Abstract
Background: Previous findings are inconsistent; yet, converging evidence suggests an association between schizophrenia (SZ) and the impairment of posttranscriptional regulation of brain development through microRNA (miRNA) systems. Methods: This study aims to (1) compare the overall frequency of 121 rare variants (RVs) in 59 genes associated with the miRNA system in genome-wide association studies (GWAS)-derived data including 768 SZ cases and 1348 healthy controls and validated in an independent GWAS data including 1802 SZ cases and 1447 controls; (2) profile genome-wide miRNA expression in blood collected from 15 early-onset SZ (EOS) cases and 15 healthy controls; and (3) construct a miRNA-messenger RNA (mRNA) regulatory network using our previous genome-wide mRNA expression data generated from a separate sample of 18 EOS cases and 12 healthy controls. Results: Our findings indicate that: (1) In genes associated with the control of miRNAs, there are approximately 50% more RVs in SZ cases than in controls (P ≤ 2.62E-10); (2) The observed lower miRNA activity in EOS patients compared with the healthy controls suggests that miRNAs are abnormally downregulated; (3) There exists a predicted regulatory network among some downregulated miRNAs and some upregulated mRNAs. Conclusions: Collectively, results from all 3 lines of evidence, suggest that the genetically based dysregulation of miRNA systems undermines miRNAs’ inhibitory effects, resulting in the abnormal upregulation of genome transcription in the development of SZ.
Key words: schizophrenia, microRNA, mRNA, rare variants, early-onset
Introduction
Schizophrenia (SZ) is a severe chronic mental disorder affecting approximately 1% of the population worldwide. Generally arising in late adolescence, it profoundly disrupts the key traits of human cognition and personality, such as language, thought, perception, emotional affect, and sense of self. When the disease manifests before age 18, it is categorized as early-onset SZ (EOS), a subcategory of SZ associated with more familial vulnerability and poor outcomes.1
Despite SZ’s wide prevalence and debilitating nature, little is known about its pathogenesis. Clinicians continue to rely on clinical symptoms for diagnosis and the evaluation of progress and treatment responses throughout the duration of the disease. Comprehension of the genomic basis of SZ would allow for more personalized treatment and an improved prediction for disease prognosis. Several earlier studies profiled gene expression in peripheral blood, while searching for biomarkers for SZ and other psychiatric disorder.2,3
To date, expression studies have identified misexpressed genes and biological pathways in both the brain4–6 and blood.7–9 However, a consistent pattern of alterations has not been established.10,11 More importantly, the mechanisms underlying the dysregulation of hundreds of genes remain unclear. Among many possibilities, microRNA (miRNA) is gaining momentum since increasing evidence suggests their involvement in SZ development.12
miRNAs, which are enriched in brain, act posttranscriptionally to regulate and fine-tune almost every biological function in the genome.13 Their coordination of gene activity within biological networks is especially vital for neurodevelopment.14,15 Therefore, it is not surprising that studies of miRNA expression in blood and postmortem brain tissue have revealed differences between SZ patients and controls.12,16 Nevertheless, the results have been inconsistent and our contemporary knowledge in this field is rather fractional. Most of these studies targeted postmortem brains or blood from chronic patients, while those investigating changes of drug-naive or early-onset patients are still lacking. Furthermore, the underlying causes of miRNA dysregulation have not been systematically scrutinized.
The biogenesis and function of miRNAs are modulated by a range of mechanisms involving many protein-protein and protein-RNA interactions.17 The miRNA processing pathway mediates the biogenesis, maturation, and transportation of miRNAs, thus exerting an effect on the abundance and functioning of miRNAs.18 Key players in this pathway include DROSHA, DGCR8, DICER1, and AGO. It can be anticipated that the alteration of miRNA levels could be at least partially due to altered expressions of miRNA machinery genes, as exemplified in the haploinsufficiency of DGCR8 in 22q11 deletion.19 Furthermore, aberrant expressions of miRNA processing genes have been observed in SZ.20,21 Thus, we hypothesize that variations in the genetic pathway controlling miRNA processing may lead to the misexpression of miRNAs observed in SZ patients.
Although many genome-wide association studies (GWAS) have identified a large number of disease susceptibility loci, the vast majority of total heritability and the underlying causal variants of psychiatric disease have yet to be identified.22,23 Most genetic association studies have used the common disease-common variant (CDCV) paradigm. Yet the relatively low reproducibility of effects identified using the CDCV approach to date has led many researchers to adopt a common disease-rare variant (CDRV) approach. Under the CDRV paradigm, complex disease traits are assumed to arise from small changes at many different loci, based on the rationale observed from empirical data indicating that RVs are more likely than common variants to be damaging.24 It has been shown that the sum total of RVs can produce substantial effects.25
While evidence is growing that RVs play a crucial role in shaping the genetic architecture of human phenotypes,25–27 their rarity makes it nearly impossible, to identify the effect of any single variant, using traditional single-marker tests. Under the premise that testing multiple RVs simultaneously might enrich their association signals and reduce the burden of multiple testing, researchers have begun to collapse multiple RVs from the same genomic region or pathway into sets.28–33
In an earlier study, we analyzed associations between 967 single nucleotide polymorphisms (SNPs) located in 59 miRNA machinery genes and disease states, in 768 SZ cases and 1348 healthy controls.34 Although this analysis failed to reveal any association between the typed SNPs and SZ, we adopted the CDRV view and reasoned that, the genetic-based dysregulation of miRNAs may reflect the collective effects of many different rare SNPs within the pathway. Altogether, these RVs would lead to a reduction in miRNAs, misexpression of the transcriptome, and disease symptoms and onset (figure 1). To empirically test our hypothesized link between the miRNA pathway and disease, we profiled miRNA expression in peripheral blood mononuclear cells derived from a cohort of EOS patients and evaluated the associations between RVs in the miRNA machinery pathway and SZ.
Fig. 1.
A plausible link between the abnormal miRNA system and SZ. miRNA, microRNA; SZ, schizophrenia.
Methods
Subjects
All participants were unrelated Han Chinese recruited from the north of China. The discovery sample consisted of 768 unrelated patients with SZ (360 males and 408 females) and 1348 control subjects (658 males and 690 females), derived from a previous study.34 The validation sample included 1802 cases (968 males and 834 females), and 1447 healthy controls (349 males and 1098 females) (Yue W, unpublished data). For miRNA profiling, we recruited 15 first-onset SZ patients (8 males and 7 females, aged 13.80±1.93 years ranging from 12 to17 years) and 15 healthy controls (7 males and 8 females, aged 14.07±1.82 years ranging from 9 to16 years). Consensus diagnoses were made by at least 2 experienced psychiatrists independently according to the Diagnosis and Statistical Manual of Mental Disorders Fourth Edition (DSM-IV) criteria for SZ. Patients with unanimous diagnosis were enrolled into the study. Healthy teenager controls were recruited from several middle schools with an interview performed by psychiatrists; individuals with a history of mental health or neurological diseases were all excluded. The study was approved by the Medical Research Ethics Committee of First Hospital of Shanxi Medical University. Informed consent was signed by the teenager participants and their parents.
SNP Data Derivation
We defined rare SNPs as those with the minor allele frequency < 0.01 in either controls or SZ patients. In the discovery stage, we derived rare SNPs within 59 miRNA machinery genes from our GWAS data.35 miRNA machinery genes were curated from the literature36,37 and the Patrocles database,38 as described elsewhere.34 There were 158 rare SNPs, among them 37 SNPs were nonpolymorphic and filtered out, thus there remained 121 rare SNPs within 38 genes (some genes lack typed RVs), with a calling rate of 99.86%. In the validation stage, we derived relevant data from another GWAS (Yue W, unpublished data), in which 112 SNPs were typed, while other 9 SNPs were lacking, with the calling rate of 99.99%.
miRNA Profiling
Peripheral blood samples were collected from the participants. Total RNA was harvested using TRIzol (Invitrogen) and miRNeasy mini kit (QIAGEN) according to the manufacturer’s instructions. After passing the RNA quantity measurement using the NanoDrop 1000, the samples were labeled using the miRCURY Hy3/Hy5 Power labeling kit and hybridized on the miRCURY LNA Array (v.18.0). Following the washing steps, the slides were scanned using the Axon GenePix 4000B microarray scanner. Scanned images were then imported into GenePix Pro 6.0 software (Axon) for grid alignment and data extraction. Replicated miRNAs were averaged and miRNAs with intensities ≥50 in all samples were chosen for calculating normalization factor. Expressed data were normalized using the median normalization, followed by log2 transformation. After normalization, the log2 ratio distribution was roughly the same across all samples (supplementary figure 1). Differentially expressed (DE) miRNAs were identified through fold change (FC) and P value filtering. The data have been deposited in NCBI’s Gene Expression Omnibus and are accessible through GEO Series accession number GSE54578 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE54578).
Statistical Analysis and In Silico Prediction
PLINK39 was used to conduct the genetic association tests. Pearson’s Chi-squared test was used for comparison of rare alleles between SZ cases and controls. SNPinfo40 and F-SNP41 were used to predict the potential functions of the 121 rare SNPs. R42 was used to perform the data processing and analyses of the miRNA data. Student t test was used to compare expression levels between the patient and control groups. Clustering heatmaps were plotted using gplots.43 We predicted miRNA targets using miRBase44 and miRanda,45 both implemented in R package RmiR.Hs.miRNA.46 miRNA target network was plotted using Cytoscape.47
Results
Collective Association of RVs With SZ
There were 121 and 112 SNPs within 38 miRNA pathway genes in the discovery (supplementary table 1) and validation (supplementary table 2) samples, respectively. No SNPs deviated from Hardy-Weinberg equilibrium in both the case and the control groups (P > .001). As shown in table 1, there was a 45.5% and 50.0% increase of rare alleles in SZ cases when compared with controls in the discovery and validation sample, respectively, and the collective frequencies of rare SNPs were associated with SZ in both the discovery (P = 2.62E-10) and the validation sample (P < 2.2E-16), with an average OR of 1.42 and 1.47, in the discovery and validation sample, respectively. In-silico function predictions of rare SNPs showed that the majority of these RVs were predicted to influence transcription factor binding, splicing, miRNA binding, protein coding, regulatory potential, or conservation (supplementary table 3).
Table 1.
Collective Frequencies of Rare Allele in SZ Patients and Controls
| Sample | SZ (RVF [%]) | Control (RVF [%]) | OR (95% CI) | P |
|---|---|---|---|---|
| Discovery | 589/184685 (0.32) | 731/325335 (0.22) | 1.42 (1.27–1.58) | 2.62E-10 |
| Validation | 1441/402167 (0.36) | 786/323288 (0.24) | 1.47 (1.35–1.61) | <2.2E-16 |
Note: RVF, rare variants frequency; SZ, schizophrenia.
miRNA Profiles
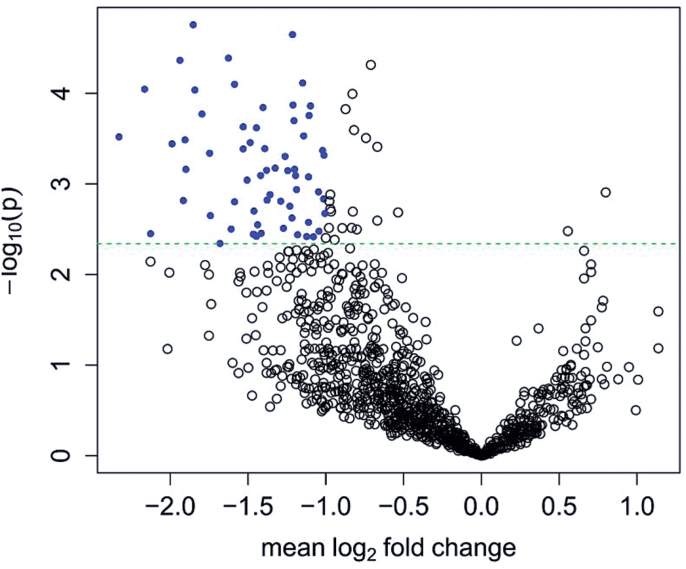
A total of 1070 miRNAs were detected using microarrays in our samples. There are 63 DE miRNAs according to FC ≥ 2 and P adjusted ≤ .05. Adaptive Benjamini and Hochberg method48 was adopted to correct the multiple tests. All 63 miRNAs were downregulated in SZ patients compared with healthy control subjects (figure 2, supplementary table 4, also see supplementary figure 2 for the results of cluster analysis).
Fig. 2.
Volcano plot of miRNA expressions. Plotted along the x-axis is the mean of log2 fold change, along the y-axis the negative logarithm of the P values. Blue denotes the 63 underexpressed genes. The horizontal green line is the negative logarithm of the adaptive Benjamini and Hochberg-adjusted P value threshold (4.55E-3).
miRNA Target Prediction
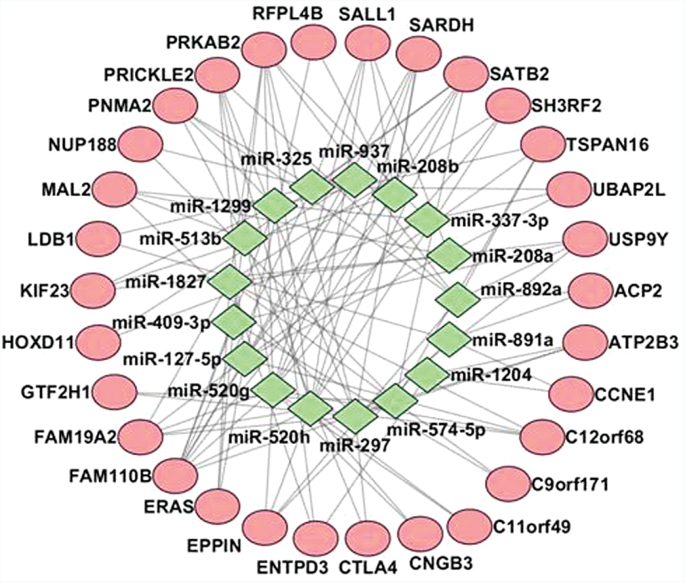
In a previous messenger RNA (mRNA) profiling study, we detected 84 DE mRNAs at the genome-wide level (FC ≥ 2 and P adjusted ≤ .05) in a separate sample of 18 EOS cases and 12 healthy controls, of which, 82 were upregulated and 2 downregulated in SZ patients compared with healthy control subjects (Yue et al, submitted). We predicted whether the 84 DE genes were targeted by the 63 DE miRNAs, which yielded 107 miRNA target pairs, involving 15 miRNAs and 47 genes (figure 3, supplementary table 5).
Fig. 3.
Predicted miRNA target relationship. Red denotes genes and green denotes miRNAs. miRNA, microRNA.
Discussion
SZ’s heritability is estimated to be approximately 80%; however, the majority of identified robust signals lacked direct biological relevance, suggesting that most efforts to find a genetic basis for the disease have not proven to be as informative as expected.49 Nevertheless, mounting evidence continues to implicate genetic dysregulation, perhaps caused by the disruption of regulatory functions of miRNAs, in the etiology of SZ. In particular, (1) the microdeletion 22q11.2 harboring the DGCR8 gene appears to be a major locus for SZ susceptibility in humans,50,51 and haploinsufficiency of DGCR8 leading to abnormal miRNA biogenesis is associated with SZ-like behavioral and neuronal deficits19,52–54; and (2) GWAS data analysis has revealed a strong association with SZ near MIR137, in a region with multiple predicted miR-137 targets.55,56
miRNA is currently thought to play a pivotal role in both the neurodegeneration and the etiology of psychiatric disease.57–59 Growing evidence confirms that dysfunction within a single monoamine system cannot alone account for complex neuropsychiatric diseases such as SZ, which result from the wider disruption of an entire cellular network.60 miRNAs’ diverse effects and coordinating functions make them an appropriate target of interest for SZ research, and many different lines of evidence have now confirmed the association between aberrant expression of miRNA and the development of psychiatric diseases and other human traits.57–59,61 Earlier studies, however, which have primarily focused on the brain, have produced widely divergent or conflicting results, in large part due to the variations in sample status and in the tissues used.57–59,61 It is not possible to determine, for instance, whether the alterations in miRNA in postmortem brain tissue are stochastic, causative, or the consequence of long-term treatment, and physical complexities. Recent evidence indicates that the dynamic process of miRNA expression in the brain is highly influenced by age,62 and a dynamic shift in miRNA expression has also been observed during brain maturation.63
To acquire a better understanding of the neuropathology of SZ, we sought to conduct our miRNA profile examinations in a cohort of drug-naive teenage patients with first-onset SZ. The study group was homogeneous in age, all were students, and all were in good health without other major physical diagnosis. More importantly, all study subjects were untreated, precluding any confounding effects of therapeutic drugs. All patients were in the early stage of disease and given their ages, their brains were still developing. We therefore speculate that alterations in transcription identified in these patient cohorts are likely to be relevant to the disease process.
At a genome-wide false discovery rate of 0.05, we detected 63 DE miRNAs with lower levels in SZ patients than in controls. This observation accords with those of Gardiner et al64 who identified 83 downregulated miRNAs in SZ patients. A comparison of the 2 results revealed that 2 miRNAs, miR-337-3p and miR-409-3p, were underexpressed in both studies. The discrepancies between the 2 studies may be due to the heterogeneity of SZ, the different populations involved, and the different disease statuses, since the patients in Gardiner et al were chronic cases, with the mean treatment duration of more than 15 years, while in our study, all patients were drug-naive teenage cases. Although there is little overlap between DE miRNAs identified by the 2 studies, both confirm an association between SZ and a global downregulation of miRNAs.
Several miRNAs have been implicated in SZ, such as miR-124, miR-132, miR-134, and miR-137.12 However, among these miRNAs, only miR-124-3p was DE in our study, while other miRNAs, though enriched in brain, were expressed at low levels in our samples, possible due to the incongruities between brain and blood. Many of the 63 DE miRNAs have not been reported by previous studies. We noted that most of the DE miRNAs were recently curated miRNAs, and there are no clinical studies for these new miRNAs (id > 700).
In light of wide discrepancies concerning individual miRNAs involved across studies, we speculate that a change in direction may provide more valuable information for the field. It seems unlikely that SZ could result from one or several specific miRNAs, but may very likely arise from a panel or group of miRNAs, none of which would be indispensable or adequate, given the low specificity of miRNA binding in that each miRNA can bind hundreds of targets, while each mRNA may be regulated by multiple miRNAs. Our observation suggests that the dosage deficiency of miRNAs may well contribute to the neuropathogenesis of SZ disease.
Since miRNAs generally inhibit mRNA transcription and translation, miRNA underexpression should result in mRNA upregulation and overexpression. This phenomenon, however, has yet to be convincingly observed. We have detected predominant overexpression of mRNAs in another sample of patients with EOS compared with controls, with 82 out of 84 DE genes showing increased activity. To test the plausible link between the reduction in the miRNAs’ inhibitory effect and the upregulation of mRNAs, we conducted prediction tests to determine whether the 63 DE miRNAs target the 84 DE genes identified in our SZ cohort. Our prediction yielded 107 miRNA target pairs involving 15 miRNAs and 48 genes. Considering that our miRNA study was conducted in a different cohort from our other mRNA study, these results are satisfactory.
Rather than pinpointing specific deficiencies in gene products in the origin of SZ, our results, collectively, implicate an overall dysregulation of transcription. We postulate that SZ is not the result of abnormalities in 1 or 2 pathways or biological systems (such as the dopaminergic or glutamatergic systems), but arises rather from disruption throughout an entire cellular network.60 This hypothesis also takes into account the apparently heterogeneous origins of SZ and other mental disorders.
While miRNA deregulation can, at least in part, explain the altered expression of mRNAs in SZ patients, the consistent reduction in miRNA levels remains unexplained. Based on the evidence from 2 cancer studies,36,65 we suggest here that the miRNA machinery pathway may in fact control the expression of miRNAs. In line with this theory, disturbances during miRNA biogenesis would reduce the overall number of miRNAs produced, such as in the haploinsufficiency of DGCR8.19
Empirical evidence strongly suggests that RVs are crucial players in the formation of SZ phenotypes.26,27,66 Therefore, we examined the RVs’ collective effects in miRNA pathways in schizophrenic disease. We found the frequency of rare alleles to be approximately 50% higher in SZ patients than in healthy controls. Thus, in SZ cases, our findings suggest that RVs are significantly overrepresented. RVs tend to be deleterious and their effect sizes are relatively large. The low population frequency of RVs is overcome by their large numbers throughout the genome. It can be expected that the 121 SNPs reported in this study represent only the tip of the iceberg. Therefore, it is not unreasonable to speculate that it is their collective effect that disrupts the biogenesis of miRNAs in SZ. Predictions further demonstrate that most variants are potentially functional, and given the complexity of genetic regulatory networks, much of their functional relevance is undoubtedly yet to be discovered.
Together, the RVs within the miRNA pathway genes may exert crucial detrimental effects on the biogenesis of miRNAs, which may consequently cause the deregulation of the entire genome. There may be a cascade amplification effect between each step of the link from RVs to SZ, since one miRNA pathway gene may influence most miRNAs, while each miRNA can bind to multiple targets.
A caveat in mind was that our miRNA results were derived from blood samples; thus, due care should be taken when extrapolating these results into the brain. A close association between the state of the immune system—in lymphocytes particularly—and major psychiatric disorders including SZ has been suggested.67 Lymphocytes have been suggested as a neural probe for studies of psychiatric disorders, due to the bidirectional communication between the immune and nervous systems.2 A comparison of gene expression in blood and brain showed that whole blood shares significant gene expression similarities with multiple brain tissues, with the median correlation between transcripts present in both whole blood and central nervous system being around 0.5.68 Mamdani et al69 reported that whole-blood gene expression changes may track treatment response to citalopram in depressed patients. It has been suggested that peripheral biomarkers may in part mirror similar pathological processes in the brain, represent distinct molecular changes in the blood that are highly specific to the primary pathophysiology, or reflect the molecular response of the peripheral cells secondary to the disease.70 Thus, abnormalities in blood, whether or not they are mimicked in brain, are useful indicators of disease pathology.
Noteworthy, this study also has several other limitations. As a result of the rarity of drug-naive patients with EOS, the sample size in the expression experiment was relatively small. It is also far from ideal to derive RVs from a preexisting GWAS dataset, and bias due to the design of the commercial arrays is unavoidable. We did not further validate the DE miRNAs or biologically validate the miRNA binding due to the constraints of the fund. Although, in our study, we focused on RVs and did not exclude the potential effects of common variants in this pathway since CDCV and CDRV are not mutually exclusive. Nevertheless, we did not investigate the effect of other sources of genetic variations, such as structural genomic variations and epigenetic variations, all of which may contribute to the disease and warrant study in the future.
In summary, the considered converging lines of evidence all point to the genetically determined dysregulation of the miRNA system as a key feature in the development of SZ. Our data support the notion that SZ may result from the dysregulation of the whole genome or disturbances of the entire cellular network. This big picture scope appears to be intimidating; however, our encouraging message is that miRNA appears to be a pivotal regulator in the disease development process. Therefore, we can target the limited number of miRNA pathway genes, even though we can hardly manipulate numerous RVs or thousands of transcripts. In this sense, our findings may provide evidence for a promising avenue toward treatment by monitoring the expression of miRNAs. Clearly, longitudinal research is needed to elucidate how the dynamics of miRNA expression affect—and are affected by—both the disease and the treatment process.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
National Natural Science Foundation of China (81278412, 81471364, 91232305, 81361120395, 81221002, 81222017); research grants from the National Basic Research Program of China (2011CB707805); the National Science and Technology Project (2012BAI01B06). Y.Y.S., H.C., and W.G. were supported by the Division of Intramural Research Programs of National Institute of Mental Health, National Institutes of Health (Project MH002929-03).
Supplementary Material
Acknowledgments
We sincerely thank all the subjects for their support and participation and all the medical staff involved in collecting blood samples. The views expressed in this presentation do not necessarily represent the views of the NIMH, NIH, HHS, or the US Government. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1.Clemmensen L, Vernal DL, Steinhausen HC. A systematic review of the long-term outcome of early onset schizophrenia. BMC Psychiatry. 2012;12:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gladkevich A, Kauffman HF, Korf J. Lymphocytes as a neural probe: potential for studying psychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:559–576. [DOI] [PubMed] [Google Scholar]
- 3.Schwarz E, Izmailov R, Spain M, et al. Validation of a blood-based laboratory test to aid in the confirmation of a diagnosis of schizophrenia. Biomark Insights. 2010;5:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fillman SG, Cloonan N, Catts VS, et al. Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry. 2013;18:206–214. [DOI] [PubMed] [Google Scholar]
- 5.Guillozet-Bongaarts AL, Hyde TM, Dalley RA, et al. Altered gene expression in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry. 2014;19:478–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mistry M, Gillis J, Pavlidis P. Genome-wide expression profiling of schizophrenia using a large combined cohort. Mol Psychiatry. 2013;18:215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanders AR, Göring HH, Duan J, et al. ; MGS. Transcriptome study of differential expression in schizophrenia. Hum Mol Genet. 2013;22:5001–5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sainz J, Mata I, Barrera J, et al. Inflammatory and immune response genes have significantly altered expression in schizophrenia. Mol Psychiatry. 2013;18:1056–1057. [DOI] [PubMed] [Google Scholar]
- 9.Schwarz E, Guest PC, Rahmoune H, et al. Identification of a biological signature for schizophrenia in serum. Mol Psychiatry. 2012;17:494–502. [DOI] [PubMed] [Google Scholar]
- 10.Horváth S, Janka Z, Mirnics K. Analyzing schizophrenia by DNA microarrays. Biol Psychiatry. 2011;69:157–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumarasinghe N, Tooney PA, Schall U. Finding the needle in the haystack: a review of microarray gene expression research into schizophrenia. Aust N Z J Psychiatry. 2012;46:598–610. [DOI] [PubMed] [Google Scholar]
- 12.Mellios N, Sur M. The emerging role of microRNAs in schizophrenia and autism spectrum disorders. Front Psychiatry. 2012;3:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao FB. Posttranscriptional control of neuronal development by microRNA networks. Trends Neurosci. 2008;31:20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li X, Jin P. Roles of small regulatory RNAs in determining neuronal identity. Nat Rev Neurosci. 2010;11:329–338. [DOI] [PubMed] [Google Scholar]
- 15.Im HI, Kenny PJ. MicroRNAs in neuronal function and dysfunction. Trends Neurosci. 2012;35:325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beveridge NJ, Cairns MJ. MicroRNA dysregulation in schizophrenia. Neurobiol Dis. 2012;46:263–271. [DOI] [PubMed] [Google Scholar]
- 17.Winter J, Jung S, Keller S, Gregory RI, Diederichs S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol. 2009;11:228–234. [DOI] [PubMed] [Google Scholar]
- 18.Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11:597–610. [DOI] [PubMed] [Google Scholar]
- 19.Stark KL, Xu B, Bagchi A, et al. Altered brain microRNA biogenesis contributes to phenotypic deficits in a 22q11-deletion mouse model. Nat Genet. 2008;40:751–760. [DOI] [PubMed] [Google Scholar]
- 20.Santarelli DM, Beveridge NJ, Tooney PA, Cairns MJ. Upregulation of dicer and microRNA expression in the dorsolateral prefrontal cortex Brodmann area 46 in schizophrenia. Biol Psychiatry. 2011;69:180–187. [DOI] [PubMed] [Google Scholar]
- 21.Beveridge NJ, Gardiner E, Carroll AP, Tooney PA, Cairns MJ. Schizophrenia is associated with an increase in cortical microRNA biogenesis. Mol Psychiatry. 2010;15:1176–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee SH, Wray NR, Goddard ME, Visscher PM. Estimating missing heritability for disease from genome-wide association studies. Am J Hum Genet. 2011;88:294–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh S, Kumar A, Agarwal S, Phadke SR. Genetic insight of schizophrenia: past and future perspectives. Gene. 2014; 535:97–100. [DOI] [PubMed] [Google Scholar]
- 24.Gorlov IP, Gorlova OY, Sunyaev SR, Spitz MR, Amos CI. Shifting paradigm of association studies: value of rare single-nucleotide polymorphisms. Am J Hum Genet. 2008;82:100–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rivas MA, Beaudoin M, Gardet A, et al. Deep resequencing of GWAS loci identifies independent rare variants associated with inflammatory bowel disease. Nat Genet. 2011;43:1066–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen JC, Kiss RS, Pertsemlidis A, Marcel YL, McPherson R, Hobbs HH. Multiple rare alleles contribute to low plasma levels of HDL cholesterol. Science. 2004;305:869–872. [DOI] [PubMed] [Google Scholar]
- 27.Nejentsev S, Walker N, Riches D, Egholm M, Todd JA. Rare variants of IFIH1, a gene implicated in antiviral responses, protect against type 1 diabetes. Science. 2009;324:387–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dickson SP, Wang K, Krantz I, Hakonarson H, Goldstein DB. Rare variants create synthetic genome-wide associations. PLoS Biol. 2010;8:e1000294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Price AL, Kryukov GV, de Bakker PI, et al. Pooled association tests for rare variants in exon-resequencing studies. Am J Hum Genet. 2010;86:832–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Madsen BE, Browning SR. A groupwise association test for rare mutations using a weighted sum statistic. PLoS Genet. 2009;5:e1000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ladouceur M, Dastani Z, Aulchenko YS, Greenwood CM, Richards JB. The empirical power of rare variant association methods: results from sanger sequencing in 1,998 individuals. PLoS Genet. 2012;8:e1002496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu MC, Lee S, Cai T, Li Y, Boehnke M, Lin X. Rare-variant association testing for sequencing data with the sequence kernel association test. Am J Hum Genet. 2011;89:82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin DY, Tang ZZ. A general framework for detecting disease associations with rare variants in sequencing studies. Am J Hum Genet. 2011;89:354–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang F, Chen Y, Liu C, et al. Systematic association analysis of microRNA machinery genes with schizophrenia informs further study. Neurosci Lett. 2012;520:47–50. [DOI] [PubMed] [Google Scholar]
- 35.Yue WH, Wang HF, Sun LD, et al. Genome-wide association study identifies a susceptibility locus for schizophrenia in Han Chinese at 11p11.2. Nat Genet. 2011;43:1228–1231. [DOI] [PubMed] [Google Scholar]
- 36.Ryan BM, Robles AI, Harris CC. Genetic variation in microRNA networks: the implications for cancer research. Nat Rev Cancer. 2010;10:389–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang H, Dinney CP, Ye Y, Zhu Y, Grossman HB, Wu X. Evaluation of genetic variants in microRNA-related genes and risk of bladder cancer. Cancer Res. 2008;68:2530–2537. [DOI] [PubMed] [Google Scholar]
- 38.Hiard S, Charlier C, Coppieters W, Georges M, Baurain D. Patrocles: a database of polymorphic miRNA-mediated gene regulation in vertebrates. Nucleic Acids Res. 2010;38:D640–D651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu Z, Taylor JA. SNPinfo: integrating GWAS and candidate gene information into functional SNP selection for genetic association studies. Nucleic Acids Res. 2009;37:W600–W605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee PH, Shatkay H. F-SNP: computationally predicted functional SNPs for disease association studies. Nucleic Acids Res. 2008;36:D820–D824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. http://www.R-project.org/. [Google Scholar]
- 43.Warnes GR, Bolker B, Bonebakker L, et al. gplots: Various R Programming Tools for Plotting Data; 2013. http://CRAN.R-project.org/package=gplots. [Google Scholar]
- 44.Kozomara A, Griffiths-Jones S. miRBase: integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res. 2011;39:D152–D157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Betel D, Wilson M, Gabow A, Marks DS, Sander C. The microRNA.org resource: targets and expression. Nucleic Acids Res. 2008;36:D149–D153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Favero F. RmiR.Hs.miRNA: Various Databases of MicroRNA Targets; 2012. R package version 1.0.6. [Google Scholar]
- 47.Shannon P, Markiel A, Ozier O, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Benjamini Y, Hochberg Y. On the adaptive control of the false discovery rate in multiple testing with independent statistics. J Educ Behav Stat. 2000;25:60–83. [Google Scholar]
- 49.Bergen SE, Petryshen TL. Genome-wide association studies of schizophrenia: does bigger lead to better results? Curr Opin Psychiatry. 2012;25:76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karayiorgou M, Simon TJ, Gogos JA. 22q11.2 microdeletions: linking DNA structural variation to brain dysfunction and schizophrenia. Nat Rev Neurosci. 2010;11:402–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Insel TR. Rethinking schizophrenia. Nature. 2010;468:187–193. [DOI] [PubMed] [Google Scholar]
- 52.Schofield CM, Hsu R, Barker AJ, Gertz CC, Blelloch R, Ullian EM. Monoallelic deletion of the microRNA biogenesis gene Dgcr8 produces deficits in the development of excitatory synaptic transmission in the prefrontal cortex. Neural Dev. 2011;6:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ouchi Y, Banno Y, Shimizu Y, et al. Reduced adult hippocampal neurogenesis and working memory deficits in the Dgcr8-deficient mouse model of 22q11.2 deletion-associated schizophrenia can be rescued by IGF2. J Neurosci. 2013;33:9408–9419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brzustowicz LM, Bassett AS. miRNA-mediated risk for schizophrenia in 22q11.2 deletion syndrome. Front Genet. 2012;3:291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schizophrenia Psychiatric Genome-Wide Association Study C. Genome-wide association study identifies five new schizophrenia loci. Nat Genet. 2011;43:969–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wright C, Turner JA, Calhoun VD, Perrone-Bizzozero N. Potential impact of miR-137 and its targets in schizophrenia. Front Genet. 2013;4:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miller BH, Wahlestedt C. MicroRNA dysregulation in psychiatric disease. Brain Res. 2010;1338:89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu B, Karayiorgou M, Gogos JA. MicroRNAs in psychiatric and neurodevelopmental disorders. Brain Res. 2010;1338:78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Welberg L. Neurodegeneration: export disrupts transport. Nat Rev Neurosci. 2010;11:74. [DOI] [PubMed] [Google Scholar]
- 60.Hunsberger JG, Austin DR, Chen G, Manji HK. MicroRNAs in mental health: from biological underpinnings to potential therapies. Neuromolecular Med. 2009;11:173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hébert SS, De Strooper B. Alterations of the microRNA network cause neurodegenerative disease. Trends Neurosci. 2009;32:199–206. [DOI] [PubMed] [Google Scholar]
- 62.Somel M, Guo S, Fu N, et al. MicroRNA, mRNA, and protein expression link development and aging in human and macaque brain. Genome Res. 2010;20:1207–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Beveridge NJ, Santarelli DM, Wang X, et al. Maturation of the human dorsolateral prefrontal cortex coincides with a dynamic shift in microRNA expression. Schizophr Bull. 2014;40:399–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gardiner E, Beveridge NJ, Wu JQ, et al. Imprinted DLK1-DIO3 region of 14q32 defines a schizophrenia-associated miRNA signature in peripheral blood mononuclear cells. Mol Psychiatry. 2012;17:827–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lin J, Horikawa Y, Tamboli P, Clague J, Wood CG, Wu X. Genetic variations in microRNA-related genes are associated with survival and recurrence in patients with renal cell carcinoma. Carcinogenesis. 2010;31:1805–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ji W, Foo JN, O’Roak BJ, et al. Rare independent mutations in renal salt handling genes contribute to blood pressure variation. Nat Genet. 2008;40:592–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schwarz MJ, Müller N, Riedel M, Ackenheil M. The Th2-hypothesis of schizophrenia: a strategy to identify a subgroup of schizophrenia caused by immune mechanisms. Med Hypotheses. 2001;56:483–486. [DOI] [PubMed] [Google Scholar]
- 68.Sullivan PF, Fan C, Perou CM. Evaluating the comparability of gene expression in blood and brain. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:261–268. [DOI] [PubMed] [Google Scholar]
- 69.Mamdani F, Berlim MT, Beaulieu MM, Labbe A, Merette C, Turecki G. Gene expression biomarkers of response to citalopram treatment in major depressive disorder. Transl Psychiatry. 2011;1:e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Harris LW, Pietsch S, Cheng TM, Schwarz E, Guest PC, Bahn S. Comparison of peripheral and central schizophrenia biomarker profiles. PLoS One. 2012;7:e46368. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.