Abstract
A limited number of studies have evaluated sexual functioning in patients with schizophrenia. Most patients show an interest in sex that differs little from the general population. By contrast, psychiatric symptoms, institutionalization, and psychotropic medication contribute to frequently occurring impairments in sexual functioning. Women with schizophrenia have a better social outcome, longer lasting (sexual) relationships, and more offspring than men with schizophrenia. Still, in both sexes social and interpersonal impairments limit the development of stable sexual relationships. Although patients consider sexual problems to be highly relevant, patients and clinicians not easily discuss these spontaneously, leading to an underestimation of their prevalence and contributing to decreased adherence to treatment. Studies using structured interviews or questionnaires result in many more patients reporting sexual dysfunctions. Although sexual functioning can be impaired by different factors, the use of antipsychotic medication seems to be an important factor. A comparison of different antipsychotics showed high frequencies of sexual dysfunction for risperidone and classical antipsychotics, and lower frequencies for clozapine, olanzapine, quetiapine, and aripiprazole. Postsynaptic dopamine antagonism, prolactin elevation, and α1-receptor blockade may be the most relevant factors in the pathogenesis of antipsychotic-induced sexual dysfunction. Psychosocial strategies to treat antipsychotic-induced sexual dysfunction include psychoeducation and relationship counseling. Pharmacological strategies include lowering the dose or switching to a prolactin sparing antipsychotic. Also, the addition of a dopamine agonist, aripiprazole, or a phosphodiesterase-5 inhibitor has shown some promising results, but evidence is currently scarce.
Key words: antipsychotic, sexual dysfunction, schizophrenia, dopamine, prolactin, negative symptoms
Introduction
Sexual dysfunction in patients with schizophrenia may be related to the disease itself (eg negative symptoms, decreased initiative, and motivation), psychosocial factors, somatic health and the use of psychotropic medications.1–4
The aim of this article is to provide an overview of the literature on all aspects of sexual functioning that are relevant for clinicians who treat patients with psychotic disorders, including both the theoretical background and the practical implications. Different psychotropic medications, like antipsychotics and antidepressants, can cause sexual side effects in patients with schizophrenia. In this article, the focus will be on antipsychotics as these are most prescribed in these patients. Antipsychotic drugs have been associated with sexual dysfunction, such as decreased sexual desire, erectile dysfunction, anorgasmia, and delayed or retrograde ejaculation.5,6 Studies using structured interviews or self report questionnaires have shown that 16% to 60% of the patients using antipsychotics experience sexual dysfunctions.7 Sexual side effects have a considerable impact on quality of life and are probably a major factor in nonadherence to prescribed antipsychotic drugs.6,8–10
We will subsequently describe the relationship between schizophrenia and sexual dysfunction, psychosocial aspects of sexual functioning, the burden of antipsychotic-induced sexual dysfunction as weighed by patients and doctors, the prevalence and different types of antipsychotic-induced sexual side effects, which mechanisms may be involved, and which treatment strategies for the sexual side of antipsychotics effects are available. No recent reviews have been published which cover these aspects from a clinical perspective.
A PubMed search was performed using the following search terms: “schizophrenia,” “psychosis,” “clinical,” “sexual,” “sexual function,” “sexual dysfunction,” “prevalence,” “social functioning,” “severity of illness,” “antidepressant,” “antipsychotic,” “neuroleptic,” “adherence,” “nonadherence,” “treatment strategies,” “psychosocial,” “libido,” “sexual desire,” “arousal,” “erection,” “priapism,” “vaginal lubrication,” ”orgasm,” “ejaculation,” “neurotransmitter,” “receptor,” “dopamine,” “noradrenalin,” “norepinephrine,” “serotonin,” “prolactin,” “acetylcholine,” “histamine.” Articles were selected by the first author (MB). In case of uncertainty inclusion was discussed with the last author (HK). All retrieved studies were checked for cross-references that were retreived when they fulfilled the search criteria. Finally, we structured, summarized, and integrated the information into a balanced clinically oriented review article.
Schizophrenia and Sexual Functioning
In the early 20th century, it was believed that schizophrenia was caused by deficiencies in sex hormones.11 Early psychoanalytic theories suggested that psychosis might derive from unconscious homosexual tendencies.12 Erotic sexual behaviors were also seen as possible causal factors for schizophrenia in pre-schizophrenic patients.13 As these ideas were not based on any empirical research, they have gradually been abandoned.
According to case reports, some patients suffering from psychotic symptoms experience coenesthetic hallucinations (ie the sensation of bodily functions that are usually undetectable) of a sexual nature, erotomanic delusions, delusions related to sexual identity, the sexual act, or pregnancy. Also, hypersexuality can occur during an acute psychotic episode. Such psychotic manifestations related to sexuality are uncommon and usually disappear after starting antipsychotic medication.11,14,15
According to Skopec et al16 most patients with schizophrenia do not differ from controls in terms of actual sexual behavior. On the other hand, relationships of people with serious mental illness are characterized by less intimacy and commitment than in the general population.17
Schizophrenia may influence sexual behavior in men and women in different ways.18 This may be related to differences in the age of onset of schizophrenia. Women often develop schizophrenia later in life, possibly related to the effects of sex hormones on the brain.19 Women with schizophrenia have better social outcomes, as they date, have sex, marry, and raise children more often than men.20 McEvoy et al21 studied sexual activity and attitudes in chronic inpatients with schizophrenia. A majority of female patients with chronic schizophrenia continued to be interested in sex. About one-half of them wanted to become pregnant, but at the same time many of them were unaware of the limitations to their parenting abilities.21
Viewed from a social perspective, the sexual behavior of patients with schizophrenia was restricted in many ways before the 1950s. Extramarital sexual intercourse was socially frowned upon,22 and institutionalization in the Western world reinforced sexual inactivity by discouraging or even prohibiting sexual relationships. In the United States, eg, illegitimate pregnancies did occur among patients in psychiatric facilities, but only at one-fifth of the rate found among the general population.23
Around the time that oral contraceptive medication became available, psychiatric institutions started to change their regulations, allowing more sexually mixed social activities and home passes.23 Particularly in the United States, the United Kingdom, and Italy, deinstitutionalization accelerated after the 1960s. In North America, beds in public psychiatric hospitals had decreased by 80% within 10 years.24 Parallel to de-institutionalization, the relative fertility of women with a major mental illness increased,25 as living in the community yielded more opportunities for sexual encounters.26
According to studies from the 1980s, patients with schizophrenia viewed institutionalization as an obstacle to sexuality; it took them longer to have a first date, first kiss, first coitus, and first marriage.27 In a population with institutionalized patients, both males and females with chronic schizophrenia showed diminished interest in sexual activity, decreased frequency of intercourse, and loss of satisfaction from sexual interactions, compared with controls.28 The lower frequency and lesser degree of satisfaction with sexual intercourse were related to the severity of psychopathology and the length of institutionalization. Interestingly, sexual dreams and fantasies among these patients did not differ significantly from dreams and fantasies in the control group.28
In summary, only a limited number of studies have evaluated sexual functioning in patients with schizophrenia. To which degree schizophrenia is linked to problematic sexual behavior has yet to be established. It is clear that institutionalization and psychotropic medication often impair sexual functioning. Still, most patients with schizophrenia show an interest in sex that differs little from the general population. In comparison to men, women with schizophrenia tend to have a better social outcome, as reflected not only in longer lasting (sexual) relationships but also in more often having offspring. On the whole, however, due to social and interpersonal impairments, patients with schizophrenia are not frequently involved in long and stable sexual relationships.
The Burden of Sexual Complaints From the Patient’s and Doctor’s Perspective
Sexual side effects are of major importance to patients and lead to decreased adherence to therapy and reduced quality of life.6,9,10 Nonetheless, patients and clinicians not easily start to discuss sexual functioning.29 Most patients, when asked, are willingly and relieved to discuss sexual problems.14,30 Studies suggest that talking about sexuality does not destabilize patients.31–33 Even those with treatment-resistant schizophrenia may wish to talk about these topics.34 In contrast, clinicians often do not ask and underestimate the rates of sexual dysfunctions35 as well as their negative impact on the lives of the patients. Some clinicians may not know enough about the issue14 or feel ashamed of talking about these sensitive subjects.
In a study by Finn et al, 41 patients with schizophrenia were asked to compare the subjective burden caused by their psychotic symptoms with that caused by other symptoms or side effects.10 The burden of symptoms could be rated from 1 (mild) to 5 (most serious). Patients-rated prosecutory hallucinations as a heavy burden (frequency in patients reporting the symptom 66%, and the corresponding burden 4.3). Significantly, the burden of erectile dysfunction (frequency 34%, burden 4.5) and absence of ejaculation or painful ejaculation (frequency 12%, burden 4.0) was rated in the same range.
Strauss and Gross interviewed 86 psychiatrists on the importance of sexual side effects of psychopharmacological treatment.29 Most psychiatrists considered sexual side effects to be clinically relevant in 2 out of 3 patients, and felt that these side effects were likely to influence treatment adherence in a negative way. Although the psychiatrists were convinced that most patients would not discuss sexual side effects spontaneously, only 10% had actually asked their patients about this.
Studies using spontaneous accounts of patients report low incidences of sexual dysfunction related to treatment with antipsychotics; actually less than 10% of the patients report sexual dysfunction spontaneously when asked about side effects. On the other hand, studies using structured interviews or self report questionnaires tend to report a prevalence of 30–60% for sexual side effects related to treatment with antipsychotics.5,36–39 This emphasizes that direct questioning about sexual functioning including sexual side effects is necessary to avoid underestimating their frequency among patients with schizophrenia and related psychotic disorders.
In summary, patients with schizophrenia consider sexual problems to be highly relevant. It affects their quality of life and treatment adherence negatively. Still, both clinicians and patients not easily start to discuss sexual functioning, leading to an underestimation of the frequency and impact of sexual problems.
Prevalence of Antipsychotic-Induced Sexual Dysfunction in Patients with Schizophrenia
When comparing studies on antipsychotic-induced sexual dysfunction, some aspects have to be taken into account. First, in part of the studies it is not totally clear if sexual dysfunction is caused by the use of antipsychotic medication, or that other factors could also be involved. A second aspect is the difference between sexual “problem” and “dysfunction.” Sexual problems are common, also in the general population,40 but sexual dysfunction comes along with subjective distress, which is often not asked for. Finally, there are many methodological differences between studies. Keeping in mind these shortcomings in the comparison between studies, we give a general overview on the prevalence of antipsychotic-induced sexual dysfunction based on available reviews and a meta-analysis.
A review comparing different antipsychotics with regard to sexual dysfunction concluded that risperidone induces sexual dysfunction most frequently, followed by typical antipsychotics (haloperidol), olanzapine, quetiapine; the lowest frequency is found for aripiprazole.6 Knegtering et al found a comparable order (risperidone, typical antipsychotics, clozapine, olanzapine, quetiapine, aripiprazole), based on previous studies by this research group, all of which used the same design and questionnaire.5,36,41–43
In 2011, a meta-analysis was published about sexual dysfunction in psychiatric populations of patients taking antipsychotics. In contrast to other findings, it was found that quetiapine, ziprasidone, perphenazine, aripiprazole, olanzapine, risperidone, haloperidol, clozapine, and thioridazine had an incrementally increasing impact on sexual function ranging from 16% (quetiapine) to 60% (thioridazine). It was noted that the quality of the included studies showed large variation, and the findings related to aripiprazole, clozapine, perphenazine, and thioridazine should be considered with caution as replication was very limited.7
Also in 2011, a large multicenter randomized trial was published by Malik et al,4 the European First Episode Schizophrenia Trial (EUFEST) study. Rates of sexual dysfunction at baseline were comparable with rates after 12 months. In contrast to other studies, there were no significant differences in rates of sexual dysfunction between the different medications. A higher score of negative symptoms as measured with the Positive and Negative Syndrome Scale (PANSS), was associated with libido reduction, although some other studies did not find this association.4 In a Japanese study (352 outpatients with schizophrenia), no differences in rates of sexual dysfunction were found between medication groups (risperidone, olanzapine, aripiprazole, haloperidol).3
Although not all studies agree, sexual side effects may not subside over time.4 This emphasizes the importance of treatment strategies for sexual side effects.
In summary, studies using questionnaires suggest that 16%–60% of the patients report sexual dysfunction, possibly related to the use of antipsychotics. In most studies, frequencies of sexual dysfunction differ between different types of medication. Conflicting results between studies may result from differences in the study design and questionnaires used.
Types of Sexual Dysfunction in Patients with Schizophrenia
A commonly used classification of stages in sexual response is: sexual desire (thoughts, interest), sexual arousal/plateau (feeling sexually excited as well as physiological effects, eg erection or lubrication), orgasm (peak in pleasure; mentally as well as physiologically), and resolution/refraction.44 In the following section, an overview will be given of clinical studies on different types of sexual dysfunction reported during use of antipsychotics.
Sexual Desire
Sexual desire is a term commonly defined as interest in sexual objects or sexual experiences. There is no objective physiological criterion for desire. It is generally inferred from the self-reported frequency of sexual thoughts, fantasies, dreams, wishes and interest in initiating, and/or engaging in sexual experiences.44
A meta-analysis shows that 12–38% of patients using antipsychotics experience a reduction of sexual desire (ranging from 12% for aripiprazole to 38% for clozapine).7 Knegtering et al report 6–50% (ranging from 6% for aripiprazole to 50% for risperidone).5,43
Medicated as well as unmedicated patients with schizophrenia often report a decrease in sexual desire. In a study by Aizenberg1, patients with schizophrenia reported significantly more frequent sexual desire reduction vs unaffected controls, while sexual desire was reduced in patients using antipsychotics as well as in those not using antipsychotics.1 The “disease-related” sexual desire reduction might be induced by an unknown underlying process, the patients’ psychotic symptoms or as part of the general loss of initiative and activity level (ie negative symptoms).
Sexual Arousal
Sexual arousal is closely connected to sexual desire. It is defined both in subjective terms, like feeling sexually excited, and objective physiological terms like erection and lubrication. Patients being treated with antipsychotics often report being less easily sexually aroused.1
Sexual Arousal: Erectile Dysfunction
Erection describes the nonflaccid state of the penis and is in most cases the physiological expression of sexual arousal. Erectile dysfunction refers to the inability of men to achieve and/or maintain erection. In clinical practice patients often report problems related to a delayed, shortened, or diminished ability to reach full erection. Although erections often co-occur with the subjective feelings of sexual excitement, they can also occur without arousal, for instance during Rapid Eye Movement (REM) sleep.
In many studies assessing arousal, questionnaires are used to evaluate quantitative (duration, frequency) and qualitative (rigidity) aspects of erection. More objective ways to study erection include for instance a mechanical strain gauge that measures the penile circumference. Such objective evaluations are complex, and given the taboos surrounding sexuality, not readily accepted in routine clinical practice or clinical trials evaluating side effects. This explains why studies evaluating sexual side effects in patients using antipsychotics on the whole relied on questionnaires.
The meta-analysis of Serretti and Chiesa7 shows that 7–46% of patients using antipsychotics experience dysfunction of arousal, like erection and lubrication (ranging from 7% for aripiprazole to 46% for thioridazine). Knegtering et al report 0–39% (ranging from 0% for aripiprazole to 39% for risperidone).5,43
Aizenberg et al found that in patients with schizophrenia who had a sexual partner, erection was reduced in quality or time during coitus. Patients using antipsychotics experienced significantly more erection disturbance compared to patients without antipsychotics, both during sexual intercourse and during masturbation. At the same time, no change occurred in waking erections (an expression of REM sleep correlated erections) in these patients.1
Sexual Arousal: Priapism
Priapism is a painful, prolonged, and sustained erection of the penis and is a urologic emergency. How priapism exactly results from treatment with antipsychotics remains unclear, but α-adrenergic receptors may be involved.45,46 The onset can be sexual stimulation, but the condition itself persists long after sexual excitement has subsided. Priapism is an emergency that needs immediate attention as it can lead to long-term devastating consequences such as erectile dysfunction, urinary retention and gangrene. Even with treatment, 40%–50% of patients can develop erectile dysfunction owing to ischemia and fibrosis of the corpora cavernosa.45–47
Priapism related to treatment with antipsychotics is a rarely occurring effect and has shown up only in case reports, which relate priapism to many different antipsychotics, such as haloperidol, clozapine, risperidone, olanzapine, aripirazole, and quetiapine. Antipsychotics with strong α1- and α2-antagonistic properties seem to induce priapism most frequently.45,46 Risperidone has a high affinity, followed by clozapine and quetiapine. Olanzapine has the lowest affinity for the adrenergic receptors. The only exception is ziprasidone with a high affinity, but there are just a few case reports in the literature. A possible explanation may be that this drug has not been available as long as other atypical antipsychotics.46 Most sexual side effects of medications are reversible. In contrast, although priapism is rare, it should be treated immediately as it may result in irreversible sexual dysfunctions.
Clitoral priapism is an even less frequently reported side effect. In antipsychotics, it has only been reported with olanzapine.48
Sexual Arousal: Vaginal Lubrication
Vaginal lubrication is the excretion of a lubricating fluid by the vaginal wall that facilitates sexual intercourse. It is associated with increased vaginal blood flow and sexual arousal. Although vaginal lubrication in women may be viewed as the physiological equivalent of erection, it has hardly been studied in relation to schizophrenia or antipsychotic medication. Lubrication can be evaluated indirectly by measuring vaginal blood flow using for instance photopletysmography, or by indirect measures of heat dissipation and Doppler techniques.49 Measuring vaginal blood flow requires the insertion of a tampon shaped device into the vagina. These instruments are too intrusive to be used in routine clinical practice or clinical research.
Studies suggest that women report diminished lubrication in frequencies that are comparable to the frequency of erectile dysfunction reported by men treated with the same antipsychotics.5,7
Orgasm
Orgasm is characterized by a peak in sexual pleasure accompanied by rhythmic contractions of the genital and reproductive organs, cardiovascular and respiratory changes and a release of sexual tension.44 Physiological measurements of orgasm, like fluctuations in rectal pressure, are infrequently described.44,50 Also, this method is too invasive for use in clinical trials on patients using antipsychotics.
The meta-analysis of Serretti and Chiesa shows that 4%–49% of patients using antipsychotics experience orgasm dysfunction (ranging from 4% for aripiprazole to 49% for thioridazine).7 Knegtering et al report 3%–46% (ranging from 3% for olanzapine to 46% for risperidone).5,43
In studies evaluating sexual dysfunctions, orgasm is evaluated as an individual item in questionnaires. Most studies assess the degree to which the patient indicates he or she is capable of experiencing an orgasm, but some studies also noted a disturbance in the quality of the orgasm.51 In the study of Aizenberg, patients using clozapine reported the quality of their orgasm had improved, unlike patients using classical antipsychotics.1
Ejaculation
Ejaculation is the emission of semen during orgasm in men. Ejaculation disturbances consist of a change in consistence or volume of the ejaculate. Most commonly reported in patients treated with antipsychotics is a decreased ejaculatory volume (DEV). Terms in the literature related to DEV are aspermia, anejaculation, dry ejaculation, or retrograde ejaculation. It has been stated that retrograde ejaculation and aspermia are often used wrongly as synonyms.52 Aspermia can be defined as the absence of ejaculate in the presence of erection, muscular ejaculation and orgasm.53 Retrograde ejaculation refers to the ejaculate being released into the bladder during orgasm as can be shown by analyzing the urine for the presence of semen after orgasm.
Decreased ejaculatory volume (DEV) is frequently (8%–58%) reported in patients treated with antipsychotics.7 Knegtering et al report 7%–40% (ranging from 0% for aripiprazole to 40% for risperidone).5,43
DEV and dry ejaculation are reportedly related to the use of several antipsychotics like thioridazine, chlorpromazine, sertindole, risperidone, and olanzapine.5,47,52–56 Although the mechanisms are not fully known, antipsychotics with α-blocking properties and possibly also calcium channel blockers are thought to be most likely to induce DEV.
Spontaneous ejaculation is a rare condition that has been described with zuclopentixol, trifluoperazine, thiothixene, and risperidone.57–59
Menstrual Disturbance, Galactorrhea, and Gynecomastia
Although menstrual disturbance, gynecomastia and galactorrhea are not in themselves sexual dysfunctions, they do tend to coincide with some of the sexual dysfunctions because they may originate at least partly from the same source: high serum prolactin levels.60 The literature suggests that lowering prolactin levels with a dopamine agonist or switching to a prolactin sparing antipsychotic will often be successful in treating antipsychotic-induced menstrual disturbance and galactorrhea.
Other Aspects of Sexual Functioning
Besides the aspects of sexual functioning discussed above, some studies have tried to evaluate the subjective judgment of patients about the overall quality of their sexual experience. Aizenberg included items in his questionnaire such as “enjoyment of sex” and “sexual satisfaction”1 and found that these were significantly higher in patients treated with clozapine than in those treated with classical antipsychotics.61 As mentioned in the section on orgasm, the study of Ghadirian et al included one item about change in the quality of orgasm.51
Some studies mention pain during orgasm51 or painful ejaculation (odynorgasmia).62,63 On the whole, pain during orgasm seems to be extremely rare and is absent as item in most studies, while the pathophysiological mechanisms in relation to treatment with antipsychotics remain unclear.
Pharmacological Mechanisms in Antipsychotic-Induced Sexual Dysfunction
The pathogenetic mechanisms of antipsychotic-associated sexual dysfunction are not fully understood. Postsynaptic dopamine antagonism, α1-antagonism, and prolactin elevation are likely to be involved.5,44 Most antipsychotics are potent postsynaptic dopamine antagonists and can cause sustained elevation of the anterior pituitary hormone, prolactin.5,51 Dopamine is an important neurotransmitter in brain areas and circuits involved in attention and salience of stimuli, and in experiencing motivation and rewards, including sexual motivation (desire) and probably also sexual reward. Sexual reward is experienced primarily during orgasm, but other stages of sexual functioning also seem to be involved in reward-related learning.44,64 However, it has to be noted that perspectives on the role of dopamine have changed in recent years: dopamine is now suggested to be more important in the anticipation of reward than reward itself.65
Dopamine blockade is probably a major factor in antipsychotic-induced sexual dysfunction.5 A second factor possibly involved in the inhibition of sexual behavior by dopamine antagonists may be the associated hyperprolactinemia.5,44 Dopaminergic activity controls the production of prolactin in the pituitary gland. While dopamine antagonists decrease levels of dopaminergic output, prolactin levels are increased. Prolactin in turn has an inhibiting effect on the tuberoinfundibular dopaminergic neurons, thereby completing the feedback mechanism between dopamine and prolactin.66
Antipsychotic-induced hyperprolactinemia has been associated with a number of side effects including galactorrhea, menstrual disturbances, amenorrhea, and sexual dysfunction,5,67–70 although some studies only confirm this for subgroups of patients,71,72 or do not find an association between prolactin levels and sexual dysfunction.73 In contrast to risperidone and classical antipsychotics, clozapine, quetiapine, and olanzapine do not appear to cause sustained elevated levels of prolactin. The reports available suggest that patients using these antipsychotics may have comparatively lower rates of sexual dysfunction.1,5–7,41–43,74
Besides having affinity for the dopamine receptor, antipsychotics interact with many neurotransmitter systems in the brain and other parts of the body. Affinity for the serotonergic, noradrenergic, histaminic, and cholinergic/muscarinic neurotransmitter systems differs among the various groups of antipsychotics.75–77 Agonistic serotonergic effects, for instance on 5-HT2 receptors, are associated with a decreased ability to achieve orgasm. By contrast, agonism of the 5-HT1a receptors, and possibly also antagonism of the 5-HT2a and 5-HT2c receptors appear to have a stimulating effect on sexual performance.78,79 The α1 receptors are thought to be involved in erection, lubrication, and ejaculation.46,80,81 Some antipsychotics also have α2-blocking properties. In treatment with antipsychotics a clear relationship between these properties and sexual performance has not been described. In theory, as in the α2-blocking yohimbine, they may stimulate erection.82,83 Acetylcholine has a facilitatory role in erection and ejaculation when administered to the corpus cavernosum. Little is known about possible central anticholinergic effects, which often occur as adverse effect of psychotropic medication, which may contribute to inhibition of sexual functioning.84,85 Also information about the influence of histamine on sexual functioning is limited, but histamine antagonists are associated with a decrease of sexual functioning.86,87 The exact effects of central agonism or antagonism of histamine receptors on sexual functioning remains unknown. An indirect negative result of antihistaminergic medication (eg anti-allergy medication, some antipsychotics or some antidepressants77) on sexual functioning may primarily be caused by their sedating effects.
Table 1 shows the effects of neurotransmitters on sexual functioning. Table 2 shows the affinity of several antipsychotics for the neurotransmitters and represents global receptor affinity based on different studies, showing the large variation in pharmacological properties of frequently used antipsychotics which partly explain differential effects on sexual functioning.75–77,88,89
Table 1.
Main effects of antipsychotics on sexual functioning
| Effects on sexual response | |
|---|---|
| D2 agonism | Increased sexual desire (anticipation of reward) |
| D2 antagonism | Decreased sexual desire, sexual activity, erection, and ejaculation |
| 5-HT2 agonism | Delay of orgasm |
| 5-HT1a agonism | Activation of sexual behavior, facilitation of orgasm |
| 5-HT2a and 5-HT2c antagonism | Probable stimulation of sexual behaviour |
| α1 antagonism | Central effect: decrease of erection, lubrication, and ejaculation Peripheral effect: may have a stimulating effect on, eg erection |
| α2 antagonism | Stimulation of erection |
| H1 antagonism | Indirect effect on sexual performance through sedation |
| M1 antagonism | Decreased erection and lubrication |
Note: D, dopamine receptor; 5HT, serotonin receptor; α, alpha-adrenergic receptor (or alpha-adrenoceptor); H, histamine receptor; M, muscarinic receptor (a subtype of acetylcholine receptor).
Table 2.
Receptor-binding affinity profiles of most antipsychotics discussed in this article
| D1 | D2 | 5HT1a | 5HT2a | 5HT2c | α1 | α2 | H1 | M1 | |
|---|---|---|---|---|---|---|---|---|---|
| Amisulpride | ? | +++ | 0 | 0 | 0 | 0 | 0 | 0 | ? |
| Aripiprazolea | + | ++++ | +++ | ++ | ++ | ++ | ++ | ++ | 0 |
| Chlorpromazine | + | +++ | 0 | +++ | ++ | +++ | ? | +++ | + |
| Clozapine | + | + | + | +++ | ++ | +++ | + | +++ | +++ |
| Haloperidol | ++ | +++ | 0 | + | 0 | ++ | ++ | + | 0 |
| Olanzapine | ++ | ++ | 0 | +++ | ++ | ++ | + | ++++ | +++ |
| Paliperidone | + | +++ | + | +++ | ++ | +++ | ++ | +++ | + |
| Perphenazine | ? | ++++ | + | +++ | + | ++ | + | +++ | 0 |
| Pimozide | 0 | +++ | + | ++ | 0 | + | ? | 0 | 0 |
| Quetiapine | + | + | + | + | 0 | ++ | + | ++ | + |
| Risperidone | ++ | +++ | + | ++++ | ++ | +++ | +++ | +++ | 0 |
| Sertindole | ? | +++ | ++ | ++++ | ++++ | +++ | + | + | 0 |
| Thioridazine | ++ | ++ | + | ++ | ++ | +++ | ? | ++ | + |
| Ziprasidone | ++ | +++ | ++ | ++++ | ++ | +++ | + | +++ | + |
Note: D, dopamine receptor; 5HT, serotonin receptor; α, alpha-adrenergic receptor (or alpha-adrenoceptor); H, histamine receptor; M, muscarinic receptor (a subtype of acetylcholine receptor). Affinity for receptors: 0 = absent or very low (Ki value >1000); + = low (Ki value 100–1000); ++ = moderate (Ki value 10–100); +++ = high (Ki value 1–10); ++++ = very high (Ki value <1); ? = unknown.
aPartial dopamine agonist.
Other Pharmacological Mechanisms
Besides the affinity for the D2 receptor and other receptors, other pharmacological mechanisms may also influence treatment effects and side effects of antipsychotics, eg passage of the blood brain barrier, metabolization in the liver and presence of an active metabolite.
Passage of the blood brain barrier influences the ratio between the amount of the antipsychotic in the brain and peripheral blood. This may be influenced by the lipophility of a drug and the extent to which an antipsychotic may be actively moved through transporting systems in the blood brain barrier.90 In general, high lipophility of medication predicts good penetration in the brain. Antipsychotics with a poor passage of the blood brain barrier (eg risperidone, paliperidone, and amisulpride), have to be dosed relatively highly to accomplish a central effect, leading to a high peripheral level. The pituitary gland produces prolactin that is regulated by dopamine. The location of the pituitary gland outside the blood brain barrier leads to a considerable rise in prolactin blood levels for hydrophilic antipsychotics due to a higher peripheral-to-central D2 receptor occupancy. If an antipsychotic has active metabolites with an affinity to D2 receptors, it is important that they have a central-to-peripheral ratio as good as that of the parent drug, if not better.90 This is not the case for the metabolite of risperidone, 9-hydroxyrisperidone. This may explain why risperidone, through its hydrophilic metabolite, 9-OH-risperidone, elevates prolactin more than would be expected based on the affinity of the parent compound risperidone for the D2 receptor.5,91,92 Meanwhile, 9-OH-hydroxyrisperidone has been registered as a separate antipsychotic, namely paliperidone. Evidence about paliperidone and sexual dysfunction is still very scarce. It may be hypothesized that detrimental effects on sexual functioning and the increase of prolactin levels will be comparable to or exceed those of risperidone, but to date, (end 2013) there are no studies to support this.
It seems that the central-to-peripheral ratio of D2 occupancy outweighs the impact of other pharmacodynamic considerations such as the modulation of prolactin levels by the serotonin-dopamine interactions in ensuring prolactin-sparing effects.90 This leads to the conclusion that dopamine antagonism and prolactin levels are the most important factors in antipsychotic-induced sexual dysfunction.
Psychosocial Treatment Strategies and General Considerations in Clinical Guidance
It is important to educate patients with schizophrenia on the influence of the disease on different aspects in their life, including their sexuality. As mentioned previously, most patients are willingly and relieved to discuss sexual problems, when this is actively addressed by their clinician.14,30 Many factors can influence sexual functioning in patients with schizophrenia, like the primary illness, antipsychotic treatment, comorbid somatic disorders, relationship factors, the degree of social competence, previous (positive or negative) sexual experiences, or social consequences such as (self)stigmatization and discrimination.1–4,93 When patients attribute sexual dysfunction only to medication, this may increase the risk of nonadherence to medication. This underlines the importance to consider with patients which factors are important in their individual situation, in case of a relationship preferably together with their partner. Also, considering relevant influencing factors may give an indication about appropriate treatment strategies.
Additional psychosocial treatment strategies include (sex)education, patient disorder-specific interventions, and relationship counseling.94,95 In addition, education about sexual risk behavior, prevention of unwanted pregnancies, abortion, and childrearing may be relevant.96
Pharmacological Treatment Strategies for Antipsychotic-Induced Sexual Dysfunction
Knowing the underlying mechanisms of sexual (dys)function in patients with a severe mental illness being treated with antipsychotics may help to give well informed clinical guidance.
Clinicians should actively and routinely ask about undesired treatment effects of antipsychotics, including effects on sexual performance. In clinical consultation, psychological, social, symptom, and medication-related aspects of sexual performance should be disentangled. Also, it is important to try to understand the influence of sexuality on the individual’s overall quality of life, in order to assess the need for adjusting treatment. Explaining to the patient which factors may be involved and outlining the possible treatment alternatives may lead to a shared decision whether to accept a diminished sexual performance or to try to find a solution. Possible treatment alternatives should be discussed.
Knowledge about the different pharmacological properties of antipsychotics related to sexual performance may be helpful in choosing an antipsychotic with a low risk of inducing sexual side effects. A limited number of studies have focused on treatment strategies to reduce sexual dysfunction in patients treated with antipsychotics. Lowering the dose, switching to an antipsychotic with less detrimental effects on sexual functioning, or using adjunctive therapy with, eg, a dopamine agonist, aripiprazole or phosphodiesterase-5-inhibitor (PDE-5-inhibitor) are the main treatment options. The risk that psychiatric symptoms might increase during one of these treatment options should be evaluated in each individual patient. In most studies on switching strategies of antipsychotics or adding a dopamine agonist, an increase in psychotic symptoms is not frequently reported.97,98 In our clinical experience, switching medication in collaboration with the patient, with the explicit aim of finding the best tolerated antipsychotic that is still effective, nearly always leads to a better outcome, although sometimes a return to the original antipsychotic may be necessary.
The review of Nunes98 describes randomized, double-blind controlled studies of treatment aimed to improve sexual dysfunction and/or decrease prolactin levels. Improvement of erectile functioning is described for adjunctive treatment with PDE-5-inhibitors like sildenafil, lodenafil and tadalafil.101 Lowering of elevated prolactin levels and reinstatement of menstruation are reported for adjunctive therapy with aripiprazole.69 Adding selegiline102 or cyproheptadine103 did not seem to improve sexual function. The switch from risperidone to quetiapine was demonstrated in 2 randomized controlled studies, with one showing improvement in sexual functioning and lowering of prolactin levels.72,104
Open label studies98 reported improved sexual functioning for adjunctive therapy with aripiprazole,104–106 vardenafil,108 peony-glycyrrhiza-decoction,109 carbegoline,110 amantadine,111,112 shakuyaku-kanzo-to113, and imipramine.114 Open label studies described improvement in sexual performance when switching from antipsychotics that are strong dopamine antagonists (which often lead to elevated prolactin levels) to aripiprazole,105,115–117 ziprasidone,118,119 olanzapine120–123, and quetiapine124,125 (prolactin sparing antipsychotics); the switch to aripiprazole was the most studied strategy.98
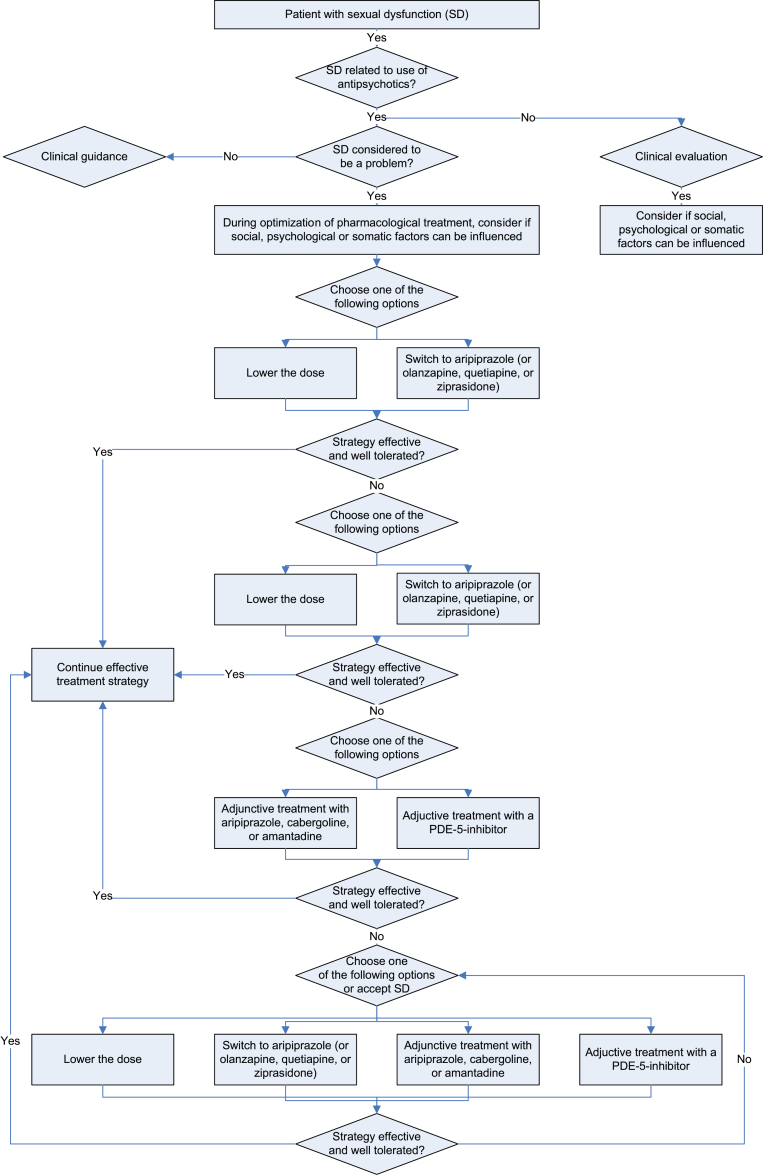
In summary, strategies to treat antipsychotic-induced sexual dysfunction include lowering the dose, switching to a prolactin sparing antipsychotic, adding a dopamine agonist, aripiprazole, or a PDE-5-inhibitor.98 These options are shown in figure 1.
Fig. 1.
Treatment strategies in antipsychotic-induced sexual dysfunction.
Conclusion
A limited number of studies have evaluated sexual functioning in patients with schizophrenia. Most patients with schizophrenia show an interest in sex that differs little from the general population. In contrast, psychiatric symptoms, institutionalization, and psychotropic medication contribute to frequently occurring impairments in sexual functioning. Women with schizophrenia have a better social outcome, longer lasting (sexual) relationships and more offspring than men. Still, in both sexes social and interpersonal impairments limit the development of stable sexual relationships.
Although patients consider sexual problems to be highly relevant, patients and clinicians are reluctant to discuss these spontaneously, leading to underestimation of their prevalence and contributing to decreased adherence to treatment. Studies using structured interviews or questionnaires result in many more patients reporting sexual dysfunctions. A comparison of different antipsychotics showed that high frequencies of sexual dysfunction were found for risperidone and classical antipsychotics, and lower frequencies for clozapine, olanzapine, quetiapine, and aripiprazole.
It is suggested that reduced sexual desire in patients with schizophrenia may also be linked to the general reduction of initiative they experience, often referred to as negative symptoms. Still, antipsychotic medication may be the most prominent cause of sexual problems including reduced sexual desire. Postsynaptic dopamine antagonism, prolactin elevation and α1-receptor blockade may be the most relevant factors in the pathogenesis of antipsychotic-induced sexual dysfunction.
Psychosocial strategies to treat antipsychotic-induced sexual dysfunction include psychoeducation and relationship counseling. Pharmacological strategies include lowering the dose or switching to a prolactin sparing antipsychotic. Also, the addition of a dopamine agonist, aripiprazole or a phosphodiesterase-5 (PDE-5) inhibitor has shown some promising results, but evidence is currently scarce.
Future Research
The fact that sexual side effects of antipsychotics may not subside over time emphasizes the importance of effective treatment strategies for these side effects. As research on this topic is limited, this should be a focus of future research. This should include psychosocial interventions for patients and partners, as well as pharmacological treatment strategies.
Second, more research is needed on the possible interaction between sexual side effects and the symptoms of schizophrenia, eg negative symptoms, lack of motivation and initiative. There may be an overlap in underlying neurobiological mechanisms.
Third, the variation in outcome between different studies could be the result of the study design and the instruments used. Future studies preferably use instruments that are validated for patients a psychotic disorder using antipsychotics. Taking into account the cognitive symptoms that many patients with a psychotic disorder experience, these questionnaires should be relatively short and are simply formulated.
Finally, when clinicians are better informed about sexual functioning in patients with severe mental illness, they will be probably more willingly to discuss this topic with the patient. We hope this article will be of help in this regard.
Acknowledgments
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1.Aizenberg D, Zemishlany Z, Dorfman-Etrog P, Weizman A. Sexual dysfunction in male schizophrenic patients. J Clin Psychiatry. 1995;56:137–141. [PubMed] [Google Scholar]
- 2.Marques TR, Smith S, Bonaccorso S, et al. Sexual dysfunction in people with prodromal or first-episode psychosis. Br J Psychiatry. 2012;201:131–136. [DOI] [PubMed] [Google Scholar]
- 3.Fujii A, Yasui-Furukori N, Sugawara N, et al. Sexual dysfunction in Japanese patients with schizophrenia treated with antipsychotics. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:288–293. [DOI] [PubMed] [Google Scholar]
- 4.Malik P, Kemmler G, Hummer M, et al. Sexual dysfunction in first-episode schizophrenia patients: Results from european first episode schizophrenia trial. J Clin Psychopharmacol. 2011;31:274–280. [DOI] [PubMed] [Google Scholar]
- 5.Knegtering H, van den Bosch R, Castelein S, Bruggeman R, Sytema S, van Os J. Are sexual side effects of prolactin-raising antipsychotics reducible to serum prolactin? Psychoneuroendocrinology. 2008;33:711–717. [DOI] [PubMed] [Google Scholar]
- 6.Baggaley M. Sexual dysfunction in schizophrenia: focus on recent evidence. Hum Psychopharmacol. 2008;23:201–209. [DOI] [PubMed] [Google Scholar]
- 7.Serretti A, Chiesa A. A meta-analysis of sexual dysfunction in psychiatric patients taking antipsychotics. Int Clin Psychopharmacol. 2011;26:130–140. [DOI] [PubMed] [Google Scholar]
- 8.Olfson M, Uttaro T, Carson WH, Tafesse E. Male sexual dysfunction and quality of life in schizophrenia. J Clin Psychiatry. 2005;66:331–338. [DOI] [PubMed] [Google Scholar]
- 9.Haddad PM, Sharma SG. Adverse effects of atypical antipsychotics: differential risk and clinical implications. CNS Drugs. 2007;21:911–936. [DOI] [PubMed] [Google Scholar]
- 10.Finn SE, Bailey JM, Schultz RT, Faber R. Subjective utility ratings of neuroleptics in treating schizophrenia. Psychol Med. 1990;20:843–848. [DOI] [PubMed] [Google Scholar]
- 11.Jacobs P, Bobek SC. Sexual needs of the schizophrenic client. Perspect Psychiatr Care. 1991;27:15–20. [DOI] [PubMed] [Google Scholar]
- 12.Norman JP. Evidence and clinical significance of homosexuality in 100 unanalyzed cases of dementia praecox. J Nerv Ment Dis. 1948;107:484–489. [DOI] [PubMed] [Google Scholar]
- 13.Arieti S. New views on the psychodynamics of schizophrenia. Am J Psychiatry. 1967;124:453–458. [DOI] [PubMed] [Google Scholar]
- 14.Akhtar S, Thomson JA., Jr Schizophrenia and sexuality: a review and a report of twelve unusual cases–Part II. J Clin Psychiatry. 1980;41:166–174. [PubMed] [Google Scholar]
- 15.Connolly FH, Gittleson NL. The relationship between delusions of sexual change and olfactory and gustatory hallucinations in schizophrenia. Br J Psychiatry. 1971;119:443–444. [DOI] [PubMed] [Google Scholar]
- 16.Skopec HM, Rosenberg SD, Tucker GJ. Sexual behavior in schizophrenia. Med Aspects Hum Sex. 1976;10:32. [PubMed] [Google Scholar]
- 17.Perry BL, Wright ER. The sexual partnerships of people with serious mental illness. J Sex Res. 2006;43:174–181. [DOI] [PubMed] [Google Scholar]
- 18.Verhulst J, Schneidman B. Schizophrenia and sexual functioning. Hosp Community Psychiatry. 1981;32:259–262. [DOI] [PubMed] [Google Scholar]
- 19.Abel KM, Drake R, Goldstein JM. Sex differences in schizophrenia. Int Rev Psychiatry. 2010;22:417–428. [DOI] [PubMed] [Google Scholar]
- 20.McGlashan TH, Bardenstein KK. Gender differences in affective, schizoaffective, and schizophrenic disorders. Schizophr Bull. 1990;16:319–329. [DOI] [PubMed] [Google Scholar]
- 21.McEvoy JP, Hatcher A, Appelbaum PS, Abernethy V. Chronic schizophrenic women’s attitudes toward sex, pregnancy, birth control, and childrearing. Hosp Community Psychiatry. 1983;34:536–539. [DOI] [PubMed] [Google Scholar]
- 22.Hilger T, Propping P, Haverkamp F. Is there an increase of reproductive rates in schizophrenics? III. An investigation in Nordbaden (SW Germany): results and discussion. Arch Psychiatr Nervenkr. 1983;233:177–186. [DOI] [PubMed] [Google Scholar]
- 23.Wignall CM, Meredith CE. Illegitimate pregnancies in state institutions. Arch Gen Psychiatry. 1968;18:580–583. [DOI] [PubMed] [Google Scholar]
- 24.Appleby L, Desai PN, Luchins DJ, Gibbons RD, Hedeker DR. Length of stay and recidivism in schizophrenia: a study of public psychiatric hospital patients. Am J Psychiatry. 1993;150:72–76. [DOI] [PubMed] [Google Scholar]
- 25.Odegård O. Fertility of psychiatric first admissions in Norway 1936-1975. Acta Psychiatr Scand. 1980;62:212–220. [DOI] [PubMed] [Google Scholar]
- 26.Nicholson J, Geller JL, Fisher WH. “Sylvia Frumkin” has a baby: a case study for policymakers. Psychiatr Serv. 1996;47:497–501. [DOI] [PubMed] [Google Scholar]
- 27.Raboch J. The sexual development and life of female schizophrenic patients. Arch Sex Behav. 1984;13:341–349. [DOI] [PubMed] [Google Scholar]
- 28.Lyketsos GC, Sakka P, Maïlis A. The sexual adjustment of chronic schizophrenics: a preliminary study. Br J Psychiatry. 1983;143:376–382. [DOI] [PubMed] [Google Scholar]
- 29.Strauss B, Gross J. [Psychotropic drug-induced changes in sexuality–frequency and relevance in psychiatric practice]. Psychiatr Prax. 1984;11:49–55. [PubMed] [Google Scholar]
- 30.Pinderhughes CA, Grace EB, Reyna LJ. Psychiatric disorders and sexual functioning. Am J Psychiatry. 1972;128:1276–1283. [DOI] [PubMed] [Google Scholar]
- 31.Buddeberg C, Furrer H, Limacher B. Sexual problems in schizophrenic patients treated by ambulatory care. Psychiatr Prax. 1988;15:187–191. [PubMed] [Google Scholar]
- 32.Lukoff D, Gioia-Hasick D, Sullivan G, Golden JS, Nuechterlein KH. Sex education and rehabilitation with schizophrenic male outpatients. Schizophr Bull. 1986;12:669–677. [DOI] [PubMed] [Google Scholar]
- 33.McCann E. The expression of sexuality in people with psychosis: breaking the taboos. J Adv Nurs. 2000;32:132–138. [DOI] [PubMed] [Google Scholar]
- 34.Kelly DL, Conley RR. Evaluating sexual function in patients with treatment-resistant schizophrenia. Schizophr Res. 2003;63:195–196. [DOI] [PubMed] [Google Scholar]
- 35.Dossenbach M, Hodge A, Anders M, et al. Prevalence of sexual dysfunction in patients with schizophrenia: international variation and underestimation. Int J Neuropsychopharmacol. 2005;8:195–201. [DOI] [PubMed] [Google Scholar]
- 36.Knegtering H, van der Moolen AE, Castelein S, Kluiter H, van den Bosch RJ. What are the effects of antipsychotics on sexual dysfunctions and endocrine functioning? Psychoneuroendocrinology. 2003;28(suppl 2):109–123. [DOI] [PubMed] [Google Scholar]
- 37.Lingjaerde O, Ahlfors UG, Bech P, Dencker SJ, Elgen K. The UKU side effect rating scale. A new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand Suppl. 1987;334:1–100. [DOI] [PubMed] [Google Scholar]
- 38.Sullivan G, Lukoff D. Sexual side effects of antipsychotic medication: evaluation and interventions. Hosp Community Psychiatry. 1990;41:1238–1241. [DOI] [PubMed] [Google Scholar]
- 39.Dossenbach M, Hodge A, Anders M, et al. Prevalence of sexual dysfunction in patients with schizophrenia: international variation and underestimation. Int J Neuropsychopharmacol. 2005;8:195–201. [DOI] [PubMed] [Google Scholar]
- 40.Mitchell KR, Mercer CH, Ploubidis GB, et al. Sexual function in Britain: findings from the third National Survey of Sexual Attitudes and Lifestyles (Natsal-3). Lancet. 2013;382:1817–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Knegtering R, Castelein S, Bous H, et al. A randomized open-label study of the impact of quetiapine versus risperidone on sexual functioning. J Clin Psychopharmacol. 2004;24:56–61. [DOI] [PubMed] [Google Scholar]
- 42.Knegtering H, Boks M, Blijd C, Castelein S, van den Bosch RJ, Wiersma D. A randomized open-label comparison of the impact of olanzapine versus risperidone on sexual functioning. J Sex Marital Ther. 2006;32:315–326. [DOI] [PubMed] [Google Scholar]
- 43.de Boer MK, Wiersma D, Bous J, et al. A randomized open-label comparison of the impact of aripiprazole versus risperidone on sexual functioning (RAS study). J Clin Psychopharmacol. 2011;31:523–525. [DOI] [PubMed] [Google Scholar]
- 44.Meston CM, Frohlich PF. The neurobiology of sexual function. Arch Gen Psychiatry. 2000;57:1012–1030. [DOI] [PubMed] [Google Scholar]
- 45.Compton MT, Miller AH. Priapism associated with conventional and atypical antipsychotic medications: a review. J Clin Psychiatry. 2001;62:362–366. [DOI] [PubMed] [Google Scholar]
- 46.Sood S, James W, Bailon MJ. Priapism associated with atypical antipsychotic medications: a review. Int Clin Psychopharmacol. 2008;23:9–17. [DOI] [PubMed] [Google Scholar]
- 47.Patel AG, Mukherji K, Lee A. Priapism associated with psychotropic drugs. Br J Hosp Med. 1996;55:315–319. [PubMed] [Google Scholar]
- 48.Bucur M, Mahmood T. Olanzapine-induced clitoral priapism. J Clin Psychopharmacol. 2004;24:572–573. [DOI] [PubMed] [Google Scholar]
- 49.Munarriz R, Kim SW, Kim NN, Traish A, Goldstein I. A review of the physiology and pharmacology of peripheral (vaginal and clitoral) female genital arousal in the animal model. J Urol. 2003;170:S40–4; discussion S44. [DOI] [PubMed] [Google Scholar]
- 50.van Netten JJ, Georgiadis JR, Nieuwenburg A, Kortekaas R. 8-13 Hz fluctuations in rectal pressure are an objective marker of clitorally-induced orgasm in women. Arch Sex Behav. 2008;37:279–285. [DOI] [PubMed] [Google Scholar]
- 51.Ghadirian AM, Chouinard G, Annable L. Sexual dysfunction and plasma prolactin levels in neuroleptic-treated schizophrenic outpatients. J Nerv Ment Dis. 1982;170:463–467. [DOI] [PubMed] [Google Scholar]
- 52.Shader RI, Elkins R. The effects of antianxiety and antipsychotic drugs and sexual behavior. Mod Probl Pharmacopsychiatry. 1980;15:91–110. [DOI] [PubMed] [Google Scholar]
- 53.Girgis SM, Etriby A, el-Hefnawy H, Kahil S. Aspermia: a survey of 49 cases. Fertil Steril. 1968;19:580–588. [DOI] [PubMed] [Google Scholar]
- 54.Kotin J, Wilbert DE, Verburg D, Soldinger SM. Thioridazine and sexual dysfunction. Am J Psychiatry. 1976;133:82–85. [DOI] [PubMed] [Google Scholar]
- 55.van Bruggen M, van Amelsvoort T, Wouters L, Dingemans P, de Haan L, Linszen D. Sexual dysfunction and hormonal changes in first episode psychosis patients on olanzapine or risperidone. Psychoneuroendocrinology. 2009;34:989–995. [DOI] [PubMed] [Google Scholar]
- 56.van Kammen DP, McEvoy JP, Targum SD, Kardatzke D, Sebree TB. A randomized, controlled, dose-ranging trial of sertindole in patients with schizophrenia. Psychopharmacology (Berl). 1996;124:168–175. [DOI] [PubMed] [Google Scholar]
- 57.Gitlin MJ. Psychotropic medications and their effects on sexual function: diagnosis, biology, and treatment approaches. J Clin Psychiatry. 1994;55:406–413. [PubMed] [Google Scholar]
- 58.Ichikawa J, Ishii H, Bonaccorso S, Fowler WL, O’Laughlin IA, Meltzer HY. 5-HT(2A) and D(2) receptor blockade increases cortical DA release via 5-HT(1A) receptor activation: a possible mechanism of atypical antipsychotic-induced cortical dopamine release. J Neurochem. 2001;76:1521–1531. [DOI] [PubMed] [Google Scholar]
- 59.Keitner GI, Selub S. Spontaneous ejaculations and neuroleptics. J Clin Psychopharmacol. 1983;3:34–36. [PubMed] [Google Scholar]
- 60.Ouwehand AJ, Mollema-Schelwald BM, Knegtering H. [The relationship between antipsychotic-induced hyperprolactinemia and menstrual disorders in women with schizophrenia; a systematic review]. Tijdschr Psychiatr. 2012;54:861–868. [PubMed] [Google Scholar]
- 61.Aizenberg D, Modai I, Landa A, Gil-Ad I, Weizman A. Comparison of sexual dysfunction in male schizophrenic patients maintained on treatment with classical antipsychotics versus clozapine. J Clin Psychiatry. 2001;62:541–544. [DOI] [PubMed] [Google Scholar]
- 62.Berger SH. Trifluoperazine and haloperidol: sources of ejaculatory pain? Am J Psychiatry. 1979;136:350. [DOI] [PubMed] [Google Scholar]
- 63.Donnellan P, Breathnach O, Crown JP. Odynorgasmia. Scand J Urol Nephrol. 2001;35:158. [DOI] [PubMed] [Google Scholar]
- 64.Giuliano F, Allard J. Dopamine and sexual function. Int J Impot Res. 2001;13(suppl 3):S18–S28. [DOI] [PubMed] [Google Scholar]
- 65.Bressan RA, Crippa JA. The role of dopamine in reward and pleasure behaviour--review of data from preclinical research. Acta Psychiatr Scand Suppl. 2005;427:14–21. [DOI] [PubMed] [Google Scholar]
- 66.Fitzgerald P, Dinan TG. Prolactin and dopamine: What is the connection? A review article. J Psychopharmacol. 2008;22(2 suppl):12–19. [DOI] [PubMed] [Google Scholar]
- 67.Dickson RA, Glazer WM. Neuroleptic-induced hyperprolactinemia. Schizophr Res. 1999;35 (suppl):S75–S86. [DOI] [PubMed] [Google Scholar]
- 68.Hummer M, Huber J. Hyperprolactinaemia and antipsychotic therapy in schizophrenia. Curr Med Res Opin. 2004;20:189–197. [DOI] [PubMed] [Google Scholar]
- 69.Shim JC, Shin JG, Kelly DL, et al. Adjunctive treatment with a dopamine partial agonist, aripiprazole, for antipsychotic-induced hyperprolactinemia: a placebo-controlled trial. Am J Psychiatry. 2007;164:1404–1410. [DOI] [PubMed] [Google Scholar]
- 70.Rettenbacher MA, Hofer A, Ebenbichler C, et al. Prolactin levels and sexual adverse effects in patients with schizophrenia during antipsychotic treatment. J Clin Psychopharmacol. 2010;30:711–715. [DOI] [PubMed] [Google Scholar]
- 71.Westheide J, Cohen S, Bender S, et al. Sexual dysfunction in psychiatric inpatients the role of antipsychotic medication. Pharmacopsychiatry. 2007;40:140–145. [DOI] [PubMed] [Google Scholar]
- 72.Nakonezny PA, Byerly MJ, Rush AJ. The relationship between serum prolactin level and sexual functioning among male outpatients with schizophrenia or schizoaffective disorder: a randomized double-blind trial of risperidone vs. quetiapine. J Sex Marital Ther. 2007;33:203–216. [DOI] [PubMed] [Google Scholar]
- 73.Howes OD, Wheeler MJ, Pilowsky LS, Landau S, Murray RM, Smith S. Sexual function and gonadal hormones in patients taking antipsychotic treatment for schizophrenia or schizoaffective disorder. J Clin Psychiatry. 2007;68:361–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Knegtering H, Bruggeman R, Castelein S, Wiersma D. [Antipsychotics and sexual functioning in persons with psychoses]. Tijdschr Psychiatr. 2007;49:733–742. [PubMed] [Google Scholar]
- 75.Leysen JE, Gommeren W. The dissociation rate of unlabelled dopamine antagonists and agonists from the dopamine-D2 receptor, application of an original filter method. J Recept Res. 1984;4:817–845. [DOI] [PubMed] [Google Scholar]
- 76.Richtand NM, Welge JA, Logue AD, Keck PE, Jr, Strakowski SM, McNamara RK. Role of serotonin and dopamine receptor binding in antipsychotic efficacy. Prog Brain Res. 2008;172:155–175. [DOI] [PubMed] [Google Scholar]
- 77.Correll CU. From receptor pharmacology to improved outcomes: individualising the selection, dosing, and switching of antipsychotics. Eur Psychiatry. 2010;25(suppl 2):S12–S21. [DOI] [PubMed] [Google Scholar]
- 78.Kennedy SH, Rizvi S. Sexual dysfunction, depression, and the impact of antidepressants. J Clin Psychopharmacol. 2009;29:157–164. [DOI] [PubMed] [Google Scholar]
- 79.Gelenberg AJ, McGahuey C, Laukes C, et al. Mirtazapine substitution in SSRI-induced sexual dysfunction. J Clin Psychiatry. 2000;61:356–360. [DOI] [PubMed] [Google Scholar]
- 80.Thompson JW, Jr, Ware MR, Blashfield RK. Psychotropic medication and priapism: a comprehensive review. J Clin Psychiatry. 1990;51:430–433. [PubMed] [Google Scholar]
- 81.Sanbe A, Tanaka Y, Fujiwara Y, et al. Alpha1-adrenoceptors are required for normal male sexual function. Br J Pharmacol. 2007;152:332–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Smith ER, Lee RL, Schnur SL, Davidson JM. Alpha 2-adrenoceptor antagonists and male sexual behavior: II. Erectile and ejaculatory reflexes. Physiol Behav. 1987;41:15–19. [DOI] [PubMed] [Google Scholar]
- 83.Tallentire D, McRae G, Spedding M, Clark R, Vickery B. Modulation of sexual behaviour in the rat by a potent and selective alpha 2-adrenoceptor antagonist, delequamine (RS-15385-197). Br J Pharmacol. 1996;118:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Floody OR. Cholinergic control of male mating behavior in hamsters: effects of systemic agonist or antagonist treatment. Pharmacol Biochem Behav. 2011;100:289–298. [DOI] [PubMed] [Google Scholar]
- 85.Canevelli M, Talarico G, Tosto G, Troili F, Lenzi GL, Bruno G. Rivastigmine in the treatment of hypersexuality in Alzheimer disease. Alzheimer Dis Assoc Disord. 2013;27:287–288. [DOI] [PubMed] [Google Scholar]
- 86.White JM, Rumbold GR. Behavioural effects of histamine and its antagonists: a review. Psychopharmacology (Berl). 1988;95:1–14. [DOI] [PubMed] [Google Scholar]
- 87.Uckert S, Wilken M, Stief C, Trottmann M, Kuczyk M, Becker A. Is there a significance of histamine in the control of the human male sexual response? Andrologia. 2012;44(suppl 1):538–542. [DOI] [PubMed] [Google Scholar]
- 88.Schotte A, Janssen PF, Gommeren W, et al. Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychopharmacology (Berl). 1996;124:57–73. [DOI] [PubMed] [Google Scholar]
- 89.Richelson E, Souder T. Binding of antipsychotic drugs to human brain receptors focus on newer generation compounds. Life Sci. 2000;68:29–39. [DOI] [PubMed] [Google Scholar]
- 90.Kapur S, Langlois X, Vinken P, Megens AA, De Coster R, Andrews JS. The differential effects of atypical antipsychotics on prolactin elevation are explained by their differential blood-brain disposition: a pharmacological analysis in rats. J Pharmacol Exp Ther. 2002;302:1129–1134. [DOI] [PubMed] [Google Scholar]
- 91.Knegtering R, Baselmans P, Castelein S, Bosker F, Bruggeman R, van den Bosch RJ. Predominant role of the 9-hydroxy metabolite of risperidone in elevating blood prolactin levels. Am J Psychiatry. 2005;162:1010–1012. [DOI] [PubMed] [Google Scholar]
- 92.Melkersson K. Differences in prolactin elevation and related symptoms of atypical antipsychotics in schizophrenic patients. J Clin Psychiatry. 2005;66:761–767. [DOI] [PubMed] [Google Scholar]
- 93.Nestoros JN, Lehmann HE, Ban TA. Sexual behavior of the male schizophrenic: the impact of illness and medications. Arch Sex Behav. 1981;10:421–442. [DOI] [PubMed] [Google Scholar]
- 94.Östman M, Björkman AC. Schizophrenia and relationships: the effect of mental illness on sexuality. Clin Schizophr Relat Psychoses. 2013;7:20–24. [DOI] [PubMed] [Google Scholar]
- 95.Ostman M. Low satisfaction with sex life among people with severe mental illness living in a community. Psychiatry Res. 2014;216:340–345. [DOI] [PubMed] [Google Scholar]
- 96.Miller LJ, Finnerty M. Sexuality, pregnancy, and childrearing among women with schizophrenia-spectrum disorders. Psychiatr Serv. 1996;47:502–506. [DOI] [PubMed] [Google Scholar]
- 97.Haddad PM, Wieck A. Antipsychotic-induced hyperprolactinaemia: mechanisms, clinical features and management. Drugs. 2004;64:2291–2314. [DOI] [PubMed] [Google Scholar]
- 98.Nunes LV, Moreira HC, Razzouk D, Nunes SO, Mari Jde J. Strategies for the treatment of antipsychotic-induced sexual dysfunction and/or hyperprolactinemia among patients of the schizophrenia spectrum: a review. J Sex Marital Ther. 2012;38:281–301. [DOI] [PubMed] [Google Scholar]
- 99.Gopalakrishnan R, Jacob KS, Kuruvilla A, Vasantharaj B, John JK. Sildenafil in the treatment of antipsychotic-induced erectile dysfunction: a randomized, double-blind, placebo-controlled, flexible-dose, two-way crossover trial. Am J Psychiatry. 2006;163:494–499. [DOI] [PubMed] [Google Scholar]
- 100.Nunes LV, Lacaz FS, Bressan RA, Nunes SO, Mari Jde J. Adjunctive treatment with lodenafil carbonate for erectile dysfunction in outpatients with schizophrenia and spectrum: a randomized, double-blind, crossover, placebo-controlled trial. J Sex Med. 2013;10:1136–1145. [DOI] [PubMed] [Google Scholar]
- 101.de Boer MK, Oolders JM, van den Heuvel ER, Wiersma D, Schoevers RA, Knegtering H. Efficacy of tadalafil on erectile dysfunction in male patients using antipsychotics: A double-blind, placebo-controlled, crossover pilot study. J Clin Psychopharmacol. 2014;34(3):380–382. [DOI] [PubMed]
- 102.Kodesh A, Weizman A, Aizenberg D, Hermesh H, Gelkopf M, Zemishlany Z. Selegiline in the treatment of sexual dysfunction in schizophrenic patients maintained on neuroleptics: a pilot study. Clin Neuropharmacol. 2003;26:193–195. [DOI] [PubMed] [Google Scholar]
- 103.Lee HS, Song DH, Kim JH, Lee YM, Han ES, Yoo KJ. Cyproheptadine augmentation of haloperidol in chronic schizophrenic patients: a double-blind placebo-controlled study. Int Clin Psychopharmacol. 1995;10:67–72. [DOI] [PubMed] [Google Scholar]
- 104.Byerly MJ, Nakonezny PA, Rush AJ. Sexual functioning associated with quetiapine switch vs. risperidone continuation in outpatients with schizophrenia or schizoaffective disorder: a randomized double-blind pilot trial. Psychiatry Res. 2008;159:115–120. [DOI] [PubMed] [Google Scholar]
- 105.Mir A, Shivakumar K, Williamson RJ, McAllister V, O’Keane V, Aitchison KJ. Change in sexual dysfunction with aripiprazole: a switching or add-on study. J Psychopharmacol. 2008;22:244–253. [DOI] [PubMed] [Google Scholar]
- 106.Chen JX, Su YA, Wang SL, et al. Aripiprazole treatment of risperidone-induced hyperprolactinemia. J Clin Psychiatry. 2009;70:1058–1059. [DOI] [PubMed] [Google Scholar]
- 107.Yasui-Furukori N, Furukori H, Sugawara N, Fujii A, Kaneko S. Dose-dependent effects of adjunctive treatment with aripiprazole on hyperprolactinemia induced by risperidone in female patients with schizophrenia. J Clin Psychopharmacol. 2010;30:596–599. [DOI] [PubMed] [Google Scholar]
- 108.Mitsonis CI, Mitropoulos PA, Dimopoulos NP, et al. Vardenafil in the treatment of erectile dysfunction in outpatients with chronic schizophrenia: a flexible-dose, open-label study. J Clin Psychiatry. 2008;69:206–212. [DOI] [PubMed] [Google Scholar]
- 109.Yuan HN, Wang CY, Sze CW, et al. A randomized, crossover comparison of herbal medicine and bromocriptine against risperidone-induced hyperprolactinemia in patients with schizophrenia. J Clin Psychopharmacol. 2008;28:264–370. [DOI] [PubMed] [Google Scholar]
- 110.Cavallaro R, Cocchi F, Angelone SM, Lattuada E, Smeraldi E. Cabergoline treatment of risperidone-induced hyperprolactinemia: a pilot study. J Clin Psychiatry. 2004;65:187–190. [DOI] [PubMed] [Google Scholar]
- 111.Correa N, Opler LA, Kay SR, Birmaher B. Amantadine in the treatment of neuroendocrine side effects of neuroleptics. J Clin Psychopharmacol. 1987;7:91–95. [PubMed] [Google Scholar]
- 112.Valevski A, Modai I, Zbarski E, Zemishlany Z, Weizman A. Effect of amantadine on sexual dysfunction in neuroleptic-treated male schizophrenic patients. Clin Neuropharmacol. 1998;21:355–357. [PubMed] [Google Scholar]
- 113.Yamada K, Kanba S, Yagi G, Asai M. Effectiveness of herbal medicine (shakuyaku-kanzo-to) for neuroleptic-induced hyperprolactinemia. J Clin Psychopharmacol. 1997;17:234–235. [DOI] [PubMed] [Google Scholar]
- 114.Aizenberg D, Shiloh R, Zemishlany Z, Weizman A. Low-dose imipramine for thioridazine-induced male orgasmic disorder. J Sex Marital Ther. 1996;22:225–229. [DOI] [PubMed] [Google Scholar]
- 115.Lee BH, Kim YK, Park SH. Using aripiprazole to resolve antipsychotic-induced symptomatic hyperprolactinemia: a pilot study. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:714–717. [DOI] [PubMed] [Google Scholar]
- 116.Lu ML, Shen WW, Chen CH. Time course of the changes in antipsychotic-induced hyperprolactinemia following the switch to aripiprazole. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1978–1981. [DOI] [PubMed] [Google Scholar]
- 117.Byerly MJ, Marcus RN, Tran QV, Eudicone JM, Whitehead R, Baker RA. Effects of aripiprazole on prolactin levels in subjects with schizophrenia during cross-titration with risperidone or olanzapine: analysis of a randomized, open-label study. Schizophr Res. 2009;107:218–222. [DOI] [PubMed] [Google Scholar]
- 118.Montejo AL, Rico-Villademoros F; Spanish Working Group for the Study of Psychotropic-Related Sexual Dysfunction. Changes in sexual function for outpatients with schizophrenia or other psychotic disorders treated with ziprasidone in clinical practice settings: a 3-month prospective, observational study. J Clin Psychopharmacol. 2008;28:568–570. [DOI] [PubMed] [Google Scholar]
- 119.Weiden PJ, Daniel DG, Simpson G, Romano SJ. Improvement in indices of health status in outpatients with schizophrenia switched to ziprasidone. J Clin Psychopharmacol. 2003;23:595–600. [DOI] [PubMed] [Google Scholar]
- 120.Kaneda Y, Kawamura I, Fujii A, Ohmori T. Impact of a switch from typical to atypical antipsychotic drugs on quality of life and gonadal hormones in male patients with schizophrenia. Neuro Endocrinol Lett. 2004;25:135–140. [PubMed] [Google Scholar]
- 121.Kinon BJ, Ahl J, Liu-Seifert H, Maguire GA. Improvement in hyperprolactinemia and reproductive comorbidities in patients with schizophrenia switched from conventional antipsychotics or risperidone to olanzapine. Psychoneuroendocrinology. 2006;31:577–588. [DOI] [PubMed] [Google Scholar]
- 122.Kim KS, Pae CU, Chae JH, et al. Effects of olanzapine on prolactin levels of female patients with schizophrenia treated with risperidone. J Clin Psychiatry. 2002;63:408–413. [DOI] [PubMed] [Google Scholar]
- 123.Lin CY, Wu PL, Pariante CM, Su KP. A crossover study of prolactin changes associated with risperidone and olanzapine. J Clin Psychiatry. 2006;67:1470. [DOI] [PubMed] [Google Scholar]
- 124.Nakajima M, Terao T, Iwata N, Nakamura J. Switching female schizophrenic patients to quetiapine from conventional antipsychotic drugs: effects on hyperprolactinemia. Pharmacopsychiatry. 2005;38:17–19. [DOI] [PubMed] [Google Scholar]
- 125.Byerly MJ, Lescouflair E, Weber MT, et al. An open-label trial of quetiapine for antipsychotic-induced sexual dysfunction. J Sex Marital Ther. 2004;30:325–332. [DOI] [PubMed] [Google Scholar]