Abstract
Eph receptor tyrosine kinases and their ephrin ligands are involved in various signalling pathways and mediate critical steps of a wide variety of physiological and pathological processes. Increasing experimental evidence demonstrates that both Eph receptor and ephrin ligands are overexpressed in a number of human tumours, and are associated with tumour growth, invasiveness and metastasis. In this regard, the Eph/ephrin system provides the foundation for potentially exciting new targets for anticancer therapies for Eph-expressing tumours. The purpose of this review is to outline current advances in the role of Eph receptors and ephrin ligands in cancer, and to discuss novel therapeutic approaches of anticancer therapies.
Keywords: Eph receptor, Ephrin, therapeutic target
Introduction
Eph receptors (Ephs) are the largest subfamily of receptor tyrosine kinases (RTKs) 1,2, with 16 members cloned 3. They are divided into two groups, EphA and EphB, depending on the types of ligands (ephrins) that they bind 4. Since the first Eph gene was cloned in 1987 5, the first ephrin ligand was also identified from cancer cells a few years later 6,7. Interactions between Ephs and the appropriate ephrin ligand activate bidirectional signalling and transducer signalling cascades. Eph receptors and ephrin ligands play critical roles in various biological functions, such as embryonic patterning, development of the nervous system and angiogenesis. However, deregulated activation of Eph/ephrin signalling in humans is thought to lead to tumorigenesis 8. A number of studies have demonstrated overexpression of Ephs and ephrins in a variety of human tumours including melanoma 9–11, neuroblastoma 12, malignant glioma 13,14 and carcinoma of the pancreas 15, breast 16–18, colon 19,20, prostate 21,22, lung 23, gastrointestinal tract 24,25, ovaries 26,27, oesophagus 28, liver 29,30 and thyroid 31. The up-regulation of Ephs and ephrins in human cancer is associated with poor prognosis and high vascularity in cancer, suggesting a detrimental role for the Eph/ephrin system in tumour progression 32. In addition, it has been suggested that up-regulated Eph expression levels could be used as molecular markers for the diagnosis of invasive and metastatic tumours 17. However, not only up-regulation but also down-regulation of Ephs and ephrins have been associated with tumour progression, and both Eph receptors and ephrin ligands can promote or suppress tumour growth. Eph receptors and ephrin ligands that are preferentially expressed in extremely invasive and metastatic tumours have provided the foundation for potentially exciting new targets for anticancer therapies for these tumours. To date, numerous strategies targeting the Eph/ephrin family have been developed for cancer treatment. This review describes the structure of Eph receptors and ephrin ligands and their signalling pathway, and summarizes the roles of Ephs/ephrins in cancer and anticancer therapies.
Structure of Eph receptors and ephrin ligands
Ephs are divided into two subclasses, EphA (EphA1-10) and EphB kinases (EphB1-6), on the basis of the sequence homology and the means by which they interact with membrane-anchored ephrin ligands. Both EphA and EphB receptors contain a single transmembrane-spanning domain. The extracellular region of Eph receptors is glycosylated, and contains a ligand-binding domain, a cysteine-rich domain and two fibronectin type III repeats. The intracellular region contains a juxtamembrane region with several conserved tyrosine residues, a tyrosine kinase domain, a sterile α motif (SAM) domain and a PDZ-binding motif within the non-catalytic region of the COOH-terminus 33,34. On the basis of their structural features and binding specificity to EphA and EphB receptors, ephrins are also divided into two subclasses, ephrinA and ephrinB. EphrinA (A1-A6) ligands are tethered to the extracellular cell membrane via a glycosylphosphatidylinositol (GPI) anchor, whereas ephrinB (B1-B3) ligands are transmembrane proteins that possess a short cytoplasmic region with a PDZ-binding motif. EphA receptors typically bind to ephrinA ligands, and EphB receptors bind to ephrinB ligands. However, this does not preclude cross-binding, as has been shown for EphA4, which can bind to ephrinA and ephrinB ligands, and EphB2 which can bind to ephrinA5 35–37 (Fig.1A).
Fig 1.
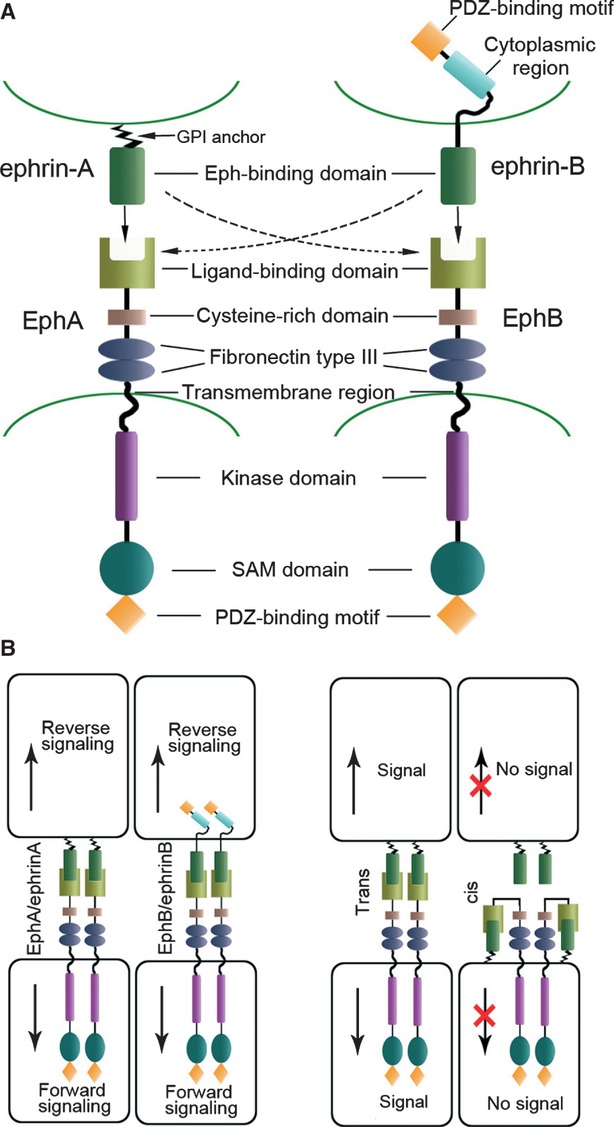
Eph/ephrin structure and signalling. (A) Domain structure of Eph receptors and ephrin ligands. The extracellular region of Eph receptors contains a ligand-binding domain, a cysteine-rich domain and two fibronectin type III repeats. The intracellular region contains a tyrosine kinase domain, a sterile α motif (SAM) domain and a PDZ-binding domain. Both ephrinA (GPI-anchored) ligands and ephrinB (transmembrane) ligands interact with the N-terminal globular domain of Eph receptor. (B) Binding Eph/ephrin molecules form heterotetramers to initiate signals. Both classes of Eph receptors and ephrins activate bidirectional signalling: forward signalling and reverse signalling. Eph receptors and ephrin ligands expressed in opposing cells interact in trans and lead to bidirectional signal transduction. EphA and ephrinA coexpressed in the same cell interact in cis. This impairs receptor activation and inhibits trans interaction.
Interaction between Eph receptors and their ephrin ligands
Specificity of the binding of ephrins to their Ephs is mediated by the N-terminal glycosylated ligand-binding domain of Ephs 38. Eph receptors and ephrins expressed in opposing cells interact in trans form and activate bidirectional signalling. (Fig.1B). Eph receptors and ephrins coexpressed in the same cell interact in cis form 39, Cis interaction has been shown to inhibit trans interaction and/or signalling 40,41. Upon binding, the Eph/ephrin molecules form heterotetramers to initiate the signal. As a rule, on ephrin binding, Eph clustering leads to activation of the tyrosine kinase domain, resulting in autophosphorylation of certain intracellular tyrosine residues 42. Accordingly, these phosphotyrosine regions bind adaptor proteins, and subsequently trigger downstream signalling pathways that lead to specific biological effects. However, this classical RTK activation does not explain all Eph/ephrin signalling, and ligand-independent Eph signalling can also occur 43,44. For example, EphA8 receptor results in mitogen-activated protein kinase (MAPK) activation in a neural cell line 45, and promotes integrin-mediated cell attachment in a tyrosine kinase activity-independent fashion 46. A previous study has also indicated that EphB4 can affect cancer cell behaviour in an ephrin-independent manner 47.
Bidirectional signalling
Bidirectional signalling is an important feature of Eph-ephrin signalling and arises dues to activation of signalling pathways in both the receptor-expression and the ligand-expression cells 48. Forward and reverse signalling are activated by Ephs and ephrins respectively 39 (Fig.2).
Fig 2.
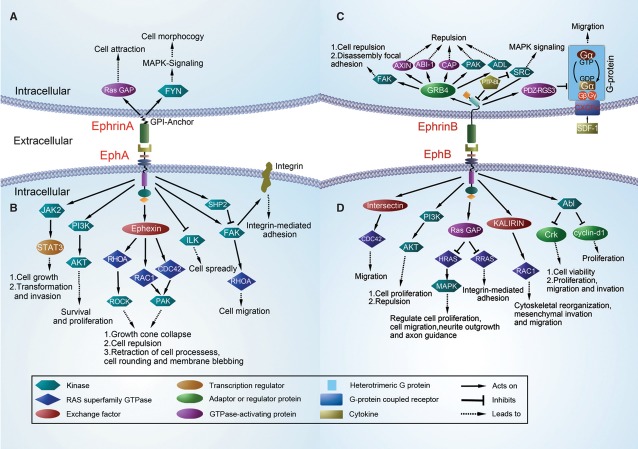
EphA/ephrinA bidirectional signalling. (A) Stimulation of ephrinA5 recruits and activates the Src-family kinase, FYN. Subsequently, FYN induces a change in the cellular architecture and adhesion of ephrinA5-expressing cells 68 and results in mitogen-activated protein kinase (MAPK) activation 74. (B) EphA4 activates signal transducers and STAT3 50. EphA receptors directly activate GTPases of the Rho family (RHOA, RAC1 and CDC42) through the exchange factor Ephexin 51,52. This pathway involves EphA2 and PI3 kinase in endothelial cells 151. EphA2 inhibits Akt 190,191 and inactivates focal adhesion kinase (FAK) through the SHP2 phosphatase 48. EphA2 activates RHOA through FAK 192,193. EphA1 inhibits integrin-linked kinase (ILK)194. EphB/ephrinB bidirectional signalling. (C) Growth Factor Receptor Bound protein 4 (GRB4) contains a SH2 domain and can link ephrinB ligands to a signalling network that modifies cell morphology 77. EphrinB1 disrupts focal adhesions through GRB4 48. The phosphatase PTP-BL is recruited to the ephrinB carboxy-terminal tail. PTP-BL dephosphorylates ephrinB and inactivates Src 35. PDZ-RGS3 binds constitutively to ephrinB and catalyses the hydrolysis of GTP to GDP in the G-Alpha subunit of heterotrimeric GPCR. It also inhibits SDF1–mediated cell chemotaxis through the CXCR4 195. (D) EphB forward signalling activates RAC1 and CDC42 exchange factors 48,146,193. EphB2 activates Ras GAP to inhibit the H-RAS and R-RAS 48,196. EphB2 regulates cell positioning via PI3K 64. The EphB4 receptor suppresses breast cancer cell tumorigenicity through an Abl-Crk pathway 66. EphB2 regulates cell proliferation through an Abl-cyclin D1 pathway 64.
Forward signalling
Eph receptors can regulate biological effects through different kinase-mediated forward signalling molecules and pathways, including small GTPases of Rho members (Rho, Rac and Cdc42), focal adhesion kinase (FAK), the PI3 kinase pathway and Jak/Stat pathway 49,50. Small GTPases of the Rho family, which are activated by EphA receptors, control cell shape and movement by promoting the formation of stress fibres (Rho), lamellipodia (Rac) and filopodia (Cdc42) 51,52. The EphA receptor activates GTPases through the exchange factor, ephexin. Ephexin, which is preferentially expressed in the nervous system, binds the kinase domain of EphA. EphrinA-induced signals initiate growth cone collapse through the activation of Rho and its downstream effectors 52. In melanoma and 293T cells, ephrinA-induced recruitment of Crk to EphA3 and a rapid, transient increase in activated Rho causes the retraction of cell processes, cell rounding and membrane blebbing. In addition, SH3 mutant Crk ablates Rho activation and ephrinA-induced cell morphological changes 53. EphB receptors appear to associate with the exchange factors intersectin 54 and kalirin 55. Intersectin could activate the Rho-family GTPase Cdc42 and its activity is enhanced by EphB receptor. Activation of the EphB receptor induces translocation of kalirin, an exchange factor for Rac, to synapses and leads to increased local Rac1 activation. The EphB-intersectin-Cdc42 and EphB-kalirin-Rac pathways have been proposed to regulate the EphB-receptor-mediated cytoskeletal reorganization, mesenchymal invasion and migration 54,55. Ephs also regulate the activity of small GTPases of the Ras family, including H-Ras and R-Ras 56,57, H-Ras can in turn activate a MAPK pathway that is very important for transcriptional regulation, cell proliferation, cell migration, neurite outgrowth and axon guidance 58,59. Activation of Eph receptors negatively regulates the Ras-MAPK pathway in various cell types 57,60–62. For example, activation of EphA2 could down-regulate the Ras-MAPK pathway in fibroblasts, epithelial cells, endothelial cells and tumour cells 57. EphB2 transiently down-regulates H-Ras activity and MAPK phosphorylation and leads to neurite retraction in the NG108 neuronal cell line 60,63. Eph receptor-mediated activation of R-Ras also suppresses the MAPK pathway. EphB activation can reduce integrin-mediated adhesion via negative regulation of the R-Ras-MAPK pathway 56. Focal adhesion kinase, which is a critical component of integrin signalling, may connect Eph receptors with integrins 49. In PC-3 prostate cancer cells, ligand activation of EphA2 causes dissociation of FAK. EphB2-regulated cell positioning via PI3K, independently of kinase activity, because of this, PI3K activity is important for conveying positional information 64. In contrast, EphB signalling could drive cell proliferation by promoting cell cycle entry. Cyclin D1 is a regulator of cell cycle entry 65. In Genander's study, EphB2 activity drove cells proliferation through Abl, resulting in post-transcriptional regulation of cyclin D1 protein levels 64. In breast cancer cells, Abl binds to EphB4 in an activity-dependent manner. EphB4 activates an anti-oncogenic pathway involving Abl family tyrosine kinases and the Crk adaptor protein. This Abl-Crk pathway inhibits breast cancer cell viability and proliferation in addition to motility and invasion 66. Autophosphorylation of EphA4 could lead to the activation of Jak2, which in turn phosphorylated Stat1 and Stat3, and promoted transcriptional activity and cells proliferation 50. Taken together, Jak/Stat proteins were considered to be downstream targets of EphA4 signalling.
Reverse signalling
The interaction between Ephs receptors and their ephrins not only induces forward signalling by the Eph receptor but also leads to reverse signalling by the ephrin ligand 67. EphrinA ligands can transmit signals despite lacking a cytoplasmic domain. The mechanisms of reverse signalling of ephrinA ligands are thought to be associated with ephrinA clustering and recruitment of regulatory proteins 68. EphrinA ligands are anchored to the membrane by covalent linkage to GPI, and depend on transmembrane coreceptors for transmitting signals intracellularly 69. It has been shown that signalling through ephrinA ligands is mediated by the recruitment of adapter proteins. The ephrinA ligands are targeted to lipid rafts, which contain some signalling molecules such as caveolin proteins and G proteins. This indicates that ephrinA ligands may activate a number of signalling pathways 70. Src-family tyrosine kinases are important regulators of signalling through GPI-anchored proteins 71,72. Davy et al. 68 demonstrated that activation of Src kinase family members is important for signalling downstream of ephrinA5. Interaction of ephrinA5 with EphA-Fc fusion proteins has been shown to recruit and activate the Src-family kinase, Fyn. Subsequently, Fyn was shown to increase tyrosine phosphorylation of p80, and induce a change in the cellular architecture and adhesion of the ephrinA5-expressing cells. Bonanomi et al. 69 showed that Ret, which is also a RTK, is required for motor axon attraction mediated by ephrinA reverse signalling. Because of this, Ret is a transmembrane coreceptor and is dependent on ephrinA ligand for transmitting signals. Recent data demonstrate that activation of ephrinA2 and ephrinA5 by EphA3 leads to a β1-integrin-dependent increase in the adhesion of ephrinA-expressing cells to laminin 73. This may be because of p120 (120-kDa raft membrane protein), which plays a role in coupling ephrinA activation to integrin activation. Blocking the p120 can abolish the increase in cell adhesion. EphrinA5 engagement activates MAPK, through both integrin-dependent and integrin-independent pathways, which in turn regulate cell architecture and morphology 74. These studies indicate that ephrinA can influence cellular biological behaviour by transducing signals. EphrinB ligands are similar to Ephs in that they contain a single transmembrane domain, a cytoplasmic region and a PDZ-binding motif. EphrinB reverse signalling also involves Src kinases family that are responsible for ephrinB phosphorylation following Eph receptor binding 48,67,75,76. Phosphorylated ephrinB can initiate reverse signalling through SH2 or PDZ domain-containing proteins 77,78. The adaptor protein, Grb4, contains an SH2 domain and can link ephrinB ligands to a signalling network that modifies cell morphology 77. The GTPase-activating protein PDZ-RGS3 binds to the cytoplasmic C terminus of ephrinB through its PDZ domain. PDZ-RGS3 can mediate signalling that is induced by ephrinB1 78. EphrinB also plays significant roles in boundary formation. In Zebrafish embryo studies, bidirectional signalling between ephrinB2 and EphB2 at rhombomere boundaries restricts cell intermingling 79. Loss of the ephrinB2 cytoplasmic domain in mice results in defects in vasculogenesis and angiogenesis, which are very similar to those observed in ephrinB2-knockout mice 80. EphrinB signalling was also shown to play critical roles in vascular development by a corneal micropocket assay 81. Therefore, this suggests that reverse signalling is required for vascular development of vasculature.
Ephs and ephrins in developing and adult tissues
The Eph/ephrin system is associated with various signalling pathways, and participates in diverse biological processes such as cell proliferation and viability, cytoskeletal organization, cell migration and embryonic development. It plays key roles in the development of the nervous system, for example, in axon guidance 82, axon fasciculation 83 and neural crest cells migration 84. EphB receptors and ephrinB ligands regulate synaptogenesis, including the establishment and modification of the post-synaptic specialization 85,86. Eph/ephrin signalling is also essential for formation of villi and crypts in the intestinal epithelium 87.
Most Eph receptors and ephrin ligands are not only expressed during development but are also expressed in adult tissues 88. Hafner et al. 19 investigated the expression of 12 Eph receptors (EphA1–A8 and EphB1–B6) and 8 ephrin ligands (ephrinA1–A5 and ephrinB1–B3) in 13 different type of healthy human tissue, including brain, lung, liver, spleen, colon, small intestine, kidney, bladder, prostate, testis, uterus, thymus and bone marrow. They reported that except for EphA8 and ephrinA2, all members of the family were expressed in all investigated normal tissues. However, Eph and ephrin proteins are expressed at much lower levels in adult compared with embryonic tissue 89. Some articles demonstrate that low-level expression of Eph and ephrin in the adult gut 87, vasculature 90 and kidney 91, and could continue play a role in tissue architecture. In contrast, the high-level expression of Eph and ephrin proteins has been studied in very few normal tissues. For example, the expression of Eph and ephrin is relatively strong in the brain, where Eph may participate in the processes of synaptic plasticity, learning and memory 92.
Ephs and ephrins in cancer
It is generally recognized that Eph receptors and ephrin ligands play considerable roles in carcinogenesis, cancer progression and neovascularization of various human malignancies. The expression of Eph receptors and ephrin ligands has been identified in multiple types of human tumours, including melanoma 9–11, neuroblastoma 12, malignant glioma 13,14 and carcinoma of the pancreas 15, breast 16–18,74, colon 19,20, prostate 21,22, lung 23, gastrointestinal tract 24,25, ovaries 26,27, oesophagus 28, liver 29,30 and thyroid 31. The expression of Ephs and ephrins is often reported to be up-regulated in human tumours 93. Thus, these up-regulated Ephs and ephrins may provide molecular markers for the diagnosis of invasive and metastatic tumours 17. There are also reports describing the down-regulation of the Eph/ephrin family in cancer 18. For instance, EphA6 was down-regulated in colorectal and renal cell carcinoma, and EphB2 staining was reduced in hepatic cell cancer compared with the surrounding benign liver tissue 19. Data from recent studies demonstrate that both Eph receptors and ephrins have roles in tumour promotion and tumour suppression. The role of Ephs and ephrins in certain cancer is discussed in further detail below (Table1).
Table 1.
Expression of Eph receptors and ephrin ligands in cancers compared with normal tissues
| Cancer type | Expression | Ephs/ephrins | References |
|---|---|---|---|
| Breast cancer | Up | EphA2, EphB4 | 16,141,142,197–199 |
| Down | – | – | |
| Colorectal cancer | Up | EphA1, EphA2, EphA3, EphA8, EphB4, ephrinA1, ephrinB2 | 5,28,104,109,110,200,201 |
| Down | EphA6, EphA7, EphB1, EphB2 | 20 | |
| Prostate cancer | Up | EphA2, EphA3, EphA5, EphA6, EphA7, EphA8, EphA10, EphB3, ephrinA2 | 202 |
| Down | – | – | |
| Brain tumour, GBM | Up | EphA2, EphA3, EphA4, EphA7, EphB2, EphB4, ephrinB3 | 19,116,117,120,189 |
| Down | – | – | |
| Melanoma | Up | EphA2, EphA3, EphB3, ephrinA1 | 10,121,125,189,203 |
| Down | EphA4 | 204 | |
| Lung cancer | Up | EphA2, EphB3 | 23,127 |
| Down | – | – | |
| Hepatocellular cancer | Up | EphA3, ephrinA1 | 30,129 |
| Down | – | – | |
| Gastric cancer | Up | EphA1, EphA2, EphA3, EphB2 | 24,130,131,133,135 |
| Down | ephrinB1 | 25 |
Breast cancer
There is a significant relationship between Eph expression levels and invasiveness and aggressiveness in breast cancer 94. EphA2 overexpression has been found to cause oncogenic transformation and to promote tumourigenesis and metastasis in murine models of breast cancer 16,95,96. Elevated expression of EphA2 may promote tumour cell malignancy by interacting with both ErbB2 and epidermal growth factor receptor (EGFR) 97,98. Hypermethylated in cancer 1 (HIC1), a tumour suppressor gene, encodes a transcriptional repressor that is silenced in many human tumours. Recent research on breast cancer identified EphA2 as a direct target gene of HIC1. Infection of breast cancer cell lines with a retrovirus expressing HIC1 was shown to reduce EphA2 mRNA and protein 99. Thus, deregulation of the EphA2 pathway by silencing HIC1 might play an important role in the progression of breast cancer.
EphB4 provides a survival advantage in breast cancer by attenuating the inherent cell death pathways and up-regulating antiapoptotic proteins. EphB4 knockdown has been found to inhibit breast cancer cell viability, migration and invasion in vitro and tumour growth in vivo 100. Furthermore, EphB4 receptor signalling is also able to suppress breast tumour cell growth and motility 66. Recently, a retrospective study demonstrated that EphA2, EphA4, EphA7, EphB4 and EphB6 were significantly correlated with poor prognosis of breast cancer patients 101. This suggests that these Eph family members may become useful targets for therapeutic intervention and potential indicators for clinical assessment of tumour prognosis.
Colorectal cancer
Several studies have identified a role for Ephs and ephrins in colorectal cancer. A recent study demonstrated significant up-regulation of EphA1 in over 50% of colorectal cancer cases (P = 0.005), whereas many of the remaining patients showed down-regulation of EphA1 102. In addition, EphA1 overexpression was more in stage II compared to stage III colorectal cancer. Low EphA1 expression has been significantly correlated with poor survival. Similar to EphA1, overexpression of EphA2 and ephrinA1 was more common in the early stage than in the late stage of cancer. On the other hand, reduced expression of ephrinA1 inhibits growth of HT29 colorectal cancer cells 103. Recently, we reported that EphA3 expression positively correlated with tumour size, histological grade, depth of invasion, lymph node metastasis, distant metastasis and pTNM stage. In addition, patients with high expression of EphA3 had the lowest survival rate (P = 0.001) 104. Therefore, EphA3 may play an important role in the progression of tumours, and appeared as one of the specific molecular markers for assessment of tumour biological behaviour and prognosis. Loss of EphB expression was correlated with colorectal cancer progression, suggesting that reduction of EphB activity could promote tumorigenesis 20. Supporting this suggestion is the converse finding that highly elevated expression of EphB2 is associated with a longer survival time in colorectal cancer 20,105,106. Reduced expression of the EphB2 gene, as well as high expression of EphA4 gene, has also been suggested to promote liver metastasis in colorectal cancer 107. Overexpression of the EphA4 gene and reduced expression of the EphB2 gene may thus be a useful predictor of liver metastasis in patients with colorectal cancer. In addition, the overexpression of EphB3 enhanced cell-cell contacts and suppressed tumour growth in HT29 colorectal cancer 108. Furthermore, EphB4 and ephrinB2 were highly expressed in colorectal cancer compared to the normal mucosa 109,110. This suggests that EphB4 and ephrinB2 may play a role in the progression of colorectal cancer.
Prostate cancer
A number of Eph receptors and ephrin ligands have been detected in prostate cancer. Walker-Daniels et al. 22 reported that EphA2 is overexpressed in human prostate cancer compared with benign prostate tissues, and overexpression of EphA2 has been linked with metastasis. EphA2 has also been shown to induce an inactive conformation of integrins and inhibit cell spreading, migration and integrin-mediated adhesion through rapid recruitment of the protein tyrosine phosphatase SHP2, and subsequent dephosphorylation and inactivation of FAK 49. A recent experiment in prostate cancer tissue and cell lines showed that the frequency of EphA7 methylation was higher in cancer with higher Gleason scores 21. In addition, ectopic expression of EphA7 in DU145 cells was able to inhibit cell colony formation, but not cell growth. As far as metastatic cancer is concerned, for example, Astin and colleagues 111 analysed the dynamics of prostate cancer cell lines co-cultured with fibroblasts, and demonstrated that the unimpeded migration of metastatic PC-3 cells towards fibroblasts was dependent on activation of EphB3 and EphB4 by ephrinB2.
Brain tumours
Eph receptors and ephrin ligands are involved in the development of the central nervous system 82–84,112–114. EphA2 overexpression in glioblastoma multiforme (GBM) has been indicated to be a critical mediator of invasiveness and thus also represents an attractive molecular target for the development of therapeutics against GBM 115. Overexpression of EphA4 enhances cell proliferation and migration through promoting the FGFR1 signalling pathway 116. EphA7 protein is also overexpressed in GBM, and is correlated with poor survival of GBM patients 117. This may because of the fact that EphA7 could promote tumour neovascularization. Therefore, the local release of EphA7 inhibitors in to GBM could restrain tumour angiogenesis and improve patient outcome. Recent work demonstrated that EphA7 is an important mediator of neural progenitor apoptosis during brain development 118. EphB2 expression is higher in glioblastomas, especially in invasive ones, than in low-grade astrocytomas or normal brain tissue 119. EphB2 tyrosine phosphorylation can promote glioma migration and invasion, whereas blocking EphB2 could inhibit these aspects of tumorigenesis. Together, these data suggest that EphB2 has potential value for therapeutic intervention. Expression of ephrinB family members was determined in invading glioblastoma cells and glioma cell lines including U87, T98G, U251 and SNB19. EphrinB3 mRNA was up-regulated in all of these cells and promoted RAC1-dependent invasion of glioma cell lines 120. Furthermore, ephrinB3 expression and phosphorylation were correlated with increasing human glioma grade.
Melanoma
Initial studies reported that some members of the Eph receptors family are abnormally expressed in melanoma cells compared with melanocytes. In addition, EphA2 expression was significantly higher in metastatic cell than that in primary melanoma cells 121,122. EphA2 forward signalling in malignant melanoma can promote vasculogenic mimicry 123. Moreover, Udayakumar et al. verified that EphA2 is an important oncogene in melanoma by analysing EphA2 levels in a panel of melanoma cell lines 124. EphrinA1, a ligand of the EphA2 receptor, is not only a growth factor for melanoma cells 125 but is also angiogenic and a chemoattractant for endothelial cells. In addition, ephrinA1 was found to be expressed in 67% of metastatic melanomas, and 43% of advanced primary melanomas, but only in the occasional lesions 10. Together, these studies suggest that ephrinA1 may play a role in promoting melanocytic cell growth and inducing vascularization in advanced melanomas.
Lung cancer
There are relatively few studies of Eph/ephrin family in lung cancer. EphA2 was found to be overexpressed in patients who subsequently developed brain metastases, whereas low expression of EphA2 was related to disease-free survival or contralateral lung metastasis 23. The above data suggest that high levels of EphA2 could be used to identify the patients that are at risk of lung cancer metastasis to the brain. EphA2 mutations were demonstrated to be present in lung squamous cell carcinoma and were associated with increases in tumour invasion and survival. Whether or not EphA2 mutations could serve as a potential therapeutic target for lung squamous cell carcinoma requires further study 126. In addition, Ji et al. 127 found that overexpression of EphB3 in NSCLC cell lines promoted cell growth and migration. Recently, this research group reported that they identified a novel EphB3-binding protein, the receptor for activated C-kinase 1 (RACK1). RACK1 regulates the assembly of signal complexes including protein phosphatase 2A, Akt and itself in response to EphB3 activation, resulting in inhibition of NSCLC metastasis 128.
Hepatocellular cancer
EphA3 was previously found to be expressed at higher level in hepatocellular carcinoma (HCC) than that in corresponding healthy tissue 129. Remarkably, one novel missense mutation, a GAC to GTC transition (D219V) was found in the extracellular domain of EphA3, and two genetic alterations in the intracellular SAM domain of EphA3 appear to be polymorphisms 29. In addition, the overexpression of ephrinA1 was more frequently detected in poorly differentiated HCC than in well differentiated HCC. This indicates that ephrinA1 may be associated with the malignant phenotype of HCC.
Gastric cancer
Wang et al. 130 found that EphA1 protein was significantly associated with depth of invasion and cancer stage. Furthermore, patients with EphA1 up-regulation had a shorter survival time than those with absence or downregulation of EphA1. EphA2 has been associated with malignant transformation and was positively correlated with tumour invasion, lymph node metastasis and TNM stage 24,131. Knockdown of EphA2 expression could inhibit gastric cancer cell proliferation and invasion in vitro and in vivo 132. This indicates that the specific inhibition of EphA2 may be useful for gastric cancer therapy. More recently, we found that increased EphA3 expression was positively correlated with vascular endothelial growth factor (VEGF), microvessel density (MVD) and patient survival. Thus, EphA3 may play important roles in the angiogenesis and prognosis of gastric cancer. The combined detection of EphA3, VEGF and the determination of MVD, to some extent, can reflect the biological behaviour of gastric cancer and could be used to guide the choice of chemotherapy and molecular targeting therapy 133.
EphB2 mutations have been identified in human gastric tumours 134. Reduced expression of EphB2 was significantly associated with advanced disease stage, poor histological differentiation and poor survival rate 135. EphrinB1 are frequently overexpressed in gastric cancer, and the expression of ephrinB1 is especially high in poorly differentiated invasive tumour cells 25. Accumulating evidence demonstrates that expression of B-type ephrins is closely associated with tumour cell invasion. Reduction of ephrinB1 expression inhibits migration and invasion of scirrhous gastric cancer cells in vitro without affecting tumour cell proliferation or apoptosis 136.
Functions in tumour angiogenesis
Angiogenesis, the formation and growth of new blood vessels by sprouting from existing vessels 137,138, is critical for tumour growth and metastasis by supplying the tumour with nutrients, growth factors and oxygen 139. Several Eph receptors and ephrins play an important role in tumour angiogenesis by mediating communication of vascular cells with other vascular cells, as well as tumour cells 140. There is considerable evidence to support the tumour-promoting role of the Eph/ephrin family in angiogenesis 141.
Forward signalling induced by EphA2 is known to promote angiogenesis 142. In vitro and in vivo experiments also found that EphA2 forward signalling increases vascular permeability though phosphorylation of claudins 143,144. Additional studies revealed the expression of both EphA2 and ephrinA1 in tumour cells of two xenograft models from human breast cancer and Kaposi sarcoma, as well as in human cancer specimens 141. A further study indicated that EphA2, which was positively correlated with VEGF expression, was overexpressed in squamous cell carcinoma of the tongue and was also implicated in angiogenesis 145. EphB4 and its cognitive ligand ephrinB2 not only play an essential role in embryonic vessel development and vascular remodelling but also participate in tumour angiogenesis. It has been suggested that ephrinB2 promotes the vascular formation and remodelling in EphB4-positive tumour tissues 146. In addition, Martiny-Baron et al. demonstrated that EphB4 forward signalling is an important mediator of VEGF-induced angiogenesis, because of that VEGF-induced angiogenesis could be inhibited by the inhibition of EphB4 forward signalling 147. EphrinB2 reverse signalling is also required for VEGF-induced angiogenesis through regulation of VEGFR2 endocytosis 148.
Eph receptors and ephrin ligands as tumour therapeutics targets
Expression of Eph receptors and ephrin ligands are often up-regulated in various human malignant tumours. Decreases in Eph receptor levels can effectively suppress tumour growth in animal models. Therefore, Eph receptors and ephrin ligands represent probable new targets for anticancer therapies. To date, numerous strategies targeting Eph/ephrin family have been developed for cancer treatment, which we will elaborate below (Table2).
Table 2.
Strategies targeting Eph receptors and ephrin ligands for cancer therapy
| Treatment | Target | Tumour | References |
|---|---|---|---|
| Inhibitors of Eph/ephrin interaction | |||
| EphA2-FC, EphA3-FC | ephrinA | Breast cancer Pancreatic cancer | 142,149,150 |
| EphB4-FC | EphB4 | Melanoma | 156 |
| Mab2H9 antagonistic antibody | EphB2 | Colorectal cancer | 157 |
| TNYL-RAW peptide | EphB4 | Breast cancer | 158 |
| SNEW peptide | EphB2 | Breast cancer | 158 |
| KYL, APY, VTM peptide | EphA4 | Angiogenesis | 159,161 |
| 2,5-dimethylpyrroly benzoic acid derivatives | EphA4 | Angiogenesis | 161 |
| Disalicylic Acid-furanyl derivative | EphA2 | Prostate cancer | 205 |
| Activators of Eph forward signalling | |||
| EA2,B233,3F2-WT antibody | EphA2 | Breast cancer | 162 |
| EA5 antibody | EphA2 | Ovarian cancer | 163 |
| mAB208 | EphA2 | Renal cell cancer | 164 |
| Dimerized IIIA4 antibody | EphA3 | Malignant Melanoma | 183 |
| YSA, SWL peptides | EphA2 | Breast cancer | 206 |
| Kinase inhibitor | |||
| Dasatinib | EphA2 | Prostate cancer Ovarian cancer Pancreatic caner | 15,173,207 |
| Benzenesulphonamide derivative | EphB4 | Angiogenesis | 165 |
| Xanthine derivatives | EphB4 | Hepatocellular cancer | 168 |
| Inhibitor of Eph expression | |||
| EphA2 siRNA | EphA2 | Pancreatic cancer Ovarian cancer | 174 176,177 |
| EphB4 siRNA | EphB4 | Breast cancer | 100 |
| Oligonucleotides | EphB4 | Breast cancer Bladder cancer | 100,208 |
| Imaging agent | |||
| 64CU –DOTA-1C1 antibody | EphA2 | Colorectal cancer, Prostate cancer, Ovarian cancer, Glioblastoma, Malignant Melanoma | 182 |
| 111Indium-labelled IIIA4 antibody | EphA3 | Malignant Melanoma | 183 |
| Antibody-Drug/toxin conjugation | |||
| ephrinA1-PE38QQR | EphA2 | Glioblastoma | 13 |
| 1C1-maleimidocaproyl-MMAF conjugate | EphA2 | Prostate cancer | 179 |
| 2H9 antibody-vc-MMAE conjugate | EphB2 | Colorectal cancer | 157 |
| Immunotherapy | |||
| bscEphA2 × CD3 bispecific single-chain antibody | EphA2/CD3 | Breast cancer Colorectal cancer | 184 |
| EphA2-DCs | EphA2 | Colon cancer | 187 |
| EphA2833–891 peptide | EphA2 | Malignant gliomas | 14 |
| EphA3- and EphB6-derived peptides | EphA3/EphB6 | Glioma | 188,189 |
Preventing receptor-ligand interactions
The activation of Eph receptors by ephrin ligands relies on direct contact between cells that express Ephs and ephrins to induce signalling. Preventing receptor-ligand interactions may be useful to inhibit Eph/ephrin function. A large number of molecules can be used for this purpose. As function-blocking antagonists, soluble Eph and ephrin exdomains that bind their corresponding partner can inhibit Eph function during tumour progression and neovascularization 142,149,150. Soluble EphA2-Fc can inhibit EphA forward signalling, but promote reverse signalling, whereas EphB4-Fc can inhibit both forward and reverse signalling. Both EphA2-Fc and EphB4-Fc can suppress tumour growth in mouse models by inhibiting tumour angiogenesis 150–154. Furthermore, it was reported that soluble monomeric EphB4 significantly suppressed tumour growth in a mouse model 155,156. Scehnet et al. used the extracellular domain of EphB4 fused with human serum albumin to block ephrinB2 in Kaposi sarcoma cells in vitro. This block of ephrinB2 resulted in the inhibition of migration and invasion of Kaposi sarcoma cells in response to various growth factors 154. Antagonistic antibodies (MAb 2H9) 157 and peptides (TNYL-RAW, SNEW, KYL, etc.) 158,159 that compete with ephrins for binding to Eph receptors are also useful for blocking these interactions. Recently, Lamberto et al. 160 have reported that KYL, APY and VTM antagonistic peptides could selectively target EphA4 to inhibit ephrin binding to EphA4. Two isomeric small molecules that selectively inhibit ephrin binding to EphA2 and EphA4 have been identified 161. The peptides and small molecules could be used to develop pharmaceuticals that selectively targeting Eph receptors with high affinity. Agonistic antibodies have also been used to suppress tumour growth in mouse models. These agonists are activators of Eph-ephrin signalling that stimulate Eph forward signalling and could be used to negatively regulate tumour cell growth and to induce the degradation of Eph receptors in cancer cells 66,162–164. By stimulating its ephrinB2 ligand, EphB4 activates an anti-oncogenic pathway (Abl-Crk pathway) that can inhibit breast cancer cell survival, proliferation, motility and invasion 66. Coffman et al. targeted EphA2 on cancer cells using agonistic antibodies that simulate the effect of ligand binding. They showed that agonistic EphA2 antibodies can decrease tumour growth in vivo through protein degradation 162. Therefore, we suggest that Eph agonistic antibodies could be useful in cancer treatment in combination with chemotherapy. There are a great many approaches to identify inhibitors to the Eph kinase domain 147,165–169. For example, several 2,5-dimethylpyrroly benzoic acid derivatives are inhibitors of EphA4 receptors and 2,4-bis-anilinopyrimidines are inhibitors of EphB4 receptors. In addition, ALW-II-49–7 was reported to inhibit EphB2 tyrosine kinase activity. Furthermore, NVP-BHG712, which was originally described as an inhibitor of EphB4 kinase, can inhibit VEGF-driven angiogenesis in vivo 147. However, NVP-BHG712 can inhibit many kinases and is also non-selective for different Eph receptor kinases. Dasatinib is a small molecular inhibitor of multiple tyrosine kinases containing Src, BCR-ABL and c-Kit, multiple Eph kinases and platelet-derived growth factor-beta receptor kinases 170. It is used to suppress proliferation of haematological malignancies 170,171. In addition, dasatinib can inhibit growth, migration and invasion of breast cancer cells 172. Dasatinib can also inhibit invasion, and induce cell apoptosis of ovarian cancer, which was highly sensitive to dasatinib 173. It was also reported that the potency of dasatinib may be because of an ability to decrease EphA2 phosphorylation 15. Small interfering RNA (siRNA) that specifically induces destruction of specific mRNA is a powerful tool in the analysis of protein function and targeted therapeutics. Duxbury et al. demonstrated that EphA2 siRNA suppresses EphA2 expression, cellular invasiveness, anoikis resistance and FAK phosphorylation in vitro, and inhibited tumour growth and metastasis in a pancreatic cancer xenograft model 174. In addition, knockdown of EphA2 inhibited endothelial expression of EphA2, suppressed ephrinA1- and VEGF- induced cell migration, inhibited cell proliferation and induced cell apoptosis in human glioma cells 175,176. It was demonstrated that targeted knockdown of EphB4 expression by siRNA (and antisense oligodeoxynucleotides (ODNs)) led to poor survival of breast cancer cells, and increased apoptosis 100. Furthermore, antisense ODN-mediated EphB4 knockdown resulted in the suppression of tumour growth in a murine tumour xenograft model. Previous studies have indicated that siRNA targeting the oncoprotein EphA2 was incorporated into the neutral liposome 1,2-dioleoyl-sn-glycero-3-phosphatidylcholine (DOPC) for efficient in vivo siRNA delivery 177. Treatment with EphA2-targeting siRNA-DOPC resulted in significantly decreased tumour cell proliferation and tumour growth in an orthotopic mouse model of ovarian cancer 178.
Drug/toxin-conjugated antibody targeting of Eph-positive cancers
Monoclonal antibodies that selectively bind tumour cells provide a vehicle for targeted delivery of cytotoxins. A number of recent studies have provided insight into drug/toxin-conjugated Eph antibodies capable of killing tumour cells that express high levels of Eph receptors 157,179,180. Organic compounds that are suitable for conjugation and delivery by antibodies have been identified. It has been described that auristatins, derivatives of the tubulin polymerization inhibitor, were used as potent cytotoxic agents delivered by conjugated antibodies 181. Pseudomonas aeruginosa exotoxin A, which is a novel cytotoxin composed of ephrinA1 ligand conjugated to a bacterial toxin, was also used to kill GBM cells overexpressing EphA2 13. Jackson et al. demonstrated that the anti-EphA2 antibody-drug conjugate (1C1–maleimidocaproyl-MMAF (mcMMAF)) induces degradation of the EphA2 receptor and inhibits tumour growth in vivo 179. In addition, an EphB2 antibody conjugated to monomethylauristatin E specifically killed EphB2-expressing colorectal cancer cells in vitro and in vivo 157. However, EphB2 is also expressed in normal tissues, and the potential for this method to destroy normal cells needs to be further investigated. Therefore, advanced technology, drug potency and conjugation methods are urgently needed to develop safe and effective antibody-drug/toxin conjugates for the treatment of cancer.
Antibodies conjugated to imaging agents could be used for PET imaging. EphA2 labelled with 64Cu using the chelating agent 1,4,7,10-tetraazacyclododecane N,N’,N’’,N’’’-tetraacetic acid (DOTA) was used for quantitative radioimmunoPET imaging of EphA2-expression tumour-bearing mice. The tumour uptake value of 64Cu-DOTA-1C1 obtained from PET imaging correlated very well with the tumour expression level of EphA2 in vivo 182. In addition, both IIIA4 monoclonal antibodies and ephrinA5 that were labelled with 111Indium were successfully used for γ-camera imaging in solid tumour-bearing xenografts 183.
Targets for cancer immunotherapy
Dendritic cell-based tumour vaccines can induce protective antitumour immunity in tumour models, by inducing both the tumour-specific cytotoxic T-lymphocyte and helper-T cell response. There is some evidence that Eph receptors may be useful targets for cancer immunotherapy 14,184–188. A bispecific single-chain antibody (bscAb) that simultaneously targets EphA2 on tumour cells and the T cell receptor/CD3 complex on T cells can lyse EphA2-expressing tumour cells in vitro and in vivo 184. In an experimental approach, EphA2-derived peptides that induce specific, tumour-reactive CD8+ or CD4+ T cell responses might be able to serve as agents for immunotherapy of renal cell carcinoma 185. Yamaguchi et al. investigated the effectiveness of vaccination dendritic cells (DCs) loaded with EphA2-derived peptides (Eph-DCs) in a murine colon cancer model. They demonstrated that immunization with Eph-DCs suppressed MC38 tumour (with EphA2 overexpression) growth compared with the control group, and in contrast, Eph-DC vaccination had no influence on BL6 tumour (without EphA2 expression) growth 187. Furthermore, a previous study showed that the synthetic EphA2883–891 peptide induces an antigen-specific cytotoxic T-lymphocyte response in human leucocyte antigen A2+ patient-derived peripheral blood mononuclear cells from EphA2-expression malignant gliomas 186. EphA3- and EphB6-derived peptides were also suggested to be recognized by cancer-specific cytotoxic T cells 188,189.
Conclusion
The Eph receptors that comprise the largest subgroup of tyrosine kinase, and their ephrin ligands, form a cell-cell system that is associated with various important biological processes, including nervous system development, angiogenesis and tumorigenesis. They regulate cell-to-cell adhesion, cell proliferation and viability, cytoskeletal organization and cell migration. Increasing experimental evidence indicates deregulated activation of Eph/ephrin signalling in cancer. Our understanding of the Eph/ephrin pathway has improved tremendously. Ephs/ephrins can influence tumour behaviour by bidirectional signalling as well as other signalling modalities. Every tumour cell has to integrate and translate the signals that it receives into corresponding cellular responses, to achieve its overall biological function. However, varying surface densities of Eph receptors and ephrin ligands on tumour cells may influence Eph receptor signalling and the cellular response. The binding characteristic formation of multimers, bidirectional signalling and cross-talk with other molecules and signalling pathways further contribute to the complexity of the Eph/ephrin system. Therefore, a detailed understanding of Eph/ephrin signalling is important to regulate Eph-mediated tumour cell responses, to exploit the tumour-specific expression of Eph receptors and ephrin ligands, and to provide potentially novel targets for anticancer therapies for Eph-expressing tumours.
To date, a number of strategies targeting the Eph/ephrin system have been developed for cancer treatment, such as the prevention of receptor-ligand interactions, targeted delivery of drugs and immunotherapy. However, the complexity of their signalling and biological functions complicates the development of effective therapeutic agents. Strategies targeting the Eph/ephrin system might be useful in tumours in which Eph receptors promote tumorigenesis, and ineffective or even detrimental in tumours in which Eph receptors suppress tumorigenesis. In addition, the side effects of Eph/ephrin-targeting agents on normal tissues expression these family members are not well documented. Further examination of changes in Eph/ephrin expression in tumours and cancer-related Eph/ephrin gene mutations, as well as the underlying molecular mechanisms of bidirectional signalling of the Eph/ephrin system are needed to develop successful, safe and effective therapeutic strategies.
Acknowledgments
This work was supported by the National Nature Science Foundation of China (no. 81172368), and the Committee of Science and Technology of Beijing, China (no. Z111107058811047). The funding bodies had no role in study design, data collection or analysis, decision to publish, or preparation of the manuscript.
Disclosures
The authors confirm that there are no conflicts of interest.
References
- Wilkinson DG. Multiple roles of EPH receptors and ephrins in neural development. Nat Rev Neurosci. 2001;2:155–64. doi: 10.1038/35058515. [DOI] [PubMed] [Google Scholar]
- Himanen JP, Nikolov DB. Eph receptors and ephrins. Int J Biochem Cell Biol. 2003;35:130–4. doi: 10.1016/s1357-2725(02)00096-1. [DOI] [PubMed] [Google Scholar]
- Clifford N, Smith LM, Powell J, et al. The EphA3 receptor is expressed in a subset of rhabdomyosarcoma cell lines and suppresses cell adhesion and migration. J Cell Biochem. 2008;105:1250–9. doi: 10.1002/jcb.21926. [DOI] [PubMed] [Google Scholar]
- Anon. Unified nomenclature for Eph family receptors and their ligands, the ephrins. Eph Nomenclature Committee. Cell. 1997;90:403–4. doi: 10.1016/s0092-8674(00)80500-0. [DOI] [PubMed] [Google Scholar]
- Hirai H, Maru Y, Hagiwara K, et al. A novel putative tyrosine kinase receptor encoded by the eph gene. Science. 1987;238:1717–20. doi: 10.1126/science.2825356. [DOI] [PubMed] [Google Scholar]
- Bartley TD, Hunt RW, Welcher AA, et al. B61 is a ligand for the ECK receptor protein-tyrosine kinase. Nature. 1994;368:558–60. doi: 10.1038/368558a0. [DOI] [PubMed] [Google Scholar]
- Beckmann MP, Cerretti DP, Baum P, et al. Molecular characterization of a family of ligands for eph-related tyrosine kinase receptors. EMBO J. 1994;13:3757–62. doi: 10.1002/j.1460-2075.1994.tb06685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer A, Klein R. Multiple roles of ephrins in morphogenesis, neuronal networking, and brain function. Genes Dev. 2003;17:1429–50. doi: 10.1101/gad.1093703. [DOI] [PubMed] [Google Scholar]
- Hafner C, Becker B, Landthaler M, et al. Expression profile of Eph receptors and ephrin ligands in human skin and downregulation of EphA1 in nonmelanoma skin cancer. Mod Pathol. 2006;19:1369–77. doi: 10.1038/modpathol.3800660. [DOI] [PubMed] [Google Scholar]
- Easty DJ, Hill SP, Hsu MY, et al. Up-regulation of ephrin-A1 during melanoma progression. Int J Cancer. 1999;84:494–501. doi: 10.1002/(sici)1097-0215(19991022)84:5<494::aid-ijc8>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Vogt T, Stolz W, Welsh J, et al. Overexpression of Lerk-5/Eplg5 messenger RNA: a novel marker for increased tumorigenicity and metastatic potential in human malignant melanomas. Clin Cancer Res. 1998;4:791–7. [PubMed] [Google Scholar]
- Tang XX, Zhao H, Robinson ME, et al. Implications of EPHB6, EFNB2, and EFNB3 expressions in human neuroblastoma. Proc Natl Acad Sci U S A. 2000;97:10936–41. doi: 10.1073/pnas.190123297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wykosky J, Gibo DM, Debinski W. A novel, potent, and specific ephrinA1-based cytotoxin against EphA2 receptor expressing tumor cells. Mol Cancer Ther. 2007;6:3208–18. doi: 10.1158/1535-7163.MCT-07-0200. [DOI] [PubMed] [Google Scholar]
- Hatano M, Eguchi J, Tatsumi T, et al. EphA2 as a glioma-associated antigen: a novel target for glioma vaccines. Neoplasia. 2005;7:717–22. doi: 10.1593/neo.05277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Q, Jorgensen C, Pawson T, et al. Effects of dasatinib on EphA2 receptor tyrosine kinase activity and downstream signalling in pancreatic cancer. Br J Cancer. 2008;99:1074–82. doi: 10.1038/sj.bjc.6604676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelinski DP, Zantek ND, Stewart JC, et al. EphA2 overexpression causes tumorigenesis of mammary epithelial cells. Cancer Res. 2001;61:2301–6. [PubMed] [Google Scholar]
- Fox BP, Kandpal RP. Invasiveness of breast carcinoma cells and transcript profile: Eph receptors and ephrin ligands as molecular markers of potential diagnostic and prognostic application. Biochem Biophys Res Commun. 2004;318:882–92. doi: 10.1016/j.bbrc.2004.04.102. [DOI] [PubMed] [Google Scholar]
- Berclaz G, Flutsch B, Altermatt HJ, et al. Loss of EphB4 receptor tyrosine kinase protein expression during carcinogenesis of the human breast. Oncol Rep. 2002;9:985–9. [PubMed] [Google Scholar]
- Hafner C, Schmitz G, Meyer S, et al. Differential gene expression of Eph receptors and ephrins in benign human tissues and cancers. Clin Chem. 2004;50:490–9. doi: 10.1373/clinchem.2003.026849. [DOI] [PubMed] [Google Scholar]
- Batlle E, Bacani J, Begthel H, et al. EphB receptor activity suppresses colorectal cancer progression. Nature. 2005;435:1126–30. doi: 10.1038/nature03626. [DOI] [PubMed] [Google Scholar]
- Guan M, Xu C, Zhang F, et al. Aberrant methylation of EphA7 in human prostate cancer and its relation to clinicopathologic features. Int J Cancer. 2009;124:88–94. doi: 10.1002/ijc.23890. [DOI] [PubMed] [Google Scholar]
- Walker-Daniels J, Coffman K, Azimi M, et al. Overexpression of the EphA2 tyrosine kinase in prostate cancer. Prostate. 1999;41:275–80. doi: 10.1002/(sici)1097-0045(19991201)41:4<275::aid-pros8>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Kinch MS, Moore MB, Harpole DH., Jr Predictive value of the EphA2 receptor tyrosine kinase in lung cancer recurrence and survival. Clin Cancer Res. 2003;9:613–8. [PubMed] [Google Scholar]
- Nakamura R, Kataoka H, Sato N, et al. EPHA2/EFNA1 expression in human gastric cancer. Cancer Sci. 2005;96:42–7. doi: 10.1111/j.1349-7006.2005.00007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka H, Tanaka M, Kanamori M, et al. Expression profile of EFNB1, EFNB2, two ligands of EPHB2 in human gastric cancer. J Cancer Res Clin Oncol. 2002;128:343–8. doi: 10.1007/s00432-002-0355-0. [DOI] [PubMed] [Google Scholar]
- Landen CN, Kinch MS, Sood AK. EphA2 as a target for ovarian cancer therapy. Expert Opin Ther Targets. 2005;9:1179–87. doi: 10.1517/14728222.9.6.1179. [DOI] [PubMed] [Google Scholar]
- Herath NI, Spanevello MD, Sabesan S, et al. Over-expression of Eph and ephrin genes in advanced ovarian cancer: ephrin gene expression correlates with shortened survival. BMC Cancer. 2006;6:144. doi: 10.1186/1471-2407-6-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki T, Kato H, Fukuchi M, et al. EphA2 overexpression correlates with poor prognosis in esophageal squamous cell carcinoma. Int J Cancer. 2003;103:657–63. doi: 10.1002/ijc.10860. [DOI] [PubMed] [Google Scholar]
- Bae HJ, Song JH, Noh JH, et al. Low frequency mutation of the Ephrin receptor A3 gene in hepatocellular carcinoma. Neoplasma. 2009;56:331–4. doi: 10.4149/neo_2009_04_331. [DOI] [PubMed] [Google Scholar]
- Iida H, Honda M, Kawai HF, et al. Ephrin-A1 expression contributes to the malignant characteristics of {alpha}-fetoprotein producing hepatocellular carcinoma. Gut. 2005;54:843–51. doi: 10.1136/gut.2004.049486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karidis NP, Giaginis C, Tsourouflis G, et al. Eph-A2 and Eph-A4 expression in human benign and malignant thyroid lesions: an immunohistochemical study. Med Sci Monit. 2011;17:BR257–65. doi: 10.12659/MSM.881929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyokawa E, Takai S, Tanaka M, et al. Overexpression of ERK, an EPH family receptor protein tyrosine kinase, in various human tumors. Cancer Res. 1994;54:3645–50. [PubMed] [Google Scholar]
- Bruckner K, Pablo Labrador J, Scheiffele P, et al. EphrinB ligands recruit GRIP family PDZ adaptor proteins into raft membrane microdomains. Neuron. 1999;22:511–24. doi: 10.1016/s0896-6273(00)80706-0. [DOI] [PubMed] [Google Scholar]
- Torres R, Firestein BL, Dong H, et al. PDZ proteins bind, cluster, and synaptically colocalize with Eph receptors and their ephrin ligands. Neuron. 1998;21:1453–63. doi: 10.1016/s0896-6273(00)80663-7. [DOI] [PubMed] [Google Scholar]
- Kullander K, Klein R. Mechanisms and functions of Eph and ephrin signalling. Nat Rev Mol Cell Biol. 2002;3:475–86. doi: 10.1038/nrm856. [DOI] [PubMed] [Google Scholar]
- Himanen JP, Chumley MJ, Lackmann M, et al. Repelling class discrimination: ephrin-A5 binds to and activates EphB2 receptor signaling. Nat Neurosci. 2004;7:501–9. doi: 10.1038/nn1237. [DOI] [PubMed] [Google Scholar]
- Gale NW, Holland SJ, Valenzuela DM, et al. Eph receptors and ligands comprise two major specificity subclasses and are reciprocally compartmentalized during embryogenesis. Neuron. 1996;17:9–19. doi: 10.1016/s0896-6273(00)80276-7. [DOI] [PubMed] [Google Scholar]
- Kalo MS, Pasquale EB. Multiple in vivo tyrosine phosphorylation sites in EphB receptors. Biochemistry. 1999;38:14396–408. doi: 10.1021/bi991628t. [DOI] [PubMed] [Google Scholar]
- Egea J, Klein R. Bidirectional Eph-ephrin signaling during axon guidance. Trends Cell Biol. 2007;17:230–8. doi: 10.1016/j.tcb.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Yin Y, Yamashita Y, Noda H, et al. EphA receptor tyrosine kinases interact with co-expressed ephrin-A ligands in cis. Neurosci Res. 2004;48:285–96. doi: 10.1016/j.neures.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Carvalho RF, Beutler M, Marler KJ, et al. Silencing of EphA3 through a cis interaction with ephrinA5. Nat Neurosci. 2006;9:322–30. doi: 10.1038/nn1655. [DOI] [PubMed] [Google Scholar]
- Himanen JP, Nikolov DB. Eph signaling: a structural view. Trends Neurosci. 2003;26:46–51. doi: 10.1016/s0166-2236(02)00005-x. [DOI] [PubMed] [Google Scholar]
- Boyd AW, Lackmann M. Signals from Eph and ephrin proteins: a developmental tool kit. Sci STKE. 2001;2001:1–6. doi: 10.1126/stke.2001.112.re20. [DOI] [PubMed] [Google Scholar]
- Matsuoka H, Obama H, Kelly ML, et al. Biphasic functions of the kinase-defective Ephb6 receptor in cell adhesion and migration. J Biol Chem. 2005;280:29355–63. doi: 10.1074/jbc.M500010200. [DOI] [PubMed] [Google Scholar]
- Gu C, Shim S, Shin J, et al. The EphA8 receptor induces sustained MAP kinase activation to promote neurite outgrowth in neuronal cells. Oncogene. 2005;24:4243–56. doi: 10.1038/sj.onc.1208584. [DOI] [PubMed] [Google Scholar]
- Gu C, Park S. The EphA8 receptor regulates integrin activity through p110gamma phosphatidylinositol-3 kinase in a tyrosine kinase activity-independent manner. Mol Cell Biol. 2001;21:4579–97. doi: 10.1128/MCB.21.14.4579-4597.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noren NK, Yang NY, Silldorff M, et al. Ephrin-independent regulation of cell substrate adhesion by the EphB4 receptor. Biochem J. 2009;422:433–42. doi: 10.1042/BJ20090014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquale EB. Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol. 2005;6:462–75. doi: 10.1038/nrm1662. [DOI] [PubMed] [Google Scholar]
- Miao H, Burnett E, Kinch M, et al. Activation of EphA2 kinase suppresses integrin function and causes focal-adhesion-kinase dephosphorylation. Nat Cell Biol. 2000;2:62–9. doi: 10.1038/35000008. [DOI] [PubMed] [Google Scholar]
- Lai KO, Chen Y, Po HM, et al. Identification of the Jak/Stat proteins as novel downstream targets of EphA4 signaling in muscle: implications in the regulation of acetylcholinesterase expression. J Biol Chem. 2004;279:13383–92. doi: 10.1074/jbc.M313356200. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Shamah SM, Lin MZ, Goldberg JL, et al. EphA receptors regulate growth cone dynamics through the novel guanine nucleotide exchange factor ephexin. Cell. 2001;105:233–44. doi: 10.1016/s0092-8674(01)00314-2. [DOI] [PubMed] [Google Scholar]
- Lawrenson ID, Wimmer-Kleikamp SH, Lock P, et al. Ephrin-A5 induces rounding, blebbing and de-adhesion of EphA3-expressing 293T and melanoma cells by CrkII and Rho-mediated signalling. J Cell Sci. 2002;115:1059–72. doi: 10.1242/jcs.115.5.1059. [DOI] [PubMed] [Google Scholar]
- Irie F, Yamaguchi Y. EphB receptors regulate dendritic spine development via intersectin, Cdc42 and N-WASP. Nat Neurosci. 2002;5:1117–8. doi: 10.1038/nn964. [DOI] [PubMed] [Google Scholar]
- Penzes P, Beeser A, Chernoff J, et al. Rapid induction of dendritic spine morphogenesis by trans-synaptic ephrinB-EphB receptor activation of the Rho-GEF kalirin. Neuron. 2003;37:263–74. doi: 10.1016/s0896-6273(02)01168-6. [DOI] [PubMed] [Google Scholar]
- Zou JX, Wang B, Kalo MS, et al. An Eph receptor regulates integrin activity through R-Ras. Proc Natl Acad Sci USA. 1999;96:13813–8. doi: 10.1073/pnas.96.24.13813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao H, Wei BR, Peehl DM, et al. Activation of EphA receptor tyrosine kinase inhibits the Ras/MAPK pathway. Nat Cell Biol. 2001;3:527–30. doi: 10.1038/35074604. [DOI] [PubMed] [Google Scholar]
- Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–2. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- Elowe S, Holland SJ, Kulkarni S, et al. Downregulation of the Ras-mitogen-activated protein kinase pathway by the EphB2 receptor tyrosine kinase is required for ephrin-induced neurite retraction. Mol Cell Biol. 2001;21:7429–41. doi: 10.1128/MCB.21.21.7429-7441.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt RL, Kinch MS. Activation of the EphA2 tyrosine kinase stimulates the MAP/ERK kinase signaling cascade. Oncogene. 2002;21:7690–9. doi: 10.1038/sj.onc.1205758. [DOI] [PubMed] [Google Scholar]
- Zisch AH, Pazzagli C, Freeman AL, et al. Replacing two conserved tyrosines of the EphB2 receptor with glutamic acid prevents binding of SH2 domains without abrogating kinase activity and biological responses. Oncogene. 2000;19:177–87. doi: 10.1038/sj.onc.1203304. [DOI] [PubMed] [Google Scholar]
- Tong J, Elowe S, Nash P, et al. Manipulation of EphB2 regulatory motifs and SH2 binding sites switches MAPK signaling and biological activity. J Biol Chem. 2003;278:6111–9. doi: 10.1074/jbc.M208972200. [DOI] [PubMed] [Google Scholar]
- Genander M, Halford MM, Xu NJ, et al. Dissociation of EphB2 signaling pathways mediating progenitor cell proliferation and tumor suppression. Cell. 2009;139:679–92. doi: 10.1016/j.cell.2009.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg J, Genander M, Halford MM, et al. EphB receptors coordinate migration and proliferation in the intestinal stem cell niche. Cell. 2006;125:1151–63. doi: 10.1016/j.cell.2006.04.030. [DOI] [PubMed] [Google Scholar]
- Noren NK, Foos G, Hauser CA, et al. The EphB4 receptor suppresses breast cancer cell tumorigenicity through an Abl-Crk pathway. Nat Cell Biol. 2006;8:815–25. doi: 10.1038/ncb1438. [DOI] [PubMed] [Google Scholar]
- Bruckner K, Pasquale EB, Klein R. Tyrosine phosphorylation of transmembrane ligands for Eph receptors. Science. 1997;275:1640–3. doi: 10.1126/science.275.5306.1640. [DOI] [PubMed] [Google Scholar]
- Davy A, Gale NW, Murray EW, et al. Compartmentalized signaling by GPI-anchored ephrin-A5 requires the Fyn tyrosine kinase to regulate cellular adhesion. Genes Dev. 1999;13:3125–35. doi: 10.1101/gad.13.23.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonanomi D, Chivatakarn O, Bai G, et al. Ret is a multifunctional coreceptor that integrates diffusible- and contact-axon guidance signals. Cell. 2012;148:568–82. doi: 10.1016/j.cell.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh P, Schnitzer JE. Segregation of heterotrimeric G proteins in cell surface microdomains. G(q) binds caveolin to concentrate in caveolae, whereas G(i) and G(s) target lipid rafts by default. Mol Biol Cell. 2001;12:685–98. doi: 10.1091/mbc.12.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanova I, Horejsi V, Ansotegui IJ, et al. GPI-anchored cell-surface molecules complexed to protein tyrosine kinases. Science. 1991;254:1016–9. doi: 10.1126/science.1719635. [DOI] [PubMed] [Google Scholar]
- Murray EW, Robbins SM. Antibody cross-linking of the glycosylphosphatidylinositol-linked protein CD59 on hematopoietic cells induces signaling pathways resembling activation by complement. J Biol Chem. 1998;273:25279–84. doi: 10.1074/jbc.273.39.25279. [DOI] [PubMed] [Google Scholar]
- Huai J, Drescher U. An ephrin-A-dependent signaling pathway controls integrin function and is linked to the tyrosine phosphorylation of a 120-kDa protein. J Biol Chem. 2001;276:6689–94. doi: 10.1074/jbc.M008127200. [DOI] [PubMed] [Google Scholar]
- Davy A, Robbins SM. Ephrin-A5 modulates cell adhesion and morphology in an integrin-dependent manner. EMBO J. 2000;19:5396–405. doi: 10.1093/emboj/19.20.5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland SJ, Gale NW, Mbamalu G, et al. Bidirectional signalling through the EPH-family receptor Nuk and its transmembrane ligands. Nature. 1996;383:722–5. doi: 10.1038/383722a0. [DOI] [PubMed] [Google Scholar]
- Arvanitis D, Davy A. Eph/ephrin signaling: networks. Genes Dev. 2008;22:416–29. doi: 10.1101/gad.1630408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan CA, Henkemeyer M. The SH2/SH3 adaptor Grb4 transduces B-ephrin reverse signals. Nature. 2001;413:174–9. doi: 10.1038/35093123. [DOI] [PubMed] [Google Scholar]
- Lu Q, Sun EE, Klein RS, et al. Ephrin-B reverse signaling is mediated by a novel PDZ-RGS protein and selectively inhibits G protein-coupled chemoattraction. Cell. 2001;105:69–79. doi: 10.1016/s0092-8674(01)00297-5. [DOI] [PubMed] [Google Scholar]
- Xu Q, Mellitzer G, Robinson V, et al. In vivo cell sorting in complementary segmental domains mediated by Eph receptors and ephrins. Nature. 1999;399:267–71. doi: 10.1038/20452. [DOI] [PubMed] [Google Scholar]
- Adams RH, Diella F, Hennig S, et al. The cytoplasmic domain of the ligand ephrinB2 is required for vascular morphogenesis but not cranial neural crest migration. Cell. 2001;104:57–69. doi: 10.1016/s0092-8674(01)00191-x. [DOI] [PubMed] [Google Scholar]
- Huynh-Do U, Vindis C, Liu H, et al. Ephrin-B1 transduces signals to activate integrin-mediated migration, attachment and angiogenesis. J Cell Sci. 2002;115:3073–81. doi: 10.1242/jcs.115.15.3073. [DOI] [PubMed] [Google Scholar]
- Tessier-Lavigne M. Eph receptor tyrosine kinases, axon repulsion, and the development of topographic maps. Cell. 1995;82:345–8. doi: 10.1016/0092-8674(95)90421-2. [DOI] [PubMed] [Google Scholar]
- Winslow JW, Moran P, Valverde J, et al. Cloning of AL-1, a ligand for an Eph-related tyrosine kinase receptor involved in axon bundle formation. Neuron. 1995;14:973–81. doi: 10.1016/0896-6273(95)90335-6. [DOI] [PubMed] [Google Scholar]
- Wang HU, Anderson DJ. Eph family transmembrane ligands can mediate repulsive guidance of trunk neural crest migration and motor axon outgrowth. Neuron. 1997;18:383–96. doi: 10.1016/s0896-6273(00)81240-4. [DOI] [PubMed] [Google Scholar]
- Dalva MB, Takasu MA, Lin MZ, et al. EphB receptors interact with NMDA receptors and regulate excitatory synapse formation. Cell. 2000;103:945–56. doi: 10.1016/s0092-8674(00)00197-5. [DOI] [PubMed] [Google Scholar]
- Ethell IM, Irie F, Kalo MS, et al. EphB/syndecan-2 signaling in dendritic spine morphogenesis. Neuron. 2001;31:1001–13. doi: 10.1016/s0896-6273(01)00440-8. [DOI] [PubMed] [Google Scholar]
- Batlle E, Henderson JT, Beghtel H, et al. Beta-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell. 2002;111:251–63. doi: 10.1016/s0092-8674(02)01015-2. [DOI] [PubMed] [Google Scholar]
- Frisen J, Holmberg J, Barbacid M. Ephrins and their Eph receptors: multitalented directors of embryonic development. EMBO J. 1999;18:5159–65. doi: 10.1093/emboj/18.19.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herath NI, Boyd AW. The role of Eph receptors and ephrin ligands in colorectal cancer. Int J Cancer. 2010;126:2003–11. doi: 10.1002/ijc.25147. [DOI] [PubMed] [Google Scholar]
- Adams RH. Vascular patterning by Eph receptor tyrosine kinases and ephrins. Semin Cell Dev Biol. 2002;13:55–60. doi: 10.1006/scdb.2001.0289. [DOI] [PubMed] [Google Scholar]
- Ogawa K, Wada H, Okada N, et al. EphB2 and ephrin-B1 expressed in the adult kidney regulate the cytoarchitecture of medullary tubule cells through Rho family GTPases. J Cell Sci. 2006;119:559–70. doi: 10.1242/jcs.02777. [DOI] [PubMed] [Google Scholar]
- Gerlai R. Eph receptors and neural plasticity. Nat Rev Neurosci. 2001;2:205–9. doi: 10.1038/35058582. [DOI] [PubMed] [Google Scholar]
- Brantley-Sieders D, Schmidt S, Parker M, et al. Eph receptor tyrosine kinases in tumor and tumor microenvironment. Curr Pharm Des. 2004;10:3431–42. doi: 10.2174/1381612043383160. [DOI] [PubMed] [Google Scholar]
- Walker-Daniels J, Hess AR, Hendrix MJ, et al. Differential regulation of EphA2 in normal and malignant cells. Am J Pathol. 2003;162:1037–42. doi: 10.1016/S0002-9440(10)63899-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brantley-Sieders DM, Fang WB, Hicks DJ, et al. Impaired tumor microenvironment in EphA2-deficient mice inhibits tumor angiogenesis and metastatic progression. FASEB J. 2005;19:1884–6. doi: 10.1096/fj.05-4038fje. [DOI] [PubMed] [Google Scholar]
- Fang WB, Brantley-Sieders DM, Parker MA, et al. A kinase-dependent role for EphA2 receptor in promoting tumor growth and metastasis. Oncogene. 2005;24:7859–68. doi: 10.1038/sj.onc.1208937. [DOI] [PubMed] [Google Scholar]
- Brantley-Sieders DM, Zhuang G, Hicks D, et al. The receptor tyrosine kinase EphA2 promotes mammary adenocarcinoma tumorigenesis and metastatic progression in mice by amplifying ErbB2 signaling. J Clin Invest. 2008;118:64–78. doi: 10.1172/JCI33154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen AB, Pedersen MW, Stockhausen MT, et al. Activation of the EGFR gene target EphA2 inhibits epidermal growth factor-induced cancer cell motility. Mol Cancer Res. 2007;5:283–93. doi: 10.1158/1541-7786.MCR-06-0321. [DOI] [PubMed] [Google Scholar]
- Foveau B, Boulay G, Pinte S, et al. The receptor tyrosine kinase EphA2 is a direct target gene of hypermethylated in cancer 1 (HIC1) J Biol Chem. 2012;287:5366–78. doi: 10.1074/jbc.M111.329466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar SR, Singh J, Xia G, et al. Receptor tyrosine kinase EphB4 is a survival factor in breast cancer. Am J Pathol. 2006;169:279–93. doi: 10.2353/ajpath.2006.050889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brantley-Sieders DM, Jiang A, Sarma K, et al. Eph/ephrin profiling in human breast cancer reveals significant associations between expression level and clinical outcome. PLoS ONE. 2011;6:1–9. doi: 10.1371/journal.pone.0024426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herath NI, Doecke J, Spanevello MD, et al. Epigenetic silencing of EphA1 expression in colorectal cancer is correlated with poor survival. Br J Cancer. 2009;100:1095–102. doi: 10.1038/sj.bjc.6604970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potla L, Boghaert ER, Armellino D, et al. Reduced expression of EphrinA1 (EFNA1) inhibits three-dimensional growth of HT29 colon carcinoma cells. Cancer Lett. 2002;175:187–95. doi: 10.1016/s0304-3835(01)00613-9. [DOI] [PubMed] [Google Scholar]
- Xi HQ, Zhao P. Clinicopathological significance and prognostic value of EphA3 and CD133 expression in colorectal carcinoma. J Clin Pathol. 2011;64:498–503. doi: 10.1136/jcp.2010.087213. [DOI] [PubMed] [Google Scholar]
- Guo DL, Zhang J, Yuen ST, et al. Reduced expression of EphB2 that parallels invasion and metastasis in colorectal tumours. Carcinogenesis. 2006;27:454–64. doi: 10.1093/carcin/bgi259. [DOI] [PubMed] [Google Scholar]
- Jubb AM, Zhong F, Bheddah S, et al. EphB2 is a prognostic factor in colorectal cancer. Clin Cancer Res. 2005;11:5181–7. doi: 10.1158/1078-0432.CCR-05-0143. [DOI] [PubMed] [Google Scholar]
- Oshima T, Akaike M, Yoshihara K, et al. Overexpression of EphA4 gene and reduced expression of EphB2 gene correlates with liver metastasis in colorectal cancer. Int J Oncol. 2008;33:573–7. [PubMed] [Google Scholar]
- Chiu ST, Chang KJ, Ting CH, et al. Over-expression of EphB3 enhances cell-cell contacts and suppresses tumor growth in HT-29 human colon cancer cells. Carcinogenesis. 2009;30:1475–86. doi: 10.1093/carcin/bgp133. [DOI] [PubMed] [Google Scholar]
- Liu W, Ahmad SA, Jung YD, et al. Coexpression of ephrin-Bs and their receptors in colon carcinoma. Cancer. 2002;94:934–9. doi: 10.1002/cncr.10122. [DOI] [PubMed] [Google Scholar]
- Stephenson SA, Slomka S, Douglas EL, et al. Receptor protein tyrosine kinase EphB4 is up-regulated in colon cancer. BMC Mol Biol. 2001;2:15. doi: 10.1186/1471-2199-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astin JW, Batson J, Kadir S, et al. Competition amongst Eph receptors regulates contact inhibition of locomotion and invasiveness in prostate cancer cells. Nat Cell Biol. 2010;12:1194–204. doi: 10.1038/ncb2122. [DOI] [PubMed] [Google Scholar]
- Flanagan JG, Vanderhaeghen P. The ephrins and Eph receptors in neural development. Annu Rev Neurosci. 1998;21:309–45. doi: 10.1146/annurev.neuro.21.1.309. [DOI] [PubMed] [Google Scholar]
- Holder N, Klein R. Eph receptors and ephrins: effectors of morphogenesis. Development. 1999;126:2033–44. doi: 10.1242/dev.126.10.2033. [DOI] [PubMed] [Google Scholar]
- Martinez A, Soriano E. Functions of ephrin/Eph interactions in the development of the nervous system: emphasis on the hippocampal system. Brain Res Brain Res Rev. 2005;49:211–26. doi: 10.1016/j.brainresrev.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Wykosky J, Gibo DM, Stanton C, et al. EphA2 as a novel molecular marker and target in glioblastoma multiforme. Mol Cancer Res. 2005;3:541–51. doi: 10.1158/1541-7786.MCR-05-0056. [DOI] [PubMed] [Google Scholar]
- Fukai J, Yokote H, Yamanaka R, et al. EphA4 promotes cell proliferation and migration through a novel EphA4-FGFR1 signaling pathway in the human glioma U251 cell line. Mol Cancer Ther. 2008;7:2768–78. doi: 10.1158/1535-7163.MCT-07-2263. [DOI] [PubMed] [Google Scholar]
- Wang LF, Fokas E, Juricko J, et al. Increased expression of EphA7 correlates with adverse outcome in primary and recurrent glioblastoma multiforme patients. BMC Cancer. 2008;8:1–9. doi: 10.1186/1471-2407-8-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depaepe V, Suarez-Gonzalez N, Dufour A, et al. Ephrin signalling controls brain size by regulating apoptosis of neural progenitors. Nature. 2005;435:1244–50. doi: 10.1038/nature03651. [DOI] [PubMed] [Google Scholar]
- Nakada M, Niska JA, Miyamori H, et al. The phosphorylation of EphB2 receptor regulates migration and invasion of human glioma cells. Cancer Res. 2004;64:3179–85. doi: 10.1158/0008-5472.can-03-3667. [DOI] [PubMed] [Google Scholar]
- Nakada M, Drake KL, Nakada S, et al. Ephrin-B3 ligand promotes glioma invasion through activation of Rac1. Cancer Res. 2006;66:8492–500. doi: 10.1158/0008-5472.CAN-05-4211. [DOI] [PubMed] [Google Scholar]
- Kinch MS, Carles-Kinch K. Overexpression and functional alterations of the EphA2 tyrosine kinase in cancer. Clin Exp Metastasis. 2003;20:59–68. doi: 10.1023/a:1022546620495. [DOI] [PubMed] [Google Scholar]
- Hendrix MJ, Seftor EA, Hess AR, et al. Molecular plasticity of human melanoma cells. Oncogene. 2003;22:3070–5. doi: 10.1038/sj.onc.1206447. [DOI] [PubMed] [Google Scholar]
- Hess AR, Seftor EA, Gruman LM, et al. VE-cadherin regulates EphA2 in aggressive melanoma cells through a novel signaling pathway: implications for vasculogenic mimicry. Cancer Biol Ther. 2006;5:228–33. doi: 10.4161/cbt.5.2.2510. [DOI] [PubMed] [Google Scholar]
- Udayakumar D, Zhang G, Ji Z, et al. Epha2 is a critical oncogene in melanoma. Oncogene. 2011;30:4921–9. doi: 10.1038/onc.2011.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easty DJ, Herlyn M, Bennett DC. Abnormal protein tyrosine kinase gene expression during melanoma progression and metastasis. Int J Cancer. 1995;60:129–36. doi: 10.1002/ijc.2910600119. [DOI] [PubMed] [Google Scholar]
- Faoro L, Singleton PA, Cervantes GM, et al. EphA2 mutation in lung squamous cell carcinoma promotes increased cell survival, cell invasion, focal adhesions, and mammalian target of rapamycin activation. J Biol Chem. 2010;285:18575–85. doi: 10.1074/jbc.M109.075085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji XD, Li G, Feng YX, et al. EphB3 is overexpressed in non-small-cell lung cancer and promotes tumor metastasis by enhancing cell survival and migration. Cancer Res. 2011;71:1156–66. doi: 10.1158/0008-5472.CAN-10-0717. [DOI] [PubMed] [Google Scholar]
- Li G, Ji XD, Gao H, et al. EphB3 suppresses non-small-cell lung cancer metastasis via a PP2A/RACK1/Akt signalling complex. Nat Commun. 2012;3:1–10. doi: 10.1038/ncomms1675. [DOI] [PubMed] [Google Scholar]
- Nam SW, Park JY, Ramasamy A, et al. Molecular changes from dysplastic nodule to hepatocellular carcinoma through gene expression profiling. Hepatology. 2005;42:809–18. doi: 10.1002/hep.20878. [DOI] [PubMed] [Google Scholar]
- Wang J, Dong Y, Wang X, et al. Expression of EphA1 in gastric carcinomas is associated with metastasis and survival. Oncol Rep. 2010;24:1577–84. doi: 10.3892/or_00001020. [DOI] [PubMed] [Google Scholar]
- Yuan W, Chen Z, Wu S, et al. Expression of EphA2 and E-cadherin in gastric cancer: correlated with tumor progression and lymphogenous metastasis. Pathol Oncol Res. 2009;15:473–8. doi: 10.1007/s12253-008-9132-y. [DOI] [PubMed] [Google Scholar]
- Yuan W, Chen Z, Wu S, et al. Silencing of EphA2 inhibits invasion of human gastric cancer SGC-7901 cells in vitro and in vivo. Neoplasma. 2012;59:105–13. doi: 10.4149/neo_2012_014. [DOI] [PubMed] [Google Scholar]
- Xi HQ, Wu XS, Wei B, et al. Aberrant expression of EphA3 in gastric carcinoma: correlation with tumor angiogenesis and survival. J Gastroenterol. 2012;47:785–94. doi: 10.1007/s00535-012-0549-4. [DOI] [PubMed] [Google Scholar]
- Davalos V, Dopeso H, Velho S, et al. High EPHB2 mutation rate in gastric but not endometrial tumors with microsatellite instability. Oncogene. 2007;26:308–11. doi: 10.1038/sj.onc.1209780. [DOI] [PubMed] [Google Scholar]
- Yu G, Gao Y, Ni C, et al. Reduced expression of EphB2 is significantly associated with nodal metastasis in Chinese patients with gastric cancer. J Cancer Res Clin Oncol. 2011;137:73–80. doi: 10.1007/s00432-010-0861-4. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Kamata R, Takigahira M, et al. Phosphorylation of ephrin-B1 regulates dissemination of gastric scirrhous carcinoma. Am J Pathol. 2007;171:68–78. doi: 10.2353/ajpath.2007.070033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–64. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- Iruela-Arispe ML, Dvorak HF. Angiogenesis: a dynamic balance of stimulators and inhibitors. Thromb Haemost. 1997;78:672–7. [PubMed] [Google Scholar]
- Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82:4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- Pasquale EB. Eph receptors and ephrins in cancer: bidirectional signalling and beyond. Nat Rev Cancer. 2010;10:165–80. doi: 10.1038/nrc2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa K, Pasqualini R, Lindberg RA, et al. The ephrin-A1 ligand and its receptor, EphA2, are expressed during tumor neovascularization. Oncogene. 2000;19:6043–52. doi: 10.1038/sj.onc.1204004. [DOI] [PubMed] [Google Scholar]
- Brantley DM, Cheng N, Thompson EJ, et al. Soluble Eph A receptors inhibit tumor angiogenesis and progression in vivo. Oncogene. 2002;21:7011–26. doi: 10.1038/sj.onc.1205679. [DOI] [PubMed] [Google Scholar]
- Miao H, Wang B. Eph/ephrin signaling in epithelial development and homeostasis. Int J Biochem Cell Biol. 2009;41:762–70. doi: 10.1016/j.biocel.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson J, Schomberg S, Schroeder W, et al. Endothelial EphA receptor stimulation increases lung vascular permeability. Am J Physiol Lung Cell Mol Physiol. 2008;295:L431–9. doi: 10.1152/ajplung.90256.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Z, Zhang WF, Chen XM, et al. Expression of EphA2 and VEGF in squamous cell carcinoma of the tongue: correlation with the angiogenesis and clinical outcome. Oral Oncol. 2008;44:1110–7. doi: 10.1016/j.oraloncology.2008.01.018. [DOI] [PubMed] [Google Scholar]
- Noren NK, Lu M, Freeman AL, et al. Interplay between EphB4 on tumor cells and vascular ephrin-B2 regulates tumor growth. Proc Natl Acad Sci USA. 2004;101:5583–8. doi: 10.1073/pnas.0401381101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martiny-Baron G, Holzer P, Billy E, et al. The small molecule specific EphB4 kinase inhibitor NVP-BHG712 inhibits VEGF driven angiogenesis. Angiogenesis. 2010;13:259–67. doi: 10.1007/s10456-010-9183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawamiphak S, Seidel S, Essmann CL, et al. Ephrin-B2 regulates VEGFR2 function in developmental and tumour angiogenesis. Nature. 2010;465:487–91. doi: 10.1038/nature08995. [DOI] [PubMed] [Google Scholar]
- Cheng N, Brantley D, Fang WB, et al. Inhibition of VEGF-dependent multistage carcinogenesis by soluble EphA receptors. Neoplasia. 2003;5:445–56. doi: 10.1016/s1476-5586(03)80047-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrzanski P, Hunter K, Jones-Bolin S, et al. Antiangiogenic and antitumor efficacy of EphA2 receptor antagonist. Cancer Res. 2004;64:910–9. doi: 10.1158/0008-5472.can-3430-2. [DOI] [PubMed] [Google Scholar]
- Ireton RC, Chen J. EphA2 receptor tyrosine kinase as a promising target for cancer therapeutics. Curr Cancer Drug Targets. 2005;5:149–57. doi: 10.2174/1568009053765780. [DOI] [PubMed] [Google Scholar]
- Wykosky J, Debinski W. The EphA2 receptor and ephrinA1 ligand in solid tumors: function and therapeutic targeting. Mol Cancer Res. 2008;6:1795–806. doi: 10.1158/1541-7786.MCR-08-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijper S, Turner CJ, Adams RH. Regulation of angiogenesis by Eph-ephrin interactions. Trends Cardiovasc Med. 2007;17:145–51. doi: 10.1016/j.tcm.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Scehnet JS, Ley EJ, Krasnoperov V, et al. The role of Ephs, Ephrins, and growth factors in Kaposi sarcoma and implications of EphrinB2 blockade. Blood. 2009;113:254–63. doi: 10.1182/blood-2008-02-140020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz N, Krasnoperov V, Reddy R, et al. The soluble extracellular domain of EphB4 (sEphB4) antagonizes EphB4-EphrinB2 interaction, modulates angiogenesis, and inhibits tumor growth. Blood. 2006;107:2330–8. doi: 10.1182/blood-2005-04-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martiny-Baron G, Korff T, Schaffner F, et al. Inhibition of tumor growth and angiogenesis by soluble EphB4. Neoplasia. 2004;6:248–57. doi: 10.1593/neo.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao W, Luis E, Ross S, et al. EphB2 as a therapeutic antibody drug target for the treatment of colorectal cancer. Cancer Res. 2004;64:781–8. doi: 10.1158/0008-5472.can-03-1047. [DOI] [PubMed] [Google Scholar]
- Koolpe M, Burgess R, Dail M, et al. EphB receptor-binding peptides identified by phage display enable design of an antagonist with ephrin-like affinity. J Biol Chem. 2005;280:17301–11. doi: 10.1074/jbc.M500363200. [DOI] [PubMed] [Google Scholar]
- Murai KK, Nguyen LN, Koolpe M, et al. Targeting the EphA4 receptor in the nervous system with biologically active peptides. Mol Cell Neurosci. 2003;24:1000–11. doi: 10.1016/j.mcn.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Lamberto I, Qin H, Noberini R, et al. Distinctive binding of three antagonistic peptides to the ephrin-binding pocket of the EphA4 receptor. Biochem J. 2012;445:47–56. doi: 10.1042/BJ20120408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noberini R, Koolpe M, Peddibhotla S, et al. Small molecules can selectively inhibit ephrin binding to the EphA4 and EphA2 receptors. J Biol Chem. 2008;283:29461–72. doi: 10.1074/jbc.M804103200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman KT, Hu M, Carles-Kinch K, et al. Differential EphA2 epitope display on normal versus malignant cells. Cancer Res. 2003;63:7907–12. [PubMed] [Google Scholar]
- Landen CN, Jr, Lu C, Han LY, et al. Efficacy and antivascular effects of EphA2 reduction with an agonistic antibody in ovarian cancer. J Natl Cancer Inst. 2006;98:1558–70. doi: 10.1093/jnci/djj414. [DOI] [PubMed] [Google Scholar]
- Wesa AK, Herrem CJ, Mandic M, et al. Enhancement in specific CD8+ T cell recognition of EphA2+ tumors in vitro and in vivo after treatment with ligand agonists. J Immunol. 2008;181:7721–7. doi: 10.4049/jimmunol.181.11.7721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki Y, Nakano M, Sato H, et al. Design and effective synthesis of novel templates, 3,7-diphenyl-4-amino-thieno and furo-[3,2-c] pyridines as protein kinase inhibitors and in vitro evaluation targeting angiogenetic kinases. Bioorg Med Chem Lett. 2007;17:250–4. doi: 10.1016/j.bmcl.2006.09.050. [DOI] [PubMed] [Google Scholar]
- Bardelle C, Coleman T, Cross D, et al. Inhibitors of the tyrosine kinase EphB4. Part 2: structure-based discovery and optimisation of 3,5-bis substituted anilinopyrimidines. Bioorg Med Chem Lett. 2008;18:5717–21. doi: 10.1016/j.bmcl.2008.09.087. [DOI] [PubMed] [Google Scholar]
- Choi Y, Syeda F, Walker JR, et al. Discovery and structural analysis of Eph receptor tyrosine kinase inhibitors. Bioorg Med Chem Lett. 2009;19:4467–70. doi: 10.1016/j.bmcl.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafleur K, Huang D, Zhou T, et al. Structure-based optimization of potent and selective inhibitors of the tyrosine kinase erythropoietin producing human hepatocellular carcinoma receptor B4 (EphB4) J Med Chem. 2009;52:6433–46. doi: 10.1021/jm9009444. [DOI] [PubMed] [Google Scholar]
- Qiao L, Choi S, Case A, et al. Structure-activity relationship study of EphB3 receptor tyrosine kinase inhibitors. Bioorg Med Chem Lett. 2009;19:6122–6. doi: 10.1016/j.bmcl.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keam SJ. Dasatinib: in chronic myeloid leukemia and Philadelphia chromosome-positive acute lymphoblastic leukemia. BioDrugs. 2008;22:59–69. doi: 10.2165/00063030-200822010-00007. [DOI] [PubMed] [Google Scholar]
- Buettner R, Mesa T, Vultur A, et al. Inhibition of Src family kinases with dasatinib blocks migration and invasion of human melanoma cells. Mol Cancer Res. 2008;6:1766–74. doi: 10.1158/1541-7786.MCR-08-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichot CS, Hartig SM, Xia L, et al. Dasatinib synergizes with doxorubicin to block growth, migration, and invasion of breast cancer cells. Br J Cancer. 2009;101:38–47. doi: 10.1038/sj.bjc.6605101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konecny GE, Glas R, Dering J, et al. Activity of the multikinase inhibitor dasatinib against ovarian cancer cells. Br J Cancer. 2009;101:1699–708. doi: 10.1038/sj.bjc.6605381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duxbury MS, Ito H, Zinner MJ, et al. EphA2: a determinant of malignant cellular behavior and a potential therapeutic target in pancreatic adenocarcinoma. Oncogene. 2004;23:1448–56. doi: 10.1038/sj.onc.1207247. [DOI] [PubMed] [Google Scholar]
- Cheng N, Brantley DM, Liu H, et al. Blockade of EphA receptor tyrosine kinase activation inhibits vascular endothelial cell growth factor-induced angiogenesis. Mol Cancer Res. 2002;1:2–11. [PubMed] [Google Scholar]
- Zhou Z, Yuan X, Li Z, et al. RNA interference targeting EphA2 inhibits proliferation, induces apoptosis, and cooperates with cytotoxic drugs in human glioma cells. Surg Neurol. 2008;70:562–8. doi: 10.1016/j.surneu.2008.04.031. ; discussion 8-9. [DOI] [PubMed] [Google Scholar]
- Landen CN, Jr, Chavez-Reyes A, Bucana C, et al. Therapeutic EphA2 gene targeting in vivo using neutral liposomal small interfering RNA delivery. Cancer Res. 2005;65:6910–8. doi: 10.1158/0008-5472.CAN-05-0530. [DOI] [PubMed] [Google Scholar]
- Shahzad MM, Lu C, Lee JW, et al. Dual targeting of EphA2 and FAK in ovarian carcinoma. Cancer Biol Ther. 2009;8:1027–34. doi: 10.4161/cbt.8.11.8523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson D, Gooya J, Mao S, et al. A human antibody-drug conjugate targeting EphA2 inhibits tumor growth in vivo. Cancer Res. 2008;68:9367–74. doi: 10.1158/0008-5472.CAN-08-1933. [DOI] [PubMed] [Google Scholar]
- Lee JW, Han HD, Shahzad MM, et al. EphA2 immunoconjugate as molecularly targeted chemotherapy for ovarian carcinoma. J Natl Cancer Inst. 2009;101:1193–205. doi: 10.1093/jnci/djp231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doronina SO, Toki BE, Torgov MY, et al. Development of potent monoclonal antibody auristatin conjugates for cancer therapy. Nat Biotechnol. 2003;21:778–84. doi: 10.1038/nbt832. [DOI] [PubMed] [Google Scholar]
- Cai W, Ebrahimnejad A, Chen K, et al. Quantitative radioimmunoPET imaging of EphA2 in tumor-bearing mice. Eur J Nucl Med Mol Imaging. 2007;34:2024–36. doi: 10.1007/s00259-007-0503-5. [DOI] [PubMed] [Google Scholar]
- Vearing C, Lee FT, Wimmer-Kleikamp S, et al. Concurrent binding of anti-EphA3 antibody and ephrin-A5 amplifies EphA3 signaling and downstream responses: potential as EphA3-specific tumor-targeting reagents. Cancer Res. 2005;65:6745–54. doi: 10.1158/0008-5472.CAN-05-0758. [DOI] [PubMed] [Google Scholar]
- Hammond SA, Lutterbuese R, Roff S, et al. Selective targeting and potent control of tumor growth using an EphA2/CD3-Bispecific single-chain antibody construct. Cancer Res. 2007;67:3927–35. doi: 10.1158/0008-5472.CAN-06-2760. [DOI] [PubMed] [Google Scholar]
- Tatsumi T, Herrem CJ, Olson WC, et al. Disease stage variation in CD4+ and CD8+ T-cell reactivity to the receptor tyrosine kinase EphA2 in patients with renal cell carcinoma. Cancer Res. 2003;63:4481–9. [PubMed] [Google Scholar]
- Alves PM, Faure O, Graff-Dubois S, et al. EphA2 as target of anticancer immunotherapy: identification of HLA-A*0201-restricted epitopes. Cancer Res. 2003;63:8476–80. [PubMed] [Google Scholar]
- Yamaguchi S, Tatsumi T, Takehara T, et al. Immunotherapy of murine colon cancer using receptor tyrosine kinase EphA2-derived peptide-pulsed dendritic cell vaccines. Cancer. 2007;110:1469–77. doi: 10.1002/cncr.22958. [DOI] [PubMed] [Google Scholar]
- Jin M, Komohara Y, Shichijo S, et al. Erythropoietin-producing hepatocyte B6 variant-derived peptides with the ability to induce glioma-reactive cytotoxic T lymphocytes in human leukocyte antigen-A2+ glioma patients. Cancer Sci. 2008;99:1656–62. doi: 10.1111/j.1349-7006.2008.00866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiari R, Hames G, Stroobant V, et al. Identification of a tumor-specific shared antigen derived from an Eph receptor and presented to CD4 T cells on HLA class II molecules. Cancer Res. 2000;60:4855–63. [PubMed] [Google Scholar]
- Miao H, Li DQ, Mukherjee A, et al. EphA2 mediates ligand-dependent inhibition and ligand-independent promotion of cell migration and invasion via a reciprocal regulatory loop with Akt. Cancer Cell. 2009;16:9–20. doi: 10.1016/j.ccr.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menges CW, McCance DJ. Constitutive activation of the Raf-MAPK pathway causes negative feedback inhibition of Ras-PI3K-AKT and cellular arrest through the EphA2 receptor. Oncogene. 2008;27:2934–40. doi: 10.1038/sj.onc.1210957. [DOI] [PubMed] [Google Scholar]
- Parri M, Taddei ML, Bianchini F, et al. EphA2 reexpression prompts invasion of melanoma cells shifting from mesenchymal to amoeboid-like motility style. Cancer Res. 2009;69:2072–81. doi: 10.1158/0008-5472.CAN-08-1845. [DOI] [PubMed] [Google Scholar]
- Klein R. Bidirectional modulation of synaptic functions by Eph/ephrin signaling. Nat Neurosci. 2009;12:15–20. doi: 10.1038/nn.2231. [DOI] [PubMed] [Google Scholar]
- Yamazaki T, Masuda J, Omori T, et al. EphA1 interacts with integrin-linked kinase and regulates cell morphology and motility. J Cell Sci. 2009;122:243–55. doi: 10.1242/jcs.036467. [DOI] [PubMed] [Google Scholar]
- Henkemeyer M, Itkis OS, Ngo M, et al. Multiple EphB receptor tyrosine kinases shape dendritic spines in the hippocampus. J Cell Biol. 2003;163:1313–26. doi: 10.1083/jcb.200306033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dail M, Richter M, Godement P, et al. Eph receptors inactivate R-Ras through different mechanisms to achieve cell repulsion. J Cell Sci. 2006;119:1244–54. doi: 10.1242/jcs.02842. [DOI] [PubMed] [Google Scholar]
- Kouros-Mehr H, Werb Z. Candidate regulators of mammary branching morphogenesis identified by genome-wide transcript analysis. Dev Dyn. 2006;235:3404–12. doi: 10.1002/dvdy.20978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan M. Overexpression of EphA2 gene in invasive human breast cancer and its association with hormone receptor status [ASCO Annual Meeting Proceedings] J Clin Oncol. 2005;23:9583. [Google Scholar]
- Wu Q, Suo Z, Risberg B, et al. Expression of Ephb2 and Ephb4 in breast carcinoma. Pathol Oncol Res. 2004;10:26–33. doi: 10.1007/BF02893405. [DOI] [PubMed] [Google Scholar]
- Maru Y, Hirai H, Takaku F. Overexpression confers an oncogenic potential upon the eph gene. Oncogene. 1990;5:445–7. [PubMed] [Google Scholar]
- Kataoka H, Igarashi H, Kanamori M, et al. Correlation of EPHA2 overexpression with high microvessel count in human primary colorectal cancer. Cancer Sci. 2004;95:136–41. doi: 10.1111/j.1349-7006.2004.tb03194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox BP, Tabone CJ, Kandpal RP. Potential clinical relevance of Eph receptors and ephrin ligands expressed in prostate carcinoma cell lines. Biochem Biophys Res Commun. 2006;342:1263–72. doi: 10.1016/j.bbrc.2006.02.099. [DOI] [PubMed] [Google Scholar]
- Dodelet VC, Pasquale EB. Eph receptors and ephrin ligands: embryogenesis to tumorigenesis. Oncogene. 2000;19:5614–9. doi: 10.1038/sj.onc.1203856. [DOI] [PubMed] [Google Scholar]
- Easty DJ, Mitchell PJ, Patel K, et al. Loss of expression of receptor tyrosine kinase family genes PTK7 and SEK in metastatic melanoma. Int J Cancer. 1997;71:1061–5. doi: 10.1002/(sici)1097-0215(19970611)71:6<1061::aid-ijc24>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Noberini R, De SK, Zhang Z, et al. A disalicylic Acid-furanyl derivative inhibits ephrin binding to a subset of eph receptors. Chem Biol Drug Des. 2011;78:667–78. doi: 10.1111/j.1747-0285.2011.01199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolpe M, Dail M, Pasquale EB. An ephrin mimetic peptide that selectively targets the EphA2 receptor. J Biol Chem. 2002;277:46974–9. doi: 10.1074/jbc.M208495200. [DOI] [PubMed] [Google Scholar]
- Wang XD, Reeves K, Luo FR, et al. Identification of candidate predictive and surrogate molecular markers for dasatinib in prostate cancer: rationale for patient selection and efficacy monitoring. Genome Biol. 2007;8:R255. doi: 10.1186/gb-2007-8-11-r255. . doi: 10.1186/gb-2007-8-11-r255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia G, Kumar SR, Stein JP, et al. EphB4 receptor tyrosine kinase is expressed in bladder cancer and provides signals for cell survival. Oncogene. 2006;25:769–80. doi: 10.1038/sj.onc.1209108. [DOI] [PubMed] [Google Scholar]