Abstract
Objective: Cognitive remediation (CR) approaches have demonstrated to be effective in improving cognitive functions in schizophrenia. However, there is a lack of integrated CR approaches that target multiple neuro- and social-cognitive domains with a special focus on the generalization of therapy effects to functional outcome. Method: This 8-site randomized controlled trial evaluated the efficacy of a novel CR group therapy approach called integrated neurocognitive therapy (INT). INT includes well-defined exercises to improve all neuro- and social-cognitive domains as defined by the Measurement And Treatment Research to Improve Cognition in Schizophrenia (MATRICS) initiative by compensation and restitution. One hundred and fifty-six outpatients with a diagnosis of schizophrenia or schizoaffective disorder according to DSM-IV-TR or ICD-10 were randomly assigned to receive 15 weeks of INT or treatment as usual (TAU). INT patients received 30 bi-weekly therapy sessions. Each session lasted 90min. Mixed models were applied to assess changes in neurocognition, social cognition, symptoms, and functional outcome at post-treatment and at 9-month follow-up. Results: In comparison to TAU, INT patients showed significant improvements in several neuro- and social-cognitive domains, negative symptoms, and functional outcome after therapy and at 9-month follow-up. Number-needed-to-treat analyses indicate that only 5 INT patients are necessary to produce durable and meaningful improvements in functional outcome. Conclusions: Integrated interventions on neurocognition and social cognition have the potential to improve not only cognitive performance but also functional outcome. These findings are important as treatment guidelines for schizophrenia have criticized CR for its poor generalization effects.
Introduction
Treatment of schizophrenia patients has moved well beyond the reduction of symptoms to the goal of functional recovery.1 In addition to symptom remission, functional recovery demands an adequate level of functional outcome in terms of living, work and interpersonal relationships.2,3 However, despite advances in antipsychotic medications and psychological therapies, functional recovery rates have not changed substantially over the last 25 years.4,5 This has prompted the search for factors contributing to poor functional outcome in schizophrenia.
A wealth of studies provides evidence for the link between neuro-cognitive and functional impairments.6–8 Neuro-cognitive functions can be defined as processes of linking and appraising information9 and have been considered a core feature of schizophrenia.10 Against this background, the NIMH-Measurement And Treatment Research to Improve Cognition in Schizophrenia (MATRICS) initiative has been founded to identify cognitive domains relevant to schizophrenia research and to develop a standardized cognitive battery to foster the evaluation of cognitive enhancing interventions.11–13 For this purpose, 6 neuro-cognitive domains were defined: speed of processing, attention, verbal learning and memory, visual learning and memory, working memory, and reasoning and problem solving.14 More recently, considerable research has focused on social cognition in schizophrenia that refers to the mental operations underlying social interactions such as the perception, interpretation, and generation of responses to the intentions, dispositions, and behaviors of others.15–17 The MATRICS-initiative initially defined 5 social-cognitive domains: emotion processing, social perception, theory of mind, social attributions, and social schema.16,17 Studies reported that social-cognitive functions are related to both neurocognition18,19 and functional outcome.20–22
These findings have fueled the interest in cognitive remediation therapy (CR) that aims to improve cognitive processes with the goal of durability and generalization of these cognitive benefits to functional outcome.23 The efficacy of CR has been summarized in several meta-analyses23–26 with small to moderate effects on cognitive domains and functional outcome at post-treatment and follow-up. The effect of CR on symptoms was small and disappeared at follow-up. However, these beneficial results should not mask that CR approaches vary widely with regard to their intervention targets with most CR approaches targeting either neuro- or social-cognitive functions.27 Although the mediating role of social cognition suggests that an integrated treatment of neuro- and social cognition may produce better generalization effects on functional outcome than neuro- or social-cognitive therapy alone,28 few approaches have been developed that combine neuro- and social-CR.29–32 The importance of integrated interventions is also supported by meta-analyses on the effectiveness of the Integrated Psychological Therapy (IPT)33–35 that demonstrated that a combined treatment of neuro- and social-cognitive IPT-subprograms produced larger effects on functional outcome than neuro-cognitive subprograms alone.36–38 As there is no evidence that boosting one cognitive domain might improve functional outcome more than another,39 an approach targeting multiple neuro- and social-cognitive domains may be of benefit for most schizophrenia patients. Nevertheless, none of the contemporary CR approaches integrates all neuro- and social-cognitive MATRICS-domains.24,26,35 Moreover, there is still a lack of well-controlled studies assessing the durability and generalizability of treatment effects of CR.40,41
Against this background, we developed a CR group approach called Integrated Neurocognitive Therapy (INT) that combines neuro- and social-CR by targeting all 11 MATRICS-domains.42–44 We conducted a randomized controlled trial to evaluate the efficacy of INT after therapy and after follow-up compared to treatment as usual (TAU). Based on the results of IPT36–38 and current meta-analyses,23–26 we hypothesized that INT would produce significant effects in neuro- and social-cognitive domains, symptoms, and functional outcome after therapy and at follow-up.
Methods
Participants
The sample comprised 156 patients with a diagnosis of schizophrenia or schizoaffective disorder according to DSM-IV-TR or ICD-10. Diagnosis was confirmed by their treating psychiatrist or clinical psychologist. Additional inclusion criteria were current enrollment in an outpatient treatment, age between 18 and 50 years, and illness duration of more than 2 years. In accordance with the recommendations of the working group conference on multisite trial design for CR in schizophrenia,45 study subjects should have IQ scores greater than 80 (Reduced Wechsler Intelligence Test46). Exclusion criteria were neurological disorders, substance dependence and/or abuse according to DSM-IV-TR or ICD-10 within 6 months before baseline assessments, and hospitalization or changes in medication doses within 2 months before baseline assessments. All participants provided written informed consent prior to participation under protocols approved by the ethics committee at the University of Bern.
Procedures
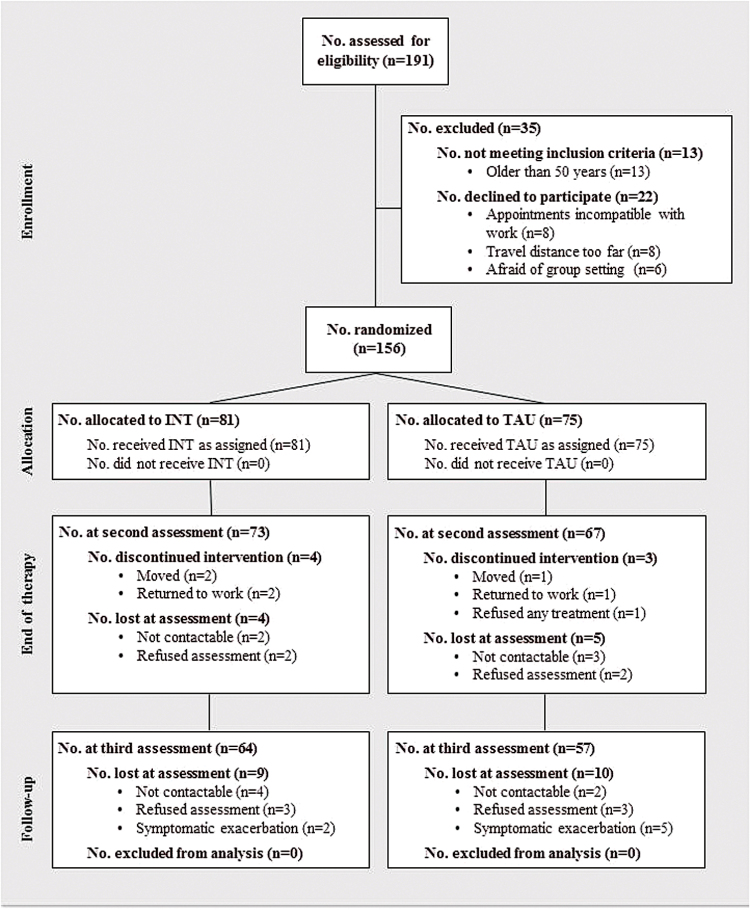
Patients were recruited from 8 outpatient-treatment facilities in Switzerland, Germany, and Austria. They were randomly assigned to INT or to TAU at each treatment site. An independent statistician carried out the randomization procedure for all treatment sites. First, computer-generated random numbers were used to generate 2 groups. Afterwards, these 2 groups were randomly assigned to INT or TAU. The main therapy outcomes were neuro- and social-cognitive functions; secondary outcomes were symptoms and functional outcome. A comprehensive battery of these measures was administered at baseline prior to randomization, after therapy (ie, after 15 weeks), and after a follow-up period of 9 months. Trained research assistants who were blind to group allocation carried out all assessments. The assessors reported no breaks of blinding. Figure 1 shows the flowchart of subject progress.
Fig. 1.
Flow diagram of subject progress through phases of the randomized controlled trial for the integrated Neurocognitive Therapy (INT) and Treatment As Usual (TAU) group.
Interventions
Integrated Neurocognitive Therapy.
INT9,42–44 is a manualized CR group approach that consists of 30 sessions that were administered by a therapist and a co-therapist in groups of 6–8 patients. Sessions took place bi-weekly and each session lasted 90min. Participants received no compensation for attending treatment sessions.
All neuro- and social-cognitive MATRICS-domains are divided into 4 therapy modules with increasing complexity and emotional strain during the course of INT (table 1). Each of the 4 modules consists of neuro- and social-cognitive domains. The same didactic structure is applied to each domain: (1) INT starts with introductory sessions that intend to enhance patients’ understanding and awareness of the relevance of the respective cognitive domain in everyday life and to increase patients’ insight into their own cognitive resources and deficits. (2) In the consecutive sessions, patients learn individual coping strategies in order to compensate for cognitive impairments, and apply these strategies in interactive group exercises. (3) In restitution sessions, patients practice their coping strategies in repeated exercises to foster automatization of these compensatory processes. These exercises are partially computer-based using the Cogpack program (version 5.1, Marker Software, Ladenburg, Germany). (4) Finally, homework assignments and in vivo exercises serve to promote transfer of the acquired cognitive skills into patients’ daily living context and to maintain treatment effects. Thereby, INT represents both a strategy-based learning and a drill-and-practice approach.
Table 1.
Therapy Contents of Integrated Neurocognitive Therapy
| Module A | |
| Speed of processing and attention | Introduction/education |
| Education and self-perception in the target domains (performance-based individual cognitive profile)a | |
| Reference to everyday life and personal experiencesa | |
| Focus on under-stimulation at work, in leisure, and while reading a book | |
| Factors influencing the cognitive performance: eg, alertness, medication, interests and motivation | |
| Compensation | |
| Development of individual coping strategies (strategy learning)a to improve speed of processing and attention; eg, reduce distraction, self-empowerment and self- verbalization, taking a break | |
| Restitution and in vivo exercises | |
| Practice coping strategies in repeated group exercises, computerized exercises (rehearsal learning)a and goal-related in vivo exercises in the focused cognitive domain (to support transfer to everyday life)a | |
| Emotion processing | Introduction/education |
| Emotional influences on perception (filter model) | |
| Identification and definition of basic emotions and their prototypical characteristics | |
| Compensation | |
| Affect recognition training following three steps: 1) facial expressions, 2) gestures, 3) sequences of emotions | |
| Restitution and in vivo exercises | |
| Repeated training of affect decoding to rely on facts instead of assumptions | |
| Module B | |
| Verbal and visual learning, and memory | Introduction/education: |
| Distinguishing between different types of memory: short term memory, long term memory, prospective memory (in contrast to working memory) | |
| Memory contents: verbal memory (eg, letters, names) and visual/spatial memory (eg, faces, signs) | |
| Compensation | |
| Learning and individualizing coping strategies; eg, chunking, using all senses, external memory aids, gathering more information, categorizing, mnemonic rhyme, internal image | |
| Restitution and in vivo exercises | |
| Practice strategies in repeated exercises in the lab and in daily life | |
| Social perception and theory of mind | Introduction/education |
| Identifying key social stimuli (social perception) and taking the perspective of others (ToM) | |
| Compensation | |
| Social perception training following three steps: 1) gathering information, 2) interpretation and discussion, 3) assigning a title | |
| ToM strategies: Distinguish between facts and assumptions when interpreting social information, role change, reference to own and others experiences | |
| Restitution and in vivo exercises | |
| Training of communication skills | |
| Module C | |
| Reasoning and problem solving | Introduction/education: |
| Focus on cognitive flexibility and concept formation and implementing a problem solving model | |
| Compensation | |
| Planning and problem solving skills training (strategy learning) | |
| How to find the right words in a conversation (communication training) | |
| Restitution and in vivo exercises | |
| Practice strategies in repeated exercises in the lab and in daily life | |
| Social schema | Introduction/education |
| Influence of norms and roles on social behavior and automatic use of social knowledge | |
| Compensation | |
| Training of social actions dependent on norms and behavioral sequences (social scripts) | |
| Changing own behavior if it deviates from the norm | |
| Coping with social stigma | |
| Restitution and in vivo exercises | |
| Practice strategies in role plays and in daily life | |
| Module D | |
| Working memory | Introduction/education: |
| Focus on selective attention and distractibility in terms of over-stimulation in social contexts | |
| Compensation | |
| Behavioral shift (to change from one action to another) and adaptive behavior | |
| Selective attention skills training while being confronted with emotional strain and distraction | |
| Learning how to avoid distraction during a conversation | |
| Restitution and in vivo exercises | |
| Practice strategies in repeated exercises and daily life | |
| Social attributions and emotion regulation | Introduction/education |
| Relationship of one`s own attribution style to emotional strain and over-stimulation | |
| Compensation | |
| Analyzing one`s own attribution style and the consequences of internal and external attributions | |
| Reattribution: Finding alternative explanations | |
| Stress-inoculation training and emotion regulation training | |
| Restitution and in vivo exercise | |
| Practice strategies in repeated exercises in daily life | |
aThese contents are part of the interventions on each neuro- and social-cognitive domain in all 4 modules.
All therapists were trained in cognitive-behavior therapy methods. Therapist training in INT was provided systematically by the developers in a 2-day seminar at each treatment site. During therapy and follow-up, supervision was available personally or via telephone or email. Supervision was carried out at least fortnightly. Fidelity checklists were administered in each session and assured adherence to the therapy protocol at all treatment sites. Those assigned to INT attended on average 81.1% (SD = 13.0%) of the therapy sessions.
INT represents a further development of the cognitive parts of IPT but differs from it in that it targets all 11 neuro- and social-cognitive MATRICS-domains while IPT focuses predominantly on the domains of speed of processing, attention, reasoning and problem solving as well as on emotion and social perception. Furthermore, INT uses computerized neuro-cognitive exercises for restitution, and has been exclusively designed for outpatient settings. Therefore, the exercises of INT are more cognitively and emotionally demanding and are more closely linked to personal experiences and everyday life. In comparison to INT, IPT seems especially appropriate for inpatients with substantial cognitive and psychosocial impairments and severe negative symptoms.43,44
Treatment as Usual.
INT effects were compared to those of TAU. TAU was defined as standard care including a broad array of interventions used in clinical practice for schizophrenia patients (eg, medication, individual therapy, case-management). Both INT and TAU patients were not allowed to take part in specific group therapies that primarily applied other CR techniques, cognitive-behavioral therapy, social skills therapy or supported employment because these interventions may also have substantial beneficial effects on neuro- and social-cognitive functions and functional outcome. However, INT and TAU patients could receive all other kinds of psychosocial interventions administered in a group setting including music therapy, art therapy, movement/dance therapy, case-management, psychoeducation, supportive and vocational counseling, and leisure time-groups.
Measures
While commonly used measures in schizophrenia research are only described briefly, more detailed information and references are provided for rather new assessments in schizophrenia patients.
Neurocognition.
The neuro-cognitive test battery included the following measures commonly applied in schizophrenia research: (1) Speed of processing was assessed using the Trail Making Test47 (TMT) Part A (time to completion) and the Controlled Oral Word Association Test48 (COWAT) (mean value of produced words per minute); (2) Attention using the Continuous Performance Test49 (CPT) (total number of commission errors during the test) and the d250 (number of correctly marked items). The d2 is a paper-and-pencil cancellation test that has proven to be a reliable and valid measure of selective attention;50 (3) Verbal learning and memory using the Auditory Verbal Learning Test51 (AVLT) (summary score of correctly remembered words after each trial); (4) Visual learning and memory with the Wechsler Memory Scale-Revised Third Edition52 (WMS-R) (total number of correctly recognized items); (5) Working memory with the Letter-Number Span53 (LNS) (total number of correctly remembered items); (6) Reasoning and problem solving with the Wisconsin Card Sorting Test54 (WCST) (total number of perseverative answers).
Social Cognition.
We assessed emotion perception through the Picture of Facial Affect Test55 (PFA) and the Emotion Recognition Questionnaire56 (Emorec). Both measures require the participant to view photographs of faces and to identify specific basic emotions (PFA) or to rate the intensity of the perceived emotion on a 5-point Likert scale (Emorec). Friesen and Ekman developed this series of photographs.57 The Emorec has proven to be a reliable and valid measure of emotion perception.56 The test score of both measures was the total number of correct judgments. (2) We administered the Schema Component Sequencing Task-Revised58 (SCST-R) as a computerized measure of social schema. The task is to order the component actions of 12 social situations in the right sequence using verbal stimuli. The dependent variable was the total number of the correctly juxtaposed pairs over all situations. (3) The Ambiguous Intentions Hostility Questionnaire59 (AIHQ) measures 5 social-cognitive attribution biases (hostility, anger, blame, intention, and aggression). We applied the 5 ambiguous situations only as they seem to be most sensitive for attribution biases.59 Two independent raters coded the written answers and revealed high average intra-class reliability (ICCs = 0.93–0.99).
Symptoms and Functional Outcome.
The Positive and Negative Syndrome Scale (PANSS)60 was administered to rate negative and positive symptom severity and the Global Assessment of Functioning Scale (GAF) of the DSM-IV to measure functional outcome. The sample of this study comprised schizophrenia outpatients with the symptom ratings being in the medium range (table 2). Studies indicate that the GAF scale is a valid measure of global functional outcome given that patients are clinically stable.61 All raters received specific training and revealed high inter-rater reliability (ICC = 0.91 for PANSS and ICC = 0.92 for GAF).
Table 2.
Sample Characteristics of INT and TAU Group
| INT group (n = 81) | TAU group (n = 75) | t/χ2 a | P | |
|---|---|---|---|---|
| Age (y), mean (SD) | 34.6 (8.5) | 33.8 (8.7) | 0.6 | 0.5 |
| Gender (male %) | 64.2 | 74.7 | 2.0 | 0.2 |
| IQb mean (SD) | 105.6 (10.0) | 102.2 (12.3) | 1.7 | 0.1 |
| Duration of illness (y), mean (SD) | 10.2 (7.5) | 9.9 (7.0) | 0.3 | 0.8 |
| Number of hospitalizations (n) | 3.7 (3.3) | 4.6 (4.9) | −1.2 | 0.2 |
| Education (y), mean (SD) | 11.2 (4.1) | 10.8 (4.4) | 0.6 | 0.6 |
| Marital status (%) | ||||
| Single | 77.5 | 93.4 | ||
| Married | 9.9 | 1.6 | ||
| Divorced | 12.7 | 4.9 | 3.6 | 0.3 |
| Chlorpromazine equivalent doses, mean (SD) | 422.3 (420.9) | 456.0 (380.2) | −0.5 | 0.7 |
Note: INT, integrated neurocognitive therapy; TAU, treatment as usual.
a t-Tests for normally distributed variables; χ2-tests for categorical variables.
bReduced Wechsler Intelligence Test 46 (WIP).
Statistical Analyses
All analyses were conducted using SPSS 21.0 (SPSS Inc). Raw data were checked for normality and outliers. Group comparisons between INT and TAU as well as between completers and noncompleters of therapy at baseline were performed using chi-square and t-tests. With regard to therapy outcomes, we computed composite scores with equal weights using z-transformations for “global neurocognition,” “global social cognition,” “global symptoms,” and for cognitive domains that were assessed by more than one test, that is, “speed of processing,” “attention,” and “emotion perception.” Changes in the global composite scores and the assessed cognitive domains were analyzed using linear mixed modeling with maximum likelihood estimation including all subjects in an intent-to-treat analysis. Models included group (INT and TAU) and time (baseline, post-therapy, follow-up) as well as their interaction (group × time) as fixed effects. Additionally, models comprised random intercepts and slopes for subjects and treatment sites. A diagonal covariance structure was used as the repeated covariance type and a variance components matrix was selected for the random effects. Significant interactions were interpreted as a differential treatment effect.
Following recent recommendations, we also focused on effect sizes.62 Cohen’s d was calculated at post-therapy and follow-up using the difference of the respective group means divided by their pooled standard deviation.63 Additionally, we calculated the number-needed-to-treat (NNT) for all significant interaction effects at various levels of change (10%–60%) in cognitive, symptomatic, and functional outcomes as assessed by single tests.64,65
Results
Baseline Analyses
INT and TAU neither differed significantly in demographic variables or antipsychotic medication (table 2) nor in any outcome variable (table 3). All but 4 patients were taking antipsychotic medication (91% atypical neuroleptics, 7% typical neuroleptics, 2% mixed), and 7 patients (4%) received antidepressants (SSRIs) as concomitant medication. There were no significant group differences in medication doses at baseline, post-therapy, and follow-up. Moreover, both groups did not demonstrate significant changes in their medication doses from baseline to post-therapy and follow-up. Completers and noncompleters did not differ significantly in demographic and outcome variables at post-therapy and follow-up.
Table 3.
Measures of Neurocognition, Social Cognition, Negative Symptoms, and Functional Outcome at Baseline, After Therapy/After 15 Weeks, and at 9-Month Follow-Up
| Baseline | After therapy/after 15 weeks | Follow-up/after 37 weeks | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| INT, Mean (SD) | TAU, Mean (SD) | t a (P values) | INT, Mean (SD) | TAU, Mean (SD) | F b (P values) | Cohen`s d; NNT | INT, Mean (SD) | TAU, Mean (SD) | F b (P values) | Cohen`s d; NNT | |
| Neurocognition | −0.05 (0.52) | −0.15 (0.58) | 1.10 (0.27) | 0.27 (0.48) | 0.03 (0.62) | 4.45* (0.04) | 0.43 | 0.14 (0.44) | 0.13 (0.44) | 2.26 (0.09) | 0.02 |
| Speed of processing: TMT A & COWAT | −0.31 (1.58) | −0.51 (1.69) | 0.76 (0.45) | 0.72 (1.53) | 0.07 (1.66) | 5.41* (0.02) | 0.41 | −0.15 (1.14) | −0.03 (1.66) | 1.40* (0.25) | −0.08 |
| Attention: CPT and d2 | −0.07 (0.98) | −0.08 (2.44) | 0.03 (0.97) | 0.12 (0.93) | −0.14 (1.56) | 0.26 (0.61) | 0.20 | 0.39 (0.97) | 0.30 (1.10) | 6.78* (0.02) | 0.09 |
| Visual memory: WMS-R | 6.09 (1.69) | 6.07 (1.59) | 0.07 (0.94) | 6.49 (1.62) | 6.46 (1.74) | 0.00 (0.99) | 0.01 | 6.78 (1.54) | 6.45 (1.88) | 0.84 (0.43) | 0.19 |
| Verbal memory: AVLT | 44.78 (10.47) | 45.34 (11.23) | −0.32 (0.75) | 45.2 (10.89) | 45.40 (12.73) | 0.06 (0.81) | 0.04 | 50.20 (10.49) | 47.91 (11.12) | 3.61* (0.03) | 0.26; |
| 10%: 10.1 | |||||||||||
| 20%: 10.4 | |||||||||||
| 30%: 13.1 | |||||||||||
| 40%: 16.6 | |||||||||||
| 50%: 44.4 | |||||||||||
| 60%: 60.9 | |||||||||||
| Working memory: LNS | 13.12 (3.81) | 12.36 (4.24) | 1.17 (0.24) | 13.50 (2.89) | 12.52 (3.42) | 0.29 (0.59) | 0.26 | 13.36 (2.90) | 12.54 (3.30) | 0.21 (0.81) | 0.16 |
| Reasoning & problem solving: WCST | −36.49 (25.93) | −36.36 (30.74) | 0.03 (0.98) | −28.83 (25.93) | −36.7 (29.37) | 4.57* (0.03) | 0.32; | −28.56 (22.64) | −29.08 (20.78) | 3.23 (0.05) | 0.03 |
| 10%: 12.8 | |||||||||||
| 20%: 8.0 | |||||||||||
| 30%: 8.2 | |||||||||||
| 40%: 8.9 | |||||||||||
| 50%: 9.7 | |||||||||||
| 60%: 11.3 | |||||||||||
| Social Cognition | −0.22 (2.40) | −0.16 (2.42) | −0.13 (0.90) | 0.71 (2.23) | −0.06 (2.59) | 7.05** (<0.01) | 0.32 | 0.63 (2.26) | 0.08 (2.39) | 5.04** (<0.01) | 0.24 |
| Emotion perception: PFA & Emorec | −0.20 (1.78) | −0.10 (1.64) | −0.36 (0.72) | 0.41 (1.65) | −0.10 (1.69) | 10.97** (<0.01) | 0.31 | 0.42 (1.63) | 0.01 (1.79) | 6.63** (0.02) | 0.24 |
| Social schema: SCST-R | 61.94 (11.34) | 59.76 (14.71) | −0.13 (0.90) | 64.10 (11.85) | 59.56 (15.80) | 3.92* (0.04) | 0.33; | 64.26 (11.22) | 62.44 (12.83) | 1.57 (0.21) | 0.15 |
| 10%: 6.4 | |||||||||||
| 20%: 6.3 | |||||||||||
| 30%: 6.6 | |||||||||||
| 40%: 9.0 | |||||||||||
| 50%: 11.4 | |||||||||||
| 60%: 17.9 | |||||||||||
| Attributions: AIAQ | −0.40 (3.78) | −0.17 (4.40) | −0.35 (0.73) | 0.26 (3.23) | 0.32 (3.51) | 0.12 (0.74) | −0.02 | 0.17 (3.66) | −0.16 (3.75) | 0.34 (0.71) | 0.09 |
| Symptoms | −0.16 (1.62) | −0.42 1.64 | 0.97 (0.33) | 0.78 (1.48) | −0.19 1.72 | 9.15** (<0.01) | 0.60 | 0.79 (1.62) | 0.06 (1.64) | 5.19** (<0.01) | 0.45 |
| Negative: PANSS | −16.44 (5.87) | −17.84 (5.99) | −1.46 (0.15) | −14.09 (5.27) | −17.56 (5.38) | 9.89** (<0.01) | 0.66; | −13.68 (5.04) | −16.29 (6.12) | 5.09** (<0.01) | 0.48; |
| 10%: 3.9 | 10%: 6.1 | ||||||||||
| 20%: 3.9 | 20%: 5.0 | ||||||||||
| 30%: 4.0 | 30%: 5.3 | ||||||||||
| 40%: 4.1 | 40%: 5.4 | ||||||||||
| 50%: 5.8 | 50%: 5.6 | ||||||||||
| 60%: 9.4 | 60%: 5.8 | ||||||||||
| Positive: PANSS | −15.48 (5.06) | −15.67 (4.45) | 0.24 (0.81) | −13.13 (4.14) | −14.79 (5.07) | 4.49* (0.04) | 0.36; | −12.82 (4.67) | 14.07 (4.06) | 2.75 (0.07) | 0.28 |
| 10%: 10.0 | |||||||||||
| 20%: 33.3 | |||||||||||
| 30%: 11.1 | |||||||||||
| 40 %: 50.0 | |||||||||||
| 50%: 20.0 | |||||||||||
| 60% 100.0 | |||||||||||
| Functional Outcome: GAF | 49.12 (8.12) | 48.40 (8.90) | 0.52 (0.60) | 52.60 (8.30) | 49.57 (8.77) | 4.71* (0.03) | 0.40; | 55.41 (7.33) | 50.72 (9.42) | 8.17** (<0.01) | 0.71; |
| 10%: 6.4 | 10%: 3.8 | ||||||||||
| 20%: 7.2 | 20%: 3.6 | ||||||||||
| 30%: 8.7 | 30%: 3.9 | ||||||||||
| 40%: 9.9 | 40%: 4.3 | ||||||||||
| 50%: 15.2 | 50%: 5.3 | ||||||||||
| 60%: 41.5 | 60%: 8.8 | ||||||||||
Note: AIHQ, Ambiguous Intentions Hostility Questionnaire 59 ; AVLT, Auditory Verbal Learning Test 51 ; COWAT, Controlled Oral Word Association Test 48 ; CPT, Continuous Performance Test 49 ; d2, Aufmerksamkeits-Belastungs-Test 50 ; Emorec, Emotion Recognition Questionnaire 56 ; GAF, Global Assessment of Functioning Scale; INT, Integrated Neurocognitive Therapy; LNS, Letter-Number Span 53 ; PANSS, Positive and Negative Syndrome Scale, negative symptom subscale 60 ; PFA, Picture of Facial Affect Test 57 ; SCST-R, Social Component Sequencing Task-Revised 58 ; TAU, Treatment As Usual; TMT, Trail Making Test, Part A 47 ; WCST, Wisconsin Card Sorting Test 54 ; WMS-R, Wechsler Memory Scale-Revised. 52 Bold letters indicate significant group × time interaction effects; All effect sizes and test scores are presented in a way that higher scores indicate more improvements or better performance in the respective measure. Cohen`s d has been calculated using the following formula after therapy and at follow-up 63,66 : Effect size (d) = (MINT–MTAU)/SDpooled; MINT indicates the mean for the INT group; MTAU indicates the mean for the TAU-group; SDpooled indicates the pooled standard deviation for the two groups. 63 Alpha coefficients for cognitive composite scores: attention (0.54), emotion perception (0.60), speed (0.55), neurocognition (0.53), social cognition (0.49).
a t Tests for normally distributed variables.
b F- and P-values for group × time interaction effects.
*P < 0.05; **P < 0.01.
Treatment Effects on Neurocognition
Means, SDs, and effect sizes of all therapy outcomes are presented in table 3. INT demonstrated significant improvements in global neurocognition after therapy (F 1,172 = 4.45, P = 0.04, Cohen’s d = 0.43) but not at follow-up (F 2,264 = 2.40, P = 0.09, d = 0.02). Using the TMT and the COWAT as a measure of speed of processing, group differences favoring INT were significant after therapy (F 1,146 = 5.41, P = 0.02, d = 0.41). Moreover, INT demonstrated significant improvements in reasoning and problem solving at post-therapy (F 1,155 = 4.57, P = 0.03, d = 0.32, NNT ≥ 8.0) while significant treatment effects at follow-up could be detected in verbal memory (F 2,149 = 3.61, P = 0.03, d = 0.26, NNT ≥ 10.1) and attention (F 2,106 = 6.78, P = 0.02, d = 0.09). No significant group-by-time interactions were found for visual and working memory either at post-therapy or follow-up (table 3).
Treatment Effects on Social Cognition
INT produced significantly larger effects in global social cognition than TAU at both post-therapy (F 1,130 = 7.05, P = 0.009, d = 0.32) and at 9-month follow-up (F 2,122 = 5.04, P = 0.008, d = 0.24) (table 3). With regard to the assessed social-cognitive domains, INT demonstrated significantly larger improvements than TAU after therapy in emotion perception (F 1,222 = 10.97, P = 0.001, d = 0.31) and social schema (F 1,148 = 3.94, P = 0.04, d = 0.33, NNT ≥ 17,9). These beneficial effects could be maintained at follow-up for emotion perception (F 2,124 = 6.63, P = 0.02; d = 0.24) but not for social schema. Both groups did not differ in their attribution styles after therapy and at-follow-up.
Treatment Effects on Symptoms and Functional Outcome
After therapy, INT demonstrated significant reductions in negative symptoms (F 1,151 = 9.98, P = 0.02, d = 0.66, NNT ≥ 3.9) and positive symptoms (F 1,141 = 4.49, P = 0.04, d = 0.36, NNT ≥ 10.0). These favorable effects on negative symptoms could be maintained at follow-up (F 2,170 = 5.09, P = 0.01, d = 0.40, NNT ≥ 5.0) but the effect on positive symptoms was no longer significant. This is reflected by an overall significant global symptom score (F 1,141 = 9.15, P = 0.002, d = 0.60) that slightly decreased at follow-up (F 2,146 = 5.19, P = 0.007, d = 0.45). Notably, INT also produced significant improvements in functional outcome (F 1,150 = 5.09, P = 0.03, d = 0.40, NNT ≥ 5.0) compared to TAU that even increased until follow-up (F 2,187 = 8.17, P < 0.01, d = 0.71, NNT ≥ 3.6).
All effect sizes (Cohen’s d) for cognitive, symptomatic and functional outcomes were in the small to moderate range (table 3).66 Notably, there was no substantial variability between treatment sites in all outcome variables as indicated by nonsignificant Wald-tests (all Z ≤ 1.64, all P > 0.05).
Discussion
This international multisite trial evaluated a novel group therapy for schizophrenia outpatients that targets all neuro- and social-cognitive domains as defined by the MATRICS-initiative.14,16,17 As hypothesized, we found that INT showed significant gains in some neuro- and social-cognitive domains, symptoms, and functional outcome compared with TAU.
After treatment, INT resulted in significant improvements in both global neurocognition and social cognition as well as in the directly targeted neuro- and social-cognitive domains of speed of processing, reasoning and problem solving, emotion perception, and social schema. However, the respective effects sizes were only in the small to moderate range. The positive effects on these neuro-cognitive domains and on emotion perception are in accordance with recent meta-analyses.24–27 At 9-month follow-up, additional significant improvements in verbal memory and attention could be detected. In contrast, no significant effect on working and visual memory was evident either after treatment or at follow-up. This may be due to a “ceiling” effect as our sample demonstrated mean baseline values in the LNS that were as high as those after therapy in the normative MATRICS-sample.12,13 Alternatively, a longer therapy duration may be necessary to improve these memory domains because treatments that produced significant effects on working31,67 and visual memory32 completed substantially more hours of practicing exercises than INT. Moreover, one of these successful therapy programs67 additionally targeted early perceptual processes because they were found to have impact on higher order neuro-cognitive functions.68,69 This may have optimized the effect of neuro-CR in particular on memory functions and may be a complementary future treatment target for INT.
Regarding social cognition, our positive effects on emotion perception replicate the results of other studies.27,41 The significant improvements in social schema extend the current literature and are of considerable significance because they suggest that even more complex social-cognitive operations are amenable to treatment. Furthermore, this effect on social schema is of special interest as social schema was found to be a more powerful mediator between neurocognition and functional outcome than other social-cognitive domains.23
Another important finding of our study was that INT produced robust and durable generalization effects on functional outcome as assessed by the GAF that even increased over time. The effect size at follow-up was generally larger than in a previous meta-analysis24 and was associated with low NNT values: As few as 5 patients need to receive INT to improve long-term functional outcome by 10%–50%. These generalization effects may reflect the benefit of integrated interventions. This seems to be due to the fact that intact neuro-cognitive functions represent a necessary prerequisite for social cognition and, in turn, for functional outcome.70 Thereby, neuro-cognitive interventions may potentiate the impact of social-cognitive interventions on functional outcome. Importantly, neuro-CR alone is not sufficient to improve functional outcome, most likely because social cognition has an even stronger relationship to functional outcome than neurocognition,18 and explains additional variance of functional outcome beyond neurocognition.23 Therefore, intact social-cognitive functions may be necessary to generalize neuro-cognitive improvements to functional outcome. As a certain time interval must elapse for one variable to have an effect on another in order to imply a change mechanism,71,72 the temporal result pattern we found, ie, significant neuro-, social-cognitive and functional improvements at post-therapy that could be maintained for social cognition and that even increased for functional outcome at follow-up, are in line with our assumption that neuro-cognitive improvements produced during therapy could have functioned as a mediator for social-cognitive and functional improvements at follow-up. However, this result pattern is also in line with the alternative explanation that social cognition and other mediators may have driven functional improvements independently from neuro-cognitive improvements. Therefore, these 2 explanations need to be tested by the means of longitudinal structural equation modeling in future studies and require the assessment of all outcome variables at several times to disentangle the complex change mechanisms of INT and CR in general.
The significantly reduced severity of negative symptoms found in INT after therapy and at follow-up may also be the result of neuro- and social-cognitive improvements because negative symptoms seem to mediate the relationship between cognitive functions and functional outcome.73,74 This is in line with our own results that demonstrated that social cognition, negative symptoms, and social skills functioned as important mediators between neurocognition and functional outcome in the INT group which resulted in a significant indirect effect. Thus, these mediators may have potentiated the beneficial effect of neuro-cognitive improvements on functional outcome.75 Alternatively, it is possible that the group participation had a salutary effect on negative symptoms because these reductions in negative symptoms were also found in other integrated group therapy approaches76,77 but not in computerized integrated treatments.31,32 Furthermore, other studies78 showed that self-efficacy beliefs about cognitive abilities can have an impact on motivation to perform tasks and can change with successful performance. Other recent research by Grant and Beck79 has demonstrated that defeatist performance beliefs also act as mediator between neurocognition, negative symptoms, and functional outcome. Therefore, improvements in self-efficacy and defeatist beliefs during INT may also have contributed to improvements in negative symptoms and functional outcome which needs to be investigated in future studies. The fact that positive symptoms improved only at post-therapy but not at follow-up may indicate that these improvements in negative symptoms are not only a by-product of a general symptomatic remission but a change mechanism in itself.
Our clinical impressions suggest that INT is a feasible and well-accepted treatment as indicated by the relatively high attendance rate of 81.1% over 30 sessions and the low drop-out rate of 10.0% at the end of the intervention. This point of view is also supported by the fact that INT patients received no travel compensation and were not paid for attending therapy sessions. The high acceptability of INT may be due to the fact that INT puts strong emphasis on the enhancement and maintenance of intrinsic motivation, by taking patients’ daily-living experiences into account, and by using engaging therapy materials with various degrees of difficulty. INT-therapists continuously support group processes to foster group cohesion which may also have prevented dropouts.43 Furthermore, therapy effects of INT did not differ in all outcome variables between the 8 treatment sites. Thus, high treatment fidelity can be assumed.
Our study had several limitations. As we have not formally assessed medication adherence, we cannot rule out its potential impact on the outcomes. We used only chart reviews for study inclusion. However, the reliability of the psychiatric diagnoses could have been enhanced by combining chart reviews with structured diagnostic interviews.80 Moreover, our participants were generally younger and more intelligent than other schizophrenia samples which may limit the generalization effects of INT and suggests that the efficacy of INT needs to be investigated in other samples in the future. We administered the GAF scale as a measure of functional outcome. Although it is widely used and seems appropriate in samples of stable patients,61 it may be confounded with symptom severity and may not be very sensitive for psychosocial changes.81 Thus, the GAF scale is not appropriate to assess functional recovery. Therefore, it would have been useful to include more measures of this domain and it should be complemented or replaced by a purer functional measure. Moreover, we did not use the MATRICS Consensus Cognitive Battery (MCCB) because it was published after we had started this study. Our social-cognitive assessments were restricted to emotion perception, social schema, and attributions due to the poor suitability of measures with adequate psychometric properties and sufficient sensitivity to change17 when we designed the study. Another limitation is the absence of an active control group. However, a previous meta-analysis found no impact of the control type on the effect size of improvements.26 While active control groups are crucial to identify essential treatment elements, the comparison with TAU is a necessary first step to evaluate the efficacy of a novel therapy like INT.24 However, the group modality, additional therapeutic attention, and alliance with the INT therapists could have indirectly influenced the outcomes of interest. Mixed modeling allowed us to take the hierarchical nature of the data into account and revealed no substantial variability between treatment sites, which supports the effectiveness of INT. However, it would be valuable to collect data on the group (eg, group composition) and therapist (eg, expertise) level in future studies, and to enter them into the analyses.
In summary, despite these limitations, our results suggest that INT is a feasible and effective new group therapy approach with the potential to improve functional outcome in schizophrenia outpatients. A design with active and passive control conditions, a direct comparison of individual and group setting and of various durations of CR may further elucidate factors that are crucial for transfer to functional outcome.
Funding
Swiss National Science Foundation (3200B0-108133/1).
Acknowledgments
The authors thank the following centers for their participation in the study: Psychiatrische Universitätsklinik Zürich (Dr.med. A. Theodoridou), Psychiatriezentrum Biel (Dr.med. A. Rausch), Psychiatrische Universitäts- und Poliklinik Bern, Ev. Krankenhaus Bielefeld, Klinik für Psychiatrie u. Psychotherapie Bethel (Prof.Dr.med. M. Driessen, Dipl.-Psych. C. Barenbrock), Rehabilitationszentrum für psychisch Kranke Peiting-Herzogsägmühle (Dr.phil. S. Queri; Dr.med. A. Gabrecht), ARBEWE-Rehabilitationszentrum Nürnberg (Dipl.-Psych. A. Baumann, Dipl.-Psych. G. Fischer), Rehabilitationszentrum Vitos Eltville (Dipl.-Psych. G. Deutschle, Dipl.-Soz.-Päd. B. Franke), Landeskrankenhaus Schwarzach/St. Veit (Dr.med. M. Keglevic). The authors also like to thank M.Sc. A. Eugster, M.Sc. J. Emmerich, Ph.D. M. Lächler, M.Sc. D. Speiser, and M.Sc. J. Weiss who carried out the assessments. The study’s sponsor had no role in study design, data collection, or analysis, or in interpretation, writing, or submission of the report. There are no conflicts of interest and no financial disclosures have been reported.
References
- 1.Kern RS, Glynn SM, Horan WP, Marder SR. Psychosocial treatments to promote functional recovery in schizophrenia. Schizophr Bull. 2009;35:347–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kopelowicz A, Liberman RP, Ventura J, Zarate R, Mintz J. Neurocognitive correlates of recovery from schizophrenia. Psychol Med. 2005;35:1165–1173. [DOI] [PubMed] [Google Scholar]
- 3.Liberman RP, Kopelowicz A. Recovery from schizophrenia: a concept in search of research. Psychiatr Serv. 2005;56:735–742. [DOI] [PubMed] [Google Scholar]
- 4.Jääskeläinen E, Juola P, Hirvonen N, et al. A systematic review and meta-analysis of recovery in schizophrenia. Schizophr Bull. 2013;39:1296–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shivashankar S, Telfer S, Arunagiriraj J, et al. Has the prevalence, clinical presentation and social functioning of schizophrenia changed over the last 25 years? Nithsdale schizophrenia survey revisited. Schizophr Res. 2013;146:349–356. [DOI] [PubMed] [Google Scholar]
- 6.Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia. Schizophr Bull. 2000;26:119–136. [DOI] [PubMed] [Google Scholar]
- 7.Green MF, Kern RS, Heaton RK. Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophr Res. 2004;72:41–51. [DOI] [PubMed] [Google Scholar]
- 8.Shamsi S, Lau A, Lencz T, et al. Cognitive and symptomatic predictors of functional disability in schizophrenia. Schizophr Res. 2011;126:257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roder V, Mueller DR. Integrated neurocognitive therapy for schizophrenia patients. Berlin, Springer International Publishing, the Netherlands; 2015. [Google Scholar]
- 10.Kraepelin E. Psychiatrie. Ein Lehrbuch für Studierende und Ärzte. 3rd ed. 3. Band: Kinische Psychiatrie. Leipzig: Barth; 1913. [Google Scholar]
- 11.Green MF, Nuechterlein KH. The MATRICS initiative: developing a consensus cognitive battery for clinical trials. Schizophr Res. 2004;72:1–3. [DOI] [PubMed] [Google Scholar]
- 12.Kern RS, Nuechterlein KH, Green MF, et al. The MATRICS Consensus Cognitive Battery, part 2: co-norming and standardization. Am J Psychiatry. 2008;165:214–220. [DOI] [PubMed] [Google Scholar]
- 13.Nuechterlein KH, Green MF, Kern RS, et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165:203–213. [DOI] [PubMed] [Google Scholar]
- 14.Nuechterlein KH, Barch DM, Gold JM, Goldberg TE, Green MF, Heaton RK. Identification of separable cognitive factors in schizophrenia. Schizophr Res. 2004;72:29–39. [DOI] [PubMed] [Google Scholar]
- 15.Brothers L. The social brain: a project for integrating primate behavior and neurophysiology in a new domain. Concepts Neurosci. 1990;1:27–51. [Google Scholar]
- 16.Green MF, Olivier B, Crawley JN, Penn DL, Silverstein S. Social cognition in schizophrenia: recommendations from the measurement and treatment research to improve cognition in schizophrenia new approaches conference. Schizophr Bull. 2005;31:882–887. [DOI] [PubMed] [Google Scholar]
- 17.Green MF, Penn DL, Bentall R, et al. Social cognition in schizophrenia: an NIMH workshop on definitions, assessment, and research opportunities. Schizophr Bull. 2008;34:1211–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fett AK, Viechtbauer W, Dominguez MD, Penn DL, van Os J, Krabbendam L. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neurosci Bio behav Rev. 2011;35:573–588. [DOI] [PubMed] [Google Scholar]
- 19.Ventura J, Wood RC, Hellemann GS. Symptom domains and neurocognitive functioning can help differentiate social cognitive processes in schizophrenia: a meta-analysis. Schizophr Bull. 2013;39:102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mancuso F, Horan WP, Kern RS, Green MF. Social cognition in psychosis: multidimensional structure, clinical correlates, and relationship with functional outcome. Schizophr Res. 2011;125:143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horan WP, Green MF, DeGroot M, et al. Social cognition in schizophrenia, Part 2: 12-month stability and prediction of functional outcome in first-episode patients. Schizophr Bull. 2012;38:865–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Irani F, Seligman S, Kamath V, Kohler C, Gur RC. A meta-analysis of emotion perception and functional outcomes in schizophrenia. Schizophr Res. 2012;137:203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wykes T, Huddy V, Cellard C, McGurk SR, Czobor P. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am J Psychiatry. 2011;168:472–485. [DOI] [PubMed] [Google Scholar]
- 24.Grynszpan O, Perbal S, Pelissolo A, et al. Efficacy and specificity of computer-assisted cognitive remediation in schizophrenia: a meta-analytical study. Psychol Med. 2011;41:163–173. [DOI] [PubMed] [Google Scholar]
- 25.McGurk SR, Twamley EW, Sitzer DI, McHugo GJ, Mueser KT. A meta-analysis of cognitive remediation in schizophrenia. Am J Psychiatry. 2007;164:1791–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurtz MM, Richardson CL. Social cognitive training for schizophrenia: a meta-analytic investigation of controlled research. Schizophr Bull. 2012;38:1092–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keefe RSE, Vinogradov S, Medalia A, et al. Report from the working group conference on multisite trial design for cognitive remediation in schizophrenia. Schizophr Bull. 2011;37:1057–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmidt SJ, Mueller DR, Roder V. Social cognition as a mediator variable between neurocognition and functional outcome in schizophrenia: empirical review and new results by structural equation modeling. Schizophr Bull. 2011;37(suppl 2):41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eack SM, Greenwald DP, Hogarty SS, et al. Cognitive enhancement therapy for early-course schizophrenia: effects of a two-year randomized controlled trial. Psychiatr Serv. 2009;60:1468–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horan WP, Kern RS, Tripp C, et al. Efficacy and specificity of social cognitive skills training for outpatients with psychotic disorders. J Psychiatr Res. 2011;45:1113–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindenmayer JP, McGurk SR, Khan A, et al. Improving social cognition in schizophrenia: a pilot intervention combining computerized social cognition training with cognitive remediation. Schizophr Bull. 2013;39:507–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sacks S, Fisher M, Garrett C, et al. Combining computerized social cognitive training with neuroplasticity-based auditory training in schizophrenia. Clin Schizophr Relat Psychoses. 2013;7:78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roder V, Brenner HD, Kienzle N, Hodel B.Integriertes Psychologisches Therapieprogram fur schizophrene Patienten (IPT). Munich: Psychologie Verlags Union; 1988. [Google Scholar]
- 34.Brenner HD, Roder V, Hodel B, Kienzle N, Reed D, Liberman RP.Integrated psychological therapy for schizophrenic patients (IPT). Seattle, WA: Hogrefe & Huber Publishers; 1994. [Google Scholar]
- 35.Roder V, Mueller DR, Brenner HD, Spaulding W.Psychological Therapy (IPT) for the Treatment of Neurocognition, Social Cognition and Social Competency in Schizophrenia Patients. Seattle, WA: Hogrefe & Huber; 2010. [Google Scholar]
- 36.Roder V, Mueller DR, Mueser KT, Brenner HD. Integrated psychological therapy (IPT) for schizophrenia: is it effective? Schizophr Bull. 2006;32(suppl 1):81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roder V, Mueller DR, Schmidt SJ. Effectiveness of integrated psychological therapy (IPT) for schizophrenia patients: a research update. Schizophr Bull. 2011;37(suppl 2):71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mueller DR, Schmidt SJ, Roder V. Integrated psychological therapy: effectiveness in schizophrenia inpatient settings related to patients’ age. Am J Geriatr Psychiatry. 2013;21:231–241. [DOI] [PubMed] [Google Scholar]
- 39.Wykes T, Huddy V. Cognitive remediation for schizophrenia: it is even more complicated. Curr Opin Psychiatry. 2009;22:161–167. [DOI] [PubMed] [Google Scholar]
- 40.Dixon LB, Dickerson F, Bellack AS, et al. The 2009 schizophrenia PORT psychosocial treatment recommendations and summary statements. Schizophr Bull. 2010;36:48–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fiszdon JM, Reddy LF. Review of social cognitive treatments for psychosis. Clin Psychol Rev. 2012;32:724–740. [DOI] [PubMed] [Google Scholar]
- 42.Mueller DR, Roder V. Integrated psychological therapy and integrated neurocognitive therapy (INT). In: Roder V, Medalia A, eds. Neurocognition and Social Cognition in Schizophrenia Patients. Basic Concepts and Treatment. Basel, Switzerland: Karger; 2010:118–144. [Google Scholar]
- 43.Mueller DR, Schmidt SJ, Roder V. Integrated neurocognitive therapy. In: Roberts DL, Penn DL, eds. Social cognition in schizophrenia. From evidence to treatment. New York: Oxford; 2013:311–334. [Google Scholar]
- 44.Roder V, Mueller DR.INT—Integrierte neurokognitive Therapie bei schizophren Erkrankten. Berlin, Heidelberg: Springer; 2013. [Google Scholar]
- 45.Keefe RS, Vinogradov S, Medalia A, et al. Report from the working group conference on multisite trial design for cognitive remediation in schizophrenia. Schizophr Bull. 2011;37:1057–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dahl G.WIP: Handbuch zum Reduzierten Wechsler-Intelligenztest; Anwendung, Auswertung, statistische Analysen, Normwerte. Weinheim, Germany: Beltz; 1986. [Google Scholar]
- 47.Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Motor Skill. 1958;8:271–276. [Google Scholar]
- 48.Benton AL. Differential behavioral effects in frontal lobe disease. Neuropsychologia. 1968;6:53–60. [Google Scholar]
- 49.Knye M, Roth N, Westhus W, Heine A.Continuous Performance Test (CPT). Göttingen, Germany: Hogrefe; 2003. [Google Scholar]
- 50.Brickenkamp R, Liepmann D, Schmidt-Atzert L.Test d2-Revision: Aufmerksamkeits-und Konzentrationstest. Göttingen, Germany: Hogrefe; 2010. [Google Scholar]
- 51.Lezak MD.Neuropsychological Assessment. 2nd ed. New York, NY: Oxford University Press; 2004. [Google Scholar]
- 52.Härting C, Markowitsch H, Neufeld H, Calabrese P, Deisinger K, Kessler J.WMS-R Wechsler Gedächtnistest—Revidierte Fassung. Bern, Switzerland: Hans Huber; 2000. [Google Scholar]
- 53.Gold JM, Carpenter C, Randolph C, Goldberg TE, Weinberger DR. Auditory working memory and Wisconsin Card Sorting Test performance in schizophrenia. Arch Gen Psychiatry. 1997;54:159–165. [DOI] [PubMed] [Google Scholar]
- 54.Loong J.W.K. (1989). Wisconsin Card Sorting Test (WCST). La Luna Court, San Luis Obisbo: Wang Neuropsychological Laboratory. [Google Scholar]
- 55.Frommann N, Streit M, Wölwer W. Remediation of facial affect recognition impairment in patient with schizophrenia: A new training program. Psychiatry Res. 2003;117;281–284. [DOI] [PubMed] [Google Scholar]
- 56.Bähler M.Evaluation eines neu entwickelten Fragebogen zur Emotionserkennung bei schizophren Erkrankten. Masterarbeit: Université de Fribourg, Suisse; 2012. [Google Scholar]
- 57.Ekman P, Friesen WV, Press CP.Pictures of facial affect. Palo Alto: Consulting Psychologists Press; 1975. [Google Scholar]
- 58.Vauth R, Rüsch N, Wirtz M, Corrigan PW. Does social cognition influence the relation between neurocognitive deficits and vocational functioning in schizophrenia? Psychiatry Res. 2004;128:155–165. [DOI] [PubMed] [Google Scholar]
- 59.Combs DR, Penn DL, Wicher M, Waldheter E. The Ambiguous Intentions Hostility Questionnaire (AIHQ): a new measure for evaluating hostile social-cognitive biases in paranoia. Cogn Neuropsychiatry. 2007;12:128–143. [DOI] [PubMed] [Google Scholar]
- 60.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 61.Startup M, Jackson MC, Bendix S. The concurrent validity of the Global Assessment of Functioning (GAF). Br J Clin Psychol. 2002;41:417–422. [DOI] [PubMed] [Google Scholar]
- 62.Nakagawa S. A farewell to Bonferroni: the problem of low statistical power and publication bias. Behav Ecol. 2004;15:1044–1045. [Google Scholar]
- 63.Rustenbach SJ.Metaanalyse. Eine anwendungsorientierte Einführung. Bern, Switzerland: Huber; 2003. [Google Scholar]
- 64.Bowie CR, McGurk SR, Mausbach B, Patterson TL, Harvey PD. Combined cognitive remediation and functional skills training for schizophrenia: effects on cognition, functional competence, and real-world behavior. Am J Psychiatry. 2012;169:710–718. [DOI] [PubMed] [Google Scholar]
- 65.Furukawa TA, Leucht S. How to obtain NNT from Cohen’s d: comparison of two methods. PLoS One. 2011;6:e19070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cohen J.Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, MI: Routledge; 1988. [Google Scholar]
- 67.Fisher M, Holland C, Merzenich MM, Vinogradov S. Using neuroplasticity-based auditory training to improve verbal memory in schizophrenia. Am J Psychiatry. 2009;166:805–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dias EC, Butler PD, Hoptman MJ, Javitt DC. Early sensory contributions to contextual encoding deficits in schizophrenia. Arch Gen Psychiatry. 2011;68:654–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.De Jong JJ, de Gelder B, Hodiamont PG. Sensory processing, neurocognition, and social cognition in schizophrenia: towards a cohesive cognitive model. Schizophr Res. 2013;146:209–216 http://dx.doi.org/10.1016/j.schres.2013.02.034. [DOI] [PubMed] [Google Scholar]
- 70.Fanning JR, Bell MD, Fiszdon JM. Is it possible to have impaired neurocognition but good social cognition in schizophrenia? Schizophr Res. 2012;135:68–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cole DA, Maxwell SE. Testing mediational models with longitudinal data: questions and tips in the use of structural equation modeling. J Abnorm Psychol. 2003;112:558–577. [DOI] [PubMed] [Google Scholar]
- 72.Little TD.Longitudinal Structural Equation Modeling. New York, London: The Guilford Press; 2013. [Google Scholar]
- 73.Rassovsky Y, Horan WP, Lee J, Sergi MJ, Green MF. Pathways between early visual processing and functional outcome in schizophrenia. Psychol Med. 2011;41:487–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Green MF, Hellemann G, Horan WP, Lee J, Wynn JK. From perception to functional outcome in schizophrenia: modeling the role of ability and motivation. Arch Gen Psychiatry. 2012;69:1216–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schmidt SJ, Müller DR, Roder V. The importance of cognition, negative symptoms and subjective parameters for functional recovery in schizophrenia. 13th International Congress on Schizophrenia Research ICOSR, Colorado Springs, 2–6 April 2011. Schizophr Bull. 2011;37(suppl 1):281–282. [Google Scholar]
- 76.Roberts DL, Penn DL. Social cognition and interaction training in outpatients with schizophrenia: a preliminary study. Psychiatry Res. 2009;166:141–147. [DOI] [PubMed] [Google Scholar]
- 77.Eack SM, Mesholam-Gately RI, Greenwald DP, Hogarty SS, Keshavan MS. Negative symptom improvement during cognitive rehabilitation: results from a 2-year trial of Cognitive Enhancement Therapy. Psychiatry Res. 2013;209:21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Choi KH, Saperstein AM, Medalia A. The relationship of trait to state motivation: the role of self-competency beliefs. Schizophr Res. 2012;139:73–77. [DOI] [PubMed] [Google Scholar]
- 79.Grant PM, Beck AT. Defeatist beliefs as a mediator of cognitive impairment, negative symptoms, and functioning in schizophrenia. Schizophr Bull. 2009;35:798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Basco MR, Bostic JQ, Davis D, Rush AJ, Witte B, Hendrickse W, Barnett V. Methods to improve diagnostic accuracy in a community mental health setting. Am J Psychiatry. 2000;157:1599–1605. [DOI] [PubMed] [Google Scholar]
- 81.Robertson DA, Hargreaves A, Kelleher EB, et al. Social dysfunction in schizophrenia: an investigation of the GAF scale’s sensitivity to deficits in social cognition. Schizophr Res. 2013;146:363–365. [DOI] [PubMed] [Google Scholar]