Abstract
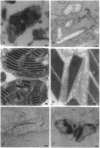
Overaccumulation of abnormally organized mitochondria in so-called "ragged-red" skeletal muscle fibers is a morphological hallmark of mitochondrial myopathies, in particular of mitochondrial encephalomyopathies. Characteristic for the abnormal mitochondria is the occurrence of highly ordered crystalline inclusions. Immuno-electron microscopy revealed that these inclusions react heavily with specific antibodies against mitochondrial creatine kinase (Mi-CK). Image processing of selected crystalline inclusions, sectioned along the crystallographic b, c planes, resulted in an averaged picture displaying an arrangement of regular, square-shaped particles with a central cavity. The overall appearance, dimensions, and symmetry of these building blocks are very reminiscent of single isolated Mi-CK octamers. Taking these findings together, it is concluded that Mi-CK octamers indeed represent the major, if not the only, component of these mitochondrial inclusions.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bessman S. P., Carpenter C. L. The creatine-creatine phosphate energy shuttle. Annu Rev Biochem. 1985;54:831–862. doi: 10.1146/annurev.bi.54.070185.004151. [DOI] [PubMed] [Google Scholar]
- Biermans W., Bakker A., Jacob W. Contact site between inner and outer mitochondrial membrane: a dynamic microcompartment for creatine kinase activity. Biochim Biophys Acta. 1990 Jul 25;1018(2-3):225–228. doi: 10.1016/0005-2728(90)90254-2. [DOI] [PubMed] [Google Scholar]
- Bindoli A. Lipid peroxidation in mitochondria. Free Radic Biol Med. 1988;5(4):247–261. doi: 10.1016/0891-5849(88)90018-4. [DOI] [PubMed] [Google Scholar]
- Brdiczka D. Contact sites between mitochondrial envelope membranes. Structure and function in energy- and protein-transfer. Biochim Biophys Acta. 1991 Nov 13;1071(3):291–312. doi: 10.1016/0304-4157(91)90018-r. [DOI] [PubMed] [Google Scholar]
- Dalakas M. C., Illa I., Pezeshkpour G. H., Laukaitis J. P., Cohen B., Griffin J. L. Mitochondrial myopathy caused by long-term zidovudine therapy. N Engl J Med. 1990 Apr 19;322(16):1098–1105. doi: 10.1056/NEJM199004193221602. [DOI] [PubMed] [Google Scholar]
- DiMauro S., Bonilla E., Zeviani M., Nakagawa M., DeVivo D. C. Mitochondrial myopathies. Ann Neurol. 1985 Jun;17(6):521–538. doi: 10.1002/ana.410170602. [DOI] [PubMed] [Google Scholar]
- Eppenberger-Eberhardt M., Riesinger I., Messerli M., Schwarb P., Müller M., Eppenberger H. M., Wallimann T. Adult rat cardiomyocytes cultured in creatine-deficient medium display large mitochondria with paracrystalline inclusions, enriched for creatine kinase. J Cell Biol. 1991 Apr;113(2):289–302. doi: 10.1083/jcb.113.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrants G. W., Hovmöller S., Stadhouders A. M. Two types of mitochondrial crystals in diseased human skeletal muscle fibers. Muscle Nerve. 1988 Jan;11(1):45–55. doi: 10.1002/mus.880110109. [DOI] [PubMed] [Google Scholar]
- Fitch C. D., Jellinek M., Mueller E. J. Experimental depletion of creatine and phosphocreatine from skeletal muscle. J Biol Chem. 1974 Feb 25;249(4):1060–1063. [PubMed] [Google Scholar]
- Gori Z., De Tata V., Pollera M., Bergamini E. Mitochondrial myopathy in rats fed with a diet containing beta-guanidine propionic acid, an inhibitor of creatine entry in muscle cells. Br J Exp Pathol. 1988 Oct;69(5):639–650. [PMC free article] [PubMed] [Google Scholar]
- Hanzlíková V., Schiaffino S. Mitochondrial changes in ischemic skeletal muscle. J Ultrastruct Res. 1977 Jul;60(1):121–133. doi: 10.1016/s0022-5320(77)80048-8. [DOI] [PubMed] [Google Scholar]
- Jacobus W. E. Respiratory control and the integration of heart high-energy phosphate metabolism by mitochondrial creatine kinase. Annu Rev Physiol. 1985;47:707–725. doi: 10.1146/annurev.ph.47.030185.003423. [DOI] [PubMed] [Google Scholar]
- Kottke M., Adams V., Wallimann T., Nalam V. K., Brdiczka D. Location and regulation of octameric mitochondrial creatine kinase in the contact sites. Biochim Biophys Acta. 1991 Jan 30;1061(2):215–225. doi: 10.1016/0005-2736(91)90287-i. [DOI] [PubMed] [Google Scholar]
- Morgan-Hughes J. A., Schapira A. H., Cooper J. M., Holt I. J., Harding A. E., Clark J. B. The molecular pathology of respiratory-chain dysfunction in human mitochondrial myopathies. Biochim Biophys Acta. 1990 Jul 25;1018(2-3):217–222. doi: 10.1016/0005-2728(90)90252-y. [DOI] [PubMed] [Google Scholar]
- Müller M., Moser R., Cheneval D., Carafoli E. Cardiolipin is the membrane receptor for mitochondrial creatine phosphokinase. J Biol Chem. 1985 Mar 25;260(6):3839–3843. [PubMed] [Google Scholar]
- Ohira Y., Kanzaki M., Chen C. S. Intramitochondrial inclusions caused by depletion of creatine in rat skeletal muscles. Jpn J Physiol. 1988;38(2):159–166. doi: 10.2170/jjphysiol.38.159. [DOI] [PubMed] [Google Scholar]
- Rojo M., Hovius R., Demel R. A., Nicolay K., Wallimann T. Mitochondrial creatine kinase mediates contact formation between mitochondrial membranes. J Biol Chem. 1991 Oct 25;266(30):20290–20295. [PubMed] [Google Scholar]
- Rojo M., Hovius R., Demel R., Wallimann T., Eppenberger H. M., Nicolay K. Interaction of mitochondrial creatine kinase with model membranes. A monolayer study. FEBS Lett. 1991 Apr 9;281(1-2):123–129. doi: 10.1016/0014-5793(91)80374-c. [DOI] [PubMed] [Google Scholar]
- Saks V. A., Kuznetsov A. V., Kupriyanov V. V., Miceli M. V., Jacobus W. E. Creatine kinase of rat heart mitochondria. The demonstration of functional coupling to oxidative phosphorylation in an inner membrane-matrix preparation. J Biol Chem. 1985 Jun 25;260(12):7757–7764. [PubMed] [Google Scholar]
- Schlegel J., Wyss M., Schürch U., Schnyder T., Quest A., Wegmann G., Eppenberger H. M., Wallimann T. Mitochondrial creatine kinase from cardiac muscle and brain are two distinct isoenzymes but both form octameric molecules. J Biol Chem. 1988 Nov 15;263(32):16963–16969. [PubMed] [Google Scholar]
- Schlegel J., Zurbriggen B., Wegmann G., Wyss M., Eppenberger H. M., Wallimann T. Native mitochondrial creatine kinase forms octameric structures. I. Isolation of two interconvertible mitochondrial creatine kinase forms, dimeric and octameric mitochondrial creatine kinase: characterization, localization, and structure-function relationships. J Biol Chem. 1988 Nov 15;263(32):16942–16953. [PubMed] [Google Scholar]
- Schnyder T., Gross H., Winkler H., Eppenberger H. M., Wallimann T. Structure of the mitochondrial creatine kinase octamer: high-resolution shadowing and image averaging of single molecules and formation of linear filaments under specific staining conditions. J Cell Biol. 1991 Jan;112(1):95–101. doi: 10.1083/jcb.112.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnyder T., Winkler H., Gross H., Eppenberger H. M., Wallimann T. Crystallization of mitochondrial creatine kinase. Growing of large protein crystals and electron microscopic investigation of microcrystals consisting of octamers. J Biol Chem. 1991 Mar 15;266(8):5318–5322. [PubMed] [Google Scholar]
- Scholte H. R. The biochemical basis of mitochondrial diseases. J Bioenerg Biomembr. 1988 Apr;20(2):161–191. doi: 10.1007/BF00768393. [DOI] [PubMed] [Google Scholar]
- Sluga E., Monneron A. Uber die Feinstruktur und Topochemie von Riesenmitochondrien und deren Einlagerungen bei Myopathien. Virchows Arch A Pathol Pathol Anat. 1970;350(3):250–260. [PubMed] [Google Scholar]
- Smeitink J., Stadhouders A., Sengers R., Ruitenbeek W., Wevers R., ter Laak H., Trijbels F. Mitochondrial creatine kinase containing crystals, creatine content and mitochondrial creatine kinase activity in chronic progressive external ophthalmoplegia. Neuromuscul Disord. 1992;2(1):35–40. doi: 10.1016/0960-8966(92)90024-z. [DOI] [PubMed] [Google Scholar]
- Stadhouders A. M., Sengers R. C. Morphological observations in skeletal muscle from patients with a mitochondrial myopathy. J Inherit Metab Dis. 1987;10 (Suppl 1):62–80. doi: 10.1007/BF01812848. [DOI] [PubMed] [Google Scholar]
- Wallace D. C. Diseases of the mitochondrial DNA. Annu Rev Biochem. 1992;61:1175–1212. doi: 10.1146/annurev.bi.61.070192.005523. [DOI] [PubMed] [Google Scholar]
- Wallimann T., Wyss M., Brdiczka D., Nicolay K., Eppenberger H. M. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the 'phosphocreatine circuit' for cellular energy homeostasis. Biochem J. 1992 Jan 1;281(Pt 1):21–40. doi: 10.1042/bj2810021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegmann G., Huber R., Zanolla E., Eppenberger H. M., Wallimann T. Differential expression and localization of brain-type and mitochondrial creatine kinase isoenzymes during development of the chicken retina: Mi-CK as a marker for differentiation of photoreceptor cells. Differentiation. 1991 Mar;46(2):77–87. doi: 10.1111/j.1432-0436.1991.tb00868.x. [DOI] [PubMed] [Google Scholar]
- Wegmann G., Zanolla E., Eppenberger H. M., Wallimann T. In situ compartmentation of creatine kinase in intact sarcomeric muscle: the acto-myosin overlap zone as a molecular sieve. J Muscle Res Cell Motil. 1992 Aug;13(4):420–435. doi: 10.1007/BF01738037. [DOI] [PubMed] [Google Scholar]
- Wyss M., Smeitink J., Wevers R. A., Wallimann T. Mitochondrial creatine kinase: a key enzyme of aerobic energy metabolism. Biochim Biophys Acta. 1992 Sep 25;1102(2):119–166. doi: 10.1016/0005-2728(92)90096-k. [DOI] [PubMed] [Google Scholar]
- Wyss M., Wallimann T. Metabolite channelling in aerobic energy metabolism. J Theor Biol. 1992 Sep 7;158(1):129–132. doi: 10.1016/s0022-5193(05)80650-2. [DOI] [PubMed] [Google Scholar]
- Zeviani M., Bonilla E., DeVivo D. C., DiMauro S. Mitochondrial diseases. Neurol Clin. 1989 Feb;7(1):123–156. [PubMed] [Google Scholar]
- van Deursen J., Heerschap A., Oerlemans F., Ruitenbeek W., Jap P., ter Laak H., Wieringa B. Skeletal muscles of mice deficient in muscle creatine kinase lack burst activity. Cell. 1993 Aug 27;74(4):621–631. doi: 10.1016/0092-8674(93)90510-w. [DOI] [PubMed] [Google Scholar]