Abstract
Genome-wide association studies (GWAS) of schizophrenia have identified multiple risk variants with robust association signals for schizophrenia. However, these variants could explain only a small proportion of schizophrenia heritability. Furthermore, the effect size of these risk variants is relatively small (eg, most of them had an OR less than 1.2), suggesting that additional risk variants may be detected when increasing sample size in analysis. Here, we report the identification of a genome-wide significant schizophrenia risk locus at 22q13.1 by combining 2 large-scale schizophrenia cohort studies. Our meta-analysis revealed that 7 single nucleotide polymorphism (SNPs) on chromosome 22q13.1 reached the genome-wide significance level (P < 5.0×10–8) in the combined samples (a total of 38441 individuals). Among them, SNP rs6001946 had the most significant association with schizophrenia (P = 2.04×10–8). Interestingly, all 7 SNPs are in high linkage disequilibrium and located in the MKL1 gene. Expression analysis showed that MKL1 is highly expressed in human and mouse brains. We further investigated functional links between MKL1 and proteins encoded by other schizophrenia susceptibility genes in the whole human protein interaction network. We found that MKL1 physically interacts with GSK3B, a protein encoded by a well-characterized schizophrenia susceptibility gene. Collectively, our results revealed that genetic variants in MKL1 might confer risk to schizophrenia. Further investigation of the roles of MKL1 in the pathogenesis of schizophrenia is warranted.
Key words: schizophrenia, MKL1, genetic association, protein-protein interaction, GSK3B
Introduction
Schizophrenia is a severe mental disorder characterized by delusions, hallucinations, disorganized thinking, and cognitive deficits.1,2 Accumulating evidence indicates that both genetic and environmental factors are involved in the pathogenesis of schizophrenia.3–7 Though schizophrenia has a strong genetic component (with an estimated heritability of approximately 0.80),8 the genetic architecture and etiology of schizophrenia remain largely unknown. To identify schizophrenia susceptibility genes, numerous genetic linkage and association studies have been conducted and many candidate genes and loci have been identified, including COMT,9–11 DISC1,12–14 NRG1,15–18 BDNF,19,20 MHC locus,21,22 among others.23
With the dramatic increase in sample size and genotyping throughput, recent genome-wide association studies (GWAS) of schizophrenia have identified multiple convincing risk variants and loci that show robust association with schizophrenia.21,24–30 However, these identified risk variants only explain a small proportion of schizophrenia heritability, because the effect size of these identified variants is relatively small, with most of them having an odds ratio (OR) less than 1.2.24 Accumulating evidence suggests that common polygenic variation contributes to risk of schizophrenia,21,24 implying that novel risk variants are likely to be discovered with the increase of sample size.
To identify additional genetic risk variants that might contribute to schizophrenia susceptibility, we analyzed 2 large schizophrenia cohort datasets. We found that common genetic variants in the Megakaryoblastic leukemia 1 (MKL1) gene were significantly associated with schizophrenia in both datasets. Further analyses in the combined sample (a total of 38441 subjects) identified 7 SNPs in MKL1 with genome-wide significance (P < 5.0×10–8), and those SNPs were in high linkage disequilibrium (LD). Our results provide genetic evidence that supports the association of the MKL1 gene with schizophrenia, suggesting that the MKL1 gene might be involved in the pathogenesis of schizophrenia.
Materials and Methods
Schizophrenia Studies
The first study (case-control based, PGC + SWE samples) consisted of 13833 cases and 18310 controls and has been reported by Ripke et al.24 This sample set is composed of a large-scale GWAS analysis of the Swedish national sample (SWE samples, including 5001 cases and 6243 controls) and independent samples from the schizophrenia Psychiatric Genomics Consortium (PGC samples, including 8832 cases and 12067 controls). All of the samples were genotyped by Affymetrix, Illumina, or Perlegen high-throughput genotyping platforms. The association was performed by using logistic regression of imputed dosages with sample identifiers and 3 principal components as covariates. Briefly, genetic association information of 13833 cases and 18310 controls from the combined PGC + SWE samples were used in this study. Detailed information on sample ascertainment and diagnosis, genotyping quality control, genomic control, and statistical analyses can be found in the original work.24
The second study (family-based) contained 6289 individuals (including 3286 schizophrenia cases) from 1811 nuclear families and was reported by Aberg et al.31 Three independent samples were recruited in this family-based replication sample: a European family-based sample (2740 individuals, 794 families, and 1420 schizophrenia cases), an Asian family-based sample (2296 individuals, 579 families, and 1222 schizophrenia cases), and an African family-based sample (1262 individuals, 438 families, and 644 schizophrenia cases). More detailed information regarding sample description (recruitment of schizophrenic patient and healthy controls and diagnosis), genotyping, quality control, population stratification analysis, and statistical analysis can be found in the original article.31 There is no overlap between individuals from this family-based study and the subjects from the case-control based PGC + SWE sample (Ripke et al.24).
Extraction of Genetic Association Information and Meta-analysis
Because the family-based genetic association analysis is complementary to the case-control association study, we hypothesized that authentic schizophrenia risk variants may be identified if they simultaneously show significant association with schizophrenia in case-control and family-based samples. Moreover, with the increase of sample size, the statistical power would be expected to increase when family-based and case-control samples were combined. Based on this hypothesis, we first extracted all single nucleotide polymorphism (SNPs) from the family-based study.31 Since our goal is to identify the promising genetic risk variants, only SNPs with a P value less than 0.01 from the study of Aberg et al.31 were considered. As many of these SNPs are located in chromosomal regions that were reported by Ripke et al.,24 we focused on chromosome 22, a chromosome where no genome-wide significant finding was reported by Ripke et al.24 We then explored the association between these SNPs in PGC + SWE case-control samples. Finally, meta-analysis was performed with a fixed-effect model by using PLINK (a whole genome association analysis toolset, http://pngu.mgh.harvard.edu/~purcell/plink/)32 or “Metafor” from the R package.33 To test whether there is heterogeneity for the analyzed SNPs in case-control samples and family-based samples, we also performed heterogeneity tests by using PLINK and the Metafor package.33
Linkage Disequilibrium Analysis
To assess the LD of the SNPs located on chromosome 22, we downloaded the genotypic data of Europeans from the 1000 Genomes project.34 The LD between these SNPs was then calculated and visualized by using the Haploview program.35
MKL1 Expression Analysis in Human Brains
We explored the expression pattern of MKL1 in diverse human tissues using the gene enrichment profiler36 and the genotype-tissue expression Portal (GTEx).37 In addition, we examined the spatiotemporal expression pattern of MKL1 in developing human brains using the BrainCloud.38 To further characterize the expression profiling of MKL1 in schizophrenia patients and healthy controls, we examined MKL1 expression using the whole transcriptome sequencing (RNA-seq) from post-mortem human brain tissue.39 Briefly, brain tissues [from the anterior cingulate cortex (Brodmann region 24)] of 35 schizophrenia patients, 35 bipolar disorder, and 35 healthy controls were obtained from the Stanley Medical Research Institute (SMRI; http://www.stanleyresearch.org/dnn/) for transcriptome sequencing. Total RNA was isolated by Trizol reagent (Invitrogen). RNA samples that passed the stringent quality control were used for deep sequencing using Illumine sequencer. More detailed about the dissection of brain tissues, RNA extraction, sequencing, and data analysis can be found in reference.39
MKL1 Expression Analysis in Developing and Adult Mouse Brains
We also investigated the expression of MKL1 in developing and adult mouse brains using real-time quantitative PCR. In brief, C57BL/6J mice were used and embryos were designated as embryonic day 0.5 (E0.5) at noon on the day at which vaginal plugs were observed. All animal procedures used in this study were approved by The University Committee of Animal Resources (UCAR) at the University of Rochester. Whole brains from 2 embryonic (E12.5 and E14.5) and 7 postnatal stages (P1, P2, P4, P7, P20, P40, and P90) were isolated and total RNA was extracted by Trizol reagent (Invitrogen). RNA was quantified and equal amount of total RNA (3 µg) was treated with DNase I (Fermentas) to remove the potential DNA contamination. DNase I treated RNA was then reversed transcribed by SuperScript III Reverse Transcriptase (Invitrogen) using oligo dT primers. The synthesized cDNA was used to conduct real-time quantitative PCR using SsoAdvanced SYBR Green Supermix (Bio-Rad) and the CFX Real-Time PCR Systems (BioRad). The expression of MKL1 was normalized to the expression of GAPDH and the fold change in expression was calculated using the ΔΔCt (threshold cycle) method. The primer sequences of GAPDH are: Forward: 5′-AGGTCGGTG TGAACGGATTTG-3′; Reverse: 5′- TGTAG ACCAT GTAGT TGAG GTCA-3′. The primers of MKL1 are Forward: 5′- AGGAC CGAGGAC TATTTG AAAC G-3′; Reverse: 5′- CCACAAT GATAGCCTCCTTCAG-3′. For each stage, at least 3 animals were used and triplicate assays were performed.
Frequency Distribution of the Risk Variants in Global Populations
We examined the frequency distribution of the risk alleles of the identified risk variants in global populations using genotype information from the Human Genome Diversity Project (HGDP) selection browser (http://hgdp.uchicago.edu/cgi-bin/gbrowse/HGDP/),40 which contains the allele frequency data of 53 worldwide populations.
Functional Prediction of the Significant SNPs
We performed bioinformatic analyses to predict the potential functional consequences of the significant SNPs using the Regulome DB (http://www.regulomedb.org).41 Multiple types of data (eg, ChIP-seq, DNase-seq, and eQTLs) from the Encyclopedia of DNA Elements (ENCODE) project42 were used by Regulome DB to annotate SNPs of interest.
Protein-Protein Interaction Analysis
Proteins often have their function through interacting with (eg, binding to) other proteins or other molecules in the cellular system. One protein interaction example is the protein complex—in schizophrenia, dysbindin, an essential component of the biogenesis of lysosome-related organelles complex 1 (BLOC-1), interacts with all 7 other components of BLOC-1.43 Therefore, the malfunction of a member of the protein complex may lead to cascading functional consequences. Consistent with this, numerous studies have shown that disease-associated genes tend to interact more with each other than random proteins in the protein-protein interaction (PPI) network, and proteins located at the same genomic locus tend to interact within the PPI network.44,45 Accumulating data have suggested that PPIs play pivotal roles in the identification and prioritization of schizophrenia candidate genes.46,47 In fact, our recent study suggests that proteins encoded by schizophrenia susceptibility genes significantly and physically interact and encode a highly interconnected protein-protein interaction network.48 Therefore, investigating the protein interaction may help to prioritize and identify schizophrenia risk genes.
To test whether MKL1 physically interacts with proteins encoded by other schizophrenia risk genes, we first extracted the known schizophrenia susceptibility genes using well-characterized databases of schizophrenia.23,49–51 In addition, we also carefully curated high-confidence schizophrenia susceptibility genes, including genes identified by recent GWAS of schizophrenia and convergent functional genomics (CFG) studies of schizophrenia.52 Finally, top-prioritized schizophrenia susceptibility genes from copy number variation studies of schizophrenia were also included. The detailed list of schizophrenia susceptibility genes is available in our previous study48 and supplementary table S1. We then downloaded the well-defined PPI data from InWeb53,54 and CytoScape55 (iRefScape and GeneMANIA). To test whether proteins in the PPI network significantly interact with other proteins, a permutation test was performed using Disease Association Protein-Protein Link Evaluator (DAPPLE, http://www.broadinstitute.org/mpg/dapple/dapple.php)56
Results
Common Variants in MKL1 Gene were Significantly Associated with Schizophrenia
Under the hypothesis that true schizophrenia risk variants might be identified if they show significant association in both case-control and family-based schizophrenia samples, we obtained the genetic association data of the case-control PGC + SWE samples24 and the family-based samples.31 In total, 8107 SNPs were available from the family-based samples (see “Materials and Methods” section). Because we focused on the most promising risk variants, only SNPs with a P value less than 0.01 were considered for further investigation. A total of 117 SNPs with a P value less than 0.01 from the family-based sample were extracted (table 1). A detailed examination of these SNPs showed that most of them were reported by Ripke et al.24 Interestingly, we noted that 28 of these SNPs were located on chromosome 22q13.1 (table 1), a chromosome region where no genome-wide significant finding had been previously reported by Ripke et al.24
Table 1.
Association of 7 SNPs in MKL1 Gene with Schizophrenia
| SNP | Chr | Position | A12 c | ORa | P valueb | ||||
|---|---|---|---|---|---|---|---|---|---|
| PGC + SWEd | Family- Basede | Combinedf | PGC + SWE | Family-Based | Combined | ||||
| rs10918670 | 1 | 167216210 | GA | 1.054 | 1.212 | 1.068 | 0.013 | 0.0032 | 0.0011 |
| rs11584337 | 1 | 167248664 | TG | 1.054 | 1.213 | 1.068 | 0.013 | 0.0032 | 0.0011 |
| rs2132303 | 1 | 2223258 | TC | 1.060 | 0.847 | 1.031 | 0.017 | 0.010 | 0.181 |
| rs2296268 | 1 | 36603165 | TC | 1.029 | 1.152 | 1.048 | 0.14 | 0.0013 | 0.008 |
| rs2636319 | 1 | 243501047 | CA | 0.932 | 0.882 | 0.926 | 4.15×10–5 | 0.0083 | 2.03×10–6 |
| rs2636320 | 1 | 243501056 | CA | 0.931 | 0.882 | 0.925 | 3.6×10–5 | 0.0083 | 1.57×10–6 |
| rs2643904 | 1 | 2236697 | GA | 0.943 | 1.341 | 0.978 | 0.018 | 6.54×10–5 | 0.346 |
| rs3006925 | 1 | 243609927 | TC | 0.905 | 0.825 | 0.898 | 1.42×10–6 | 0.0045 | 5.13×10–8 |
| rs3767432 | 1 | 167383472 | GA | 1.060 | 1.239 | 1.075 | 0.0072 | 0.0017 | 4.5×10–4 |
| rs3767434 | 1 | 167371151 | GA | 1.060 | 1.235 | 1.074 | 0.0063 | 0.0024 | 4.6×10–4 |
| rs4915419 | 1 | 200251050 | CA | 0.943 | 1.127 | 0.966 | 6.3×10–4 | 0.0064 | 0.031 |
| rs589249 | 1 | 37162352 | GA | 1.062 | 1.138 | 1.073 | 9.7×10–4 | 0.0040 | 3.6×10–5 |
| rs7532692 | 1 | 167250080 | TC | 1.053 | 1.196 | 1.067 | 0.015 | 0.0048 | 0.0014 |
| rs10193188 | 2 | 152551210 | GA | 0.953 | 0.882 | 0.939 | 0.034 | 0.0059 | 0.0018 |
| rs10445792 | 2 | 201078821 | CA | 1.107 | 1.179 | 1.116 | 8.09×10–6 | 0.0047 | 2.19×10–7 |
| rs11687313 | 2 | 201146399 | GA | 0.878 | 0.783 | 0.862 | 8.91 × 10 –8 | 0.00024 | 3.58×10 −10 |
| rs11688415 | 2 | 201143409 | TC | 0.876 | 0.788 | 0.865 | 4.98×10–8 | 3.5×10–4 | 2.14×10−10 |
| rs11694369 | 2 | 200741720 | GA | 0.885 | 0.796 | 0.871 | 1.29×10–8 | 6.0×10–4 | 1.19×10−10 |
| rs13010104 | 2 | 208369213 | TC | 1.117 | NA | 1.125 | 7.23×10–7 | NA | 6.34×10–8 |
| rs13010676 | 2 | 208369294 | GA | 1.116 | NA | 1.095 | 8.29×10–7 | NA | 2.78×10–5 |
| rs1861228 | 2 | 103208945 | GA | 0.971 | 0.898 | 0.961 | 0.091 | 0.015 | 0.013 |
| rs4664494 | 2 | 152499580 | TC | 1.040 | 1.146 | 1.060 | 0.089 | 0.0030 | 0.0043 |
| rs6713162 | 2 | 152496526 | GA | 1.041 | 1.121 | 1.056 | 0.082 | 0.040 | 0.008 |
| rs6739563 | 2 | 201134216 | TG | 1.114 | 1.189 | 1.125 | 1.77 × 10 –6 | 0.0016 | 1.95 × 10 −08 |
| rs1010471 | 3 | 180691092 | GA | 1.074 | 0.875 | 1.044 | 5.72×10–5 | 0.0027 | 0.0093 |
| rs12496073 | 3 | 25033188 | GA | 1.033 | 1.154 | 1.049 | 0.084 | 0.0019 | 0.0057 |
| rs13076055 | 3 | 12341996 | GA | 0.963 | 1.129 | 0.986 | 0.048 | 0.0083 | 0.42 |
| rs17203055 | 3 | 119084331 | GA | 1.126 | NA | 1.085 | 2.38×10–5 | NA | 0.0024 |
| rs4684846 | 3 | 12338849 | GA | 1.039 | 0.885 | 1.014 | 0.048 | 0.0078 | 0.43 |
| rs6445062 | 3 | 172192239 | TC | 1.061 | 0.870 | 1.034 | 6.1×10–4 | 0.0015 | 0.040 |
| rs13120709 | 4 | 169435779 | TC | 1.027 | 1.121 | 1.040 | 0.14 | 0.012 | 0.021 |
| rs17001561 | 4 | 77096118 | GA | 1.040 | 1.207 | 1.056 | 0.11 | 0.0072 | 0.017 |
| rs29357 | 4 | 114209691 | GA | 1.021 | NA | 1.002 | 0.45 | NA | 0.95 |
| rs566091 | 4 | 152852168 | GC | 0.973 | 0.855 | 0.953 | 0.25 | 0.0046 | 0.029 |
| rs1986252 | 5 | 60754419 | TC | 0.931 | 0.879 | 0.925 | 2.1×10–5 | 0.008 | 1.06×10–6 |
| rs4604142 | 5 | 60642595 | TC | 1.082 | 1.138 | 1.088 | 2.41×10–6 | 0.0084 | 1.21×10–7 |
| rs6898746 | 5 | 60843706 | TA | 0.929 | 0.869 | 0.923 | 1.4×10–5 | 0.0036 | 4.48×10–7 |
| rs7734879 | 5 | 60711632 | CA | 0.943 | 0.883 | 0.935 | 7.8×10–4 | 0.010 | 4.94×10–5 |
| rs156743 | 6 | 27967089 | TC | 1.163 | 1.174 | 1.165 | 1.47×10–8 | 0.0079 | 4.20×10−10 |
| rs16897515 | 6 | 27278020 | CA | 1.157 | 1.255 | 1.166 | 8.20×10−10 | 0.0026 | 1.39×10–11 |
| rs2077580 | 6 | 32020844 | GA | 0.962 | 1.276 | 0.993 | 0.23 | 0.0086 | 0.82 |
| rs2239523 | 6 | 31089507 | GC | 0.983 | 0.820 | 0.965 | 0.34 | 4.8×10–4 | 0.046 |
| rs2857605 | 6 | 31524851 | GA | 1.007 | 1.185 | 1.026 | 0.73 | 0.0039 | 0.20 |
| rs3130287 | 6 | 32050544 | TC | 0.949 | 0.826 | 0.936 | 0.024 | 0.0085 | 0.0032 |
| rs3130349 | 6 | 32147696 | GA | NA | 1.207 | 1.207 | NA | 0.0043 | 0.0043 |
| rs3132935 | 6 | 32171075 | GA | NA | 0.832 | 0.832 | NA | 0.0013 | 0.0013 |
| rs3132947 | 6 | 32176782 | TG | NA | 0.834 | 0.834 | NA | 0.0016 | 0.0016 |
| rs3134605 | 6 | 32159956 | GA | NA | 0.858 | 0.858 | NA | 0.0043 | 0.0043 |
| rs3134954 | 6 | 32071893 | GA | 1.057 | 1.217 | 1.071 | 0.018 | 0.0071 | 0.0021 |
| rs354391 | 6 | 66319480 | CA | 1.030 | 1.137 | 1.093 | 0.13 | 0.0068 | 0.015 |
| rs3800316 | 6 | 27256102 | CA | 0.894 | 0.831 | 0.888 | 1.85×10–8 | 0.0033 | 4.20×10−10 |
| rs6923836 | 6 | 113393530 | GA | 1.045 | 0.873 | 1.021 | 0.011 | 0.0022 | 0.21 |
| rs6940698 | 6 | 25821580 | TC | 0.867 | 0.700 | 0.853 | 3.54×10–6 | 6.0×10–4 | 6.06×10–8 |
| rs1229761 | 7 | 114223723 | TG | 1.03 | 0.827 | 1.013 | 0.11 | 0.0034 | 0.47 |
| rs13274028 | 8 | 8729193 | CA | 1.041 | 0.850 | 1.016 | 0.025 | 7.1×10–4 | 0.352 |
| rs3739396 | 8 | 18388041 | GA | 1.092 | 1.184 | 1.104 | 2.6×10–4 | 0.0058 | 1.05×10–5 |
| rs10115971 | 9 | 101024887 | GA | 1.187 | 0.843 | 1.136 | 0.021 | 0.0026 | 0.493 |
| rs12343574 | 9 | 129440840 | CA | 1.079 | 0.825 | 1.043 | 0.0081 | 0.010 | 0.12 |
| rs1004467 | 10 | 104594507 | TC | 1.157 | 1.126 | 1.149 | 3.00 × 10 –7 | 0.016 | 1.77 × 10 –8 |
| rs10883757 | 10 | 104400133 | TC | 0.894 | 0.892 | 0.894 | 2.2×10–4 | 0.021 | 1.31×10–5 |
| rs11190913 | 10 | 103059038 | GA | 0.941 | 1.110 | 0.959 | 0.0013 | 0.051 | 0.018 |
| rs11191499 | 10 | 104764271 | TC | 1.203 | 1.158 | 1.193 | 5.20×10−10 | 0.0064 | 1.32×10–11 |
| rs11191514 | 10 | 104773364 | TC | 0.828 | 0.865 | 0.837 | 2.64×10−10 | 0.007 | 8.29×10–12 |
| rs11191548 | 10 | 104846178 | TC | 1.209 | 1.139 | 1.193 | 2.68×10−10 | 0.02 | 2.49×10–11 |
| rs11191560 | 10 | 104869038 | TC | 1.209 | 1.143 | 1.194 | 2.05×10−10 | 0.016 | 1.70×10–11 |
| rs11594111 | 10 | 14945406 | GA | 0.990 | NA | 1.007 | 0.70 | NA | 0.76 |
| rs11818043 | 10 | 104391627 | GA | 0.888 | 0.887 | 0.888 | 7.77×10–5 | 0.014 | 3.47×10−06 |
| rs12221064 | 10 | 104677126 | TC | 0.834 | 0.866 | 0.841 | 9.83×10−10 | 0.0075 | 2.93×10–11 |
| rs12413046 | 10 | 104871204 | GA | 0.826 | 0.879 | 0.838 | 1.68×10−10 | 0.020 | 1.63×10–11 |
| rs17094683 | 10 | 104851301 | TC | 0.827 | 0.868 | 0.836 | 2.07×10−10 | 0.0088 | 8.58×10–12 |
| rs17114803 | 10 | 104386934 | TC | 1.125 | 1.122 | 1.124 | 8.62×10–5 | 0.018 | 4.81×10–6 |
| rs2298278 | 10 | 104390303 | GA | 1.126 | 0.893 | 1.056 | 8.09×10–5 | 0.019 | 0.034 |
| rs3740387 | 10 | 104849468 | TC | 0.897 | 0.905 | 0.898 | 2.26×10−10 | 0.018 | 1.29×10–11 |
| rs3740390 | 10 | 104638480 | GA | 1.195 | 1.161 | 1.187 | 2.21×10–9 | 0.0054 | 4.59×10–11 |
| rs4409766 | 10 | 104616663 | TC | 1.159 | 1.156 | 1.158 | 2.26 × 10 –7 | 0.0035 | 2.82 × 10 –9 |
| rs4919666 | 10 | 104384029 | GA | 1.126 | 1.124 | 1.125 | 8.71×10–5 | 0.017 | 4.45×10–6 |
| rs7897654 | 10 | 104662458 | TC | 1.113 | 1.121 | 1.114 | 6.52×10–9 | 0.0085 | 1.72×10−10 |
| rs7923415 | 10 | 104418656 | TC | 0.972 | 0.879 | 0.959 | 0.11 | 0.0053 | 0.013 |
| rs1059440 | 11 | 63991801 | GA | 1.052 | 1.141 | 1.065 | 0.025 | 0.011 | 0.0022 |
| rs11231727 | 11 | 64011854 | TC | 0.961 | 0.895 | 0.952 | 0.022 | 0.011 | 0.002 |
| rs11605738 | 11 | 64013406 | GA | 0.933 | 0.879 | 0.924 | 0.0021 | 0.013 | 0.00014 |
| rs3219243 | 12 | 109542392 | TC | 0.961 | 0.868 | 0.945 | 0.062 | 0.0041 | 0.0043 |
| rs17065458 | 13 | 44704662 | TC | 1.073 | 1.177 | 1.091 | 0.010 | 0.0047 | 4.1×10–4 |
| rs2036130 | 13 | 82606614 | TC | 0.952 | 0.805 | 0.925 | 0.13 | 0.0029 | 0.0096 |
| rs1261117 | 18 | 52949657 | TC | 1.153 | 1.225 | 1.166 | 9.3×10–4 | 0.026 | 8.21×10–5 |
| rs1564483 | 18 | 60794654 | GA | 1.038 | 1.150 | 1.054 | 0.069 | 0.0045 | 0.0056 |
| rs4892046 | 18 | 70484496 | GA | 1.021 | 1.151 | 1.036 | 0.21 | 0.0025 | 0.027 |
| rs12611334 | 19 | 40229409 | GC | 1.038 | 1.152 | 1.048 | 0.037 | 0.0087 | 0.005 |
| rs16939 | 19 | 46276056 | CA | 1.031 | 1.113 | 1.041 | 0.076 | 0.0147 | 0.011 |
| rs10483204 | 22 | 40870794 | TC | 1.134 | 1.156 | 1.138 | 5.90×10–6 | 0.0093 | 1.91×10–7 |
| rs10483205 | 22 | 40883599 | TC | 0.877 | 0.856 | 0.874 | 3.99×10–6 | 0.014 | 1.86×10–7 |
| rs12159787 | 22 | 40870699 | GA | 0.883 | 0.861 | 0.878 | 7.51×10–6 | 0.0062 | 1.56×10–7 |
| rs138866 | 22 | 50205903 | GA | 0.933 | 0.865 | 0.925 | 0.0010 | 0.015 | 8.94×10–5 |
| rs138880 | 22 | 50218611 | CA | 1.072 | 1.187 | 1.086 | 0.0010 | 0.0029 | 4.01×10–5 |
| rs16985899 | 22 | 40963402 | TC | 0.874 | 0.854 | 0.871 | 1.67×10–6 | 0.010 | 5.64×10–8 |
| rs17001977 | 22 | 40880213 | GA | 1.139 | 1.163 | 1.143 | 4.81×10–6 | 0.014 | 2.27×10–7 |
| rs17001993 | 22 | 40900077 | GA | 1.139 | 1.168 | 1.144 | 4.24×10–6 | 0.012 | 1.64×10–7 |
| rs17001997 | 22 | 40905072 | GA | 1.139 | 1.168 | 1.144 | 4.54×10–6 | 0.012 | 1.84×10–7 |
| rs17002024 | 22 | 40975268 | TC | 0.878 | 0.853 | 0.873 | 2.46×10–6 | 0.0065 | 5.81×10–8 |
| rs17002026 | 22 | 40982581 | TC | 0.878 | 0.854 | 0.873 | 2.21×10–6 | 0.0067 | 5.34×10–8 |
| rs17002027 | 22 | 40984571 | TC | 1.145 | 1.178 | 1.151 | 1.40 × 10 –6 | 0.0076 | 3.98 × 10 –8 |
| rs17002030 | 22 | 40989374 | TC | 1.147 | 1.173 | 1.151 | 9.35 × 10 –7 | 0.0096 | 2.92 × 10 –8 |
| rs17002034 | 22 | 40996367 | TG | 0.873 | 0.850 | 0.869 | 1.40 × 10 –6 | 0.0082 | 4.08 × 10 –8 |
| rs17002038 | 22 | 41000964 | TC | 0.874 | 0.853 | 0.870 | 1.53×10–6 | 0.0099 | 5.39×10–8 |
| rs3827381 | 22 | 40881402 | TC | 1.131 | 1.154 | 1.136 | 1.55×10–5 | 0.010 | 5.26×10–7 |
| rs3827382 | 22 | 40881403 | GA | 1.138 | 1.163 | 1.143 | 7.94×10–6 | 0.014 | 3.61×10–7 |
| rs5749683 | 22 | 34252374 | TC | 0.924 | 0.868 | 0.915 | 2.2×10–4 | 0.0053 | 7.11×10–6 |
| rs5995867 | 22 | 40888136 | TG | 1.135 | 1.160 | 1.140 | 5.37×10–6 | 0.0076 | 1.37×10–7 |
| rs5995871 | 22 | 40922332 | GA | 0.879 | 0.850 | 0.873 | 2.87×10–6 | 0.0054 | 6.31×10–8 |
| rs5995886 | 22 | 41033801 | GA | 1.102 | 1.139 | 1.108 | 4.02×10–6 | 0.0076 | 1.21×10–7 |
| rs6001912 | 22 | 40828361 | TC | 1.138 | 1.158 | 1.142 | 4.74×10–6 | 0.0092 | 1.4×10–7 |
| rs6001930 | 22 | 40876234 | TC | 1.133 | 1.154 | 1.137 | 6.84×10–6 | 0.010 | 2.23×10–7 |
| rs6001931 | 22 | 40877514 | GA | 1.133 | 1.155 | 1.138 | 6.88×10–6 | 0.0099 | 2.24×10–7 |
| rs6001946 | 22 | 40903421 | GA | 0.882 | 0.876 | 0.880 | 6.38 × 10 –6 | 0.0009 | 2.04 × 10 –8 |
| rs6001974 | 22 | 40979164 | GA | 0.877 | 0.850 | 0.872 | 2.18 × 10 –6 | 0.0053 | 4.16 × 10 –8 |
| rs6001980 | 22 | 41004384 | TC | 1.140 | 1.172 | 1.146 | 2.02 × 10 –6 | 0.0061 | 4.60 × 10 –8 |
| rs6001981 | 22 | 41017425 | TC | 0.876 | 0.854 | 0.872 | 1.79 × 10 –6 | 0.0069 | 4.66 × 10 –8 |
aThe OR is based on reference alleles.
bTwo-tailed P values.
cA12, reference and alternative alleles.
dPGC + SWE case-control samples consisted of 13833 cases and 18310 controls.
eThe family-based samples are from 3 different subsamples with a total of 6283 subjects.
fMeta-analysis results for the case-control and family-based samples. Meta-analysis was performed based on a fixed-effects model. SNPs reached genome-wide significance level in PGC + SWE case-control sample are in italics. Genome-wide significant P values (P < 5.0 × 10–8) in the combined samples are shown in bold.
We further explored the association between the 117 SNPs and schizophrenia in the case-control PGC + SWE samples (13833 cases and 18310 controls). The results from the family-based and case-control PCG + SWE samples were highly concordant, with 88% (103 out of 117) of the OR having the same direction of effect (table 1). Among the 117 SNPs, 15 (marked by underline in table 1) reached genome-wide significance level (P < 5.0×10−10) in the case-control PGC + SWE samples and have been reported by Ripke et al.24 Intriguingly, we found that all of the 28 SNPs from chromosome 22 showed moderate association with schizophrenia in the PGC + SWE samples (table 1). More importantly, we noticed that the effect direction (risk allele) of these 28 SNPs in the family-based sample (from Aberg et al.’s study)31 were the same as in the case-control samples (PGC + SWE) (table 1), strongly suggesting that these SNPs may be authentic risk variants.
Next, we evaluated the association between the 117 SNPs and schizophrenia in the combined samples from the 2 studies (a total of 38441 subjects) by using meta-analysis, which was performed with a fixed-effect model using PLINK32 or “metafor” package implemented in R.33 In addition to the 15 genome-wide significant SNPs (underlined in table 1) reported by Ripke et al.,24 we identified 11 new SNPs (marked in bold in table 1) that reached genome-wide significance level (P < 5.0×10−8) in the combined samples (table 1). Among the 11 newly identified SNPs, rs11687313 and rs6739563 are located on chromosome 2, rs1004467 and rs4409766 are located on chromosome 10, and the remaining 7 SNPs are located on chromosome 22. We further tested if the 11 newly identified SNPs represent independent association signals by using LD information (Europeans) from the 1000 Genomes project.34 We found that rs11687313 and rs6739563 are highly linked with rs11688415 (r 2 = 1 and 0.79, respectively, supplementary figure 1), a SNP that has been reported by Ripke et al.24 In addition, rs1004467 is highly linked with rs11191514 (r 2 = 0.82, supplementary figure 2), a SNP reached genome-wide significance level in study of Ripke et al.24 These results suggested that the significant SNPs from chromosome 2 and 10 were likely due to the linkage of these SNPs with the genome-wide significant SNPs reported by Ripke et al.24
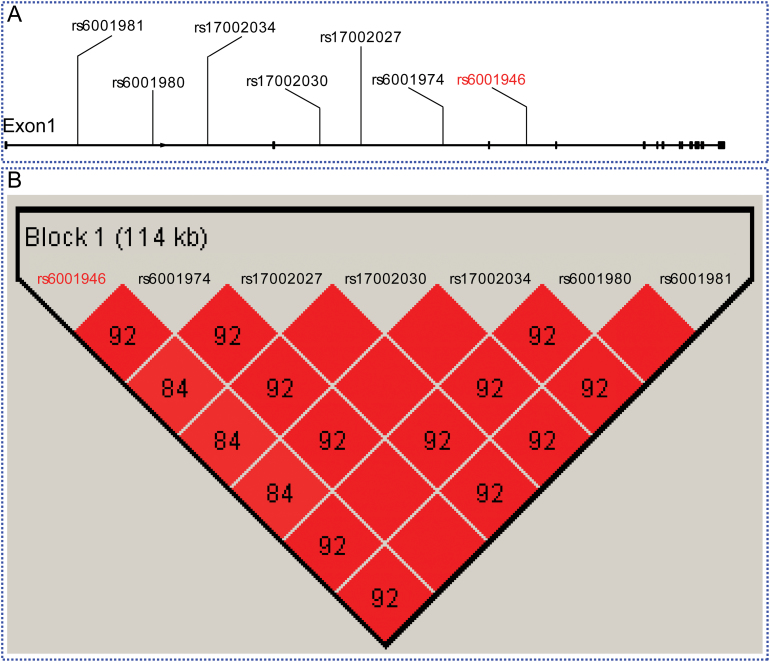
Intriguingly, the meta-analysis revealed that 7 out of the 28 SNPs on chromosome 22q13.1 reached the genome-wide significance level (P < 5.0×10–8) (table 1) in the combined samples. Among them, SNP rs6001946 had the most significant association with schizophrenia (P = 2.04×10–8) (table 1). To determine the genomic location of the 7 SNPs that reached the genome-wide significance level (P < 5.0×10–8), we mapped these SNPs to the human reference genome (hg19). All 7 of these significant SNPs are located in the MKL1 gene: rs6001981, rs6001980, and rs17002034 reside in intron 1; rs17002027, rs17002030 and rs6001974 are located in intron 2; and rs6001946 is located in intron 3 of the MKL1 gene (figure 1A). Despite the fact that these 7 SNPs reached genome-wide significance level, it should be noted that the OR of these 7 SNPs are relatively small, with all of them having an OR less than 1.2.
Fig. 1.
Genomic locations and linkage disequilibrium of the 7 genome-wide significant single nucleotide polymorphism (SNPs) identified in this study. (A) All of these 7 SNPs are located in introns of the MKL1 gene. Of note, rs6001946 (marked by red), which showed the most significant association with schizophrenia, is located in intron 3 of MKL1. (B) These 7 SNPs are highly linked in Europeans based on the genotype data from the 1000 Genomes project. Linkage disequilibrium values (r 2) are showed in the red rectangles.
Genome-Wide Significant SNPs in the MKL1 Gene are Highly Linked
We analyzed the LD pattern among the 7 SNPs (in MKL1) that reached genome-wide significance level by using the genotypic data (Phase I) of Europeans from the 1000 Genomes project.57 The LD analysis revealed that the 7 genome-wide significant SNPs from chromosome 22 are in high-LD. In fact, we found that all 7 significant SNPs are located in 1 haplotype block (figure 1B). Because all the significant SNPs are located in introns of MKL1 gene, we therefore explored whether these SNPs have potential functional consequences, eg, influence transcription factor binding or gene expression. We found that 4 SNPs have RegulomeDB scores (supplementary table S2). Of note, the RegulomeDB score of rs6001974 is relatively high and transcription factor USF2 binds to the region containing SNP rs6001974. These results suggested that these SNPs may have potential functional consequences. However, further work is needed to test whether these SNPs can regulate or affect the expression of MKL1.
Expression Profiling of the MKL1 Gene in the Human Brain
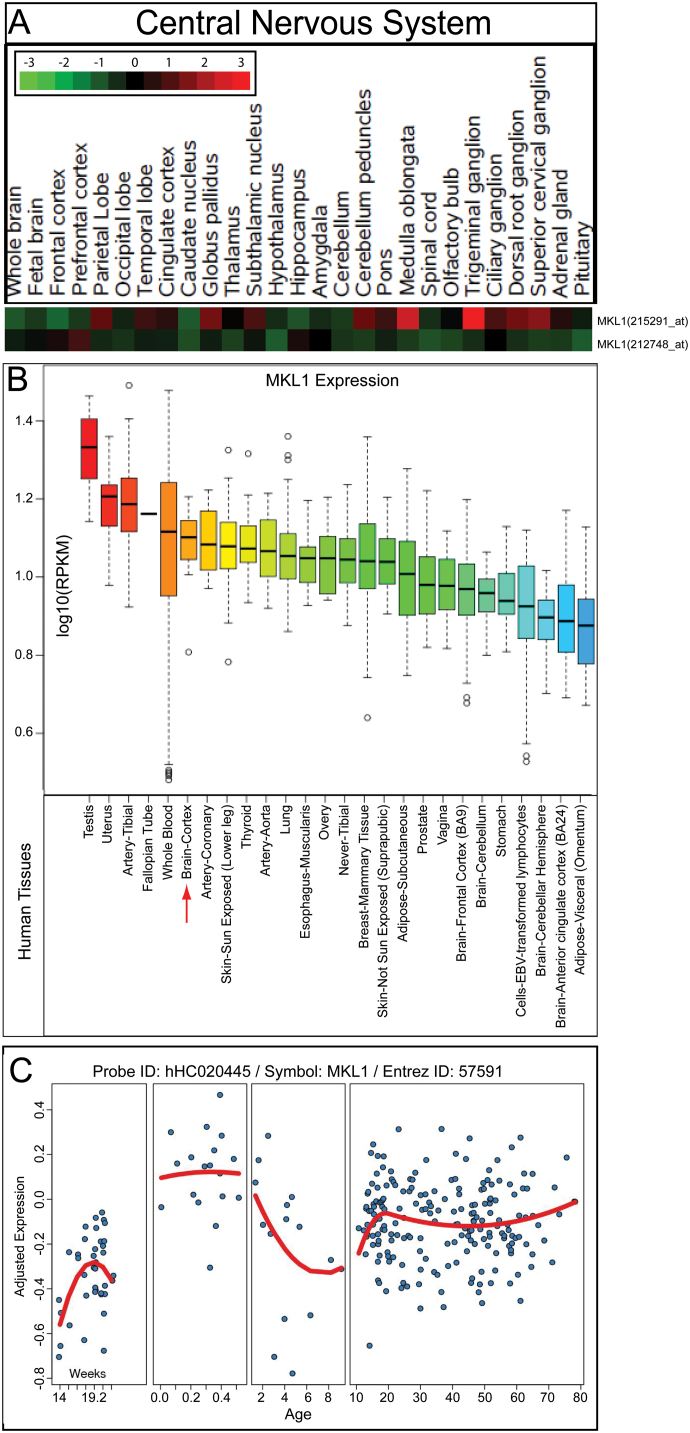
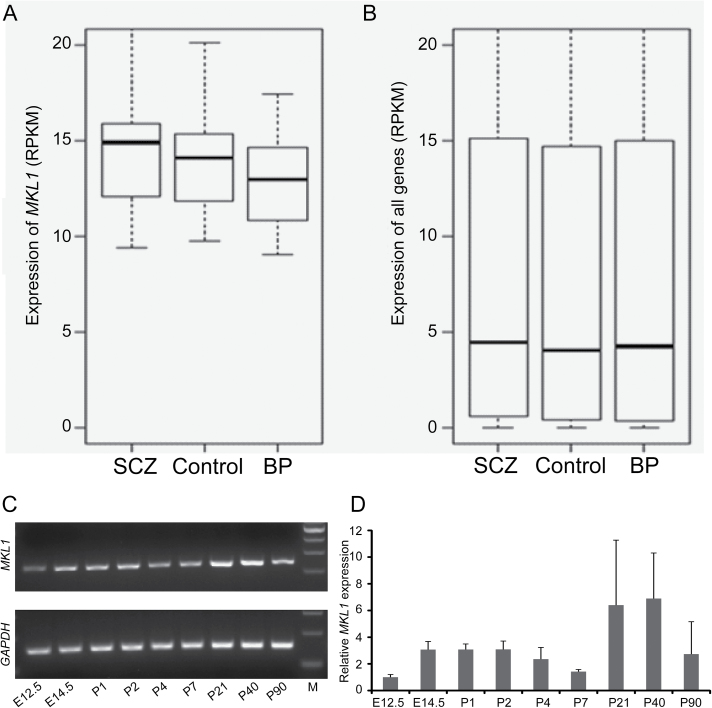
Schizophrenia is a mental disorder that mainly originates from the dysfunction of brain. If MKL1 contributes to schizophrenia risk, it might be interesting to explore whether it expresses in brain tissues. To characterize the expression pattern of MKL1, we examined the expression profiling of MKL1 in diverse human tissues using the gene enrichment profiler.36 We found that MKL1 is widely expressed in human brain tissues, with the highest expression in trigeminal ganglion (figure 2A). Using RNA sequencing-based expression data from the GTEx, we confirmed that MKL1 is highly expressed in the human brain (figure 2B). We also explored the temporal expression pattern of MKL1 in developing and adult human brains using the BrainCloud. The expression of MKL1 is relatively low in prenatal human brains; however, MKL1 is expressed at a high level in human brains after birth (figure 2B). Finally, we investigated whether MKL1 is differentially expressed in schizophrenia patients and healthy controls using brain tissues (the anterior cingulate cortex (Brodmann region 24)) from the Stanley Medical Research Institute (see Materials and Methods).39 We found that MKL1 is highly expressed in human brains compared with all other human genes (figure 3A, B). In addition, expression of MKL1 is slightly higher in schizophrenia brain tissue samples than others (control or bipolar disorder tissue samples) (figure 3A). Nevertheless, MKL1 did not show significant differential expression in schizophrenia cases and healthy controls. These collective results indicated that MKL1 is widely expressed in brain at high level, suggesting that it may also be involved in brain development and schizophrenia pathogenesis.
Fig. 2.
Spatiotemporal expression pattern of MKL1 in human brain. (A) MKL1 is widely expressed in human brain tissues, with the highest expression level in the trigeminal ganglion. (B) RNA sequencingbased expression data showed that MKL1 is highly expressed in human cortex. (C) Temporal expression profiling of MKL1 in the prefrontal cortex of humans. The expression level of MKL1 is relatively low in the early developmental stage. After birth, the expression level of MKL1 is increased in the human brain.
Fig. 3.
MKL1 is highly expressed in human and mouse brains. (A, B) Expression profiling of MKL1 in the anterior cingulate cortex of schizophrenia patients, bipolar disorder patients and healthy controls. Compared with all other human genes (average RPKM value < 5) (B), MKL1 showed high expression level in human brains (RPKM > 10) (A). In addition, MKL1 expression level in the schizophrenia patients is slightly higher than in healthy controls (A). (C, D) MKL1 is highly expressed in developing and adult mouse brain. (C) RT-PCR revealed the expression of MKL1 in developing mouse brain from E12.5 to adult (P90). (D) Real-time quantitative PCR showed that the expression of MKL1 peaks at P21-P40. The GAPDH was used as the internal control. Data are expressed as mean ± SD (n = 3). RPKM: reads per kilobase per million reads, a standard measure of gene expression in RNA-seq data. BP, bipolar disorder; M, DNA marker; SCZ, schizophrenia.
MKL1 is Highly Expressed in Developing and Adult Mouse Brain
We further investigated MKL1 expression in developing and adult mouse brain using real-time quantitative PCR. Consistent with the findings from human brain, MKL1 is highly expressed in mouse brain at different developing stages (figure 3C, D). At E12.5, expression of MKL1 is relatively low. MKL1 is abundantly expressed in mouse brain from E14.5, with the highest expression level at P40 (figure 3D). These results further confirmed that MKL1 is highly expressed in central nervous system, suggesting that it may play a role in brain development and schizophrenia pathogenesis.
The Risk Variants in MKL1 Showed Dramatic Allele Frequency Difference in World-Wide Populations
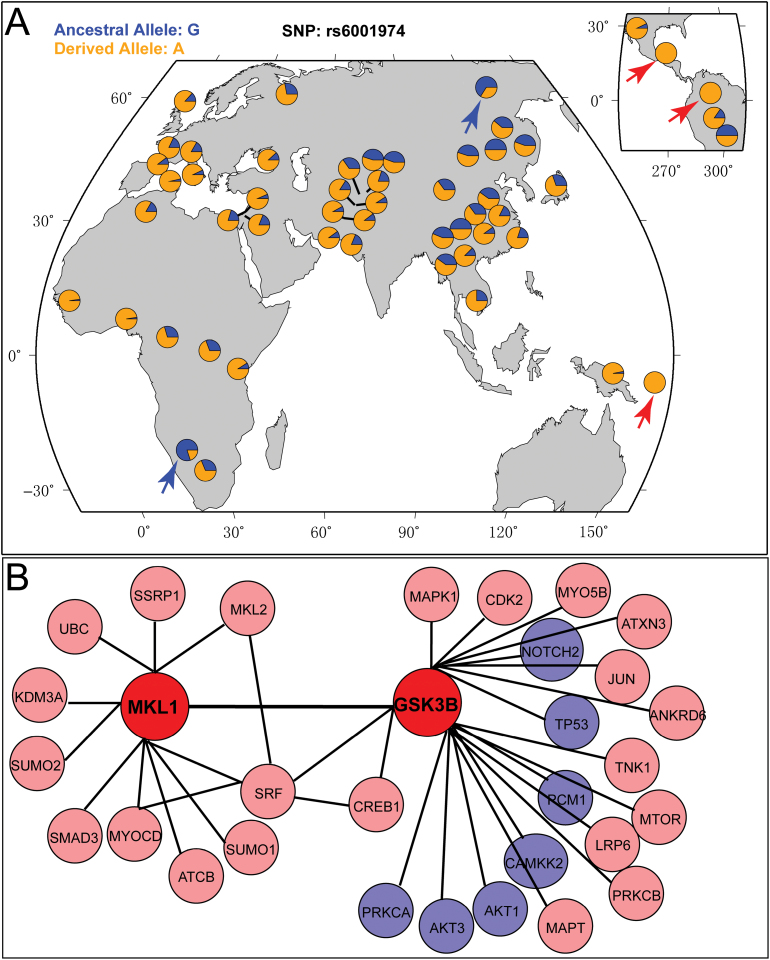
Recent studies suggest that genetic variants conferring risk of psychiatric disorders show significant frequency differences among worldwide populations.58,59 Thus, we examined the allele frequency distribution of the risk variants identified in this study in global populations. We utilized the genotype data from the HGDP selection database.40 Because the 7 significant SNPs are in high LD, we first focused on rs6001946, which showed the most significant association with schizophrenia (table 1). Nevertheless, rs6001946 was not found in the HGDP database. Therefore, we checked rs6001974, which is highly linked with rs6001946 (r 2 = 0.92) (figure 1B). We found that the risk allele (A allele) of rs6001974 showed drastic frequency differences in worldwide populations (figure 4A). Interestingly, the risk allele (A) of rs6001974 is common in most world populations (eg, fixed in some populations, red arrows in figure 4A), while the frequency of A allele is relatively low in some populations (blue arrows in figure 4A).
Fig. 4.
Frequency distribution of the risk allele of SNP rs17002034 in global populations and protein-protein interaction (PPI) analysis. (A) The risk allele (T) of rs6001974 showed drastic differences in its frequency among global populations. Of note, the A allele (risk allele) is fixed in some populations. (B) MKL1 physically interacts with GSK3B, a protein encoded by a well-known schizophrenia risk gene. Circles represent proteins and edges represent PPIs. MKL1 and GSK3B are marked by red circles. Blue circles represent proteins encoded by schizophrenia susceptibility genes.
MKL1 Physically Interacts with GSK3B
Our recent studies have shown that proteins encoded by schizophrenia susceptibility genes tend to interact with each other.48,60 If MKL1 confers risk to schizophrenia, then it may physically interact with other proteins encoded by schizophrenia susceptibility genes. Using high-confidence protein-protein interactions from the well-characterized PPI databases, we investigated the interactions between MKL1 and other proteins encoded by schizophrenia risk genes. We found that MKL1 physically interacts with GSK3B (figure 4B), a protein encoded by a promising schizophrenia susceptibility gene, GSK3B. 61–64 Several lines of evidence suggest that GSK3B may represent a promising schizophrenia candidate gene. First, previous studies have shown that genetic variants in GSK3B were significantly associated with schizophrenia.61–63 Second, AKT1-GSK3B signaling was impaired in schizophrenia.61 Third, expression of GSK3B was down-regulated in schizophrenia patients compared with healthy controls.64 Fourth, a recent study revealed that GSK3B expression-correlated genetic variation is associated with prefrontal cortical thickness, prefrontal physiology and schizophrenia.62 Fifth, GSK3B is the target of lithium,65 a mood-stabilizing agent, ie used as an adjunctive treatment to antipsychotic for schizophrenia.66,67 Finally, GSK3B plays pivotal role in brain development.68,69 These lines of evidence suggested that GSK3B may represent a promising schizophrenia candidate gene. Considering the importance of GSK3B signaling in brain development68–70 and schizophrenia pathogenesis, the interaction between MKL1 and GSK3B further supports the role of MKL1 gene in schizophrenia pathogenesis.
Discussion
MKL1 is a major coactivator of the serum response factor (SRF),71 an important transcription factor (TF) that regulates activity-driven gene expression in neurons.72 It has been well-established that SRF is a multifaceted TF in the brain, and it plays essential roles in neurodevelopment and brain function,73 including in neuronal migration,72,74 neuronal circuit assembly,75 hippocampal lamination and dendrite development,76 learning and memory,77 axon outgrowth and projection,78,79 and axon regeneration.80 Interactions with other coactivator, such as MLK1, is necessary for the function of SRF.71 As one of the most important coactivators of SRF, MKL1 has been reported to play important roles in brain function and neurodevelopment. MKL1 is widely expressed in diverse tissues, with the highest expression levels in the testis and brain.81 Inactivation of MKL1 leads to neuronal migration defects and aberrant neurite outgrowth during development.74 In addition, the inhibition of MKL1 by RNAi causes a decreased number of dendritic processes and dendritic length.71 Intriguingly, recent studies have also shown that MKL1 can regulate neuronal plasticity through influencing the expression of BDNF,82–84 a protein, ie encoded by a well-studied schizophrenia susceptibility gene.85–87 These lines of convergent evidence strongly suggest that MKL1 plays a pivotal role in brain development. Considering that accumulating evidence supports that schizophrenia is a neurodevelopmental disorder,88–97 our results suggest that MKL1 might be involved in schizophrenia pathogenesis through influencing brain development. Further investigation is thus warranted.
Our findings provide genetic evidence of common variants in the MKL1 gene conferring the risk of schizophrenia. First, given that 2 large complementary samples were used (ie case-control and family-based samples) in this study, the significant association between SNPs in the MKL1 gene and schizophrenia in both samples support that the MKL1 gene likely represents a novel risk gene for schizophrenia. Second, the risk alleles of these 7 SNPs in the family-based study are the same as those in the case-control study (PGC + SWE); this striking feature provides further evidence that these identified variants might represent authentic schizophrenia risk variants. Third, expression data indicates that MKL1 is highly expressed in the human brain, implying its potential roles in neurodevelopment and cognitive function. Fourth, protein-protein interaction analysis further confirmed the potential role of the MKL1 gene in the pathogenesis of schizophrenia. Collectively, our study supports that MKL1 is likely a novel, promising schizophrenia susceptibility gene. Our results also support the common polygenic model of schizophrenia,21,24 which further suggests that additional risk variants are likely to be identified with the increase of sample size and better analysis strategies. Further work is needed to replicate our results and to elucidate the role of MKL1 in the etiology of schizophrenia.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
Ministry of Science and Technology of China (2011CB910900 to Y.G.Y.); Strategic Priority Research Program (B) of the Chinese Academy of Sciences (XDB02020300 to Y.G.Y.); National Institutes of Health (R01LM011177).
Supplementary Material
Acknowledgments
X.J.L. was supported by the “100-Talents Scheme” (Bairen Jihua) from the Kunming Institute of Zoology, Chinese Academy of Sciences. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We thank Dr Stephan Ripke and the members of the schizophrenia working group in the Psychiatric Genomics Consortium for making their results publicly available, and Dr Hui Yu for technical assistance in checking MKL1 gene expression from RNA-seq data of schizophrenia, bipolar disorder, and control samples. All authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Tandon R, Nasrallah HA, Keshavan MS. Schizophrenia, “just the facts” 4. Clinical features and conceptualization. Schizophr Res. 2009;110:1–23. [DOI] [PubMed] [Google Scholar]
- 2.Andreasen NC, Flaum M. Schizophrenia: the characteristic symptoms. Schizophr Bull. 1991;17:27–49. [DOI] [PubMed] [Google Scholar]
- 3.Tsuang M. Schizophrenia: genes and environment. Biol Psychiatry. 2000;47:210–220. [DOI] [PubMed] [Google Scholar]
- 4.Tsuang MT, Stone WS, Faraone SV. Genes, environment and schizophrenia. Br J Psychiatry Suppl. 2001;40:s18–s24. [DOI] [PubMed] [Google Scholar]
- 5.van Os J, Rutten BP, Poulton R. Gene-environment interactions in schizophrenia: review of epidemiological findings and future directions. Schizophr Bull. 2008;34:1066–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siever LJ, Davis KL. The pathophysiology of schizophrenia disorders: perspectives from the spectrum. Am J Psychiatry. 2004;161:398–413. [DOI] [PubMed] [Google Scholar]
- 7.Harrison PJ. The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain. 1999;122 (Pt 4):593–624. [DOI] [PubMed] [Google Scholar]
- 8.Sullivan PF, Kendler KS, Neale MC. Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch Gen Psychiatry. 2003;60:1187–1192. [DOI] [PubMed] [Google Scholar]
- 9.Egan MF, Goldberg TE, Kolachana BS, et al. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A. 2001;98:6917–6922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shifman S, Bronstein M, Sternfeld M, et al. A highly significant association between a COMT haplotype and schizophrenia. Am J Hum Genet. 2002;71:1296–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glatt SJ, Faraone SV, Tsuang MT. Association between a functional catechol O-methyltransferase gene polymorphism and schizophrenia: meta-analysis of case-control and family-based studies. Am J Psychiatry. 2003;160:469–476. [DOI] [PubMed] [Google Scholar]
- 12.Millar JK, Wilson-Annan JC, Anderson S, et al. Disruption of two novel genes by a translocation co-segregating with schizophrenia. Hum Mol Genet. 2000;9:1415–1423. [DOI] [PubMed] [Google Scholar]
- 13.Hennah W, Varilo T, Kestilä M, et al. Haplotype transmission analysis provides evidence of association for DISC1 to schizophrenia and suggests sex-dependent effects. Hum Mol Genet. 2003;12:3151–3159. [DOI] [PubMed] [Google Scholar]
- 14.Hodgkinson CA, Goldman D, Jaeger J, et al. Disrupted in schizophrenia 1 (DISC1): association with schizophrenia, schizoaffective disorder, and bipolar disorder. Am J Hum Genet. 2004;75:862–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stefansson H, Sigurdsson E, Steinthorsdottir V, et al. Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet. 2002;71:877–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams NM, Preece A, Spurlock G, et al. Support for genetic variation in neuregulin 1 and susceptibility to schizophrenia. Mol Psychiatry. 2003;8:485–487. [DOI] [PubMed] [Google Scholar]
- 17.Addington AM, Gornick MC, Shaw P, et al. Neuregulin 1 (8p12) and childhood-onset schizophrenia: susceptibility haplotypes for diagnosis and brain developmental trajectories. Mol Psychiatry. 2007;12:195–205. [DOI] [PubMed] [Google Scholar]
- 18.Georgieva L, Dimitrova A, Ivanov D, et al. Support for neuregulin 1 as a susceptibility gene for bipolar disorder and schizophrenia. Biol Psychiatry. 2008;64:419–427. [DOI] [PubMed] [Google Scholar]
- 19.Krebs MO, Guillin O, Bourdell MC, et al. Brain derived neurotrophic factor (BDNF) gene variants association with age at onset and therapeutic response in schizophrenia. Mol Psychiatry. 2000;5:558–562. [DOI] [PubMed] [Google Scholar]
- 20.Muglia P, Vicente AM, Verga M, et al. Association between the BDNF gene and schizophrenia. Mol Psychiatry. 2003;8:146–147. [DOI] [PubMed] [Google Scholar]
- 21.Purcell SM, Wray NR, Stone JL, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jia P, Wang L, Fanous AH, et al. A bias-reducing pathway enrichment analysis of genome-wide association data confirmed association of the MHC region with schizophrenia. J Med Genet. 2012;49:96–103. [DOI] [PubMed] [Google Scholar]
- 23.Jia P, Sun J, Guo AY, Zhao Z. SZGR: a comprehensive schizophrenia gene resource. Mol Psychiatry. 2010;15:453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ripke S, O’Dushlaine C, Chambert K, et al. Genome-wide association analysis identifies 13 new risk loci for schizophrenia. Nat Genet. 2013;45:1150–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Donovan MC, Craddock N, Norton N, et al. Identification of loci associated with schizophrenia by genome-wide association and follow-up. Nat Genet. 2008;40:1053–1055. [DOI] [PubMed] [Google Scholar]
- 26.Rietschel M, Mattheisen M, Degenhardt F, et al. Association between genetic variation in a region on chromosome 11 and schizophrenia in large samples from Europe. Mol Psychiatry. 2012;17:906–917. [DOI] [PubMed] [Google Scholar]
- 27.Shi Y, Li Z, Xu Q, et al. Common variants on 8p12 and 1q24.2 confer risk of schizophrenia. Nat Genet. 2011;43:1224–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yue WH, Wang HF, Sun LD, et al. Genome-wide association study identifies a susceptibility locus for schizophrenia in Han Chinese at 11p11.2. Nat Genet. 2011;43:1228–1231. [DOI] [PubMed] [Google Scholar]
- 29.Stefansson H, Ophoff RA, Steinberg S, et al. Common variants conferring risk of schizophrenia. Nature. 2009;460:744–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steinberg S, de Jong S, Andreassen OA, et al. Common variants at VRK2 and TCF4 conferring risk of schizophrenia. Hum Mol Genet. 2011;20:4076–4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aberg KA, Liu Y, Bukszár J, et al. A comprehensive family-based replication study of schizophrenia genes. JAMA Psychiatry. 2013;70:573–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Viechtbauer W. Conducting Meta-Analyses in R with the metafor Package. J Stat Softw. 2010;36. [Google Scholar]
- 34.Abecasis GR, Altshuler D, Auton A, et al. A map of human genome variation from population-scale sequencing. Nature. 2010;467:1061–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. [DOI] [PubMed] [Google Scholar]
- 36.Benita Y, Cao Z, Giallourakis C, et al. Gene enrichment profiles reveal T-cell development, differentiation, and lineage-specific transcription factors including ZBTB25 as a novel NF-AT repressor. Blood. 2010;115:5376–5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.The GTEx Consortium. The Genotype-Tissue Expression (GTEx) project. Nat Genet. 2013;45:580–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Colantuoni C, Lipska BK, Ye T, et al. Temporal dynamics and genetic control of transcription in the human prefrontal cortex. Nature. 2011;478:519–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao Z, Xu J, Chen J, et al. Transcriptome sequencing and genome-wide association analyses reveal lysosomal function and actin cytoskeleton remodeling in schizophrenia and bipolar disorder [published online ahead of print August 12, 2014]. Mol Psychiatry. doi: 10.1038/mp.2014.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pickrell JK, Coop G, Novembre J, et al. Signals of recent positive selection in a worldwide sample of human populations. Genome Res. 2009;19:826–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boyle AP, Hong EL, Hariharan M, et al. Annotation of functional variation in personal genomes using RegulomeDB. Genome Res. 2012;22:1790–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.The ENCODE Project Consortium*. An integrated encyclopedia of DNA elements in the human genome. Nature. 489:57–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guo AY, Sun J, Riley BP, et al. The dystrobrevin-binding protein 1 gene: features and networks. Mol Psychiatry. 2009;14:18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oti M, Snel B, Huynen MA, Brunner HG. Predicting disease genes using protein-protein interactions. J Med Genet. 2006;43:691–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oti M, Brunner HG. The modular nature of genetic diseases. Clin Genet. 2007;71:1–11. [DOI] [PubMed] [Google Scholar]
- 46.Moreau Y, Tranchevent LC. Computational tools for prioritizing candidate genes: boosting disease gene discovery. Nat Rev Genet. 2012;13:523–536. [DOI] [PubMed] [Google Scholar]
- 47.Jia P, Zhao Z. Network.assisted analysis to prioritize GWAS results: principles, methods and perspectives. Hum Genet. 2014;133:125–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luo X, Huang L, Jia P, et al. Protein-protein interaction and pathway analyses of top schizophrenia genes reveal schizophrenia susceptibility genes converge on common molecular networks and enrichment of nucleosome (chromatin) assembly genes in schizophrenia susceptibility loci. Schizophr Bull. 2014;40:39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Allen NC, Bagade S, McQueen MB, et al. Systematic meta-analyses and field synopsis of genetic association studies in schizophrenia: the SzGene database. Nat Genet. 2008;40:827–834. [DOI] [PubMed] [Google Scholar]
- 50.Lewis CM, Levinson DF, Wise LH, et al. Genome scan meta-analysis of schizophrenia and bipolar disorder, part II: Schizophrenia. Am J Hum Genet. 2003;73:34–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ng MY, Levinson DF, Faraone SV, et al. Meta-analysis of 32 genome-wide linkage studies of schizophrenia. Mol Psychiatry. 2009;14:774–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ayalew M, Le-Niculescu H, Levey DF, et al. Convergent functional genomics of schizophrenia: from comprehensive understanding to genetic risk prediction. Mol Psychiatry. 2012;17:887–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lage K, Hansen NT, Karlberg EO, et al. A large-scale analysis of tissue-specific pathology and gene expression of human disease genes and complexes. Proc Natl Acad Sci USA. 2008;105:20870–20875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lage K, Karlberg EO, Størling ZM, et al. A human phenome-interactome network of protein complexes implicated in genetic disorders. Nat Biotechnol. 2007;25:309–316. [DOI] [PubMed] [Google Scholar]
- 55.Shannon P, Markiel A, Ozier O, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rossin EJ, Lage K, Raychaudhuri S, et al. Proteins encoded in genomic regions associated with immune-mediated disease physically interact and suggest underlying biology. PLoS Genet. 2011;7:e1001273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Abecasis GR, Auton A, Brooks LD, et al. An integrated map of genetic variation from 1,092 human genomes. Nature. 2012;491:56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li M, Luo XJ, Xiao X, et al. Allelic differences between Han Chinese and Europeans for functional variants in ZNF804A and their association with schizophrenia. Am J Psychiatry. 2011;168:1318–1325. [DOI] [PubMed] [Google Scholar]
- 59.Li M, Luo XJ, Rietschel M, et al. Allelic differences between Europeans and Chinese for CREB1 SNPs and their implications in gene expression regulation, hippocampal structure and function, and bipolar disorder susceptibility. Mol Psychiatry. 2014;19:452–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Luo XJ, Huang L, Li M, Gan L. Protein-protein interaction analysis reveals common molecular processes/pathways that contribute to risk of schizophrenia. Schizophr Res. 2013;143:390–392. [DOI] [PubMed] [Google Scholar]
- 61.Emamian ES, Hall D, Birnbaum MJ, Karayiorgou M, Gogos JA. Convergent evidence for impaired AKT1-GSK3beta signaling in schizophrenia. Nat Genet. 2004;36:131–137. [DOI] [PubMed] [Google Scholar]
- 62.Blasi G, Napolitano F, Ursini G, et al. Association of GSK-3β genetic variation with GSK-3β expression, prefrontal cortical thickness, prefrontal physiology, and schizophrenia. Am J Psychiatry. 2013;170:868–876. [DOI] [PubMed] [Google Scholar]
- 63.Li M, Mo Y, Luo XJ, et al. Genetic association and identification of a functional SNP at GSK3β for schizophrenia susceptibility. Schizophr Res. 2011;133:165–171. [DOI] [PubMed] [Google Scholar]
- 64.Kozlovsky N, Belmaker RH, Agam G. Low GSK-3beta immunoreactivity in postmortem frontal cortex of schizophrenic patients. Am J Psychiatry. 2000;157:831–833. [DOI] [PubMed] [Google Scholar]
- 65.Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci USA. 1996;93:8455–8459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miller FT, Libman H. Lithium carbonate in the treatment of schizophrenia and schizo-affective disorder: review and hypothesis. Biol Psychiatry. 1979;14:705–710. [PubMed] [Google Scholar]
- 67.Delva NJ, Letemendia FJ. Lithium treatment in schizophrenia and schizo-affective disorders. Br J Psychiatry. 1982;141:387–400. [DOI] [PubMed] [Google Scholar]
- 68.Jiang H, Guo W, Liang X, Rao Y. Both the establishment and the maintenance of neuronal polarity require active mechanisms: critical roles of GSK-3beta and its upstream regulators. Cell. 2005;120:123–135. [DOI] [PubMed] [Google Scholar]
- 69.Hur EM, Zhou FQ. GSK3 signalling in neural development. Nat Rev Neurosci. 2010;11:539–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grimes CA, Jope RS. The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Prog Neurobiol. 2001;65:391–426. [DOI] [PubMed] [Google Scholar]
- 71.Kalita K, Kuzniewska B, Kaczmarek L. MKLs: co-factors of serum response factor (SRF) in neuronal responses. Int J Biochem Cell Biol. 2012;44:1444–1447. [DOI] [PubMed] [Google Scholar]
- 72.Alberti S, Krause SM, Kretz O, et al. Neuronal migration in the murine rostral migratory stream requires serum response factor. Proc Natl Acad Sci USA. 2005;102:6148–6153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Knöll B, Nordheim A. Functional versatility of transcription factors in the nervous system: the SRF paradigm. Trends Neurosci. 2009;32:432–442. [DOI] [PubMed] [Google Scholar]
- 74.Mokalled MH, Johnson A, Kim Y, Oh J, Olson EN. Myocardin-related transcription factors regulate the Cdk5/Pctaire1 kinase cascade to control neurite outgrowth, neuronal migration and brain development. Development. 2010;137:2365–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Knöll B, Kretz O, Fiedler C, et al. Serum response factor controls neuronal circuit assembly in the hippocampus. Nat Neurosci. 2006;9:195–204. [DOI] [PubMed] [Google Scholar]
- 76.Stritt C, Knöll B. Serum response factor regulates hippocampal lamination and dendrite development and is connected with reelin signaling. Mol Cell Biol. 2010;30:1828–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Etkin A, Alarcón JM, Weisberg SP, et al. A role in learning for SRF: deletion in the adult forebrain disrupts LTD and the formation of an immediate memory of a novel context. Neuron. 2006;50:127–143. [DOI] [PubMed] [Google Scholar]
- 78.Wickramasinghe SR, Alvania RS, Ramanan N, et al. Serum response factor mediates NGF-dependent target innervation by embryonic DRG sensory neurons. Neuron. 2008;58:532–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lu PP, Ramanan N. Serum response factor is required for cortical axon growth but is dispensable for neurogenesis and neocortical lamination. J Neurosci. 2011;31:16651–16664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stern S, Haverkamp S, Sinske D, et al. The transcription factor serum response factor stimulates axon regeneration through cytoplasmic localization and cofilin interaction. J Neurosci. 2013;33:18836–18848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ishikawa M, Shiota J, Ishibashi Y, et al. Identification, expression and characterization of rat isoforms of the serum response factor (SRF) coactivator MKL1. FEBS Open Bio. 2013;3:387–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kalita K, Kharebava G, Zheng JJ, Hetman M. Role of megakaryoblastic acute leukemia-1 in ERK1/2-dependent stimulation of serum response factor-driven transcription by BDNF or increased synaptic activity. J Neurosci. 2006;26:10020–10032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.O’Sullivan NC, Pickering M, Di Giacomo D, Loscher JS, Murphy KJ. Mkl transcription cofactors regulate structural plasticity in hippocampal neurons. Cereb Cortex. 2010;20:1915–1925. [DOI] [PubMed] [Google Scholar]
- 84.Ishikawa M, Nishijima N, Shiota J, et al. Involvement of the serum response factor coactivator megakaryoblastic leukemia (MKL) in the activin-regulated dendritic complexity of rat cortical neurons. J Biol Chem. 2010;285:32734–32743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Angelucci F, Brenè S, Mathé AA. BDNF in schizophrenia, depression and corresponding animal models. Mol Psychiatry. 2005;10:345–352. [DOI] [PubMed] [Google Scholar]
- 86.Qian L, Zhao J, Shi Y, et al. Brain-derived neurotrophic factor and risk of schizophrenia: an association study and meta-analysis. Biochem Biophys Res Commun. 2007;353:738–743. [DOI] [PubMed] [Google Scholar]
- 87.Ho BC, Andreasen NC, Dawson JD, Wassink TH. Association between brain-derived neurotrophic factor Val66Met gene polymorphism and progressive brain volume changes in schizophrenia. Am J Psychiatry. 2007;164:1890–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fatemi SH, Folsom TD. The neurodevelopmental hypothesis of schizophrenia, revisited. Schizophr Bull. 2009;35:528–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rapoport JL, Giedd JN, Gogtay N. Neurodevelopmental model of schizophrenia: update 2012. Mol Psychiatry. 2012;17:1228–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Owen MJ, O’Donovan MC, Thapar A, Craddock N. Neurodevelopmental hypothesis of schizophrenia. Br J Psychiatry. 2011;198:173–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Piper M, Beneyto M, Burne TH, et al. The neurodevelopmental hypothesis of schizophrenia: convergent clues from epidemiology and neuropathology. Psychiatr Clin North Am. 2012;35:571–584. [DOI] [PubMed] [Google Scholar]
- 92.McGrath JJ, Féron FP, Burne TH, Mackay-Sim A, Eyles DW. The neurodevelopmental hypothesis of schizophrenia: a review of recent developments. Ann Med. 2003;35:86–93. [DOI] [PubMed] [Google Scholar]
- 93.Mjellem N, Kringlen E. Schizophrenia: a review, with emphasis on the neurodevelopmental hypothesis. Nord J Psychiatry. 2001;55:301–309. [DOI] [PubMed] [Google Scholar]
- 94.Kozlovsky N, Belmaker RH, Agam G. GSK-3 and the neurodevelopmental hypothesis of schizophrenia. Eur Neuropsychopharmacol. 2002;12:13–25. [DOI] [PubMed] [Google Scholar]
- 95.Bassett AS, Chow EW, O’Neill S, Brzustowicz LM. Genetic insights into the neurodevelopmental hypothesis of schizophrenia. Schizophr Bull. 2001;27:417–430. [DOI] [PubMed] [Google Scholar]
- 96.Marenco S, Weinberger DR. The neurodevelopmental hypothesis of schizophrenia: following a trail of evidence from cradle to grave. Dev Psychopathol. 2000;12:501–527. [DOI] [PubMed] [Google Scholar]
- 97.Lafargue T, Brasic J. Neurodevelopmental hypothesis of schizophrenia: a central sensory disturbance. Med Hypotheses. 2000;55:314–318. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.