Abstract
Background: Relational models of psychopathology propose that symptoms are dynamically connected and hypothesize that genetic and environmental influences moderate the strength of these symptom connections. Previous findings suggest that the interplay between hallucinations and delusions may play a crucial role in the development of psychotic disorder. The current study examined whether the connection between hallucinations and delusions is impacted by proxy genetic and environmental risk factors. Methods: Hallucinations and delusions at baseline and at 3-year follow-up were assessed in a sample of 1054 healthy siblings and 918 parents of 1109 patients with psychosis, and in 589 healthy controls (no familial psychosis risk). Environmental factors assessed were cannabis use, childhood trauma, and urbanicity during childhood. Logistic regression analyses tested whether familial psychosis risk predicted increased risk of delusions, given presence of hallucinations. Moderating effects of environmental factors on the hallucination-delusion association were tested in a similar fashion, restricted to the control and sibling groups. Results: The risk of delusions, given hallucinations, was associated with proxy genetic risk: 53% in parents, 47% in siblings, and 36% in controls. The hallucination-delusion association was stronger in those reporting cannabis use (risk difference: 32%) and childhood trauma (risk difference: 15%) although not all associations were statistically conclusive (respectively: p = .037; p = .054). A directionally similar but nonsignificant effect was found for urb anicity during childhood (risk difference: 14%, p =.357). Conclusion: The strength of the connection between delusions and hallucinations is associated with familial and environmental risks for psychotic disorder, suggesting that specific symptom connections in the early psychosis psychopathology network are informative of underlying mechanisms.
Key words: hallucinations, delusions, psychosis
Introduction
The occurrence of psychotic experiences in the general population may be viewed as the behavioral expression of underlying psychosis liability.1 General population studies have consistently shown that psychotic experiences occur in around 8%2 of the population, the majority never converting to clinical psychosis. The relative risk for transition to clinical psychotic disorder, given presence of baseline subthreshold psychotic symptoms, is around 3.5, which translates to a yearly incidence rate of less than 1%.3 The dynamics underlying transition to clinical outcome have been captured by the psychosis proneness-persistence-impairment model, which proposes that normally transient subclinical expression of psychosis, under the influence of environmental and familial risk factors, becomes more persistent over time. Persistence of symptoms, in turn, increases risk of impairment and clinical outcome.1
For the study of early psychosis, psychopathology can be represented as categories of mental disorder (groupings based on clustering of individuals), symptom dimensions (scores on a factor of clustered symptoms),4 or symptom networks (symptoms impacting on each other as part of a circuit or network).5 An example of the latter is that delusions with a threatening content (eg, delusions of persecution) may provoke feelings of anxiety; anxiety in turn may lead to greater alertness for threatening cues (eg, people giving unfriendly looks) supportive of the notion that others are malevolent and threatening, thus strengthening the delusional ideation. Symptoms thus can impact on each other and become interconnected, suggesting that psychopathology can be modeled as a network of mutually impacting experiences.6–8 Research has indicated that risk for transition to clinical outcomes may be related to dynamic relationships between subthreshold symptoms,9 impacting on each other over time. Thus, associations between subthreshold psychotic experiences on the one hand, and co-occurring symptoms of anxiety and depression, as well as negative symptoms on the other, impact on each other’s severity over time, thus increasing risk for functional impairment and transition to clinical psychotic disorder.10–12 In addition, psychotic experiences impact on themselves over time, showing more or less persistence, more persistence being associated with greater probability of clinical transition.13
A relatively novel area in empirical models of symptom connections involves the interplay between hallucinations and delusions in the early stages of psychosis. This is of particular interest, given theories on how delusions may arise secondarily to hallucinatory experiences, as described in the work of Maher on anomalous perceptual experiences14 and Kapur on aberrant salience.15 In this work, the differentiation between primary delusions (ie, delusions that arise spontaneously and are the result of reasoning bias) and secondary or explanatory delusions (ie, delusions formed as an explanation, which can be understood in the light of a persons’ background) represents a crucial distinction. The early phases of psychosis may be marked by “perceptual changes” or “hallucinatory experiences,” which give rise to a puzzling or bewildered feeling that warrants an (odd) explanation. The formation of secondary delusional ideation following hallucinatory experiences increases the risk of deficits in social functioning and subsequent onset of clinical needs.14,15 Many models have, at least in part, adopted the notion that delusions combine with hallucinations in the early stages of psychosis.16–21
The association between subthreshold hallucinatory and delusional experiences has recently been the object of empirical analysis. One general population study distinguishing between groups, reporting (1) no psychotic experiences, (2) only hallucinations, (3) only delusions, and (4) both hallucinations and delusions, found that having both symptoms, as opposed to having either one in isolation, resulted in increased symptom severity and persistence, as well as increased risk of clinical outcome and need for care.22 This finding was replicated in 2 general population studies,23,24 as well as a study from the World Health Organization.25 Further findings from these studies indicated that combined hallucinations and delusions, as opposed to either symptom in isolation, are associated with (1) greater environmental etiological load (childhood trauma, cannabis use, urbanicity),22,23,25 (2) familial liability to psychosis,22,23 and (3) affective dysregulation,22,23,25 all known risk factors for psychotic disorder.12,26–28 These findings are compatible with suggestions that hallucinations and delusions are dynamically associated with each other, giving rise to a “hallucinatory-delusional” state in which hallucinations and delusions are connected under the influence of environmental risks, affective dysregulation and familial liability, increasing probability of need for care, and clinical outcome.
Further evidence for the importance of an early “hallucinatory-delusional” state comes from studies focusing on the sequence in which symptoms arise. A virtual reality study investigated which factors (intellectual functioning, emotional processes, reasoning styles, social factors, and anomalous perceptual experiences) were predictive of increased levels of state anxiety and paranoia in healthy controls.29 Findings showed that the only specific predictor for state paranoia was proneness to anomalous perceptual experiences,29 indicating that hallucination proneness is predictive of paranoid ideation in healthy controls. These results are further underscored by 2 longitudinal studies in children measuring hallucinations at baseline and delusional ideation at 5-year follow-up. Results showed that persistent hallucinations at baseline and follow-up predict development of delusions over follow-up.30,31 Similar effects were found for adults in a general population longitudinal study, indicating that baseline hallucinatory experiences, when complicated by delusions at follow-up, resulted in increased risk for clinical psychosis, as compared to baseline hallucinations without subsequent delusional ideation.32 These findings suggest that the pathway from hallucinatory experiences to clinical psychosis may be mediated, at least in part, by secondary formation of delusions.
Given the fact that co-occurrence of hallucinations and delusions in the general population is associated with familial risk for psychosis,22,23 the current study aimed to investigate the influence of familial liability on co-occurrence of hallucinations and delusions, in more detail than before, in a sample with increased genetic risk for psychosis. Data from the Genetic Risk and Outcome of Psychosis (GROUP)33 study were used, including healthy siblings of affected families (hereafter: healthy siblings OAF), their parents, and healthy controls. As healthy siblings of affected patients share 50% of their genes and a proportion of environmental exposure with patients, have a degree of subclinical expression of psychosis, but are free of illness-related factors (alterations in functioning due to clinical psychopathology or medication effects), sibling status represents a suitable index for investigating the effects of familial liability on patterns of subclinical psychotic symptomatology. Studies have shown that siblings are at increased risk of psychosis34,35 and display higher rates of schizotypal traits,36–39 suggesting expression of familial liability to psychosis. In the current study, it was hypothesized that controls would exhibit the lowest rate of co-occurring hallucinations and delusions, and healthy siblings OAF and parents, having increased genetic risk, would show higher rates of co-occurring hallucinations and delusions.
In addition, following previous findings that the co-occurrence of hallucinations and delusions show a stronger association with environmental factors22,23,25 than isolated hallucinations or delusions, the second hypothesis under investigation was that environmental factors (growing up in an urban environment, childhood trauma, and cannabis use) moderate the strength of the connection between hallucinations and delusions.
Methods
Data pertain to baseline (T0) and follow-up (T1) measurements of an ongoing longitudinal study (GROUP) in the Netherlands and Belgium including patients, their parents, their healthy siblings, and a group of healthy controls. In selected representative geographical areas, patients were identified through representative clinicians whose caseload was screened for inclusion criteria. Subsequently, a group of patients presenting consecutively at these services either as out- or inpatients were recruited for the study. Controls were selected through a system of random mailings to addresses in the catchment areas of the cases. The baseline sample and the sampling procedure have been described in detail previously.33 Baseline inclusion criteria for the patient group were (1) age between 16 and 60 years, (2) mastery of the Dutch language, (3) the ability and willingness to sign informed consent, and (4) a diagnosis of nonaffective psychosis based on the Comprehensive Assessment of Symptoms and History Interview (CASH).40 The following additional criteria were used for the control and healthy siblings OAF group: (1) no lifetime history of any psychotic disorder (healthy siblings OAF and controls) and (2) no first-degree relative with any lifetime psychotic disorder (controls only). The T1 measurements were carried out 2.9 years after baseline with the exception of parental measurements, as parents were only assessed at baseline. At both T0 and T1, instruments regarding symptoms, diagnosis, and environmental risk were administered. Trained research assistants administered all interviews and were instructed to prompt with follow-up questions in case of a positive answer to the CASH items in order to identify false positives. All diagnoses and psychopathology ratings were assessed in consensus meetings chaired by a consultant psychiatrist. The project was approved by the local Ethics Committee and all participants gave written informed consent prior to participating in the study.
The full GROUP sample at T0 comprised 1119 patients with nonaffective psychosis, 1057 healthy siblings OAF, 918 parents, and 589 unrelated healthy controls. In the current analyses, only data from the control, healthy sibling OAF, and parent groups were used. Participants from one site, which did not use the CASH40 to assess psychotic symptoms, were excluded from the analyses.
Measurements
The Comprehensive Assessment of Symptoms and History.
The hallucination section of the CASH40 was used to assess auditory, visual, olfactory, and somatic hallucinations at both the T0 and T1 interview. Dichotomous measures were created indicating the reported presence (1) or absence (0) of hallucinations at baseline (hereafter any baseline hallucination(s) as measured by the T0 lifetime version of the CASH) and at follow-up (ie, any T1 hallucination(s)). A variable was creating that indicated the presence or absence of any hallucination(s) at the T0 and T1 interview (0: “no hallucinations at baseline or at T1” and 1: “any hallucination(s) at baseline and or T1”), which was used in the current analyses.
Delusions.
CASH delusion items (section 6) included the following types of delusions: persecution, jealousy, guilt, grandiosity, religious, somatic, ideas of reference, being controlled by an external force, being able to read other persons’ thoughts, thoughts being read by others or thought broadcasting, and thoughts being inserted or withdrawn by an external source. Each item was assessed individually, and an overall score representing absence (0) or presence (1) for any baseline delusion(s) (T0) and any T1 delusion(s) (T1) was created. Again, a variable representing presence of any delusion(s) was created by combining scores of any baseline delusion(s) and any T1 delusion(s) (0: “absent” and 1: “present”), which was used in the current analyses.
Environmental Factors
Urbanicity
Urbanicity before the age of 15 years was obtained via a questionnaire which inquired about each home address before the age of 15. For each reported address, a population density record was calculated with the aid of historical population density records. Next, the average population density over the period 0–14 years of age was calculated in accordance with the Dutch CBS urbanicity rating (1 = <500/km2; 2 = 500–1000/km2; 3 = 1000–1500/km2; 4 = 1500–2500/km2; 5 = 2500+/km2), or Belgium equivalent, as described in more detail elsewhere.41–43 A median split in the healthy control group data was used to indicate above (1) or below (0) median exposure to urbanicity before the age of 15 years.
Childhood Trauma
The variable for Childhood trauma was constructed with the Childhood Trauma Questionnaire – Short Form (CTQ- SF; Dutch version),44,45 measuring exposure to physical, emotional, and sexual abuse, and physical and emotional neglect during childhood. Each item was rated on a 6-point scale (0: “never true” and 5: “very often true”). A mean item score was calculated for the CFQ-SF after which the median split based on control group data was used to divide the groups in high (1) and low childhood trauma exposure (0).
Cannabis
At baseline, recent cannabis use was measured by urine analysis by the National Alcohol and Drug Use Jellinek Laboratory in the Netherlands. The cutoff level used to determine cannabis use as present or absent was 50ng/ml. Given the conservative cutoff level of 50 ng/ml, the timeframe for detecting cannabis use was approximately 1 month. A measure for recent cannabis use was created (negative, 0: “absent” and positive, 1: “present”).
Analyses
All analyses were conducted in STATA version 1146 with GROUP data release version 3.02. The variable Group had 3 levels and was coded as 0 (controls), 1 (healthy siblings OAF), and 2 (parents). Data were analyzed in the “long format” (STATA terminology) with each subject contributing 2 (T0 and T1) rows of observations to the analysis, conforming to previous work in this area,22,23 except for parents, who were only assessed at T0. Therefore, the n reported throughout the paper refers to number of observations rather than number of subjects (ie, subjects contributing multiple observations). This means that the analyses reported below were cross-sectional in nature. Since there were multiple observations per subject, and some families contributed more than one relative, all analyses were controlled for clustering of data at both the family and the subject level by adding family and subject ID to the models as random intercepts.
Demographic differences were assessed with ANOVA analyses (for continuous variables) and logistic regression (for dichotomous variables), quantifying possible differences in the sample between groups and between T0 and T1, which were deemed significant if p ≤.05.
Hypothesis I: Do Hallucinations and Delusions Co-occur and Is This Association Stronger for Those With Higher Familial Psychosis Risk?
The co-occurrence of hallucinations and delusions was assessed by calculating the proportion with any delusion(s) per group (0 = controls, 1 = siblings, 2 = parents), given the presence or absence of hallucinations. These proportions are presented for descriptive purposes in the results section.
The analyses carried out to investigate Hypothesis I involved 2 different steps. The first step was to investigate whether there was an interaction effect of any hallucination × group on delusional outcome. This was done with the aid of multilevel logistic regression, tested with the aid of the XTMELOGIT command in STATA, controlling for age, sex, and clustering of data at the subject and family level by adding family and subject ID to the model as random intercepts.
The second part consisted of an investigation of the simple effects of group on delusional outcome, given the presence or absence of hallucinations. Investigating the simple effects allows for a more detailed account of the association between group and presence or absence of any hallucination on delusional outcomes. These analyses investigated 2 main factors. First, it was investigated whether the risk of having delusions (dependent variable) was greater for individuals with compared to those without hallucinations (independent variable) within groups. This was done with the aid of 3 different post hoc comparisons (ie, Comparison 1: controls without hallucinations vs controls with hallucinations; Comparison 2: healthy siblings OAF without hallucinations vs healthy siblings OAF with hallucinations; Comparison 3: parents without hallucinations vs parents with hallucinations). The second factor was whether the risk of having delusions, given the presence of hallucinations differed as a function of familial liability, that is whether this risk differed across groups (ie, group: controls, healthy siblings OAF, and parents). Again, 3 different post hoc comparisons were used to test whether there was difference in risk between groups (Comparison 1: controls with hallucinations vs healthy siblings OAF with hallucinations; Comparison 2: controls with hallucinations vs parents with hallucinations; Comparison 3: healthy siblings OAF with hallucinations vs parents with hallucinations).
An alternate way in which the within- and between-group effects described above was investigated is by constructing 3 different models, with each model comparing the effect of presence or absence of hallucinations on delusional outcome in 2 different categories of group (Model 1: controls vs healthy siblings OAF; Model 2: controls vs parents; Model 3: healthy siblings OAF vs parents). A more detailed description of these models can be found in the supplementary section S1.
Hypothesis II: Do Environmental Factors Moderate the Strength of the Connection Between Hallucinations and Delusions?
The analysis investigating whether the strength of the connection between hallucinations and delusions was moderated by the selected environmental factors was carried out in the control and healthy siblings OAF data only, since environmental data in the parent group were not assessed at T0. Multilevel logistic regression models assessed the main interaction effect between hallucinations and environmental factors on delusion occurrence in data combining the control and the healthy siblings OAF groups. For each type of exposure (respectively urbanicity, childhood trauma, and cannabis use), an analysis including an interaction term of environmental exposure × hallucinations was carried out. Comparable to the analyses of the first hypothesis, in the second phase of the analysis, the simple effects of environmental exposure and presence of hallucinations on delusional outcome were investigated. For each environmental exposure, a separate analysis was carried out. The model included any hallucination(s) (at either T0 or T1), together with the investigated environmental risk factor (respectively urbanicity, childhood trauma, and recent cannabis use) and tested the simple effect of any hallucination(s) and the environmental factor, with any delusion(s) as outcome variable. The interaction between any hallucination(s) and the environmental factors was tested in a similar fashion as described for the first hypothesis. For each environmental exposure, a dummy variable was created (hallucination by urbanicity, hallucination by trauma, and hallucination by cannabis). Each of these variables contained 4 levels (Level 1: 0-0, no hallucination(s), environmental risk factor absent; Level 2: 1-0, any hallucination(s), environmental risk factor absent; Level 3: 0-1, no hallucination(s), environmental risk factor present; Level 4: 1-1, any hallucination(s), environmental risk factor present). Chi squares (χ2) were available for each level of the any hallucination(s) and environmental exposure interaction, with Level 1 as reference category. For each analysis, Level 2 vs 4 comparisons were made in order to assess the effect of the environmental exposure and co-occurrence of delusions, given the presence of hallucinations. All analyses were controlled for age, sex, and clustering of observations at the family- and subject-level by adding family and subject ID to the model as random intercepts. All analyses were conducted in the combined T0 and T1 data, each individual contributing 2 rows (T0 and T1).
Results
Sample
The T0 sample consisted of 1057 healthy siblings OAF, 918 parents, and 589 unrelated healthy controls. The T1 sample included 810 healthy siblings OAF and 462 controls. Follow-up rates for the healthy siblings OAF and the control groups were 76.6% and 78.4%, respectively. Mean time to follow-up was 2.9 years. Descriptive statistics of the sample are given in table 1. At both time points, controls were more likely to be older, white, female, and of higher educational level than healthy siblings OAF. Participants who completed both T0 and T1 measurements, on average, had a higher educational level and more often were of White ethnic origin than those who only participated at T0.
Table 1.
Descriptive Statistics of the GROUP Sample at Baseline and Follow-up
| T0 | T1 | ||||
|---|---|---|---|---|---|
| Parents (n = 918) | Siblings (n = 1054) | Controls (n = 589) | Siblings (n = 810) | Controls (n = 462) | |
| Mean age (SD) | 54.8 (6.8) | 27.8 (8.3) | 30.4a
(10.6) |
30.5 (7.9) | 34.2 (10.6)b |
| Male sex | 42.6% (n = 391) | 45.6% (n = 481) | 45.7% (n = 269) | 44.3% (n = 359) | 43.8% (n = 202) |
| Educationc ,d | |||||
| No education/primary school | 5.4% (n = 16) |
7.4% (n = 285) | 2.7% (n = 16) |
1.6% (n = 13) |
0.2% (n = 1) |
| Lower (secondary/vocational) education | 47% (n = 432) | 41.4% (n = 438) | 30.2% (n = 178) | 39% (n = 316) | 28.4% (n = 131) |
| Higher vocational education | 6.6% (n = 61) | 19.8% (n = 209) | 31.6% (n = 186) | 16.3% (n = 132) | 19.7% (n = 91) |
| Higher (secondary) education | 34.7% (n = 319) | 29.3% (n = 309) | 35.1% (n = 207) | 42.6% (n = 345) | 51.5% (n = 238) |
| Unknown | 6.2% (n = 57) |
2.2% (n = 23) |
0.3% (n = 2)a |
0.5% (n = 4) |
0.2% (n = 1)b |
| Ethnic groupd | |||||
| White | 88.3% (n = 811) | 83.% (n = 877) | 90.0% (n = 530) | 86.8% (n = 703) | 91.1% (n = 421) |
| Other | 11.8% (n = 108) | 17% (n = 180) | 10.0% (n = 59)a | 13.2% (n = 107) | 8.9% (n = 41)b |
| Diagnosise | |||||
| Schizophrenia | 0% (n = 0) | 0% (n = 0) |
0% (n = 0) | 0.7% (n = 6) | 0% (n = 0) |
| Other psychosis | 0% (n = 0) | 0% (n = 0) |
0% (n = 0) | 0.3 (n = 2) | 0% (n = 0) |
| Mood disorders | 18.8% (n = 173) | 11.7% (n = 124) | 8.7% (n = 51) | 17.5% (n = 142) | 13% (n = 60) |
| Substance abuse/dependence | 0.1% (n = 1) | 0.3% (n = 3) | 0% (n = 0) | 0.1% (n = 1) | 0.2% (n = 1) |
| Anxiety disorders | 0.5% (n = 5) | 0% (n = 0) |
0.2% (n = 1) | 0.6% (n = 5) | 0.4% (n = 2) |
| Personality disorders | 0% (n = 0) | 0.2% (n = 2) | 0% (n = 0) | 0.1% (n = 1) | 0% (n = 0) |
| Other diagnosis | 0.1% (n = 1) | 0.7% (n = 7) | 0.3% (n = 2) | 1% (n = 8) | 0.2% (n = 1) |
| Postponed diagnosis | 0.1 (n = 1) | 0.5% (n = 5) | 0.5% (n = 3) | 0.3% (n = 2) | 0.9% (n = 4) |
| No diagnosis | 80.3% (n = 738) | 86.7% (n = 916) | 89.8 (n = 532) | 79.4% (n = 643) | 84.9% (n = 392) |
| Missing | 0 | 0 | 0 | 0 | 0% (n = 0) |
Notes: aControl group differs from healthy siblings OAF group at T0 (p < .05).
bControl group differs from healthy siblings OAF group at T1 (p < .05).
cEducation: no/primary: no or only primary school, lower secondary education: LBO/HH/LHNO/VBO, MAVO/VMBO, higher vocational education: MBO, higher secondary education: HAVO, VW0, HBO, and University.
dT0 sample differs significantly from T1 sample (p <.05).
eOther diagnoses include cognitive disorder, developmental disorder, anorexia, and adjustment disorder.
The number of individuals with valid CASH data at baseline was 1942, who over baseline and follow-up contributed a total of 2598 observations (764 in controls, 1165 in healthy siblings OAF, and 669 in parents).
Hypothesis I: Do Hallucinations and Delusions Co-occur and Is This Association Stronger for Those With Higher Psychosis Risk?
First, co-occurrence rates for hallucinations and delusions were investigated per group (table 2). In the control group, 92.0% (n = 703) reported no psychotic symptoms, 4.3% (n = 33) reported only delusions, 2.4% (n = 18) reported only hallucinations, and 1.3% (n = 10) reported both. In the healthy siblings OAF group, 86.3% (n = 1005) reported no symptoms, 7.0% (n = 82) reported only delusions, 3.5% (n = 41) reported only hallucination(s), and 3.2% (n = 37) reported hallucinations as well as delusions. For parents, this was, respectively, 88.0% (n = 589), 6.6% (n = 44), 2.5% (n = 17), and 2.8% (n = 19).
Table 2.
Reported Symptoms per Group
| Group | N a | No Psychotic Symptoms, % (n)a | Only Hallucinations, % (n)a | Only Delusions, % (n)a | Both Hallucinations and Delusions, % (n)a |
|---|---|---|---|---|---|
| Controls | 764 | 92.0 (703) | 2.4 (18) | 4.3 (33) | 1.3 (10) |
| Siblings | 1165 | 86.3 (1005) | 3.5 (41) | 7.0 (82) | 3.2 (37) |
| Parents | 669 | 88.0 (589) | 2.5 (17) | 6.6 (44) | 2.8 (19) |
Note: aRefers to repeated observations in individuals (except parent group that was only assessed once at baseline).
Second, it was investigated whether reporting any delusion(s) differed as a function of presence or absence of any hallucination(s) (ie, reporting only delusions vs co-occurring delusions and hallucinations). The results showed that for controls, siblings, and parents, the probability of reporting delusions, given the presence of any hallucination(s), was higher as compared to absence of hallucinations (table 3).
Table 3.
The Association Between Any T0 or T1 Hallucination and Any T0 or T1 Delusion, Reported Separately for the Different Groupsa
| Group | N | Proportion Reporting Any Hallucination, % (n) | Proportion Reporting Delusions Given Absence of Hallucinations, % (n) | Proportion Reporting Delusions Given Presence of Hallucinations, % (n) | Difference (%) | Association χ2, p a, b |
|---|---|---|---|---|---|---|
| Controls | 764 | 3.7 (28) | 4.5 (33/736) | 35.7 (10/28) | 31.2 | χ2 = 22.71, p <.001 |
| Siblings | 1165 | 6.7 (78) | 7.5 (82/1083) | 47.4 (37/78) | 39.9 | χ2 = 28.66, p <.001 |
| Parents | 669 | 5.4 (36) | 7.0 (44/625) | 52.8 (19/36) | 45.8 | χ2 = 28.66, p <.001 |
Notes: aAll analyses refer to multilevel logistic regression analyses controlled for age, sex, group, and clustering of observations within subjects, with delusions as dependent variable and “any T0 or T1 hallucination” as independent variable.
bRepresents the within-group effect of the presence of hallucinations on the probability of delusions. The proportions in the table, and differences between them, are presented for descriptive purposes only.
No significant differences were found in the occurrence of any delusion(s) between the healthy controls, healthy siblings OAF, and parent groups, given absence of any hallucination(s). The main interaction effect of group × any hallucination on delusional outcome was investigated. Results showed no significant interaction of group (control [reference] vs healthy sibling OAF: p = .747, control [reference] vs parents: p = .757). However, the probability of reporting any delusion(s), given the presence of any hallucination(s), was stronger for groups with increased psychosis risk (figure 1). The regression analyses investigating the effect of hallucinations and proxy genetic risk categories on delusions showed that parents had an increased probability of delusions, given presence of hallucinations, compared to controls (χ2 = 5.04, p = .03), and to healthy siblings OAF (χ2 = 4.18, p = .04). The difference in probability of delusions, given presence of hallucinations, between healthy siblings OAF and controls was directionally similar to that observed in the parent group (figure 1), albeit not significant (χ2 = 0.52, p = .47). When these contrasts were investigated with the aid of 3 different models, the results remained by and large the same, except for the fact that the found difference between parents and healthy siblings OAF was no longer significant, caused by the change in random effect estimators (see supplementary table 1).
Fig. 1.
Multilevel logistic regression analyses of the probability of delusional ideation as a function of hallucinations, showing differences in the strength of the connection between delusions and hallucinations across 3 groups with variable psychosis risk. 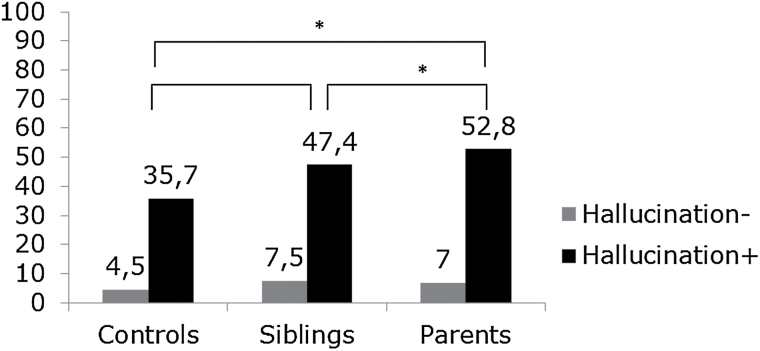
*p < .05. Hallucination (−)= “no hallucination(s)” and hallucination (+) = “any hallucination.” Grey bars represent the proportion of individuals reporting delusions in the absence of hallucinations; black bars: the proportion of individuals reporting delusions in the presence of any hallucination. Analyses controlled for age, sex, and clustering of observations at subject and family level.
Hypothesis II: Do Environmental Factors Moderate the Strength of the Connection Between Hallucinations and Delusions?
Multilevel logistic regression models tested the interaction between presence of any hallucination(s) and each of the 3 environmental factors (ie, recent cannabis use, urbanicity, and childhood trauma). Results showed no significant main interaction effects for urbanicity × any hallucination (p = .14) and for childhood trauma × any hallucination (p = .28) but did find a significant interaction for the recent cannabis use × any hallucination term (p = .02). The model testing the simple effects of environmental risk factors and hallucination on any delusion showed associations in the expected direction (ie, stronger association between hallucinations and delusions in the exposed vs nonexposed groups) (for results, see table 4). The moderating effect was significant or borderline significant for cannabis use (p = .037) and childhood trauma (p = .054), but not for urbanicity (p = .357).
Table 4.
The Moderating Effect of Environmental Factors on the Risk of Delusions Given Hallucinations
| Exposure type | n a | Proportion exposed, % (n) |
Proportion with delusions given absence of hallucinations, % (n) | Proportion with delusions given presence of hallucinations, % (n) | Difference (%) | Effect size environmental factor (%) | Interaction χ2, p |
|---|---|---|---|---|---|---|---|
| Cannabis urinalysis + | 1827 | 6.2 (114) |
10 (10) | 73.3 (11) | 63.3 | 31.6 | χ2 = 4.3, p = .037 |
| Cannabis urinalysis − | 93.8 (1713) |
6.2 (100) | 37.9 (33) | 31.7 | |||
| Childhood trauma + | 2628 | 51.5 (1352) | 8.1 (62) | 44.6 (25) | 36.5 | 15.2 | χ2 = 3.7, p = .054 |
| Childhood trauma − | 48.6 (1276) |
6.8 (33) | 28.1 (9) | 21.3 | |||
| Urbanicity <age 15 + | 3072 | 47.5 (1612) | 5.6 (47) | 52.1 (25) | 46.5 | 14.4 | χ2 = 0.9, p = .357 |
| Urbanicity <age 15 − | 52.5 (1460) |
7.0 (60) | 39.1 (18) | 32.1 |
Note: aAll analyses carried out in combined data of the control and sibling group and refer to multilevel logistic regression analyses controlled for age, sex, group, and clustering of observations within subjects, with delusions as dependent variable and the “any T0 or T1 hallucination × environmental factor” interaction as independent variable.
Discussion
Findings
Previous investigations have consistently reported that hallucinations and delusions cluster more often than would be expected by chance.22,23,25 The current study extends this finding from the general population to a sibling and parent sample of affected families. There was no main interaction of any hallucinations × group in the model of delusional ideation. Interaction analyses, however, have low power, given the low rates of co-occurrence in particularly the controls. When examining the simple effects of any hallucination and group on delusional outcome, the findings suggested that, given the presence of hallucinations, a dose-response relationship exists such that greater psychosis risk was associated with greater levels of co-occurrence of hallucinations and delusions. A dose-response effect was not apparent for hallucinations or delusions occurring in isolation, indicating that it is not the mere presence of a symptom, but rather the co-occurrence of both that serves as a discriminating factor between healthy and affected families. Furthermore, the findings indicate that environmental risk factors (cannabis exposure and childhood trauma) are associated with increased co-occurrence of hallucinations and delusions. Although not all reported differences were statistically conclusive, the pattern of the results nevertheless was consistent with previous work.22,23 The findings indicate that the pattern of co-occurring hallucinations and delusions between controls and healthy siblings OAF is in the expected direction (siblings showing increased co-occurrence), however this pattern was not statistically significant. Several explanations could be given for the finding that healthy siblings OAF appear to resemble controls more than was a priori expected. First, healthy siblings of patients, more than parents, may answer defensively to questions about psychopathology.47,48 Additionally, the healthy siblings OAF who took part in the study may have represented the group of siblings with relatively low expression of psychotic experiences. These factors may have resulted in a degree of underestimation of the association between hallucinations and delusions in this group. Therefore, the addition of a large sample of parents in the current study can be considered a strength.
The current findings form a replication of previous findings indicating that the co-occurrence of hallucinations and delusions is associated with familial and environmental risk.22,23 Furthermore, studies have consistently shown that clustering of hallucinations and delusions leads to greater symptom severity and persistence of symptoms,22–25 as predicted by the psychosis proneness-persistence-impairment model.1 This finding, combined with evidence from previous findings that the psychotic state deepens when hallucinations become complicated by delusions22–25,32 is supportive of the theoretical frameworks proposed by Maher14 and Kapur15 in which early psychosis is marked by a phase in which anomalous perceptual experiences connect with secondary (explanatory) delusional ideation. The theoretical framework, in which hallucinations and delusions interact with each other, is in line with recent network theories of psychopathology that state that symptoms are not a mere passive expression of underlying liability but interact with each other and have an active role in disorder onset.5,8,9 The interaction between hallucinations and delusions thus can be viewed as a crucial connection in the symptom network underlying psychosis. Taken together, the findings lend credence to the notion of a “hallucinatory-delusional state” in which hallucinations and delusions become connected under the influence of environmental and familial risk factors, resulting in greater severity and persistence of symptoms, and a deepening of the psychotic state.
Given the fact that the findings on the connection between hallucinations and delusions in (sub)clinical psychosis are robust and replicable,22–25 a search for biological substrates of this connection is warranted. Dopaminergic dysregulation may be the starting point of the psychotic cascade15,49 which is supported by the finding that presynaptic dopaminergic abnormalities associated with psychotic disorder are not present in healthy individuals reporting auditory hallucinations,50 but instead appear as early psychosis progresses to the clinical state.51 Furthermore, findings suggest that in populations at high risk for psychosis, antipsychotic medication dampens experiences of aberrant salience, whereas cognitive behavioral therapy changes delusional ideation and associated biases.18 These findings indicate that dopaminergic alterations may mediate the process in which meaning is attached to perceptual abnormalities, giving way to a delusional interpretation of the perceptual changes.
Methodological Considerations for Future Studies
The following methodological issues should be taken into consideration for future studies. Firstly, the current study used analyses that were cross-sectional in nature to investigate the association between hallucinations and delusions across groups. Therefore, no conclusions can be drawn on the temporality of the connection between hallucinations and delusions. Future studies might benefit from more frequent and fine-grained follow-up in order to investigate the temporality of symptom onset. The current study found lower rates of co-occurrence of hallucinations and delusions in the healthy controls (1.3%) than previous studies, which reported co-occurrence of hallucinations and delusions in 2.0%–2.5% in general population samples.22,23,25 As a recent review found strong evidence that assessment type accounts for a large part of the variability in rates of psychotic experiences between studies,2 the different rates of co-occurrence may reflect random deviance around the population mean and it may reflect differences in assessment methods between the current study, using structured clinical interview, and previous studies, which were all based on the Composite International Diagnostic Interview.22–25 A further consideration related to the interview method is that symptom data were based on dichotomous measures representing information on lifetime and interval-time (T0-T1) presence vs absence. Future studies may benefit from continuous scales to assess psychotic symptoms, allowing for the investigation of severity of reported symptoms. Furthermore, the current study included relatives of patients with nonaffective psychosis. Future studies may extend the investigation of symptom connections in other groups, particularly patients with affective psychosis or sample by presence of psychotic symptoms rather than a specific diagnostic category. Although the inclusion of both parents and healthy siblings OAF may be considered a strength, it also creates methodological issues that require further consideration. Any group differences need to be interpreted with caution, since the design was unbalanced (N individuals with genetic risk vs N in the healthy control group), affecting statistical power. Additionally, possible cohort effects need to be taken into consideration, in that parents have lived longer through the period of risk, increasing the probability of onset of symptoms in isolation as well as in combination with each other. One other factor, as mentioned above, may be that healthy siblings OAF and parents may adapt different answering styles to overt questions about psychosis, siblings possibly answering more “defensibly” than parents. This could cause an underestimation of symptoms in healthy siblings OAF.47,48 Furthermore, the current study investigated the association between cannabis use, as an environmental risk, and co-occurring hallucinations and delusions with the aid of a measure limited to current cannabis use. This does not exclude an effect of cannabis exposure at an earlier age.52–54 Additionally, as each environmental risk factor was modeled separately, any comparisons between them can only be descriptive and not direct as the risk factors’ covariance was not accounted for.
Studies of relational models of psychopathology in psychosis can be usefully combined with neuroimaging paradigms in order to test to what degree the mechanism of progressive connection between perceptual alterations with delusional ideation is associated with underlying changes in biology, eg, presynaptic dopaminergic changes. Longitudinal high-risk samples using neuroimaging measures may be of particular interest, as these permit assessment of incident psychopathology and the temporal order of interaction between environmental risks and neural substrates underlying psychopathology. This type of study would allow investigating neural substrates of the temporal onset of symptoms (ie, hallucinations and delusions). In addition, this type of study can be used to investigate the possible causal or mediating role of environmental exposures (eg, childhood trauma, cannabis use) in symptom onset and provide information about the neural and biological mechanisms through which these factors impact on psychosis risk. Furthermore, studies comparing coping styles and attribution processes related to hallucinations in individuals with only hallucinations to those with both hallucinations and delusions may offer new insights on protective mechanisms. These studies may also offer insight in the role of cognitive biases and psychological processes in the transition from unusual perceptual experience and aberrant salience to delusions, as well as in resilience for delusions in spite of dopamine dysregulation. Additionally, future studies may benefit from distinguishing qualitatively different durations of onset of psychotic symptoms (ie, insidious or acute) and the relationship between hallucinations and delusions.55
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
This work was supported by the Geestkracht program of the Dutch Health Research Council (ZON-MW , grant number 10-000-1002) and matching funds from participating universities and mental health care organizations (Site Amsterdam: Academic Psychiatric Centre AMC, Ingeest, Arkin, Dijk en Duin, Rivierduinen, Erasmus MC, GGZ Noord Holland Noord; Site Utrecht: University Medical Centre Utrecht, Altrecht, Symfora, Meerkanten, Riagg Amersfoort, Delta; Site Groningen: University Medical Centre Groningen, Lentis, GGZ Friesland, GGZ Drenthe, Dimence, Mediant, GGNet Warnsveld, Yulius Dordrecht and Parnassia Psycho-Medical Centre; Site Maastricht: Maastricht University Medical Centre, GGZ Eindhoven, GGZ Midden-Brabant, GGZ Oost-Brabant, GGZ Noord- Midden Limburg, Mondriaan Zorggroep, Prins Clauscentrum Sittard, RIAGG Roermond, Universitair Centrum Sint-Jozef Kortenberg, CAPRI University of Antwerp, PC Ziekeren Sint-Truiden, PZ Sancta Maria Sint-Truiden, GGZ Overpelt, OPZ Rekem). The analyses were supported by unrestricted grants from Jansen-Cilag, Eli Lilly and Company, Astra-Zeneca, and Lundbeck. The research leading to these results has received funding from the European Community’ s Seventh Framework Program under grant agreement No. HEALTH-F2-2009–241909 (Project EU-GEI).
Supplementary Material
Acknowledgments
We are grateful for the generosity of time and effort by the families who make the GROUP project possible. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1.van Os J, Linscott RJ, Myin-Germeys I, Delespaul P, Krabbendam L. A systematic review and meta-analysis of the psychosis continuum: evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. Psychol Med. 2009;39:179–195. [DOI] [PubMed] [Google Scholar]
- 2.Linscott RJ, van Os J. An updated and conservative systematic review and meta-analysis of epidemiological evidence on psychotic experiences in children and adults: on the pathway from proneness to persistence to dimensional expression across mental disorders. Psychol Med. 2013;43:1–17. [DOI] [PubMed] [Google Scholar]
- 3.Kaymaz N, Drukker M, Lieb R, et al. Do subthreshold psychotic experiences predict clinical outcomes in unselected non-help-seeking population-based samples? A systematic review and meta-analysis, enriched with new results. Psychol Med. 2012;42:2239–2253. [DOI] [PubMed] [Google Scholar]
- 4.Bilder RM, Mukherjee S, Rieder RO, Pandurangi AK. Symptomatic and neuropsychological components of defect states. Schizophr Bull. 1985;11:409–419. [DOI] [PubMed] [Google Scholar]
- 5.Borsboom D, Cramer AO. Network analysis: an integrative approach to the structure of psychopathology. Annu Rev Clin Psychol. 2013;9:91–121. [DOI] [PubMed] [Google Scholar]
- 6.Kendler KS, Zachar P, Craver C. What kinds of things are psychiatric disorders? Psychol Med. 2011;41:1–8. [DOI] [PubMed] [Google Scholar]
- 7.Borsboom D, Cramer AO, Schmittmann VD, Epskamp S, Waldorp LJ. The small world of psychopathology. PLoS ONE. 2011;6:e27407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wichers M. The dynamic nature of depression: a new micro-level perspective of mental disorder that meets current challenges. Psychol Med. 2013;44:1–12. [DOI] [PubMed] [Google Scholar]
- 9.van Os J. The dynamics of subthreshold psychopathology: implications for diagnosis and treatment. Am J Psychiatry. 2013;170:695–698. [DOI] [PubMed] [Google Scholar]
- 10.Dominguez MD, Saka MC, can Saka M, Lieb R, Wittchen HU, van Os J. Early expression of negative/disorganized symptoms predicting psychotic experiences and subsequent clinical psychosis: a 10-year study. Am J Psychiatry. 2010;167:1075–1082. [DOI] [PubMed] [Google Scholar]
- 11.Werbeloff N, Drukker M, Dohrenwend BP, et al. Self-reported attenuated psychotic symptoms as forerunners of severe mental disorders later in life. Arch Gen Psychiatry. 2012;69:467–475. [DOI] [PubMed] [Google Scholar]
- 12.Wigman JT, van Nierop M, Vollebergh WA, et al. Evidence that psychotic symptoms are prevalent in disorders of anxiety and depression, impacting on illness onset, risk, and severity–implications for diagnosis and ultra-high risk research. Schizophr Bull. 2012;38:247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dominguez MD, Wichers M, Lieb R, Wittchen HU, van Os J. Evidence that onset of clinical psychosis is an outcome of progressively more persistent subclinical psychotic experiences: an 8-year cohort study. Schizophr Bull. 2011;37:84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maher BA. Delusional thinking and perceptual disorder. J Individ Psychol. 1974;30:98–113. [PubMed] [Google Scholar]
- 15.Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003;160:13–23. [DOI] [PubMed] [Google Scholar]
- 16.Garety PA, Kuipers E, Fowler D, Freeman D, Bebbington PE. A cognitive model of the positive symptoms of psychosis. Psychol Med. 2001;31:189–195. [DOI] [PubMed] [Google Scholar]
- 17.Freeman D. Suspicious minds: the psychology of persecutory delusions. Clin Psychol Rev. 2007;27:425–457. [DOI] [PubMed] [Google Scholar]
- 18.van der Gaag M. A neuropsychiatric model of biological and psychological processes in the remission of delusions and auditory hallucinations. Schizophr Bull. 2006;32(suppl 1):113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frith C. The neural basis of hallucinations and delusions. C R Biol. 2005;328:169–175. [DOI] [PubMed] [Google Scholar]
- 20.Larøi F, Van der Linden M. Metacognitions in proneness towards hallucinations and delusions. Behav Res Ther. 2005;43:1425–1441. [DOI] [PubMed] [Google Scholar]
- 21.Bentall RP, Fernyhough C, Morrison AP, Lewis S, Corcoran R. Prospects for a cognitive-developmental account of psychotic experiences. Br J Clin Psychol. 2007;46:155–173. [DOI] [PubMed] [Google Scholar]
- 22.Smeets F, Lataster T, Dominguez MD, et al. Evidence that onset of psychosis in the population reflects early hallucinatory experiences that through environmental risks and affective dysregulation become complicated by delusions. Schizophr Bull. 2012;38:531–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smeets F, Lataster T, van Winkel R, de Graaf R, Ten Have M, van Os J. Testing the hypothesis that psychotic illness begins when subthreshold hallucinations combine with delusional ideation. Acta Psychiatr Scand. 2013;127:34–47. [DOI] [PubMed] [Google Scholar]
- 24.Binbay T, Drukker M, Elbi H, et al. Testing the psychosis continuum: differential impact of genetic and nongenetic risk factors and comorbid psychopathology across the entire spectrum of psychosis. Schizophr Bull. 2012;38:992–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nuevo R, Van Os J, Arango C, Chatterji S, Ayuso-Mateos JL. Evidence for the early clinical relevance of hallucinatory-delusional states in the general population. Acta Psychiatr Scand. 2013;127:482–493. [DOI] [PubMed] [Google Scholar]
- 26.Varese F, Smeets F, Drukker M, et al. Childhood adversities increase the risk of psychosis: a meta-analysis of patient-control, prospective- and cross-sectional cohort studies. Schizophr Bull. 2012;38:661–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vassos E, Pedersen CB, Murray RM, Collier DA, Lewis CM. Meta-analysis of the association of urbanicity with schizophrenia. Schizophr Bull. 2012;38:1118–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arseneault L, Cannon M, Witton J, Murray RM. Causal association between cannabis and psychosis: examination of the evidence. Br J Psychiatry. 2004;184:110–117. [DOI] [PubMed] [Google Scholar]
- 29.Freeman D, Gittins M, Pugh K, Antley A, Slater M, Dunn G. What makes one person paranoid and another person anxious? The differential prediction of social anxiety and persecutory ideation in an experimental situation. Psychol Med. 2008;38:1121–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bartels-Velthuis AA, van de Willige G, Jenner JA, Wiersma D, van Os J. Auditory hallucinations in childhood: associations with adversity and delusional ideation. Psychol Med. 2012;42:583–593. [DOI] [PubMed] [Google Scholar]
- 31.Escher S, Romme M, Buiks A, Delespaul P, van Os J. Formation of delusional ideation in adolescents hearing voices: a prospective study. Am J Med Genet. 2002;114:913–920. [DOI] [PubMed] [Google Scholar]
- 32.Krabbendam L, Myin-Germeys I, Hanssen M, et al. Hallucinatory experiences and onset of psychotic disorder: evidence that the risk is mediated by delusion formation. Acta Psychiatr Scand. 2004;110:264–272. [DOI] [PubMed] [Google Scholar]
- 33.Korver N, Quee PJ, Boos HB, Simons CJ, de Haan L; GROUP investigators. Genetic Risk and Outcome of Psychosis (GROUP), a multi-site longitudinal cohort study focused on gene-environment interaction: objectives, sample characteristics, recruitment and assessment methods. Int J Methods Psychiatr Res. 2012;21:205–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lichtenstein P, Yip BH, Björk C, et al. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373:234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arajärvi R, Ukkola J, Haukka J, et al. Psychosis among “healthy” siblings of schizophrenia patients. BMC Psychiatry. 2006;6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vollema MG, Sitskoorn MM, Appels MC, Kahn RS. Does the Schizotypal Personality Questionnaire reflect the biological-genetic vulnerability to schizophrenia? Schizophr Res. 2002;54:39–45. [DOI] [PubMed] [Google Scholar]
- 37.Kendler KS, Gardner CO. The risk for psychiatric disorders in relatives of schizophrenic and control probands: a comparison of three independent studies. Psychol Med. 1997;27:411–419. [DOI] [PubMed] [Google Scholar]
- 38.Kendler KS, McGuire M, Gruenberg AM, O’, Hare A, Spellman M, Walsh D. The Roscommon Family Study. III. Schizophrenia-related personality disorders in relatives. Arch Gen Psychiatry. 1993;50:781–788. [DOI] [PubMed] [Google Scholar]
- 39.Fanous A, Gardner C, Walsh D, Kendler KS. Relationship between positive and negative symptoms of schizophrenia and schizotypal symptoms in nonpsychotic relatives. Arch Gen Psychiatry. 2001;58:669–673. [DOI] [PubMed] [Google Scholar]
- 40.Andreasen NC, Flaum M, Arndt S. The Comprehensive Assessment of Symptoms and History (CASH). An instrument for assessing diagnosis and psychopathology. Arch Gen Psychiatry. 1992;49:615–623. [DOI] [PubMed] [Google Scholar]
- 41.Frissen A, Lieverse R, Drukker M, et al. for Genetic Risk and Outcome in Psychosis (GROUP). Evidence that childhood urban environment is associated with a blunted increase in negative affect associated with stress across groups of psychotic patients, relatives and controls. Soc Psychiatry Psychiatr Epidemiol. 2014. [DOI] [PubMed]
- 42.Centraal Bureau voor Statistiek. Bevolking der Gemeenten van Nederland. The Hague, the Netherlands: Central Bureau of Statistics publications; 1993. [Google Scholar]
- 43.Gent University. Historische Databank van Lokale Statistieken-LOKSTAT. Universiteit Gent, Vakgroep Geschiedenis o.l.v. Eric Vanhaute en Sven Vrielinck. [Google Scholar]
- 44.Bernstein DP, Fink L, Handelsman L, et al. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry. 1994;151:1132–1136. [DOI] [PubMed] [Google Scholar]
- 45.Thombs BD, Bernstein DP, Lobbestael J, Arntz A. A validation study of the Dutch Childhood Trauma Questionnaire-Short Form: factor structure, reliability, and known-groups validity. Child Abuse Negl. 2009;33:518–523. [DOI] [PubMed] [Google Scholar]
- 46.Statacorp. Statistical Software: Release 11.0 [Computer Program]. College station, TX: TXS Stata Corporation; 2009. [Google Scholar]
- 47.Lataster T, Verweij K, Viechtbauer W; GROUP. Effect of illness expression and liability on familial associations of clinical and subclinical psychosis phenotypes. Acta Psychiatr Scand. 2014;129:44–53. [DOI] [PubMed] [Google Scholar]
- 48.Appels MC, Sitskoorn MM, Vollema MG, Kahn RS. Elevated levels of schizotypal features in parents of patients with a family history of schizophrenia spectrum disorders. Schizophr Bull. 2004;30:781–790. [DOI] [PubMed] [Google Scholar]
- 49.Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III–the final common pathway. Schizophr Bull. 2009;35:549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Howes OD, Shotbolt P, Bloomfield M, et al. Dopaminergic function in the psychosis spectrum: an [18F]-DOPA imaging study in healthy individuals with auditory hallucinations. Schizophr Bull. 2013;39:807–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Howes O, Bose S, Turkheimer F, et al. Progressive increase in striatal dopamine synthesis capacity as patients develop psychosis: a PET study. Mol Psychiatry. 2011;16:885–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arseneault L, Cannon M, Poulton R, Murray R, Caspi A, Moffitt TE. Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. BMJ. 2002;325:1212–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Semple DM, McIntosh AM, Lawrie SM. Cannabis as a risk factor for psychosis: systematic review. J Psychopharmacol (Oxford). 2005;19:187–194. [DOI] [PubMed] [Google Scholar]
- 54.Stefanis NC, Delespaul P, Henquet C, Bakoula C, Stefanis CN, Van Os J. Early adolescent cannabis exposure and positive and negative dimensions of psychosis. Addiction. 2004;99:1333–1341. [DOI] [PubMed] [Google Scholar]
- 55.Morgan C, Abdul-Al R, Lappin JM, et al. ; AESOP Study Group. Clinical and social determinants of duration of untreated psychosis in the AESOP first-episode psychosis study. Br J Psychiatry. 2006;189:446–452. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


