Abstract
Mesenchymal stem cells (MSCs) have been shown to improve the outcome of acute renal injury models; but whether MSCs can delay renal failure in chronic kidney disease (CKD) remains unclear. In the present study, the were cultured in media containing various concentrations of basic fibroblast growth factor, epidermal growth factor and ascorbic acid 2-phosphate to investigate whether hepatocyte growth factor (HGF) secretion could be increased by the stimulation of these growth factors. Then, TGF-β1-treated renal interstitial fibroblast (NRK-49F), renal proximal tubular cells (NRK-52E) and podocytes were co-cultured with conditioned MSCs in the absence or presence of ascorbic acid 2-phosphate to quantify the protective effects of conditioned MSCs on renal cells. Moreover, male Sprague-Dawley rats were treated with 1 × 106 conditioned MSCs immediately after 5/6 nephrectomy and every other week through the tail vein for 14 weeks. It was found that basic fibroblast growth factor, epidermal growth factor and ascorbic acid 2-phosphate promoted HGF secretion in MSCs. Besides, conditioned MSCs were found to be protective against TGF-β1 induced epithelial-to-mesenchymal transition of NRK-52E and activation of NRK-49F cells. Furthermore, conditioned MSCs protected podocytes from TGF-β1-induced loss of synaptopodin, fibronectin induction, cell death and apoptosis. Rats transplanted with conditioned human MSCs had a significantly increase in creatinine clearance rate, decrease in glomerulosclerosis, interstitial fibrosis and increase in CD4+CD25+Foxp3+ regulatory T cells counts in splenocytes. Together, our studies indicated that conditioned MSCs preserve renal function by their anti-fibrotic and anti-inflammatory effects. Transplantation of conditioned MSCs may be useful in treating CKD.
Keywords: mesenchymal stem cells, chronic kidney disease, epithelial-to-mesenchymal transition, hepatocyte growth factor, immunomodulation
Introduction
Tubulointerstitial fibrosis is the final common pathway to end stage renal disease regardless of disease aetiology 1. The pathophysiology of renal fibrosis involves macrophage infiltration, elaboration of pro-inflammatory cytokines, fibroblast activation and an imbalance in extracellular matrix deposition and degradation 2–4. Epithelial-to-mesenchymal transition (EMT) is a process in which epithelial cells lose their epithelial characteristics and acquire phenotypic features characteristic for mesenchymal cells. Growing evidence has implicated that EMT as one of the pathways leading to interstitial fibrosis in diseased kidneys 5–8.
Transforming growth factor-β1 (TGF-β1) is a strong EMT inducer in renal tubule cells in a dose-dependent manner by up-regulating de novo α-smooth muscle actin expression (SMA) and the down-regulation of the epithelial adhesion molecule E-cadherin 9,10. Hepatocyte growth factor (HGF) was first identified as a mitogen for mature hepatocyte. Subsequent in vitro and in vivo studies have found that HGF specifically counteracts the actions of TGF-β1 11,12. Hepatocyte growth factor administration and HGF gene therapy have potent therapeutic effects in cases of liver cirrhosis 13, lung fibrosis 14,15 and chronic renal fibrosis 16.
Mesenchymal stem cells (MSCs) possess multi-differentiation capabilities 17–19. It is also well established that MSCs possess immuno-modulatory properties. MSCs favouring polarization from a Th1 phenotype towards a Th2 phenotype and from a pro-inflammatory towards an anti-inflammatory environment by affecting dendritic cells, cytotoxic T lymphocytes, and NK cells 20. Results from pre-clinical studies on experimental autoimmune disease such as autoimmune encephalomyelitis 21, collagen-induced arthritis 22, myasthenia gravis 23 or autoimmune myocarditis 24 as well in clinical trials such as graft-versus-host disease 25 and systemic lupus erythematous 26 all indicate that activation and expansion of CD4+CD25+Foxp3+ regulatory T cells (Tregs) is an important mechanism of MSC-mediated immuno-modulation. MSCs also have anti-fibrotic properties by secreting a variety of anti-scarring factors such as HGF, basic fibroblast growth factor (bFGF) and adrenomedullin 27. It has been shown that cardiac fibroblasts are less proliferative when cultured in MSCs-conditioned medium 28.
It is well known that MSCs improve the outcome of acute renal injury models by paracrine action, but their role in chronic kidney diseases (CKD) remains unknown 29,30. Recently, it has been demonstrated that MSCs reduce interstitial fibrosis, but do not delay the progression of CKD in collagen α3-deficient mice 31. Therefore, it is imperative to enhance the production of anti-fibrotic factors in MSCs to get optimal therapeutic effects on CKD. In this study, we hypothesize that increasing the secretion of HGF, the most critical anti-fibrotic factor, may enhance the therapeutic potential of MSCs. In the present study, we culture MSCs supplemented with different growth factors and ascorbic acid 2-phosphate to increase the secretion of HGF. The mechanisms of the therapeutic effects of conditioned MSCs on CKD were assessed by in vitro co-culture of TGF-β1 treated rat tubular epithelial cells, myofibroblast, and podocytes with conditioned MSCs as well as in vivo 5/6 nephrectomy model in rats.
Materials and methods
Isolation and culture of MSCs
Isolation and characterization of MSCs from human bone marrow were carried out as previously reported 32,33. Bone marrow aspirates was obtained during fracture surgeries from normal donor aged 22 years (male donor) and 56 years (female donor) with informed consent. An approval from the institutional review board of the Taipei Veterans General Hospital was obtained before commencing the study. MSCs were single cell-derived and clonally expanded, and their surface immunophenotype and multi-lineage differentiation potentials into osteoblasts, adipocytes, chondrocytes, were confirmed before they were used for further experiments 32,33.
Cytokine and ascorbic acid 2-phosphate treatment in MSCs
Subconfluent MSCs (passage 11–13) were trypsinized and seeded in 6-well plastic dishes at a starting cell density of 5 × 104. The basal culture medium consists of Iscove modified Dulbecco medium (IMDM; Gibco, Grand Island, NY, USA) supplied with 10% foetal bovine serum (Invitrogen, Grand Island, NY, USA), 100 U penicillin, 1000 U streptomycin and 2 mM L-glutamine (Gibco). To examine the effect of bFGF, epidermal growth factor (EGF) and ascorbic acid 2-phosphate on HGF secretion, the basal culture media was supplemented with bFGF (R&D Systems, Inc., Minneapolis, MN, USA) or EGF (R&D Systems, Inc.) at concentrations of 0, 1, 2, 4, 8, or 10 ng/ml in the absence or presence of ascorbic acid 2-phosphate (0.1 or 1 mM, Sigma-Aldrich, St. Louis, MO, USA). The supernatant was collected 72 hrs later and frozen at −20°C for ELISA assay of HGF expression. Representative wells were harvested by trypsin digestion and cell numbers determined by trypan blue exclusion and hematocytometer counts. Data were expressed as the secreted HGF per 106 cells at time of harvest.
HGF immunoassay
Concentration of HGF in the conditioned media of cultured MSCs was measured by using a sandwich ELISA according to manufacturer's instructions (R&D Systems, Inc.). Supernatants were centrifuged before testing. Samples were run in duplicates. A standard curve was constructed using known concentrations of recombinant human HGF (0–8000 pg/ml).
Preparation for the conditioned MSCs
MSCs were cultured in basal culture media supplemented with 10 ng/ml bFGF and 10 ng/ml EGF.
Culture of NRK-52E and NRK-49F cells
Rat renal proximal tubular cells (NRK-52E) and normal rat kidney interstitial fibroblast cells (NRK-49F) were purchased from the Bioresource Collection and Research Center of the Food Industry Research Institute, Taiwan. Normal rat kidney interstitial fibroblast cells cells are believed to be proximal tubular origin on the basis of patterns of collagen secretion, C-type natriuretic peptide secretion, and the presence of EGF receptors 34. Normal rat kidney interstitial fibroblast cells cells are an adherent fibroblast cell line that is derived from rat kidneys. Normal rat kidney interstitial fibroblast cells are found to express EGF and multiplication stimulating activity receptor 35. The cells were cultured in 95% Dulbecco's modified Eagle's medium (Sigma-Aldrich) with 4 mM L-glutamine adjusted to contain 1.5 g/l sodium bicarbonate and 4.5 g/l glucose supplemented with 5% bovine calf serum (Thermo Scientific HyClone, Logan, UT, USA) at 37°C in a 5% CO2 atmosphere.
Culture of podocytes
Conditionally immortalized podocytes were kindly provided by Dr Peter Mundel (L. Miller School of Medicine, Miami, FL, USA) and were cultured as previously reported in the literature 36. Briefly, frozen podocytes were first maintained under permissive conditions at 33°C in RPMI-1640 media (Gibco) containing 10% foetal bovine serum, 10 U/ml γ-interferon (R&D Systems, Inc.) and 100 U/ml of penicillin/streptomycin in collagen-I (Advanced BioMatrix, San Diego, CA, USA) coated flasks. To induce differentiation, podocytes were cultured in a non-permissive condition at 37°C without γ-interferon. Podocytes maintained under non-permissive conditions for at least 12 days were used for experiments.
Co-culture of kidney cells and conditioned MSCs
To investigate whether conditioned MSCs would inhibit EMT induced by TGF-β1 in NRK-52E, NRK-49F cells and podocytes, conditioned MSCs were co-cultured with TGF-β1-treated NRK-52E, NRK-49F and podocytes in the absence or presence of ascorbic acid 2-phosphate. Recombinant human TGF-β1 (PeproTech, Rocky Hill, NJ, USA) was added to the co-culture media at the concentration of 15 ng/ml. The co-culture was set up in a transwell system with renal cells in the bottom wells for 3 days using Dulbecco's modified Eagle's medium for NRK-52E and NRK-49F; RPMI-1640 for podocytes. The cell lysates were used for Western blot analysis. Co-culture supernatants were collected for HGF concentration detecting by ELISA.
Western blot analysis of α-SMA, fibronectin and synaptopodin
Expression of α-SMA and fibronectin in NRK-49F, α-SMA in NRK-52E cells and synaptopodin protein in podocyte lysates was analysed using Western blotting as previously described 34,37. Culture cells were plated in 6-well plates and washed once with ice-cold phosphate–buffered saline (PBS). Cells were lysed by the addition of 100 μl of CelLytic Reagent (Sigma-Aldrich) containing protease inhibitor cocktail (Sigma-Aldrich) for 15 min. at 4°C and the cells were collected using a disposable scraper. Lysates were cleared by centrifugation for 10 min. at 12,000 × g at 4°C. Protein concentration was determined using a Bio-Rad Dye Reagent (Bio-Rad Laboratories, Inc., Hercules, CA, USA). A quantity of 20 μg of protein was mixed with sample buffer, boiled for 5 min., electrophoresed on 8% SDS polyacrylamide gel and electroblotted onto nitrocellulose membranes. The membrane was blocked in PBS containing 5% skimmed milk powder, 1% FCS, and 0.02% Tween 20 for 1 hr at room temperature. The membrane was incubated with 1A4 anti-α-SMA (Clone1A4, 1:1000 dilution; Sigma-Aldrich), fibronectin (H-300, 1:400 dilution; Santa Cruz, CA, USA) or synaptopodin (H-140, 1:1000 dilution; Santa Cruz) mAb. After washing, the membrane was incubated with a secondary antibody in PBS containing 5% skimmed milk powder, and 1% FCS. Membranes were washed with PBS buffer and the signals were visualized using the chemiluminescence (ECL) system with an ECL-detecting reagent. The β-tubulin (Sigma-Aldrich) or β-actin (Sigma-Aldrich) was used for loading controls.
RNA isolation and quantitative polymerase chain reaction (PCR) for E-cadherin and fibronectin
Total RNA of NRK-52E and podocytes was harvested by using the Trizol reagents (Invitrogen) according to manufacturer's instructions. A quantity of 2 μg total RNA was reverse transcribed using a cDNA synthesis kit (MMLV; Epicentre, Madison, WI, USA). TaqMan and SYBR green I technology was used for quantitative PCR reactions for E-cadhein and fibronectin, respectively. E-cadherin, fibronectin and house-keeping gene β-actin and glyceraldehydes-3-phosphate dehydrogenase (GAPDH) were amplified with specific primers designed by Universal ProbeLibrary Assay Design Center (http://www.roche-applied-science.com) (Table1). The reaction condition was one cycle of 95°C for 30 sec. followed by 40 cycles of 95°C for 1 sec. and 60°C for 15 sec. The effects of conditioned MSCs in the absence or presence of ascorbic acid 2-phosphate on level of E-cadherin and fibronectin mRNA expression were normalized to β-actin and GAPDH compared to fold-change relative to normal NRK-52E and podocytes respectively.
Table 1.
Primers used in quantitative PCR analysis
| mRNA | Forward primer | Reverse primer |
|---|---|---|
| E-cadherin | GATCCTGGCCCTCCTGAT | TCTTTGACCACCGTTCTCCT |
| Fibronectin | GGGTCACGTACCTCTTCAAAGTCT | CCGTCAGAGGATTGCTTTCC |
| β-actin | CCCGCGAGTACAACCTTCT | CGTCATCCATGGCGAACT |
| GAPDH | GGGAAGCCCATCACCATCT | CGGCCTCACCCCATTTG |
MTT assays for podocytes viability
To analyse the effects of MSCs on viability of TGF-β1-treated podocytes, TGF-β1-treated podocytes were co-cultured without or with conditioned MSCs at 1:1 ratios in the absence or presence of ascorbic acid 2-phosphate for 3 days and pulsed with 3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT, Promega, Madison, WI, USA) for 4 hr. MTT is reduced by viable cells to a coloured, water-insoluble formazan salt. The absorption at 570 nm of formazan product was measured using a microplate reader.
Evaluation of apoptosis in podocytes by flow cytometry
Apoptosis was quantitatively measured using the fluorescein isothiocyanate (FITC)/allophycocyanin-conjugated annexin V and propidium iodide (PI) apoptosis assay kit (Clontech, Mountain View, CA, USA). The staining process was performed according to the manufacturer's instructions. TGF-β1-treated podocytes in the presence or absence of conditioned MSCs with or without ascorbic acid 2-phosphate treatment were simultaneously stained with Annexin V-FITC and dye exclusion. Data were obtained using flow cytometric analysis with FACScan within 1 hr.
5/6 nephrectomy CKD model in rats
Male Sprague-Dawley rats (250–275 g, n = 12) were obtained from BioLASCO Taiwan Co., Ltd. The animal experiments in this study were approved by the Ethics Committee for Animal Research of the Taipei Veterans General Hospital. All rats used in the analysis were housed in the same room and fed with identical diet. At approximately 8 weeks of age, 5/6 nephrectomy was performed in a two-step surgical procedure under anaesthesia by intraperitoneal injection of Zoletil 50® (Virbac SA, Carros Cedex, France). At first, the 2/3 cortex of the left kidney was ablated by placing suture around each pole of left kidney at its 1/3 position. Then the 1/3 kidney on each end of left kidney was excised. One week later the right kidney was completely removed.
MSCs treatment protocol and animal groups
The rats undergoing 5/6 nephrectomy were randomly divided into two groups in our experiment. Group I (n = 6) was 5/6 nephrectomy alone. Group II (n = 6) was 5/6 nephrectomy and treated with conditioned MSCs transplantation. First, we administrated 1 × 106 conditioned MSCs into the vena cava intraoperatively post-5/6 nephrectomy and then intravenously through tail vein every other week for 14 weeks, with a total of eight episodes of cell transplantation (intraoperative, second, fourth, sixth, eighth, tenth, twelfth and fourteenth weeks post-operation). All rats were killed at 16 weeks post-operation to evaluate the therapeutic effectiveness of conditioned MSCs transplantation.
Measurement of renal function parameters
Body weight measurement and blood samples were taken from the tail vein every other week after the surgery. The presence of proteinuria was monitored by urinary protein/urinary creatinine ratios of 24-hrs-urine collection in a metabolic cage every other week after surgery. Renal function was assessed by measuring serum creatinine and blood urea nitrogen (BUN) and creatinine clearance (CCr). BUN concentration was determined by the diacetylmonoxime method using a commercially available kit (Wako BUN Test, Wako Pure Chemical Co., Osaka, Japan) and creatinine concentration was measured by using the Jaffé method using a commercially kit (Wako CRN Test, Wako Pure Chemical Co.). The CCr was calculated as urine creatinine × urine volume/serum creatinine. Remnant kidney tissues from killed animals were excised at the end of study, weighed, fixed and further processed for histology, and PCR experiment to evaluate the engraftment of transplanted conditioned MSCs.
Renal histomorphometric analyses for glomerulosclerosis and interstitial fibrosis
Fragments of the renal cortex were fixed overnight in 10% neutral phosphate-buffered formalin, dehydrated in alcohol, and then embedded in paraffin. The sections (3 μm thick) are stained with haematoxylin and eosin, Masson's trichrome stain and periodic acid- silver methenamine stain. In each rat, 75–100 glomeruli were examined. The percentage of glomeruli with glomerulosclerosis was calculated by dividing the number of sclerotic glomeruli by the total number of glomeruli examined. A semiquantitative score ranged from 0 to 4+ was used to evaluate the severity of interstitial fibrosis where 0, no change; 1+, fibrosis to <10% of the interstitial area; 2+, fibrosis to 10–25% of the interstitial area; 3+, fibrosis to 25–50% of the interstitial area; 4+ fibrosis to >50% of the interstitial area.
Flow cytometric analysis of Treg cells
Splenocytes harvested from rats were analysed using flow cytometry. Cells were equally distributed into tubes and washed once in phosphate-buffered saline. For the Tregs analysis, cells were incubated with FITC anti-rat CD4 and phycoerythrin (PE) anti-rat CD25, and Anti-Mouse/Rat Foxp3 PE at 4°C for 30 min. Following surface staining, cells were fixed and permeabilized according to the manufacturer's protocol of Foxp3 buffer set (eBioscience, San Diego, CA, USA). FACS analysis was performed using FACS Calibur and CellQuest software.
PCR experiment for engraftment of conditioned MSCs
To track the existence of conditioned human MSCs in rat kidneys, genomic DNA from rat kidneys was analysed at the end of the experiments by human and rat-specific β2-microglobulin PCR to evaluate engraftment. The primers for human β2-microglobulin and rat β2-microglobulin were as follows: Human—forward, GTGTCTGGGTTTCATCCATC and reverse, GGCAGGCATACTCATCTTTT. Rat—forward, CCAGTTTAACTCCAGATCCGG, and reverse, TTCTGATCAAAACACTCATTGAAGC, respectively 38. Amplification for β2-microglobulin was performed with initial denaturation at 95°C for 1 min., followed by 35 cycles of 30 sec. at 95°C, 1 min. at 57.1°C, and 30 sec. at 72°C, with a final extension at 70°C for 5 min.
Statistical analysis
Data were presented as mean ± SD. We used chi-square for linear-by-linear association to examine the dose effect of bFGF, EGF and ascorbic acid 2-phosphate on secretion of HGF. For in vitro experimental studies, we compared four groups by means of one-way ANOVA. If the ANOVA analyses were significant, Tukey's post hoc analyses were performed. Differences in serological parameters and quantitative histopathologic analyses between the control and MSCs-treated groups were analysed with independent t-tests. Data analysis was performed using the SPSS 18.0 software (Chicago, IL, USA). P < 0.05 was considered to be statistically significant.
Results
Synergistic enhancement of HGF secretion in MSCs by bFGF, EGF and Ascorbic acid 2-phosphate
Concentration response curve for the effect of bFGF, EGF and ascorbic acid 2-phosphate on HGF secretion by MSCs were shown in Figure1. Medium with bFGF and EGF could significantly increase HGF secretion in a dose-dependent fashion. (P < 0.001, linear-by-linear association, Fig.1A and B). In the presence of 10 ng/ml of bFGF and EGF, HGF level increased to 1243.97 ± 62.06 pg/ml/106 cells (2.59-fold increase relative to the baseline). In addition, ascorbic acid 2-phosphate synergistically enhances bFGF- and EGF-induced HGF secretion in a dose-dependent manner (P < 0.001, linear-by-linear association, Fig.1C).
Fig 1.
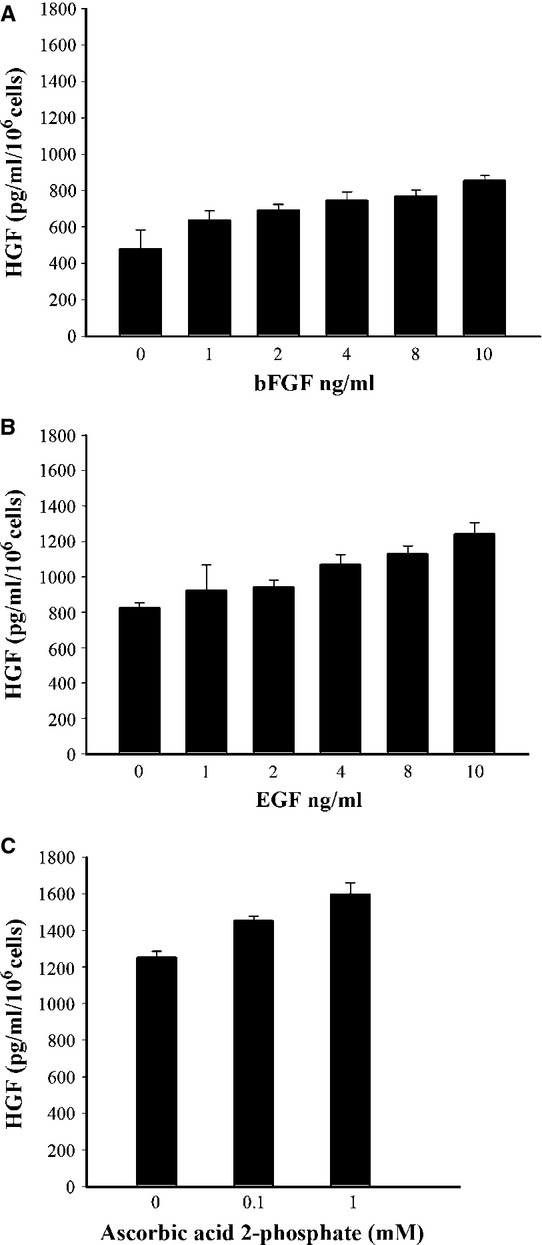
Effect of bFGF, EGF and ascorbic acid 2-phosphate on HGF secretion by MSCs. (A) MSCs were incubated without or with the indicated concentrations of bFGF, (B) presence of 10 ng/ml bFGF with indicated concentration of EGF (C) and medium supplemented with 10 ng/ml bFGF, 10 ng/ml EGF in the absence or presence of ascorbic acid 2-phosphate for 72 hrs. HGF in the conditioned medium was determined by an ELISA. The values represent the mean ± S.D. MSCs secreted significantly increased HGF in medium supplemented with bFGF, EGF and ascorbic acid 2-phosphate (P < 0.05, linear-by-linear association).
Conditioned MSCs prevented TGF-β1 induced activation of EMT in NRK-49F and NRK-52E cells
As shown in Figure2, presence of conditioned MSCs normalized the levels of the EMT related α-SMA, and fibronectin in TGF-β1 treated NRK-49F. In addition TGF-β1 induced increase in the expression of α-SMA protein in NRK-52E cells, which was also inhibited by the addition of conditioned MSCs in the absence or presence of ascorbic acid 2-phospate. Quantitative PCR revealed that conditioned MSCs in the absence or presence of ascorbic acid 2-phospate has significant reversal of the level of E-cadherin mRNA, which was reduced by the TGF-β1 treatment (Fig.3) in NRK-52E.
Fig 2.
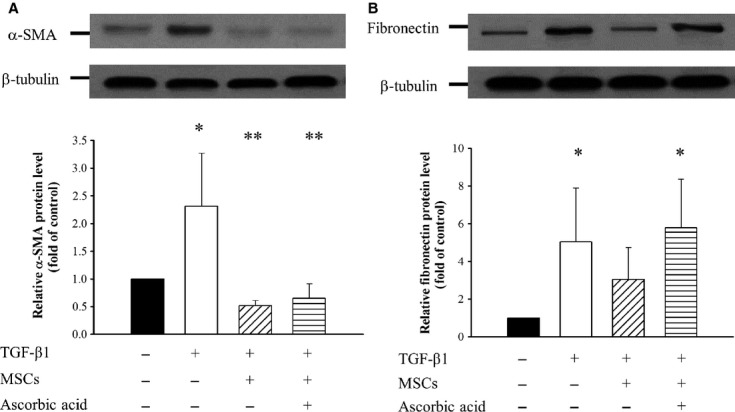
Effects of conditioned MSCs on expression of α-SMA and fibronectin in renal interstitial fibroblast NRK-49F. NRK-49F cells were cultured without or with 15 ng/ml TGF-β1 alone or co-culture with conditioned MSCs and/or ascorbic acid 2- phosphate for 3 days. (A) Representative Western blot analysis and relative bar graph analysis for α-SMA and β-tubulin level. (B) Representative Western blot and relative bar graph analysis of fibronectin protein level in NRK-49F after various treatments. *P < 0.05 versus normal control; **P < 0.05 versus TGF-β1 treated.
Fig 3.
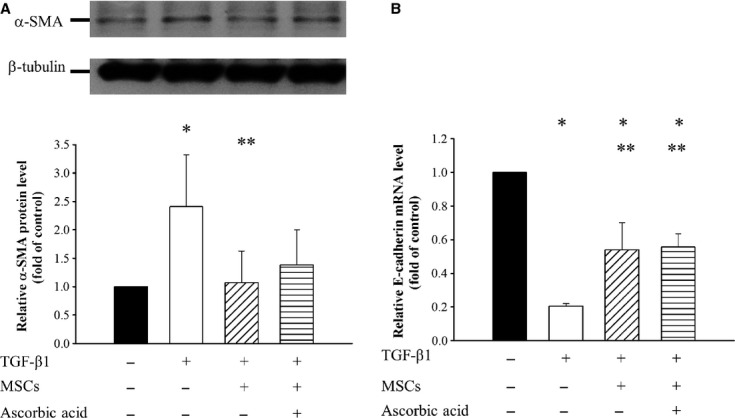
Effects of conditioned MSCs on protein expression of α-SMA and mRNA level of E-cadherin in renal tubular epithelial NRK-52E. (A) Representative Western blot analysis and relative bar graph analysis α-SMA and (B) Bar graph analysis of quantitative PCR analysis of relative E-cadherin expression to β-actin normalized to control after various treatments. Quantitative data are presented as mean ± S.D. *P < 0.05 versus normal control; **P < 0.05 versus TGF-β1 treated.
Conditioned MSCs suppressed TGF-β1-induced podocytes synaptopodin loss and fibronectin induction
TGF-β1 reduced the synaptopodin expression as compared to control podocytes, whereas conditioned MSCs in the absence or presence of ascorbic acid 2-phospate prevented such a loss in synaptopodin (Fig.4A). As shown in Figure4B, quantitative PCR revealed that TGF-β1 induced fibronectin expression in podocytes to 2.41 ± 0.25-fold compared to that of normal podocytes group (P < 0.05). Conditioned MSCs in the absence or presence of ascorbic acid 2-phosphate significantly suppressed the TGF-β1-induced fibronectin mRNA expression to 1.67 ± 0.30 and 1.50 ± 0.36-fold respectively (P < 0.05).
Fig 4.
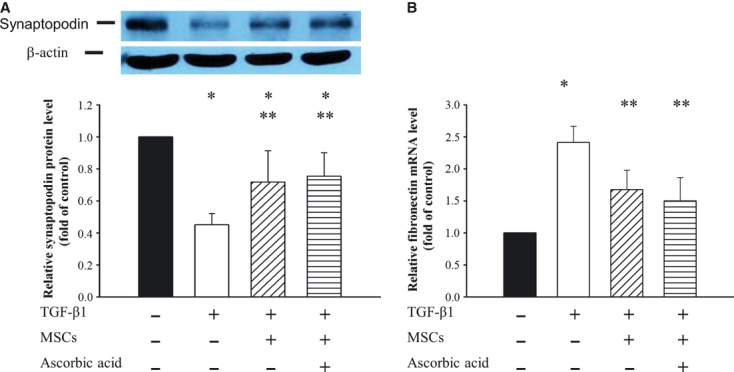
Effects of conditioned MSCs on expression of synaptopodin and fibronectin in podocytes. Podocytes were incubated without or with 15 ng/ml TGF-β1, conditioned MSCs and/or ascorbic acid for 72 hrs. Cell lysates were analysed with Western blotting. (A) Representative Western blot and bar graph analysis of relative protein level of synaptopodin, which were normalized to control. (B) Bar graph analysis of quantitative PCR analysis of relative fibronectin expression to GAPDH normalized to control. *P < 0.05 as compared with control; **P < 0.05 versus TGF-β1 treated.
Conditioned MSCs prevented death of podocytes induced by TGF-β1
Detachment of podocytes leads to proteinuria and development of glomerulosclerosis 39,40. We measured viable number of podocyte after TGF-β1 exposure in the absence or presence of conditioned MSCs and ascorbic acid 2-phospate by MTT assay. As shown in Figure5, viability was significantly different among various conditions (P < 0.001). After exposure to TGF-β1 alone, podocyte viability was reduced. However, when TGF-β1-treated podocytes co-cultured with conditioned MSCs in the absence or presence of ascorbic acid 2-phosphate, cell viabilities were 94.6 ± 12.8% and 102.3 ± 13.3%, respectively, both significantly higher than in TGF-β1 treated podocytes alone (P < 0.05). There was no difference in viability between conditioned MSCs-treated podocytes in the presence of ascorbic acid 2-phosphate and the control group. These results suggest that conditioned MSCs maintained podocytes viability in the presence of TGF-β1.
Fig 5.
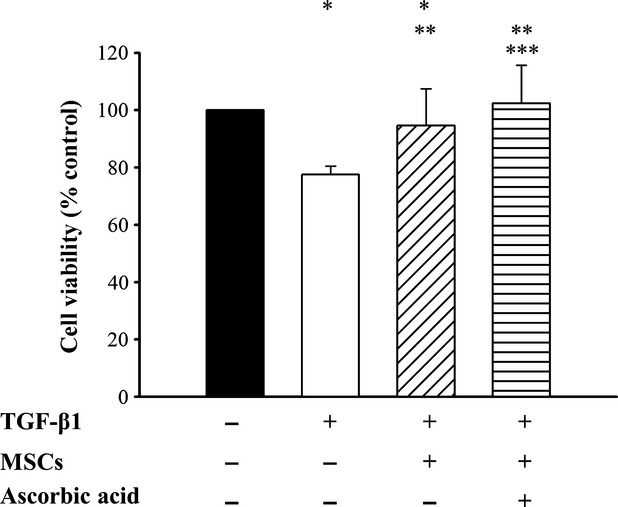
MTT assays of TGF-β1 treated podocytes. Podocytes viability was determined by MTT assay. *P < 0.05 as compared with control; **P < 0.05 versus TGF-β1 treated; ***P < 0.05 versus conditioned MSCs treated alone.
Conditioned MSCs protected podocytes against TGF-β1 induced apoptosis
To determine whether conditioned MSCs protected TGF-β1-induced podocytes apoptosis in vitro, podocytes were co-cultured with conditioned MSCs for 72 hrs. As shown in Figure6A, TGF-β1 enhanced the early apoptosis rate of podocytes (22.3 ± 4.7% versus 7.6 ± 1.0% in the control group, P < 0.05, Fig.6B). In contrast, conditioned MSCs in the absence or presence of ascorbic acid 2-phospate significantly reduced TGF-β1 induced apoptosis (P < 0.05 versus without MSCs). There were no statistically significant differences in apoptosis among those co-cultured with conditioned MSCs alone and those co-cultured with conditioned MSCs in the presence of ascorbic acid 2-phosphate.
Fig 6.
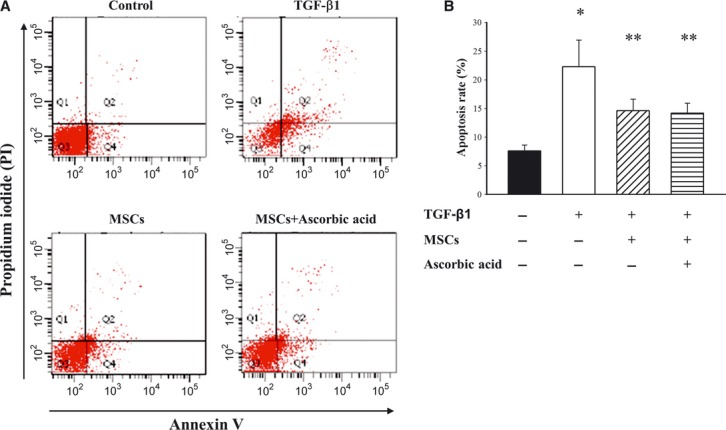
Effect of conditioned MSCs on TGF-β1 induced podocytes apoptosis. Podocytes apoptosis was determined by flow cytometry analysis using annexin V/propidium iodide staining. Cells in the lower right were considered as early apoptic cells. (A) Representative contour plots and (B) the percentage of apoptosis were measured. *P < 0.05 as compared with control; **P < 0.05 versus TGF-β1 treated.
Production of HGF in supernatants from the co-culture system
To investigate the possible mechanism responsible for the attenuation of EMT of conditioned MSCs, we analysed the HGF levels in supernatants of NRK-52E, NRK-49F and podocytes with conditioned MSCs. The amounts of HGF in co-culture supernatants are shown in Figure7. HGF levels were significantly increased in 72 hrs co-culture supernatants following co-culture of NRK-52E, NRK-49F and podocytes with conditioned MSCs in the absence or presence of ascorbic acid 2-phosphate compared with control values (P < 0.05). The data suggested that conditioned MSCs attenuated TGF-β1-induced renal EMT and cell fibrosis through their anti-fibrotic effect, at least in part, by HGF secretion.
Fig 7.
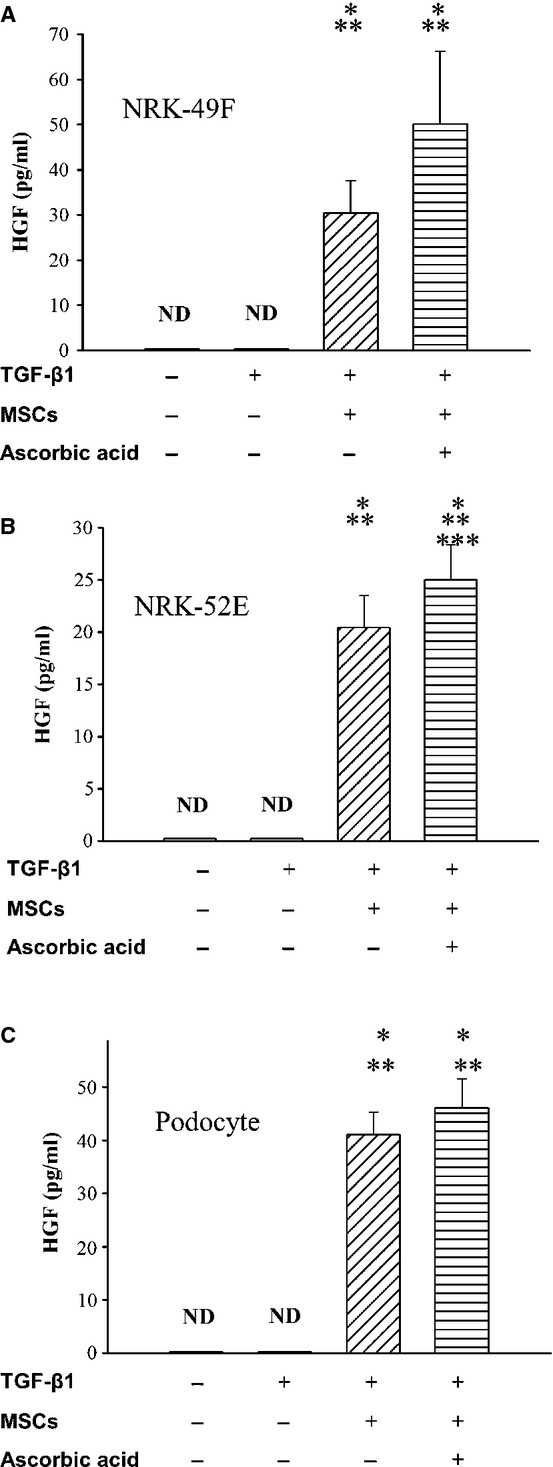
HGF concentration in co-culture supernatants. The concentration of HGF was significantly increased in co-culture supernatants of (A) NRK-49 F, (B) NRK-52E and (C) podocytes with conditioned MSCs in the absence of presence of ascorbic acid 2-phosphate. *P < 0.05 versus normal control; **P < 0.05 versus TGF-β1 treated, ***P < 0.05 versus conditioned MSCs treated alone.
Conditioned MSCs transplantation reduced progression of renal function deterioration
Figure8 shows the effects of conditioned MSCs on body weight and renal functional parameters. The body weight of group I rats were significantly lower than that of the group II at 2, 4, 6 and 8 post-operative week (P < 0.05). Following 5/6 nephrectomy rats developed renal dysfunction as evidence by elevated serum BUN (Fig.8B), creatinine (Fig.8C), fall of CCr (Fig.8D) and severity of proteinuria (Fig.8E). Compared to group I, rats in group II had a lower BUN and creatinine; however, there were no significant differences over the follow-up period. In group I rats, the CCr dropped progressively with time. Compared to group I, conditioned MSCs treatments significantly inhibited the drop of CCr at 6, 12, 14 and 16 post-operative week (P < 0.05). The weight of left remnant kidney was 2.17 ± 0.29 g in group I, compared with 2.67 ± 0.39 g in group II (P < 0.05). Both groups displayed high urine protein to creatinine ratio, but a significant difference was not found.
Fig 8.
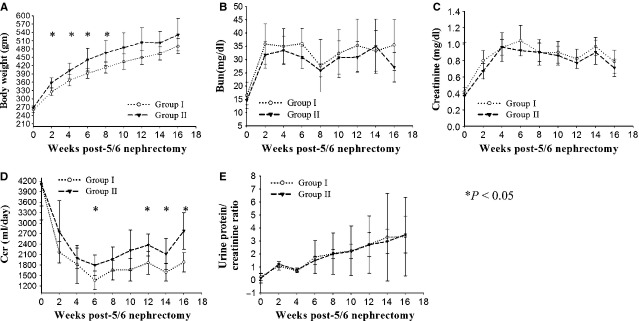
Analysis of body weight and renal function in CKD rats. (A) Body weight. (B) Plasma blood urea nitrogen concentration (C) Plasma creatinine concentration. (D) Creatinine clearance rate (E) Urinary protein-creatinine ratio. *P < 0.05.
Conditioned MSCs administration reduced glomerulosclerosis and interstitial fibrosis
Renal histological sections revealed moderate glomerulosclerosis and interstitial fibrosis in the group I rats. (Fig.9A–C). In contrast, rats treated with conditioned MSCs had milder changes (Fig.9D–F). Figure9G and H showed the comparison of histopathological scores of glomerulosclerosis and interstitial fibrosis between untreated and conditioned MSCs treated rats. At 16 weeks post-5/6 nephrecomy, the percent of glomeruli with focal glomerulosclerosis was 13.6 ± 3.56% in group II and 26.65 ± 3.73% in untreated 5/6 nephrectomy rats (P < 0.001). Mean tubulointerstitial fibrosis scores were 2.0 ± 0.63 and 1.0 ± 0.63 for groups I and II respectively (P < 0.05) (Fig.9H).
Fig 9.
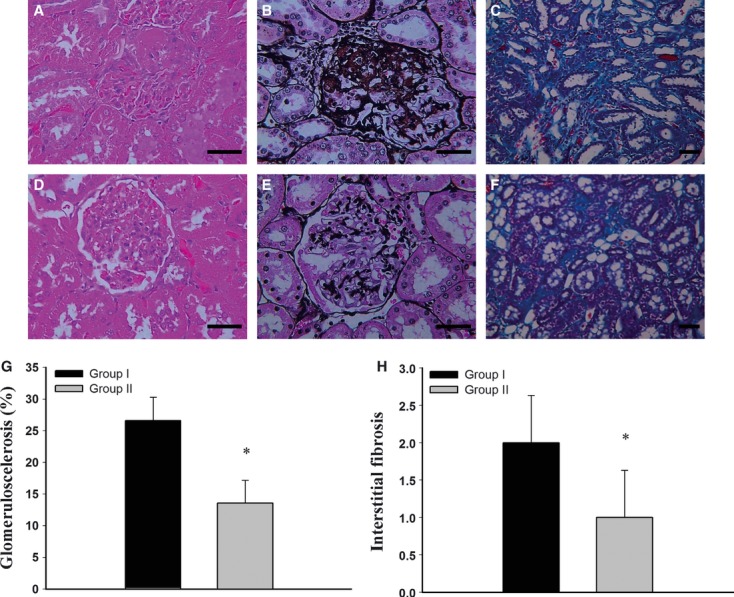
Histopathological findings for the rat remnant kidneys. Renal tissue sections from group I were stained (A) haematoxylin and eosin (400×), (B) periodic acid- silver methylamine stain (400×) and (C) Masson's trichrome stain (200×). Kidneys from group II were stained with (D) haematoxylin and eosin (400×), (E) periodic acid- silver methenamine stain (400×) for glomerulosclerosis and (F) Masson's trichrome stain for interstitial fibrosis (200×). (G) Comparisons of histopathological scores of glomerulosclerosis and (H) interstitial fibrosis between MSCs treated and group I control rats. (Scale bar 50 μm) *P < 0.05.
Conditioned MSCs inducted Tregs in spleen
To investigate the effect of MSCs in Tregs regulation, we study the percentage of CD4+CD25+Foxp3+ cells in spleen using flow cytometric analysis (Fig.10). The percentage of CD4+CD25+Foxp3+ at 16 weeks post-5/6 nephrectomy in group I and group II were 4.08 ± 0.49% and 5.21 ± 0.86%, respectively. Treatment with conditioned MSCs significantly increased the number of CD4+CD25+Foxp3+ cells in spleen of kidney remnant rats (P < 0.05).
Fig 10.
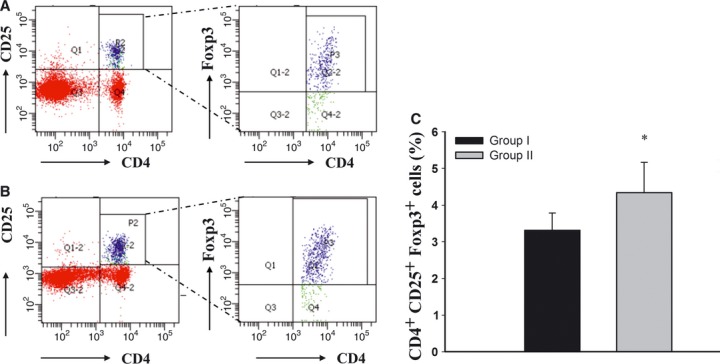
Flow cytometric analyses of CD4+CD25+Foxp3+ cells in splenocytes. The population of CD4+CD25+Foxp3+ in splenocytes was analysed by flow cytometry. (A) Representative contour plots analysis of CD4+CD25+Foxp3+ cells for the presence of CD4+CD25+ surface staining (left column) and intracellular transcription factor Foxp3+ (right column) in splenocytes in group I and (B) group II. (C) Percentages of CD4+CD25+Foxp3+ in splenocytes in different groups. *P < 0.05.
Human MSCs did not engraft into rat kidneys
To assess engraftment of transplanted conditioned human MSCs to rat kidneys, PCR analysis for human specific β2-micorglobulin showed that human cells did not exist in kidney tissue at the end of study (data not shown).
Discussion
MSCs-cell based therapies are a promising approach for a number of diseases based on their multi-lineage differential potential and paracrine effects on neighbouring cells by secretion of soluble factors 41. Since then MSCs were first isolated from the bone marrow by Friedenstein et al. 42, basal media supplemented with different growth factors were used to culture MSCs to potentiate their migration, proliferation and paracrine anti-fibrotic effects. Varying concentrations of bFGF 43 and other growth factors such as platelet-derived growth factor 44, insulin-like growth factor-I and EGF 45, are often used to supplement the expansion media of MSCs. It has widely been reported that bFGF and EGF are important growth factors for MSCs expansion 44–46 by increasing the growth rate and life span. In agreement with previous studies, our results suggest that bFGF and EGF promote HGF production by MSCs in a dose-dependent manner. It has been found that the addition of ascorbic acid 2-phosphate increased the level of HGF in both undifferentiated adipose-derived stem cells 47 and foreskin fibroblast 48. Our experiments confirm ascorbic acid 2-phosphate synergizes with bFGF and EGF to further increase HGF secretion in MSCs.
Effective migration and ability to home to injured site play a fundamental role in therapeutic effects of intravenously infused MSCs. HGF exerts a strong chemo-attractive effect on MSCs and induces migration of MSCs in vitro 49. Blocking HGF bioactivity results in significant reduction of MSCs migration. Therefore, the conditioned MSCs may not only increase secretion of HGF but also attract more MSCs to promote therapeutic potential.
Irrespective of the causes, interstitial fibrosis is the common irreversible pathological pathway that eventually leads to end-stage renal disease. Accumulating evidence indicates that EMT is a critical process in renal interstitial fibrosis characterized by fibroblast activation and imbalance between generation and degradation of extracellular matrix 5–8,50. Tubular epithelial cells and fibroblast undergo EMT characterized by de novo activation of α-SMA, vimentin, and fibronectin after injury 9,10,34. Of the many factors that regulate EMT, TGF-β1 acts as the major inducer of EMT, whereas HGF acts as an EMT inhibitor 51. Current in vitro study demonstrated that MSCs not only inhibited α-SMA and fibronectin expression in NRK-49F but also restored the E-cadherin of TGF-β1 treated NRK-52E in transwell co-culture chamber. These results suggest that conditioned MSCs significantly protect renal cells from EMT and this provides a novel strategy for therapeutic intervention of EMT induced renal fibrosis.
Podocytes are specialized, terminally differentiated visceral epithelial cells that cover the outer layer of the glomerular basement membrane 52. The key function of podocytes is to maintain the integrity of the glomerular filtration barrier. Depending on the severity of the injury, podocytes undergo foot process effacement, EMT, apoptosis, detachment from glomerular basement membrane and ‘podocytopenia’, which ultimately lead to progressive glomerular filtration barrier failure and glomerulosclerosis 53,54. Synaptopodin is an actin-associated protein specifically expressed in differentiated podocytes and is critical for the integrity of podocyte actin cytoskeleton. Loss of normal podocytes function is associated with decreased expression of the synaptopodin 55. Similar to tubular cells, recent studies demonstrate that podocytes in culture, upon incubation with TGF-β1, lose epithelial markers (E-cadherin), and increase in the expression of mesenchymal marker desmin, and fibronectin 56. Indeed podocytes injury plays a central role in the pathogenesis of glomerulosclerosis. Accordingly, therapies targeting limiting podocytes injury and stabilizing podocyte number may slow the progression glomerulosclerosis. Our in vitro studies demonstrate that conditioned MSCs significantly preserve podocyte-specific protein synaptopodin, which was down-regulated in TGF-β1 as well as rescued podocytes viability and protected podocytes from apoptosis.
HGF is a heterodimeric molecule consisting of α chain and β chain. In the kidney, HGF is expressed in interstitial cells, probably endothelial cells, macrophages and mesangial cells 57. Physiologically, HGF has both mitogenic and anti-apoptotic activities on renal cells. HGF is inversely related to TGF-β1 expression in chronic renal fibrosis 58. In vivo animal models of CKD have demonstrated HGF infusion halted TGF-β1 mediated myofibroblastic activation, progression of proteinuria and renal fibrosis 59–61. These results indicate that HGF is one of the most important protective and anti-fibrotic factors in renal fibrosis. To confirm the paracrine effects of conditioned MSCs in vitro, NRK-49F, NRK-52E and podocytes were co-cultured with conditioned MSCs. Significant secretion of HGF was detectable in all co-culture supernatants of NRK-49F NRK-52E and podocytes. Although ascorbic acid-2 phosphate significantly increase secretion of HGF by conditioned MSCs and increased the level of HGF in co-culture supernatants of NRK-52E, the in vitro studies did not demonstrate conditioned MSCs in the presence of ascorbic acid 2-phosphate confer significant benefits except superior effects on viability of TGF-β1-treated podocytes. This inconsistency may be related to experimental design and condition which have masked such additive effects. On the basis of these in vitro co-culture experiments, we infer that conditioned human MSCs have the potential to improve chronic kidney injury by the secretion of HGF.
MSCs are multipotent stem cells with strong immunomodulatory properties 62. It is well known that MSCs improve the outcome of acute renal injury models by paracrine action 29,63. The role of MSCs in the process of renal fibrogenesis of CKD is less obvious. Recently, encouraging data have established that allogenic MSCs improve either interstitial fibrosis with delayed progression of CKD or decrease proteinuia in different CKD animal models 64–67. However, the therapeutic benefit and mechanism through engraftment of MSCs or paracrine fashion remains controversial. In the current xenogenic study, the administration of the conditioned human MSCs decreased the rate of progression of CKD measured by CCr, severity of glomerulosclerosis and interstitial fibrosis. However, urine analysis did not showed significant attenuation of proteinuria although conditioned MSCs reduced apoptosis of podocytes treated with TGF-β1 in vitro. There is no significant difference between plasma creatinine concentrations between two groups, in spite of higher CCr in MSCs treated group. CCr is superior to plasma creatinine analysis for estimating renal function especially those with moderate renal impairment and reduced muscle mass 68. Future experiments by knockdown of HGF in the MSCs or blocking HGF by an anti-HGF neutralizing antibody is needed to directly assess whether or not HGF is a key mediator of MSC-derived renoprotection. Genetically modified MSCs may be used as cellular delivery vehicles for the delivery of therapeutic gene products to target sites. Further study may need to focus on transfecting MSCs with HGF to carry more HGF into fibrotic kidney and to maximize the beneficial effect of MSCs-based repair strategies.
Tregs are generally thought to play critical roles for the maintenance of peripheral tolerance. Tregs manifest their function through a myriad of mechanisms that include the secretion of immunosuppressive soluble factors such as TGF-β with various amounts of IL-4 and IL-10 69,70. Several in vivo and in vitro studies indicate that Tregs activation and induction plays an important role in MSC-mediated immunomodulation 71. The potential of Tregs to protect against the pathogenic immune responses in kidney disease has recently been shown in a murine model of crescentic glomerulonephritis 72. Moreover, mice reconstituted with CD4+CD25+ T cells had significantly reduced glomerulosclerosis, tubular damage and interstitial infiltrates in adriamycin induced nephropathy 73. Together, Tregs were shown to be protective in nephritis. Previous study showed MSCs administration decrease pro-inflammatory cytokine (IL-6, TNF-α) and increase IL-4 and IL-10 expression levels in rat kidney injury model 67. In accordance with the previous study, we demonstrated that MSCs treatment caused an increase in splenocyte Tregs. We anticipated conditioned MSCs delay progression of CKD by up-regulation of Tregs which, in turn, could shift cytokine balance in the favour of normal phenotype 67. A limitation of the present study is that the percentage of peripheral blood Tregs was not measured even though a study in the markers and phenotype of Tregs of rats has demonstrated Tregs were identical in spleen compared with blood except only slightly lower percentage of Tregs cells in blood 74.
MSCs are known to be hypoimmunogenic and can evade the host immune elimination 75. In animal experiments, human bone marrow-derived MSCs administered intravenously are able to engraft in animals with or without a pre-transplant total body irradiation 76,77. The therapeutic potential of human MSCs was demonstrated in immunodeficient NOD/SCID mice with cisplatin-induced acute renal failure 78. There are a few reports showing human MSCs avoid immune reaction and engraft in xenogenic immunocompetent models for acute myocardial infarction 79,80. In contrast, following xenotransplantation, xenogeneic stem cells may elicit donor-specific T cell proliferation and macrophage-mediated cellular response. Transplant rejection can occur in a xenogenic model 81. The therapeutic potential of bone marrow-derived MSCs of human origin on rat CKD model has not been reported so far. On the basis of these facts, we used conditioned human MSCs to treat rat CKD. Initial studies carried out by Morigi et al. reported that MSCs transdifferentiated into tubular epithelium in acute renal failure model 82. Nevertheless, follow-up studies revealed that the direct engraftment of MSCs is not the predominant mechanism to enhance renal repair 63. Our PCR analysis for human specific β2-micorglobulin showed that human cells did not exist in kidney tissue at the end of study following repeated administration of MSCs; and the results are consistent with the previous findings that the cytoprotective effect of systemically infused MSCs was mediated via paracrine, endocrine mechanisms and by immune-modulatory effect.
Conclusion
The current study has documented the constitutive and inducible secretion of HGF by MSCs in vitro. Conditioned MSCs transplantation attenuated interstitial fibrosis and progression of CKD in a 5/6 nephrectomy rat model, at least in part, through paracrine effects by secretion of HGF and immune modulation by induction of the Treg subset rather than engraft and differentiate into glomerular cells. Systemic transplantation of conditioned MSCs with high HGF secretion may therefore serve as a potential alternative for the treatment of renal fibrosis in CKD.
Acknowledgments
The authors gratefully acknowledge financial support from the Taipei Veterans General Hospital (VGHV100C-055) and the National Science Council (NSC-99-2314-B-075-019, and NSC-100-2314-B-075-064). This study was also supported by a grant from the Ministry of Education, ‘Aim for the Top University Plan’.
Conflict of interest
All authors of this article declare that there are no conflicts of interest with regard to this study or in reporting the findings described in this article. The authors alone are responsible for the content and the writing of this article.
References
- Nath KA. The tubulointerstitium in progressive renal disease. Kidney Int. 1998;54:992–4. doi: 10.1046/j.1523-1755.1998.00079.x. [DOI] [PubMed] [Google Scholar]
- Taal MW, Omer SA, Nadim MK, et al. Cellular and molecular mediators in common pathway mechanisms of chronic renal disease progression. Curr Opin Nephrol Hypertens. 2000;9:323–31. doi: 10.1097/00041552-200007000-00001. [DOI] [PubMed] [Google Scholar]
- Boucher A, Droz D, Adafer E, et al. Characterization of mononuclear cell subsets in renal cellular interstitial infiltrates. Kidney Int. 1986;29:1043–9. doi: 10.1038/ki.1986.105. [DOI] [PubMed] [Google Scholar]
- Sean Eardley K, Cockwell P. Macrophages and progressive tubulointerstitial disease. Kidney Int. 2005;68:437–55. doi: 10.1111/j.1523-1755.2005.00422.x. [DOI] [PubMed] [Google Scholar]
- Lopez-Novoa JM, Nieto MA. Inflammation and EMT: an alliance towards organ fibrosis and cancer progression. EMBO Mol Med. 2009;1:303–14. doi: 10.1002/emmm.200900043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Liu Y. Dissection of key events in tubular epithelial to myofibroblast transition and its implications in renal interstitial fibrosis. Am J Pathol. 2001;159:1465–75. doi: 10.1016/S0002-9440(10)62533-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwano M, Plieth D, Danoff TM, et al. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341–50. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutz F, Zeisberg M. Renal fibroblasts and myofibroblasts in chronic kidney disease. J Am Soc Nephrol. 2006;17:2992–8. doi: 10.1681/ASN.2006050420. [DOI] [PubMed] [Google Scholar]
- García-Sánchez O, López-Hernández FJ, López-Novoa JM. An integrative view on the role of TGF-beta in the progressive tubular deletion associated with chronic kidney disease. Kidney Int. 2010;77:950–5. doi: 10.1038/ki.2010.88. [DOI] [PubMed] [Google Scholar]
- Böttinger EP, Bitzer M. TGF-beta signaling in renal disease. J Am Soc Nephrol. 2002;13:2600–10. doi: 10.1097/01.asn.0000033611.79556.ae. [DOI] [PubMed] [Google Scholar]
- Yang J, Dai C, Liu Y. A novel mechanism by which hepatocyte growth factor blocks tubular epithelial to mesenchymal transition. J Am Soc Nephrol. 2005;16:68–78. doi: 10.1681/ASN.2003090795. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Nakamura T. Hepatocyte growth factor in renal regeneration, renal disease, and potential therapeutics. Curr Opin Nephrol Hypertension. 2000;9:395–402. doi: 10.1097/00041552-200007000-00011. [DOI] [PubMed] [Google Scholar]
- Tsai PC, Fu TW, Chen YM, et al. The therapeutic potential of the human umbilical mesenchymal stem cells in Wharton's Jelly on treatment of rat liver fibrosis model. Liver Transpl. 2009;15:484–95. doi: 10.1002/lt.21715. [DOI] [PubMed] [Google Scholar]
- Kiyama S, Yamada T, Iwata H, et al. Reduction of fibrosis in a rat model of non-alcoholic steatohepatitis cirrhosis by human HGF gene transfection using electroporation. J Gastroenterol Hepatol. 2008;23:e471–6. doi: 10.1111/j.1440-1746.2007.05111.x. . doi: 10.1111/j.1440-1746.2007.0511.x. [DOI] [PubMed] [Google Scholar]
- Shukla MN, Rose JL, Ray R, et al. Hepatocyte growth factor inhibits epithelial to myofibroblast transition in lung cells via Smad7. Am J Respir Cell Mol Biol. 2009;40:643–53. doi: 10.1165/rcmb.2008-0217OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. Hepatocyte growth factor in kidney fibrosis: therapeutic potential and mechanisms of action. Am J Physiol Renal Physiol. 2004;287:F7–16. doi: 10.1152/ajprenal.00451.2003. [DOI] [PubMed] [Google Scholar]
- Woodbury D, Schwarz EJ, Prockop DJ, et al. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61:364–70. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Liu JW, Dunoyer-Geindre S, Serre-Beinier V, et al. Characterization of endothelial-like cells derived from human mesenchymal stem cells. J Thromb Haemost. 2007;5:826–34. doi: 10.1111/j.1538-7836.2007.02381.x. [DOI] [PubMed] [Google Scholar]
- Weng YS, Lin HY, Hsiang YJ, et al. The effects of different growth factors on human bone marrow stromal cells differentiating into hepatocyte-like cells. Adv Exp Med Biol. 2003;534:119–28. doi: 10.1007/978-1-4615-0063-6_9. [DOI] [PubMed] [Google Scholar]
- Aggarwal S, Pittenger MF, Aggarwal S, et al. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–22. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- Zappia E, Casazza S, Pedemonte E, et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106:1755–61. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
- Augello A, Tasso R, Negrini S, et al. Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur J Immunol. 2005;35:1482–90. doi: 10.1002/eji.200425405. [DOI] [PubMed] [Google Scholar]
- Kong QF, Sun B, Bai SS, et al. Administration of bone marrow stromal cells ameliorates experimental autoimmune myasthenia gravis by altering the balance of Th1/Th2/Th17/Treg cell subsets through the secretion of TGF-beta. J Neuroimmunol. 2009;207:83–91. doi: 10.1016/j.jneuroim.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Ohnishi S, Yanagawa B, Tanaka K, et al. Transplantation of mesenchymal stem cells attenuates myocardial injury and dysfunction in a rat model of acute myocarditis. J Mol Cell Cardiol. 2007;42:88–97. doi: 10.1016/j.yjmcc.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Sato K, Ozaki K, Mori M, et al. Mesenchymal stromal cells for graft-versus-host disease: basic aspects and clinical outcomes. J Clin Exp Hematop. 2010;50:79–89. doi: 10.3960/jslrt.50.79. [DOI] [PubMed] [Google Scholar]
- Aggarwal S, Pittenger MF. Allogenic mesenchymal stem cells transplantation in refractory systemic lupus erythematosus: a pilot clinical study. Ann Rheum Dis. 2010;69:1423–9. doi: 10.1136/ard.2009.123463. [DOI] [PubMed] [Google Scholar]
- Suga H, Eto H, Shigeura T, et al. Fibroblast growth factor-2-induced hepatocyte growth factor secretion by adipose-derived stromal cells inhibits postinjury fibrogenesis through a c-Jun N-terminal kinase-dependent mechanism. Stem Cells. 2009;27:238–49. doi: 10.1634/stemcells.2008-0261. [DOI] [PubMed] [Google Scholar]
- Li L, Zhang Y, Li Y, et al. Mesenchymal stem cell transplantation attenuates cardiac fibrosis associated with isoproterenol-induced global heart failure. Transpl Int. 2008;21:1181–9. doi: 10.1111/j.1432-2277.2008.00742.x. [DOI] [PubMed] [Google Scholar]
- Humphreys BD, Bonventre JV. Mesenchymal stem cells in acute kidney injury. Annu Rev Med. 2008;59:311–25. doi: 10.1146/annurev.med.59.061506.154239. [DOI] [PubMed] [Google Scholar]
- Bussolati B, Tetta C, Camussi G. Contribution of stem cells to kidney repair. Am J Nephrol. 2008;28:813–22. doi: 10.1159/000137681. [DOI] [PubMed] [Google Scholar]
- Ninichuk V, Gross O, Segerer S, et al. Multipotent mesenchymal stem cells reduce interstitial f fibrosis but do not delay progression of chronic kidney disease in collagen4A3-deficient mice. Kidney Int. 2006;70:121–9. doi: 10.1038/sj.ki.5001521. [DOI] [PubMed] [Google Scholar]
- Lee KD, Kuo TK, Whang-Peng J, et al. In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology. 2004;40:1275–84. doi: 10.1002/hep.20469. [DOI] [PubMed] [Google Scholar]
- Kuo TK, Hung SP, Chuang CH, et al. Stem cell therapy for liver disease: parameters governing the success of using bone marrow mesenchymal stem cells. Gastroenterology. 2008;134:2111–21. doi: 10.1053/j.gastro.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan JM, Ng YY, Hill PA, et al. Transforming growth factor-beta regulates tubular epithelial-myofibroblast transdifferentiation in vitro. Kidney Int. 1999;56:1455–67. doi: 10.1046/j.1523-1755.1999.00656.x. [DOI] [PubMed] [Google Scholar]
- de-Larco JE, Todaro GJ. Epithelioid and fibroblastic rat kidney cell clones: epidermal growth factor (EGF) receptors and the effect of mouse sarcoma virus transformation. J Cell Physiol. 1978;94:335–42. doi: 10.1002/jcp.1040940311. [DOI] [PubMed] [Google Scholar]
- Shankland SJ, Pippin JW, Reiser J, et al. Podocytes in culture: past, present, and future. Kidney Int. 2007;72:26–36. doi: 10.1038/sj.ki.5002291. [DOI] [PubMed] [Google Scholar]
- Yokoi H, Mukoyama M, Sugawara A, et al. Role of connective tissue growth factor in fibronectin expression and tubulointerstitial fibrosis. Am J Physiol Renal Physiol. 2002;282:933–42. doi: 10.1152/ajprenal.00122.2001. [DOI] [PubMed] [Google Scholar]
- Friedl G, Schmidt H, Rehak I, et al. Undifferentiated human mesenchymal stem cells (hMSCs) are highly sensitive to mechanical strain: transcriptionally controlled early osteo-chondrogenic response in vitro. Osteoarthritis Cartilage. 2007;15:1293–300. doi: 10.1016/j.joca.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Macconi D, Bonomelli M, Benigni A, et al. Pathophysiologic implications of reduced podocyte number in a rat model of progressive glomerular injury. Am J Pathol. 2006;168:42–54. doi: 10.2353/ajpath.2006.050398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankland SJ. The podocyte's response to injury: role in proteinuria and glomerulosclerosis. Kidney Int. 2006;69:2131–47. doi: 10.1038/sj.ki.5000410. [DOI] [PubMed] [Google Scholar]
- Horwitz EM, Dominici M. How do mesenchymal stromal cells exert their therapeutic benefit? Cytotherapy. 2008;10:771–4. doi: 10.1080/14653240802618085. [DOI] [PubMed] [Google Scholar]
- Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- Choi SC, Kim SJ, Choi JH, et al. Fibroblast growth factor-2 and -4 promote the proliferation of bone marrow mesenchymal stem cells by the activation of the PI3K-Akt and ERK1/2 signaling pathways. Stem Cells Dev. 2008;17:725–36. doi: 10.1089/scd.2007.0230. [DOI] [PubMed] [Google Scholar]
- Bocelli-Tyndall C, Zajac P, Di Maggio N, et al. Fibroblast growth factor 2 and platelet-derived growth factor, but not platelet lysate, induce proliferation-dependent, functional class II major histocompatibility complex antigen in human mesenchymal stem cells. Arthritis Rheum. 2010;62:3815–25. doi: 10.1002/art.27736. [DOI] [PubMed] [Google Scholar]
- Tamama K, Fan VH, Griffith LG, et al. Epidermal growth factor as a candidate for ex vivo expansion of bone marrow-derived mesenchymal stem cells. Stem Cells. 2006;24:686–95. doi: 10.1634/stemcells.2005-0176. [DOI] [PubMed] [Google Scholar]
- Tamama K, Kawasaki H, Wells A. Epidermal growth factor (EGF) treatment on multipotential stromal cells (MSCs). possible enhancement of therapeutic potential of MSC. J Biomed Biotechnol. 2010;2010:795385. doi: 10.1155/2010/795385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilroy GE, Foster SJ, Wu X, et al. Cytokine profile of human adipose-derived stem cells: expression of angiogenic, hematopoietic, and pro-inflammatory factors. J Cell Physiol. 2007;212:702–9. doi: 10.1002/jcp.21068. [DOI] [PubMed] [Google Scholar]
- Wu YL, Gohda E, Iwao M, et al. Stimulation of hepatocyte growth factor production by ascorbic acid and its stable 2-glucoside. Growth Horm IGF Res. 1998;8:421–8. doi: 10.1016/s1096-6374(98)80313-4. [DOI] [PubMed] [Google Scholar]
- Forte G, Minieri M, Cossa P, et al. Hepatocyte growth factor effects on Mesenchymal stem cells: proliferation, migration, and differentiation. Stem Cells. 2006;24:23–33. doi: 10.1634/stemcells.2004-0176. [DOI] [PubMed] [Google Scholar]
- Liu Y. New insights into epithelial-mesenchymal transition in kidney fibrosis. J Am Soc Nephrol. 2010;21:212–22. doi: 10.1681/ASN.2008121226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int. 2006;69:213–7. doi: 10.1038/sj.ki.5000054. [DOI] [PubMed] [Google Scholar]
- Mundel P, Kriz W. Structure and function of podocytes: an update. Anat Embryol. 1995;192:385–97. doi: 10.1007/BF00240371. [DOI] [PubMed] [Google Scholar]
- Schiffer M, Bitzer M, Roberts IS, et al. Apoptosis in podocytes induced by TGF-beta and Smad7. J Clin Invest. 2001;108:807–16. doi: 10.1172/JCI12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharram BL, Goyal M, Wiggins JE, et al. Podocyte depletion causes glomerulosclerosis: diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol. 2005;16:2941–52. doi: 10.1681/ASN.2005010055. [DOI] [PubMed] [Google Scholar]
- Turk T, Leeuwis JW, Gray J, et al. BMP signaling and podocyte markers are decreased in human diabetic nephropathy in association with CTGF overexpression. J Histochem Cytochem. 2009;57:623–31. doi: 10.1369/jhc.2009.953224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Kang YS, Dai C, et al. Epithelial-to-mesenchymal transition is a potential pathway leading to podocyte dysfunction and proteinuria. Am J Pathol. 2008;172:299–308. doi: 10.2353/ajpath.2008.070057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K, Nakamura T. Renotropic role and therapeutic potential of HGF in the kidney. Nephrol Dial Transplant. 2002;17:59–61. doi: 10.1093/ndt/17.suppl_9.59. [DOI] [PubMed] [Google Scholar]
- Mizuno S, Matsumoto K, Kurosawa T, et al. Reciprocal balance of hepatocyte growth factor and transforming growth factor-β1 in renal fibrosis in mice. Kidney Int. 2000;57:937–48. doi: 10.1038/sj.ki.4491416. [DOI] [PubMed] [Google Scholar]
- Azuma H, Takahara S, Matsumoto K, et al. Hepatocyte growth factor prevents the development of chronic allograft nephropathy in rats. J Am Soc Nephrol. 2001;12:1280–92. doi: 10.1681/ASN.V1261280. [DOI] [PubMed] [Google Scholar]
- Mizuno S, Matsumoto K, Nakamura T. Hepatocyte growth factor prevents renal fibrosis and dysfunction in a mouse model of chronic renal disease. Kidney Int. 2001;59:1304–14. doi: 10.1046/j.1523-1755.2001.0590041304.x. [DOI] [PubMed] [Google Scholar]
- Gong R, Rifai A, Tolbert EM, et al. Hepatocyte growth factor ameliorates renal interstitial inflammation in rat remnant kidney by modulating tubular expression of macrophage chemoattractant protein-1 and RANTES. J Am Soc Nephrol. 2004;15:2868–81. doi: 10.1097/01.ASN.0000141962.44300.3A. [DOI] [PubMed] [Google Scholar]
- Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- Tögel F, Hu Z, Weiss K, et al. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289:F31–42. doi: 10.1152/ajprenal.00007.2005. [DOI] [PubMed] [Google Scholar]
- Kunter U, Rong S, Boor P, et al. Mesenchymal stem cells prevent progressive experimental renal failure but maldifferentiate into glomerular adipocytes. J Am Soc Nephrol. 2007;18:1754–64. doi: 10.1681/ASN.2007010044. [DOI] [PubMed] [Google Scholar]
- Caldas HC, Fernandes IM, Gerbi F, et al. Effect of whole bone marrow cell infusion in the progression of experimental chronic renal failure. Transplant Proc. 2008;40:853–5. doi: 10.1016/j.transproceed.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Kirpatovskii VI, Kazachenko AV, Plotnikov EY, et al. Experimental intravenous cell therapy of acute and chronic renal failure. Bull Exp Biol Med. 2007;143:160–5. doi: 10.1007/s10517-007-0039-5. [DOI] [PubMed] [Google Scholar]
- Semedo P, Correa-Costa M, Antonio Cenedeze M, et al. Mesenchymal stem cells attenuate renal fibrosis through immune modulation and remodeling properties in a rat remnant kidney model. Stem Cells. 2009;27:3063–73. doi: 10.1002/stem.214. [DOI] [PubMed] [Google Scholar]
- Levey AS, Perrone RD, Madias NE. Serum creatinine and renal function. Annu Rev Med. 1988;39:465–90. doi: 10.1146/annurev.me.39.020188.002341. [DOI] [PubMed] [Google Scholar]
- Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–32. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corthay A. How do regulatory T cells work? Scand J Immunol. 2009;70:326–36. doi: 10.1111/j.1365-3083.2009.02308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghannam S, Pene J, Torcy-Moquet G, et al. Mesenchymal stem cells inhibit human Th17 cell differentiation and function and induce a T regulatory cell. J Immunol. 2010;185:302–12. doi: 10.4049/jimmunol.0902007. [DOI] [PubMed] [Google Scholar]
- Paust HJ, Ostmann A, Erhardt A, et al. Regulatory T cells control the Th1 immune response in murine crescentic glomerulonephritis. Kidney Int. 2011;80:154–64. doi: 10.1038/ki.2011.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng G, Wang Y, Mahajan D, et al. The role of tubulointerstitial inflammation. Kidney Int. 2005;67:S96–100. doi: 10.1111/j.1523-1755.2005.09423.x. [DOI] [PubMed] [Google Scholar]
- Stephens LA, Barclay AN, Mason D. Phenotypic characterization of regulatory CD4+CD25+ T cells in rats. Int Immunol. 2004;16:365–75. doi: 10.1093/intimm/dxh033. [DOI] [PubMed] [Google Scholar]
- Locatelli F, Giorgiani G, Di-Cesare-Merlone A, et al. The changing role of stem cell transplantation in childhood. Bone Marrow Transplant. 2008;41:S3–7. doi: 10.1038/bmt.2008.45. [DOI] [PubMed] [Google Scholar]
- François S, Mouiseddine M, Mathieu N, et al. Human mesenchymal stem cells favor healing of the cutaneous radiation syndrome in a xenogenic transplant model. Ann Hematol. 2007;86:1–8. doi: 10.1007/s00277-006-0166-5. [DOI] [PubMed] [Google Scholar]
- Allers C, Sierralta WD, Neubauer S, et al. Dynamic of distribution of human bone marrow-derived mesenchymal stem cells after transplantation into adult unconditioned mice. Transplantation. 2004;78:503–8. doi: 10.1097/01.tp.0000128334.93343.b3. [DOI] [PubMed] [Google Scholar]
- Morigi M, Introna M, Imberti B, et al. Human bone marrow mesenchymal stem cells accelerate recovery of acute renal injury and prolong survival in mice. Stem Cells. 2008;26:2075–82. doi: 10.1634/stemcells.2007-0795. [DOI] [PubMed] [Google Scholar]
- Saito T, Kuang JQ, Bittira B, et al. Xenotransplant cardiac chimera: immune tolerance of adult stem cells. Ann Thorac Surg. 2002;74:19–24. doi: 10.1016/s0003-4975(02)03591-9. [DOI] [PubMed] [Google Scholar]
- Buja LM, Vela D. Immunologic and inflammatory reactions to exogenous stem cells implications for experimental studies and clinical trials for myocardial repair. J Am Coll Cardiol. 2010;56:1693–700. doi: 10.1016/j.jacc.2010.06.041. [DOI] [PubMed] [Google Scholar]
- Grinnemo KH, Mansson A, Dellgren G, et al. Xenoreactivity and engraftment of human mesenchymal stem cells transplanted into infarcted rat myocardium. J Thorac Cardiovasc Surg. 2004;127:1293–300. doi: 10.1016/j.jtcvs.2003.07.037. [DOI] [PubMed] [Google Scholar]
- Morigi M, Imberti B, Zoja C, et al. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J Am Soc Nephrol. 2004;15:1794–804. doi: 10.1097/01.asn.0000128974.07460.34. [DOI] [PubMed] [Google Scholar]


