Abstract
Renal interstitial cells play an important role in the physiology and pathology of the kidneys. As a novel type of interstitial cell, telocytes (TCs) have been described in various tissues and organs, including the heart, lung, skeletal muscle, urinary tract, etc. (www.telocytes.com). However, it is not known if TCs are present in the kidney interstitium. We demonstrated the presence of TCs in human kidney cortex interstitium using primary cell culture, transmission electron microscopy (TEM) and in situ immunohistochemistry (IHC). Renal TCs were positive for CD34, CD117 and vimentin. They were localized in the kidney cortex interstitial compartment, partially covering the tubules and vascular walls. Morphologically, renal TCs resemble TCs described in other organs, with very long telopodes (Tps) composed of thin segments (podomers) and dilated segments (podoms). However, their possible roles (beyond intercellular signalling) as well as their specific phenotype in the kidney remain to be established.
Keywords: telocytes, telopodes, human kidney cortex, interstitial cells, CD34, CD117
Introduction
Telocytes (TCs) are a distinct interstitial cell population, first described in 2010 1,2. They are found in several mammalian and human organs 3–20. The main characteristic of TCs is the presence of telopodes (Tps), visible using electron microscopy. Tps are composed of alternating thin segments (podomers) and thick segments (podoms). Renal interstitial cells are involved in many diseases, such as acute interstitial nephritis 21, chronic allograft rejection 22 and ischaemia reperfusion injury 23. Traditionally, fibroblasts and immune cells are considered the main interstitial cells in kidneys 24,25. However, renal stromal cells have great morphological plasticity. In the past, these cells were arbitrarily called ‘fibroblasts’, despite the fact that the main function of fibroblasts is structural (to produce collagen and collagen fibrils). In normal kidney cortex interstitium, collagen is not at all abundant. This could suggest that the kidney interstitial cells are heterogeneous in their phenotype. Up to date, there are no reports regarding the presence of TCs in the kidney interstitium. This study aimed to investigate the presence of TCs in the human kidney cortex, using primary cell culture, vital cell staining with Janus Green B, mitochondrial labelling using Mito Tracker Green, immunocytochemistry, transmission electron microscopy (TEM), and in situ immunohistochemistry (IHC).
Materials and methods
Tissue samples
Human kidney cortex samples were obtained from five patients, aged 40–64 years, who suffered from malignant kidney tumours and consented to radical nephrectomy. The non-neoplastic parts of the resection specimens were confirmed by morphopathology and used for this study.
Cell culture
Sterile samples were minced into small pieces of about 1 mm3 and washed three times with phosphate-buffered saline (PBS, Gibco, Portland, Oregon, USA). Samples were further digested for 4 hrs on an orbital shaker at 37°C, with 10 mg/ml collagenase type II (Sigma-Aldrich, St. Louis, Missouri, USA) and 2000 U/ml deoxyribonuclease I (Sigma-Aldrich) in PBS, without Ca2+ and Mg2+. Dispersed cells were collected by centrifugation at 284 × g and separated by filtration through 40 μm diameter cell strainers (BD Falcon, San Jose, CA, USA). Cells were cultured in DMEM/F12 (Gibco, Portland, Oregon, USA), supplemented with 10% FBS. The medium was changed every 48 hrs and a phase contrast microscope (Olympus 1x51; Olympus, Tokyo, Japan) was used to observe the growth of TCs.
Staining and mitochondrial labelling of cultured cells
Cells were washed in pre-warmed phenol red-free DMEM, and incubated in 0.02% Janus Green B (Sigma-Aldrich) for 30 min. Mito Tracker Green FM (Beyotime, Haimen, China), a lipophilic, selective dye that can be concentrated by active mitochondria, was used for mitochondrial labelling. Cells were incubated in 100 nmol/l Mito Tracker Green for 30 min. TCs were examined and photographed using fluorescence microscopy (450–490 nm excitation light, 520 nm barrier filter, Olympus 1x51; Olympus).
Immunocytochemistry
Cells grown on cover slips were fixed in 4% paraformaldehyde for 10 min., cold preserved over night at 4°C, then incubated in PBS for another 30 min. at room temperature. Cells were then incubated in 5% BSA in PBS for 1 hr at room temperature. The cells were incubated with the primary antibodies for 60 min. at 37°C, using rat anti-human CD34 (1:100; Santa Cruz, Santa Cruz, CA, USA), rabbit anti-human CD117 (1:10; Abcam, Cambridge, UK), and rabbit anti-human vimentin (1:200; Abcam). After three serial rinses with PBS, the primary antibodies were detected with secondary antibodies conjugated to fluorescein isothiocyanate (FITC) or tetraethyl rhodamine isothiocyanate (TRITC; Jackson, Lancaster, PA, USA). Finally, the nuclei were counterstained with 1 mg/ml 4′, 6-diamidino-2-phenylindole (DAPI) (Sigma-Aldrich). Negative controls were obtained by following the same protocol while omitting the primary antibodies. Samples were examined under a phase contrast microscope (Olympus BX51; Olympus) equipped with appropriate fluorescence filters.
Transmission electron microscopy
Human kidney samples were obtained using a lateral, cutting through-type autobiopsy gun (MG15-22; Bard, Murray Hill, NJ, USA) before the renal vascular pedicle was clamped during radical nephrectomy. Tissue samples were initially fixed in 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.2), then post-fixed in 1.0% OsO4 (Polysciences Inc., Niles, Illinois, USA) for 1 hr. Samples were then rinsed extensively in ddH20, dehydrated in a graded series of ethanol, and embedded in Eponate 812 resin (Ted Pella Inc., Redding, CA, USA). The embedded samples were dried by heat with a graded temperature. Then, sections of 50 nm were cut using a Leica Ultracut UCT ultramicrotome (Leica Microsystems Inc, LKB-II, Germany), stained with a 3% solution of uranyl acetate and lead citrate, and mounted on formvar-coated 50 mesh grids. Prepared tissue samples were observed using a TEM (Philips model CM120; Philips, Amsterdam, Holland). Digital pictures (2048 × 2048 pixels, 4 MB, and uncompressed grayscale TIFF files) were obtained using a high resolution ratio digital camera connected to the TEM.
In situ immunohistochemistry
Samples were fixed in 4% formalin and embedded in paraffin according to routine histology protocols. Sequential 5 μm sections were deparaffinized in xylene, hydrated in alcohol series, and washed in phosphate-buffered solution at pH 7.4. Antigen retrieval was performed by heating in TRIS-citrate buffer for 40 min. at 96°C. The sections were set aside to reach room temperature, and then washed in PBS. Endogenous peroxidase blocking was completed using 3% H2O2-methanol, and then the sections were incubated in 5% BSA for 30 min. The primary antibodies used were the following: CD117 (1:50; Abcam), vimentin (1:500; Abcam), CD34 (1:200; Santa Cruz), CD31 (1:100; Dako, Glostrup, Denmark), D2–40 (1:200; Dako, Glostrup, Denmark), and tryptase (1:100; Abcam). CD31 and D2–40 were used to exclude endothelial staining of CD34 26,27 and tryptase to exclude mast cell staining of CD117 28.
Sections were incubated with primary antibody at 4°C overnight and HRP-conjugated secondary antibodies for 60 min. at room temperature. Then, they were stained with 3, 3′–diaminobenzidine followed by haematoxylin counterstaining. The same areas of serial sections were observed and photographed using an Olympus microscope (1 × 71) equipped with a digital camera (Olympus dp72; Olympus). Negative controls were obtained by following the same protocol while omitting the primary antibodies. Positive controls were obtained using gastrointestinal stromal tumour samples expressing CD117, CD34 and vimentin.
Results
Cell culture
Under phase contrast microscope, we observed spindle-shaped cells with long, thin, and moniliform prolongations. As shown in Figure1A, a characteristic cell has one long, thin, and moniliform prolongation consisting of alternating thin and thick segments (podomers and podoms respectively). Another cell with several such prolongations was also observed (Fig.1B). The shapes of cell bodies changed from pyriform (Fig.1A) to polygonous (Fig.1B) depending on the number of prolongations. These cells closely resemble the TCs described in other organs, suggesting the presence of TCs in the human kidney cortex.
Fig 1.
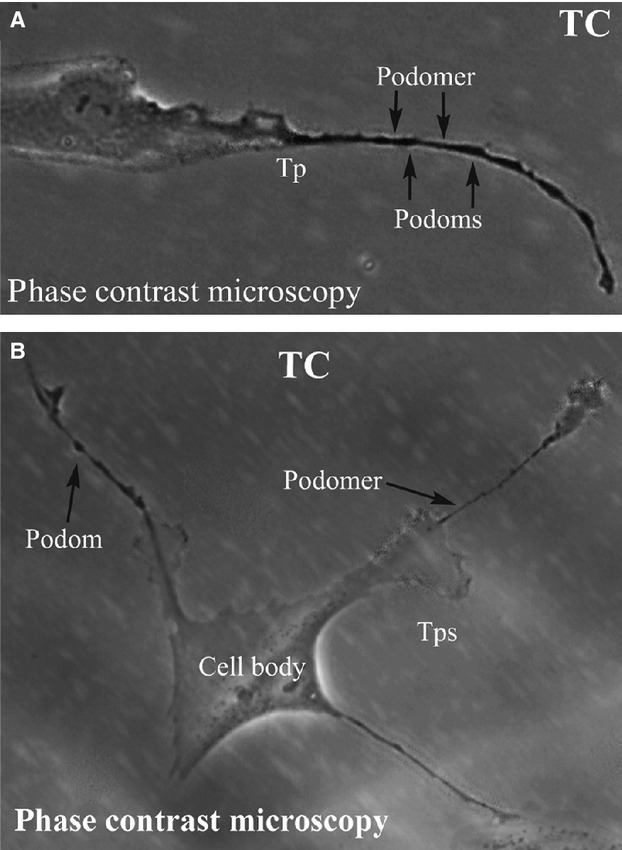
(A) and (B) Phase contrast microscopy of kidney TCs in primary culture. Note the typically very long Tps (over 50 μm). Also, the specific structure of Tps is obvious: alternation of dilations (podoms) with thin segments (podomers). Direct magnification: 400×. TC: Telocyte; Tp: Telopode.
Vital cell staining and mitochondrial labelling
Both Janus Green B (Fig.2A) and Mito Tracker Green (Fig.2B) illustrated the high concentration of mitochondria in the cultured cells. When compared to the thin segments (podomers), the dilated segments (podoms) of the prolongations have brighter fluorescent signals (Fig.2B). Our evaluation of the human kidney cortex demonstrated a greater concentration of mitochondria within the thick segments of prolongations and around the nucleus (Fig.2), as previously demonstrated by Popesu et al. in TCs from different organs 6,8,9. This similarity of mitochondrial distribution may suggest possible roles for kidney TCs.
Fig 2.
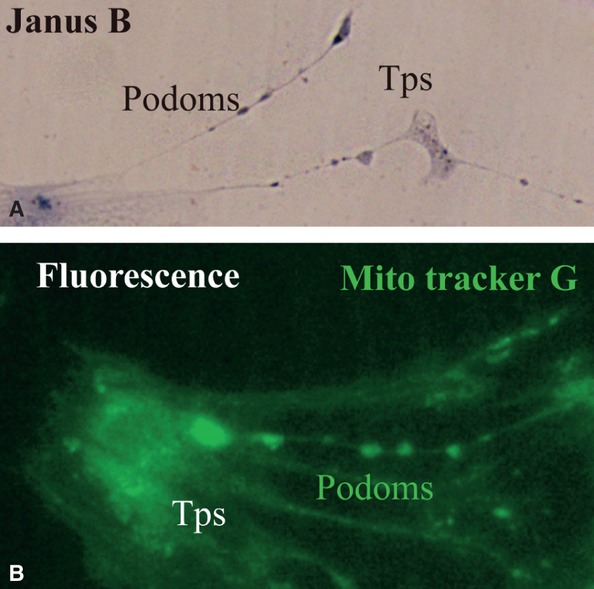
(A) and (B) Kidney TCs in primary culture. (A) Vital staining for mitochondria with Janus Green B; note the podoms coloured blue-brown because of the presence of mitochondria. (B) Fluorescence microscopy using Mito Tracker Green as molecular probe for the presence of mitochondria; note the moniliform aspect of Tps as a result of accumulation of mitochondria in podoms. TC: Telocyte; Tp: Telopode; Mito Tracker G: Mito Tracker Green.
Immunocytochemistry
The cultured cells positively expressed CD117, CD34 and vimentin (Fig.3). The alternating thick and thin segments of their prolongations are shown by fluorescence (Fig.3A and C). Staining with CD34 (Fig.3B) did not have the same colour as staining with CD117 (Fig.3A), when both were labelled with FITC, as the fluorescence colour appeared yellowish compared with CD117 staining. This phenomenon might be related to expression differences between CD34 and CD117.
Fig 3.
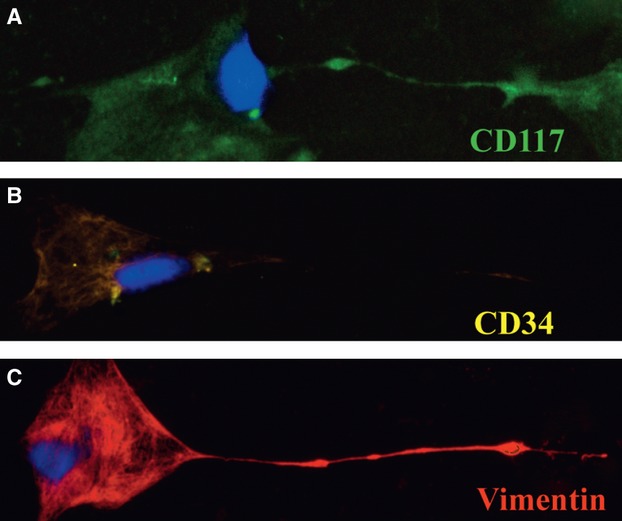
Immunofluorescence labelling for CD117, CD34 and vimentin of cultured cells. (A) FITC labelling for CD117 (green). (B) FITC labelling for CD34 (yellow). (C) TRITC labelling for vimentin (red). DAPI staining for nuclei (blue) in (A), (B), and (C). FITC: fluorescein isothiocyanate; TRITC: tetraethyl rhodamine isothiocyanate; DAPI: 4′, 6-diamidino-2-phenylindole.
Transmission electron microscopy
Cells with long, thin, and moniliform prolongations in the human kidney cortex interstitium are shown in Figures4A and 5A. These cells are located around tubules and vessels with their prolongations wrapping around the endothelium (Figs4 and 5). The prolongations suddenly emerge from cell bodies and are extremely long and very sinuous. The dilated segment of the prolongation is clearly displayed in Figure5C. The mitochondria, lamellar body, microfilaments, caveolae and vesicle are seen in high magnification images (Figs4B–D and 5B, C). Other organelles, such as the rough endoplasmic reticulum, Golgi body and ribosomes, are rarely seen in our images. These cells tend to have more than one prolongation in vivo. Their shapes change from spindle to triangular, depending on the number of prolongations.
Fig 4.
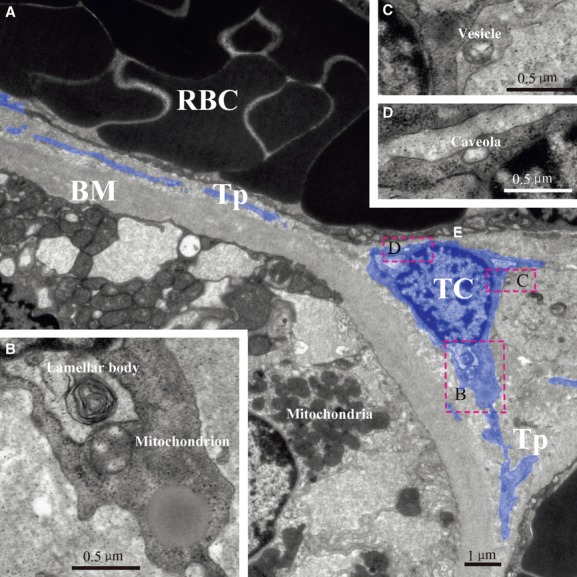
TEM image of kidney telocyte. There is a typical telocyte between a tubule and a vessel (A). Mitochondrion and lamellar body are found near nuclear (B). Shed vesicle and caveola could also be found after magnification (C) and (D). TC: telocyte; Tp: telopode; RBC: red blood cell; E: endothelium; BM: basement membrane.
Fig 5.
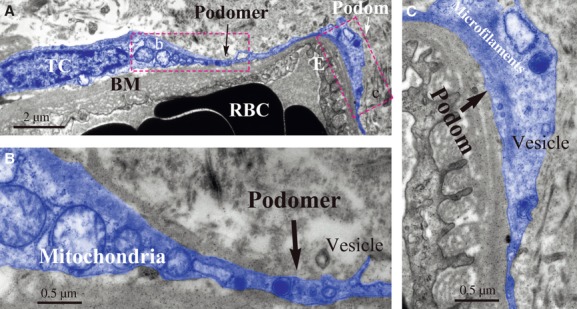
TEM image of kidney telocyte. A typical telocyte with Tps is around a blood capillary located in kidney interstitium (A). Note Tps which prolong along the basement membrane of vessel. Details of its podomer and podom are presented (B) and (C). Shed vesicle is next to podomer and obvious mitochondria are next to nuclear (B). In podom, there are microfilaments and vesicle (C). TC: telocyte; RBC: red blood cell; E: endothelium; BM: basement membrane.
In situ immunohistochemistry
Immunostaining of CD117, CD34, vimentin, CD31, D2–40 and tryptase of the same area on serial sections of cortex are shown in Figure6. According to our results, cells with a typical TC morphology, and with positive expression for CD117 (Fig.6A), CD34 (Fig.6B) and vimentin (Fig.6C) are located in the interstitium of the kidney cortex. These cells, together with their prolongations, surround renal epithelial tubules and/or vessels. They were negative for expression of CD31 (Fig.6D), D2–40 (Fig.6E) and tryptase (Fig.6F), which excluded endothelia and mast cell staining. The distribution of these cells, as observed by IHC, was also confirmed by TEM.
Fig 6.
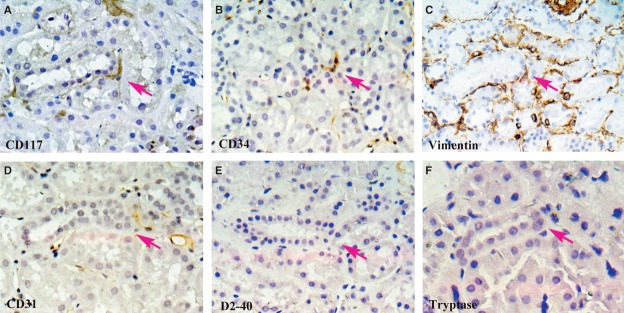
In situ IHC of CD117, CD34, vimentin, CD31, D2–40 and Tryptase on serial slides. One cell with positive expression of CD117 can be seen in the kidney interstitium (A; magenta arrow). It has three long and thin Tps. Positive expressions of CD34 and vimentin can be observed in the same area of serial slides (B) and (C). Staining results of CD31, D2–40 and tryptase are presented (D)–(F) on serial slides. Their negative expressions in same area help us exclude mast cell staining of CD117 and endothelial cell staining of CD34. Magnification: 400×.
Discussion
The presence of TCs was previously documented in different organs, including the epicardium 3, endocardium 4, myocardium 5, term placenta 6, duodenum 7, skeletal muscle 8, trachea and lung 9, vasculature 10, pleura 11, trigeminal ganglion 12, myometrium 13, endometrium 14, skin 15, urinary tract 16, parotid glands 17, meninges and choroid plexus 18, pancreas 19 and jejunum 20. Yet, the presence of TCs has not been reported in the mammalian kidney cortex.
Our study aimed to establish whether or not TCs are present in kidney interstitium, using cell culture, TEM and IHC. Previous studies 1–20,29 established six distinct features of TCs as the following: (1) localization within the interstitium, (2) the shape of the cell body could be pyriform, fusiform, triangular, or quadrangular, in accordance with the number of cellular prolongations (Tps), (3) having Tps: (i) typical prolongations, being very long and very thin, with a moniliform silhouette (alternation of podomers–thin segments–and podoms–dilated segments); (ii) a dichotomic branching pattern; (iii) inter-connected by homo-cellular junctions (but also connected with other neighbouring cells, by heterocellular junctions); (iv) forming a labyrinthine system, (4) podoms harbour the so-called ‘calcium uptake/releasing unit’, (5) positive for CD117, CD34 and vimentin, and (6) extremely low expression of microRNA-193.
Using cell culture, TEM, and in situ IHC, we documented that cells with a morphology similar to that described for TCs in other organs were present in the human kidney cortex interstitium. Morphologically, the cells that we isolated and identified met all the criteria previously established for TCs and for their Tps. The specific alternation of thin segments and dilated segments was observed (Fig.1). The vital staining with Mito Tracker Green confirmed the existence of mitochondria within the dilations (Fig.2B). These cells had positive expression of CD117, CD34 and vimentin (Fig.3). TEM images established that these cells were located proximal to vessels and tubules in the interstitium. In some instances, their prolongations extended along the basement membrane (Figs4A and 5A). In situ IHC results suggested the same distribution in the human kidney cortex (Fig.6A–C). Negative expression of CD31, D2–40 and tryptase further confirmed the presence of TCs by excluding endothelial and mast cells (Fig.6D–F).
Previous studies stated that kidney interstitial cells only consisted of fibroblasts, dendritic cells, macrophages and lymphocytes 24,25. The cells described here were also located in the kidney interstitium; although, they had characteristic prolongations (Tps) and specific biomarkers (CD117, CD34 and vimentin), which can distinguish them from fibroblasts and other cells in both morphology and phenotype. During our research, we also obtained samples from the medulla of human kidneys and examined them through cell culture, immunocytochemistry, in situ IHC and TEM following the same protocols as those for the cortex. Results were contradictory. During cell culture, we found cells with the suggestive appearance of TCs. However, no typical TCs were observed in our TEM images and in situ IHC showed no positive expression of CD117 in the medulla. We speculate that the quantity of TCs in the medulla was insufficient for TEM and in situ IHC to be detected.
In conclusion, our study established the presence of TCs in the human kidney cortex interstitium, mainly locating around renal tubules and vessels. Yet, the precise function of TCs remains to be established. In several studies on TCs, potential roles have been proposed 30–32. The specific distribution of TCs might be related to their function. Previous studies demonstrated close relationships between TCs and other cells 33–39. In both heart 40 and skeletal muscle 41, TCs were found in stem cell niches. Furthermore, TCs were found accompanying cardiomyocyte progenitor cells in different stages of development 40. Our results demonstrated that TCs are located close to tubules and blood capillaries in the kidney cortex. The presence of extracellular vesicles is considered an important pathway of cell interaction 42–44. Extracellular vesicles, in very close vicinity to the cellular bodies of TCs or at different levels of Tps, have been described by Popescu et al. in other organs 3,8,10,11,14,16,19. This suggests the interactions of TCs with neighbouring cells. Our TEM images also show extracellular vesicles of TCs within the kidney interstitium, suggesting that kidney TCs are also involved in cell-to-cell communication, protection, information exchange and so on 45, through a paracrine/juxtacrine mechanism.
Acknowledgments
This study was funded by the National Nature Science Foundation of China (81070595, 81170695, 81100533, 81100524), Science and Technology Commission of Shanghai Municipality (09411952000, 10DZ2212000), Foundation of Ministry of Health of the People's Republic of China (IHECC07-001), Special Funds of 211 works of Fudan University (211Med-XZZD02). This study was approved by the Bioethics Committee of Zhongshan Hospital of Fudan University, Shanghai, according to generally accepted international standards (no. 2011-209). The authors thank the Department of Electronic Microscopy, Shanghai Medical College, Fudan University, for their technical assistance in TEM; Professor L.M. Popescu and Dr. Catalin G. Manole at the Department of Molecular Medicine, ‘Victor Babe’ National Institute of Pathology, Bucharest, Romania for their generous and valuable suggestions.
Funding: This study was funded by the National Nature Science Foundation of China (81070595, 81170695, 81100533, 81100524), Science and Technology Commission of Shanghai Municipality (09411952000, 10DZ2212000), Foundation of Ministry of Health of the People's Republic of China (IHECC07-001), Special Funds of 211 works of Fudan University (211Med-XZZD02).
Conflict of interest
The authors declare no conflict of interest.
References
- Popescu LM, Faussone-Pellegrini MS. TELOCYTES - a case of serendipity: the winding way from Interstitial Cells of Cajal (ICC), via Interstitial Cajal-Like Cells (ICLC) to TELOCYTES. J Cell Mol Med. 2010;14:729–40. doi: 10.1111/j.1582-4934.2010.01059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostin S. Myocardial telocytes: a specific new cellular entity. J Cell Mol Med. 2010;14:1917–21. doi: 10.1111/j.1582-4934.2010.01111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu LM, Manole CG, Gherghiceanu M, et al. Telocytes in human epicardium. J Cell Mol Med. 2010;14:2085–93. doi: 10.1111/j.1582-4934.2010.01129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gherghiceanu M, Manole CG, Popescu LM. Telocytes in endocardium: electron microscope evidence. J Cell Mol Med. 2010;14:2330–4. doi: 10.1111/j.1582-4934.2010.01133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suciu L, Nicolescu MI, Popescu LM. Cardiac telocytes: serial dynamic images in cell culture. J Cell Mol Med. 2010;14:2687–92. doi: 10.1111/j.1582-4934.2010.01185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suciu L, Popescu LM, Gherghiceanu M, et al. Telocytes in human term placenta: morphology and phenotype. Cells Tissues Organs. 2010;192:325–39. doi: 10.1159/000319467. [DOI] [PubMed] [Google Scholar]
- Cantarero Carmona I, Luesma Bartolome MJ, Junquera Escribano C. Identification of telocytes in the lamina propria of rat duodenum: transmission electron microscopy. J Cell Mol Med. 2011;15:26–30. doi: 10.1111/j.1582-4934.2010.01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu LM, Manole E, Serboiu CS, et al. Identification of telocytes in skeletal muscle interstitium: implication for muscle regeneration. J Cell Mol Med. 2011;15:1379–92. doi: 10.1111/j.1582-4934.2011.01330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Li H, Manole CG, et al. Telocytes in trachea and lungs. J Cell Mol Med. 2011;15:2262–8. doi: 10.1111/j.1582-4934.2011.01404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantarero I, Luesma MJ, Junquera C. The primary cilium of telocytes in the vasculature: electron microscope imaging. J Cell Mol Med. 2011;15:2594–600. doi: 10.1111/j.1582-4934.2011.01312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinescu ME, Gherghiceanu M, Suciu L, et al. Telocytes in pleura: two- and three-dimensional imaging by transmission electron microscopy. Cell Tissue Res. 2011;343:389–97. doi: 10.1007/s00441-010-1095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusu MC, Pop F, Hostiuc S, et al. The human trigeminal ganglion: c-kit positive neurons and interstitial cells. Ann Anat. 2011;193:403–11. doi: 10.1016/j.aanat.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Cretoiu SM, Simionescu AA, Caravia L, et al. Complex effects of imatinib on spontaneous and oxytocin-induced contractions in human non-pregnant myometrium. Acta Physiol Hung. 2011;98:329–38. doi: 10.1556/APhysiol.98.2011.3.10. [DOI] [PubMed] [Google Scholar]
- Hatta K, Huang ML, Weisel RD, et al. Culture of rat endometrial telocytes. J Cell Mol Med. 2012;16:1392–6. doi: 10.1111/j.1582-4934.2012.01583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceafalan L, Gherghiceanu M, Popescu LM, et al. Telocytes in human skin - are they involved in skin regeneration? J Cell Mol Med. 2012;16:1405–20. doi: 10.1111/j.1582-4934.2012.01580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevaert T, De Vos R, Van Der Aa F, et al. Identification of telocytes in the upper lamina propria of the human urinary tract. J Cell Mol Med. 2012;16:2085–93. doi: 10.1111/j.1582-4934.2011.01504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolescu MI, Bucur A, Dinca O, et al. Telocytes in parotid glands. Anat Rec (Hoboken) 2012;295:378–85. doi: 10.1002/ar.21540. [DOI] [PubMed] [Google Scholar]
- Popescu BO, Gherghiceanu M, Kostin S, et al. Telocytes in meninges and choroid plexus. Neurosci Lett. 2012;516:265–9. doi: 10.1016/j.neulet.2012.04.006. [DOI] [PubMed] [Google Scholar]
- Nicolescu MI, Popescu LM. Telocytes in the interstitium of human exocrine pancreas: ultrastructural evidence. Pancreas. 2012;41:949–56. doi: 10.1097/MPA.0b013e31823fbded. [DOI] [PubMed] [Google Scholar]
- Cretoiu D, Cretoiu SM, Simionescu AA, et al. Telocytes, a distinct type of cell among the stromal cells present in the lamina propria of jejunum. Histol Histopathol. 2012;27:1067–78. doi: 10.14670/HH-27.1067. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Nangaku M. Pathogenesis of tubular interstitial nephritis. Contrib Nephrol. 2011;169:297–310. doi: 10.1159/000314577. [DOI] [PubMed] [Google Scholar]
- Vitalone MJ, Naesens M, Sigdel T, et al. The dual role of epithelial-to-mesenchymal transition in chronic allograft injury in pediatric renal transplantation. Transplantation. 2011;92:787–95. doi: 10.1097/TP.0b013e31822d092c. [DOI] [PubMed] [Google Scholar]
- Lee S, Huen S, Nishio H, et al. Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol. 2011;22:317–26. doi: 10.1681/ASN.2009060615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaissling B, Le Hir M. The renal cortical interstitium: morphological and functional aspects. Histochem Cell Biol. 2008;130:247–62. doi: 10.1007/s00418-008-0452-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boor P, Floege J. The renal (myo-)fibroblast: a heterogeneous group of cells. Nephrol Dial Transplant. 2012;27:3027–36. doi: 10.1093/ndt/gfs296. [DOI] [PubMed] [Google Scholar]
- Berg EL, Mullowney AT, Andrew DP, et al. Complexity and differential expression of carbohydrate epitopes associated with L-selectin recognition of high endothelial venules. Am J Pathol. 1998;152:469–77. [PMC free article] [PubMed] [Google Scholar]
- Nielsen JS, McNagny KM. Novel functions of the CD34 family. J Cell Sci. 2008;121:3683–92. doi: 10.1242/jcs.037507. [DOI] [PubMed] [Google Scholar]
- Lebduska P, Korb J, Tumova M, et al. Topography of signaling molecules as detected by electron microscopy on plasma membrane sheets isolated from non-adherent mast cells. J Immunol Methods. 2007;328:139–51. doi: 10.1016/j.jim.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Cismasiu VB, Radu E, Popescu LM. miR-193 expression differentiates telocytes from other stromal cells. J Cell Mol Med. 2011;15:1071–4. doi: 10.1111/j.1582-4934.2011.01325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manole CG, Cismasiu V, Gherghiceanu M, et al. Experimental acute myocardial infarction: telocytes involvement in neo-angiogenesis. J Cell Mol Med. 2011;15:2284–96. doi: 10.1111/j.1582-4934.2011.01449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Bai C, Wang X. Telocyte morphologies and potential roles in diseases. J Cell Physiol. 2012;227:2311–7. doi: 10.1002/jcp.23022. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Bai C, Wang X. Potential significance of telocytes in the pathogenesis of lung diseases. Expert Rev Respir Med. 2012;6:45–9. doi: 10.1586/ers.11.91. [DOI] [PubMed] [Google Scholar]
- Faussone-Pellegrini MS, Bani D. Relationships between telocytes and cardiomyocytes during pre- and post-natal life. J Cell Mol Med. 2010;14:1061–3. doi: 10.1111/j.1582-4934.2010.01074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bani D, Formigli L, Gherghiceanu M, et al. Telocytes as supporting cells for myocardial tissue organization in developing and adult heart. J Cell Mol Med. 2010;14:2531–8. doi: 10.1111/j.1582-4934.2010.01119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Zhang Y, Wen X, et al. Telocytes accompanying cardiomyocyte in primary culture: two- and three-dimensional culture environment. J Cell Mol Med. 2010;14:2641–5. doi: 10.1111/j.1582-4934.2010.01186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gherghiceanu M, Popescu LM. Heterocellular communication in the heart: electron tomography of telocyte-myocyte junctions. J Cell Mol Med. 2011;15:1005–11. doi: 10.1111/j.1582-4934.2011.01299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu LM, Gherghiceanu M, Suciu LC, et al. Telocytes and putative stem cells in the lungs: electron microscopy, electron tomography and laser scanning microscopy. Cell Tissue Res. 2011;345:391–403. doi: 10.1007/s00441-011-1229-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gherghiceanu M, Popescu LM. Cardiac telocytes - their junctions and functional implications. Cell Tissue Res. 2012;348:265–79. doi: 10.1007/s00441-012-1333-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusu MC, Pop F, Hostiuc S, et al. Telocytes form networks in normal cardiac tissues. Histol Histopathol. 2012;27:807–16. doi: 10.14670/HH-27.807. [DOI] [PubMed] [Google Scholar]
- Gherghiceanu M, Popescu LM. Cardiomyocyte precursors and telocytes in epicardial stem cell niche: electron microscope images. J Cell Mol Med. 2010;14:871–7. doi: 10.1111/j.1582-4934.2010.01060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojin FM, Gavriliuc OI, Cristea MI, et al. Telocytes within human skeletal muscle stem cell niche. J Cell Mol Med. 2011;15:2269–72. doi: 10.1111/j.1582-4934.2011.01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy AS, Peer WA. Vesicle Trafficking: ROP-RIC Roundabout. Curr Biol. 2012;22:R576–8. doi: 10.1016/j.cub.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Guduric-Fuchs J, O'Connor A, Camp B, et al. Selective extracellular vesicle-mediated export of an overlapping set of microRNAs from multiple cell types. BMC Genomics. 2012;13:357. doi: 10.1186/1471-2164-13-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner MP. Endocytic traffic: vesicle fusion cascade in the early endosomes. Curr Biol. 2012;22:R597–8. doi: 10.1016/j.cub.2012.06.009. [DOI] [PubMed] [Google Scholar]
- Nieuwland R, Sturk A. Why do cells release vesicles? Thromb Res. 2010;125(Suppl. 1):S49–51. doi: 10.1016/j.thromres.2010.01.037. [DOI] [PubMed] [Google Scholar]


