (consistent with clozapine being a third line treatment). Consequently one would expect that the true association between clozapine and reduced mortality risk would be at least as strong or stronger than that which we observed. In addition we adjusted for indicators of severity of illness including diagnosis, symptoms, physical illness, and functional status; however, a reliable assessment of duration of psychiatric illness was not available. Ultimately, the only means of excluding residual confounding is through randomized controlled trial evidence, which we do not believe is likely to be forthcoming to address this particular question.
Adverse lifestyle choices (other than drinking problems and opiate use), such as smoking, poor diet, and physical inactivity which may also contribute to the increased risk of mortality in individuals with SMI38–41 were not controlled for in this analysis, although it is not clear that those prescribed clozapine are likely to differ in these respects compared to people prescribed other antipsychotics. Also general practice data and glucose or cholesterol data were not available for inclusion in this analysis. It is possible that the increased clinical contact received by those prescribed clozapine might provide greater opportunities for clinicians to influence the lifestyle choices of their patients. However, this is unlikely to account for the association between clozapine and reduced risk of mortality, since this association persisted after adjustment for level of clinical contact. Specialist mental healthcare is provided at no cost to consumers as part of the UK National Health Service (NHS), so the only missing mental health service contacts would be from individuals seeking exclusively private healthcare.37
The results of this investigation have important implications for clinical practice. Our results suggest that the observed protective effect of clozapine is not due to the extra clinical contact, as previously suggested, nor due to confounding by the broad range of other covariates that we examined. Clozapine appears to reduce the risk of both natural and unnatural mortality in patients with SMI. Current guidelines restricting the use of clozapine to those with treatment resistant SMI may need revising.
Funding
This work was supported by the Clinical Records Interactive Search (CRIS) system funded and developed by the National Institute for Health Research (NIHR) Mental Health Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London and a joint infrastructure grant from Guy’s and St Thomas’ Charity and the Maudsley Charity (grant number BRC-2011–10035). RH is funded by a Medical Research Council Population Health Scientist Fellowship (grant number MR/J01219X/1). All other authors receive salary support from the National Institute for Health Research (NIHR) Mental Health Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
RH, C-KC, RJ, HS, MB and RS have received research funding from Roche, Pfizer, J&J and Lundbeck. Other authors have no conflict of interest.
Acknowledgments
Acknowledgments
All the authors listed have made substantial contributions to: the process of hypothesis generation, data collection, statistical analyses, or interpretation of data; manuscript preparation; and given final approval to the manuscript. The corresponding author has access to reported and unreported data and has complete freedom to direct the analysis and reporting without influence, editorial direction or censorship from the sponsors.
References
- 1.Harris EC, Barraclough B. Excess mortality of mental disorder. Br J Psychiatry. 1998;173:11–53. [DOI] [PubMed] [Google Scholar]
- 2.Chang CK, Hayes RD, Broadbent M, et al. All-cause mortality among people with serious mental illness (SMI), substance use disorders, and depressive disorders in southeast London: a cohort study. BMC Psychiatry. 2010;10:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoang U, Stewart R, Goldacre MJ. Mortality after hospital discharge for people with schizophrenia or bipolar disorder: retrospective study of linked English hospital episode statistics, 1999–2006. BMJ. 2011;343:d5422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang CK, Hayes RD, Perera G, et al. Life expectancy at birth for people with serious mental illness from a secondary mental health care case register in London, UK PloS ONE 2011;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayes RD, Chang CK, Fernandes AC, et al. Functional status and all-cause mortality in serious mental illness. PLoS One. 2012;7:e44613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kilian R, Becker T, Krüger K, Schmid S, Frasch K. Health behavior in psychiatric in-patients compared with a German general population sample. Acta Psychiatr Scand. 2006;114:242–248. [DOI] [PubMed] [Google Scholar]
- 7.Osborn DP, Nazareth I, King MB. Physical activity, dietary habits and Coronary Heart Disease risk factor knowledge amongst people with severe mental illness: a cross sectional comparative study in primary care. Soc Psychiatry Psychiatr Epidemiol. 2007;42:787–793. [DOI] [PubMed] [Google Scholar]
- 8.Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med. 2004;350:1013–1022. [DOI] [PubMed] [Google Scholar]
- 9.Ray WA, Chung CP, Murray KT, Hall K, Stein CM. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med. 2009;360:225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stahl SM, Mignon L, Meyer JM. Which comes first: atypical antipsychotic treatment or cardiometabolic risk? Acta Psychiatr Scand. 2009;119:171–179. [DOI] [PubMed] [Google Scholar]
- 11.Crump C, Winkleby MA, Sundquist K, Sundquist J. Comorbidities and mortality in persons with schizophrenia: a Swedish national cohort study. Am J Psychiatry. 2013;170:324–333. [DOI] [PubMed] [Google Scholar]
- 12.Kiviniemi M, Suvisaari J, Koivumaa-Honkanen H, et al. Antipsychotics and mortality in first-onset schizophrenia: prospective Finnish register study with 5-year follow-up. Schizophr Res. 2013;150:274–280. [DOI] [PubMed] [Google Scholar]
- 13.Walker AM, Lanza LL, Arellano F, Rothman KJ. Mortality in current and former users of clozapine. Epidemiology. 1997;8:671–677. [DOI] [PubMed] [Google Scholar]
- 14.Hennen J, Baldessarini RJ. Suicidal risk during treatment with clozapine: a meta-analysis. Schizophr Res. 2005;73:139–145. [DOI] [PubMed] [Google Scholar]
- 15.Tiihonen J, Lonnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374:620–627. [DOI] [PubMed] [Google Scholar]
- 16.Nielsen J, Kane JM, Correll CU. Real-world effectiveness of clozapine in patients with bipolar disorder: results from a 2-year mirror-image study. Bipolar Disord. 2012;14:863–869. [DOI] [PubMed] [Google Scholar]
- 17.Kumra S, Kranzler H, Gerbino-Rosen G, et al. Clozapine and “high-dose” olanzapine in refractory early-onset schizophrenia: a 12-week randomized and double-blind comparison. Biol Psychiatry. 2008;63:524–529. [DOI] [PubMed] [Google Scholar]
- 18.Howes OD, Vergunst F, Gee S, et al. Adherence to treatment guidelines in clinical practice: study of antipsychotic treatment prior to clozapine initiation. Br J Psychiatry. 2012;201:481–485. [DOI] [PubMed] [Google Scholar]
- 19.Cullen BA, McGinty EE, Zhang Y, et al. Guideline-concordant antipsychotic use and mortality in schizophrenia. Schizophr Bull. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meltzer HY. Clozapine: balancing safety with superior antipsychotic efficacy. Clin Schizophr Relat Psychoses. 2012;6:134–144. [DOI] [PubMed] [Google Scholar]
- 21.Stewart R, Soremekun M, Perera G, et al. The South London and Maudsley NHS Foundation Trust Biomedical Research Centre (SLAM BRC) case register: development and descriptive data. BMC Psychiatry. 2009;9:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayes RD, Chang CK, Fernandes A, et al. Associations between symptoms and all-cause mortality in individuals with serious mental illness J Psychosom Res. 2012;72:114–119 [DOI] [PubMed] [Google Scholar]
- 23.Hayes RD, Chang CK, Fernandes A, et al. Associations between substance use disorder sub-groups, life expectancy and all-cause mortality in a large British specialist mental healthcare service. Drug Alcohol Depend. 2011;118:56–61. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organisation. Manual of the international statistical classification of diseases and realted health problems 10 revision (ICD-10). 2000. [Google Scholar]
- 25.Moncrieff J, Thomas P, Crawford M, Henderson C. Modernising mental health services. Psychiatrists should oppose community treatment orders. BMJ. 1999;318:807. [PubMed] [Google Scholar]
- 26.Wing JK, Beevor AS, Curtis RH, et al. Health of the Nation Outcome Scales (HoNOS). Research and development. Br J Psychiatry. 1998;172:11–18. [DOI] [PubMed] [Google Scholar]
- 27.Wing J, Curtis RH, Beevor A. Health of the Nation Outcome Scales (HoNOS). Glossary for HoNOS score sheet. Br J Psychiatry. 1999;174:432–434. [DOI] [PubMed] [Google Scholar]
- 28.Orrell M, Yard P, Handysides J, Schapira R. Validity and reliability of the Health of the Nation Outcome Scales in psychiatric patients in the community. Br J Psychiatry. 1999;174:409–412. [DOI] [PubMed] [Google Scholar]
- 29.Pirkis JE, Burgess PM, Kirk PK, et al. A review of the psychometric properties of the Health of the Nation Outcome Scales (HoNOS) family of measures. Health Qual Life Outcomes. 2005;3:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hunter R, Cameron R, Norrie J. Using patient-reported outcomes in schizophrenia: the Scottish Schizophrenia Outcomes Study. Psychiatr Serv. 2009;60:240–245. [DOI] [PubMed] [Google Scholar]
- 31.Noble M, McLennan D, Wilkinson K, et al. The English indices of deprivation 2007. London: Communities and Local Government; 2008. [Google Scholar]
- 32.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res. 2011;46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taylor DM, Douglas-Hall P, Olofinjana B, Whiskey E, Thomas A. Reasons for discontinuing clozapine: matched, case-control comparison with risperidone long-acting injection. Br J Psychiatry. 2009;194:165–167. [DOI] [PubMed] [Google Scholar]
- 34.Allison DB, Mentore JL, Heo M, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry. 1999;156:1686–1696. [DOI] [PubMed] [Google Scholar]
- 35.Newcomer JW, Haupt DW. The metabolic effects of antipsychotic medications. Can J Psychiatry. 2006;51:480–491. [DOI] [PubMed] [Google Scholar]
- 36.Kelly DL, McMahon RP, Liu F, et al. Cardiovascular disease mortality in patients with chronic schizophrenia treated with clozapine: a retrospective cohort study. J Clin Psychiatry. 2010;71:304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keown P, Mercer G, Scott J. Retrospective analysis of hospital episode statistics, involuntary admissions under the mental health act 1983, and number of psychiatric beds in England 1996–2006. BMJ. 2008;337:a1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Auquier P, Lancon C, Rouillon F, Lader M, Holmes C. Mortality in schizophrenia. Pharmacoepidemiol Drug Saf. 2006;15:873–879. [DOI] [PubMed] [Google Scholar]
- 39.Robson D, Gray R. Serious mental illness and physical health problems: a discussion paper. Int J Nurs Stud. 2007;44:457–466. [DOI] [PubMed] [Google Scholar]
- 40.Fagiolini A, Goracci A. The effects of undertreated chronic medical illnesses in patients with severe mental disorders. J Clin Psychiatry. 2009;70Suppl 3:22–29. [DOI] [PubMed] [Google Scholar]
- 41.Brown S, Birtwistle J, Roe L, Thompson C. The unhealthy lifestyle of people with schizophrenia. Psychol Med. 1999;29:697–701. [DOI] [PubMed] [Google Scholar]