SUMMARY
SETTING
The impact of the human immunodeficiency virus (HIV) on multidrug-resistant tuberculosis (MDR-TB) treatment outcomes in sub-Saharan Africa, where extensive rollout of highly active antiretroviral therapy (HAART) has occurred, remains unclear.
OBJECTIVE
To compare the time to initial culture conversion among patients with and those without HIV infection in a setting of individualized MDR-TB care in Botswana.
DESIGN
Prospective cohort study of MDR-TB patients receiving ambulatory, integrated TB-HIV care at two public clinics in Botswana. The time to culture conversion was compared by HIV status using Cox proportional hazard ratios (HRs).
RESULTS
A total of 40 HIV-infected and 30 non-HIV-infected patients with MDR-TB and follow-up cultures were identified. The median time to initial culture conversion was 78 days (interquartile range [IQR] 42–186) for HIV-infected and 95 days (IQR 70–133) for non-HIV-infected individuals (log rank P > 0.5; unadjusted HR 0.9, 95%CI 0.5–1.5). Adjusting for age, sex, treatment history and number of active anti-tuberculosis drugs did not change this result (adjusted HR 0.8, 95%CI 0.4–1.4).
CONCLUSION
We found no difference in the proportion of or time to initial sputum culture conversion between an HIV-infected and a non-infected cohort of MDR-TB patients in Botswana, suggesting that outcomes may be comparable in similar settings with access to individualized anti-tuberculosis treatment and HAART.
Keywords: HIV, tuberculosis, sub-Saharan Africa
WHILE THE TREATMENT of drug-susceptible tuberculosis (TB) has excellent clinical outcomes even in the setting of human immunodeficiency virus (HIV) infection,1 the treatment of multidrug-resistant TB (MDR-TB), defined as resistance to at least isoniazid (INH) and rifampin (RMP), results in longer time to culture conversion, less frequent cure rates, higher mortality, and more frequent toxicities compared with conventional treatment for drug-susceptible TB.2–4 Moreover, treatment outcomes in MDR-TB are further compromised by co-infection with HIV. In the era before highly active antiretroviral therapy (HAART), HIV-infected patients with MDR-TB had significantly poorer treatment responses and higher mortality compared to non-HIV-infected MDR-TB patients.5–14 Few studies have examined MDR-TB treatment outcomes in HIV-infected patients with less advanced HIV or who are on HAART, particularly in sub-Saharan Africa. Sputum culture conversion is an accepted surrogate endpoint in MDR-TB treatment,15,16 and two recent studies from Southern Africa, one using individualized17 and the other using standardized18 treatment approaches, have demonstrated high rates of culture conversion among HIV-infected individuals who survived during anti-tuberculosis treatment. Together they included fewer than 100 HIV-infected patients, and the study from Lesotho17 suggested worse survival among HIV-infected individuals. Further data evaluating outcomes in HIV-infected patients with MDR-TB are thus needed.
Recent estimates place Botswana as having the second highest HIV prevalence in the world, at 25%,19 and one of the highest incidences of TB disease, at 503 cases per 100 000 population.20 Although MDR-TB rates have historically been low in Botswana, national surveys of drug resistance reveal an increase in the prevalence of MDR-TB during 1995–2002: from 0.2% to 0.8% in treatment-naïve and from 6.1% to 10.4% in previously treated patients.21 Since the nationwide roll-out of HAART began in Botswana in 2003, HIV-infected individuals with TB, including MDR-TB, have had broad access to HAART. We therefore assessed early treatment outcomes in patients with and without HIV infection in a pilot setting of ambulatory, individualized MDR-TB care with widespread access to HAART.
METHODS
Study design
Patients with MDR-TB receiving ambulatory, individualized care—defined as TB treatment guided by culture and drug susceptibility testing (DST)—at two public clinics in Botswana were prospectively identified, and clinical and demographic data were collected and entered into a database by the study clinician (JH) on behalf of the Division of Disease Control of the Ministry of Health of Botswana. Data from this database were subsequently abstracted and retrospectively analyzed for the purposes of this study. Individuals with HIV (defined by two parallel HIV enzyme-linked immunosorbent assays [ELISAs]) were compared to those without HIV (defined by a negative ELISA at the start of treatment or by a referring clinician’s report of a recent negative test) with respect to time from the date of initiating appropriate treatment for MDR-TB to the date of initial sputum culture clearance. Initial sputum culture clearance was defined as two consecutive negative sputum cultures at least 1 month apart.22 An appropriate MDR-TB treatment regimen was defined as containing at least four active drugs based on DST and/or treatment history, and the date of treatment initiation was defined as the first day a regimen fulfilling these criteria was used.
In addition, we retrospectively collected adverse event data from patient charts to describe the proportion of patients in whom ototoxicity, peripheral neuropathy and renal toxicity were detected. Ototoxicity was defined as evidence of diminished hearing on physical examination. As hearing loss was assessed by physical examination and recorded as ‘present’ or ‘absent’, all cases were clinically apparent and were not graded or determined subclinically by audiology. Peripheral neuropathy was defined as the detection of diminished peripheral sensation on physical examination or the patient’s subjective report of peripheral neuropathic symptoms. Renal toxicity was defined as a persistent elevation of the serum creatinine above baseline following the start of MDR-TB treatment.
Ethics approval was obtained from the Botswana Ministry of Health and the University of Pennsylvania.
Study sites and data collection
The study was conducted at two public TB clinics in Botswana: one at Princess Marina Hospital (PMH) in the capital city of Gaborone, and the other at Ghanzi Primary Hospital (Ghanzi), in a village located 673 km from Gaborone, where a targeted MDR-TB treatment program was being piloted prior to planned expanded scale-up throughout the country. These were the only stand-alone, ambulatory, integrated MDR-TB treatment clinics at the time in Botswana.
Patients at the sites received free anti-tuberculosis treatment and, if HIV-infected, free HAART, which was made available to the patients at the same site by the same provider where TB was being treated. Patients seen within the public sector from the southern half of Botswana were referred to these two TB treatment clinics if there were concerns about TB drug resistance. There, they received care from an interdisciplinary team consisting of a physician, a nurse and a pharmacist—all with training in the management of TB and HIV. At each clinic, patients had sputum collected and sent for smear microscopy and mycobacterial culture at least monthly. Sputum specimens were processed at the National TB Reference Laboratory using auramine-rhodamine staining and were routinely cultured on Löwenstein-Jensen medium. DST for INH, RMP, ethambutol and streptomycin was routinely performed on all Mycobacterium tuberculosis isolates.
All specimens exhibiting resistance to INH and RMP were routinely sent for second-line DST to South Africa (Medical Research Council Laboratory in Pretoria and/or other private laboratories in Gauteng Provence) where they were tested for susceptibility to kanamycin, ofloxacin and ethionamide. Treatment for drug-resistant TB was individualized by using at least four drugs, including an injectable aminoglycoside and a fluoroquinolone, to which the isolate was either likely (i.e., based on treatment history) or proven to be susceptible. All patients in stable condition received directly observed therapy (DOT) on an ambulatory basis at their local clinic and were seen at least monthly for review in the MDR-TB clinics. MDR-TB directed treatment was started by the specialist physician at each clinic either when DST data indicated the presence of MDR-TB or empirically when a patient was felt to be failing first-line anti-tuberculosis agents (i.e., continued positive culture or persistent clinical symptoms).
Subject population
The subject population included all confirmed MDR-TB patients who were started on anti-MDR-TB treatment at PMH and Ghanzi from October 2005. Subjects were excluded from analysis if they had no quantifiable culture follow-up time after the start of treatment. Patients meeting these criteria were observed to the end of January 2009.
Data collection
Patient data at the clinics were recorded prospectively in an electronic medical record and on paper charts containing physician notes. These sources were reviewed, and demographic, clinical and laboratory data, including date of birth, sex, HIV status, CD4+ count, HAART regimen, anti-tuberculosis regimen and sputum microbiology were collected on standardized TB data collection forms and entered into a Microsoft Excel® spreadsheet (Microsoft, Redmond, WA, USA).
Descriptive analysis
Baseline characteristics of those with and those without HIV co-infection were compared. Categorical variables were summarized by frequencies and proportions, and continuous variables were summarized by means and medians. Differences between groups were assessed using χ2 testing for categorical data and t-tests or Wilcoxon rank-sum tests for continuous data.
Unadjusted analysis
Time from the date of initiation of MDR-TB treatment to date of initial sputum culture conversion was based on two consecutive negative cultures separated by at least 30 days, and using the date of the first of the two consecutive cultures as the date of the endpoint. The time to sputum culture conversion by HIV status was compared using the log-rank test and Kaplan-Meier curves. The primary unadjusted association was also evaluated in a Cox proportional hazard model and expressed as a hazard ratio (HR) with a 95% confidence interval (CI). Patients whose sputum cultures had not converted by the end of the follow-up period due to death or lack of follow-up are described, but were right-censored from the primary analysis.
Adjusted analysis
Adjusted analyses were performed using Cox proportional hazard models controlling for age,23 sex,24 number of active anti-tuberculosis agents administered at the time of MDR-TB treatment initiation25 and TB treatment history26 as potential confounders. All analyses were performed using SAS 9.2 (SAS Institute Inc, Cary, NC, USA) and STATA 11.0 (Stata Corp, College Station, TX, USA).
Power calculation
Given the fixed sample size of 70, and using a power of 80% with an alpha level of 0.05, the minimum detectable increased time to culture clearance among patients with HIV vs. those without HIV was >2, 3, 4 and 5 months if the mean time to culture clearance among those without HIV was respectively 2, 3, 4.8 and 6.7 months.
RESULTS
Baseline characteristics
A total of 74 patients with culture-confirmed MDR-TB were identified in the clinic population. Four patients (3 with HIV and 1 without) were excluded from the final analysis due to inadequate culture follow-up time. Of the remaining 70 patients, the median age was 38 years (interquartile range [IQR] 26–46), 51 (73%) were male, 40 (57%) were HIV-infected, and 30 (43%) were HIV-negative. Twenty-three patients (33%) tested negative for HIV at or following the start of MDR-TB treatment, and 7 (10%) were referred from outside clinics with a recent negative HIV test prior to the start of treatment. Baseline characteristics by HIV status are shown in Table 1.
Table 1.
Baseline characteristics by HIV status
| Characteristic | HIV-positive (n = 40) n (%) |
HIV-negative (n = 30) n (%) |
P value |
|---|---|---|---|
| Age, years, median [IQR] | 39 [30–45] | 33 [24–59] | 0.4 |
| Sex | |||
| Male | 29 (73) | 22 (73) | >0.5 |
| Female | 11 (28) | 8 (27) | |
| TB treatment history | |||
| Any first-line treatment* | 29 (73) | 23 (77) | >0.5 |
| Any second-line treatment† (plus first-line) | 7 (18) | 3 (10) | |
| Unknown | 4 (10) | 4 (13) | |
| Number of resistant drugs at baseline, median [IQR]‡ | 5 [4–5] | 5 [5–5] | >0.5 |
| Ethambutol resistance | |||
| Yes | 24 (60) | 23 (77) | 0.14 |
| No | 16 (40) | 7 (23) | |
| Unknown | 0 | 0 | |
| Streptomycin resistance | |||
| Yes | 25 (63) | 19 (63) | >0.5 |
| No | 13 (33) | 9 (30) | |
| Unknown | 2 (5) | 2 (7) | |
| Kanamycin resistance | |||
| Yes | 1 (3) | 3 (10) | 0.4 |
| No | 18 (45) | 12 (40) | |
| Unknown | 21 (53) | 15 (50) | |
| Ofloxacin resistance | |||
| Present | 2 (5) | 3 (10) | >0.5 |
| Absent | 16 (40) | 10 (33) | |
| Unknown | 22 (55) | 17 (57) | |
| Ethionamide resistance | |||
| Present | 7 (18) | 6 (20) | >0.5 |
| Absent | 12 (30) | 9 (30) | |
| Unknown | 21 (53) | 15 (50) | |
| Number of active drugs, median [IQR]§ | 4 [4–4] | 4 [4–4] | >0.5 |
First-line treatment indicates exposure to >1 month of one or more of the following drugs: isoniazid, rifampin, ethambutol, pyrazinamide or streptomycin.
Second-line treatment indicates exposure to >1 month of one or more of the following drugs: amikacin, ciprofloxacin, cycloserine, ethionamide or PAS.
Median number of anti-tuberculosis drugs to which the patient was resistant on baseline DST.
Number of anti-tuberculosis drugs in baseline regimen to which the patient had no evidence of resistance on DST or to which the patient had no prior exposure.
HIV = human immunodeficiency virus; IQR = interquartile range; TB = tuberculosis; PAS = para-aminosalicylic acid; DST = drug susceptibility testing.
Among the HIV-infected patients with available CD4+ counts, the median CD4+ count measured prior to the start of MDR-TB treatment was 158 (IQR 88–347, n = 27); this increased to 262 (IQR 129–382, n = 14) after a median of 3 months of follow-up; 28 (69%) were on HAART prior to the start of MDR-TB treatment, and 36 (90%) were on HAART during MDR-TB treatment. The majority of the patients on HAART received a regimen consisting of two nucleoside reverse transcriptase inhibitors (NRTI) plus a non-nucleoside reverse transcriptase inhibitor (NNRTI). The most common NRTI backbones were as follows: 19 (53%) zidovudine/lamivudine, 8 (22%) stavudine/lamivudine, 8 (22%) tenofovir/lamivudine and 4 (11%) abacavir/lamivudine.
All of the patients had been treated previously for TB before enrolling in the MDR-TB clinic: 52 (74%) had received >1 month of first-line treatment only, and 10 (14%) had received both second- and first-line anti-tuberculosis agents (Table 1). The median number of anti-tuberculosis agents to which patients were resistant at the time of MDR-TB treatment initiation was 5 (IQR 5–5); this did not differ by HIV status (P > 0.5). The type and frequency of anti-tuberculosis drug use also did not differ by HIV status (Table 2). The median number of active anti-tuberculosis drugs employed in the baseline regimen was 4 (IQR 4–4) for the cohort overall, and also did not differ by HIV status (P > 0.5). Of note, two subjects—one with HIV and one without—were identified as having extensively drug-resistant TB during treatment follow-up. The median duration of use of an aminoglycoside was approximately 8 months, and did not differ by HIV status (P = 0.48). Four patients died following enrollment in the clinic: two following sputum culture conversion (both HIV-infected) and two prior to culture conversion (both non-HIV-infected). None of the subjects included in this cohort defaulted from care during the study period.
Table 2.
Anti-tuberculosis drugs used by HIV status
| Drug | HIV-positive (n = 40) n (%) |
HIV-negative (n = 30) n (%) |
P value |
|---|---|---|---|
| Ethambutol | 12 (30) | 8 (27) | >0.5 |
| Pyrazinamide | 38 (95) | 29 (97) | >0.5 |
| Ciprofloxacin | 37 (93) | 27 (90) | >0.5 |
| Ofloxacin | 0 | 1 (3) | 0.4 |
| Moxifloxacin | 2 (5) | 3 (10) | 0.4 |
| Streptomycin | 2 (5) | 1 (3) | >0.5 |
| Amikacin | 35 (88) | 20 (83) | >0.5 |
| Capreomycin | 2 (5) | 3 (10) | 0.4 |
| Cycloserine | 27 (67) | 20 (67) | >0.5 |
| Terizidone | 0 | 1 (3) | 0.4 |
| Para-aminosalicylic acid | 2 (5) | 3 (10) | 0.4 |
| Amoxacillin/clavulanate | 2 (5) | 6 (20) | 0.05 |
| Clarithromycin | 3 (8) | 1 (3) | 0.5 |
HIV = human immunodeficiency virus.
Proportion with sputum culture conversion
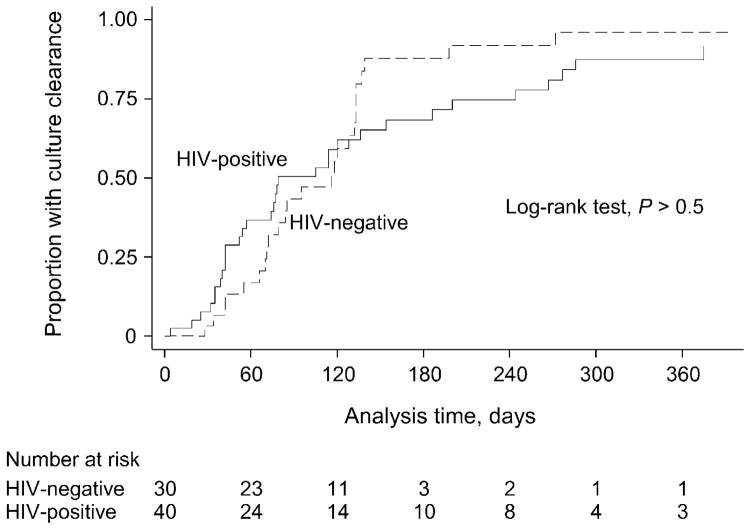
During a median treatment follow-up time of 82 days (IQR 52–133), 59/70 (84%) patients overall, 34/40 (85%) HIV-infected patients and 25/30 (83%) non-HIV-infected patients achieved sputum culture clearance. By 6 months of follow-up, there was no significant difference in the proportion of those with HIV infection (15/30, 50%) and those without (21/40, 53%; P > 0.5) who had achieved sputum culture clearance. Of note, however, the number of patients contributing to survival time by 6 months had diminished to 3 HIV-negative and 10 HIV-positive patients (Figure).
Figure.
Kaplan-Meier curves comparing time to culture clearance by HIV status. HIV = human immunodeficiency virus.
Time to sputum culture conversion
The median time to sputum culture clearance was 78 days (IQR 42–186) for HIV-infected patients and 95 days (IQR 70–133) for non-HIV-infected patients (log-rank P > 0.5, Kaplan-Meier curve, Figure). The unadjusted HR was 0.9 (95%CI 0.5–1.5), and did not change after adjusting for age, sex, TB treatment history and number of active anti-tuberculosis drugs given at time of treatment initiation (adjusted HR 0.8, 95%CI 0.4–1.4).
Toxicity
Neuropathy at any time point after follow-up occurred in 16/40 (40%) HIV-infected and 3/30 (10%) non-HIV-infected patients (P < 0.01). Similarly, nephropathy occurred in 10/40 (25%) HIV-infected and 2/30 (7%) non-HIV-infected patients (P = 0.04). No differences were detected in the proportion experiencing ototoxicity between those with and those without HIV: respectively 21/40 (53%) and 21/30 (70%; P = 0.14). Neuropsychiatric toxicity (e.g., seizures or psychosis) occurred in six individuals, 3/40 (8%) HIV-infected and 3/30 (10%) non-HIV-infected (P > 0.5), all of whom required discontinuation of cycloserine, to which the symptoms were attributed.
DISCUSSION
In this cohort of adults with MDR-TB where nearly all HIV-infected patients were treated with HAART, the time to initial sputum culture conversion after initiating MDR-TB treatment was comparable among those with and those without HIV infection. These results add to the limited data17 indicating that, in resource-limited settings with broad access to HAART and individualized MDR-TB care, microbiologic outcomes in HIV-infected individuals may be comparable to those observed in patients without HIV.
The majority of patients in this study converted their sputum culture within 3 months of TB treatment initiation, which is similar to the rates and timing of culture conversion observed in HIV-infected individuals with similar access to HAART in South Africa and Lesotho,17,18 and in non-HIV-infected individuals in Latvia.16 Morever, similar to the clinical setting described in Lesotho, patients in Botswana likely benefited from having access to individualized care, which enabled clinicians to tailor MDR-TB regimens more closely to mirror resistance patterns. Another reason for the excellent microbiologic outcomes in this study is likely related to the availability of second-line DST and second-line drugs for the treatment of MDR-TB in Botswana, which enabled the use of several active agents in each patient’s MDR-TB care. Individualized care has been shown to improve treatment outcomes in non-HIV-infected patients with MDR-TB,27 and may be equally important in those with HIV.
Another explanation for the favorable early microbiologic outcomes in those with HIV in this study may be related to the model of MDR-TB-HIV care employed in Botswana. Similar to clinical sites in Lesotho17 and South Africa,18,28 we provided ambulatory, DOT-based TB treatment as well as integrated HIV care (i.e., HAART, cotrimoxazole prophylaxis, monitoring of CD4+ count and HIV viral load) to all co-infected patients. The clinicians also monitored for overlapping drug toxicities, evaluated for immune reconstitution inflammatory syndrome and screened for intercurrent opportunistic infections. Integration of HIV and TB care has been shown to be important for patients with drug-susceptible TB,29,30 and is likely also critical in MDR-TB, where response to anti-tuberculosis drugs is more delayed. Efforts to scale up individualized treatment of MDR-TB and to integrate HAART into MDR-TB treatment in high-burden settings are thus urgently needed.
Patients with HIV were significantly more likely than those without HIV to have renal dysfunction and peripheral neuropathy. Although we did not have sufficient power to rigorously evaluate risk factors for neuropathy, and although we cannot exclude some degree of detection bias in this result due to collection of the data under routine clinical conditions, increased risk of this adverse event in those with HIV is likely related in part to use of NRTIs, particularly stavudine, and to the HIV itself.31 Toxicities are an important cause of morbidity in patients with MDR-TB, and in many cases limited treatment options necessitate continuation of second-line agents and HAART, despite ongoing development of serious adverse events. A striking example is ototoxicity, which results from aminoglycoside use and was experienced by over half of the individuals in this study. Further research is needed to develop less toxic therapies for treatment of this disease.
Our study had a number of limitations. We had adequate statistical power to detect a difference in time to culture clearance of ≥3 months. Although our point estimates of the time to culture clearance do not suggest a longer time to culture conversion among those with HIV, larger studies may uncover smaller differences in time to culture conversion that may be of public health interest. In addition, only short-term microbiologic outcomes were assessed, and further follow-up is needed to determine if early favorable microbiologic outcomes predict equally favorable clinical recovery over the longer term. Finally, although we observed very low rates of mortality in our cohort relative to earlier observational studies of MDR-TB in the setting of HIV, we realize that inadequate follow-up or underreporting of deaths from district sites could bias our results.
Like many sub-Saharan African countries, TB culture and DST in Botswana is reserved primarily for patients who fail to respond to first-line anti-tuberculosis treatment, which is assessed most often by failure to convert sputum smears from positive to negative by 2 months. As we do not know the proportion of patients with and without HIV who survived long enough to undergo TB culture and DST and be successfully referred to the MDR-TB treatment clinics, we cannot exclude potential selection bias. Nonetheless, this study demonstrates that patients with HIV who survive to successful referral for MDR-TB treatment do not appear to have worse short-term microbiologic outcomes compared to patients without HIV.
Finally, although this study indicates encouraging early microbiologic outcomes in Botswana, these results should be viewed in light of the fact that numerous practical challenges were faced while establishing the clinical structure. These included challenges in integrating HIV and TB care, establishing a consistent supply of second-line TB drugs, ensuring that specimens for culture and DST were routinely collected and evaluated at the national laboratory, obtaining second-line DST in South Africa, arranging transportation for patients throughout the district, and establishing proper infection control measures within the clinic.
In conclusion, these results suggest that short-term outcomes may be similar amongst HIV-infected and non-infected MDR-TB patients in the setting of widespread use of HAART. Further studies are needed to determine the impact of HIV on longer-term clinical outcomes in the treatment of MDR-TB.
Acknowledgments
The authors thank the staff at the Infectious Disease Clinic at Princess Marina Hospital, the National TB Reference Laboratory in Gaborone and the Botswana Ministry of Health for their support. In particular, they thank L Molapisi for her assistance with patient care and data retrieval during the course of the study, and V Gammino for her thoughtful comments with regards to the study design and analysis. The authors also thank T Steen and H Moffat for their ongoing advice, support, and encouragement during the piloting of this clinic. This work was supported by the National Institutes of Health (T32 AI 055435).
Footnotes
Conflict of interest: none declared.
References
- 1.Maartens G, Wilkinson RJ. Tuberculosis. Lancet. 2007;370:2030–2043. doi: 10.1016/S0140-6736(07)61262-8. [DOI] [PubMed] [Google Scholar]
- 2.Mukherjee JS, Rich ML, Socci AR, et al. Programmes and principles in treatment of multidrug-resistant tuberculosis. Lancet. 2004;363:474–481. doi: 10.1016/S0140-6736(04)15496-2. [DOI] [PubMed] [Google Scholar]
- 3.Orenstein EW, Basu S, Shah NS, et al. Treatment outcomes among patients with multidrug-resistant tuberculosis: systematic review and meta-analysis. Lancet Infect Dis. 2009;9:153–161. doi: 10.1016/S1473-3099(09)70041-6. [DOI] [PubMed] [Google Scholar]
- 4.Rajbhandary SS, Marks SM, Bock NN. Costs of patients hospitalized for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2004;8:1012–1016. [PMC free article] [PubMed] [Google Scholar]
- 5.Goble M, Iseman MD, Madsen LA, Waite D, Ackerson L, Horsburgh CR., Jr Treatment of 171 patients with pulmonary tuberculosis resistant to isoniazid and rifampin. N Engl J Med. 1993;328:527–532. doi: 10.1056/NEJM199302253280802. [DOI] [PubMed] [Google Scholar]
- 6.Fischl MA, Daikos GL, Uttamchandani RB, et al. Clinical presentation and outcome of patients with HIV infection and tuberculosis caused by multiple-drug-resistant bacilli. Ann Intern Med. 1992;117:184–190. doi: 10.7326/0003-4819-117-3-184. [DOI] [PubMed] [Google Scholar]
- 7.Busillo CP, Lessnau KD, Sanjana V, et al. Multidrug-resistant Mycobacterium tuberculosis in patients with human immunodeficiency virus infection. Chest. 1992;102:797–801. doi: 10.1378/chest.102.3.797. [DOI] [PubMed] [Google Scholar]
- 8.Turett GS, Telzak EE, Torian LV, et al. Improved outcomes for patients with multidrug-resistant tuberculosis. Clin Infect Dis. 1995;21:1238–1244. doi: 10.1093/clinids/21.5.1238. [DOI] [PubMed] [Google Scholar]
- 9.Salomon N, Perlman DC, Friedmann P, Buchstein S, Kreiswirth BN, Mildvan D. Predictors and outcome of multidrug-resistant tuberculosis. Clin Infect Dis. 1995;21:1245–1252. doi: 10.1093/clinids/21.5.1245. [DOI] [PubMed] [Google Scholar]
- 10.Park MM, Davis AL, Schluger NW, Cohen H, Rom WN. Outcome of MDR-TB patients, 1983–1993. Prolonged survival with appropriate therapy. Am J Respir Crit Care Med. 1996;153:317–324. doi: 10.1164/ajrccm.153.1.8542137. [DOI] [PubMed] [Google Scholar]
- 11.Frieden TR, Sherman LF, Maw KL, et al. A multi-institutional outbreak of highly drug-resistant tuberculosis: epidemiology and clinical outcomes. JAMA. 1996;276:1229–1235. [PubMed] [Google Scholar]
- 12.Franzetti F, Gori A, Iemoli E, et al. Outcome of multidrug-resistant tuberculosis in human immunodeficiency virus-infected patients. Clin Infect Dis. 1999;29:553–560. doi: 10.1086/598633. [DOI] [PubMed] [Google Scholar]
- 13.Telzak EE, Chirgwin KD, Nelson ET, et al. Predictors for multidrug-resistant tuberculosis among HIV-infected patients and response to specific drug regimens. Int J Tuberc Lung Dis. 1999;3:337–343. [PubMed] [Google Scholar]
- 14.Flament-Saillour M, Robert J, Jarlier V, Grosset J. Outcome of multidrug-resistant tuberculosis in France: a nationwide case-control study. Am J Respir Crit Care Med. 1999;160:587–593. doi: 10.1164/ajrccm.160.2.9901012. [DOI] [PubMed] [Google Scholar]
- 15.Diacon AH, Pym A, Grobusch M, et al. The diarylquinoline TMC207 for multidrug-resistant tuberculosis. N Engl J Med. 2009;360:2397–2405. doi: 10.1056/NEJMoa0808427. [DOI] [PubMed] [Google Scholar]
- 16.Holtz TH, Sternberg M, Kammerer S, et al. Time to sputum culture conversion in multidrug-resistant tuberculosis: predictors and relationship to treatment outcome. Ann Intern Med. 2006;144:650–659. doi: 10.7326/0003-4819-144-9-200605020-00008. [DOI] [PubMed] [Google Scholar]
- 17.Seung KJ, Omatayo DB, Keshavjee S, Furin JJ, Farmer PE, Satti H. Early outcomes of MDR-TB treatment in a high HIV-prevalence setting in southern Africa. PLoS ONE. 2009;4:e7186. doi: 10.1371/journal.pone.0007186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brust JC, Lygizos M, Chaiyachati K, et al. Culture conversion among HIV co-infected multidrug-resistant tuberculosis patients in Tugela Ferry, South Africa. PLoS ONE. 2011;6:e15841. doi: 10.1371/journal.pone.0015841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joint United Nations Programme on HIV/AIDS. UNAIDS report on the global AIDS epidemic 2010. Geneva, Switzerland: UNAIDS; 2010. [Google Scholar]
- 20.World Health Organization. WHO report 2011. Geneva, Switzerland: WHO; 2011. Global tuberculosis control. WHO/HTM/TB/2011.16. [Google Scholar]
- 21.Nelson LJ, Talbot EA, Mwasekaga MJ, et al. Anti-tuberculosis drug resistance and anonymous HIV surveillance in tuberculosis patients in Botswana, 2002. Lancet. 2005;366:488–490. doi: 10.1016/S0140-6736(05)67062-6. [DOI] [PubMed] [Google Scholar]
- 22.Laserson KF, Thorpe LE, Leimane V, et al. Speaking the same language: treatment outcome definitions for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2005;9:640–645. [PubMed] [Google Scholar]
- 23.Jacobson KR, Tierney DB, Jeon CY, Mitnick CD, Murray MB. Treatment outcomes among patients with extensively drug-resistant tuberculosis: systematic review and meta-analysis. Clin Infect Dis. 2010;51:6–14. doi: 10.1086/653115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeon DS, Shin DO, Park SK, et al. Treatment outcome and mortality among patients with multidrug-resistant tuberculosis in tuberculosis hospitals of the public sector. J Korean Med Sci. 2011;26:33–41. doi: 10.3346/jkms.2011.26.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwon YS, Kim YH, Suh GY, et al. Treatment outcomes for HIV-uninfected patients with multidrug-resistant and extensively drug-resistant tuberculosis. Clin Infect Dis. 2008;47:496–502. doi: 10.1086/590005. [DOI] [PubMed] [Google Scholar]
- 26.Kliiman K, Altraja A. Predictors of extensively drug-resistant pulmonary tuberculosis. Ann Intern Med. 2009;150:766–775. doi: 10.7326/0003-4819-150-11-200906020-00004. [DOI] [PubMed] [Google Scholar]
- 27.Laniado-Laborin R. Multidrug-resistant tuberculosis: standardized or individualized treatment? The question has already been answered. Expert Rev Respir Med. 2010;4:143–146. doi: 10.1586/ers.10.6. [DOI] [PubMed] [Google Scholar]
- 28.Brust JCM, Shah NS, Scott M, Chaiyachati K, et al. Integrated, home-based treatment for MDR-TB and HIV in rural South Africa: an alternate model of care. Int J Tuberc Lung Dis. 2012;16:998–1004. doi: 10.5588/ijtld.11.0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abdool Karim SS, Naidoo K, Grobler A, et al. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med. 2010;362:697–706. doi: 10.1056/NEJMoa0905848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lawn SD, Campbell L, Kaplan R, Little F, Morrow C, Wood R. Delays in starting antiretroviral therapy in patients with HIV-associated tuberculosis accessing non-integrated clinical services in a South African township. BMC Infect Dis. 2011;11:258. doi: 10.1186/1471-2334-11-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Evans SR, Ellis RJ, Chen H, et al. Peripheral neuropathy in HIV: prevalence and risk factors. AIDS. 2011;25:919–928. doi: 10.1097/QAD.0b013e328345889d. [DOI] [PMC free article] [PubMed] [Google Scholar]