Abstract
Mesenchymal stem/stromal cells (MSC) are currently the best candidate therapeutic cells for regenerative medicine related to osteoarticular, muscular, vascular and inflammatory diseases, although these cells remain heterogeneous and necessitate a better biological characterization. We and others recently described that MSC originate from two types of perivascular cells, namely pericytes and adventitial cells and contain the in situ counterpart of MSC in developing and adult human organs, which can be prospectively purified using well defined cell surface markers. Pericytes encircle endothelial cells of capillaries and microvessels and express the adhesion molecule CD146 and the PDGFRβ, but lack endothelial and haematopoietic markers such as CD34, CD31, vWF (von Willebrand factor), the ligand for Ulex europaeus 1 (UEA1) and CD45 respectively. The proteoglycan NG2 is a pericyte marker exclusively associated with the arterial system. Besides its expression in smooth muscle cells, smooth muscle actin (αSMA) is also detected in subsets of pericytes. Adventitial cells surround the largest vessels and, opposite to pericytes, are not closely associated to endothelial cells. Adventitial cells express CD34 and lack αSMA and all endothelial and haematopoietic cell markers, as for pericytes. Altogether, pericytes and adventitial perivascular cells express in situ and in culture markers of MSC and display capacities to differentiate towards osteogenic, adipogenic and chondrogenic cell lineages. Importantly, adventitial cells can differentiate into pericyte-like cells under inductive conditions in vitro. Altogether, using purified perivascular cells instead of MSC may bring higher benefits to regenerative medicine, including the possibility, for the first time, to use these cells uncultured.
Keywords: stem cell, progenitor cell, pericyte, adventitial cell, mesenchymal stem cell
Introduction
Despite an ever developing interest in the promising properties of mesenchymal stem cells (MSC), the true nature and identity of these cells has always been elusive. MSC, which were originally extracted from adult bone marrow 1, are now obtained from many tissues and are defined by their ability to adhere to plastic in culture, expression of a set of surface markers and capacity to differentiate into mesodermal cell lineages. Yet, these cells remain heterogeneous and not well characterized 2–4. Is the heterogeneity of cultured MSC reflective of the existence of distinct MSC progenitors in situ? We have hypothesized that because of their presence in virtually all organs, blood vessels represent a reservoir of these mesodermal stem cells. The presence of related progenitors, named mesoangioblasts because of their ability to differentiate into endothelial cells and other mesodermal lineages, has been already demonstrated in the embryonic dorsal aorta 5,6. Moreover, haematopoietic stem cells are generated from a hemogenic endothelium in the embryonic aortic wall 7. Finally, the identification of mesodermal progenitors in the tunica adventitia suggests that the outmost layer of blood vessels also contributes to tissue homeostasis and repair 8. These results led us to further investigate the presence of MSC within the vascular wall in various developing and adult human organs. Small blood vessels are formed by endothelial and mural cells. Charles Marie Benjamin Rouget described for the first time mural cells of capillaries as ‘non-pigmented adventitial cells’ or ‘intramural pericytes’, very closely associated with the endothelium and extending numerous ramified protoplasmic processes 9. His name was given to these cells—Rouget cells—until 1923, when Zimmermann renamed them pericytes, described as being mainly associated with vessel contractile function 10. Electron microscopy described pericytes as cellular structures encapsulated under the basal membrane of microvessels (diameter: 10–100 μm) and capillaries (diameter <10 μm) where they form a single layer around and in close contact with the endothelium. In this respect, the presence of pericytes in vessels devoid of basement membrane is by definition not expected, as for example in hepatic sinusoids 11. Thus, pericytes are clearly distinguishable from other perivascular cells, smooth muscle cells and adventitial cells, found around larger blood vessels and outside the basal membrane. Pericyte morphology is also different, these being large, stellate cells. In situ, pericytic cytoplasmic protuberances run parallels to the longitudinal axis of capillaries, but circular around microvessels 12. Other than pericytes, adventitial cells represent another perivascular cell type. The wall of all blood vessels, but capillaries, is constituted by three layers named tunica intima, tunica media and tunica adventitia. The tunica adventitia is the outmost layer of the vascular wall, originally described as an unorganized structure composed by fibroblasts, collagen and nerves playing a role in maintaining vessel structural integrity. More recently, a number of studies have reported a dynamic role for the tunica adventitia in vascular remodelling 8,13–16 inflammation and immune response 17–20. Although pericytes and adventitial perivascular cells have been described for more than a century, it is only recently that the blood vessel wall was demonstrated as a reservoir of progenitor cells. We showed that perivascular cells, i.e. pericytes and adventitial cells, are the in vivo counterpart of MSC obtained in culture from various organs 21,22. Pericytes and adventitial cells are two perivascular cell compartments with distinct phenotypes and anatomical locations in situ as well as distinguishable behaviours in culture. These perivascular cells can be prospectively purified by flow cytometry using a well-defined surface marker combination, common in all human organs tested (Table1). Importantly, pericytes and adventitial cells dissociated from vessel wall contain multipotent precursors with robust regeneration properties similar to those of classic heterogeneous MSC. Some studies show that human MSC may accumulate chromosomal aberrations 23,24 in contrast to other reports which strongly support their chromosomal stability 25,26.
Table 1.
| Pericytes | Adventitial cells | |
|---|---|---|
| Perivascular location | Capillaries and microvessels | Large vessels |
| Human tissue origin | Adult, foetal and embryonic skeletal muscle and pancreas, adult WAT, foetal skin, small intestine, brain, foetal and embryonic BM, term and mid-term placenta | Adult WAT, foetal skeletal muscle, lung and BM |
| FACS selection | CD146+CD34-CD56-CD45- | CD34+CD31-CD146-CD45- |
| Markers in vitro | CD146, NG2, PDGFRβ, αSMA, CD90, CD73, CD105, CD44, ALP, nestin, vimentin | CD34, CD90, CD73, CD105, CD44, vimentin |
| Markers in vivo | CD146, NG2, PDGFRβ, α SMA, CD90, CD73, CD105, CD44, ALP | CD34, CD90, CD73, CD105, CD44 |
| Documented differentiation potential | Osteogenic, adipogenic, chondrogenic, myogenic | Osteogenic, adipogenic, chondrogenic, pericytic |
Our own experiments demonstrated that cultured human pericytes and adventitial cells injected into immunodeficient mice are not tumorigenic 21.
Pericyte characterization
Markers of pericytes
In vivo, the main criterion to identify pericytes remains their anatomical localization and morphology. Beside localization, defining the molecular phenotype of pericytes has been a challenge. The 3G5 antigen, first suggested to be expressed by pericytes of the retina and adipose tissue, was later documented as a ubiquitous pericyte marker 27,28. Other pericyte markers have been described such as the melanoma-associated antigen, Thy1.1, the ephrin receptor and its ligands, neuropilin-1 and -2 and the Notch receptor and its ligands, Jagged-1 and Jagged-2. Although none of these antigens is present exclusively on pericytes 29. Vimentin and desmin are expressed by most chick pericytes as well as smooth muscle cells 30. Alkaline phosphatase (ALP) has been used to identify and isolate pericytes from adult human skeletal muscle 31,32. The presence of ALP on pericytes from other tissues was next confirmed 21. Finally, we have demonstrated that CD146, NG2 and PDGFRβ can be used to purify human pericytes from foetal and adult human tissues 21.
CD146
Perivascular and endothelial cell marker, CD146, aka MUC18, MCAM, Mel-CAM or S-Endo1, is a transmembrane glycoprotein and a member of the immunoglobulin superfamily. Perivascular and endothelial cell marker, CD146 expression is mainly associated with blood vessels, being present on vascular endothelium, pericytes and smooth muscle cells 21,33–35. In the embryo, CD146 marks other cell types present in the neural crest, notochord, mesonephros, ectoderm, somites and skeletal muscle rudiment 36,37.
However, cells in the paraxial mesoderm do not express CD146 before segmentation; only once somites are specified to the muscle cell lineage is CD146 expressed. In humans, CD146 expression is correlated with the development of the trophoblast as early as 12 days post fecundation 14. The exact role of CD146 is less obvious. In zebrafish, absence of CD146 inhibits lumen formation in intersomitic capillaries preventing establishment of the blood flow and causing defaults in the vascular system 36. In human adult BM, CD146 is found on adventitial reticular cells identified as sinusoidal pericytes. These osteoprogenitors are able to self renew in serial transplantation and restore the perivascular cell compartment 38–40. In the absence of CD146, no self-renewal has been observed.
PDGFRβ
Platelet-derived growth factor, PDGFRβ is expressed on pericytes 21,31,35,41. In the absence of PDGFβ production by endothelial cells, pericytes of newborn rats detached from blood vessels 42. Moreover, mouse embryos deficient in PDGFβ and PDGFRβ are viable, but die after birth lacking most pericytes and developing haematological, renal and placental abnormalities at late embryonic stages 43–45. A compensatory mechanism set to recruit small subsets of pericytes via the PDGFRα subunit may explain the absence of embryonic lethality because complete loss of PDGF signalling is lethal at embryonic day 9.5 46.
αSMA and NG2
Alpha smooth muscle actin (αSMA) is a universal marker of smooth muscle cells in large vessels, as seen in the human umbilical cord at term (Fig.1a). Pericytes of microvessels also express αSMA, in contrast to most pericytes surrounding capillaries 21,47,48. It is suggested that the presence of αSMA in subsets of pericytes, and absence from others, is correlated with one of the main functions of pericytes that is to contract and control blood pressure 49.
Fig 1.
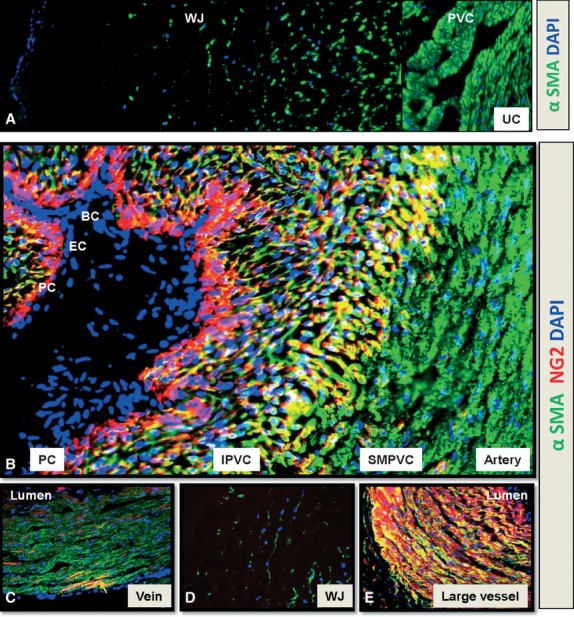
NG2 and αSMA expression in blood vessels of human umbilical cord (hUC). Transversal frozen sections of term (a–d) and mid-term (e) hUC. (a) αSMA (green) is expressed by perivascular cells (PVC) and by most of single cells of the Wharton's jelly (WJ) (b) from lumen to the outsider layer, the artery is composed by blood (BC, blue) and endothelial cells (EC, blue) NG2-αSMA-, pericytes (PC, red) NG2+αSMA-, intermediate PVC (IPVC, yellow) NG2+αSMA+ and smooth muscle perivascular cells (SMPVC, green) NG2-αSMA+. (c) The vascular wall of the vein mostly expresses αSMA (green). Rare cells coexpress both NG2 and αSMA (arrow, yellow). (d) NG2 expression is not detected in the WJ. (e) the vascular wall of veins and arteries of the mid-gestation hUC coexpress NG2 and αSMA (yellow) except pericytes (PC, red) which are NG2+αSMA-. All nuclei are stained by DAPI (blue). Immunostainings were performed according to our established protocol and hUC were used according to University of Pittsburgh regulations 21. Magnifications: 100× (a), 200× (c, d, e) and 400× (b).
The NG2 proteoglycan (neural glial antigen 2), or chondroitin sulphate proteoglycan 4, was first described in the nervous system 50 and later discovered on pericytes and smooth muscle cells 21,51–53. Not all pericytes express NG2, which is circumscribed to the arterial system 21,54,55. A phenotypic transition from pericytes to smooth muscle cells has been already proposed 56,57. Indeed, NG2 expression distinguishes three subsets of human pericytes, associated with: capillaries (NG2+αSMA-), venules (NG2-αSMA+) and arterioles (NG2+αSMA+) 21,55. Interestingly, all three phenotypes can be seen simultaneously around big vessels such as the human umbilical cord artery (Fig.1b), or surrounding large vessels in the developing human placenta 58. The human umbilical cord at term is composed of three large vessels (two veins and one artery) surrounded by stromal cells and embedded in a mostly acellular substance named Wharton's jelly. The mid-gestation umbilical cord contains an additional vein. Pericyte marker, NG2 is not expressed in either vein of the human umbilical cord, which confirms NG2 as a marker of arterial/venous polarity. Whereas all perivascular cells express αSMA within venules, only rare cells within the venular wall co-express NG2 and αSMA (Fig.1c). Wharton's jelly does not contain NG2-expressing cells (Fig.1d). In contrast to the human umbilical cord at term, arteries and veins of the mid-gestation cord do not show a differential distribution of NG2. Mural cells in all large vessels co-express NG2 and αSMA, except for the monolayer immediately in contact with endothelial cells (Fig.1e). Whether or not the latter can be named pericytes of large vessels is less obvious although their presence in adipose tissue was also proposed 59. Compared to NG2, CD146 is equally distributed, in the large vessels of the human term umbilical cord, within the arterial wall and has a distribution similar to that of NG2 in the vein 60,61. In conclusion, our data define human pericytes as CD146+PDGFRβ+CD34-CD56-CD45- cells (Fig.2a). Subsets of perivascular cells within this phenotype can be distinguished by NG2 and αSMA differential expression 21,55.
Fig 2.
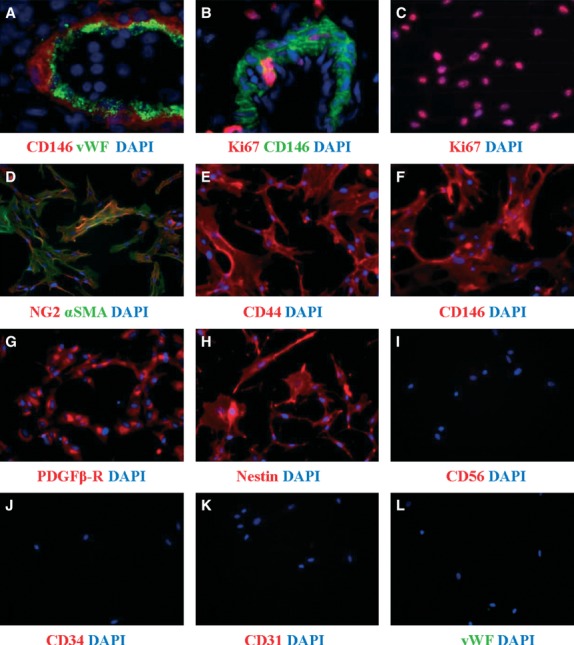
Immunofluorescence of human pericytes before and after long-term culture. Immunohistochemistry on frozen section of adult pancreas (a) and foetal skeletal muscle (b) and immunocytochemistry on cultured pericytes (c–l) show the expression of CD146, NG2, αSMA, CD44, PDGFRβ and nestin by pericytes before and after culture. Endothelial (vWF, CD34, CD31) and neural and myogenic (CD56) markers are absent. Few pericytes express Ki67, marker of proliferation, in situ (b) compare to cultured pericytes (c). All nuclei are stained by DAPI (blue). Pericyte culture and immunostainings were performed according to our established protocol and human developing and adult tissues were used according to University of Pittsburgh regulations 21. Magnifications: 600× (a); 400× (b) and 200× (c–l).
Pericyte long-term culture
Because of their implications in pathological processes, tissues such as the retina, brain, lung, skin and kidney have been used to establish methods to culture pericytes. These protocols include the growth of pericytes from microvessels of the bovine retina, a method adapted to various tissues such as human placenta and adipose tissue 62–65. These cultures, albeit enriched in pericytes, remain heterogeneous in term of cell composition. For this reason, we set up to prospectively purify and culture human pericytes (Table1). When seeded in culture, purified human pericytes do not attach rapidly, but sit for several hours then spread on the pre-coated plastic dish and divide very slowly during the first 2 to 4 passages to eventually proliferate and expand. Foetal muscle derived pericytes for example can be expanded up to 40 population doublings 21. This high capacity to proliferate is only observed in culture. Indeed, most cultured pericytes tested at passage 5 express Ki67, in contrast to pericytes in situ, of which only a few proliferate (Fig.2b and c). After around 30 doublings, human pericytes grow more slowly and eventually undergo senescence, similar to conventional MSC 21,66–68. In terms of phenotype, long-term cultured pericytes retain expression of discriminating markers (CD146, NG2, PDGFRβ), and, importantly, exhibit MSC phenotype and multipotency 21. Cultured pericytes express NG2, αSMA, CD44, CD146, PDGFRβ and nestin (Fig.2d–h), but lack the myogenic and neural cell marker CD56 (Fig.2i) and the endothelial and haematopoietic cell markers CD34, CD31 and vWF (Fig.2j–l). As of morphology, cultured pericytes are large, in general more than 50 μm, with an irregular stellate shape. Pericytes do not show contact inhibition; at confluence, they retract and fuse to eventually form ball like structures reminiscent of neurospheres. Nestin expressing mesospheres exhibiting MSC multipotency and myogenic myospheres were similarly isolated from adult murine BM and skeletal muscle respectively 69–72.
Adventitial cell characterization
Markers of adventitial cells and long-term culture
The phenotype of progenitors residing in the tunica adventitia of blood vessels was first described in the mouse. Hu et al. identified a population of Sca1+ progenitors abundant in the tunica adventitia, but absent from the tunica media or intima 73. Upon proper stimulation, purified Sca1+ progenitors differentiated into smooth muscle cells in vitro and in vivo. The authors also demonstrated that Sca1+ adventitial progenitors actively participate in the formation of atherosclerotic lesions by migrating into the tunica media and differentiating in smooth muscle cells. In agreement with these results Passman et al. described a population of Sca1+CD34+ckit-CD140b+ progenitors localized in a subregion of the tunica adventitia and highly active for sonic hedgehog signalling 74. Sca1+ adventitial progenitors were described to retain the potential to differentiate not only into smooth muscle cells but also into endothelial-like cells and osteoblasts, thus confirming that the tunica adventitia represents a niche for multipotent progenitor cells. Adventitial progenitors with similar phenotype and potential were also described in humans. Mesenchymal stem cell progenitor, CD34+CD31– cells endowed with the ability to give rise to endothelial-like cells were detected in the proximity of the tunica adventitia of adult human thoracic arteries and in the adipose-derived stromal vascular fraction 75–77. Mesenchymal stem cell progenitor, CD34+CD31– adventitial cells from the human saphenous vein were shown to associate with endothelial cells in vitro and in vivo and to promote neo-angiogenesis through paracrine mechanisms 78. Altogether, these studies indicate CD34+CD31- cells as a promising cell product for therapeutic angiogenesis, but the potential of adventitial progenitors is not limited to angiogenesis. A number of studies have indeed demonstrated that, in mice and in humans, the tunica adventitia harbours CD34+CD31– mesenchymal stem cell progenitors that may play a role in extravascular tissue homeostasis and repair 22,77,79–81. We have previously reported pericytes and adventitial cells as two anatomically and phenotypically distinct populations of perivascular cells expressing MSC markers in situ and able to generate MSC-like cells in culture 21,22. Mesenchymal stem cells, MSC generated from pericytes or adventitial cells were undistinguishable when assessed for expression of conventional MSC surface markers (CD90, CD73, CD105, CD44) and ability to differentiate into bone, fat and cartilage (Table1). However, similar to murine Sca1+ cells, human adventitia-derived MSC do not express any of the smooth muscle cell markers αSMA, NG2, CD146 and PDGFRβ that are instead homogeneously expressed by pericyte-derived MSC. Moreover, adventitial MSC could acquire a pericyte-like phenotype when stimulated with angiogenic factors 22. Despite similar morphologies, adventitial cells and pericytes have different abilities to grow in culture. Differently from pericytes, freshly sorted adventitial cells rapidly attach and start proliferating in tissue culture plates without the need for gelatin coating. Adventitial cells retain a proliferative advantage over pericytes over the long term, as showed by a significantly lower population doubling time 22. Clonal cultures can be grown from both adventitial cells and pericytes 21,22. As for other cell types (haematopoietic cells, endothelial cells), expression of CD34 is rapidly down-regulated once cells are cultured in vitro, and CD34 is undetectable after 1 or 2 passages. Finally, similar to pericytes, adventitial cells cultured in a low-attachment plate which maintain cells in a suspended, unattached state, can form spheres that can be serially passage in vitro. Interestingly, similar CD34+CD31- spheroidal colonies have been also isolated from pericyte progenitors of the postnatal rat aorta 82. Taken together, these studies indicate the co-existence in situ and in culture of distinct perivascular multipotent progenitors able to contribute to distinct subsets of MSC, possibly organized in a hierarchical fashion. Current studies are aimed to investigate the contribution of individual or combined pericytes and adventitial cells to tissue repair.
Adipose tissue is an abundant source of perivascular MSC
Although pericytes and adventitial cells are ubiquitous multipotent progenitors, isolation of these cells for clinical purposes is not feasible from all human tissues. Based on availability, dispensability, harvesting procedure and progenitor frequency, human adipose tissue emerges as an abundant and convenient source of therapeutic perivascular progenitors. Indeed, the adipose stromal vascular fraction (SVF) is highly enriched in blood vessels and SVF-derived perivascular cells give rise in vitro to MSC, previously derived indirectly in primary culture of the unsorted SVF as ADSC (adipose-derived stem cells) 21. In 2001, Gronthos et al. demonstrated that ADSC express all MSC markers: CD105, CD106, CD166, CD44 and importantly the CD146, perivascular and endothelial cell marker 83. At the same time, Zuk et al. demonstrated that ADSC have similar capacity as MSC to differentiate into adipogenic, osteogenic, chondrogenic and myogenic cell lineages, at a clonal level 84,85. CD146+CD34-CD31-CD45 –pericytes and CD34+CD146-CD31-CD45– adventitial cells represent around 15% and 20% of the stromal vascular fraction respectively 21,22 whereas perivascular cells in adult bone marrow are less than 0.5% of total mononuclear cells. Identification of two frequent multipotent progenitor cell subsets in human adipose tissue represents a critical step towards the clinical use of autologous stem cells. Purification and combination of pericytes and adventitial cells from lipoaspirates would yield clinically relevant numbers of progenitor cells devoid of bystander cells or negative regulators (endothelial cells), that could be in some indications directly transplanted without ex vivo expansion. Such an approach would significantly improve the efficacy and safety of current cell therapy strategies making use of cultured total stromal cells (James et al., 2012, In press).
Perivascular cells as stem/progenitor cells for regenerative medicine
Perivascular cells for cardiovascular repair and regeneration
Despite the multiple roles of pericytes in the pathophysiology of the cardiovascular system, their application in cardiovascular regenerative medicine remains to be tested. We recently investigated the therapeutic potential of pericytes in ischaemic heart repair. Upon transplantation into acutely infarcted hearts of NOD/SCID mice, human muscle-derived and cultured pericytes significantly improved cardiac function when compared to control injections. Pericytes exhibited cardio-reparative effects such as promotion of angiogenesis, reduction of scar and inhibition of chronic inflammation, likely because of their secretion of a variety of trophic factors (Chen et al., submitted). Dar et al. very recently described the production of pericytes from spontaneously differentiating embryoid bodies derived from human pluripotent stem cells (hPSC) 86. These CD105+CD90+CD73+CD31- multipotent mesodermal precursors express the pericyte markers CD146, NG2 and PDGFRβ but not the smooth muscle cell marker, αSMA. hPSC-derived pericytes transplanted into immunodeficient mice with ligature induced limb ischaemia not only induced vascular regeneration but also promoted muscle repair, by incorporating into the damaged muscle and vasculature 86. Pericytes have been also used to engineer vascular grafts: a cylindrical synthetic scaffold seeded with human muscle pericytes and transplanted into the sectioned rat aorta supported the development of a structurally and functionally normal blood vessel 87. These results suggest that pericytes can serve as a cell source for cardiovascular therapy. The use of adventitial cells in cardiovascular repair and regeneration has been also investigated. Campagnolo et al. showed that CD34+CD31- adventitial cells interact with endothelial cells and promote the formation and stabilization of capillary-like structures. A significant pro-angiogenic effect of adventitial cells was observed after administration in mouse ischaemic limbs, as shown by full recovery of blood flow as early as 7 days post treatment.
These results indicate the therapeutic capacity of adventitial cells in post injury angiogenesis/vasculogenesis 78. Very recently, the same group reported that transplantation of adventitial cells improves repair of the mouse infarcted heart through angiogenesis involving microRNA-132 (miR-132). Adventitial cells treatment enhanced cardiac repair in multiple aspects, augmenting cardiac contractility, attenuating LV dilatation, reducing cardiomyocyte apoptosis and interstitial fibrosis, increasing myocardial blood flow and neovascularization and decreasing vascular permeability. The paracrine function of adventitial cells activates endogenous repair responses, including pro-angiogenic and pro-survival Akt/eNOS/Bcl-2 signalling. Furthermore, adventitial cells constitutively express and secrete miR-132 and markedly up-regulate its expression upon stimulation, which in turn acts as a paracrine activator of cardiac healing 88. Together these data indicate the therapeutic potential of pericytes and adventitial cells in ischaemic tissue repair.
Perivascular cells for the regeneration of other tissues
The effect of pericytes on muscle regeneration was examined in immunodeficient mouse models of skeletal muscle injury and dystrophy 21. Pericytes (CD146+CD34-CD45-CD56-) purified using flow cytometry from human skeletal muscle biopsies were injected into the hind-limb muscles of SCID-non-obese diabetic (NOD/SCID) mice after injury by intramuscular injection of cardiotoxin. Pericyte-derived muscle fibres were detected by the presence of human-specific spectrin and centrally located human nuclei 21. Both freshly sorted and long-term cultured pericytes generated more human myofibres than human CD56+ skeletal myoblasts and total unsorted muscle cells. This ruled out the possibility that the myogenic potential observed in pericytes results from a contamination by myoblasts in culture. Interestingly, myogenic potential also exists in pericytes residing in other human organs, including placenta, white adipose tissue and pancreas.
Pericytes purified from those non-muscular organs not only exhibited myogenic potential in culture but also regenerated human dystrophin- or spectrin-positive myofibres upon injection into mdx/SCID or cardiotoxin-treated NOD/SCID mouse muscles, in which they also promoted angiogenesis 21,64. These results confirmed that pericytes sorted from healthy and dystrophic human skeletal muscle biopsies by ALP expression regenerate human myofibres in the muscles of dystrophic immunodeficient mice 31. Lately, using a transgenic labelling of alkaline phosphatase in the cre/lox inducible expression system, the same group demonstrated that pericytes residing in the postnatal skeletal muscle naturally participate in the regeneration of the injured/dystrophic skeletal muscle 32. New results further suggest a connection between muscle-residing pericytes and NF-κB activation in human muscle regenerative response following eccentric contractions 89. These results document the role of pericytes in muscle regeneration and suggest their future applications in skeletal muscle therapy. Human pericytes can also make bone. When cultured in standard osteogenic medium, pericytes exhibited alkaline phosphatase expression and mineral deposition. Pericytes seeded onto Gelfoam scaffolds and implanted into skeletal muscle pockets in immunodeficient mice developed into bony nodules 21. Furthermore, when cultured in the presence of an osteoinductive growth factor, Nell-1, pericytes exhibited robust osteogenic differentiation on either culture plastic ware or human cancellous bone chip (hCBC) scaffold. Upon implantation into a muscle pouch in the nude mouse, pericytes seeded on hCBC formed significantly more new bone than hCBC scaffold alone. Nell-1 significantly increased pericyte proliferation as well as osteogenic differentiation in vitro and in vivo 90. These and other more recent results suggest pericytes as novel therapeutic cells for skeletal regenerative medicine (James et al. 2012, In press). Overall, the superior regenerative capacity of pericytes can be attributed to multiple factors, including intrinsic multilineage developmental potential, robust paracrine function and efficient migration in response to stimuli 21,60,64,91–93.
We and others have demonstrated that adventitial cells, regardless of their tissue of origin also display developmental features typical of MSC 17,22,73,78,79,88,94. Although myogenic potential remains to be determined, the ability of adventitial cells to differentiate into major mesodermal cell lineages, including bone and cartilage, suggests a contribution of these cells in mesodermal organ development and post injury regeneration. Altogether, these reports suggest that beyond a mere structural constituent of the vascular wall, the adventitia is a dynamic reservoir of stem/progenitor cells that participate in vascular remodelling and regeneration of surrounding tissues.
Conclusion
Mesenchymal stem cells are one of the most promising stem/progenitor cell populations for regenerative medicine. Despite this potential, MSC are heterogeneous and their origin in situ has long remained unknown. We and others recently demonstrated that two main types of perivascular cells, pericytes and adventitial cells, are the native counterparts of MSC in developing and adult human organs. Studies on prospectively purified subsets of MSC will reveal important facts regarding their stemness, developmental biology, migratory capacity and regenerative potential. This will provide a better understanding of perivascular/MSC behaviour in health and disease and allow their optimal utilization in regenerative medicine.
Acknowledgments
Mihaela Crisan is grateful for the award of an EMBO Long term Fellowship and NWO Veni grants. Mirko Corselli is supported by a California Institute for Regenerative Medicine (CIRM) training grant. W.C. was supported in part by a Predoctoral Fellowship from the American Heart Association. Experiments described have been in part supported by funds from the University of Pittsburgh and University of California at Los Angeles and by grants from the National Institute of Health, Commonwealth of Pennsylvania, Pittsburgh Foundation, CIRM and Medical Research Council to B.P.
Conflict of interest
The authors confirm that there are no conflicts of interest.
References
- Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–50. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- Pevsner-Fischer M, Levin S, Zipori D. The origins of mesenchymal stromal cell heterogeneity. Stem Cell Rev. 2011;7:560–8. doi: 10.1007/s12015-011-9229-7. [DOI] [PubMed] [Google Scholar]
- da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204–13. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–4. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- De Angelis L, Berghella L, Coletta M, et al. Skeletal myogenic progenitors originating from embryonic dorsal aorta coexpress endothelial and myogenic markers and contribute to postnatal muscle growth and regeneration. J Cell Biol. 1999;147:869–78. doi: 10.1083/jcb.147.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minasi MG, Riminucci M, De Angelis L, et al. The meso-angioblast: a multipotent, self-renewing cell that originates from the dorsal aorta and differentiates into most mesodermal tissues. Development. 2002;129:2773–83. doi: 10.1242/dev.129.11.2773. [DOI] [PubMed] [Google Scholar]
- Tavian M, Zheng B, Oberlin E, et al. The vascular wall as a source of stem cells. Ann N Y Acad Sci. 2005;1044:41–50. doi: 10.1196/annals.1349.006. [DOI] [PubMed] [Google Scholar]
- Sartore S, Chiavegato A, Faggin E, et al. Contribution of adventitial fibroblasts to neointima formation and vascular remodeling: from innocent bystander to active participant. Circ Res. 2001;89:1111–21. doi: 10.1161/hh2401.100844. [DOI] [PubMed] [Google Scholar]
- Ashton N, de Oliveira F. Nomenclature of pericytes. Intramural and extramural. Br J Ophthalmol. 1966;50:119–23. doi: 10.1136/bjo.50.3.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allt G, Lawrenson JG. Pericytes: cell biology and pathology. Cells Tissues Organs. 2001;169:1–11. doi: 10.1159/000047855. [DOI] [PubMed] [Google Scholar]
- Hampton JC. An electron microscope study of the hepatic uptake and excretion of submicroscopic particles injected into the blood stream and into the bile duct. Acta Anat. 1958;32:262–91. doi: 10.1159/000141328. [DOI] [PubMed] [Google Scholar]
- Shepro D, Morel NM. Pericyte physiology. FASEB J. 1993;7:1031–8. doi: 10.1096/fasebj.7.11.8370472. [DOI] [PubMed] [Google Scholar]
- Siow RC, Churchman AT. Adventitial growth factor signalling and vascular remodelling: potential of perivascular gene transfer from the outside-in. Cardiovasc Res. 2007;75:659–68. doi: 10.1016/j.cardiores.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Shih IM. The role of CD146 (Mel-CAM) in biology and pathology. J Pathol. 1999;189:4–11. doi: 10.1002/(SICI)1096-9896(199909)189:1<4::AID-PATH332>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Gutterman DD. Adventitia-dependent influences on vascular function. Am J Physiol. 1999;277:H1265–72. doi: 10.1152/ajpheart.1999.277.4.H1265. [DOI] [PubMed] [Google Scholar]
- Li G, Chen SJ, Oparil S, et al. Direct in vivo evidence demonstrating neointimal migration of adventitial fibroblasts after balloon injury of rat carotid arteries. Circulation. 2000;101:1362–5. doi: 10.1161/01.cir.101.12.1362. [DOI] [PubMed] [Google Scholar]
- Maiellaro K, Taylor WR. The role of the adventitia in vascular inflammation. Cardiovasc Res. 2007;75:640–8. doi: 10.1016/j.cardiores.2007.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick G, Romen M, Amberger A, et al. Atherosclerosis, autoimmunity, and vascular-associated lymphoid tissue. FASEB J. 1997;11:1199–207. doi: 10.1096/fasebj.11.13.9367355. [DOI] [PubMed] [Google Scholar]
- Bush E, Maeda N, Kuziel WA, et al. CC chemokine receptor 2 is required for macrophage infiltration and vascular hypertrophy in angiotensin II-induced hypertension. Hypertension. 2000;36:360–3. doi: 10.1161/01.hyp.36.3.360. [DOI] [PubMed] [Google Scholar]
- Capers Q, Alexander RW, Lou P, et al. Monocyte chemoattractant protein-1 expression in aortic tissues of hypertensive rats. Hypertension. 1997;30:1397–402. doi: 10.1161/01.hyp.30.6.1397. [DOI] [PubMed] [Google Scholar]
- Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–13. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Corselli M, Chen CW, Sun B, et al. The tunica adventitia of human arteries and veins as a source of mesenchymal stem cells. Stem Cells Dev. 2012;21:1299–308. doi: 10.1089/scd.2011.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosland GV, Svendsen A, Torsvik A, et al. Long-term cultures of bone marrow-derived human mesenchymal stem cells frequently undergo spontaneous malignant transformation. Cancer Res. 2009;69:5331–9. doi: 10.1158/0008-5472.CAN-08-4630. [DOI] [PubMed] [Google Scholar]
- Ben-David U, Mayshar Y, Benvenisty N. Large-scale analysis reveals acquisition of lineage-specific chromosomal aberrations in human adult stem cells. Cell Stem Cell. 2011;9:97–102. doi: 10.1016/j.stem.2011.06.013. [DOI] [PubMed] [Google Scholar]
- Bernardo ME, Zaffaroni N, Novara F, et al. Human bone marrow derived mesenchymal stem cells do not undergo transformation after long-term in vitro culture and do not exhibit telomere maintenance mechanisms. Cancer Res. 2007;67:9142–9. doi: 10.1158/0008-5472.CAN-06-4690. [DOI] [PubMed] [Google Scholar]
- Sensebe L, Tarte K, Galipeau J, et al. Limited acquisition of chromosomal aberrations in human adult mesenchymal stromal cells. Cell Stem Cell. 2012;10:9–10. doi: 10.1016/j.stem.2011.12.005. ; author reply -1. [DOI] [PubMed] [Google Scholar]
- Nayak RC, Berman AB, George KL, et al. A monoclonal antibody (3G5)-defined ganglioside antigen is expressed on the cell surface of microvascular pericytes. J Exp Med. 1988;167:1003–15. doi: 10.1084/jem.167.3.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zannettino AC, Paton S, Arthur A, et al. Multipotential human adipose-derived stromal stem cells exhibit a perivascular phenotype in vitro and in vivo. J Cell Physiol. 2008;214:413–21. doi: 10.1002/jcp.21210. [DOI] [PubMed] [Google Scholar]
- Gale NW, Baluk P, Pan L, et al. Ephrin-B2 selectively marks arterial vessels and neovascularization sites in the adult, with expression in both endothelial and smooth-muscle cells. Dev Biol. 2001;230:151–60. doi: 10.1006/dbio.2000.0112. [DOI] [PubMed] [Google Scholar]
- Fujimoto T, Singer SJ. Immunocytochemical studies of desmin and vimentin in pericapillary cells of chicken. J Histochem Cytochem. 1987;35:1105–15. doi: 10.1177/35.10.3305702. [DOI] [PubMed] [Google Scholar]
- Dellavalle A, Sampaolesi M, Tonlorenzi R, et al. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 2007;9:255–67. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- Dellavalle A, Maroli G, Covarello D, et al. Pericytes resident in postnatal skeletal muscle differentiate into muscle fibres and generate satellite cells. Nat Commun. 2011;2:499. doi: 10.1038/ncomms1508. [DOI] [PubMed] [Google Scholar]
- Bardin N, Frances V, Lesaule G, et al. Identification of the S-Endo 1 endothelial-associated antigen. Biochem Biophys Res Commun. 1996;218:210–6. doi: 10.1006/bbrc.1996.0037. [DOI] [PubMed] [Google Scholar]
- Sers C, Riethmuller G, Johnson JP. MUC18, a melanoma-progression associated molecule, and its potential role in tumor vascularization and hematogenous spread. Cancer Res. 1994;54:5689–94. [PubMed] [Google Scholar]
- Schwab KE, Gargett CE. Co-expression of two perivascular cell markers isolates mesenchymal stem-like cells from human endometrium. Hum Reprod. 2007;22:2903–11. doi: 10.1093/humrep/dem265. [DOI] [PubMed] [Google Scholar]
- Chan B, Sinha S, Cho D, et al. Critical roles of CD146 in zebrafish vascular development. Dev Dyn. 2005;232:232–44. doi: 10.1002/dvdy.20220. [DOI] [PubMed] [Google Scholar]
- Pujades C, Guez-Guez B, Dunon D. Melanoma Cell Adhesion Molecule (MCAM) expression in the myogenic lineage during early chick embryonic development. Int J Dev Biol. 2002;46:263–6. doi: 10.1387/ijdb.011493. [DOI] [PubMed] [Google Scholar]
- Bianco P. Back to the future: moving beyond “mesenchymal stem cells”. J Cell Biochem. 2011;112:1713–21. doi: 10.1002/jcb.23103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschaseaux F, Pontikoglou C, Sensebe L. Bone regeneration: the stem/progenitor cells point of view. J Cell Mol Med. 2010;14:103–15. doi: 10.1111/j.1582-4934.2009.00878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchetti B, Funari A, Michienzi S, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–36. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- Winkler EA, Bell RD, Zlokovic BV. Pericyte-specific expression of PDGF beta receptor in mouse models with normal and deficient PDGF beta receptor signaling. Mol Neurodegener. 2010;5:32. doi: 10.1186/1750-1326-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin LE, Hemo I, Keshet E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development. 1998;125:1591–8. doi: 10.1242/dev.125.9.1591. [DOI] [PubMed] [Google Scholar]
- Hellstrom M, Kalen M, Lindahl P, et al. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 1999;126:3047–55. doi: 10.1242/dev.126.14.3047. [DOI] [PubMed] [Google Scholar]
- Leveen P, Pekny M, Gebre-Medhin S, et al. Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes Dev. 1994;8:1875–87. doi: 10.1101/gad.8.16.1875. [DOI] [PubMed] [Google Scholar]
- Lindahl P, Johansson BR, Leveen P, et al. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–5. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- French WJ, Creemers EE, Tallquist MD. Platelet-derived growth factor receptors direct vascular development independent of vascular smooth muscle cell function. Mol Cell Biol. 2008;28:5646–57. doi: 10.1128/MCB.00441-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschi KK, D'Amore PA. Pericytes in the microvasculature. Cardiovasc Res. 1996;32:687–98. [PubMed] [Google Scholar]
- Nehls V, Drenckhahn D. Heterogeneity of microvascular pericytes for smooth muscle type alpha-actin. J Cell Biol. 1991;113:147–54. doi: 10.1083/jcb.113.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papetti M, Shujath J, Riley KN, et al. FGF-2 antagonizes the TGF-beta1-mediated induction of pericyte alpha-smooth muscle actin expression: a role for myf-5 and Smad-mediated signaling pathways. Invest Ophthalmol Vis Sci. 2003;44:4994–5005. doi: 10.1167/iovs.03-0291. [DOI] [PubMed] [Google Scholar]
- Miller B, Sheppard AM, Bicknese AR, et al. Chondroitin sulfate proteoglycans in the developing cerebral cortex: the distribution of neurocan distinguishes forming afferent and efferent axonal pathways. J Comp Neurol. 1995;355:615–28. doi: 10.1002/cne.903550410. [DOI] [PubMed] [Google Scholar]
- Grako KA, Stallcup WB. Participation of the NG2 proteoglycan in rat aortic smooth muscle cell responses to platelet-derived growth factor. Exp Cell Res. 1995;221:231–40. doi: 10.1006/excr.1995.1371. [DOI] [PubMed] [Google Scholar]
- Ozerdem U, Grako KA, Dahlin-Huppe K, et al. NG2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Dev Dyn. 2001;222:218–27. doi: 10.1002/dvdy.1200. [DOI] [PubMed] [Google Scholar]
- Ozerdem U, Monosov E, Stallcup WB. NG2 proteoglycan expression by pericytes in pathological microvasculature. Microvasc Res. 2002;63:129–34. doi: 10.1006/mvre.2001.2376. [DOI] [PubMed] [Google Scholar]
- Murfee WL, Skalak TC, Peirce SM. Differential arterial/venous expression of NG2 proteoglycan in perivascular cells along microvessels: identifying a venule-specific phenotype. Microcirculation. 2005;12:151–60. doi: 10.1080/10739680590904955. [DOI] [PubMed] [Google Scholar]
- Crisan M, Chen CW, Corselli M, et al. Perivascular multipotent progenitor cells in human organs. Ann N Y Acad Sci. 2009;1176:118–23. doi: 10.1111/j.1749-6632.2009.04967.x. [DOI] [PubMed] [Google Scholar]
- Diaz-Flores L, Gutierrez R, Madrid JF, et al. Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histol Histopathol. 2009;24:909–69. doi: 10.14670/HH-24.909. [DOI] [PubMed] [Google Scholar]
- Diaz-Flores L, Gutierrez R, Varela H, et al. Microvascular pericytes: a review of their morphological and functional characteristics. Histol Histopathol. 1991;6:269–86. [PubMed] [Google Scholar]
- Robin C, Bollerot K, Mendes S, et al. Human placenta is a potent hematopoietic niche containing hematopoietic stem and progenitor cells throughout development. Cell Stem Cell. 2009;5:385–95. doi: 10.1016/j.stem.2009.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallone T, Realini C, Bohmler A, et al. Adult human adipose tissue contains several types of multipotent cells. J Cardiovasc Transl Res. 2011;4:200–10. doi: 10.1007/s12265-011-9257-3. [DOI] [PubMed] [Google Scholar]
- Montemurro T, Andriolo G, Montelatici E, et al. Differentiation and migration properties of human foetal umbilical cord perivascular cells: potential for lung repair. J Cell Mol Med. 2011;15:796–808. doi: 10.1111/j.1582-4934.2010.01047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schugar RC, Chirieleison SM, Wescoe KE, et al. High harvest yield, high expansion, and phenotype stability of CD146 mesenchymal stromal cells from whole primitive human umbilical cord tissue. J Biomed Biotechnol. 2009;2009:789526. doi: 10.1155/2009/789526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin JD, D'Amore PA. Culture of retinal capillary cells using selective growth media. Microvasc Res. 1983;26:74–80. doi: 10.1016/0026-2862(83)90056-0. [DOI] [PubMed] [Google Scholar]
- Maier CL, Shepherd BR, Yi T, et al. Explant outgrowth, propagation and characterization of human pericytes. Microcirculation. 2010;17:367–80. doi: 10.1111/j.1549-8719.2010.00038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park TS, Gavina M, Chen CW, et al. Placental perivascular cells for human muscle regeneration. Stem Cells Dev. 2011;20:451–63. doi: 10.1089/scd.2010.0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YI, Kim HI, Choi MY, et al. Ex vivo organ culture of adipose tissue for in situ mobilization of adipose-derived stem cells and defining the stem cell niche. J Cell Physiol. 2010;224:807–16. doi: 10.1002/jcp.22188. [DOI] [PubMed] [Google Scholar]
- Kotobuki N, Hirose M, Takakura Y, et al. Cultured autologous human cells for hard tissue regeneration: preparation and characterization of mesenchymal stem cells from bone marrow. Artif Organs. 2004;28:33–9. doi: 10.1111/j.1525-1594.2004.07320.x. [DOI] [PubMed] [Google Scholar]
- Bruder SP, Jaiswal N, Haynesworth SE. Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J Cell Biochem. 1997;64:278–94. doi: 10.1002/(sici)1097-4644(199702)64:2<278::aid-jcb11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Ksiazek K. A comprehensive review on mesenchymal stem cell growth and senescence. Rejuvenation Res. 2009;12:105–16. doi: 10.1089/rej.2009.0830. [DOI] [PubMed] [Google Scholar]
- Mendez-Ferrer S, Michurina TV, Ferraro F, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–34. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarig R, Baruchi Z, Fuchs O, et al. Regeneration and transdifferentiation potential of muscle-derived stem cells propagated as myospheres. Stem Cells. 2006;24:1769–78. doi: 10.1634/stemcells.2005-0547. [DOI] [PubMed] [Google Scholar]
- Westerman KA, Penvose A, Yang Z, et al. Adult muscle ‘stem’ cells can be sustained in culture as free-floating myospheres. Exp Cell Res. 2010;316:1966–76. doi: 10.1016/j.yexcr.2010.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Li Y, Chen C, et al. Human skeletal muscle-derived stem cells retain stem cell properties after expansion in myosphere culture. Exp Cell Res. 2011;317:1016–27. doi: 10.1016/j.yexcr.2011.01.019. [DOI] [PubMed] [Google Scholar]
- Hu Y, Xu Q. Adventitial biology: differentiation and function. Arterioscler Thromb Vasc Biol. 2011;31:1523–9. doi: 10.1161/ATVBAHA.110.221176. [DOI] [PubMed] [Google Scholar]
- Passman JN, Dong XR, Wu SP, et al. A sonic hedgehog signaling domain in the arterial adventitia supports resident Sca1+ smooth muscle progenitor cells. Proc Natl Acad Sci USA. 2008;105:9349–54. doi: 10.1073/pnas.0711382105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zengin E, Chalajour F, Gehling UM, et al. Vascular wall resident progenitor cells: a source for postnatal vasculogenesis. Development. 2006;133:1543–51. doi: 10.1242/dev.02315. [DOI] [PubMed] [Google Scholar]
- Sengenes C, Miranville A, Maumus M, et al. Chemotaxis and differentiation of human adipose tissue CD34+/CD31- progenitor cells: role of stromal derived factor-1 released by adipose tissue capillary endothelial cells. Stem Cells. 2007;25:2269–76. doi: 10.1634/stemcells.2007-0180. [DOI] [PubMed] [Google Scholar]
- Pasquinelli G, Tazzari PL, Vaselli C, et al. Thoracic aortas from multiorgan donors are suitable for obtaining resident angiogenic mesenchymal stromal cells. Stem Cells. 2007;25:1627–34. doi: 10.1634/stemcells.2006-0731. [DOI] [PubMed] [Google Scholar]
- Campagnolo P, Cesselli D, Al Haj Zen A, et al. Human adult vena saphena contains perivascular progenitor cells endowed with clonogenic and proangiogenic potential. Circulation. 2010;121:1735–45. doi: 10.1161/CIRCULATIONAHA.109.899252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino A, Chiba H, Nagai K, et al. Human vascular adventitial fibroblasts contain mesenchymal stem/progenitor cells. Biochem Biophys Res Commun. 2008;368:305–10. doi: 10.1016/j.bbrc.2008.01.090. [DOI] [PubMed] [Google Scholar]
- Zimmerlin L, Donnenberg VS, Pfeifer ME, et al. Stromal vascular progenitors in adult human adipose tissue. Cytometry A. 2010;77:22–30. doi: 10.1002/cyto.a.20813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong JJ, Chandrakanthan V, Xaymardan M, et al. Adult cardiac-resident MSC-like stem cells with a proepicardial origin. Cell Stem Cell. 2011;9:527–40. doi: 10.1016/j.stem.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howson KM, Aplin AC, Gelati M, et al. The postnatal rat aorta contains pericyte progenitor cells that form spheroidal colonies in suspension culture. Am J Physiol Cell Physiol. 2005;289:C1396–407. doi: 10.1152/ajpcell.00168.2005. [DOI] [PubMed] [Google Scholar]
- Gronthos S, Franklin DM, Leddy HA, et al. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol. 2001;189:54–63. doi: 10.1002/jcp.1138. [DOI] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–28. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–95. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dar A, Domev H, Ben-Yosef O, et al. Multipotent vasculogenic pericytes from human pluripotent stem cells promote recovery of murine ischemic limb. Circulation. 2012;125:87–99. doi: 10.1161/CIRCULATIONAHA.111.048264. [DOI] [PubMed] [Google Scholar]
- He W, Nieponice A, Soletti L, et al. Pericyte-based human tissue engineered vascular grafts. Biomaterials. 2010;31:8235–44. doi: 10.1016/j.biomaterials.2010.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katare R, Riu F, Mitchell K, et al. Transplantation of human pericyte progenitor cells improves the repair of infarcted heart through activation of an angiogenic program involving micro-RNA-132. Circ Res. 2011;109:894–906. doi: 10.1161/CIRCRESAHA.111.251546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyldahl RD, Xin L, Hubal MJ, et al. Activation of nuclear factor-kappaB following muscle eccentric contractions in humans is localized primarily to skeletal muscle-residing pericytes. FASEB J. 2011;25:2956–66. doi: 10.1096/fj.10-177105. [DOI] [PubMed] [Google Scholar]
- Zhang X, Peault B, Chen W, et al. The Nell-1 growth factor stimulates bone formation by purified human perivascular cells. Tissue Eng Part A. 2011;17:2497–509. doi: 10.1089/ten.tea.2010.0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CW, Montelatici E, Crisan M, et al. Perivascular multi-lineage progenitor cells in human organs: regenerative units, cytokine sources or both? Cytokine Growth Factor Rev. 2009;20:429–34. doi: 10.1016/j.cytogfr.2009.10.014. [DOI] [PubMed] [Google Scholar]
- Corselli M, Chen CW, Crisan M, et al. Perivascular ancestors of adult multipotent stem cells. Arterioscler Thromb Vasc Biol. 2010;30:1104–9. doi: 10.1161/ATVBAHA.109.191643. [DOI] [PubMed] [Google Scholar]
- Tottey S, Corselli M, Jeffries EM, et al. Extracellular matrix degradation products and low-oxygen conditions enhance the regenerative potential of perivascular stem cells. Tissue Eng Part A. 2011;17:37–44. doi: 10.1089/ten.tea.2010.0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majesky MW, Dong XR, Hoglund V, et al. The adventitia: a dynamic interface containing resident progenitor cells. Arterioscler Thromb Vasc Biol. 2011;31:1530–9. doi: 10.1161/ATVBAHA.110.221549. [DOI] [PMC free article] [PubMed] [Google Scholar]


