Summary
Lymphedema is a chronic disorder that, in developed countries, occurs most commonly after lymph node dissection for cancer treatment. Although the pathophysiology of lymphedema is unknown, the disease is characterized histologically by fibrosis and abnormal adipose deposition. Clinical studies have provided evidence that obesity and postoperative weight gain are significant risk factors for the development of lymphedema. In fact, recent studies have shown that extreme obesity can result in markedly impaired lymphatic function and primary lymphedema. The aim of this Special Topic article is to review evidence linking obesity and lymphedema. In addition, the authors review recent studies that have analyzed the cellular mechanisms that may be responsible for this relationship, with a goal of highlighting areas of research that may have significant translational potential.
Lymphedema is a chronic, incurable condition caused by the anomalous development of the lymphatic system (primary lymphedema) or injury to the lymphatic vasculature (secondary lymphedema). It is estimated that nearly 5 million Americans suffer from lymphedema of the extremities or genitalia.1 In these cases, chronic interstitial fluid accumulation leads to fibrosis, persistent inflammation, and adipose deposition, often resulting in massive hypertrophy of the affected area. Adipose deposition in late-stage lymphedema decreases the potential for response to mechanical treatments such as manual lymphatic massage or compressive garments and is associated with severe infections, functional disability, skin changes, psychosocial morbidity, and malignant transformation (Fig. 1).2
Fig. 1.
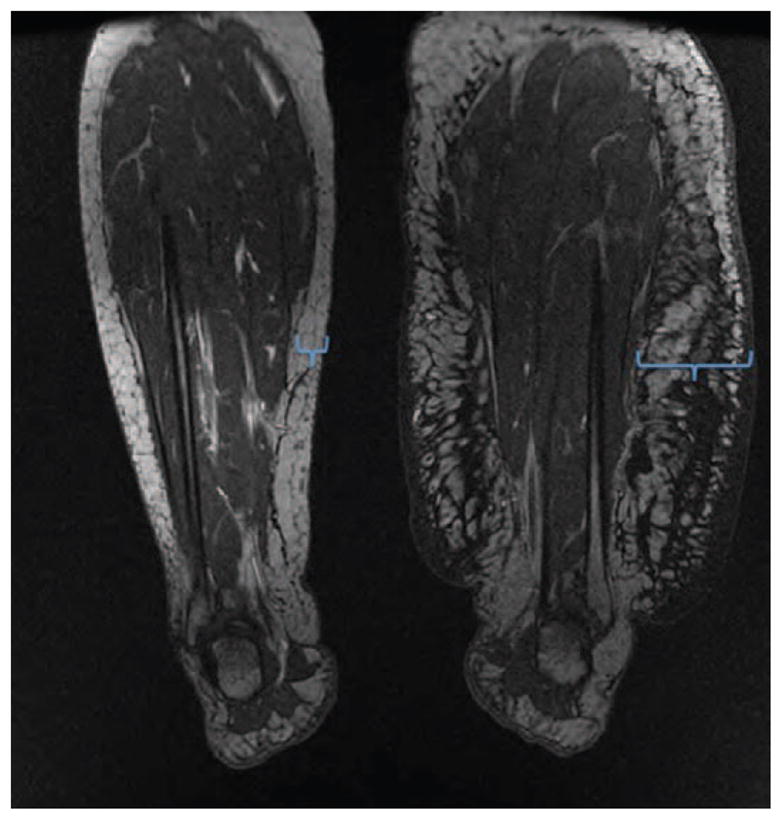
End-stage lymphedema is associated with subcutaneous adipose deposition and “regional” obesity. (Left) Bilateral magnetic resonance imaging scan of a patient with severe left leg lymphedema after groin lymph node dissection for melanoma 15 years previously. Note significant adipose deposition in the subcutaneous compartment of the affected leg (blue brackets) compared with the normal limb. (Right) Photograph of a young patient with grade II lymphedema of the left leg 2 years after treatment for cervical cancer. Note adipose deposition in the entire left leg.
Lymphedema and its management are of significant interest to plastic surgeons. This is because the majority of patients in the United States who suffer from lymphedema are breast cancer survivors, many of whom have, at some point, undergone breast reconstruction. In addition, liposuction performed by plastic surgeons has been used extensively as a means of debulking lymphedematous limbs because the end stage of the disease is characterized by abnormal adipose tissue deposition.3 More recently, interest in micro-surgical reconstruction of lymphedema, either by lymphovenous bypass procedures or microvascular lymph node transplantation, has gained favor and has been the subject of intense study. This interest is reflected by the publication of nearly 40 articles in Plastic and Reconstructive Surgery on this topic in the past 5 years. With this interest in mind, the purpose of this review is to summarize recent advances in understanding the link between lymphedema and its major risk factor, obesity.
In this Special Topic article, we have reviewed the existing clinical and basic science literature on the effect of obesity on lymphatic function and lymphedema. Specifically, we performed a review of the literature on the clinical association of obesity and lymphedema by searching the English literature for articles identifying risk factors for lymphedema in general, and articles analyzing the effects of interventions such as weight loss or modifications of diet on existing lymphedema. We also sought to identify clinical studies that analyzed the effects of obesity on lymphatic transport function. Similarly, to develop hypotheses designed to explain the clinical association between obesity and lymphedema, we reviewed the scientific literature on this topic, searching for articles analyzing the effects of dietary changes on the lymphatic system and the effects of genetic models of lymphatic insufficiency on obesity. Although this was not a systematic review, our article highlights the important clinical studies relating obesity and lymphedema and the effects of dietary or exercise interventions. We have highlighted these studies with a few patient samples from our own clinical practice and provide a comprehensive model of the clinical and pathologic features of lymphedema and adipose deposition.
CLINICAL EVIDENCE LINKING LYMPHEDEMA AND OBESITY
Obesity Is a Major Risk Factor for Developing Secondary Lymphedema
Several studies have shown that obesity can increase the risk of secondary lymphedema following damage to the lymphatic vasculature. As early as 1957, it was noted that the greater the weight of the patient, the more likely the individual was to develop lymphedema following breast cancer treatment.4 In a prospective level II study of 137 patients with breast cancer, individuals with a body mass index greater than 30 had three times the risk of developing upper extremity lymphedema compared with patients with a body mass index less than 25.5 Similarly, in another level II prospective clinical trial with 936 patients, McLaughlin et al. found that patients who developed lymphedema had a higher baseline and current body mass index compared with those who did not.6 Another level II investigation of 282 patients found that body mass index was the variable most closely associated with arm lymphedema after breast cancer treatment, and that the greater the body mass index, the higher the frequency of lymphedema.7 The 5-year incidence of lymphedema in women with breast cancer with a body mass index greater than 29 was 36 percent, compared with 12 percent for patients with lower body mass indexes. Although the majority of studies linking elevated body mass index with an increased risk of developing secondary lymphedema have involved the upper extremity following breast cancer treatment, these findings likely are translatable to patients at risk for lower extremity lymphedema following inguinal lymphadenectomy.
Body mass index at the time of breast cancer diagnosis appears to be a stronger risk factor for developing lymphedema than weight gain following treatment. For example, in a prospective level II study of 138 patients, Ridner et al. reported that patients with a body mass index greater than 30 at the time of management were 3.6 times more likely to develop upper extremity lymphedema within 30 months of surgery compared with women with a body mass index less than 30.8 In contrast, patients whose body mass index increased to greater than 30 during the period after treatment did not have an increased risk of developing lymphedema. However, these results should be viewed with caution and require further study because the follow-up time after weight gain in this study was limited and because these findings contradict other studies demonstrating a link between postoperative weight changes and lymphedema. This concept is best supported by a level I, randomized clinical trial demonstrating significant reductions in upper extremity lymphedema and arm volumes in patients who lost weight by dieting for 12 weeks (reduction of body mass index of 1.3 ± 1.1 kg/m2) compared with a matched control group that did not lose weight.9 More recently, a number of additional level I studies have shown that monitored exercise programs can also decrease the severity of lymphedema (reviewed by Kwan et al.), suggesting that changes in body composition can influence adipose deposition and limb volumes in these patients.10 These findings are critical because they represent the only effective noncompressive treatment of lymphedema that has been reported to date. In addition, when considered together, these findings suggest that lymphedema is a form of regional obesity or lipodystrophy of the affected extremity that may be modulated by changes in diet and body type.
Extreme Obesity Can Cause Lymphedema Independently of Surgery
Not only does obesity increase the risk of lymphedema in patients with lymphatic injury, recent studies have shown that superobese individuals can develop lymphedema even without antecedent surgery or injury. One retrospective level IV study of patients presenting to an obesity clinic10 found that approximately one-third of patients had abnormalities on lymphoscintigraphy, a technique that is 100 percent specific and 92 percent sensitive for lymphedema.11 These findings were supported by Arngrim et al., who reported decreased adipose tissue clearance of macromolecules in obese patients compared with lean controls in a prospective level II study.12 Another retrospective case series (level IV) showed that a body mass index threshold appears to exist at which point lower extremity lymphedema occurs (Fig. 2).13 In this study, the lymphatic function of 15 obese patients referred to a lymphedema center with lower extremity “lymphedema” was assessed using lymphoscintigraphy. None of the patients in the cohort had any risk factors for lymphedema (e.g., inguinal irradiation, lymphadenectomy, penetrating trauma, primary lymphedema). All 10 patients with a body mass index between 30 and 53 had normal lower extremity lymphatic function, whereas the five patients with a body mass index greater than 59 had abnormal lymphatic drainage consistent with lymphedema.13 Consequently, a body mass index threshold appears to exist between 53 and 59 at which point lower extremity lymphatic dysfunction occurs. This threshold may be higher for the upper extremity because obesity-induced lymphedema was confirmed in only a single massively obese individual (body mass index of 105) but not in patients with a body mass index less than 90. Although the causes of this observation remain unknown, this finding may be related to a variety of differences between the upper and lower extremities, including (1) preferential adipose deposition in the lower extremities compared with the upper extremities and (2) dependent positioning of the lower extremity with resultant impaired lymphatic transport against gravity.
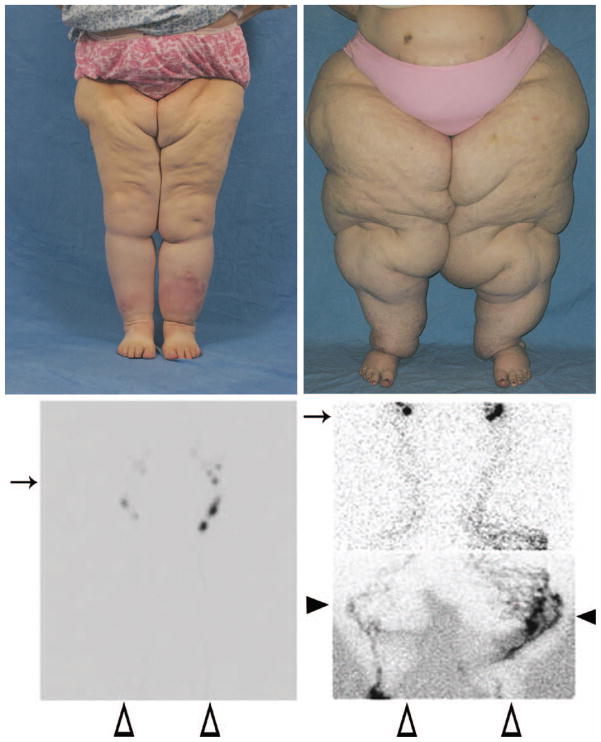
Fig. 2.
Obesity-induced lower extremity lymphedema. A body mass index threshold appears to exist between 53 and 59 when lymphatic dysfunction occurs. (Above, left) A woman with a body mass index of 53.3. (Above, right) A woman with a body mass index of 78.3. (Below, left) Lymphoscintigram from the patient shown above, left with a body mass index of 53.3. Note normal transit of technetium to inguinal nodes 20 minutes after injection. Arrows indicate inguinal nodes, black arrowheads show tortuous lymphatic channels and dermal backflow, and white arrowheads mark the feet where the radiolabeled tracer was injected. (Below, right). Lymphoscintigram from the superobese patient shown above, right (body mass index of 78.3). Note the delayed transit of tracer to inguinal nodes 3 hours after injection, tortuous collateral lymphatic channels, and dermal backflow consistent with lymphedema.
The link between superobesity and lymphedema is clinically significant because both of these conditions are prevalent. Currently, one-third of the U.S. population is obese (body mass index >30), 6 percent is superobese (body mass index >40), and the proportion of the population that is obese is increasing between 2.1 and 4.7 percent every 10 years.14 Lymphedema affects approximately one in 1000 Americans and as many as 200 million people worldwide.1,15 Consequently, the relationship between obesity and lymphedema has major implications on public health and health care expenditures and may result in significantly increased rates of primary lymphedema.
CELLULAR MECHANISMS LINKING OBESITY AND LYMPHEDEMA
Although it is clear that obesity and lymphedema are related in patients who have undergone surgery, the cellular mechanisms that regulate this effect remain largely unknown. For example, we currently do not know whether obesity increases the risk of lymphedema as a result of increased production of lymph from an enlarging limb that overwhelms the capacity of the lymphatic system, as a consequence of external compression of lymphatics by adipose tissues, or even as a result of direct injury to the lymphatic endothelium by changes in body weight or diet. This gap in our knowledge is important because it precludes development of novel preventative or treatment options. Therefore, mechanistic studies aiming to understand how obesity and lymphedema are related are important.
Obesity and Lymphedema Have a Reciprocal Relationship
Recent studies suggest that the interaction between obesity and lymphedema is reciprocal. That is, not only is it now clear that obesity can lead to impaired lymphatic function, it is also evident that impaired lymphatic function can lead to adipose deposition and obesity. For example, histologic studies on clinical samples and animal models of lymphedema have shown that adipose deposition in the lymphedematous tissues is, in many ways, identical to fat depots in obese patients.16 Similar to obesity, lymphedema-associated fat deposition results from both proliferation and hypertrophy of local adipocytes, and the resulting adipose depots are chronically inflamed and infiltrated by macrophages and lymphocytes.16 In addition, lymphedema tissues exhibit evidence of adipocyte death and phagocytosis by macrophages, resulting in the appearance of so-called “crown-like structures.” This is important because production of inflammatory cytokines by these structures is associated with both an increased risk and a more aggressive behavior of a variety of malignancies in obese patients.17
Using a mouse model of lymphedema, Aschen et al. have shown that impaired lymphatic flow results in inflammation and massive up-regulation of adipocyte differentiation genes such as peroxisome proliferator-activated receptor gamma (PPAR-γ) and CCAAT/enhancer-binding protein alpha (CEPB-α), and increased expression of adipokines (hormones produced by adipose tissues).18 This finding is important because it suggests that accumulation of interstitial fluid can lead to activation of adipocyte differentiation and proliferation, thus providing a mechanism for local adipose deposition that is noted in patients with lymphedema. Activation of these genes even occurs with mild disturbances in lymphatic function, such as after axillary lymph node dissection, and is maintained chronically even 6 weeks after the operation when all visible evidence of swelling has resolved. Thus, impaired lymphatic function in patients who have undergone lymph node dissection or in those with abnormal lymphatic development may sensitize and promote adipose deposition in the subcutaneous space by increasing activation of adipose differentiation genes.
Further evidence linking lymphatic dysfunction and obesity can be derived from transgenic mice with a variety of mutations in their lymphatic system. For example, mice with spontaneous inactivating heterozygous mutations of the Fms-related tyrosine kinase 4 (FLT4 or otherwise known as vascular endothelial growth factor receptor 3) gene, a model of primary (Millroy) lymphedema, exhibit abnormal subcutaneous fat deposition.19 Similarly, mice that harbor a heterozygote mutation of the Prospero homeobox protein 1 (PROX1) gene, a transcription factor that is critical for lymphatic endothelial cell differentiation and patterning, not only have impaired lymphatic development and function in their skin/subcutaneous tissues but also exhibit adult-onset obesity compared with wild-type littermates fed an identical diet.20 More recent studies have shown that the lymphatics play a critical role in transport and metabolism of cholesterol from the peripheral tissues to the plasma and that impaired lymphatic function leads to tissue accumulation of cholesterol.21
Obesity Independently Decreases Lymphatic Function
Recent studies have provided evidence that obesity and dietary changes can profoundly change lymphatic function. For example, mice that harbor defects in the apolipoprotein E (APOE) gene have abnormally high levels of circulating cholesterol and develop defects in their lymphatic system, including decreased interstitial fluid transport capacity, abnormal lymphatic valves, and impaired trafficking of immune cells.22 Similarly, our group has recently shown that wild-type mice fed a high-fat diet for 8 weeks become modestly obese and have significantly decreased lymphatic transport as assessed by lymphoscintigraphy, reduced lymph node uptake of interstitial fluid, and abnormal lymph node architecture.23 Interestingly, we found that obese mice had smaller lymph node size, loss of follicular patterning, and changes in resident lymph node inflammatory cell populations. In addition, obese mice had severely impaired dendritic cell migration to local lymph nodes, suggesting that antigen presentation from the periphery and responses to tissue inflammatory reactions are abnormal in obese mice. We found that at least some of these defects are dependent on obesity induced T- and B-cell inflammatory responses because mice genetically deficient in these cells had decreased interstitial fluid flow but maintained nearly normal rates of dendritic cell migration. In addition, preliminary studies have shown that at least some of these changes are reversible because calorie restriction in obese mice resulted in normalization of lymph node size and function (unpublished observations). This finding is important and is consistent with reports of improved lymphedema symptoms in patients after weight loss. Thus, weight management programs including nutritional counseling and surgical weight loss options may be potential means of decreasing the rates or severity of lymphedema in at-risk patients. These approaches may translate to improved lymphatic function and increased clearance of interstitial fluid.
Obesity may also impair lymphatic function as a result of changes in lymphangiogenesis. Although this hypothesis is preliminary, it is supported by studies demonstrating that obese mice have decreased numbers of capillary lymphatics in their lymph nodes. The lymphatic vessels that are present are abnormally dilated compared with lean mice.23 These findings are supported by studies by Lim et al., who found that apolipoprotein E–deficient mice also develop leaky lymphatic vessels as adults.22 Thus, the negative effects of obesity on lymphatic function may be multifactorial and dependent on changes in the extracellular matrix, inflammatory reactions, and direct injury to lymphatic endothelial cells. Obese patients may therefore be at risk for lymphedema because they have compromised lymphatic function at baseline, have abnormal inflammatory responses that can negatively impact the lymphatic system, and have impaired ability to regenerate damaged lymphatics after injury.
CONCLUSIONS
The link between obesity and lymphedema continues to be elucidated. Increasing evidence supports the concept that this relationship is reciprocal such that obesity impairs lymphatic transport capacity and impaired lymphatic function promotes adipose deposition. This association provides a mechanism for the development of primary lymphedema in superobese individuals and the increased risk of lymphedema in obese patients. Based on the studies reviewed in this article, we have provided a hypothetical model for the pathologic process of lymphedema (Fig. 3). In this model, lymphatic injury starts the cycle of lymphatic dysfunction, which leads to accumulation of interstitial fluid. Impaired lymphatic clearance results in inflammation and promotes fibroadipose deposition. In turn, fibrosis and adipose deposition further impair lymphatic function, resulting in a feedforward loop. We hypothesize that obese patients are at higher risk for lymphedema because they have baseline impaired lymphatic function. Based on clinical and laboratory studies demonstrating that the pathologic changes in the lymphatic system resulting from obesity are at least partially reversible with weight loss, we hypothesize that efforts be aimed at weight loss/management in patients who are at risk for developing primary or secondary lymphedema.
Fig. 3.
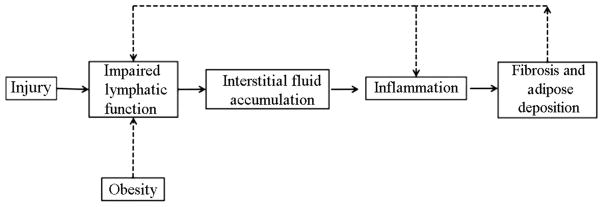
Hypothetical model of adipose deposition and effect of obesity on lymphedema.
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
References
- 1.Rockson SG, Rivera KK. Estimating the population burden of lymphedema. Ann N Y Acad Sci. 2008;1131:147–154. doi: 10.1196/annals.1413.014. [DOI] [PubMed] [Google Scholar]
- 2.Rockson SG. Lymphedema. Curr Treat Options Cardiovasc Med. 2006;8:129–136. doi: 10.1007/s11936-006-0005-y. [DOI] [PubMed] [Google Scholar]
- 3.Brorson H, Ohlin K, Olsson G, Nilsson M. Adipose tissue dominates chronic arm lymphedema following breast cancer: An analysis using volume rendered CT images. Lymphat Res Biol. 2006;4:199–210. doi: 10.1089/lrb.2006.4404. [DOI] [PubMed] [Google Scholar]
- 4.Treves N. An evaluation of the etiological factors of lymphedema following radical mastectomy; an analysis of 1,007 cases. Cancer. 1957;10:444–459. doi: 10.1002/1097-0142(195705/06)10:3<444::aid-cncr2820100306>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 5.Helyer LK, Varnic M, Le LW, Leong W, McCready D. Obesity is a risk factor for developing postoperative lymphedema in breast cancer patients. Breast J. 2010;16:48–54. doi: 10.1111/j.1524-4741.2009.00855.x. [DOI] [PubMed] [Google Scholar]
- 6.McLaughlin SA, Wright MJ, Morris KT, et al. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: Objective measurements. J Clin Oncol. 2008;26:5213–5219. doi: 10.1200/JCO.2008.16.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Werner RS, McCormick B, Petrek J, et al. Arm edema in conservatively managed breast cancer: Obesity is a major predictive factor. Radiology. 1991;180:177–184. doi: 10.1148/radiology.180.1.2052688. [DOI] [PubMed] [Google Scholar]
- 8.Ridner SH, Dietrich MS, Stewart BR, Armer JM. Body mass index and breast cancer treatment-related lymphedema. Support Care Cancer. 2011;19:853–857. doi: 10.1007/s00520-011-1089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaw C, Mortimer P, Judd PA. A randomized controlled trial of weight reduction as a treatment for breast cancer-related lymphedema. Cancer. 2007;110:1868–1874. doi: 10.1002/cncr.22994. [DOI] [PubMed] [Google Scholar]
- 10.Kwan ML, Cohn JC, Armer JM, Stewart BR, Cormier JN. Exercise in patients with lymphedema: A systematic review of the contemporary literature. J Cancer Surviv. 2011;5:320–336. doi: 10.1007/s11764-011-0203-9. [DOI] [PubMed] [Google Scholar]
- 11.Szuba A, Shin WS, Strauss HW, Rockson S. The third circulation: Radionuclide lymphoscintigraphy in the evaluation of lymphedema. J Nucl Med. 2003;44:43–57. [PubMed] [Google Scholar]
- 12.Arngrim N, Simonsen L, Holst JJ, Bülow J. Reduced adipose tissue lymphatic drainage of macromolecules in obese subjects: A possible link between obesity and local tissue inflammation? Int J Obes (Lond) 2013;37:748–750. doi: 10.1038/ijo.2012.98. [DOI] [PubMed] [Google Scholar]
- 13.Greene AK, Grant FD, Slavin SA. Lower-extremity lymphedema and elevated body-mass index. N Engl J Med. 2012;366:2136–2137. doi: 10.1056/NEJMc1201684. [DOI] [PubMed] [Google Scholar]
- 14.Flegal KM, Carroll MD, Ogden CL, et al. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 15.Moffatt CJ, Franks PJ, Doherty DC, et al. Lymphoedema: An underestimated health problem. QJM. 2003;96:731–738. doi: 10.1093/qjmed/hcg126. [DOI] [PubMed] [Google Scholar]
- 16.Zampell JC, Aschen S, Weitman ES, et al. Regulation of adipogenesis by lymphatic fluid stasis: Part I. Adipogenesis, fibrosis, and inflammation. Plast Reconstr Surg. 2012;129:825–834. doi: 10.1097/PRS.0b013e3182450b2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howe LR, Subbaramaiah K, Hudis CA, Dannenberg AJ. Molecular pathways: Adipose inflammation as a mediator of obesity-associated cancer. Clin Cancer Res. 2013;19:6074–6083. doi: 10.1158/1078-0432.CCR-12-2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aschen S, Zampell JC, Elhadad S, Weitman E, De Brot M, Mehrara BJ. Regulation of adipogenesis by lymphatic fluid stasis: Part II. Expression of adipose differentiation genes. Plast Reconstr Surg. 2012;129:838–847. doi: 10.1097/PRS.0b013e3182450b47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karkkainen MJ, Saaristo A, Jussila L, et al. A model for gene therapy of human hereditary lymphedema. Proc Natl Acad Sci USA. 2001;98:12677–12682. doi: 10.1073/pnas.221449198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harvey NL, Srinivasan RS, Dillard ME, et al. Lymphatic vascular defects promoted by Prox1 haploinsufficiency cause adult-onset obesity. Nat Genet. 2005;37:1072–1081. doi: 10.1038/ng1642. [DOI] [PubMed] [Google Scholar]
- 21.Martel C, Li W, Fulp B, et al. Lymphatic vasculature mediates macrophage reverse cholesterol transport in mice. J Clin Invest. 2013;123:1571–1579. doi: 10.1172/JCI63685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim HY, Rutkowski JM, Helft J, et al. Hypercholesterolemic mice exhibit lymphatic vessel dysfunction and degeneration. Am J Pathol. 2009;175:1328–1337. doi: 10.2353/ajpath.2009.080963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weitman ES, Aschen SZ, Farias-Eisner G, et al. Obesity impairs lymphatic fluid transport and dendritic cell migration to lymph nodes. PLoS One. 2013;8:e70703. doi: 10.1371/journal.pone.0070703. [DOI] [PMC free article] [PubMed] [Google Scholar]