Abstract
Small-conductance calcium-activated potassium (SK3) channels have been detected in human myometrium and we have previously shown a functional role of SK channels in human myometrium in vitro. The aims of this study were to identify the precise localization of SK3 channels and to quantify SK3 mRNA expression in myometrium from pregnant and non-pregnant women. Myometrial biopsies were obtained from pregnant (n = 11) and non-pregnant (n = 11) women. The expression of SK3 channels was assessed using immunohistochemistry and SK3 mRNA was determined by qRT-PCR. In non-pregnant myometrium SK3 immunoreactivity was observed in CD34 positive (CD34+) interstitial Cajal-like cells (ICLC), now called telocytes. Although CD34+ cells were also present in pregnant myometrium, they lacked SK3 immunoreactivity. Furthermore, the immunohistochemical results showed that SK3 expression in vascular endothelium was similar between the two groups. CD117 immunoreactivity was only detected in small round cells that resemble mast cells. Compared to non-pregnant myometrium we found significantly less SK3 mRNA in pregnant myometrium. We demonstrate that SK3 channels are localized solely in CD34+ cells and not in smooth muscle cells, and that the molecular expression of SK3 channels is higher in non-pregnant compared to pregnant myometrium. On the basis of our previous study and the present findings, we propose that SK3 activators reduce contractility in human myometrium by modulating telocyte function. This is the first report to provide evidence for a possible role of SK3 channels in human uterine telocytes.
Keywords: human myometrium, SK3 channels, telocytes, CD34, immunohistochemistry, qRT-PCR
Introduction
Despite our increasing understanding of the mechanism of contraction and relaxation of the myometrial smooth muscle cell, our ability to prevent, or effectively treat, preterm labour has not improved in the same way. Knowledge of uterine activity is essential if we are to discover new tocolytic treatments.
Small-conductance calcium-activated potassium (SK) channels have been shown to be present in human myometrium from pregnant and non-pregnant women 1–4. SK channel isoform 3 (SK3) is of particular interest as it has been shown to alter myometrial contractility and its expression is regulated throughout pregnancy 5–8. We have recently studied the functionality of SK channels in myometrium from pregnant and non-pregnant women and have demonstrated that SK channel activation contribute to relaxation of the human myometrium in vitro 9. However, the precise cellular localization of SK channels is still not obvious.
The myometrium is composed of smooth muscle cells (SMC) and interstitial cells. Of recent interest are the telocytes, formerly called interstitial Cajal-like cells (ICLC). Recently, Popescu et al. 10 have thoroughly described the ultrastructure and immunophenotype of the telocytes. Telocytes are distinct interstitial cells with characteristic long moniliform prolongations (telopodes) and CD34 positive (CD34+) immunoreactivity. Lately, the presence of telocytes has been identified in human and mammalian tissues and organs including heart 11–14, placenta 15, urinary tract 16, lungs 17,18, pleura 19, blood vessels 20, skin 21, pancreas 22, parotid glands 23, intestine 24 and endometrium 25.
In this study, we examined the presence of telocytes and the molecular expression and cellular immunolocalization of SK3 channels in pregnant and non-pregnant human myometrium. SK3 channels modulators regulate human myometrial contractility by modifying telocytes function through a mechanism with which we are not yet familiar.
Materials and methods
Tissue collection
All patients were recruited from the Department of Gynaecology and Obstetrics, Holbaek Hospital and the experiments were either performed at the Smooth Muscle Research Center, Koege Hospital or at the Department of Pathology, Naestved Hospital.
Myometrium was obtained, with informed written consent, from women undergoing elective caesarean section at term not in labour (gestational age between 37 and 40 weeks; mean maternal age 32.5; range 28–40 years; n = 11), or hysterectomy (mean age 46; range 35–54 years; n = 11). Tables1 and 2 describe the demographic and clinical data of pregnant and non-pregnant participants respectively. None of the women included in this study had evidence of underlying disease (hypertension, diabetes, preeclampsia, intrauterine growth restriction, etc.), and all the conceptions in the pregnant group occurred spontaneously. Biopsies were obtained from the midline of the upper lip of the uterine incision (myometrium) at elective caesarean section, or from the same part of the uterus from a macroscopic normal area at hysterectomy. The tissue specimens were immediately placed in formalin for immunohistochemical experiments and transferred at room temperature to the Department of Clinical Pathology, Naestved Hospital. Biopsies for qRT-PCR experiments were placed in RNAlater (Qiagen, Copenhagen, Denmark) immediately after dissection, and transferred to the Smooth Muscle Research Center. Upon arrival, biopsies in RNAlater were stored at 4°C overnight, and frozen at −20°C until further processing (<2 months). The study has been approved by the local ethics committee (SJ-120).
Table 1.
Demographic and clinical data of non-pregnant patients
| Patient | Age, year | Clinical indication | BMI | Uterus weight, g |
|---|---|---|---|---|
| NP01 | 47 | Metrorrhagia | 19.6 | 130 |
| Menorrhagia | ||||
| NP02 | 35 | Cervix dysplasia | 21.2 | 115 |
| Adenomyosis uteri | ||||
| NP03 | 43 | Metrorrhagia | 33 | 1100 |
| Leiomyoma | ||||
| NP04 | 41 | Metrorrhagia | 31.2 | 120 |
| NP05 | 53 | Leiomyoma | 27 | 570 |
| Ovarian cyst | ||||
| NP06 | 48 | Prolapse | 28 | 175 |
| NP07 | 54 | Metrorrhagia | 23 | 470 |
| Menorrhagia | ||||
| Adenomyosis | ||||
| NP08 | 42 | Metrorrhagia | 32.2 | 312 |
| Menorrhagia | ||||
| NP09 | 48 | Menorrhagia | 32.8 | 210 |
| Polymenorrhea | ||||
| Metrorrhagia | ||||
| NP10 | 48 | Dysmenorrhea | 29.7 | 220 |
| Menorrhagia | ||||
| Polymenorrhea | ||||
| NP11 | 47 | Dysmenorrhea | 21.7 | 240 |
| Hypermenorrhea | ||||
| Leiomyoma |
Table 2.
Demographic and clinical data of pregnant women
| Maternal age, year | 32.5 ± 3.23 |
| Body mass index, kg/m2 | 26.5 ± 6.4 |
| Gestational age at delivery, weeks | 37–40 |
| Indication for caesarean section | Maternal request, previous caesarean section or breech presentation |
| Birthweight, kg | 3.55 ± 0.3 |
| Data are presented as mean ± S.D. |
Immunohistochemistry
Immunohistochemical studies were performed on paraffin sections using anti-human KCa2.3 (SK3) (Cat. no. APC-025; Alomone Labs, Jerusalem, Israel); anti-human CD117 clone YR145 (Cat. no. 1522-1; Epitomics, Burlingame, CA, USA); anti-human CD34 clone QBEnd 10 (Cat. no. M7165; Dako, Glostrup, Denmark); anti-human smooth muscles actin clone 1A4 (SMA, cat. no. M0851; Dako); and anti-human CD31 clone JC/70A (Cat. no. M0823; Dako).
Briefly, dewaxing and antigen retrieval was performed using immersing slides in EnVision™ FLEX Target Retrieval Solution, High pH (Cat.no. K8004; Dako) and heated in the PT-module according to the manufacturer's instructions. After pre-treatment, the slides were incubated with the following primary antibodies for 30 min.: KCa2.3 (SK3) (1:700); CD117 clone YR145 (1:100); smooth muscle actin clone 1A4 (1:500); CD31 clone JC/70A (1:75); and CD34 clone QBEnd 10 (1:100). The reactions were detected using the EnVision™ FLEX/HRP Detection Reagent (Cat. no K8000; Dako) standard polymer technique. In addition, signal intensity was amplified using either EnVision™ FLEX+ Mouse (LINKER) (Cat. no. K8021; Dako) or EnVision™ FLEX+ Rabbit (LINKER) (Cat. no. K8009; Dako). Finally, sections were counterstained with haematoxylin and mounted with pertex.
Double immuno-labelling experiments
Studies were performed on paraffin sections using anti-human KCa2.3 (SK3) (1:350) in combination with either CD34 clone QBEnd 10 (1:50); smooth muscle actin clone 1A4 (1:100); or CD31 clone JC/70A (1:15). Following pre-treatment (described above), the slides were incubated with a mix of the primary antibodies for 60 min. The resulting reactions were detected as described elsewhere 26.
Controls
Human appendix was included as positive tissue control. Negative controls were performed by omitting primary antibodies, or primary antibody mix, in double immuno-labelling experiments. In addition, the specificity of the KCa2.3 (SK3) antibody was assessed using a specific blocking peptid (Alomone labs) in accordance with the manufacturer's instructions.
RNA extraction and cDNA synthesis
Tissue biopsies were ground in liquid nitrogen in a mortar, and homogenized in 1.5 ml TriZol (Invitrogen, Naerum, Denmark) using a rotor-stator homogenizer. Total RNA was extracted from (85–150 mg) ground tissue using the Trizol method (manufacturer's instruction), including bromochloropropane (VWR, Herlev, Denmark) for phase-separation. The quantity of extracted RNA was checked spectrophotometrically with a nanophotometer (Implen; VWR). To ensure RNA integrity, only samples with an A260/A280 optical density (OD) ratio of 1.7–2.0 were used. A quantity of 1 μg was loaded on a 1% agarose gel containing ethidium bromide for electrophoresis and visualized using a UV-transilluminator (Uvitec; Kem-en-tec, Taastrup, Denmark). RNA (10 μg) samples were purified by DNase treatment to remove contaminating genomic DNA. This was done using an RNAse-free DNase kit according to the manufacturer's instructions for rigorous DNA removal (50 μl reaction) (Ambion; Applied Biosystems, Foster City, CA, USA). The quantity and purity of DNase-treated RNA was checked spectrophotometrically and with the help of gel electrophoresis.
RNA was reversely transcribed into complementary DNA (cDNA) with 4.5 μl oligo(dT) primers (100 ng/μl), and reverse transcriptase (1 μl), in a 30 μl reaction volume from 600 ng DNase treated RNA. This was done using the Affinity Script qPCR cDNA synthesis kit (Stratagene, AH Diagnostics, Aarhus, Denmark) as per the manufacturer's instructions. Cycling parameters were 5 min. at 25°C; 15 min. at 42°C; 5 min. at 95°C; and 30 min. at 25°C using a Mx3000P QPCR system (Stratagene, AH Diagnostics). cDNA was stored at −20°C until further processing.
Primers
While primers for the gene of interest, KCNN3, and for the reference genes, GAPDH and ACTB, were designed using Primer3 (ncbi), primer properties were determined using Oligo Analyser (IDT) software. The primers were ordered from TAG-Copenhagen (Copenhagen, Denmark). The primers were designed to match all known splice variants of the given gene. To ensure primer specificity a BLAST search against the NCBI database was performed, and a melting curve analysis ensured that only one product was amplified. Primers for the additional reference genes B2M, YWHAZ, SF3A1, 18S, SDHA and TOP1 were included in a geNorm REF gene kit (PrimerDesign Ltd, Southampton, UK), which included the qbasePLUS software license.
Quantitative real-time polymerase chain reaction
qRT-PCR was performed using the Brilliant SYBR Green QPCR Master Mix and Mx3000P QPCR system (both from AH Diagnostics). Reactions were carried out in 25 μl reaction volumes containing 20 ng of cDNA; 12.5 μl brilliant SYBR green mastermix; 0.5 μl (10 pmol/μl) of each primer; and 9.5 μl RNase-free H2O. Amplification was detected using SYBR green fluorescent dye with the following amplification conditions: (i) one 10 min. pre-amplification cycle at 95°C; (ii) 40 amplification cycles including 30 sec. (denaturation) at 95°C; 60 sec. (annealing) at 60°C; and 60 sec. (extension) at 72°C; (iii) 60 sec. end-amplification cycle at 95°C, followed by increasing temperature from 55 to 95°C with continuous reading of fluorescence data (dissociation curve). Standard curves were performed for the stably expressed reference genes, and for the gene of interest (KCNN3), by twofold serial dilution of a cDNA pool of all samples (0.78–100 ng).
No template, and no reverse transcription, controls were performed for each sample and primer pair when appropriate to control for contamination or primer-dimers. A ΔCq > 10 between sample and noRT control ensured that the samples were not contaminated by any form of genomic DNA. All reactions were carried out in triplicate, both in the case of samples and that of standard curves. Cq values were calculated from the exponential phase of amplification when crossing threshold using MxPRO 4.1 software (AH Diagnostics). Cq values were transformed into relative quantities using the gene-specific PCR amplification efficiency by geNormPlus software (qbasePLUS, Biogazelle, PrimerDesign Ltd). A geometric mean of the two stable reference genes was employed as the normalization factor of the reference genes to calculate the relative expression level of KCNN3 within the two patient groups. While amplicons were sequenced to ensure KCNN3 primer specificity (Beckman Coulter Genomics, Essex, UK), a BLAST search against the NCBI database confirmed that only the gene of interest was amplified. All graphical presentations and statistics were prepared using GraphPad Prism 5.04 (GraphPad Software, San Diego, California, USA). Whereas values were presented as mean (95% CI) group differences were analysed using the Mann–Whitney test.
Results
Immunohistochemistry
To investigate the localization of SK3 channels we stained formalin-fixed paraffin-embedded pregnant and non-pregnant human myometrium. SK3 showed a strong positive staining of vascular endothelium (mainly large veins) and interstitial cells corresponding to telocytes in non-pregnant tissue (Fig.1A). In contrast, only vascular endothelium showed positive staining for SK3 in pregnant myometrium (Fig.2A). No, or only very weak, SK3 staining was found associated with SMC. CD117, an accepted marker for ICC in the digestive tract, did not label ICC in the myometrium of either pregnant or non-pregnant patients. CD117 immunoreactivity was only detected in small round cells that resemble mast cells (Figs1C and 2C). In both groups, interstitial cells (resembling telocytes) intermingling smooth muscle bundles, proved to be CD34+ (Figs1B and 2B). Vascular endothelium was also intensely stained with CD34.
Fig 1.
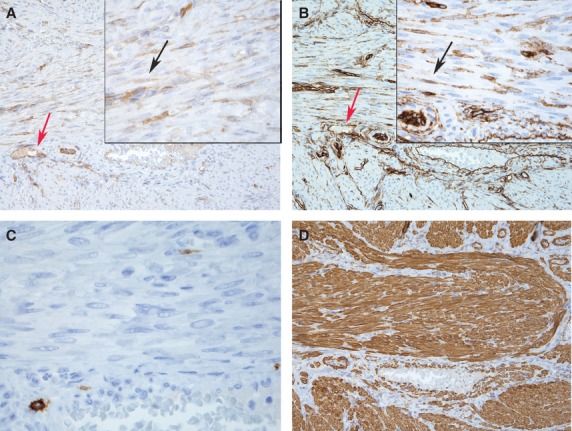
Representative immunostaining of formalin-fixed paraffin-embedded human non-pregnant myometrial section (4 μm). Strong positive staining for SK3 (A) and CD34 (B) corresponding to telocytes intermingling muscle bundles (black arrows). SK3 and CD34 were also detected in vascular endothelium (red arrows). Only normal mast cells stained for CD117 (C; magnification × 600) and section overview labelling for smooth muscles actin (D) Magnification × 200 inserts × 600.
Fig 2.
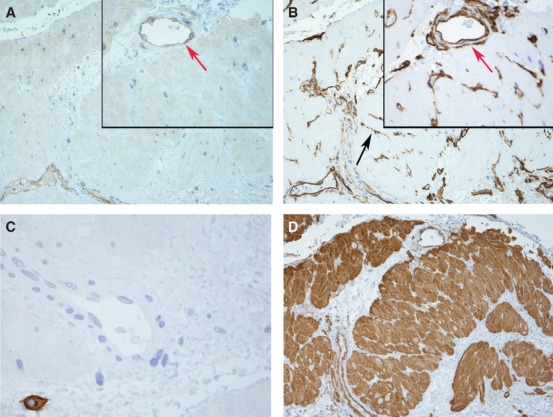
Representative sections of human pregnant myometrial preparation. Weak staining for SK3 (A), but strong positive staining for CD34 (B) corresponding to telocytes intermingling muscle bundles (black arrows). Pregnant myometrium showed loss of SK3 in telocytes. SK3 and CD34 were also detected in vascular endothelium (red arrows). Only normal mast cells stained for CD117 (C; magnification × 600). Section overview labelling for smooth muscles actin (D) Magnification × 200 inset ×600.
The double immunofluorescence labelling experiments, carried out using SK3 and CD34 antibodies, showed that telocytes displayed co-expression for SK3 and CD34 in human non-pregnant myometrium (Fig.3B); pregnant myometrium showed a lack of SK3 staining in telocytes; and vascular endothelium displayed co-expression for both SK3 and CD34 (Fig.3D). The appendiceal muscularis propria (positive tissue control) displayed the same reaction pattern: SK3+; CD34+; CD117−; and SMA- (single immunohistochemical investigations) and co- expression between SK3 and CD34 (double immunofluorescence labelling investigations) in telocytes intermingling smooth muscle bundles (Fig.3F).
Fig 3.
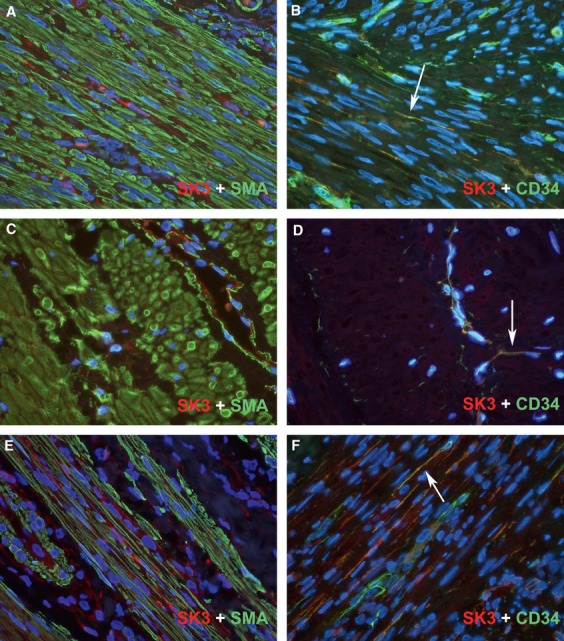
Double immune-fluorescence labelling for SK3 (red) and smooth muscles actin or CD34 (green) in human non-pregnant (A and B) and pregnant (C and D) myometrium and appendiceal muscularis propria (E and F). interstitial Cajal-like cells (ICLC) displayed co-expression for SK3 and CD34 (yellow reaction product) in human non-pregnant myometrium (B) and appendiceal muscularis propria (F) (arrows), whereas pregnant myometrium lacked SK3 in ICLC and only vascular endothelium displayed co-expression for both SK3 and CD34 (arrow-head). DAPI nuclear labelling in blue. Also, note the lower cellular density in pregnant myometrium. Magnification × 600.
Reference gene stability
To determine the relative expression of SK3 mRNA in pregnant and non-pregnant women, respectively, the stability of eight reference genes was evaluated. An M value was calculated for each candidate reference gene using the geNorm algorithm to describe the stability of the given gene in the myometrium of pregnant and non-pregnant women, independently (Fig.4). The candidate reference genes were ranked from most stable to least stable as follows: TOP1 > SDHA > YWHAZ > B2M > GAPDH > ACTB > 18S > SF3A1. Of the listed genes, TOP1, SDHA and YWHAZ were found to be stably expressed and therefore considered appropriate reference genes when comparing pregnant and non-pregnant myometrium. When the stability of reference genes in pregnant and non-pregnant myometrium was evaluated separately, the genes were ranked as follows: GAPDH > YWHAZ > ACTB > SDHA > TOP1 > SF3A1 > B2M > 18S, and SDHA > TOP1 > SF3A1 > YWHAZ > B2M > ACTB > GAPDH > 18S respectively (data not shown). In pregnant myometrium, all of the evaluated genes proved suitable as reference genes, whereas 18S was not a suitable reference gene in the case of non-pregnant myometrium. The qbasePlus software also determined the optimal number of reference genes required to calculate a normalization factor when comparing the two groups. In the case of the present experiment, in which tissue specimens from both pregnant and non-pregnant women were employed, this was achieved by the inclusion of two reference genes, namely TOP1 and SDHA.
Fig 4.
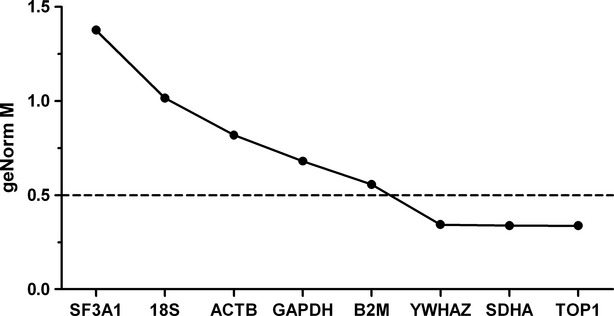
Ranking of eight candidate reference genes using geNorm. geNorm ranks the candidate reference genes based on the stability parameter M. The lower the M value, the higher the expression stability. The stability is calculated from both pregnant and non-pregnant myometrium (n = 22). A reference gene is considered stably expressed when M < 0.5.
Determination of SK3 mRNA expression levels in human myometrium
The relative amount of SK3 transcript in myometrium from pregnant and non-pregnant women was determined by normalizing to the geometric mean of SDHA and TOP1. The reference target stability measure M was 0.238 ± 0.08. The normalized amount of SK3 transcript present in pregnant and non-pregnant myometrium was found to be 0.53 (0.39–0.73) and 1.0 (0.67–1.50) respectively [mean (95% CI), n = 11]. Thus, the SK3 mRNA was down-regulated twofold in myometrium from pregnant compared to that from non-pregnant women (P = 0.016) (Fig.5A). Figure5B displays the relative amount of SK3 mRNA measured in each patient, showing a pronounced variation in the SK3 transcript levels within the non-pregnant group. In contrast, a more uniform pattern was observed within the group of pregnant women.
Fig 5.
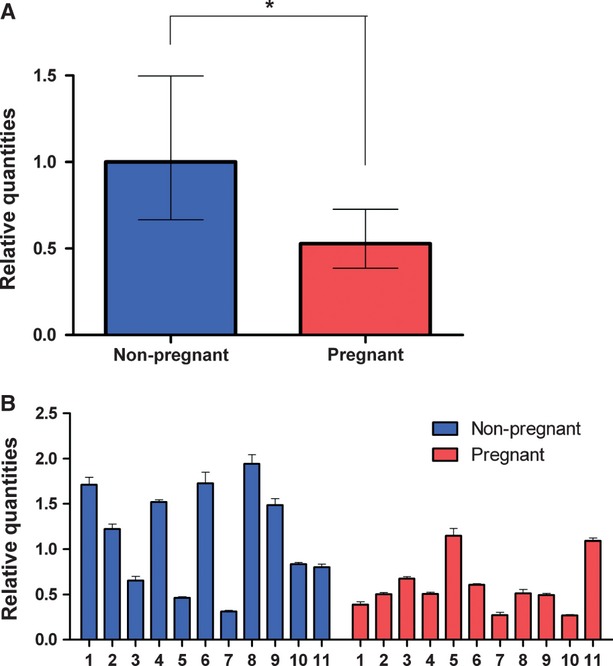
KCNN3 expression measured by qRT-PCR. The relative expression of KCNN3 is normalized to the geometric mean of the most stable reference genes, SDHA and TOP1. Bar chart showing expression of KCNN3 in non-pregnant (blue, n = 11) and pregnant (red, n = 11) (A) Bar chart showing KCNN3 expression in each patient (B) Values are presented as mean ± S.E.M. *P > 0.05.
Discussion
This study combines morphological and molecular biological data to localize and quantify SK3 channel expression in human myometrium from pregnant and non-pregnant women. To our knowledge, this is the first study to provide evidence that SK3 exclusively labels a distinct population of cells in the human myometrium, known as telocytes. This work suggests that SK3 activators reduce contractility in human myometrium by modulating ICLC function.
Our immunohistochemical data revealed that in non-pregnant myometrium SK3 immunoreactivity was observed in telocytes. The telocytes stained positive for CD34, but were negative to CD117 (ICC) and SMA (smooth muscle cells). Although CD34+ cells were also present in pregnant myometrium, they lacked SK3 immunoreactivity. Furthermore, the immunohistochemical results showed that SK3 expression in vascular endothelium was similar between the two groups.
Previous immunohistochemical studies have identified SK3 in myometrium without fully resolving the cellular localization 6. To our knowledge, we are the first group that has examined the precise cellular localization of SK3 channels in myometrium from pregnant and non-pregnant women using double immuno-labelling experiments. Furthermore, and in agreement with our observations SK3 channel protein has also been observed in CD34+ cells in the human gut 27, and Fujita et al. have shown that SK3 channels in the mouse intestine are colocalized with a fibroblast-like cell different from ICC 28.
However, previous studies showed apamin-modulated myocyte currents, which imply that SK channels are present in the myometrial SMC of pregnant rats and women 29,30. We cannot ignore the possibility that SK3 channels could be present in the SMC of human myometrium. One possible reason why we cannot detect SK channels in SMC using immunohistochemistry could be that the SK3 antibody used in our experiments is raised against the N-terminal of the SK3 channel. We are aware about the existence of different SK3 splice variants that differ in the N-terminals and it is feasible that the SK3 splice variant expressed in the myometrial SMC is different from that expressed in the ICLC. Furthermore, Tong et al. carefully reviewed much of the current knowledge regarding potassium channels in myometrium and outlines that there are some limitations when information is extrapolated from molecular data 31. qRT-PCR analysis demonstrated that SK3 channel mRNA is present in human myometrium, and that the expression is higher in non-pregnant compared to pregnant tissue. The gene expression of SK3 channels in human myometrial tissue has previously been investigated. Mazzone et al. 1,2 and Pierce & England 4 concluded that SK3 channels are down-regulated throughout pregnancy when comparing the relative level of SK3 transcript in pregnant and non-pregnant myometrium. However, they normalized SK3 transcript to 18S RNA (small subunit ribosomal RNA) and GAPDH respectively. According to our study, these reference genes are stably expressed within each group, but are not stably expressed between the two groups and are thus not optimal for normalization.
The reason why SK3 channel transcript level is lower in the uterus during pregnancy compared to that during non-pregnancy has been proposed to be the down-regulation of the SK3 channels. However, it is possible that hypertrophy and larger cell volume in the pregnant myometrium could account for these changes. Therefore, we are not sure whether the reduced amount of SK3 channel transcript is solely because of down-regulation during pregnancy, or whether it is lower in myometrium from pregnant women as a result of the decreased cell density of SMC and ICLC expressing SK3 channel.
Moreover, in the present study we found that SK3 expression varies within the non-pregnant group. This is in keeping with our immunohistochemical experiments, i.e. the myometrial preparations that showed a strong labelling of SK3, had a high expression of SK3 mRNA. Thus, the SK3 channel protein expression is in accordance with the mRNA expression. Interestingly, and in accordance with our study, Mazzone et al. also observed heterogeneity in SK3 mRNA expression within a group of human non-pregnant myometrium 2. One of the reasons for this variability could be the status of the hormonal cycle when the patients were operated and the tissue collected. There is consistent data about hormones influencing the expression of several ion channels including SK channels 3,32,33. More precisely, Pierce & England concluded that one of the mechanisms regulating SK3 channel expression occurs via transcriptional regulation through oestrogen modulation 4. However, SK3 transcript variation is not easily linked with hormonal status in our non-pregnant patients as many of their underlying disorders were associated with hormone imbalance. Also the age of the patients could be another reason for the SK3 mRNA expression variability.
Our qRT-PCR data has provided two main findings: first, and in agreement with others, that SK3 channel transcript is lower in pregnant compared to non-pregnant myometrium; and second, that the SK3 channel transcript varies greatly within the group of non-pregnant myometrium, a possibility that has previously been underappreciated. Our present study has shown that SK3 channels are expressed in CD34+ cells and are down-regulated during pregnancy. As we have recently demonstrated, SK channel activation contributes to the relaxation of human myometrium in vitro 9. Thus, although SK channels modulate uterine contractility, this does not occur directly through myocyte interaction. We therefore speculate that CD34+ cells may represent a novel mechanism controlling the excitability of the human uterine musculature. As hypothesized by Cretoiu et al. and Allix et al. a possible mechanism could be via a tyrosine-kinase independent signalling pathway 34,35. However, further studies are needed to address the functional aspects of CD34+ cells and their coupling with SMC. Such studies will lead to a better understanding of the role played by telocytes in the myometrium.
Acknowledgments
The authors would like to thank Michael Bzorek from the Department of Clinical Pathology, Naestved Hospital for his valuable help in immunohistochemical procedures and Majken M. Janssen from the Smooth Muscle Research Center, Department of Clinical Biochemistry, Koege Hospital for her outstanding technical assistance with the qRT-PCR experiments. This study was partially supported by the Region Sjaelland Research Fund.
Conflict of interest
The authors confirm that there are no conflicts of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1 Representative immunostaining of formalin-fixed paraffin-embedded human non-pregnant (A) and pregnant (B) myometrium and appendiceal muscularis propia (C) showing CD117 staining. Only normal mast cell stained for CD117 in human myometrium. In contrast, ICC show CD117+ staining in appendiceal muscularis propia (C). Magnification × 600.
References
- Mazzone JN, Kaiser RA, Buxton ILO. Calcium-activated potassium channel expression in human myometrium: effect of pregnancy. Proc West Pharmacol Soc. 2002;45:184–6. [PubMed] [Google Scholar]
- Mazzone J, Buxton IL. Changes in small conductance potassium channel expression in human myometrium during pregnancy measured by RT-PCR. Proc West Pharmacol Soc. 2003;46:74–7. [PubMed] [Google Scholar]
- Chen MX, Gorman SA, Benson B, et al. Small and intermediate conductance Ca(2+)-activated K+ channels confer distinctive patterns of distribution in human tissues and differential cellular localisation in the colon and corpus cavernosum. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:602–15. doi: 10.1007/s00210-004-0934-5. [DOI] [PubMed] [Google Scholar]
- Pierce SL, England SK. SK3 channel expression during pregnancy is regulated through estrogen and Sp factor-mediated transcriptional control of the KCNN3 gene. Am J Physiol Endocrinol Metab. 2010;299:E640–6. doi: 10.1152/ajpendo.00063.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond CT, Sprengel R, Bissonnette JM, et al. Respiration and parturition affected by conditional overexpression of the Ca2+-activated K+ channel subunit, SK3. Science. 2000;289:1942–6. doi: 10.1126/science.289.5486.1942. [DOI] [PubMed] [Google Scholar]
- Brown A, Cornwell T, Korniyenko I, et al. Myometrial expression of small conductance Ca2+-activated K+ channels depresses phasic uterine contraction. Am J Physiol Cell Physiol. 2007;292:C832–40. doi: 10.1152/ajpcell.00268.2006. [DOI] [PubMed] [Google Scholar]
- Pierce S, Kresowik J, Lamping K, et al. Overexpression of SK3 channels dampens uterine contractility to prevent preterm labor in mice. Biol Reprod. 2008;78:1058–63. doi: 10.1095/biolreprod.107.066423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell BF, Taggart MJ. Are animal models relevant to key aspects of human parturition? Am J Physiol Regul Integr Comp Physiol. 2009;297:R525–45. doi: 10.1152/ajpregu.00153.2009. [DOI] [PubMed] [Google Scholar]
- Rosenbaum ST, Larsen T, Joergensen JC, et al. Relaxant effect of a novel calcium-activated potassium channel modulator on human myometrial spontaneous contractility in vitro. Acta Physiol (Oxf) 2012;205:247–54. doi: 10.1111/j.1748-1716.2011.02384.x. [DOI] [PubMed] [Google Scholar]
- Popescu LM, Faussone-Pellegrini MS. TELOCYTES - a case of serendipity: the winding way from Interstitial Cells of Cajal (ICC), via Interstitial Cajal-Like Cells (ICLC) to TELOCYTES. J Cell Mol Med. 2010;14:729–40. doi: 10.1111/j.1582-4934.2010.01059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suciu L, Nicolescu MI, Popescu LM. Cardiac telocytes: serial dynamic images in cell culture. J Cell Mol Med. 2010;14:2687–92. doi: 10.1111/j.1582-4934.2010.01185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Zhang Y, Wen X, et al. Telocytes accompanying cardiomyocyte in primary culture: two- and three-dimensional culture environment. J Cell Mol Med. 2010;14:2641–5. doi: 10.1111/j.1582-4934.2010.01186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gherghiceanu M, Popescu LM. Cardiac telocytes - their junctions and functional implications. Cell Tissue Res. 2012;348:265–79. doi: 10.1007/s00441-012-1333-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusu MC, Pop F, Hostiuc S, et al. Telocytes form networks in normal cardiac tissues. Histol Histopathol. 2012;27:807–16. doi: 10.14670/HH-27.807. [DOI] [PubMed] [Google Scholar]
- Suciu L, Popescu LM, Gherghiceanu M, et al. Telocytes in human term placenta: morphology and phenotype. Cells Tissues Organs. 2010;192:325–39. doi: 10.1159/000319467. [DOI] [PubMed] [Google Scholar]
- Gevaert T, De Vos R, Van Der Aa F, et al. Identification of telocytes in the upper lamina propria of the human urinary tract. J Cell Mol Med. 2012;16:2085–93. doi: 10.1111/j.1582-4934.2011.01504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu LM, Gherghiceanu M, Suciu LC, et al. Telocytes and putative stem cells in the lungs: electron microscopy, electron tomography and laser scanning microscopy. Cell Tissue Res. 2011;345:391–403. doi: 10.1007/s00441-011-1229-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Li H, Manole CG, et al. Telocytes in trachea and lungs. J Cell Mol Med. 2011;15:2262–8. doi: 10.1111/j.1582-4934.2011.01404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinescu ME, Gherghiceanu M, Suciu L, et al. Telocytes in pleura: two- and three-dimensional imaging by transmission electron microscopy. Cell Tissue Res. 2011;343:389–97. doi: 10.1007/s00441-010-1095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantarero I, Luesma MJ, Junquera C. The primary cilium of telocytes in the vasculature: electron microscope imaging. J Cell Mol Med. 2011;15:2594–600. doi: 10.1111/j.1582-4934.2011.01312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceafalan L, Gherghiceanu M, Popescu LM, et al. Telocytes in human skin - are they involved in skin regeneration? J Cell Mol Med. 2012;16:1405–20. doi: 10.1111/j.1582-4934.2012.01580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolescu MI, Popescu LM. Telocytes in the interstitium of human exocrine pancreas: ultrastructural evidence. Pancreas. 2012;41:949–56. doi: 10.1097/MPA.0b013e31823fbded. [DOI] [PubMed] [Google Scholar]
- Nicolescu MI, Bucur A, Dinca O, et al. Telocytes in parotid glands. Anat Rec. 2012;295:378–85. doi: 10.1002/ar.21540. [DOI] [PubMed] [Google Scholar]
- Cretoiu D, Cretoiu SM, Simionescu AA, et al. Telocytes, a distinct type of cell among the stromal cells present in the lamina propria of jejunum. Histol Histopathol. 2012;27:1067–78. doi: 10.14670/HH-27.1067. [DOI] [PubMed] [Google Scholar]
- Hatta K, Huang ML, Weisel RD, et al. Culture of rat endometrial telocytes. J Cell Mol Med. 2012;16:1392–6. doi: 10.1111/j.1582-4934.2012.01583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzorek M, Stamp IM, Petersen BL, et al. Use of commercially available rabbit monoclonal antibodies for immunofluorescence double staining. Appl Immunohistochem Mol Morphol. 2008;16:387–92. doi: 10.1097/PAI.0b013e3181594ec6. [DOI] [PubMed] [Google Scholar]
- Vanderwinden JM, Rumessen JJ, de Kerchove d'Exaerde A, et al. Kit-negative fibroblast-like cells expressing SK3, a Ca2+-activated K+ channel, in the gut musculature in health and disease. Cell Tissue Res. 2002;310:349–58. doi: 10.1007/s00441-002-0638-4. [DOI] [PubMed] [Google Scholar]
- Fujita A, Takeuchi T, Jun H, et al. Localization of Ca2+-activated K+ channel, SK3, in fibroblast-like cells forming gap junctions with smooth muscle cells in the mouse small intestine. J Pharmacol Sci. 2003;92:35–42. doi: 10.1254/jphs.92.35. [DOI] [PubMed] [Google Scholar]
- Wang SY, Yoshino M, Sui JL, et al. Potassium currents in freshly dissociated uterine myocytes from nonpregnant and late-pregnant rats. J Gen Physiol. 1998;112:737–56. doi: 10.1085/jgp.112.6.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble K, Floyd R, Shmygol A, et al. Distribution, expression and functional effects of small conductance Ca-activated potassium (SK) channels in rat myometrium. Cell Calcium. 2010;47:47–54. doi: 10.1016/j.ceca.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Tong WC, Choi CY, Karche S, et al. A computational model of the ionic currents, Ca2+ dynamics and action potentials underlying contraction of isolated uterine smooth muscle. PLoS ONE. 2011;6:e18685. doi: 10.1371/journal.pone.0018685. . doi: 10.1371/journal.pone.0018685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum LA, Pierce SL, England SK, et al. The contribution of Kv7 channels to pregnant mouse and human myometrial contractility. J Cell Mol Med. 2011;15:577–86. doi: 10.1111/j.1582-4934.2010.01021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch MA, Kelly MJ, Rønnekleiv OK. Distribution, neuronal colocalization, and 17beta-E2 modulation of small conductance calcium-activated K(+) channel (SK3) mRNA in the guinea pig brain. Endocrinology. 2002;143:1097–107. doi: 10.1210/endo.143.3.8708. [DOI] [PubMed] [Google Scholar]
- Cretoiu SM, Simionescu AA, Caravia L, et al. Complex effects of imatinib on spontaneous and oxytocin-induced contractions in human non-pregnant myometrium. Acta Physiol Hung. 2011;98:329–38. doi: 10.1556/APhysiol.98.2011.3.10. [DOI] [PubMed] [Google Scholar]
- Allix S, Reyes-Gomez E, Aubin-Houzelstein G, et al. Uterine contractions depend on KIT-positive interstitial cells in the mouse: genetic and pharmacological evidence. Biol Reprod. 2008;79:510–7. doi: 10.1095/biolreprod.107.066373. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Representative immunostaining of formalin-fixed paraffin-embedded human non-pregnant (A) and pregnant (B) myometrium and appendiceal muscularis propia (C) showing CD117 staining. Only normal mast cell stained for CD117 in human myometrium. In contrast, ICC show CD117+ staining in appendiceal muscularis propia (C). Magnification × 600.


