Abstract
Background: It is generally believed that long-term use of antipsychotics increases mortality and, especially, the risk of cardiovascular death. However, there are no solid data to substantiate this view. Methods: We identified all individuals in Sweden with schizophrenia diagnoses before year 2006 (N = 21 492), aged 17–65 years, and persons with first-episode schizophrenia during the follow-up 2006–2010 (N = 1230). Patient information was prospectively collected through nationwide registers. Total and cause-specific mortalities were calculated as a function of cumulative antipsychotic exposure from January 2006 to December 2010. Results: Compared with age- and gender-matched controls from the general population (N = 214920), the highest overall mortality was observed among patients with no antipsychotic exposure (hazard ratio [HR] = 6.3, 95% CI: 5.5–7.3), ie, 0.0 defined daily dose (DDD)/day, followed by high exposure (>1.5 DDD/day) group (HR = 5.7, 5.2–6.2), low exposure (<0.5 DDD/day) group (HR = 4.1, 3.6–4.6), and moderate exposure (0.5–1.5 DDD/day) group (HR = 4.0, 3.7–4.4). High exposure (HR = 8.5, 7.3–9.8) and no exposure (HR = 7.6, 5.8–9.9) were associated with higher cardiovascular mortality than either low exposure (HR = 4.7, 3.7–6.0) or moderate exposure (HR = 5.6, 4.8–6.6). The highest excess overall mortality was observed among first-episode patients with no antipsychotic use (HR = 9.9, 5.9–16.6). Conclusions: Among patients with schizophrenia, the cumulative antipsychotic exposure displays a U-shaped curve for overall mortality, revealing the highest risk of death among those patients with no antipsychotic use. These results indicate that both excess overall and cardiovascular mortality in schizophrenia is attributable to other factors than antipsychotic treatment when used in adequate dosages.
Key words: schizophrenia, mortality, antipsychotic
Introduction
Patients with schizophrenia have about 10 to 20 years shorter life expectancy than the general population,1,2 but the reasons for this are not known well. It is generally believed that excess mortality is largely attributable to adverse effects of antipsychotics such as weight gain, leading to metabolic syndrome, diabetes, and ischemic heart disease. A recent pharmacoepidemiological study observed a dose-related risk of sudden cardiac death among individuals using antipsychotics.3 A systematic review suggested that long-term exposure to antipsychotics may be associated with increased mortality, while concluding that more rigorously designed, prospective studies are urgently needed.4 In fact, the majority of previous research thus far has lacked sufficient methodology to draw firm conclusions. To our knowledge, there are only 5 studies that have used large, representative, and prospectively collected datasets of actual filled prescriptions to calculate the risk of death associated with any (at least one prescription),5 current1,6–8 or cumulative1 antipsychotic exposure. All of these studies have suggested that the use of an antipsychotic is associated with a lower mortality than no use of an antipsychotic. Two of these studies investigated first-episode cohorts,6,8 which does not allow for results be generalized to older patient populations, and the study by Baandrup et al7 investigated only deaths due to natural causes. The only previous study on cumulative exposure did not investigate dosages but the proportion of time on antipsychotic treatment,1 and there are no published data on the putative net loss or gain vs cumulative antipsychotic dose in terms of mortality. Another crucially important issue is survival bias, which no study on cumulative antipsychotic exposure has addressed this far. Therefore, the putative role of long-term antipsychotic treatment in the excess mortality of patients with schizophrenia has remained unclear, and it is currently unknown how much schizophrenia per se contributes to the risk of death.
This study aimed to investigate the possible excess mortality in a nationwide cohort of patients with schizophrenia, as related to the degree of cumulative antipsychotic exposure indicated with filled prescriptions. We also aimed to study the putative effect of survival bias by conducting a sensitivity analysis among first-episode patients.
Methods
We conducted a prospective population-based cohort study of patients with schizophrenia by using nationwide register data. This research project was approved by the Regional Ethics Board of Stockholm (Decision 20071762-31).
Study Population
The cohort participants were identified from 2 nationwide health-care registers of 7 040 632 people aged 17–65 who lived in Sweden during 2005, according to Statistics Sweden. In Sweden, all individuals have their unique personal identity number, which makes it possible to track and identify each individual in all databases. Social security number is unique, and thus no person is counted twice. One of the 2 registers used was the National Patient Register, which is kept by the National Board of Health and Welfare, and contains information on all inpatient care since 1988 and visits to specialized outpatient units in Sweden since 2001 (only larger cities having specific units for psychosis patients). From that register, we selected patients who had any health care due to any psychosis (International Classification of Diseases [ICD]-10 codes F20-F29) before January 1, 2006 (N = 17 919), and who had received a diagnosis of schizophrenia (ICD-10 code F20) by the end of follow-up (December 31, 2010). The other database used was the MiDAS register, which is kept by the Social Insurance Agency of Sweden, and contains information on people granted a disability pension. From that register, all people on a disability pension due to schizophrenia (ICD-10 code F20) before January 1, 2006 were included. Altogether, 21 492 individuals were identified (corresponding to prevalence of 0.34%). The follow-up began on January 1, 2006 and ended on December 31, 2010. Thus, the follow-up time was 5 years for this patient cohort.
In order to avoid survival bias, we also conducted an analysis in a cohort of first-episode patients (N = 1230). This open cohort included all individuals in Sweden, who were between 18 and 59 years of age when entering the cohort, and who had their first-episode psychosis between 2006 and 2009 and who had also received a diagnosis of schizophrenia (in hospital treatment or outpatient treatment) by the end 2010. For these analyses, follow-up began at the date of discharge from a hospital or at the date of first psychosis diagnosis or, if the person was admitted to hospital within the first month after the first psychosis diagnosis, at the date of discharge from the hospital. In total, 2852 individuals were identified for the first-episode cohort. The individuals with disability pension due to psychosis before the beginning of follow-up were excluded (n = 501; those patients had not been in treatment contact between 1988 and 2005). We also excluded individuals who had antipsychotic medication use within 6 months before the first episode (n = 1121). After exclusion criteria, the total sample consisted of 1230 individuals.
The mortality in persons with schizophrenia was compared with a control sample from the general population. For each person with schizophrenia, we identified 10 age- and sex-matched persons without schizophrenia. The control group for persons who had received a diagnosis of schizophrenia before 2006 consisted of 214 670 persons, and for first-episode schizophrenia the control sample was 12 110 individuals. Concerning the first-episode cohort, the follow-up of the controls started on the same day as the follow-up of each patient.
Each resident in Sweden has a unique personal identity number, which enables linking to nationwide registers. Death certificates are written generally by ordinary physicians, and by forensic specialist, if death is sudden or crime is suspected. The documented causes of death are considered fairly reliable.9
Mortality and Covariates
Information on all prescribed and dispensed drugs from a pharmacy in Sweden was obtained from the Prescribed Drug Register, held by the National Board of Health and Welfare. We selected all antipsychotics dispensed in 2006–2010, except lithium, according to the Anatomical Therapeutic Chemical (ATC)10 code group N05A. The cumulative exposure of antipsychotics was estimated by using the defined daily dose (DDD, see ref for definition of DDD for each antipsychotic).10 First, we calculated the sum of dispensed medication as DDD. Next we divided the sum by the length of follow-up in days, of which the days spent in a hospital were subtracted, because antipsychotic medications that may be used in hospitals are not recorded in the Prescribed Drug Register. The identified schizophrenia patients were categorized into 4 DDD groups; (1) no antipsychotics during the follow-up, (2) small doses of antipsychotics or occasional use (0 DDD/day–0.5 DDD/day, noninclusive), (3) moderate doses of antipsychotics (0.5 DDD/day–1.5 DDD/day, inclusive), and (4) high antipsychotic doses (>1.5 DDD/day).
Date and cause of death were obtained from the Causes of Death Register, which is held by the National Board of Health and Welfare. The following specific causes of death were investigated in addition to overall mortality: cardiovascular diseases (ICD codes I00-I99), neoplasms (ICD codes C00-D48), respiratory diseases (ICD codes J00-J99), or suicide (ICD codes X60-X84).
Demographic characteristics were obtained from the LISA register held by Statistics Sweden. To study the effects of clinical and sociodemographic characteristics, patients with schizophrenia were categorized as receiving outpatient treatment for psychosis, inpatient treatment for psychosis earlier than 2005, inpatient treatment for psychosis in 2005, and as people identified only from disability pension due to schizophrenia. In addition, the association of mortality with disease duration was assessed by calculating the time from the first diagnosis of psychosis in the registers until the start of follow-up. The association of mortality with having a disability pension was studied by whether a person had been granted disability pension in year 2005.
Statistical Analyses
Cox-regression analysis was used to compare overall mortality and mortalities due to specific causes in different DDD groups with the control sample. Because controls and patients were matched according to age and sex, no covariates were used in the models.
Next, Cox-regression analyses were used for comparing the DDD groups in the cohort of patients with first episode before 2006. Initially, we checked to see if the DDD group × demographic characteristic, or DDD group × disease characteristic interactions were statistically significant. Nonsignificant interactions were removed from the analysis. Due to statistically significant interactions, the analyses were also done separately in men and women and in the 4 different treatment groups. Since no information was available on the use of medication during the hospital treatment days, these analyses were repeated by selecting individuals who spent less than 20% of follow-up time in a hospital (to exclude the possibility that medication use during long hospital treatment would substantially modify the results).
Results were confirmed in the first-episode sample. First, age × DDD group and sex × DDD group interactions were investigated, and nonsignificant interactions were removed from the analysis. The resulting small sample size limited the number of covariates and only sex and age were used as covariates. Statistical analysis of data was performed with the R-program version 2.6.2.
Results
Demographic and clinical characteristics are shown in table 1. Individuals with a high antipsychotic load had less outpatient care, a lower education level and were more often living alone and had received more hospital treatment than the other groups. We found no differences due to age or gender. Altogether, 1591 (7%) of the patients died during the 5-year follow-up in the sample of persons with schizophrenia diagnosed before year 2006. The corresponding figure among controls was 3438 (1.6%), resulting in an hazard ratio (HR) of 4.8 (95% CI: 4.5–5.1) for schizophrenia. Of persons with first-episode schizophrenia, 45 (4%) died during the follow-up.
Table 1.
Demographic and Clinical Characteristics of Patients
| Total | No Antipsychotic Exposure | Low Antipsychotic Exposure | Moderate Antipsychotic Exposure | High Antipsychotic Exposure | |
|---|---|---|---|---|---|
| n (%) | 21492 | 2077 (10%) | 4110 (19%) | 8468 (39%) | 6837 (32%) |
| Gender | |||||
| Female, n (%) | 8441 (39%) | 696 (34%) | 1780 (43%) | 3472 (41%) | 2494 (36%) |
| Male, n (%) | 13050 (61%) | 1381 (66%) | 2330 (57%) | 4996 (59%) | 4343 (64%) |
| Age, M (SD) | 45.5 (11.1) | 45.3 (11.5) | 46.0 (11.4) | 45.4 (11.2) | 45.2 (10.8) |
| Educational level, n (%) | |||||
| Low (≤9 years) | 7457 (35%) | 640 (31%) | 1224 (30%) | 2809 (33%) | 2784 (41%) |
| Medium (10–12 years) | 11257 (54%) | 1090 (52%) | 2220 (54%) | 4551 (54%) | 3396 (50%) |
| High (≥12 years) | 2310 (11%) | 284 (14%) | 583 (14%) | 963 (11%) | 481 (7%) |
| Information missing | 467 (2%) | 63 (3%) | 83 (2%) | 145 (2%) | 176 (3%) |
| Marital status, n (%) | |||||
| Married or living with a partner | 2782 (13%) | 349 (18%) | 701 (17%) | 1144 (14%) | 588 (9%) |
| Previous treatment contact | |||||
| Outpatient care due to psychosis | 3799 (18%) | 347 (17%) | 902 (22%) | 1665 (20%) | 885 (13%) |
| Previous inpatient care due to psychosis | 11294 (53%) | 946 (46%) | 2042 (50%) | 4544 (54%) | 3763 (55%) |
| In hospital during 2005 due to psychosis | 4267 (20%) | 176 (9%) | 641 (13%) | 1578 (19%) | 1872 (27%) |
| Identified only through disability pension due to schizophrenia | 2131 (10%) | 608 (30%) | 525 (13%) | 681 (8%) | 317 (5%) |
| Months since the first psychosis in the beginning of follow-up | 70.3 (38.4) | 72.8 (40.4) | 64.4 (38.7) | 68.3 (38.0) | 75.5 (38.0) |
| Receiving disability pension in 2005 | 18716 (93%) | 1625 (92%) | 3317 (91%) | 7383 (92%) | 6392 (95%) |
| First-episode patients | |||||
| n (%) | 1230 | 232 (19) | 329 (27) | 329 (27) | 340 (28) |
| Gender | |||||
| Male, n (%) | 806 (66%) | 154 (66%) | 213 (65%) | 201 (61%) | 238 (70%) |
| Female, n (%) | 424 (34%) | 78 (34%) | 116 (35%) | 128 (39%) | 102 (30%) |
| Age, M (SD) | 36.3 (11.7) | 40.2 (12.1) | 36.8 (11.5) | 35.6 (11.4) | 33.8 (11.3) |
Note: The data on chronic patients applies to December 31, 2005, and on first-episode patients, the time when entering into the cohort.
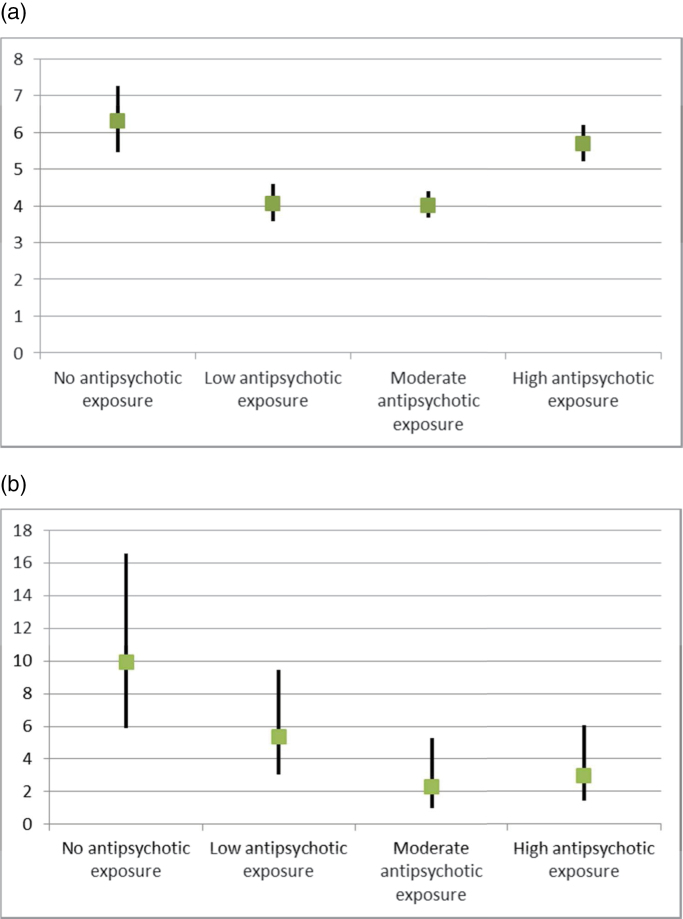
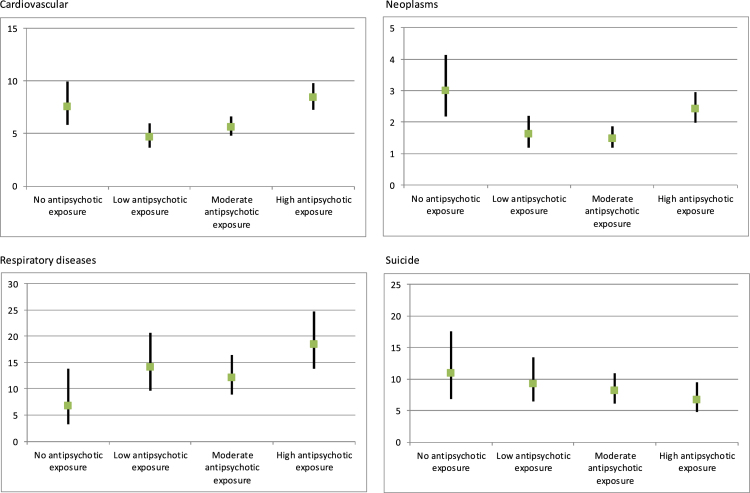
The mortality rates for different antipsychotic exposure groups are shown in table 2. The highest overall mortality is seen in patient group with no antipsychotic purchases, both in the chronic and in the first-episode groups. For patients who were hospitalized less than 20% of follow-up time, the results were similar (ie, no antipsychotic use HR = 5.5, 95% CI = 4.7–6.4; low antipsychotic use HR = 3.9, 95% CI = 3.4–4.4; moderate antipsychotic use HR = 3.9, 95% CI = 3.6–4.3; high antipsychotic use HR = 5.4, 95% CI = 4.9–5.9). Overall, cardiovascular, and cancer mortalities displayed U-shaped curves; ie, low and moderate antipsychotic exposure were associated with substantially lower risk of death than either no use or high exposure (figures 1 and 2). Respiratory disease showed a different kind of pattern, in which high-dose exposure was associated with the highest and no exposure to the lowest mortality. Suicide mortality showed a dose-response curve; ie, the higher the antipsychotic exposure, the lower the suicide risk.
Table 2.
Cox-Regression Model of Mortality Among Patients Compared With Matched Controls From the General Population
| Number of Deaths (%) | HR | 95% CI | |
|---|---|---|---|
| All cause | |||
| Chronic patients | 1591 (7.4%) | ||
| No antipsychotic exposure | 199 (9.6%) | 6.30 | 5.46–7.27 |
| Low antipsychotic exposure | 261 (6.3%) | 4.06 | 3.58–4.60 |
| Moderate antipsychotic exposure | 532 (6.3%) | 4.02 | 3.67–4.41 |
| High antipsychotic exposure | 599 (8.8%) | 5.68 | 5.21–6.20 |
| First-episode patients | 45 (3.7%) | ||
| No antipsychotic exposure | 17 (7.3%) | 9.90 | 5.91–16.59 |
| Low antipsychotic exposure | 14 (4.3%) | 5.39 | 3.08–9.45 |
| Moderate antipsychotic exposure | 6 (1.8%) | 2.31 | 1.01–5.27 |
| High antipsychotic exposure | 8 (2.4%) | 2.96 | 1.44–6.08 |
| Specific causes (chronic patients) | |||
| Cardiovascular | 520 (2.4%) | ||
| No antipsychotic exposure | 57 (2.7%) | 7.59 | 5.80–9.92 |
| Low antipsychotic exposure | 72 (1.8%) | 4.68 | 3.68–5.96 |
| Moderate antipsychotic exposure | 178 (2.1%) | 5.63 | 4.79–6.62 |
| High antipsychotic exposure | 213 (3.1%) | 8.45 | 7.27–9.83 |
| Neoplasms | 262 (1.2%) | ||
| No antipsychotic exposure | 38 (1.8%) | 2.99 | 2.17–4.13 |
| Low antipsychotic exposure | 42 (1.0%) | 1.62 | 1.19–2.20 |
| Moderate antipsychotic exposure | 79 (0.9%) | 1.48 | 1.18–1.86 |
| High antipsychotic exposure | 103 (1.5%) | 2.42 | 1.98–2.96 |
| Respiratory diseases | 175 (0.8%) | ||
| No antipsychotic exposure | 8 (0.4%) | 6.79 | 3.33–13.87 |
| Low antipsychotic exposure | 34 (0.8%) | 14.10 | 9.67–20.58 |
| Moderate antipsychotic exposure | 60 (0.7%) | 12.10 | 8.91–16.43 |
| High antipsychotic exposure | 73 (1.1%) | 18.49 | 13.87–24.63 |
| Suicide | 151 (0.7%) | ||
| No antipsychotic exposure | 19 (0.9%) | 10.92 | 6.82–17.51 |
| Low antipsychotic exposure | 33 (0.8%) | 9.27 | 6.41–13.42 |
| Moderate antipsychotic exposure | 60 (0.7%) | 8.19 | 6.13–10.95 |
| High antipsychotic exposure | 39 (0.5%) | 6.68 | 4.73–9.43 |
Note: Since control subjects were matched, no additional adjustment was done for hazard ratios (HRs).
Fig. 1.
(a) Overall mortality as hazard ratios in the chronic patient population (N = 21492) compared with mortality in the control sample. (b) Overall mortality as hazard ratios in first-episode patients (N = 1230) compared with mortality in the control sample.
Fig. 2.
Mortality expressed as hazard ratios due to specific causes in patients with schizophrenia compared with the control sample.
We conducted a secondary analysis within the patient population, to determine if demographics or clinical characteristics influenced the association between medication use and mortality (table 3). The DDD group × sex (β = .40, P = .005, HR = 1.49, 1.13–1.97) interaction was statistically significant between the moderate use group and the high use group, indicating that a high antipsychotic dosage was associated with a higher mortality in female than in male patients. In addition, DDD group (no medication vs moderate use) × previous care interaction (for inpatient treatment within 1 year vs outpatient treatment within 1 year) was statistically significant (β = 1.08, P = .03, HR = 2.94, 1.13–7.63), indicating that no medication use was associated with elevated mortality in patients who had been hospitalized within 1 year prior to start of follow-up (ie, during year 2005), but not in patients who had never had inpatient treatment. Because of statistically significant interactions, Cox regressions were used to stratify the sample by sex and in groups based on previous treatment contact. For both sexes, mortality was again higher in patients who had not used antipsychotics and in patients with high antipsychotic exposure. In all groups, based on previous treatment contact, high antipsychotic use was associated with higher mortality, and no antipsychotic use was associated with higher mortality in patients with inpatient treatment when compared with those having moderate cumulative antipsychotic exposure.
Table 3.
Number and Percentage of Deaths and Hazard Ratios (HRs) in the 4 DDD Groups Within Patients With Schizophrenia, in Separate Cox-Regression Analyses for Men and Women, and for Groups Based on Previous Treatment Contact
| No Antipsychotic Exposure | Low Antipsychotic Exposure | Moderate Antipsychotic Exposure | High Antipsychotic Exposure | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | HR | 95% CI | n | % | HR | 95% CI | n | % | HR | n | % | HR | 95% CI | |
| ALLa | 199 | 10 | 1.56 | 1.32–1.84 | 261 | 6 | 0.97 | 0.83–1.12 | 532 | 6 | 1 | 599 | 9 | 1.43 | 1.27–1.60 |
| (1.52 | 1.29–1.79) | (0.96 | 0.83–1.12) | (1.45 | 1.29–1.63) | ||||||||||
| Sexb | |||||||||||||||
| Men | 140 | 10 | 1.46 | 1.20–1.78 | 167 | 7 | 1.01 | 0.82–1.19 | 433 | 7 | 1 | 382 | 9 | 1.28 | 1.11–1.48 |
| (1.52 | 1.25–1.85) | (1.04 | 0.87–1.25) | (1.29 | 1.12–1.50) | ||||||||||
| Women | 59 | 8 | 1.64 | 1.22–2.20 | 94 | 5 | 0.93 | 0.72–1.19 | 188 | 5 | 1 | 217 | 9 | 1.83 | 1.51–2.23 |
| (1.61 | 1.20–2.16) | (0.97 | 0.76–1.24) | (1.63 | 1.34–1.98) | ||||||||||
| Previous treatment contactb | |||||||||||||||
| Outpatient treatment only | 23 | 7 | 1.44 | 0.91–2.27 | 46 | 5 | 0.90 | 0.63–1.29 | 91 | 5 | 1 | 72 | 8 | 1.50 | 1.10–2.05 |
| (1.24 | 0.78–1.95) | (0.93 | 0.65–1.32) | (1.51 | 1.11–2.05) | ||||||||||
| Earlier inpatient treatment | 95 | 10 | 1.69 | 1.35–2.14 | 128 | 6 | 0.90 | 0.73–1.11 | 299 | 7 | 1 | 334 | 9 | 1.41 | 1.21–1.65 |
| (1.58 | 1.25–1.99) | (0.95 | 0.77–1.17) | (1.37 | 1.17–1.60) | ||||||||||
| Inpatient treatment within 1 year | 34 | 19 | 3.46 | 2.34–5.12 | 37 | 6 | 0.94 | 0.65–1.38 | 95 | 6 | 1 | 150 | 8 | 1.36 | 1.05–1.76 |
| (3.62 | 2.45–5.35) | (0.96 | 0.66–1.40) | (1.34 | 1.04–1.74) | ||||||||||
| Identified only through disability pension due to schizophrenia | 47 | 8 | 1.06 | 0.71–1.59 | 50 | 10 | 1.32 | 0.88–1.96 | 47 | 7 | 1 | 43 | 14 | 2.19 | 1.45–3.31 |
| (1.13 | 0.75–1.69) | (1.40 | 0.94–2.08) | (2.03 | 1.34–3.08) | ||||||||||
Note: The identified schizophrenia patients were categorized into 4 DDD groups: (1) no antipsychotics during the follow-up, (2) small doses of antipsychotics or occasional use (0 DDD/day–0.5 DDD/day, noninclusive), (3) moderate doses of antipsychotics (0.5 DDD/day–1.5 DDD/day, inclusive), and (4) high antipsychotic doses (>1.5 DDD/day). Overall mortality (the uppermost row “ALL”) was significantly lower for moderate use when compared with no antipsychotic use (P < .001) or with high exposure (P < .001). The number of hospital days during follow-up was 20.8 for no antipsychotic exposure, 19.7 for low antipsychotic exposure, 16.5 for moderate antipsychotic exposure, and 26.6 for high antipsychotic exposure. DDD, defined daily dose; HR, hazard ratio.
aHRs are adjusted for age, gender, and treatment history (number of hospital days, number of outpatient contacts) and HRs adjusted for age and gender are shown in parentheses.
bHRs are adjusted for age and unadjusted HRs are shown in parentheses.
Results indicated with bold differ from the results for moderate antipsychotic exposure (P < .05).
In an analysis controlling for age and sex, first-episode schizophrenia patients who had not used antipsychotics had higher mortality when compared with individuals with moderate medication use (HR = 3.60, 1.40–9.23). Low exposure (HR = 2.20, 0.84–5.73) or high exposure (HR = 1.31, 0.45–3.77) were not significantly associated with mortality. The gender × DDD group interaction difference was not statistically significant.
Discussion
To our knowledge, this is the first study to investigate how cumulative exposure to antipsychotics affects the excess mortality seen in schizophrenia. The overall results of this 5-year nationwide follow-up study displayed a clear U-shaped curve, revealing the highest risk of death among patients with no antipsychotic use. Concerning cardiovascular mortality, the same pattern showed the highest mortality for high exposure to antipsychotic medications, but also no exposure was associated with a 35%–62% greater risk of cardiovascular death when compared with either the low or moderate exposure groups. Therefore, these results indicate that both excess overall and cardiovascular mortalities in schizophrenia are attributable to other factors than antipsychotic treatment when used in adequate doses.
When assessing mortality as a function of medication exposure, it is crucially important to take into account the survival bias; ie, those patients who continue to live when entering in the follow-up phase may be especially resistant to fatal adverse events of medications, which may bias the results. On the other hand, those patients who died because of a lack of antipsychotic treatment are also omitted, if they have died prior to the cohort entry date. Therefore, we conducted a sensitivity analysis among first-episode patients to avoid this survival bias. The results from this analysis revealed a 10-fold excess mortality among those patients not using any antipsychotic when compared with their matched controls. Since these are young individuals, premature deaths result into very large amount of life years lost. This indicates that a lack of antipsychotic treatment may be the most important treatment-related factor contributing to excess mortality in schizophrenia and calls for attention to improve adherence to antipsychotic treatment among these patients.
It has been observed that even low adherence to placebo is associated with poor health outcomes.11 This suggests that people who have not used antipsychotics may also have low adherence to other health guidance and medication. These patients may also suffer from alcoholism or other problems related to substance abuse, which may decrease patients’ use of antipsychotics. A total of 7% of the patients died during the 5-year follow-up. This relatively low figure is explained by the fact that patients older than 65 years were excluded from the study population.
The mean age of the first-episode patients was greater than 36 years, and the likely reason for this is a delay in setting of a diagnosis of schizophrenia as observed in other Nordic counties.8 This explanation is also supported by the fact that a large proportion of those patients who received their first time schizophrenia diagnoses during the study period had to be excluded because they had been treated with antipsychotics due to suspected psychosis before obtaining an official psychosis diagnosis. Therefore, it is possible that the 10-fold excess mortality among first-episode cohort is an underestimate, since many patients may have died before they had received schizophrenia diagnosis. This underlines the importance of setting the right diagnoses and starting of efficient treatment as early as possible.
Because the nature of this study is observational, the associations may not necessarily mean causality. The results concerning comparative mortality between different exposure groups did not change substantially when the clinical and sociodemographic characteristics were controlled in the secondary analysis within the patient population. However, it is not possible to fully adjust for the severity of the illness and lifestyle characteristics by using such databases, which do not include information on smoking or diet, for example. Disease may be more severe in patients with high medication use than in patients with moderate antipsychotic use, and therefore the higher mortality in this group may partially derive from disease severity. In fact, the HR for patients with high antipsychotic load decreased slightly (from 1.45 to 1.43 on the first row of the table 3), and the HR increased for patients with no antipsychotic use (from 1.52 to 1.56). This suggests that those patients with high antipsychotic load are more severely ill (HR decreased when previous treatment history is adjusted) and those with very few treatment contacts are less severely ill (HR increases when treatment history is adjusted). In addition to this kind of confounding by indication, also confounding by contraindication, ie, concerns about adverse effects of antipsychotics may lead to selection bias in the treatment of patients with schizophrenia. We were not able to investigate this issue, which is a limitation of our study. In addition, we were not able to study if patients actually took their medication which was dispensed. For example, it is possible that more severely ill patients use less medication than dispensed, whereas patients with milder illness may have better compliance. However, it is likely that only few patients would keep on picking up their medication time after time during the 5-year follow-up without using the previous package first. One putative limitation is that since all used DDDs of antipsychotic were summed together, it was not possible to study the interactions during concomitant use of several antipsychotics. However, this far, in all 3 studies using large databases and looking mortality during antipsychotic polypharmacy vs monotherapy, polypharmacy has been observed to be associated with slightly lower morality than monotherapy.7,8,12 Therefore, we may assume that higher mortality in high exposure group is not attributable to polypharmacy. The data on specific agents or routes of administration was not available, and if it had been, the statistical power would have been probably insufficient for multiple comparisons with our amount of patient years. Data on somatic morbidities or treatments were not available neither.
High antipsychotic use was associated with higher mortality due to natural causes; therefore, higher mortality among these patients may also be related to side effects of high antipsychotic load. Mortality risk increased more in women than in men with high antipsychotic exposure. This finding supports previous reports that suggest women require lower doses of antipsychotics and may also have a greater risk for side effects than men at higher dosage regimes.13–15 Concerning specific causes of death, the highest excess mortality was seen in respiratory diseases and, especially, among patients with high antipsychotic exposure. This is in line with a recent study suggesting that some antipsychotics may have a dose-related effect on the risk of death by pneumonia.16 The results from the study suggest that somatic health monitoring and access to health services17–19 should be improved, especially in patient populations with a high antipsychotic load.
Conclusions
Patients with low or moderate antipsychotic exposure have substantially lower overall and cardiovascular mortality than patients with no exposure or high exposure, which clearly indicates that both excess overall and cardiovascular mortality in schizophrenia is attributable to other factors than long-term antipsychotic treatment when used in adequate dosages. An alarmingly high excess mortality among first-episode patients who do not use any antipsychotics deserves more attention, in order to increase adherence to their prescribed pharmacological treatment. Despite the importance of nonadherence, clinicians spend too little time on addressing this issue.20 For example, long-acting antipsychotic injections (LAIs) are associated with about 60% lower all-cause discontinuation than corresponding oral formulations among first-episode patients.8 Thus, more use of LAIs might result in substantially lower excess mortality. Focusing more attention particularly on first-episode and recently hospitalized patients who are not using any antipsychotics, and on patients with antipsychotic doses higher than 1.5 DDD/day, is essentially important in the prevention of premature death in people with schizophrenia.
Funding
Karolinska Institutet, Stockholm, Sweden; Niuvanniemi Hospital, Kuopio, Finland; and the Sigrid Juselius Foundation, Finland, grant (129434). Funders had no role in the study design, in the collection, analysis, and interpretation of the data, in the writing of the report, and in the decision to submit the article for publication.
Acknowledgments
In the last 3 years J.T. reports serving as a consultant to Lundbeck, Organon, Janssen-Cilag, Eli Lilly, AstraZeneca, F. Hoffman-La Roche, and Bristol-Myers Squibb; he has also received fees for giving expert opinions to Bristol-Myers Squibb and GlaxoSmithKline, and lecture fees from Janssen-Cilag, Bristol-Myers Squibb, Eli Lilly, Pfizer, Lundbeck, GlaxoSmithKline, AstraZeneca, and Novartis; he is also a member on the advisory board of AstraZeneca, Janssen-Cilag, and Otsuka, and he has received a grant from the Stanley Foundation. All other authors report no financial relationships with commercial interests.
References
- 1.Tiihonen J, Lönnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374:620–627. [DOI] [PubMed] [Google Scholar]
- 2.Laursen TM, Wahlbeck K, Hällgren J, et al. Life expectancy and death by diseases of the circulatory system in patients with bipolar disorder or schizophrenia in the Nordic countries. PLoS One. 2013;8:e67133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ray WA, Chung CP, Murray KT, Hall K, Stein CM. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med. 2009;360:225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weinmann S, Read J, Aderhold V. Influence of antipsychotics on mortality in schizophrenia: systematic review. Schizophr Res. 2009;113:1–11. [DOI] [PubMed] [Google Scholar]
- 5.Crump C, Winkleby MA, Sundquist K, Sundquist J. Comorbidities and mortality in persons with schizophrenia: a Swedish national cohort study. Am J Psychiatry. 2013;170:324–333. [DOI] [PubMed] [Google Scholar]
- 6.Tiihonen J, Lönnqvist J, Wahlbeck K, Klaukka T, Tanskanen A, Haukka J. Antidepressants and the risk of suicide, attempted suicide, and overall mortality in a nationwide cohort. Arch Gen Psychiatry. 2006;63:1358–1367. [DOI] [PubMed] [Google Scholar]
- 7.Baandrup L, Gasse C, Jensen VD, et al. Antipsychotic polypharmacy and risk of death from natural causes in patients with schizophrenia: a population-based nested case-control study. J Clin Psychiatry. 2010;71:103–108. [DOI] [PubMed] [Google Scholar]
- 8.Tiihonen J, Haukka J, Taylor M, Haddad PM, Patel MX, Korhonen P. A nationwide cohort study of oral and depot antipsychotics after first hospitalization for schizophrenia. Am J Psychiatry. 2011;168:603–609. [DOI] [PubMed] [Google Scholar]
- 9.Fall K, Strömberg F, Rosell J, Andrèn O, Varenhorst E; South-East Region Prostate Cancer Group. Reliability of death certificates in prostate cancer patients. Scand J Urol Nephrol. 2008;42:352–357. [DOI] [PubMed] [Google Scholar]
- 10.ATC/DDD Index 2014 [Internet]. WHO Collat. Cent Drug Stat Methodol. 2014. http://www.whocc.no/atc_ddd_index/ Accessed January 16, 2014.
- 11.Simpson SH, Eurich DT, Majumdar SR, et al. A meta-analysis of the association between adherence to drug therapy and mortality. BMJ. 2006;333:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katona L, Czobor P, Bitter I. Real-world effectiveness of antipsychotic monotherapy vs. polypharmacy in schizophrenia: to switch or to combine? A nationwide study in Hungary. Schizophr Res. 2014;152:246–254. [DOI] [PubMed] [Google Scholar]
- 13.Leung A, Chue P. Sex differences in schizophrenia, a review of the literature. Acta Psychiatr Scand Suppl. 2000;401:3–38. [DOI] [PubMed] [Google Scholar]
- 14.Haack S, Seeringer A, Thürmann PA, Becker T, Kirchheiner J. Sex-specific differences in side effects of psychotropic drugs: genes or gender? Pharmacogenomics. 2009;10:1511–1526. [DOI] [PubMed] [Google Scholar]
- 15.Seeman MV. Schizophrenia: women bear a disproportionate toll of antipsychotic side effects. J Am Psychiatr Nurses Assoc. 2010;16:21–29. [DOI] [PubMed] [Google Scholar]
- 16.Kuo CJ, Yang SY, Liao YT, et al. Second-generation antipsychotic medications and risk of pneumonia in schizophrenia. Schizophr Bull. 2013;39:648–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marder SR, Essock SM, Miller AL, et al. Physical health monitoring of patients with schizophrenia. Am J Psychiatry. 2004;161:1334–1349. [DOI] [PubMed] [Google Scholar]
- 18.Roberts L, Roalfe A, Wilson S, Lester H. Physical health care of patients with schizophrenia in primary care: a comparative study. Fam Pract. 2007;24:34–40. [DOI] [PubMed] [Google Scholar]
- 19.Druss BG, von Esenwein SA, Compton MT, Rask KJ, Zhao L, Parker RM. A randomized trial of medical care management for community mental health settings: the Primary Care Access, Referral, and Evaluation (PCARE) study. Am J Psychiatry. 2010;167:151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kane JM, Kishimoto T, Correll CU. Non-adherence to medication in patients with psychotic disorders: epidemiology, contributing factors and management strategies. World Psychiatry. 2013;12:216–226. [DOI] [PMC free article] [PubMed] [Google Scholar]