Abstract
Background and Objectives
The safety and efficacy of stored red blood cells (RBCs) transfusion has been long debated due to retrospective clinical evidence and laboratory results, indicating a potential correlation between increased morbidity and mortality following transfusion of RBC units stored longer than 14 days. We hypothesize that storage in Optisol additive solution-5 leads to a unique metabolomics profile in the supernatant of stored RBCs.
Materials and Methods
Whole blood was drawn from five healthy donors, RBC units were manufactured, and prestorage leucoreduced by filtration. Samples were taken on days 1 and 42, the cells removed, and mass spectrometry-based metabolomics was performed.
Results
The results confirmed the progressive impairment of RBC energy metabolism by day 42 with indirect markers of a parallel alteration of glutathione and NADPH homeostasis. Moreover, oxidized pro-inflammatory lipids accumulated by the end of storage.
Conclusion
The supernatants from stored RBCs may represent a burden to the transfused recipients from a metabolomics standpoint.
Keywords: oxidative stress, red blood cell, storage, transfusion medicine
Introduction
Red blood cells (RBCs) are still the most widely transfused blood-derived therapeutic, with an annual estimate of approximately 90 million units being transfused worldwide [1, 2]. However, highly questioned retrospective clinical evidence [3] and laboratory investigations [4–6] have fueled the debate about the likely compromised safety and effectiveness of RBCs stored longer than 2 weeks. This is especially true when dealing with certain categories of recipients, such as the injured, critically ill or peri-operative patients [3]. While randomized clinical trials are currently underway [1], the application of Omics technologies (especially proteomics and metabolomics) to transfusion medicine-relevant issues has endowed researchers with unprecedented tools to further dissect the major biochemical events that evolve during prolonged storage of RBC units in the blood bank [7, 8]. Indeed, laboratory studies have highlighted the storage-dependent accumulation of the so-called storage lesions, a long list of biochemical and morphological alterations to RBC integrity and functionality [8], targeting erythrocyte morphology [9], cytosolic and membrane [4, 10] proteome stability.
The documented storage lesions include the progressive metabolic deregulation and depletion of high energy phosphate compounds ATP and DPG, dysregulated cation homeostasis (increases in intracellular Ca2+ and extracellular K+), and the accumulation of reactive oxygen species (ROS), which is partially mitigated by prestorage leucoreduction [4–6, 10]. A compromised capacity to cope with oxidative stress has been associated with the progressive fragmentation and carbonylation of cytosolic and membrane protein components [4–12]. Storage promotes the vesiculation of irreversibly damaged proteins (i.e. fragmented/aggregated or no longer functional/oxidized) [11–13]. Vesiculation is associated with the fluid phase accumulation of potential pro-inflammatory or phagocytosis- stimulating proteins [11–14] and lipid mediators, including isoprostanes and long-chain fatty acids [15]. Stored RBCs are also characterized by decreased exposure of antiphagocytic signals such as CD47 on the membrane of [16], accompanied by the increased exposure on the outer membrane leaflet of pro-phagocytosis [17] markers such as phosphatidylserine [18] and band 3 clusters [10, 19].
Despite numerous shared features, RBCs stored in the presence of different additive solutions respond differently in terms of in vitro measurements and storage lesions to the proteome and metabolome [5, 6, 20–24]. Indeed, recent mass spectrometry-based investigations on the metabolome of RBCs stored in the presence of SAGM [5, 20], AS-1 [6], MAP [21], PAGGGM [22, 23] have highlighted a common trait related to a progressive impairment of energy metabolism in stored erythrocytes. Conversely, distinctive traits were also documented including the additive solution-dependent activation of the pentose phosphate pathway as to fuel antioxidant potential via the generation of NADPH, which drives the reduction of oxidized glutathione back to its reduced form [5, 6]. Only one metabolomics study has so far documented the likely changes to the metabolome of RBC supernatants during storage duration in the presence of CPD-SAGM [5]. Although CPD-SAGM and CPDAS-5 share the same saline (NaCl 150 mm) and glucose (45 mm), AS-5 displays higher adenine (2·2 vs. 1·25 mm in SAGM) and mannitol (45·5 vs. 30 mm in SAGM) concentrations, which result in slightly lower pH (5·5 vs. 5·7) [24]. This is relevant in that differential composition of the additive solution is deemed to influence intracellular metabolism and, consistently, the metabolome of RBC supernatants, which indirectly mirrors the main intracellular storage-dependent catabolic and anabolic events. Therefore, to complement our recent proteomics observations on the very same biological matrix [13], we hypothesize that the metabolome of supernatants from RBCs stored in AS-5 will demonstrate the accumulation of complementary and unique metabolites due to the Optisol AS-5. The obtained results complement current knowledge on the metabolic alterations in the blood bank and pave the way for the designing of alternative additive solutions or provide a theoretical rationale for the implementation of innovative processing strategies [25, 26].
Materials and methods
Sample collection
One unit of whole blood (500 ± 50 ml) was collected from five healthy donors per AABB/FDA guidelines, using CPD with Optisol TM collection bag system (Teruflex; Terumo Corporation, Tokyo, Japan). Plasma was separated from RBCs by centrifugation followed by expression, employing an automated closed system, Compomat G4 (Fresenius-Kabi, Schweinfurt, Germany), and AS-5 (Optisol) was added to a final haematocrit of 50–60%. The estimated amount of residual plasma was 5–10 ml/unit [13]. RBC units were prestorage leucoreduced via filtration using a Pall BPF4 leucoreduction filter (Westbury, NY, USA) and stored at 1–6°C. Samples were obtained through sterile couplers on day (D)1 and D42 (the last day a unit can be transfused). The supernatant was isolated via centrifugation (5000 g for 7 min) followed by a second spin at 12 500 g for 6 min to sediment residual cellular material and contaminating platelets [13]. The supernatants were aliquoted and temporarily stored at −80°C prior to metabolomics analyses.
Metabolomics analyses
Extended details about the protocols adopted for metabolomics analyses are reported in Supplementary File S1. Briefly, samples were prepared using the automated MicroLab STAR® system from Hamilton Company and assayed by GC/MS and LC/MS/MS platforms (either a Thermo-Finnigan Trace DSQ fast-scanning single-quadrupole mass spectrometer using electron impact ionization or Waters ACQUITY UPLC and a Thermo-Finnigan LTQFT mass spectrometer), run either in positive and negative ion modes with adequate buffer, column, phases and gradient adjustments (Supplementary File S1). Compounds were identified by comparison to library entries of purified standards or recurrent unknown entities within a 5 ppm window range. Statistical significance was determined by calculating Welch’s two sample t-test and random forest algorithms to determine significantly (P < 0·05) altered metabolic pathways.
Results
Supernatants of leucoreduced packed RBCs stored in AS-5 were assayed at day 1 (D1) from collection and after 42 days (D42) of refrigerated storage under blood bank conditions. A total of 348 distinct biochemicals were identified either via LC-MS, LC-MS/MS or GC-MS (Table 1). Statistical analyses highlighted the significance (P < 0·05) storage-dependent increases in the concentrations of 101 metabolites, while only 16 metabolites decreased in concentration, whereas 231 remained unchanged. In Figures from 1 to 5, results are graphed as box-plots indicating median values (line), mean values ± upper/lower quartile distributions for D1 and D42 packed AS-5 RBC supernatants.
Table 1.
Concise report of the significant metabolic changes observed in RBC supernatants at storage day 1 and 42
| Welch’s Two Sample t-Test | PRBC-42 PRBC-1 |
|---|---|
| Total number of biochemicals with P ≤ 0·05 | 117 |
| Biochemicals (↑↓) | 101|16 |
| Total number of biochemicals with 0·05 < P < 0·10 | 15 |
| Biochemicals (↑↓) | 10|5 |
From a total of 348 named biochemical.
Welch’s Two Sample t-Test.
Used to determine whether the means of two populations are different.
P-value: evidence that the means are different.
P ≤ 0·05 was taken as significant.
P value trend of 0·05 < P < 0·10 identified biochemicals approaching significance.
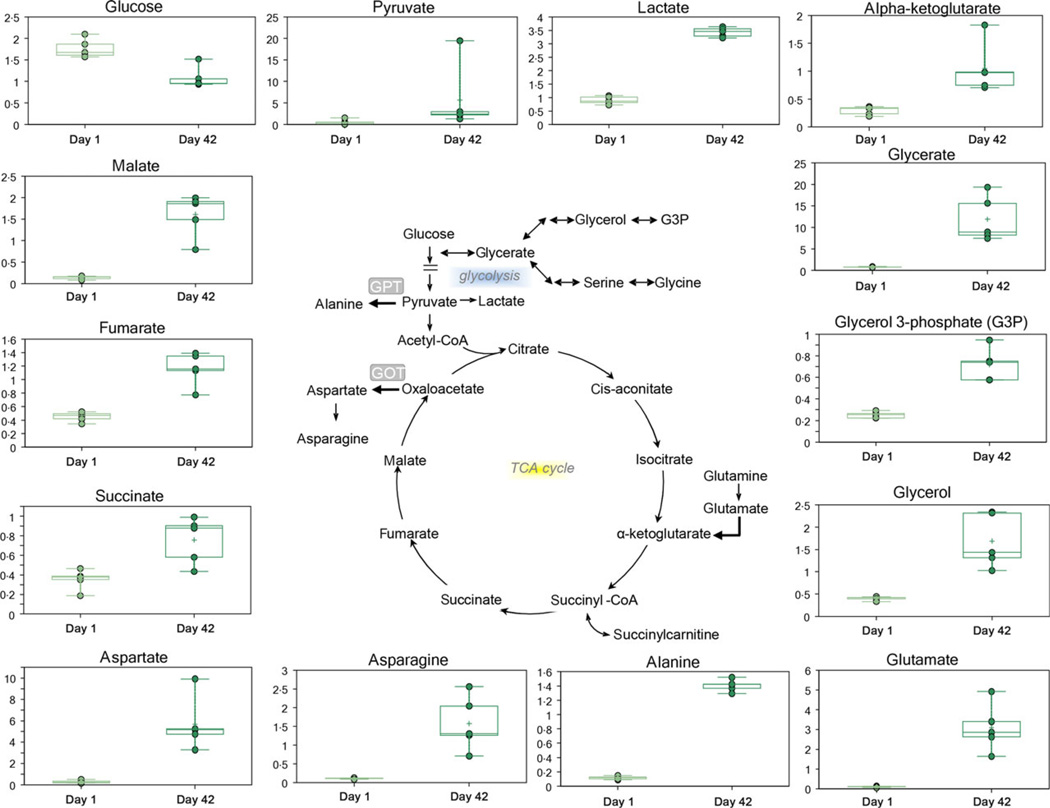
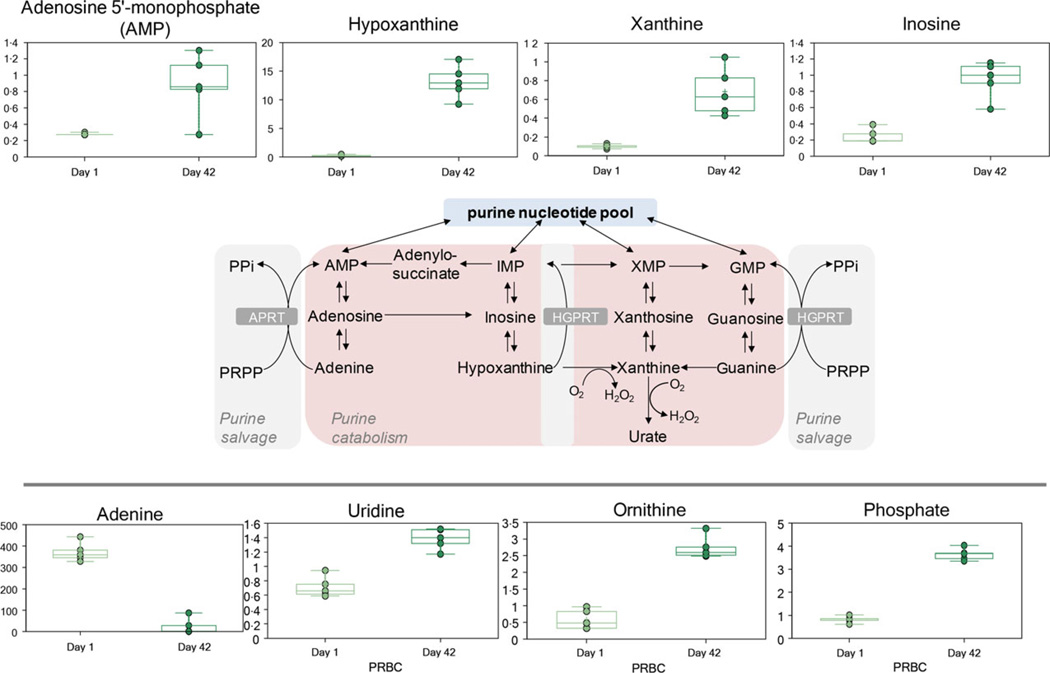
Additive solution nutritive components (glucose, Fig. 1; and adenine, Fig. 2) were progressively consumed during storage duration, while catabolic by-products were observed to accumulate in the supernatant (e.g. lactate, Fig. 1). The accumulation of ATP breakdown products AMP and free phosphate was consistent with a poorer energy state at D42 (Fig. 2). Consistently, adenine was significantly consumed by the end of the storage period, and its by-products accumulated as a result (Fig. 2). These metabolites included purine catabolites (hypoxanthine, xanthine and inosine) either derived from adenine and adenosine deamination to hypoxanthine or inosine, respectively.
Fig. 1.
An overview of glycolysis, cytosolic tricarboxylic acids, glycerol phosphate metabolism and transaminase.
Fig. 2.
An overview of purine metabolism.
By-products of transamination reactions glutamate and alanine increased in the supernatants of stored RBCs, together with glutamate precursor glutamine and glutamate-derived transamination product ketoglutarate (Fig. 1). Other tricarboxylic acids such as succinate, fumarate and malate were found to accumulate in the supernatants of stored packed RBCs (Fig. 1).
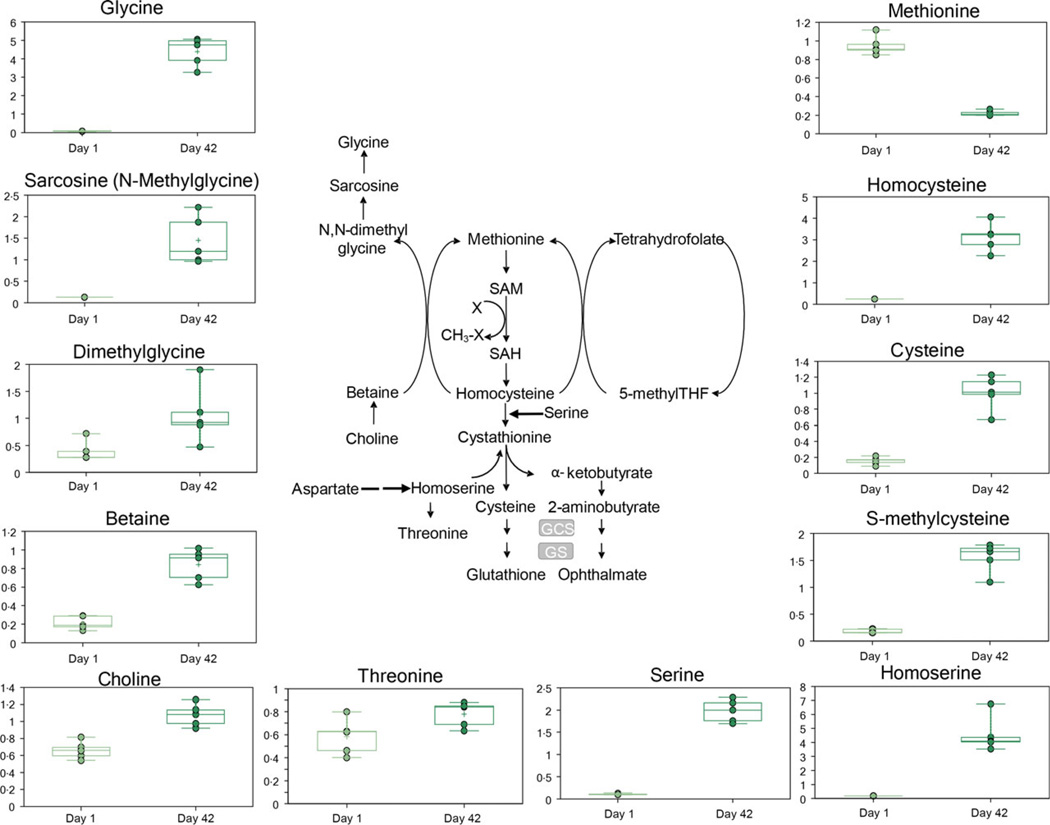
D42 supernatants also were characterized by increased levels of serine, together with its derivatives via one-carbon metabolism and cysteine biosynthesis/methionine trans-sulphuration, glycine and cysteine, respectively (Fig. 3). Several intermediates related to serine metabolism (e.g. serine, glycine, homoserine and threonine) and methionine salvage were more abundant at D42 and included homocysteine, intermediates to methyl donation by betaine (choline, betaine, dimethylglycine, sarcosine and glycine) as well as cysteine metabolites (cysteine, cystine and S-methylcysteine – Fig. 3). However, methionine was the only amino acid that decreased at the end of storage (Fig. 3).
Fig. 3.
An overview of one-carbon metabolism/serine biosynthesis and methionine metabolism.
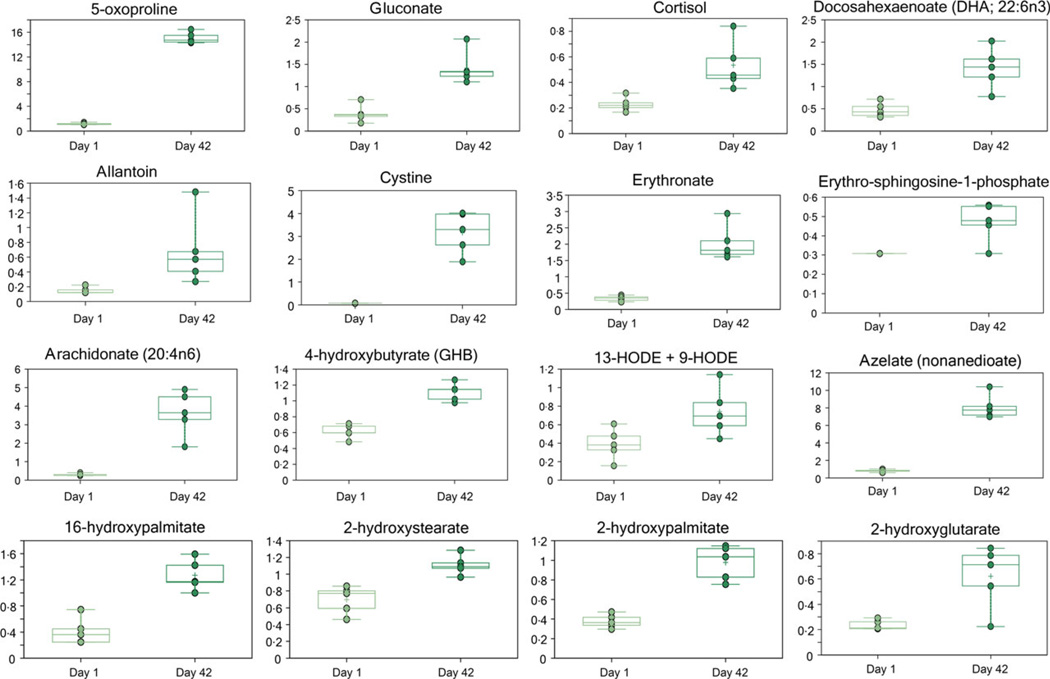
Several metabolites associated with (oxidative) stress responses were elevated at D42 of storage, including 5-oxoproline, gluconate, cortisol, allantoin, cystine, erythronate and oxidized lipids (9 + 13-HODE, 2-hydroxystearate and 2-hydroxypalmitate-Fig. 4). In addition to hydroxylipids, other signalling lipids were also more abundant at D42. The inflammation signalling lipid arachidonate was elevated more than other free fatty acids; however, none of the lysophospholipids increased significantly during RBC storage (Fig. 4, Fig. S1). Erythro-sphingosine-1-phosphate was more abundant at D42 (Fig. 4).
Fig. 4.
Oxidative stress soluble and lipid markers.
Various catabolites of membrane phospholipids were more abundant at D42 and included phospholipid head groups (ethanolamine and choline), glycerol, glycerol-3-phosphate and glycerophosphorylcholine (Fig. 1). Fatty acid catabolites including essential fatty acids, medium chain fatty acids (caproate, heptanoate, caprylate, pelargonate and caprate), long-chain fatty acids (LCFA: palmitate, margarate and stearate) and carnitine conjugates of LCFA (palmitoylcarnitine and oleoylcarnitine) were more abundant in storage fluid at D42 (Fig. S1).
Storage also promoted the progressive leaching from the plastic bags of the phthalate plasticizer (Fig. S2). Storage-dependent accumulation of heme and biliverdin was observed as well (Fig. S2).
Discussion
Storage corresponded to a progressive metabolic enrichment of the D42 RBC supernatants. This result was largely expected in the light of the simplicity of the composition of the Optisol AS-5 additive solution [24]. In this view, additive solution nutritive components (glucose – Fig. 1; and adenine – Fig. 2) were progressively consumed, while catabolic by-products accumulated in the supernatant of stored RBCs.
Energy metabolism, detoxification routes
Previous studies on stored RBC metabolomics have documented a progressive deregulation of glycolysis in response to the acidification of the intracellular environment [5]. However, although glycolysis rate decreases as pH falls, pyruvate and lactate progressively accumulate during storage [5, 6, 22–24]. Intracellular lactate accumulation would result in increased levels of lactate in the supernatant as well (Fig. 1), since H+-monocarboxylate cotransportermediated efflux of lactate from RBCs is proportional to the intracellular levels of this metabolite [27].
In stored erythrocytes, lactate generation via the Embden-Meyerhof glycolytic pathway is not sufficient to sustain ATP production and 2,3-diphosphoglycerate levels [5, 6, 20–22]. This is relevant in the light of the role of these compounds as energy tokens to be spent to promote cell survival and to stabilize the deoxygenated ‘T’ state of haemoglobin. In the present study, the accumulation of ATP breakdown products AMP and free phosphate was consistent with a poorer energy state at storage D42 (Fig. 2). Energy impairment during storage might be associated with the vesiculation of rate-limiting glycolytic enzymes (such as glyceraldehyde 3-phosphate dehydrogenase [28]). Alternatively, energy metabolism fluxes might decline in response to storage-dependent alterations of the multimeric organization of key enzymes, including glyceraldehyde 3-phosphate dehydrogenase, lactate dehydrogenase and biphosphoglycerate mutase [29].
From glycolysis to branching pathways
RBCs contain at least 2289 distinct proteins, and most are involved in energy and redox metabolism [30]. Maintenance of the redox poise in RBCs is mainly attributable to glutathione, a tripeptide of glutamate, glycine and cysteine whose synthesis is sustained in an ATP-dependent fashion to promote RBC survival in response to oxidative stress [7, 8, 20]. During prolonged storage, regeneration of reduced glutathione from its oxidized form is a key factor determining RBC survival [20]. Branching from glycolysis, the PPP generates NADPH and thus preserves glutathione homeostasis by balancing oxidized and reduced forms of the gamma-glutamylcysteine-glycine tripeptide. Fluxes to the PPP are modulated by competitive binding of glycolytic enzymes and deoxyhaemoglobin to the N-terminal cytosolic domain of band 3 [31]. Routine storage promotes a caspase and ROS-mediated fragmentation of this domain [8], thereby impairing RBC capacity to cope with oxidative stress by promoting NADPH generation via the PPP [5, 6, 28]. Therefore, stored RBCs are characterized by a significant accumulation of ROS and oxidative stress markers to proteins (carbonylation) and lipids (malondialdehyde) soon after the second week of storage [4, 5, 11].
Transaminases and the NADH reservoirs
Recent studies on RBCs stored in AS-1 (which contains more than twice as much glucose than SAGM or AS-5:111 vs. 45 mm) demonstrated that additive solutions other than SAGM might better preserve energy modulation in stored erythrocytes, while fuelling metabolic pathways branching from glycolysis other than the hexose monophosphate shunt [5]. Biosynthesis of glutathione would benefit from a sustained accumulation of its precursors: glutamate, glycine and cysteine, the rate-limiting substrate. The presented data demonstrated a significant increase in glutamate (Fig. 1). Glutamate could be produced by glutamine metabolism or by the aspartate-dependent transamination of ketoglutarate by glutamate oxaloacetate transaminase (GOT). The presented data showed increased levels of all these metabolites in older units (Fig. 1). Increased levels of ketoglutarate despite increased glutamate concentrations can be attributed to the balancing activity of another transaminase. Indeed, glutamate can also result from the activity of glutamate pyruvate transaminase (GPT), either producing alanine and ketoglutarate (both increasing in Fig. 1) from pyruvate and glutamate or vice versa. Therefore, GPT would decrease the intracellular accumulation of pyruvate and relieve the burden on lactate dehydrogenase. In parallel, this mechanism would preserve a reservoir of NADH from lactate dehydrogenase-mediated oxidation to NAD+, which is relevant to the pivotal antioxidant activity of NADH-dependent cytochrome b5 methaemoglobin reductase [7].
Although being devoid of mitochondria, RBCs still host cytosolic isoforms of NADP-dependent isocitrate dehydrogenase, and NAD-dependent malate dehydrogenase and fumarate hydratase [32]. Their activity promotes NADH and NADPH generation, and thus fuelling antioxidant pathways involving glutathione reductase and cytochrome b5 methaemoglobin reductase [7, 32]. The presented data suggest that stored RBCs could be capable of fulfilling the same goal in maintaining NADH homeostasis by coupling glycolytic fluxes to cytosolic TCA cycle enzymes. Further studies are mandatory within the framework of RBC storage for transfusion, since it is known that transaminase activities are negatively influenced by RBC age in vivo [32, 33].
These results should be also interpreted in the light of the fact that the TCA cycle is indeed present in the contaminating platelets and leucocytes, since prestorage leuco- and platelet reduction only removes 3·5 logs and ~2 logs, respectively. However, organic acids in RBC supernatants might play a role in positively influencing hypoxia-related responses as they can take part in the modulation of the life span of hypoxia inducible factor alpha (HIFα) by inhibiting its degradation via prolyl hydroxylase in the endothelial cells of the recipient [34] or rather contribute to ketoacidosis upon transfusion in trauma patients.
One-carbon and methionine metabolism to fuel cysteine and glycine generation as to sustain GSH biosynthesis
The supernatants of D42 RBCs also included increased levels of serine, as well as glycine and cysteine, which are derived from serine through one-carbon metabolism and cysteine biosynthesis/methionine trans-sulphuration pathways, respectively (Fig. 3). This finding has been reported in other cell models, such as tumour cells, in which one-carbon metabolism hyperactivity may occur in response to oxidative stress to fuel NADPH generation [35] and glutathione biosynthesis [36]. Analogously, Roback et al. [6] documented a storage-dependent increases in serine, cysteine and methionine-related metabolic pathways. Methionine may be generated by salvage from homocysteine, which uses betaine (Fig. 3) as a methyl donor or employs a reaction cycle that requires methyltetrahydrofolate and pyridoxal phosphate as cofactors in a NADPH generating pathway at the dihydrofolate reductase and methylenetetrahydrofolate reductase (MTHFR) activity steps.
Accumulation of metabolites involved in serine and cysteine metabolism (Fig. 3) may be partly attributable to decreased methionine salvage and increased cysteine synthesis, in line with the posited increase of GSH biosynthesis during RBC storage AS-5[5, 20]. Lastly, Homocysteine accumulation (hyperhomocysteinaemia) is known to be caused by the decreased activity of MTHFR enzyme, or low levels of folate, B12 or B6, and predispose patients who receive stored blood to untoward vascular consequences [37].
Nucleotide metabolism
Red blood cells rely on salvage instead of de novo synthesis pathways to sustain nucleotide metabolism [7]. Consistently, despite AS5 formula containing a twofold higher dose of adenine than in SAGM, adenine in the supernatant was significantly consumed by the end of the storage period, and its deamination by-product, hypoxanthine, accumulated as a result (Fig. 2). These results are consistent with previous reports by Zolla’s group on CPD-SAGM, RBCs stored under normoxic [5] or anaerobic conditions [38]. Purine catabolism can also contribute to oxidative stress through the activity of xanthine oxidase, which generates ROS [38].
Oxidative stress, plasticizer, haemolysis, fatty acids and vesiculation
Storage of RBCs promotes oxidative stress to the metabolome [4–6, 22–24], lipidome (isoprostanes, malondialdehyde) [5] and proteome (protein carbonylation, fragmentation, nonenzymatic glycation of haemoglobin and membrane proteins, relocation to the membrane of cytosolic antioxidant enzymes such as peroxiredoxin 2) [4, 7–11, 39, 40]. Several metabolites associated with stress responses and oxidative stress were elevated at D42 of storage, including 5-oxoproline. Oxoproline is involved in glutathione metabolism and was more abundant at D42 (Fig. 4) and recently reported to accumulate in SAGM-stored RBCs as a marker of impaired intra-cellular GSH homeostasis [20]. Gluconate accumulation reflects a ROS-dependent oxidation of glucose in the supernatant, which is consistent with previous observations in SAGM (Fig. 4) [5]. The stress steroid cortisol was more abundant at D42 (Fig. 4), probably as a result of the release from RBCs which can uptaken cortisol while circulating in vivo [41]. The observed increases in allantoin (a purine catabolite made nonenzymatically by ROS in humans), cystine (the oxidized form of cysteine), erythronate (an oxidized aminosugar possibly derived from glycated proteins) and hydroxylipid signalling molecules (9 + 13-HODE, 2-hydroxystearate and 2-hydroxypalmitate) are also consistent with oxidative stress (Fig. 4).
Erythro-sphingosine-1-phosphate, a sphingolipid signalling molecule that regulates cell survival and inflammatory responses, was more abundant at D42 (Fig. 4). Collectively these changes document a significant increase in stress responses at D42 of storage.
In addition arachidonate increased significantly during storage of LR-RBC units identical to previous work [42]. Arachidonate has pro-inflammatory properties and demonstrated the ability to prime the NADPH oxidase of neutrophils and participate with the other neutral lipids, 5-, 12-, and 15-hydroxyeicosatetraenoic acid (HETE) that accumulate during routine storage of LR-RBCs in causing TRALI in a two-event animal model. In addition, lysophosphatidylcholines did not increase during storage of leucoreduced RBCs, in agreement with previous data, and likely due to the significant platelet reduction, 2 logs, by the prestorage leucoreduction filters (Haemonetics BPF4) employed in these experiments [43].
The accumulation of lipid pro-inflammatory mediators might hold potential pitfalls to certain categories of recipients and might thus indicate the necessity to consider supernatant washing prior to the administration of longer stored units [26].
Previous studies have documented storage-dependent progressive leaching from the plastic bags and intercalation in RBC membranes of the phthalate plasticizer (Fig. S2), a potential toxic compound whose detection in the urine has been recently proposed as a diagnostic test for the detection of illicit autologous blood doping practices in endurance sports [7].
Prolonged storage also results in the increased shedding of membrane portions in the form of micro and nanovesicles [12]. Such a vesiculation process ends up affecting RBC morphology during storage duration, as erythrocytes lose their discocyte shape and acquire a spheroechinocytic phenotype [8, 9]. Such processes of membrane blebbing and vesiculation gradually compromise RBC capacity to cope with osmotic stresses [9]. Recent lipidomics studies have documented the preferential enrichment of membrane blebs with certain classes of lipids, especially ceramides (through the activity of sphingomyelinases), glycerophospholipids and sterols [15]. Glycerophospholipids, in particular, can be catabolized to generate signalling molecules and substrates that enter glycolysis in RBCs for energy production. In this study, the accumulation of several catabolites of membrane phospholipids in D42 supernatants indirectly testifies an exacerbated metabolism of these compounds, as gleaned from the accumulation of phospholipid head groups (ethanolamine and choline), glycerol, glycerol-3-phosphate and glycerophosphorylcholine (Fig. 1). These metabolites can be generated from phospholipase activity toward membrane phospholipids. Glycerate, either generated from glycerol, or as a by-product of one-carbon metabolism from glycine, was also more abundant at D42 (Fig. 1).
While RBCs are devoid of mitochondria and are thus incapable of catabolizing fatty acids, they also show an incomplete de novo long-chain fatty acid biosynthesis enzymatic machinery [7]. Storage-dependent accumulation of fatty acid catabolites (including essential fatty acids, medium chain fatty acids, long-chain fatty acids and carnitine conjugates of LCFA) was observed in RBC supernatants (Fig. S1). Since they cannot derive from de novo synthesis, these molecules are likely to be released from RBC into the supernatant by means of the vesiculation process.
Finally, heme and biliverdin accumulated during routine RBC storage (Fig. S2), as if heme metabolism was blocked downstream to biliverdin reductase. These results are consistent with the age-dependent impairment in heme metabolism [7] and are consistent with the altered native multimerization of biliverdin reductase during storage progression, as recently gleaned via native preparative native 2D-CN-SDS-PAGE analyses (Pallotta et al., paper in preparation).
Conclusion
In the present study, we performed metabolomic analyses of supernatants from RBCs stored in the presence of AS-5, as an indirect mirror of intracellular metabolism. As a result, we demonstrated the accumulation of prooxidant and pro-inflammatory compounds in the supernatants from stored units. Existing evidence regarding the progressive impairment of RBC energy metabolism by the end of the shelf life of packed RBCs was confirmed. At the same time, we present indirect evidence for altered NADPH and glutathione homeostasis, which in AS-5 RBCs could be promoted by the activity of transaminases, cytosolic isoforms of TCA cycle enzymes, serine/one-carbon and methione trans-sulphuration metabolism.
These data explain how glycolysis, nucleotide and fatty acid metabolism were consistent with the progressive depletion of energy substrates from the additive solution and the accumulation of oxidized pro-inflammatory lipid derivatives, likely originating from membrane shed vesicles. While these results had been anticipated by metabolomics investigation on the RBC cytosolic fraction, no study had hitherto addressed the metabolome of the AS-5 RBC supernatants. The presented results also document that the supernatants of stored RBCs could represent a burden to the transfused recipients not only from a proteomics standpoint, as reported [13], but also from a metabolomics perspective.
Acknowledgments
The Authors are grateful to Drs. Jan Jones and Elizabeth Kensicki (Metabolon Inc., Durham, NC) for technical assistance with metabolomics analyses. Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Numbers P50GM049222 and T32GM008315, and Grant #P50 GM049222 from NIGMS, NIH (CS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest
The authors disclose no conflict of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1 Free short, medium and long chain fatty acids.
Figure S2 Heme metabolism and phthalate levels.
Data S1 Materials and Methods extended version.
References
- 1.Flegel WA, Natanson C, Klein HG. Does prolonged storage of red blood cells cause harm? Br J Haematol. 2014;165:3–16. doi: 10.1111/bjh.12747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hess JR. Measures of stored red blood cell quality. Vox Sang. 2014;107:1–9. doi: 10.1111/vox.12130. [DOI] [PubMed] [Google Scholar]
- 3.Lelubre C, Piagnerelli M, Vincent J-L. Association between duration of storage of transfused red blood cells and morbidity and mortality in adult patients: myth or reality? Transfusion. 2009;49:1384–1394. doi: 10.1111/j.1537-2995.2009.02211.x. [DOI] [PubMed] [Google Scholar]
- 4.D’Alessandro A, D’Amici GM, Vaglio S, et al. Time-course investigation of SAGM-stored leukocyte-filtered red bood cell concentrates: from metabolism to proteomics. Haematologica. 2012;97:107–115. doi: 10.3324/haematol.2011.051789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gevi F, D’Alessandro A, Rinalducci S, et al. Alterations of red blood cell metabolome during cold liquid storage of erythrocyte concentrates in CPDSAGM. J Proteomics. 2012;76:68–80. doi: 10.1016/j.jprot.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 6.Roback JD, Josephson CD, Waller EK, et al. Metabolomics of ADSOL (AS-1) Red Blood Cell Storage. Transfus Med Rev. 2014;28:41–55. doi: 10.1016/j.tmrv.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D’Alessandro A, Zolla L. Biochemistry of red cell aging in vivo and storage lesions. Haematologica. 2013;7:389–396. [Google Scholar]
- 8.D’Alessandro A, Kriebardis A, Rinalducci S, et al. An update on red blood cell storage lesions, as gleaned through biochemistry and omics technologies. Transfusion. 2014 doi: 10.1111/trf.12804. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Blasi B, D’Alessandro A, Ramundo N, et al. Red blood cell storage and cell morphology. Transfus Med. 2012;22:90–96. doi: 10.1111/j.1365-3148.2012.01139.x. [DOI] [PubMed] [Google Scholar]
- 10.Antonelou MH, Tzounakas VL, Velentzas AD, et al. Effects of pre-storage leukoreduction on stored red blood cells signaling: a time-course evaluation from shape to proteome. J Proteomics. 2012;76:220–238. doi: 10.1016/j.jprot.2012.06.032. [DOI] [PubMed] [Google Scholar]
- 11.Delobel J, Prudent M, Rubin O, et al. Subcellular fractionation of stored red blood cells reveals a compartment-based protein carbonylation evolution. J Proteomics. 2012;76:181–193. doi: 10.1016/j.jprot.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Bosman GJCGM, Lasonder E, Groenen-Döpp YAM, et al. The proteome of erythrocyte-derived microparticles from plasma: new clues for erythrocyte aging and vesiculation. J Proteomics. 2012;76:203–210. doi: 10.1016/j.jprot.2012.05.031. [DOI] [PubMed] [Google Scholar]
- 13.Dzieciatkowska M, Silliman CC, Moore EE, et al. Proteomic analysis of the supernatant of red blood cell units: the effects of storage and leucoreduction. Vox Sang. 2013;105:210–218. doi: 10.1111/vox.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rubin O, Delobel J, Prudent M, et al. Red blood cell-derived microparticles isolated from blood units initiate and propagate thrombin generation. Transfusion. 2013;53:1744–1754. doi: 10.1111/trf.12008. [DOI] [PubMed] [Google Scholar]
- 15.Bicalho B, Holovati JL, Acker JP. Phospholipidomics reveals differences in glycerophosphoserine profiles of hypothermically stored red blood cells and microvesicles. Biochim Biophys Acta. 2013;1828:317–326. doi: 10.1016/j.bbamem.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 16.Sparrow R, Healey G. Reduced expression of CD47 on stored red blood cells. Transfusion. 2006;46:1263. doi: 10.1111/j.1537-2995.2006.00883.x. [DOI] [PubMed] [Google Scholar]
- 17.Veale MF, Healey G, Sparrow RL. Longer storage of red blood cells is associated with increased in vitro erythrophagocytosis. Vox Sang. 2014;106:219–226. doi: 10.1111/vox.12095. [DOI] [PubMed] [Google Scholar]
- 18.Kriebardis AG, Antonelou MH, Stamoulis KE, et al. Storage-dependent remodeling of the red blood cell membrane is associated with increased immunoglobulin G binding, lipid raft rearrangement, and caspase activation. Transfusion. 2007;47:1212–1220. doi: 10.1111/j.1537-2995.2007.01254.x. [DOI] [PubMed] [Google Scholar]
- 19.Lutz HU, Bogdanova A. Mechanisms tagging senescent red blood cells for clearance in healthy humans. Front Physiol. 2013;4:387. doi: 10.3389/fphys.2013.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pertinhez TA, Casali E, Lindner L, et al. Biochemical assessment of red blood cells during storage by (1)H nuclear magnetic resonance spectroscopy. Identification of a biomarker of their level of protection against oxidative stress. Blood Transfus. 2014 doi: 10.2450/2014.0305-13. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sparrow RL, Sran A, Healey G, et al. In vitro measures of membrane changes reveal differences between red blood cells stored in saline-adenine-glucose-mannitol and AS-1 additive solutions: a paired study. Transfusion. 2014;54:560–568. doi: 10.1111/trf.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishino T, Yachie-Kinoshita A, Hirayama A, et al. In silico modeling and metabolome analysis of long-stored erythrocytes to improve blood storage methods. J Biotechnol. 2009;144:212–223. doi: 10.1016/j.jbiotec.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 23.Burger P, Korsten H, De Korte D, et al. An improved red blood cell additive solution maintains 2,3-diphosphoglycerate and adenosine triphosphate levels by an enhancing effect on phosphofructokinase activity during cold storage. Transfusion. 2010;50:2386–2392. doi: 10.1111/j.1537-2995.2010.02700.x. [DOI] [PubMed] [Google Scholar]
- 24.Nishino T, Yachie-Kinoshita A, Hirayama A, et al. Dynamic simulation and metabolome analysis of long-term erythrocyte storage in adenine-guanosine solution. PLoS ONE. 2013;8:e71060. doi: 10.1371/journal.pone.0071060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sparrow RL. Time to revisit red blood cell additive solutions and storage conditions: a role for “omics” analyses. Blood Transfus. 2012;10:s7–s11. doi: 10.2450/2012.003S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sowemimo-Coker SO. Evaluation of an experimental filter designed for improving the quality of red blood cells (RBCs) during storage by simultaneously removing white blood cells and immunomodulators and improving RBC viscoelasticity and Band 3 proteins. Transfusion. 2014;54:592–601. doi: 10.1111/trf.12330. [DOI] [PubMed] [Google Scholar]
- 27.Wahl P, Yue Z, Zinner C, et al. A mathematical model for lactate transport to red blood cells. J Physiol Sci. 2011;61:93–102. doi: 10.1007/s12576-010-0125-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Messana I, Ferroni L, Misiti F, et al. Blood bank conditions and RBCs: the progressive loss of metabolic modulation. Transfusion. 2000;40:353–360. doi: 10.1046/j.1537-2995.2000.40030353.x. [DOI] [PubMed] [Google Scholar]
- 29.Pallotta V, D’Alessandro A, Rinalducci S, et al. Native protein complexes in the cytoplasm of red blood cells. J Proteome Res. 2013;12:3529–3546. doi: 10.1021/pr400431b. [DOI] [PubMed] [Google Scholar]
- 30.Goodman SR, Daescu O, Kakhniashvili DG, et al. The proteomics and interactomics of human erythrocytes. Exp Biol Med (Maywood, NJ) 2013;238:509–518. doi: 10.1177/1535370213488474. [DOI] [PubMed] [Google Scholar]
- 31.Chu H, Low PS. Mapping of glycolytic enzyme-binding sites on human erythrocyte band 3. Biochem J. 2006;400:143–151. doi: 10.1042/BJ20060792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arai T, Takahashi M, Araki K, et al. Activities of enzymes related to the malate-aspartate shuttle in the blood cells of thoroughbred horses undergoing training exercise. Vet Res Commun. 2001;25:577–583. doi: 10.1023/a:1017977200420. [DOI] [PubMed] [Google Scholar]
- 33.Robinson Y, Cristancho E, Böning D. An optimized method for the assay of the red blood cell-age-related enzyme aspartate aminotransferase. Lab Hematol. 2004;10:144–146. doi: 10.1532/LH96.04047. [DOI] [PubMed] [Google Scholar]
- 34.Koivunen P, Hirsilä M, Remes AM, et al. Inhibition of hypoxia-inducible factor (HIF) hydroxylases by citric acid cycle intermediates: possible links between cell metabolism and stabilization of HIF. J Biol Chem. 2007;282:4524–4532. doi: 10.1074/jbc.M610415200. [DOI] [PubMed] [Google Scholar]
- 35.Fan J, Ye J, Kamphorst JJ, et al. Quantitative flux analysis reveals folate-dependent NADPH production. Nature. 2014;510:298–302. doi: 10.1038/nature13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maddocks ODK, Berkers CR, Mason SM, et al. Serine starvation induces stress and p53-dependent metabolic remodelling in cancer cells. Nature. 2013;493:542–546. doi: 10.1038/nature11743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hankey GJ, Eikelboom JW. Homocysteine and vascular disease. Lancet. 1999;354:407–413. doi: 10.1016/S0140-6736(98)11058-9. [DOI] [PubMed] [Google Scholar]
- 38.D’Alessandro A, Gevi F, Zolla L. Red blood cell metabolism under prolonged anaerobic storage. Mol BioSyst. 2013;9:1196–1209. doi: 10.1039/c3mb25575a. [DOI] [PubMed] [Google Scholar]
- 39.D’Alessandro A, Mirasole C, Zolla L. Haemoglobin glycation (Hb1Ac) increases during red blood cell storage: a I-TOF mass-spectrometry-based investigation. Vox Sang. 2013;105:177–180. doi: 10.1111/vox.12029. [DOI] [PubMed] [Google Scholar]
- 40.Sparrow RL, Veale MF, Healey G, et al. Red blood cell (RBC) age at collection and storage influences RBC membrane-associated carbohydrates and lectin binding. Transfusion. 2007;47:966–968. doi: 10.1111/j.1537-2995.2007.01230.x. [DOI] [PubMed] [Google Scholar]
- 41.Farese RV, Plager JE. The in vitro red blood cell uptake of c14-cortisol; studies of plasma protein binding of cortisol in normal and abnormal states. J Clin Invest. 1962;41:53–60. doi: 10.1172/JCI104466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Silliman CC, Moore EE, Kelher MR, et al. Identification of lipids that accumulate during the routine storage of prestorage leukoreduced red blood cells and cause acute lung injury. Transfusion. 2011;51:2549–2554. doi: 10.1111/j.1537-2995.2011.03186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vetlesen A, Mirlashari MR, Akkök CA, et al. Biological response modifiers in photochemically pathogen-reduced versus untreated apheresis platelet concentrates. Transfusion. 2013;53:147–155. doi: 10.1111/j.1537-2995.2012.03681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]