Abstract
Schizophrenia is a highly heritable and polygenic disease, and identified common genetic variants have shown weak individual effects. Many studies have reported altered working memory (WM)-related brain activation in schizophrenia, preferentially in the frontal lobe. Such differences in brain activations could reflect inherited alterations possibly involved in the disease etiology, or rather secondary disease-related mechanisms. The use of polygenic risk scores (PGRS) based on a large number of risk polymorphisms with small effects is a valuable approach to examine the effect of cumulative genetic risk on brain functioning. This study examined the impact of cumulative genetic risk for schizophrenia on WM-related brain activations, assessed with functional magnetic resonance imaging. For each participant (63 schizophrenia patients and 118 healthy controls), we calculated a PGRS for schizophrenia based on 18 862 single-nucleotide polymorphism in a large multicenter genome-wide association study including 9146 schizophrenia patients and 12 111 controls, performed by the Psychiatric Genomics Consortium. As expected, the PGRS was significantly higher in patients compared with healthy controls. Further, the PGRS was related to differences in frontal lobe brain activation between high and low WM demand. Specifically, even in absence of main effects of diagnosis, increased PGRS was associated with decreased activation difference in the right middle-superior prefrontal cortex (BA 10/11) and the right inferior frontal gyrus (BA 45). This effect was seen in both cases and controls, and was not influenced by sex, age, or task performance. The findings support the notion of dysregulation of frontal lobe functioning as an inherited vulnerability factor in schizophrenia.
Key words: polygenic, schizophrenia, fMRI
Introduction
Although the estimated heritability of schizophrenia is high, the effects of specific genetic variants identified this far have been very small.1 This is likely partly due to its heterogeneous and complex phenotypic characteristics, with a highly polygenic modulation, ie, additive effects of a large number of genetic variants with individually weak effects on the phenotype.1–3 Efforts have been made to calculate polygenic risk scores (PGRS) that better capture the polygenic nature of complex disorders. A PGRS can be estimated based on a few significant hits from genome-wide association studies (GWAS) as well as on a large number of SNPs throughout the whole genome.2–4 The PGRS can then be used to examine the impact of cumulative genetic risk for the disease on various related phenotypes.5–10
In order to map the genetic architecture of complex disorders and identify biological mechanisms in the chain from genes to clinical outcome, one important approach has been to examine brain-based intermediate phenotypes that are more closely related to gene function than clinical diagnosis.11 Brain function and structure have been suggested as potentially valuable intermediate phenotypes in the search for underlying mechanisms.12,13
Impaired working memory (WM) function is a characteristic cognitive finding in schizophrenia.14 Moreover, several functional magnetic resonance imaging (fMRI) studies have shown altered prefrontal recruitment during WM processing in schizophrenia, particularly in the dorsolateral prefrontal cortex (DLPFC)15–18 although several other areas of the WM network have also been proposed.19,20
An important question is to which degree alterations in brain function seen in schizophrenia are modulated by genetic variability, and thereby possibly involved in disease etiology. To reveal this issue, brain activation of unaffected genetically close relatives of schizophrenia patients have been compared with patients and healthy controls. Some have found similar patterns of brain activation in patients and their unaffected relatives, arguing that such brain activation differences reflect genetic risk rather than clinical state.18 However, results from other studies indicate the opposite.21 Further, as unaffected relatives often show similar, although more subtle, clinical and cognitive manifestations compared with patients, this controversy has been difficult to resolve using studies of relatives alone.
In this study, we examined the effect of cumulative genetic risk for schizophrenia on WM-related brain activation using a PGRS estimated from the large-scale GWAS by the Psychiatric Genomics Consortium.2 The PGRS was estimated based on a meta-analysis of all Psychiatric Genomic Consortia (PGC) subsample data sets except the current data set.22 Following the methods of Purcell and colleagues,3 a PGRS was calculated for each patient (n = 63) and healthy control (n = 118) participating in the current fMRI study. We estimated the association between task-related brain activation and PGRS in all task-related brain areas without further constraining the search to any a priori regions of interest. We hypothesized that increased genetic risk for schizophrenia would be associated with altered brain activation in areas where case-control differences have typically been reported in the literature on schizophrenia and WM processing, including frontal lobe regions. Such a result would suggest that frontal lobe dysfunctions are directly related to genetic risk for schizophrenia and a potential vulnerability factor to the disease. We also sought to replicate previous case-control differences in frontal lobe brain activation.
Methods
Sample Characteristics
A total of 181 participants from the Thematically Organized Psychosis (TOP) study14 with satisfying genetic and functional MRI data were included. Of the 181, 63 participants were Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) diagnosed with a schizophrenia spectrum disorder23 and 118 were healthy controls. Exclusions of participants had been made based on MRI and genotyping quality control as well as scanner task behavioral criteria (hits-false alarm < 2 and/or hits < 5, n = 4). Among patients, 49 were diagnosed with schizophrenia, 9 with schizoaffective disorder and 5 with schizophreniform disorder. For further sample description, see table 1. The study was approved by the Regional Committee for Medical Research Ethics and the Norwegian Data Inspectorate, and all participants gave their written informed consent to participate. Diagnostic evaluations were carried out by trained physicians or clinical psychologists using the Structured Clinical Interview for DSM-IV Axis I Disorder.23 A representative healthy control sample was recruited from the same catchment area using national registries (Statistics Norway, www.SSB.no). For further details regarding recruitment, clinical assessment and inter-rater reliability in the TOP study, see references.14,20
Table 1.
Sample Descriptives and Behavioral Data in Patients and Controls
| SZ (n = 63) | HC (n = 118) | t/χ2 | P | |
|---|---|---|---|---|
| Age, y (SD) | 32.9 (7.9) | 34.9 (8.5) | −1.6 | .12 |
| Sex, male/female (% females) | 43/20 (31.7) | 71/47 (39.8) | 0.28 | .33 |
| Education | 12.4 (3.9) | 14.4 (2.3) | 4.3 | <.001 |
| WASI | 108.42 (13.9) | 115.5 (10.1) | −3.5 | <.001 |
| 2-back, hits | 10.8(2.4) | 12.2 (1.2) | −5.0 | <.001 |
| 2-back, hits-FA | 9.5(2.9) | 11.5 (1.6) | −4.5 | <.001 |
| Response time, 2-back hits, msa | 668 (226) | 591 (141) | 2.3 | .027 |
| 0-back, hits | 11.94 | 11.92 | 0.4 | .69 |
| 0-back, hits-FA | 11.68 | 11.75 | −0.43 | .67 |
| Response time, 0-back hits, msa | 458 (239) | 487 (161) | −.87 | .39 |
| PGRS | 0.32 (0.92) | −0.17 (1.00) | 3.30 | <.001 |
| PANSS, total positive scoreb (SD) | 12.26 (5.7) | — | — | — |
| Alcohol abuse, n (%) | 4 (6.3) | — | — | — |
| Illegal substances abuse, n (%) | 7 (11%) | — | — | — |
| GAF-S | 42.9 (10.6) | — | — | — |
| GAF-F | 44.43 (10) | — | — | — |
| Age of onset, y (SD) | 23.9 (5.7) | — | — | — |
| Antipsychotics, yes/no | 40/23 | — | — | — |
| Anti-epileptics, yes/no | 5/58 | — | — | — |
| Antidepressants, yes/no | 18/45 | — | — | — |
Note: SZ, schizophrenia; HC, healthy controls; PGRS, polygenic risk score (normalized); WASI, Wechsler Abbreviated Scale of Intelligence; PANSS, Positive and Negative Syndrome Scale, short version undertaken at the day of scanning; GAF-S, Global Assessment of Functioning-symptom score; GAF-F, Global Assessment of Functioning-function score.
Values are means (SD) if nothing else is specified. Stats; all group comparisons were made with t-test except for sex (χ2).
a n = 161 with available response time data.
b n = 35 with available PANSS data.
fMRI Paradigm
The task used during scanning was a WM N-back task, with a blocked design and 3 conditions; 0-back, 2-back, and baseline. The experiment consisted of 2 runs with 4 blocks of 0-back and 2-back, respectively. Every 0-back and 2-back block (on-blocks) were followed by a baseline block (off-blocks). During both 0-back and 2-back, stimuli consisted of consecutive presentation of pairs of numbers (1–9) shown at the top and bottom of the screen in magenta on black background. During 0-back, participants were instructed to press a button when the 2 numbers were the same. During 2-back, the 2 numbers presented were always the same, and participants were instructed to press a button when the numbers were the same as the ones presented 2 steps back in the sequence. During off-blocks, participants fixated at a cross in the middle of the screen. On-blocks consisted of 18 stimuli (presented for 300ms with a 2500-ms inter-stimulus interval), with 3–4 target stimuli and a total of 12 0-back and 13 2-back targets. On-blocks lasted for 53.04 s, off-blocks for 26.52 s, and the whole experiment for about 10min (318.24 s per run).
Image Acquisition, Processing, and Statistical Analysis
Image acquisition, quality control, and preprocessing are described in the supplementary methods. Briefly, (f)MRI data were obtained with a 1.5T Siemens scanner. The pulse sequence used for co-registration purposes was a sagittal T1-weighted magnetization prepared rapid gradient echo. Functional T2*-weighted images were scanned with 164 BOLD-sensitive whole-brain measurements per condition, using an echo-planar imaging pulse sequence.
fMRI data were preprocessed and analyzed using the fMRIB Software Library (FSL, http://www.fmrib.ox.ac.uk/fsl).24
A subject-level intermediate fixed-effects analysis was performed to calculate contrast parameter estimates (COPEs) for 2-back relative to 0-back using respective conditions’ lower-level COPE values as explanatory variables in a GLM. The 2-back > 0-back COPEs were thereafter used as the contrast of main interest to investigate associations with PGRS scores and group differences in WM-related brain activation. For completeness, we also examined the 2-back > baseline and 0-back > baseline contrasts in relation to PGRS and case-control status. Whole-brain group effects were based on a random-effect model. To correct for multiple comparisons, we performed cluster-level correction with the FSL default settings of z > 2.3 and a corrected cluster significance threshold of P = .05 for all analyses. All analyses were restricted to areas within the task network by contrast masking with respective contrasts main effect of task for the whole group.
We tested for main effects of PGRS while including diagnostic group in the model. Case-control differences were examined without the PGRS included in the model, and additional analyses were conducted with PGRS, age, and sex included as additional covariates, as well as with patients diagnosed with schizoaffective disorder or schizophreniform disorder excluded from the analyses (n = 14). Individual average COPEs in identified clusters from the PGRS analyses were entered into an ANCOVA together with diagnostic group using SPSS (IBM SPSS Statistics 21) in order to examine possible interactions between PGRS and diagnostic group on the COPEs. Further, in order to assess the potential effects of task performance intellectual abilities and medication status on the main effects, we performed post hoc ANCOVAs in SPSS on activation differences in identified clusters from the case-control and PGRS analyses while covarying for task performance (hits-false alarms during 2-back), Wechsler Abbreviated Scale of Intelligence (WASI)25 (as a proxy for IQ) and antipsychotics status (dichotomized, yes/no) with the relevant COPEs as dependent variable. Antipsychotics status was dichotomized due to non-normal distribution of defined daily dosages as assessed by visual inspection of histograms.
Further, in order to assess the spatial correlation between the effects of case-control status and associations with PGRS in healthy controls, respectively, we estimated the correlation between the unthresholded t-maps from the relevant contrasts (see supplementary methods for details).
Finally, because head motion may influence the fMRI parameters, we tested for main effects and interaction effects of task condition and diagnostic group on the amount of head motion using repeated measures ANOVA, as well as for correlation between head motion and PGRS (Pearson’s r) in SPSS.
Case-control comparisons of age, education, WASI, PGRS, and task performance were made using t-tests, and differences in sex distributions were tested using Chi.2 Associations between the PGRS and demographic and behavioral variables were examined using Pearson’s correlation. These analyses were performed in SPSS.
PGRS
PGRS for schizophrenia were computed based on imputed SNPs from the PGC following the method developed by Purcell and colleagues,3 as described in full detail in Tesli and colleagues.22 For details regarding PGRS, genotyping, and SNP imputation, see supplementary methods.
Results
Group Effects on Demographics, Task Performance, and PGRS
Table 1 summarizes relevant demographic, behavioral, and PGRS data for each group. Patients and controls did not differ significantly in sex distribution or age. Patients had lower education, showed poorer performance on the WASI, as well as lower task performance during 2-back compared with healthy controls. There were no case-control differences in performance on the 0-back task. As expected, the PGRS was significantly higher in patients than in controls (R 2 = 0.057), but did not correlate with any of the other cognitive or demographic variables (P > .05).
Functional Magnetic Resonance Imaging
Main Effect of Task.
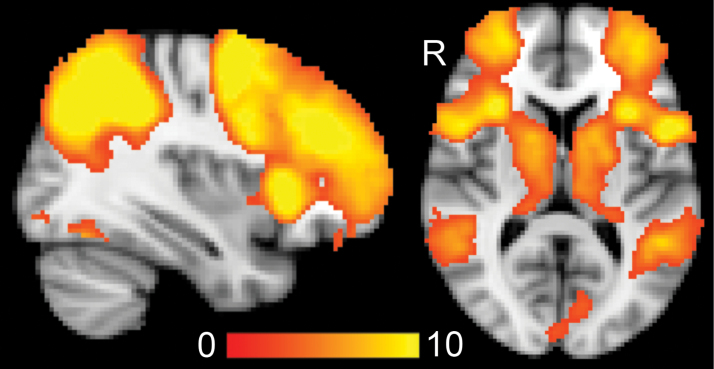
Across groups, the 2-back > 0-back contrast revealed a network of brain areas typically involved in WM processing, including the lateral and dorsal prefrontal cortex, anterior cingulate gyrus, middle temporal gyrus, thalamus, insula, caudate, inferior parietal cortex, and the occipital cortex (figure 1).
Fig. 1.
Main effect of 2-back > 0-back across all participants (n = 181). Color bar indicates z values. X = 36, z = 10 (Montreal Neurological Institute space). R = right.
Relation Between PGRS and Brain Activation.
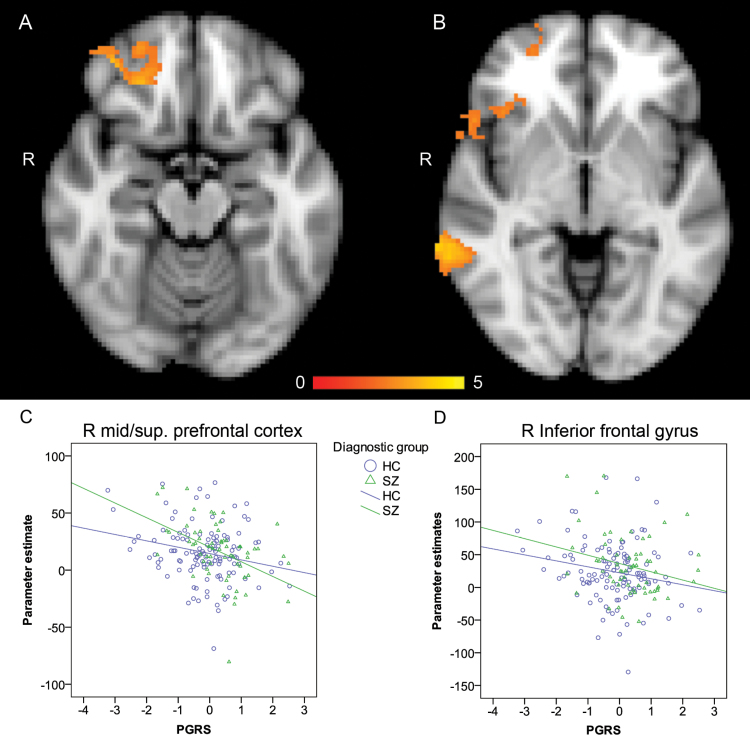
PGRS was negatively associated with COPEs from the 2-back > 0-back contrast in 2 right frontal clusters located in the inferior frontal gyrus (BA 45, z = 3.11, x, y, z = 48, 18, −10, R 2 = 0.028) and the middle/superior prefrontal cortex (BA 11/BA 10, z = 3.66, x, y, z = 38, 46, −16, R 2 = 0.035), respectively (figure 2A and 2B). A third cluster was seen in the right middle temporal gyrus (BA 22, k = 520, z = 4.0, x, y, z = 72, −40, −4, R 2 = 0.05; figure 2B). No effects were observed for the opposite contrast (0-back > 2-back). Post hoc tests revealed no case-control differences in the average COPEs in any of these clusters, and there was no interaction effect between PGRS and diagnostic group (figure 2C and 2D). Also, the effects remained when controlling for age, sex, task performance (2-back), WASI, and antipsychotics status.
Fig. 2.
A and B: Negative relation between BOLD signal change (2-back > 0-back) and PGRS. (A) z = −16, (B) z = −4 (Montreal Neurological Institute space). Color bar indicates z values. C and D Cluster mean parameter estimates (2-back > 0-back) of patients and controls as a function of PGRS. There were no significant interaction effects between diagnostic group and PGRS. PGRS = polygenic risk score, R = right.
Differences Between Schizophrenia Patients and Controls.
No significant case-control differences were observed in the frontal lobe for the 2-back > 0-back contrast. Outside the frontal lobe, patients showed higher activation in the right (k = 320, z = 4.24, x, y, z = 60, −18, 42) and left (k = 270, z = 3.85, x, y, z = −58, −26, 44) postcentral gyrus and in the right superior parietal lobe (k = 102, z = 3.31, x, y, z = −24, −50, 70) in this contrast. Results remained mainly unchanged when PGRS, age, and sex were included in the model. Additional analyses with patients diagnosed with schizoaffective disorder or schizophreniform disorder excluded from the analyses revealed highly similar results.
Complementary Contrasts.
The 2-back > baseline contrast revealed a negative association between PGRS and brain activation in the anterior cingulate cortex (ACC), right Inferior frontal gyrus (IFG) /insula, and in the bilateral postcentral gyrus (supplementary table S1). For the same contrast, schizophrenia patients had higher activation relative to healthy controls in the ACC, in the left insula, bilateral precentral cortex, and the bilateral postcentral gyrus/superior parietal lobule (supplementary table S2). Healthy controls had higher activation than schizophrenia patients in the bilateral striatum and in the left intracalcarine cortex (supplementary table S2). All PGRS and case-control effect in the frontal lobe and ACC remained significant after post hoc control analyses for task performance (2-back), IQ (WASI) and antipsychotics (P < 0.05), except for the case-control differences in the left and right striatum, which did not remain significant after controlling for antipsychotics (P > 0.05).
The 0-back > baseline contrast revealed no significant relations with PGRS. Similar to the 2-back > 0-back contrast, schizophrenia patients had higher activation relative to healthy controls in the ACC as well as in the left postcentral gyrus and in the right frontal pole, but there were no areas with significantly increased activation in controls. The ACC cluster remained significant after post hoc control analyses for task performance (2-back), IQ (WASI), and antipsychotics (P < 0.05), whereas the right frontal pole cluster did not reach the significance threshold after controlling for antipsychotics (P = 0.6, supplementary table S2).
Correlation Between Brain Activation Maps.
Correlation analyses was performed in order to test how well brain activation related to PGRS correlated with brain activation related to case-control differences across the brain. For the whole group (n = 181), PGRS-related brain activation correlated with case-control-related brain activation to r = 0.22. PGRS-related brain activation in only healthy controls correlated with case-control-related brain activation to r = 0.3.
Head Motion.
The mixed design ANOVA revealed that there were no main effects or interaction effects of task condition or diagnostic group on the amount of head motion (P > 0.05). There was no significant correlation between the amount of head motion during either condition and PGRS (P > 0.05).
Discussion
This study demonstrates a relation between polygenic risk for schizophrenia and frontal lobe brain activation differences between high and low WM load. Increased PGRS was significantly associated with decreased activation difference in 2 right frontal clusters primarily located in BA 10/11 and BA 45, respectively, areas previously linked to the disease in case-control studies.19 Notably, there were no interaction effects between diagnostic group and PGRS on brain activation difference, indicating similar effects across groups. Also, the effects of PGRS on brain activation could not be explained by age, sex, or task performance. These novel results suggest that reduced frontal lobe activation difference between high and low WM load, which may imply frontal lobe dysregulation, is a genetically modulated vulnerability factor of schizophrenia.
Among the few schizophrenia imaging genetics studies using a PGRS approach, Walton and colleagues5 examined brain activation during WM processing in relation to a schizophrenia PGRS based on about 600 SNPs. Despite a lack of case-control differences, the PGRS was positively related to DLPFC brain activation during WM processing in a mixed group of schizophrenia patients and controls,5 whereas results from this study demonstrated a negative relation between PGRS and frontal lobe brain activation. However, previous case-control studies on WM-related brain activation have yielded inconsistent results, with patients showing both frontal hyper- and hypo-activation during WM processing,19,26 as well as no differences.27 One possible source of discrepancy between studies in the direction of results is interaction effects between diagnosis and WM load on fMRI activation patterns.19,26 Lower functioning groups may show higher relative brain activation at low WM loads due to increased cognitive effort, but lower relative brain activation at more demanding tasks due to a lower maximal capacity to recruit neural resourses,28 effectively precluding interpretations of main effects of diagnosis without considering task demands and cognitive effort.
In line with the notion of interactions between cognitive effort and disease mechanisms on brain activation patterns, the effects observed in this study suggest decreased activation difference between high and low WM load with increasing genetic risk of schizophrenia, which may reflect decreased flexibility in the recruitment of neuronal resources in response to changes in task demands. Indeed, neuronal and behavioral dedifferentiation have previously been implicated as one of the hallmarks of cognitive aging and neurodegeneration,29–31 and it is possible that similar mechanisms are also involved in the pathophysiology of neurodevelopmental disorders including schizophrenia. Within healthy controls, patterns of brain activation related to PGRS across the whole brain showed a weak-moderately positive correlation with brain activation revealed by case-control differences (r = 0.3), indicating that the genetic effect on brain function is not independent from its effect on schizophrenia risk, but might also impact other traits and neurological disorders.
Imaging genetics studies of schizophrenia have long since implicated DLPFC dysfunctions, including both hyper- and hypo-activation relative to controls, as a particularly important intermediate phenotype in schizophrenia.13,15,17,32 In contrast, this relatively well-powered study did not observe any significant case-control or PGRS effects on brain activation in BA 46, which is in line with others who have also failed to replicate such an effect.27 As schizophrenia is a highly heterogeneous disorder, the etiology and underlying mechanisms of the disease are likely to vary between individuals and populations. Differences in ethnicity, environmental factors, cognitive functioning, medication, and diagnostic criteria among study groups might all impact brain imaging results.
The observed effect of polygenic risk on relative brain activation during a high vs a low load condition in the frontal lobe was seen in absence of significant case-control differences in the same sample. This might indicate a larger power to detect polygenic effects than case-control differences. Nevertheless, a similar PGRS would ideally next be examined in a sample and paradigm that also captures case-control differences in frontal lobe brain activation in order to directly compare the genetic effect with case-control differences. The current paradigm did capture case-control differences in the IFG and the ACC for the broader 2-back > baseline contrast, with effect in ACC also for 0-back > baseline, both areas previously implemented in schizophrenia.19 As this contrast is likely to also reflect other cognitive and perceptual processes than WM processing, it is however more difficult to compare the effect of case-control status with that of polygenic risk from this contrast. Here, although schizophrenia patients had higher brain activation relative to healthy controls, increased genetic risk for the disease was associated with lower relative brain activation during task blocks compared with fixation blocks in these areas. Although these unexpected results warrant replications in independent samples, they might indicate a complex relation between the effect of disease status and genetic risk for schizophrenia, where both state- and trait-related effects on brain functioning in the same region might be present with independent, and potentially opposite, effects on brain activation. Others have identified an effect of polygenic risk for bipolar disorder on brain activation in areas with a previously identified case-control effect within the same sample.10 Interestingly, the original effect of diagnostic group did not remain when controlling for the PGRS, indicating that the genetic effect might mediate the case-control differences in brain activation.10
The assessment of a PGRS to identify the neural correlates of cumulative genetic risk for mental disorders is an increasingly used strategy that might point to brain processes with a role in disease etiology. To our knowledge, this is the first schizophrenia imaging genetics study using a PGRS that significantly differs between cases and controls in the examined imaging sample. The schizophrenia PGRS used here was based on nearly 19 000 SNPs that predict schizophrenia case-control status at P = .05 in a large-scale multicenter GWAS.2 The chosen P-value threshold for SNP inclusion was the one resulting in the PGRS that explained most variance in case-control status in our clinical sample.22 The PGRS used in this study explained 5.7% of disease status in our sample, whereas, eg, the large-scale study by Purcell and colleagues3 reported an explained variance of 3% for a similar PGRS. In spite of the relatively small proportion of total variance explained, our PGRS still succeeded in capturing differences in brain activation in relevant brain areas when controlling for diagnostic group. Thus, the current results also demonstrate the utility of PGRSs in imaging genetics to examine possible genetically modulated pathophysiological mechanisms. In future work, newly developed genomic enrichment tools for gene discovery in GWAS33 will be crucial to increase the amount of explained variance in case-control status by a PGRS. This study is among the largest imaging genetics studies of schizophrenia, but still relatively small in comparison to behavioral and clinical studies. Thus, replication in independent samples is needed to confirm the present results.
In conclusion, we examined the effect of cumulative genetic risk for schizophrenia on differences in brain activation between high and low WM load by using the PGRS from a large case-control multicenter GWAS training sample. The PGRS was significantly higher in schizophrenia cases compared with controls in our fMRI sample, and increased PGRS was significantly associated with decreased activation difference between high and low WM load in areas previously implicated in schizophrenia pathophysiology. The effect of genetic risk was seen not only within schizophrenia patient but also in healthy controls. This indicates that the effect is not merely related to patient-specific factors such as severity of the disease, but rather represents a more general genetically modulated prefrontal dysregulation, which in addition to schizophrenia might also relate to other neurological diseases and traits.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
Research Council of Norway (223273 and 204966/F20), South East Health Authority (2013–123), and KG Jebsen Foundation.
Supplementary Material
Acknowledgments
We thank patients and controls for their participation, and the health professionals who facilitated our work. We also thank Thomas D. Bjella, and Anne Hilde Farstad and the staff at the Department of Radiology and Nuclear Medicine, Oslo University Hospital. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1.Ripke S, O’Dushlaine C, Chambert K, et al. Genome-wide association analysis identifies 13 new risk loci for schizophrenia. Nat Genet. 2013;45:1150–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ripke S, Sanders AR, Kendler KS, et al. Genome-wide association study identifies five new schizophrenia loci. Nat Genet. 2011;43:969–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Purcell SM, Wray NR, Stone JL, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smoller JW, Ripke S, Lee PH, et al. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 2013;381:1371–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walton E, Geisler D, Lee PH, et al. Prefrontal inefficiency is associated with polygenic risk for schizophrenia. Schizophr Bull. December 10, 2013; doi:10.1093/schbul/sbt174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walton E, Turner J, Gollub RL, et al. Cumulative genetic risk and prefrontal activity in patients with schizophrenia. Schizophr Bull. 2013;39:703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fanous AH, Zhou B, Aggen SH, et al. Genome-wide association study of clinical dimensions of schizophrenia: polygenic effect on disorganized symptoms. Am J Psychiatry. 2012;169:1309–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bigdeli TB, Bacanu SA, Webb BT, et al. Molecular validation of the schizophrenia spectrum. Schizophr Bull. 2014;40:60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McIntosh AM, Gow A, Luciano M, et al. Polygenic risk for schizophrenia is associated with cognitive change between childhood and old age. Biol Psychiatry. 2013;73:938–943. [DOI] [PubMed] [Google Scholar]
- 10.Whalley HC, Papmeyer M, Sprooten E, et al. The influence of polygenic risk for bipolar disorder on neural activation assessed using fMRI. Transl Psychiatry. 2012;2:e130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mier D, Kirsch P, Meyer-Lindenberg A. Neural substrates of pleiotropic action of genetic variation in COMT: a meta-analysis. Mol Psychiatry. 2010;15:918–927. [DOI] [PubMed] [Google Scholar]
- 12.Meyer-Lindenberg A, Weinberger DR. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nat Rev Neurosci. 2006;7:818–827. [DOI] [PubMed] [Google Scholar]
- 13.Rasetti R, Weinberger DR. Intermediate phenotypes in psychiatric disorders. Curr Opin Genet Dev. 2011;21:340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simonsen C, Sundet K, Vaskinn A, et al. Neurocognitive dysfunction in bipolar and schizophrenia spectrum disorders depends on history of psychosis rather than diagnostic group. Schizophr Bull. 2011;37:73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manoach DS, Press DZ, Thangaraj V, et al. Schizophrenic subjects activate dorsolateral prefrontal cortex during a working memory task, as measured by fMRI. Biol Psychiatry. 1999;45:1128–1137. [DOI] [PubMed] [Google Scholar]
- 16.Thermenos W, Milanovic S, Tsuang MT, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci USA. 2009;106:1279–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Potkin SG, Turner JA, Brown GG, et al. Working memory and DLPFC inefficiency in schizophrenia: the FBIRN study. Schizophr Bull. 2009;35:19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seidman LJ, Thermenos HW, Poldrack RA, et al. Altered brain activation in dorsolateral prefrontal cortex in adolescents and young adults at genetic risk for schizophrenia: an fMRI study of working memory. Schizophr Res. 2006;85:58–72. [DOI] [PubMed] [Google Scholar]
- 19.Glahn DC, Ragland JD, Abramoff A, et al. Beyond hypofrontality: a quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia. Hum Brain Mapp. 2005;25:60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brandt CL, Eichele T, Melle I, et al. Working memory networks and activation patterns in schizophrenia and bipolar disorder: comparison with healthy controls. Br J Psychiatry. 2014;204:290–298. [DOI] [PubMed] [Google Scholar]
- 21.Rasetti R, Mattay VS, Wiedholz LM, et al. Evidence that altered amygdala activity in schizophrenia is related to clinical state and not genetic risk. Am J Psychiatry. 2009;166:216–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tesli M, Espeseth T, Bettella F, et al. Polygenic risk score and the psychosis continuum model. Acta Psychiatr Scand. 2014;130:311–317. [DOI] [PubMed] [Google Scholar]
- 23.First M, Pitzer R. Structured Clinical Interview for DSM-IV Axis I Disorders: Patient Edition (SCID-P), Version 2. New York: New York State Psychiatric Institute, Biometrics Research; 1995. [Google Scholar]
- 24.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(suppl 1):S208–S219. [DOI] [PubMed] [Google Scholar]
- 25.Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI). Norwegian Manual Supplement. Stockholm: Pearson Assessment; 2007. [Google Scholar]
- 26.Callicott JH, Mattay VS, Verchinski BA, Marenco S, Egan MF, Weinberger DR. Complexity of prefrontal cortical dysfunction in schizophrenia: more than up or down. Am J Psychiatry. 2003;160:2209–2215. [DOI] [PubMed] [Google Scholar]
- 27.White T, Hongwanishkul D, Schmidt M. Increased anterior cingulate and temporal lobe activity during visuospatial working memory in children and adolescents with schizophrenia. Schizophr Res. 2011;125:118–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nyberg L, Andersson M, Kauppi K, et al. Age-related and genetic modulation of frontal cortex efficiency. J Cogn Neurosci. 2014;26:746–754. [DOI] [PubMed] [Google Scholar]
- 29.Burianová H, Lee Y, Grady CL, Moscovitch M. Age-related dedifferentiation and compensatory changes in the functional network underlying face processing. Neurobiol Aging. 2013;34:2759–2767. [DOI] [PubMed] [Google Scholar]
- 30.St-Laurent M, Abdi H, Bondad A, Buchsbaum BR. Memory reactivation in healthy aging: evidence of stimulus-specific dedifferentiation. J Neurosci. 2014;34:4175–4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Voss MW, Erickson KI, Chaddock L, et al. Dedifferentiation in the visual cortex: an fMRI investigation of individual differences in older adults. Brain Res. 2008;1244:121–131. [DOI] [PubMed] [Google Scholar]
- 32.Weinberger DR, Berman KF. Speculation on the meaning of cerebral metabolic hypofrontality in schizophrenia. Schizophr Bull. 1988;14:157–168. [DOI] [PubMed] [Google Scholar]
- 33.Andreassen OA, Thompson WK, Dale AM. Boosting the power of schizophrenia genetics by leveraging new statistical tools. Schizophr Bull. 2014;40:13–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.