Abstract
Polybrominated diphenyl ethers (PBDEs) are widely used flame retardants, and BDE-47 is a prevalent PBDE congener detected in human tissues. Exposure to PBDEs has been linked to adverse pregnancy outcomes in humans. Although the underlying mechanisms of adverse birth outcomes are poorly understood, critical roles for oxidative stress and inflammation are implicated. The present study investigated antioxidant responses in a human extravillous trophoblast cell line, HTR-8/SVneo, and examined the role of nuclear factor E2-related factor 2 (Nrf2), an antioxidative transcription factor, in BDE-47-induced inflammatory responses in the cells. Treatment of HTR-8/SVneo cells with 5, 10, 15, and 20 μM BDE-47 for 24 h increased intracellular glutathione (GSH) levels compared to solvent control. Treatment of HTR-8/SVneo cells with 20 μM BDE-47 for 24 h induced the antioxidant response element (ARE) activity, indicating Nrf2 transactivation by BDE-47 treatment, and resulted in differential expression of redox-sensitive genes compared to solvent control. Pretreatment with tert-butyl hydroquinone (tBHQ) or sulforaphane, known Nrf2 inducers, reduced BDE-47-stimulated IL-6 release with increased ARE reporter activity, reduced nuclear factor kappa B (NF-κB) reporter activity, increased GSH production, and stimulated expression of antioxidant genes compared to non-Nrf2 inducer pretreated groups, suggesting that Nrf2 may play a protective role against BDE-47-mediated inflammatory responses in HTR-8/SVneo cells. These results suggest that Nrf2 activation significantly attenuated BDE-47-induced IL-6 release by augmentation of cellular antioxidative system via upregulation of Nrf2 signaling pathways, and that Nrf2 induction may be a potential therapeutic target to reduce adverse pregnancy outcomes associated with toxicant-induced oxidative stress and inflammation.
Keywords: Polybrominated diphenyl ethers (PBDEs), antioxidant response, HTR-8/SVneo cells, human placental cells, cytokines, nuclear factor E2-related factor 2 (Nrf2)
INTRODUCTION
Polybrominated diphenyl ethers (PBDEs) are synthetic flame retardants widely used in polyurethane foam, textiles, plastics, building materials and insulation (Hites, 2004). BDE-47 (2,2′,4,4′-tetra-BDE) is one of the most prevalent congers found in human tissues and environmental samples (Hites, 2004), detected in nearly all human serum samples from the NHANES 2003–2004 biomonitoring assessment (Sjodin et al., 2008). Because of PBDEs’ environmental persistence and toxicity, the US EPA has identified PBDEs as a priority human health concern (U.S. Environmental Protection Agency, 2006). Limited studies report associations between PBDE exposure and adverse birth outcomes such as preterm birth, low birth weight or stillbirth (Breslin et al., 1989; Wu et al., 2010). Although PBDEs have been found in gestational tissues such as human placenta (Frederiksen et al., 2009), extraplacental membranes (Miller et al., 2009), amniotic fluid (Miller et al., 2012), and umbilical cord blood (Frederiksen et al., 2009), studies of mechanisms by which PBDEs act on gestational tissues during pregnancy are limited.
Improper regulation of the inflammatory networks has been associated with adverse pregnancy outcomes such as miscarriage, preeclampsia, intrauterine growth restriction (IUGR), and preterm labor (Orsi and Tribe, 2008; Tjoa et al., 2004). Specifically, increased levels of inflammatory mediators such as interleukin (IL)-6 and C-reactive protein were associated with the pathophysiology of preeclampsia and IUGR (Tjoa et al., 2003; Vince et al., 1995), and women who delivered preterm had higher rates of placental ischemia and abnormal placentation than women who delivered at term (Germain et al., 1999; Kim et al., 2003). Moreover, increased levels of IL-8 and IL-6 in cervical fluid, amniotic fluid and maternal serum have been associated with preterm birth (Goldenberg et al., 2005). It is suggested that cytokine dysregulation alters extravillous trophoblast (EVT) functions during placentation, leading to placental alterations that may compromise pregnancy (Anton et al., 2012).
A few studies reported modulation of innate immune responses by BDE-47 treatment in peripheral blood mononuclear cells or placental explants (Ashwood et al., 2009; Peltier et al., 2012). Our previous study showed that BDE-47 treatment of a human first trimester EVT cell line, HTR-8/SVneo, stimulated mRNA and protein expression of the pro-inflammatory cytokines IL-6 and IL-8 (Park et al., 2014). Furthermore, suppression of BDE-47-induced IL-6 production by antioxidant treatments implicates a role for reactive oxygen species (ROS) in the initiation and regulation of BDE-47-stimulated inflammatory responses in the cells (Park et al., 2014). Similarly, the antioxidant N-acetylcysteine (NAC) prevented LPS-stimulated parturition, fetal death in mice and LPS-induced release of pro-inflammatory cytokines from human extraplacental membranes in vitro (Buhimschi et al., 2003a; Cindrova-Davies et al., 2007). Together, these findings suggest an interaction between oxidative stress and inflammatory pathways in gestational compartments. In fact, a growing body of literature shows that ROS can function as signaling molecules in mammalian cells (Finkel, 1998; Khan and Wilson, 1995; Remacle et al., 1995) to regulate expression of genes for inflammatory cytokines, chemokines, and anti-inflammatory molecules (Reuter et al., 2010).
Nuclear factor E2-related factor 2 (Nrf2) is the master transcriptional regulator of oxidative and xenobiotic stress responses (Tjoa et al., 2003). In response to oxidative insults, Nrf2 binds to the antioxidant response element (ARE) in a promoter and activates ARE-regulated genes. A wide range of natural and synthetic small molecules such as tert-butyl hydroquinone (tBHQ) and sulforaphane induce Nrf2 activity to exert cytoprotective activities (Gharavi et al., 2007; Juge et al., 2007). Especially, the anti-inflammatory effect of Nrf2 activation have been implicated in a variety of experimental models (Khor et al., 2006; Rangasamy et al., 2004; Rangasamy et al., 2005; Thimmulappa et al., 2006). Although the mechanisms for the anti-inflammatory effects of Nrf2 are not fully understood, it is suggested that augmentation of cellular antioxidant responses via up-regulation Nrf2 signaling pathway and inhibition of proinflammatory nuclear factor kappa B (NF-κB) signaling pathway may have roles in these responses (Jin et al., 2011; Khodagholi and Tusi, 2011).
Despite its importance, there are few studies about the roles of Nrf2 in gestational tissues during pregnancy. It has been recently reported that dysregulation of Nrf2 signaling pathways is associated with adverse birth outcomes such as preeclampsia and IUGR (Chigusa et al., 2012; Kweider et al., 2012; Loset et al., 2011; Wruck et al., 2009). To our knowledge, however, there is no report about the role of Nrf2 activation in the regulation of toxicant-stimulated inflammatory responses in human first trimester placental cells. Because ROS have been implicated in the activation of inflammatory responses in gestational compartments (Buhimschi et al., 2003b; Cindrova-Davies et al., 2007) and our previous study showed that BDE-47-stimulated cytokine production was dependent on ROS formation (Park et al., 2014), we suggest that the antioxidative transcription factor Nrf2 may play a protective role on BDE-47-induced inflammatory responses in the HTR-8/SVneo cell model. The present study aimed to investigate BDE-47-stimulated antioxidant responses in HTR-8/SVneo cells and examined the roles of Nrf2 activation by Nrf2 inducers on BDE-47-induced inflammatory cytokine responses in the cells.
MATERIALS AND METHODS
Chemicals and assay kits
BDE-47 was purchased from AccuStardard (New Haven, CT, USA). Dimethyl sulfoxide (DMSO), tert-butyl hydroquinone (tBHQ), and sulforaphane were purchased from Sigma Aldrich (St. Louis, MO, USA). RPMI medium 1640, fetal bovine serum (FBS), OptiMem 1 reduced-serum medium, 10 mM non-essential amino acids in minimal essential medium, 0.25% trypsin/EDTA solution and penicillin/streptomycin were purchased from Invitrogen Life Technologies (Carlsbad, CA, USA). Sandwich enzyme-linked immunosorbent assay (ELISA) kit for human IL-6 was purchased from R & D systems (Minneapolis, MN, USA). Antioxidant Response Cignal reporter assay kit, NF-κB Cignal reporter assay kit, Attractene transfection reagent, QIAshredder columns, and RNeasy kits were purchased from Qiagen (Valencia, CA). Dual Luciferase, GSH-Glo™ Glutathione Assays were purchased from Promega (Madison, WI). The iScript cDNA synthesis kits and SsoAdvanced SYBR Green Supermix were purchased from Bio-Rad (Hercules, CA). Primers were synthesized by Integrated DNA Technologies (Coralville, IA).
Cell Culture and treatment
The human first trimester extravillous trophoblast cell line HTR-8/SVneo was kindly provided by Dr. Charles S. Graham (Queen’s University, Kingston, ON, Canada). Cells between passages 71 and 84 were cultured in RPMI 1640 medium supplemented with 10% FBS and 1% penicillin/streptomycin at 37°C in a 5% CO2 humidified atmosphere. Cells were grown to a confluence of 70–90% before treatment. Cells were washed with OptiMem 1 containing 1% FBS and 1% penicillin/streptomycin twice and acclimated with the medium for 1 h at 37 °C. From solutions of 5, 10, 15 and 20 mM BDE-47 in DMSO, exposure media containing 5, 10, 15 and 20 μM BDE-47 were made in OptiMem 1 containing 1% FBS and 1% penicillin/streptomycin immediately prior to initiating the experiment. The final concentration of DMSO in medium was 0.7 % (v/v).
Measurement of intracellular glutathione concentration
Changes in intracellular glutathione (GSH) levels by BDE-47 treatment on HTR-8/SVneo cells were quantified using the GSH-Glo Glutathione assay kit (Promega). The assay is based on the conversion of a luciferin derivative into luciferin in the presence of GSH, catalyzed by glutathione S-transferase (GST). The luminescence generated in a coupled reaction with firefly luciferase is proportional to the amount of glutathione present in the sample. Cells were seeded at a density of 10,000/well in a white, clear-bottomed 96-well plate and incubated for 24 h at 37°C. Then, cells were exposed to BDE-47 for 0.5, 4 or 24 h at 37°C. After exposure, the culture medium was removed and 100 μl of GSH-Glo™ Reagent was added to each well. After a 30 min-incubation, 100 μl of Luciferin Detection Reagent was added to each well, followed by an additional 15-min incubation. The plate was then read in a luminometer. A standard curve was generated by serial 2-fold dilutions of a 10X GSH solution. GSH levels per well were calculated based on luminescence. To examine the effect of Nrf2 induction on GSH production, cells were pretreated with tBHQ for 1 h or sulforaphane for 24 h prior to BDE-47 treatment. After treatment with BDE-47, GSH levels were measured following the manufacturer’s protocol as described above.
Oxidative stress gene array and qRT validation
Changes in mRNA expression of 84 target genes by BDE-47 treatment on HTR-8 cells were quantified using the commercial Oxidative Stress Responses PCR Array (SA biosciences; Valencia, CA). Cells were treated with DMSO (solvent control, 0.7% v/v) or BDE-47 (20 μM) for 4 or 24 h. A concentration of 20 μM BDE-47 was used because our previous studies showed that this was an effective concentration for stimulating increased ROS formation in HTR-8/SVneo cells without being cytotoxic (Park et al., 2014). After incubation, cell lysates were collected and homogenized using QIA shredder (Qiagen). Total RNA was extracted from homogenized lysates using RNeasy mini plus kit (Qiagen), and cDNA was synthesized from 1 μg of total RNA using iScript cDNA synthesis kits (Bio-Rad) following the manufacturer’s protocols. For the array, cDNA from the solvent control and BDE-47 treatment groups were analyzed using the Applied Biosystems 7900HT Sequence Detection System following the SABiosciences recommended protocol. Fold changes were calculated from ΔCT values (gene of interest CT values – Average of all housekeeping gene CT values) using the ΔΔCT method. Mean ΔCT values were compared between groups using paired t-tests from the Limma package of Bioconductor (Smyth, 2004). With qRT-PCR, we validated the findings of the array for those genes with significant mRNA expression changes that were approximately two-fold or more with 20 μM BDE-47 treatment: solute carrier family 7 (anionic amino acid transporter light chain, xc- system), member 11 (SLC7A11), heme oxygenase (decycling) 1 (HMOX1), aldehyde oxidase 1 (AOX1), sulfiredoxin 1 (SRXN1), prostaglandin-endoperoxide synthase 2 (PTGS2), sequestosome 1 (SQSTM1), prion protein (PRNP), glutathione reductase (GSR), ring finger protein 7 (RNF7), thioredoxin reductase 1 (TXNRD1), four and a half LIM domains 2 (FHL2), glutamate-cysteine ligase, modifier subunit (GCLM), glutathione peroxidase 1 (GPX1), ferritin, heavy polypeptide 1 (FTH1), and 24-dehydrocholesterol reductase (DHCR24). Sequences for primer pairs are provided in Supplemental material, Table 1. The qRT-PCR reactions were prepared with SsoAdvanced SYBR Green Supermix and primers, and run on a Bio-Rad CFX96 Real time C1000 thermal cycler following the manufacturer’s recommended protocols. The mRNA levels of each gene of interest were normalized to β-2-micoglobulin mRNA levels and presented as fold change compared to solvent controls.
Measurement of ARE reporter activity
Nrf2 activity was assessed using a commercially available reporter construct (SABiosciences, Qiagen). The reporter consists of a mixture of inducible firefly luciferase gene downstream of tandem antioxidant response element (ARE) consensus binding site repeats and constitutive Renilla luciferase gene controlled by the cytomegalovirus (CMV) promoter. HTR-8/SVneo cells were seeded at a density of 20,000/well in white, clear bottom 96-well plates containing transfection reagent complexed with negative control, positive control, or ARE reporter constructs in Opti-MEM 1 supplemented with 1% non-essential amino acid (NEAA) and 3% FBS. After transfection for 6 h at 37°C, the transfection complex was replaced with fresh medium and cells were incubated for 18 h at 37°C. Cells were then pretreated with tBHQ for 1 h or with sulforaphane for 24 h prior to treatment with BDE-47 for 24 h. Treatment solutions were prepared in OptiMEM 1 supplemented with 1% NEAA, 1% FBS and 1% P/ S. After treatment with BDE-47, medium was aspirated, and cells were passively lysed. Dual luciferase assays were performed according to manufacturer’s instructions. Luminescence was measured using a GloMax Multi Plus detection system (Promega) with two injectors. ARE firefly luciferase activity was normalized to luciferase activity of Renilla, included as an internal control accounting for cell number and transfection efficiency. Data are presented as the fold change in luciferase activity normalized to the control.
Measurement of NF-κB reporter activity
NF-κB activity was assessed using a commercially available reporter construct (SABiosciences, Qiagen). The reporter consists of a mixture of inducible firefly luciferase gene downstream of tandem NF-κB consensus binding site repeats and constitutive Renilla luciferase gene controlled by a CMV promoter. The assay was conducted as described above for ARE reporter activity. NF-κB firefly luciferase activity was normalized to luciferase activity of Renilla. Data are presented as the fold change in luciferase activity normalized to the control.
Measurement of cytokine release
The HTR-8/SVneo cells were seeded at a density of 5 × 104 cells per well in a 24-well plate and cultured for 24 h at 37 °C. Cells were washed once with OptiMem1 medium containing 1 % FBS and 1% penicillin/streptomycin, and pretreated with tBHQ for 1 h or sulforaphane for 24 h prior to 20 μM BDE-47 treatment for 24 h. The concentration of IL-6 in the medium was then analyzed by ELISA as described above, expressed as pg/ml.
Statistical analysis
Statistical analysis was performed with Sigma Plot 11.0 software (Systat Software Inc., San Jose, CA, USA). Data were analyzed either by one-way analysis of variance (ANOVA) or repeated measures two-way ANOVA. If significant effects were detected, the ANOVA was followed by Tukey post-hoc comparison of means. A P <0.05 was considered statistically different. Data were expressed as means ± SEM.
RESULTS
Effect of BDE-47 on cellular GSH
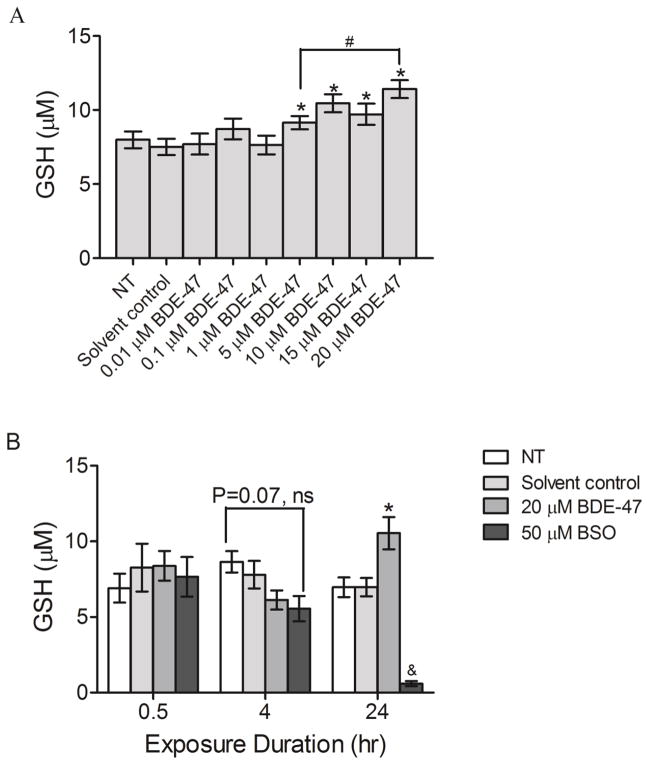
Because our previous work showed that BDE-47 increases generation of reactive oxygen species (Park et al., 2014), intracellular GSH concentration as an indicator of oxidative stress was quantified in HTR-8/SVneo cells after 24-h treatment with BDE-47 using a luminescence based assay. Treatment with 5, 10, 15 and 20 μM BDE-47 increased GSH production by 22%, 39%, 29%, and 52%, respectively, compared to the solvent control (Figure 4.1A; P<0.05). Treatment with 20 μM BDE-47 resulted in significantly increased GSH production compared to treatment with 5 μM BDE-47, also, indicating a concentration-dependent response (Figure 1A; P<0.05). To examine the temporal changes in GSH production in BDE-47-treated cells, GSH levels were measured at 0.5, 4, and 24 h after treatment with 20 μM BDE-47. BDE-47 stimulated GSH production after 24 h (51% compared to solvent control; P<0.05), as observed in the prior experiment, but there were no statistically significant changes in GSH levels at 0.5 or 4 h (Figure 1B; P<0.05). Treatment with 50 μM BSO, included as a positive control, almost completely depleted GSH after 24 h.
Figure 1.
BDE-47-stimulated intracellular GSH production in HTR-8/SVneo cells. A) Cells were non-treated control (NT), or were exposed to solvent control (0.07% v/v DMSO) or BDE-47 (0.01–20 μM) for 24 h, and then GSH levels were quantified using a luminescence-based assay. B) Time-course of GSH levels. Bars represent means±SEM (n=3 experiments). Each experiment was performed in triplicate.*P<0.05, significantly increased compared to solvent control within same time point. #P<0.05, significantly different from each other. &P<0.05, different from NT, solvent control, and BDE-47 treated groups within same time point.
Effect of BDE-47 treatment on ARE reporter activity
We investigated Nrf2 activation as a possible explanation for the increased cellular GSH concentrations observed with BDE-47 treatment, using an ARE reporter activity assay. After 24 h treatment, 10 and 20 μM BDE-47 increased ARE activity by 1.7-fold and 2-fold, respectively, compared to solvent control, indicating Nrf2 activation (Figure 2; P<0.05). We did not detect statistically significant changes at 6 h, although slight increases in ARE activity were suggested.
Figure 2.
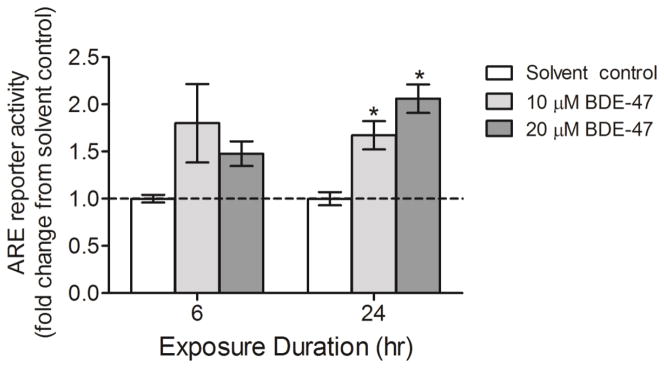
BDE-47-stimulated ARE reporter activity in HTR-8/SVneo cells. Cells were exposed to solvent control (0.7% v/v DMSO) or BDE-47 treatment for 6 or 24 h, and then ARE reporter activity was assessed as an indicator of Nrf2 activation. Data are presented as means±SEM fold change over solvent control (dashed line) for each respective time point. To derive fold changes, firefly luciferase relative light unit (RLU) values were first normalized to Renilla luciferase to compensate for cell number and transfection efficiency, then fold changes were calculated relative to solvent control for each time point (n=3 experiments). Each experiment was performed in triplicate.*P<0.05, significant compared to solvent control within same time point.
Oxidative stress PCR array
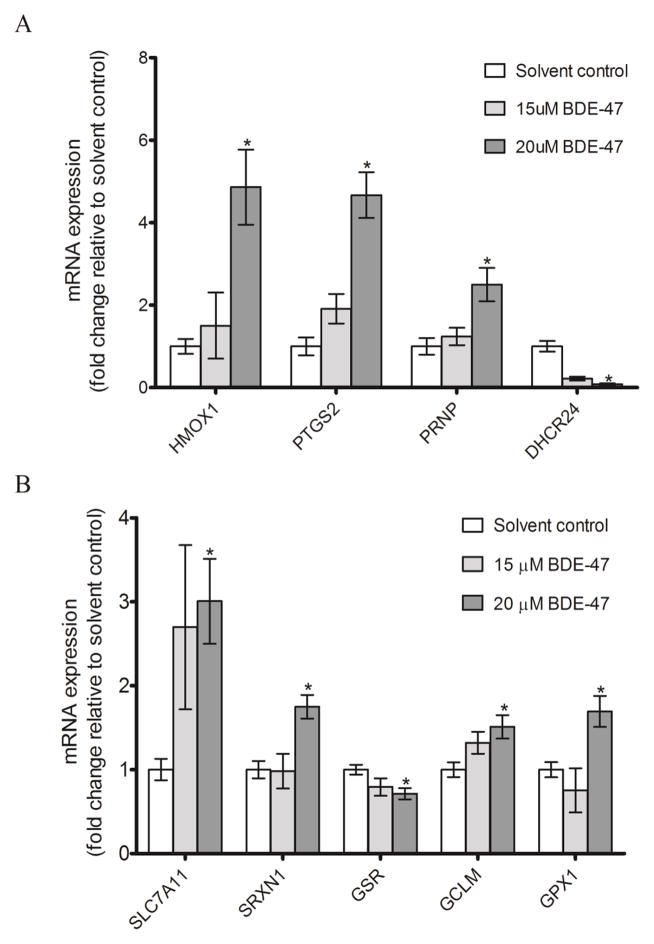
Probing BDE-47 activation of antioxidant responses further, we used a commercial Oxidative Stress PCR Array to investigate changes in expression of redox-sensitive genes. We identified 15 genes with mRNA expression significantly changed two-fold or more by 20 μM BDE-47 treatment compared to solvent control (for complete mRNA array data, see Supplemental material, Table 2). Changes in expression of the array-identified genes were then examined by qRT-PCR. Consistent with the array results, treatment with 20 μM BDE-47 for 24 h significantly increased mRNA expression of HMOX1, PTGS2, and PRNP by 4.9 fold, 4.7 fold, and 2.5 fold, respectively, and nearly abolished mRNA expression of DHCR24 to 0.08 fold relative to solvent control (Figure 3A, P<0.05). The mRNA expression of genes involved in GSH redox cycling was also induced by 20 μM BDE-47 treatment, with SLC7A11, SRXN1, GCLM, and GPX1 increased 3 fold, 1.8 fold, 1.5 fold and 1.7 fold, respectively (Figure 3B, P<0.05). BDE-47 suppressed mRNA expression of GSR to 0.7 fold relative to solvent control (Figure 3B, P<0.05). We did not observe any significant changes in mRNA expression with 15 μM BDE-47.
Figure 3.
BDE-47 effects on HTR-8 cell mRNA expression of genes previously identified with a targeted gene expression array. Cells were exposed to solvent control (0.7% v/v DMSO) or BDE-47 treatment for 24 h. Then, mRNA expression of redox-sensitive genes was quantified by qRT-PCR. A) The mRNA expression of HMOX1, PTGS2, PRNP, and DHCR24. B) The mRNA expression of SLC7A11, SRXN1, GSR, GCLM, and GPX1. Bars represent means±SEM (n=3 experiments). Each experiment was performed in triplicate.*P<0.05, significantly different compared to solvent control.
Effect of Nrf2 inducers on ARE reporter activity
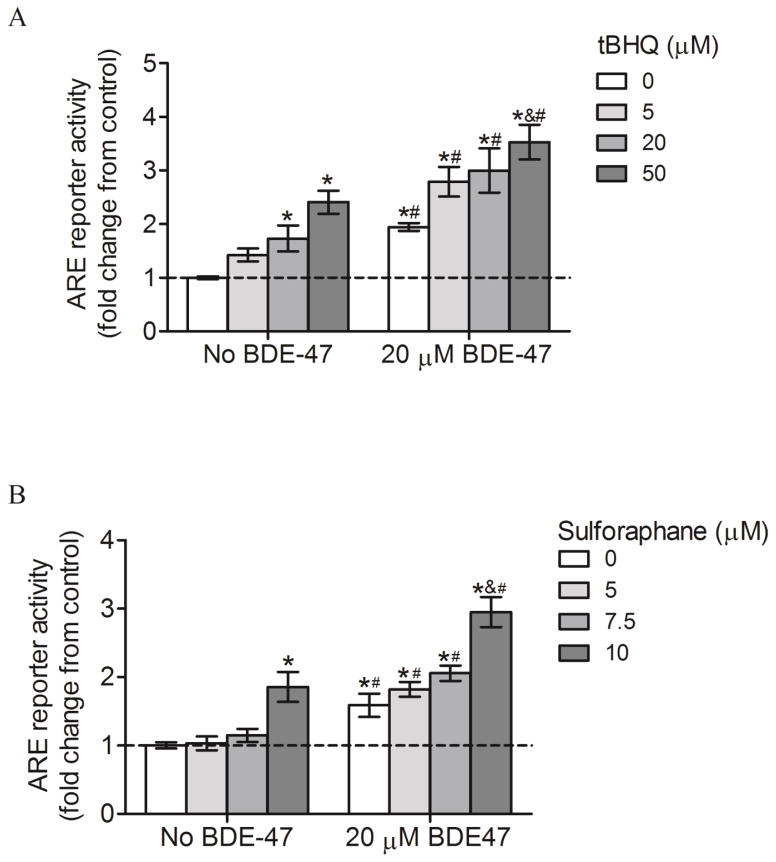
We validated the efficacy of tBHQ and sulforaphane as Nrf2 inducers in the HTR-8/SVneo cells and investigated effects of the Nrf2 inducers on BDE-47-stimulated Nrf2 activation using an ARE reporter activity assay. Treatment with 20 and 50 μM tBHQ increased ARE activity by 1.7-fold and 2.4-fold, respectively, compared with controls not exposed to tBHQ (No BDE-47 with 0 μM tBHQ, Figure 4A; P<0.05). Similarly, 10 μM sulforaphane increased ARE activity by 1.8 fold compared with controls not exposed to sulforaphane (No BDE-47 with 0 μM sulforaphane, Figure 4B; P<0.05). No statistically significant changes were observed with 5 μM tBHQ, or with 5 and 7.5 μM sulforaphane. These results identified 20 and 50 μM tBHQ, and 10 μM sulforaphane, as effective concentrations for Nrf2 activation. Because subsequent experiments would utilize co-treatments of BDE-47 with the Nrf2 inducers, we also measured ARE activity in the presence of BDE-47. Pretreatment with 0, 5, 20, and 50 μM tBHQ followed by 20 μM BDE-47 treatment increased ARE activity 1.9 fold, 2.8 fold, 3 fold, and 3.5 fold, respectively, compared to control (No BDE-47 with 0 μM tBHQ) (Figure 4A; P<0.05). Treatment with 50 μM tBHQ increased ARE activity significantly higher than 0, 5 and 10 μM tBHQ within the 20 μM BDE-47 pretreatment group, showing a concentration-dependent response. BDE-47-treated cells always showed significantly higher ARE activity compared to cells with no BDE-47 and the same tBHQ concentration (Figure 4A; P<0.05). Similarly, pretreatment with 0, 5, 7.5, and 10 μM sulforaphane followed by 20 μM BDE-47 treatment increased ARE activity 1.6 fold, 1.8 fold, 2 fold, and 3 fold, respectively, in BDE-47-pretreated cells compared to control (No BDE-47 with 20 μM tBHQ) (Figure 4B; P<0.05). Treatment with 10 μM sulforaphane resulted in significantly increased activity compared to 0, 5 and 7.5 μM sulforaphane, showing a concentration-dependent increase (Figure 4B; P<0.05). BDE-47-treated cells showed significantly higher ARE activity compared to cells with no BDE-47 and the same sulforaphane concentration (Figure 4.4B; P<0.05). These data show that pretreatment with Nrf2 inducers stimulates Nrf2 transactivation, leading to increased antioxidant capacity in HTR-8/SVneo cells.
Figure 4.
Effect of Nrf2 inducers on ARE reporter activity in HTR-8/SVneo cells without or with subsequent exposure to BDE-47. A) Cells were pretreated with tert-butyl hydroquinone (tBHQ) for 1 h prior to subsequent incubation without or with 20 μM BDE-47 for 24 h. B) Cells were pretreated with sulforaphane for 24 h prior to subsequent incubation without or with 20 μM BDE-47 for 24 h. ARE reporter activity was measured using a firefly luciferase reporter construct and expressed as fold change. To derive fold changes, firefly luciferase relative light unit (RLU) values were first normalized to Renilla luciferase to compensate for cell number and transfection efficiency, and then fold changes were calculated relative to control. Data are presented as means±SEM fold change over control, with the group not exposed to Nrf2 inducer (0 μM) and no BDE-47 pretreatment serving as control (dashed line). Each experiment was performed three times with three replicates in each experiment. *P<0.05, compared to control (dashed line). &P<0.05, compared with lower concentrations of the Nrf2 inducer within 20 μM BDE-47 treatment. #P<0.05, significantly different compared to cells in No BDE-47 group receiving the same Nrf2 inducer treatment
Effect of Nrf2 inducers on BDE-47-stimulated GSH production
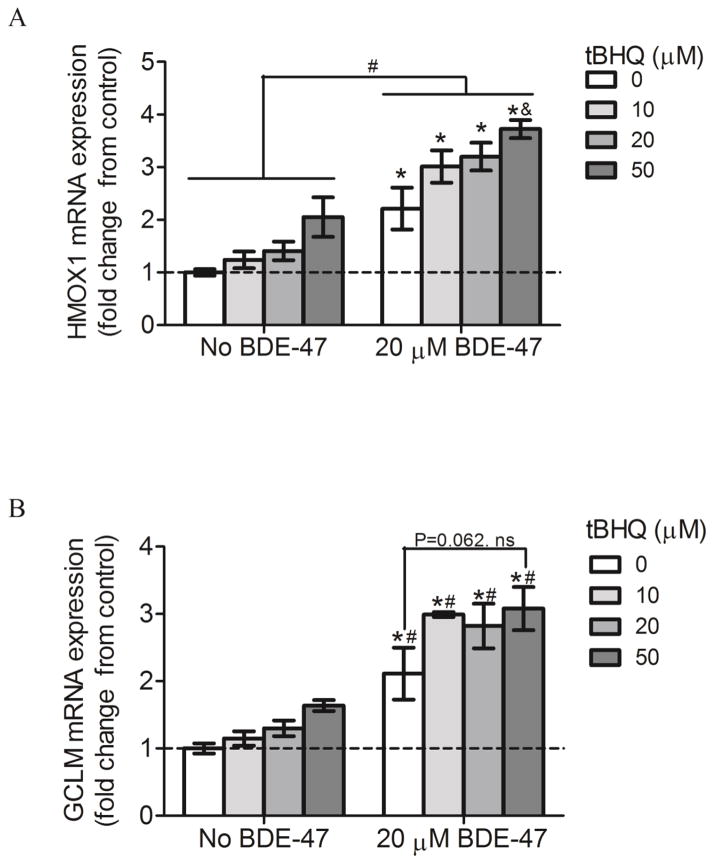
To examine the effect of Nrf2 transactivation by tBHQ on cellular antioxidative capacity, changes in intracellular antioxidant GSH concentrations were assayed. In cells without BDE-47-treatment, 20 and 50 μM tBHQ increased GSH production 20% and 37%, respectively, compared to control (No BDE-47 with 0 μM tBHQ) (Figure 5; P<0.05). Following pretreatment with 0, 10, 20 and 50 μM tBHQ, 20 μM BDE-47 increased GSH concentration by 17%, 27%, 40%, and 63%, respectively, compared to control (No BDE-47 with 0 μM tBHQ) (Figure 5; P<0.05). tBHQ induced GSH production in a concentration-dependent manner, such that pretreatment with 50 μM tBHQ significantly increased GSH compared with pretreatment with 10 and 20 μM tBHQ in those cells subsequently exposed to 20 μM BDE-47 (Figure 5; P<0.05). BDE-47-treated cells showed significantly higher GHS concentrations compared to cells not exposed to BDE-47 at the same tBHQ concentration (Figure 4A; P<0.05) (Figure 5; P<0.05). These findings suggest that cells pretreated with tBHQ may have an augmented antioxidant capacity against BDE-47 treatment.
Figure 5.
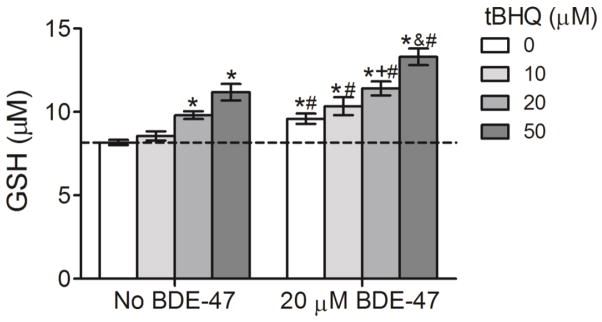
Effect of pretreatment with the Nrf2 inducer tert-butyl hydroquinone (tBHQ) on BDE-47-stimulated GSH in HTR-8/SVneo cells. Cells were pretreated with tBHQ (10–50 μM) prior to subsequent incubation without or with 20 μM BDE-47 for 24 h. GSH levels were quantified using a luminescence-based assay (n=3 experiments). Each experiment was performed in triplicate. Data are presented as means±SEM fold change over control, with the group exposed to neither tBHQ (0 μM) nor BDE-47 pretreatment (No BDE-47) serving as control (dashed line). *P<0.05, compared to control (dashed line).+P<0.05, compared to 0 μM tBHQ within 20 μM BDE-47 treatment. &P<0.05, compared to 0, 10, and 20 μM tBHQ within 20 μM BDE-47 treatment. #P<0.05, significantly different compared to cells in No BDE-47 group receiving the same Nrf2 inducer treatment
Effect of Nrf2 inducers on expression of HMOX1 and GCLM
To evaluate the role of Nrf2 in BDE-47-induced expression of antioxidant genes, mRNA expression of HMOX1 and GCLM was quantified in HTR-8/SVneo cells after pretreatment with tBHQ without and with subsequent exposure to BDE-47. Treatment with 20 μM BDE-47 significantly increased mRNA expression of HMOX1 and GCLM compared to control (No BDE-47 with 0 μM tBHQ) at all tBHQ concentrations (0, 10, 20 and 50 μM) (Figure 6A and 6B; P<0.05). Pretreatment with 50 μM tBHQ significantly increased HMOX1 mRNA expression by 68% compared to no pretreatment (0 μM tBHQ) in BDE-47-treated cells (Figure 6A; P<0.05). However, mRNA expression of GCLM was not statistically significantly increased (Figure 6B; P=0.062). We did not observe any significant changes in solvent controls (No BDE-47) with tBHQ pretreatment. Increased expression of antioxidant enzymes by tBHQ suggests that cells pretreated with tBHQ might have increased protection from BDE-47-stimulated oxidative damage.
Figure 6.
Effect of pretreatment with the Nrf2 inducer tert-butyl hydroquinone (tBHQ) on mRNA expression of the antioxidant genes HMOX1 and GCLM. HTR-8/SVneo cells were pretreated with tBHQ, and then exposed to solvent control (No BDE-47, 0.7% v/v DMSO) or BDE-47 for 24 h. Bars represent means±SEM fold change over control (dashed line, No BDE-47 with 0 μM tBHQ). The target gene expression from each sample was first normalized to the housekeeping gene B2M, and then fold changes were calculated relative to the normalized control (n=3 experiments). Each experiment was performed in triplicate. A) The mRNA expression of HMOX1. B) The mRNA expression of GCLM. *P<0.05, compared to control (dashed line). #P<0.05, significantly different compared to cells in No BDE-47 group receiving the same Nrf2 inducer treatment. &P<0.05, compared to no pretreatment (0 μM tBHQ) in BDE-47-treated cells.
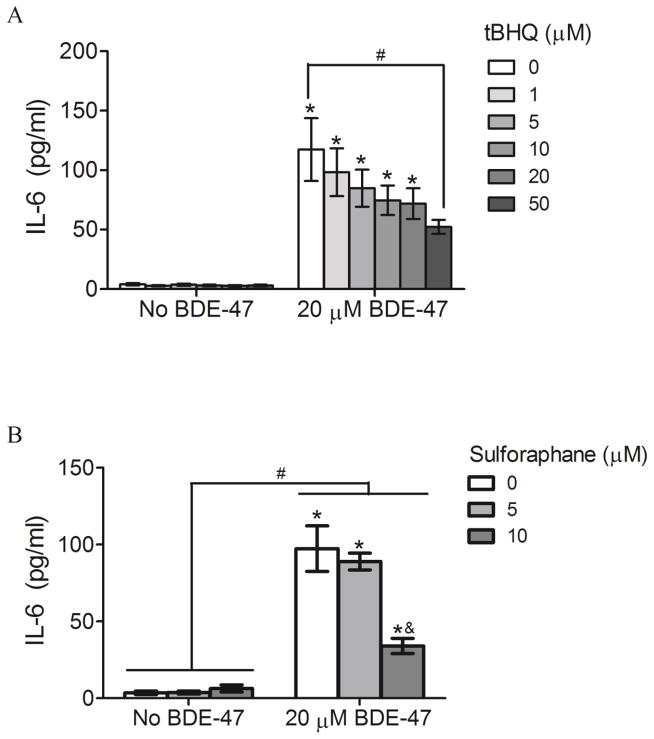
Effect of Nrf2 inducers on IL-6 production
Because IL-6 was shown to regulate trophoblast migration and invasion in vitro (Jovanovic and Vicovac, 2009), and our previous study showed that BDE-47-stimulated IL-6 production was dependent on ROS formation (Park et al., 2014), we investigated the role of the antioxidant transcription factor Nrf2 in regulation of BDE-47-stimulated IL-6 release. HTR-8/SVneo cells were pretreated with the known Nrf2 inducers tBHQ or sulforaphane prior to exposure to BDE-47. Neither tBHQ nor sulforaphane had a significant effect on IL-6 release in the absence of BDE-47 exposure (Figure 7A and 7B, respectively). Treatment with 20 μM BDE-47 increased IL-6 release compared to cells not exposed to BDE-47 for all pretreatment conditions (0–50 μM tBHQ or 0–10 μM sulforaphane, Figures 7A and 7B, respectively; P<0.05). Though still elevated compared with no BDE-47 treatment, pretreatment with the Nrf2 inducers reduced BDE-47-stimulated IL-6 release: 50 μM tBHQ significantly suppressed BDE-47-stimulated IL-6 release by 55% compared with 0 μM tBHQ (Figure 7A; P<0.05), and 10 μM sulforaphane decreased BDE-47-induced IL-6 release by 65% compared with 0 μM sulforaphane (Figure 8B; P<0.05).
Figure 7.
Effect of pretreatment with Nrf2 inducers on BDE-47-stimulated IL-6 release from HTR-8/SVneo cells. A) Cells were pretreated with tert-butyl hydroquinone (tBHQ) for 1 h prior to subsequent incubation without or with 20 μM BDE-47 for 24 h. B) Cells were pretreated with sulforaphane for 24 h prior to subsequent incubation without or with 20 μM BDE-47 for 24 h. IL-6 levels were quantified using ELISA. Bars represent means±SEM (n=3 experiments). Each experiment was performed in triplicate. *P<0.05, significantly different compared to control (0 μM tBHQ or 0 μM sulforaphane with no BDE-47 treatment). #P<0.05, significantly different from each other. &P<0.05, significantly decreased compared with 0 μM and 5 μM sulforaphane pretreatment with BDE-47 exposure.
Figure 8.
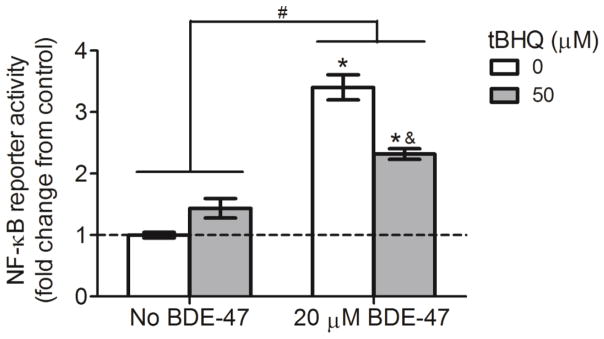
Effect of pretreatment with tert-butyl hydroquinone (tBHQ) on BDE-47-induced NF-κB reporter activity in HTR-8/SVneo cells. Cells were pretreated with tBHQ for 1 h prior to subsequent treatment without or with 20 μM BDE-47 for 24 h. NF-κB reporter activity was quantified using a firefly luciferase reporter construct and expressed as fold change. To derive fold changes, firefly luciferase relative light unit (RLU) values were first normalized to Renilla luciferase to compensate for cell number and transfection efficiency, then fold changes were calculated relative to control (n=3 experiments). Data are presented as means±SEM fold change over control (0 μM tBHQ with no BDE-47, dashed line). *P<0.05, significantly different compared to control (dashed line). #P<0.05, significantly different from each other. &P<0.05, significantly different compared to 50 μM tBHQ pretreatment with BDE-47 exposure.
Effect of tBHQ on NF-κB transactivation
Because prior studies implicated that Nrf2 activation exhibits its anti-inflammatory effect partly via suppression of the pro-inflammatory transcription factor NF-κB (Jin et al., 2011), we examined the possible involvement of Nrf2 in BDE-47-stimulated NF-κB reporter activity. Treatment with tBHQ alone had no statistically significant effect on NF-κB activation compared with solvent control (0 μM tBHQ with no BDE-47; Figure 8). Treatment with 20 μM BDE-47 increased NF-κB reporter activity 3.4-fold compared to control (0 μM tBHQ with no BDE-47) (Figure 8; P<0.05). Though elevated compared with no BDE-47 treatment, pretreatment with 50 μM tBHQ suppressed the BDE-47-stimulated activation of NF-κB by 32% (Figure 4.8; P<0.05).
DISCUSSION
PBDEs are flame retardant chemicals commonly detected in human serum, with BDE-47 among the most abundant of the PBDE congeners detected (Sjodin et al., 2008). Previously, we showed that BDE-47 directly stimulates ROS generation and proinflammatory cytokine responses in the first trimester human EVT cell line, HTR-8/SVneo (Park et al., 2014). In the present study, we show that BDE-47 activated oxidative stress response pathways, stimulating differential expression of redox-sensitive genes and augmentation of intracellular GSH, with concurrent transactivation of Nrf2 and NF-κB in HTR-8/SVneo cells. Especially, we report novel findings that induction of Nrf2 activity by Nrf2 inducers suppressed BDE-47-stimulated release of the proinflammatory cytokine IL-6 and activation of a NF-κB reporter in HTR-8/SVneo cells, implicating crosstalk between Nrf2 and NF-κB pathways.
The concentrations of BDE-47 in this study are several orders of magnitude higher (100-fold or more) than the median concentrations reported previously in human gestational fluids and tissue: 337 – 21842 pg/ml in amnionic fluid (Miller et al., 2012), 0.11–3000 ng/g lipid in placentae (Doucet et al., 2009; Frederiksen et al., 2009), and 0.46 to 504 ng/g lipid in umbilical cord blood (Frederiksen et al., 2009; Guvenius et al., 2003; Wu et al., 2010). However, the concentrations of PBDEs in placentae can be as high as ~8 μM (Doucet et al., 2009). Moreover, correcting for adsorption onto plastic, estimated at 73% (Barber et al., 2006; Mundy et al., 2004), the corrected concentrations of BDE-47 in culture medium in this study range from 2.7 nM to 5.4 μM.
Consistent with ROS generation previously described (Park et al., 2014), BDE-47 treatment resulted in differential expression of the redox-sensitive genes HMOX1, PTGS2, PRNP, DHCR24, SLC7A11, SRXN1, GSR, GCLM, and GPX1 in HTR-8/SVneo cells. Several lines of evidence suggest that the antioxidant and anti-inflammatory enzyme heme oxygenase (HO)-1 (Tjoa et al., 2003), encoded by the gene HMOX1, is a key regulator during pregnancy (Vince et al., 1995). For example, HMOX1 polymorphisms have been associated with increased incidence of idiopathic recurrent miscarriages in women (Denschlag et al., 2004), and placentas from human pathologic pregnancies including spontaneous abortion, choriocarcinoma, and hydatidiform mole, express lower levels of HO-1 compared with normal pregnancies (Zenclussen et al., 2003). PRNP, the gene for prion protein, is known to protect cells from oxidative damage and to prevent apoptosis (Liang et al., 2006; Watt et al., 2005). It was recently reported that PRNP is highly expressed in placentas from preeclamptic pregnancies (Hwang et al., 2010). We suggest that HMOX1 and PRNP may play protective roles against BDE-47-stimulated generation of ROS and pro-inflammatory mediators in placental cells.
Similarly, SLC7A11, SRXN1, GSR, GCLM, and GPX1 are genes involved in GSH redox cycling. The first step of GSH synthesis is rate-limiting and is catalyzed by glutamate-cysteine ligase composed of a glutamate-cysteine ligase catalytic subunit (GCLC) and a glutamate-cysteine ligase modifier subunit (GCLM) (Lu, 2009). Furthermore, SLC7A11 encodes an amino acid antiporter that mediates the exchange of extracellular L-cystine and intracellular L-glutamate across the cellular plasma membrane, a critical step in glutathione production and anti-oxidant protection (Lewerenz et al., 2013), and GSR encodes glutathione reductase, an antioxidant enzyme that catalyzes the reduction of GSH disulfide leading to increased availability of reduced GSH (Harvey et al., 2009). Thus, the upregulation of GCLM, SLC7A11, and GSR is consistent with the augmented GSH concentrations observed in BDE-47-stimulated HTR-8/SVneo cells. Increased expression of SRNX1 and GPX1 may implicate increased oxidation of proteins and lipids by BDE-47-stimulated ROS generation (Park et al., 2014) because SRNX1 codes for sulfiredoxin 1, which plays a role in reduction of oxidative modification on proteins (Findlay et al., 2005), and GPX1 codes for glutathione peroxidase 1, which catalyzes the reduction of hydroperoxides and lipid peroxides (Chance et al., 1979). However, further experiments beyond the scope of the present study are needed to measure oxidation of proteins and lipids. Genes involved in glutathione redox regulation have been linked with adverse pregnancy outcomes. For example, female GCLM−/−mice had compromised fertility due to early preimplantation embryo mortality (Nakamura et al., 2011). In addition, GSR activity was increased in human placentas from preterm delivery (Prokopenko et al., 2002) and GPX1 activity was decreased in human preeclamptic placentas (Mistry et al., 2010).
Among other genes, expression of PTGS2, the gene for COX-2, was highly induced in our study. COX-2 is a rate-limiting enzyme in the synthesis of prostaglandins (Shanmugam et al., 2006). Increased PTGS2 mRNA expression and prostaglandins in gestational compartments have been associated with preterm birth (Cox et al., 1993; Mijovic et al., 1998). In addition, prostaglandin E2 (PGE2) has been reported to regulate trophoblast migration and invasion that are critical for proper placentation (Biondi et al., 2006; Horita et al., 2007a; Nicola et al., 2005a). Given the critical roles for prostaglandins in pregnancy, further study could investigate the effects of BDE-47 on PGE2 production and trophoblast cellular function. In addition, expression of DHCR24, the gene for 3β-hydroxysterol-D24 reductase, was decreased in the present study. Because DHCR24 catalyzes the last step in cholesterol biosynthesis, reduced DHCR24 expression could potentially interfere with synthesis of steroid hormones, including progesterone, which plays critical roles in maintenance of pregnancy (Luu et al., 2014). Moreover, DHCR24 expression was downregulated in IUGR placentas (Diplas et al., 2009). Whether the BDE-47-stimulated DHCR24 gene responses observed in the present study are relevant to human pregnancy requires additional experiments beyond the scope of the present study. Furthermore, changes in expression of critical genes should be confirmed with protein analysis in future experiments to ascertain functional relevance.
Expression of the genes identified in the PCR array are either directly or indirectly regulated by Nrf2 (Ma, 2013; Taylor et al., 2008; Wakabayashi et al., 2010), suggesting that Nrf2 may play a critical role in the regulation of BDE-47-mediated cellular defense responses. Nrf2 is a well-known redox-sensitive transcription factor that translocates to the nucleus where it binds to ARE and activates ARE-mediated gene expression (Itoh et al., 1997; Motohashi and Yamamoto, 2004; Osburn et al., 2006). Although we did not measure Nrf2 translocation, we did observed increased ARE reporter activity in BDE-47-exposed HTR-8/SVneo cells, indicating Nrf2 activation in response to BDE-47. BDE-47 stimulates increased ROS generation (Park et al., 2014), and ROS can oxidize cysteine residues on the Nrf2 inhibitor Keap1, leading to conformational changes in Keap1, with subsequent Nrf2 release and translocation of Nrf2 to activate ARE-dependent gene expression (Rushmore et al., 1991), ultimately serving as a defensive mechanism to protect cells from ROS and inflammation. Experiments beyond the scope of this investigation, including knockdown of Nrf2 and Keap1, are needed to provide a more complete understanding of the roles of Nrf2 in the BDE-47-stimulated responses. Nonetheless, although there have been extensive studies on the protective role of Nrf2 against carcinogens and xenobiotics in vitro and in vivo (Fahey et al., 2002; Kensler et al., 2007), this is the first study to report BDE-47-stimulated activation of Nrf2 pathways in human placental cells.
In limited studies, increased Nrf2 activity was reported in cytotrophoblasts and EVTs from placentae with IUGR or preeclampsia (Kweider et al., 2012; Wruck et al., 2009). In addition, genome-wide transcriptional profiling of preeclamptic and normal pregnancies showed that the Nrf2-mediated oxidative stress response was dysregulated in preeclampsia (Chigusa et al., 2012; Loset et al., 2011). Furthermore, decreased expression of HO-1, a hallmark of Nrf2 activation, was associated with lower cell motility and trophoblast invasion (Bilban et al., 2009). Together, these data imply that Nrf2 may play a critical role in the regulation of trophoblast cellular function and invasion, and that dysregulation of Nrf2 may contribute to the etiology and progression of birth complications.
Migration and invasion of extravillous trophoblast into the spiral arteries are critical events during placentation (Brosens et al., 1967; Pijnenborg et al., 1983). It has been suggested that inflammation within the gestational compartment may lead to impaired trophoblast cellular function, contributing to the placental dysfunction seen in pregnancy-related disorders (Anton et al., 2012) such as IUGR, preeclampsia and preterm birth (Germain et al., 1999; Gomez et al., 2008; Kim et al., 2002; Ness and Sibai, 2006; Riewe et al., 2010). IL-6 has been shown to increase migration and invasion in HTR-8/SVneo cells (Jovanovic et al., 2010; Jovanovic and Vicovac, 2009) and in JEG-3 choriocarcinoma cells (Dubinsky et al., 2010). In addition, LPS reduced invasion of HTR-8/SVneo cells with increased production of IL-6 (Anton et al., 2012). Moreover, high levels of IL-6 in the cervicovaginal fluid, amniotic fluid, and maternal serum were associated with increased risk for preterm birth (Dortbudak et al., 2005; Goepfert et al., 2001; Romero et al., 2002; Wenstrom et al., 1996) with evidence of higher rates of placental ischemia and abnormal placentation than controls (Germain et al., 1999; Kim et al., 2003). These findings implicate a critical role for IL-6 in regulating trophoblast cellular function during placentation, and suggest that dysregulation of IL-6 production may contribute to adverse birth outcomes associated with abnormal placentation.
Our previous study showed that BDE-47 stimulated production of proinflammatory IL-6 in HTR-8/SVneo cells (Park et al., 2014). Because the role of Nrf2 in the regulation of trophoblast cellular function and invasion is implicated (Bilban et al., 2009) and many studies have provided evidence of an anti-inflammatory effect of Nrf2 in a variety of experimental models (Khor et al., 2006; Rangasamy et al., 2004; Rangasamy et al., 2005; Thimmulappa et al., 2006), the present study examined the hypothesis that induction of Nrf2 would suppress BDE-47-stimulated IL-6 release in HTR-8/SVneo cells. Our results showed that pretreatment with the Nrf2 inducers tBHQ or sulforaphane suppressed BDE-47-stimulated IL-6 production NF-κB transactivation in HTR-8/SVneo cells. NF-κB is a transcription factor that plays a crucial role in immune and inflammatory response (Blackwell and Christman, 1997). Although we did not assess a causal relationship between BDE-47-stimulated NF-κB activity and IL-6 release, NF-κB is well known to regulate the transcription of IL-6 (Blackwell and Christman, 1997; Reuter et al., 2010). As such, decreased NF-κB activity partially explains the anti-inflammatory effect of tBHQ in the present study. These findings are consistent with previous studies showing that tBHQ or sulforaphane decrease NF-κB activation, production of inflammatory cytokines (TNF-α, IL-1β, and IL-6), COX-2 expression, and PGE2 release in vivo and in vitro (Heiss et al., 2001; Jin et al., 2010; Khodagholi and Tusi, 2011; Koh et al., 2009). However, to the best of our knowledge, the present study is the first to report the protective role of Nrf2 activation on toxicant-stimulated inflammatory responses in human placental cells.
A wide range of natural and synthetic small molecules including tBHQ and sulforaphane induce Nrf2 activity (Ma, 2013). tBHQ-stimulates ROS formation as a consequence of redox cycling, which subsequently stimulates nuclear translocation of Nrf2 and activates ARE-dependent gene expression (Gharavi et al., 2007; Itoh et al., 1999; Sian et al., 1994). Sulforaphane is an electrophile that can react with protein thiols to form thionoacyl adducts and is believed to modify cysteine residues in Keap 1 protein to a sulfenic acid (−SOH), resulting in a conformational change of Keap1, translocation of Nrf2, and upregulation of antioxidant genes (Imhoff and Hansen, 2010; Keum, 2011). The augmented GSH concentrations and increased antioxidant gene expression observed in the present study agree with prior reports that Nrf2 inducers increase cellular antioxidative capacity (Alfieri et al., 2011; Hara et al., 2003). Consistent with augmented antioxidant responses, pretreatment with tBHQ or sulforaphane suppressed BDE-47-stimulated ROS formation (as visualized by microscopic detection of DCF fluorescence) after 24 h of exposure to BDE-47 (data not shown). Although the precise mechanism regarding the anti-inflammatory activity of Nrf2 inducers remains elusive, it is suggested that the anti-inflammatory properties might result from augmentation of cellular antioxidant systems via up-regulation of the Nrf2 signaling pathway and inhibition of the NF-κB signaling pathway (Jin et al., 2011; Khodagholi and Tusi, 2011).
The Nrf2 and NF-κB signaling pathways interact at several points through mechanisms of regulation ranging from direct effects on the transcription factors themselves to protein – protein interactions and second-messenger effects on target genes (Wakabayashi et al., 2010). It is suggested that Nrf2 may reduce available co-activator levels and promote recruitment of a co-repressor, leading to interruption of NF-κB binding to DNA. In addition, Nrf2 target genes such as HO-1, NQO1, and thioredoxin (TRX) are able to influence NF-κB activity (Wakabayashi et al., 2010). Moreover, Nrf2 interferes with NF-κB inflammatory signaling pathways through the maintenance of cellular redox status because NF-κB is activated in the oxidizing environment (Kabe et al., 2005). Our results showed suppression of BDE-47-stimulated NF-κB transactivation by tBHQ pretreatment, implicating cross talk between the Nrf2 and NF-κB signaling pathways. In addition, tBHQ stimulated mRNA expression of HMOX1, the gene for HO-1, and augmented GSH production concomitant with suppression of NF-κB reporter activity. Based on our findings and other relevant reports, the following model is suggested: that BDE-47-stimulated ROS may activate Nrf2 to restore cellular redox status to a less oxidizing environment via increased cellular antioxidant capacity, resulting in suppression of NF-κB activity, and, in turn, decreasing IL-6 production in HTR-8/SVneo cells. In addition, the increased antioxidant capacity may scavenge cellular ROS further leading to suppression of IL-6 secretion that is dependent on BDE-47-mediated ROS formation (Park et al., 2014). However, we should note that the observed anti-inflammatory effects may originate from not a single mechanism, but from multiple mechanisms involving various proteins and signaling molecules (Kabe et al., 2005). Moreover, the direct dependence of the anti-inflammatory effect on Nrf2 should be tested using genetic knockdown approaches such as iRNA.
In the present study, IL-6 was the only cytokine studied. However, overproduction of IL-6 alone may not accurately represent trophoblast inflammatory response to BDE-47, and the impact of BDE-47-stimulated inflammatory responses on trophoblast cellular function and invasion should be investigated further to confirm potential relevance of our findings to placentation and pregnancy. In addition, the present study did not examine direct anti-inflammatory effects of Nrf2 activation, but rather presented the concomitant activation of Nrf2-mediated pathways with suppression of IL-6 release when pretreated with Nrf2 inducers. Moreover, the pharmacological Nrf2 inducers tBHQ and sulforaphane may bind to proteins in a non-specific manner to affect other redox-sensitive transcription factors and protein kinases that impact cellular mechanisms other than Nrf2 signaling (Reuter et al., 2010). Nonetheless, our conclusion that Nrf2 activation may be involved in BDE-47-stimulated inflammatory responses is supported by the use of two Nrf2 inducers, tBHQ and sulforaphane, which activate Nrf2 pathways by different mechanisms yet resulted in a similar effect on IL-release in response to BDE-47.
Another limitation of the present study is that the results of in vitro experiments may not accurately reflect responses in vivo that include complex interactions between inflammatory mediators and trophoblast invasion involving a number of autocrine and paracrine factors such as growth factors, growth factor-binding proteins, proteoglycans, other cytokines/chemokines, integrins, adhesion and proteolytic molecules (Anton et al., 2012; Chakraborty et al., 2002; Lala and Chakraborty, 2003). Likewise, our experiments used a transformed cell line that may not reflect primary extravillous trophoblast cells. Although HTR-8/SVneo cells have a similar phenotype compared to their primary counterparts (Biondi et al., 2006; Graham et al., 1993; Jovanović et al., 2010), retaining migratory capability and expressing specific placental trophoblast markers (Biondi et al., 2006; Khan et al., 2011), it is reported that the cells have a different transcriptomic and epigenetic profile compared to primary extravillous trophoblast cells (Bilban et al., 2010; Novakovic et al., 2011). To address the latter issue, further investigation using primary trophoblasts or placental tissues will be needed to validate the potential relevance of our results to pregnancy.
Despite these limitations, our findings suggest potential adverse impacts of PBDE exposure during pregnancy. Invasion of EVTs into maternal spiral arteries is a key event during placentation (Brosens et al., 1967; Pijnenborg et al., 1983; Pijnenborg et al., 1980), and impaired EVT invasion has been attributed to pathologies of adverse birth outcomes with the evidence of abnormal placentation. Because IL-6 has been shown to regulate EVT proliferation, migration, and invasion during first trimester of pregnancy (Biondi et al., 2006; Horita et al., 2007b; Jovanovic et al., 2010; Jovanovic and Vicovac, 2009; Nicola et al., 2005b), overproduction of IL-6 in HTR-8/SVneo cells by BDE-47 suggests that BDE-47 exposure may disrupt trophoblast cellular function, leading to improper trophoblast invasion and abnormal placentation, thereby potentially contributing to adverse obstetrical outcomes. Ongoing research in our laboratory on the effects of PBDEs on trophoblast cellular function will lead us toward a better understanding of the mechanisms and relevant risks associated with PBDE exposures during pregnancy.
In summary, BDE-47, a predominant flame retardant chemical found in human tissues, activates Nrf2-dependent antioxidant responses in human first trimester EVTs as indicated by differential expression of oxidative stress genes, stimulated ARE reporter activity, and augmented production of GSH. Our results provide evidence that Nrf2 activation by chemical inducers suppressed BDE-47-stimulated IL-6 production in human placental cells with stimulated ARE reporter activity, reduced NF-κB reporter activity, increased GSH production, and stimulated expression of antioxidant genes compared to non-Nrf2 inducer pretreated groups. This is the first study to show that BDE-47 activated Nrf2-dependent oxidative stress pathways in human first trimester EVTs and to link PBDE-stimulated pro-inflammatory responses with Nrf2 signaling pathways. Because proper trophoblast function is necessary for placental development and successful pregnancy, and dysregulation of inflammatory responses are associated with altered trophoblast invasion and placental dysfunction, further investigation of the impact of BDE-47 on trophoblast function is warranted. In addition, further studies about the role of Nrf2 on BDE-47-stimulated responses will be needed to confirm its protective effects and to consider Nrf2 as a potential therapeutic target to prevent adverse birth outcomes.
Supplementary Material
Highlights.
BDE-47 stimulated ARE reporter activity and GSH production.
BDE-47 resulted in differential expression of redox-sensitive genes.
Nrf2 inducers upregulated Nrf2-mediated oxidative stress responses.
Nrf2 inducers reduced BDE-47-stimulated IL-6 release and NF-κB activity.
Acknowledgments
We thank the University of Michigan’s Immunology Core for its assistance with cytokine ELISA analysis. This work was supported by a grant to RLC (R01 ES014860), a project in the Superfund Research Program PROTECT Center to RL-C (P42 ES017198), and the Center for Lifestage Exposure and Adult Disease (P30 ES017885) from the National Institute of Environmental Health Sciences (NIEHS), National Institute of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIEHS or NIH.
Abbreviations
- AOX1
aldehyde oxidase 1
- ARE
antioxidant response element
- B2M
β-2-micoglobulin
- BDE-47
2,2′,4,4′-tetra-BDE
- BSO
buthionine sulphoximine
- CMV
cytomegalovirus
- COX-2
cyclooxygenase-2
- DHCR24
24-dehydrocholesterol reductase
- DMSO
Dimethyl sulfoxide
- ELISA
enzyme-linked immunosorbent assay
- EVT
extravillous trophoblast
- FBS
fetal bovine serum
- FHL2
four and a half LIM domains 2
- FTH1
ferritin, heavy polypeptide 1
- GCLM
glutamate-cysteine ligase, modifier subunit
- GPX1
glutathione peroxidase 1
- GSH
glutathione
- GSR
glutathione reductase
- GST
glutathione S-transferase
- HMOX1
heme oxygenase (decycling) 1
- HO-1
heme oxygenase-1
- IL
interleukin
- IUGR
intrauterine growth restriction
- Keap1
kelch-like ECH-associated protein 1
- NAC
N-acetylcysteine
- NF-κB
nuclear factor kappa B
- NQO1
NAD(P)H dehydrogenase, quinone 1
- Nrf2
nuclear factor E2-related factor 2
- PBDEs
polybrominated diphenyl ethers
- PGE2
prostaglandin E2
- PRNP
prion protein
- PTGS2
prostaglandin-endoperoxide synthase 2
- RNF7
ring finger protein 7
- ROS
reactive oxygen species
- SLC7A11
solute carrier family 7 (anionic amino acid transporter light chain, xc- system), member 11
- SQSTM1
sequestosome 1
- SRXN1
sulfiredoxin 1
- tBHQ
tert-butyl hydroquinone
- TRX
thioredoxin
- TXNRD1
thioredoxin reductase 1
Footnotes
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alfieri A, Srivastava S, Siow RC, Modo M, Fraser PA, Mann GE. Targeting the nrf2-keap1 antioxidant defence pathway for neurovascular protection in stroke. The Journal of physiology. 2011;589(Pt 17):4125–36. doi: 10.1113/jphysiol.2011.210294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton L, Brown AG, Parry S, Elovitz MA. Lipopolysaccharide induces cytokine production and decreases extravillous trophoblast invasion through a mitogen-activated protein kinase-mediated pathway: Possible mechanisms of first trimester placental dysfunction. Human reproduction. 2012;27(1):61–72. doi: 10.1093/humrep/der362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P, Schauer J, Pessah IN, Van de Water J. Preliminary evidence of the in vitro effects of bde-47 on innate immune responses in children with autism spectrum disorders. J Neuroimmunol. 2009;208(1–2):130–5. doi: 10.1016/j.jneuroim.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber JL, Walsh MJ, Hewitt R, Jones KC, Martin FL. Low-dose treatment with polybrominated diphenyl ethers (pbdes) induce altered characteristics in mcf-7 cells. Mutagenesis. 2006;21(5):351–60. doi: 10.1093/mutage/gel038. [DOI] [PubMed] [Google Scholar]
- Bilban M, Haslinger P, Prast J, Klinglmuller F, Woelfel T, Haider S, Sachs A, Otterbein LE, Desoye G, Hiden U, Wagner O, Knofler M. Identification of novel trophoblast invasion-related genes: Heme oxygenase-1 controls motility via peroxisome proliferator-activated receptor gamma. Endocrinology. 2009;150(2):1000–13. doi: 10.1210/en.2008-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilban M, Tauber S, Haslinger P, Pollheimer J, Saleh L, Pehamberger H, Wagner O, Knofler M. Trophoblast invasion: Assessment of cellular models using gene expression signatures. Placenta. 2010;31(11):989–96. doi: 10.1016/j.placenta.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Biondi C, Ferretti ME, Pavan B, Lunghi L, Gravina B, Nicoloso MS, Vesce F, Baldassarre G. Prostaglandin e2 inhibits proliferation and migration of htr- 8/svneo cells, a human trophoblast-derived cell line. Placenta. 2006;27(6–7):592–601. doi: 10.1016/j.placenta.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Blackwell TS, Christman JW. The role of nuclear factor-kappa b in cytokine gene regulation. Am J Respir Cell Mol Biol. 1997;17(1):3–9. doi: 10.1165/ajrcmb.17.1.f132. [DOI] [PubMed] [Google Scholar]
- Breslin WJ, Kirk HD, Zimmer MA. Teratogenic evaluation of a polybromodiphenyl oxide mixture in new zealand white rabbits following oral exposure. Fundam Appl Toxicol. 1989;12(1):151–7. doi: 10.1016/0272-0590(89)90070-5. [DOI] [PubMed] [Google Scholar]
- Brosens I, Robertson WB, Dixon HG. The physiological response of the vessels of the placental bed to normal pregnancy. The Journal of pathology and bacteriology. 1967;93(2):569–79. doi: 10.1002/path.1700930218. [DOI] [PubMed] [Google Scholar]
- Buhimschi IA, Buhimschi CS, Weiner CP. Protective effect of n-acetylcysteine against fetal death and preterm labor induced by maternal inflammation. American journal of obstetrics and gynecology. 2003a;188(1):203–8. doi: 10.1067/mob.2003.112. [DOI] [PubMed] [Google Scholar]
- Buhimschi IA, Buhimschi CS, Weiner CP. Protective effect of n-acetylcysteine against fetal death and preterm labor induced by maternal inflammation. American journal of obstetrics and gynecology. 2003b;188(1):203–8. doi: 10.1067/mob.2003.112. [DOI] [PubMed] [Google Scholar]
- Chakraborty C, Gleeson LM, McKinnon T, Lala PK. Regulation of human trophoblast migration and invasiveness. Canadian journal of physiology and pharmacology. 2002;80(2):116–24. doi: 10.1139/y02-016. [DOI] [PubMed] [Google Scholar]
- Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiological reviews. 1979;59(3):527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- Chigusa Y, Tatsumi K, Kondoh E, Fujita K, Nishimura F, Mogami H, Konishi I. Decreased lectin-like oxidized ldl receptor 1 (lox-1) and low nrf2 activation in placenta are involved in preeclampsia. The Journal of clinical endocrinology and metabolism. 2012;97(10):E1862–70. doi: 10.1210/jc.2012-1268. [DOI] [PubMed] [Google Scholar]
- Cindrova-Davies T, Yung HW, Johns J, Spasic-Boskovic O, Korolchuk S, Jauniaux E, Burton GJ, Charnock-Jones DS. Oxidative stress, gene expression, and protein changes induced in the human placenta during labor. The American journal of pathology. 2007;171(4):1168–79. doi: 10.2353/ajpath.2007.070528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox S, King M, Casey M, MacDonald P. Interleukin-1 beta, -1 alpha, and -6 and prostaglandins in vaginal/cervical fluids of pregnant women before and during labor. J Clin Endocrinol Metab. 1993;77:805–815. doi: 10.1210/jcem.77.3.8370702. [DOI] [PubMed] [Google Scholar]
- Denschlag D, Marculescu R, Unfried G, Hefler LA, Exner M, Hashemi A, Riener EK, Keck C, Tempfer CB, Wagner O. The size of a microsatellite polymorphism of the haem oxygenase 1 gene is associated with idiopathic recurrent miscarriage. Molecular human reproduction. 2004;10(3):211–4. doi: 10.1093/molehr/gah024. [DOI] [PubMed] [Google Scholar]
- Diplas AI, Lambertini L, Lee MJ, Sperling R, Lee YL, Wetmur J, Chen J. Differential expression of imprinted genes in normal and iugr human placentas. Epigenetics : official journal of the DNA Methylation Society. 2009;4(4):235–40. doi: 10.4161/epi.9019. [DOI] [PubMed] [Google Scholar]
- Dortbudak O, Eberhardt R, Ulm M, Persson GR. Periodontitis, a marker of risk in pregnancy for preterm birth. J Clin Periodontol. 2005;32(1):45–52. doi: 10.1111/j.1600-051X.2004.00630.x. [DOI] [PubMed] [Google Scholar]
- Doucet J, Tague B, Arnold DL, Cooke GM, Hayward S, Goodyer CG. Persistent organic pollutant residues in human fetal liver and placenta from greater montreal, quebec: A longitudinal study from 1998 through 2006. Environ Health Perspect. 2009;117(4):605–10. doi: 10.1289/ehp.0800205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubinsky V, Poehlmann TG, Suman P, Gentile T, Markert UR, Gutierrez G. Role of regulatory and angiogenic cytokines in invasion of trophoblastic cells. Am J Reprod Immunol. 2010;63(3):193–9. doi: 10.1111/j.1600-0897.2009.00778.x. [DOI] [PubMed] [Google Scholar]
- Fahey JW, Haristoy X, Dolan PM, Kensler TW, Scholtus I, Stephenson KK, Talalay P, Lozniewski A. Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of helicobacter pylori and prevents benzo[a]pyrene-induced stomach tumors. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(11):7610–5. doi: 10.1073/pnas.112203099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay VJ, Tapiero H, Townsend DM. Sulfiredoxin: A potential therapeutic agent? Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2005;59(7):374–9. doi: 10.1016/j.biopha.2005.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T. Oxygen radicals and signaling. Curr Opin Cell Biol. 1998;10(2):248–53. doi: 10.1016/s0955-0674(98)80147-6. [DOI] [PubMed] [Google Scholar]
- Frederiksen M, Vorkamp K, Thomsen M, Knudsen LE. Human internal and external exposure to pbdes--a review of levels and sources. Int J Hyg Environ Health. 2009;212(2):109–34. doi: 10.1016/j.ijheh.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Germain AM, Carvajal J, Sanchez M, Valenzuela GJ, Tsunekawa H, Chuaqui B. Preterm labor: Placental pathology and clinical correlation. Obstet Gynecol. 1999;94(2):284–9. doi: 10.1016/s0029-7844(99)00324-5. [DOI] [PubMed] [Google Scholar]
- Gharavi N, Haggarty S, El-Kadi AO. Chemoprotective and carcinogenic effects of tert-butylhydroquinone and its metabolites. Current drug metabolism. 2007;8(1):1–7. doi: 10.2174/138920007779315035. [DOI] [PubMed] [Google Scholar]
- Goepfert AR, Goldenberg RL, Andrews WW, Hauth JC, Mercer B, Iams J, Meis P, Moawad A, Thom E, VanDorsten JP, Caritis SN, Thurnau G, Miodovnik M, Dombrowski M, Roberts J, McNellis D. The preterm prediction study: Association between cervical interleukin 6 concentration and spontaneous preterm birth. National institute of child health and human development maternal-fetal medicine units network. Am J Obstet Gynecol. 2001;184(3):483–8. doi: 10.1067/mob.2001.109653. [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, Goepfert AR, Ramsey PS. Biochemical markers for the prediction of preterm birth. American journal of obstetrics and gynecology. 2005;192(5 Suppl):S36–46. doi: 10.1016/j.ajog.2005.02.015. [DOI] [PubMed] [Google Scholar]
- Gomez LM, Ma Y, Ho C, McGrath CM, Nelson DB, Parry S. Placental infection with human papillomavirus is associated with spontaneous preterm delivery. Hum Reprod. 2008;23(3):709–15. doi: 10.1093/humrep/dem404. [DOI] [PubMed] [Google Scholar]
- Guvenius DM, Aronsson A, Ekman-Ordeberg G, Bergman A, Noren K. Human prenatal and postnatal exposure to polybrominated diphenyl ethers, polychlorinated biphenyls, polychlorobiphenylols, and pentachlorophenol. Environ Health Perspect. 2003;111(9):1235–41. doi: 10.1289/ehp.5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara H, Ohta M, Ohta K, Kuno S, Adachi T. Increase of antioxidative potential by tert-butylhydroquinone protects against cell death associated with 6-hydroxydopamine-induced oxidative stress in neuroblastoma sh-sy5y cells. Brain research. Molecular brain research. 2003;119(2):125–31. doi: 10.1016/j.molbrainres.2003.08.021. [DOI] [PubMed] [Google Scholar]
- Harvey CJ, Thimmulappa RK, Singh A, Blake DJ, Ling G, Wakabayashi N, Fujii J, Myers A, Biswal S. Nrf2-regulated glutathione recycling independent of biosynthesis is critical for cell survival during oxidative stress. Free radical biology & medicine. 2009;46(4):443–53. doi: 10.1016/j.freeradbiomed.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss E, Herhaus C, Klimo K, Bartsch H, Gerhauser C. Nuclear factor kappa b is a molecular target for sulforaphane-mediated anti-inflammatory mechanisms. The Journal of biological chemistry. 2001;276(34):32008–15. doi: 10.1074/jbc.M104794200. [DOI] [PubMed] [Google Scholar]
- Hites RA. Polybrominated diphenyl ethers in the environment and in people: A meta-analysis of concentrations. Environmental science & technology. 2004;38(4):945–56. doi: 10.1021/es035082g. [DOI] [PubMed] [Google Scholar]
- Horita H, Kuroda E, Hachisuga T, Kashimura M, Yamashita U. Induction of prostaglandin e2 production by leukemia inhibitory factor promotes migration of first trimester extravillous trophoblast cell line, htr-8/svneo. Human reproduction. 2007a;22(7):1801–9. doi: 10.1093/humrep/dem125. [DOI] [PubMed] [Google Scholar]
- Horita H, Kuroda E, Hachisuga T, Kashimura M, Yamashita U. Induction of prostaglandin e2 production by leukemia inhibitory factor promotes migration of first trimester extravillous trophoblast cell line, htr-8/svneo. Human reproduction. 2007b;22(7):1801–9. doi: 10.1093/humrep/dem125. [DOI] [PubMed] [Google Scholar]
- Hwang HS, Park SH, Park YW, Kwon HS, Sohn IS. Expression of cellular prion protein in the placentas of women with normal and preeclamptic pregnancies. Acta obstetricia et gynecologica Scandinavica. 2010;89(9):1155–61. doi: 10.3109/00016349.2010.498497. [DOI] [PubMed] [Google Scholar]
- Imhoff BR, Hansen JM. Tert-butylhydroquinone induces mitochondrial oxidative stress causing nrf2 activation. Cell biology and toxicology. 2010;26(6):541–51. doi: 10.1007/s10565-010-9162-6. [DOI] [PubMed] [Google Scholar]
- Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y. An nrf2/small maf heterodimer mediates the induction of phase ii detoxifying enzyme genes through antioxidant response elements. Biochemical and biophysical research communications. 1997;236(2):313–22. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by nrf2 through binding to the amino-terminal neh2 domain. Genes & development. 1999;13(1):76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin W, Kong J, Wang H, Wu J, Lu T, Jiang J, Ni H, Liang W. Protective effect of tert-butylhydroquinone on cerebral inflammatory response following traumatic brain injury in mice. Injury. 2011;42(7):714–8. doi: 10.1016/j.injury.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Jin W, Ni H, Dai Y, Wang H, Lu T, Wu J, Jiang J, Liang W. Effects of tert-butylhydroquinone on intestinal inflammatory response and apoptosis following traumatic brain injury in mice. Mediators of inflammation. 2010;2010:502564. doi: 10.1155/2010/502564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic M, Stefanoska I, Radojcic L, Vicovac L. Interleukin-8 (cxcl8) stimulates trophoblast cell migration and invasion by increasing levels of matrix metalloproteinase (mmp)2 and mmp9 and integrins alpha5 and beta1. Reproduction. 2010;139(4):789–98. doi: 10.1530/REP-09-0341. [DOI] [PubMed] [Google Scholar]
- Jovanovic M, Vicovac L. Interleukin-6 stimulates cell migration, invasion and integrin expression in htr-8/svneo cell line. Placenta. 2009;30(4):320–8. doi: 10.1016/j.placenta.2009.01.013. [DOI] [PubMed] [Google Scholar]
- Juge N, Mithen RF, Traka M. Molecular basis for chemoprevention by sulforaphane: A comprehensive review. Cellular and molecular life sciences : CMLS. 2007;64(9):1105–27. doi: 10.1007/s00018-007-6484-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabe Y, Ando K, Hirao S, Yoshida M, Handa H. Redox regulation of nf-kappab activation: Distinct redox regulation between the cytoplasm and the nucleus. Antioxidants & redox signaling. 2005;7(3–4):395–403. doi: 10.1089/ars.2005.7.395. [DOI] [PubMed] [Google Scholar]
- Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the keap1-nrf2-are pathway. Annual review of pharmacology and toxicology. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- Keum YS. Regulation of the keap1/nrf2 system by chemopreventive sulforaphane: Implications of posttranslational modifications. Annals of the New York Academy of Sciences. 2011;1229:184–9. doi: 10.1111/j.1749-6632.2011.06092.x. [DOI] [PubMed] [Google Scholar]
- Khan AU, Wilson T. Reactive oxygen species as cellular messengers. Chem Biol. 1995;2(7):437–45. doi: 10.1016/1074-5521(95)90259-7. [DOI] [PubMed] [Google Scholar]
- Khan GA, Girish GV, Lala N, Di Guglielmo GM, Lala PK. Decorin is a novel vegfr-2-binding antagonist for the human extravillous trophoblast. Molecular endocrinology. 2011;25(8):1431–43. doi: 10.1210/me.2010-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodagholi F, Tusi SK. Stabilization of nrf2 by tbhq prevents lps-induced apoptosis in differentiated pc12 cells. Molecular and cellular biochemistry. 2011;354(1–2):97–112. doi: 10.1007/s11010-011-0809-2. [DOI] [PubMed] [Google Scholar]
- Khor TO, Huang MT, Kwon KH, Chan JY, Reddy BS, Kong AN. Nrf2-deficient mice have an increased susceptibility to dextran sulfate sodium-induced colitis. Cancer research. 2006;66(24):11580–4. doi: 10.1158/0008-5472.CAN-06-3562. [DOI] [PubMed] [Google Scholar]
- Kim YM, Bujold E, Chaiworapongsa T, Gomez R, Yoon BH, Thaler HT, Rotmensch S, Romero R. Failure of physiologic transformation of the spiral arteries in patients with preterm labor and intact membranes. American journal of obstetrics and gynecology. 2003;189(4):1063–9. doi: 10.1067/s0002-9378(03)00838-x. [DOI] [PubMed] [Google Scholar]
- Kim YM, Chaiworapongsa T, Gomez R, Bujold E, Yoon BH, Rotmensch S, Thaler HT, Romero R. Failure of physiologic transformation of the spiral arteries in the placental bed in preterm premature rupture of membranes. American journal of obstetrics and gynecology. 2002;187(5):1137–42. doi: 10.1067/mob.2002.127720. [DOI] [PubMed] [Google Scholar]
- Koh K, Cha Y, Kim S, Kim J. Tbhq inhibits lps-induced microglial activation via nrf2-mediated suppression of p38 phosphorylation. Biochemical and biophysical research communications. 2009;380(3):449–53. doi: 10.1016/j.bbrc.2009.01.082. [DOI] [PubMed] [Google Scholar]
- Kweider N, Huppertz B, Wruck CJ, Beckmann R, Rath W, Pufe T, Kadyrov M. A role for nrf2 in redox signalling of the invasive extravillous trophoblast in severe early onset iugr associated with preeclampsia. PLoS One. 2012;7(10):e47055. doi: 10.1371/journal.pone.0047055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lala PK, Chakraborty C. Factors regulating trophoblast migration and invasiveness: Possible derangements contributing to pre-eclampsia and fetal injury. Placenta. 2003;24(6):575–87. doi: 10.1016/s0143-4004(03)00063-8. [DOI] [PubMed] [Google Scholar]
- Lewerenz J, Hewett SJ, Huang Y, Lambros M, Gout PW, Kalivas PW, Massie A, Smolders I, Methner A, Pergande M, Smith SB, Ganapathy V, Maher P. The cystine/glutamate antiporter system x(c)(-) in health and disease: From molecular mechanisms to novel therapeutic opportunities. Antioxidants & redox signaling. 2013;18(5):522–55. doi: 10.1089/ars.2011.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Pan YL, Ning XX, Sun LJ, Lan M, Hong L, Du JP, Liu N, Liu CJ, Qiao TD, Fan DM. Overexpression of prpc and its antiapoptosis function in gastric cancer. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 2006;27(2):84–91. doi: 10.1159/000092488. [DOI] [PubMed] [Google Scholar]
- Loset M, Mundal SB, Johnson MP, Fenstad MH, Freed KA, Lian IA, Eide IP, Bjorge L, Blangero J, Moses EK, Austgulen R. A transcriptional profile of the decidua in preeclampsia. Am J Obstet Gynecol. 2011;204(1):84 e1–27. doi: 10.1016/j.ajog.2010.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SC. Regulation of glutathione synthesis. Molecular aspects of medicine. 2009;30(1–2):42–59. doi: 10.1016/j.mam.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu W, Zerenturk EJ, Kristiana I, Bucknall MP, Sharpe LJ, Brown AJ. Signaling regulates activity of dhcr24, the final enzyme in cholesterol synthesis. Journal of lipid research. 2014;55(3):410–20. doi: 10.1194/jlr.M043257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q. Role of nrf2 in oxidative stress and toxicity. Annual review of pharmacology and toxicology. 2013;53:401–26. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mijovic JE, Zakar T, Nairn TK, Olson DM. Prostaglandin endoperoxide h synthase (pghs) activity and pghs-1 and -2 messenger ribonucleic acid abundance in human chorion throughout gestation and with preterm labor. The Journal of clinical endocrinology and metabolism. 1998;83(4):1358–67. doi: 10.1210/jcem.83.4.4692. [DOI] [PubMed] [Google Scholar]
- Miller MF, Chernyak SM, Batterman S, Loch-Caruso R. Polybrominated diphenyl ethers in human gestational membranes from women in southeast michigan. Environmental science & technology. 2009;43(9):3042–6. doi: 10.1021/es8032764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MF, Chernyak SM, Domino SE, Batterman SA, Loch-Caruso R. Concentrations and speciation of polybrominated diphenyl ethers in human amniotic fluid. Sci Total Environ. 2012;417–418:294–8. doi: 10.1016/j.scitotenv.2011.11.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistry HD, Kurlak LO, Williams PJ, Ramsay MM, Symonds ME, Broughton Pipkin F. Differential expression and distribution of placental glutathione peroxidases 1, 3 and 4 in normal and preeclamptic pregnancy. Placenta. 2010;31(5):401–8. doi: 10.1016/j.placenta.2010.02.011. [DOI] [PubMed] [Google Scholar]
- Motohashi H, Yamamoto M. Nrf2-keap1 defines a physiologically important stress response mechanism. Trends in molecular medicine. 2004;10(11):549–57. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Mundy WR, Freudenrich TM, Crofton KM, DeVito MJ. Accumulation of pbde-47 in primary cultures of rat neocortical cells. Toxicol Sci. 2004;82(1):164–9. doi: 10.1093/toxsci/kfh239. [DOI] [PubMed] [Google Scholar]
- Nakamura BN, Fielder TJ, Hoang YD, Lim J, McConnachie LA, Kavanagh TJ, Luderer U. Lack of maternal glutamate cysteine ligase modifier subunit (gclm) decreases oocyte glutathione concentrations and disrupts preimplantation development in mice. Endocrinology. 2011;152(7):2806–15. doi: 10.1210/en.2011-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ness RB, Sibai BM. Shared and disparate components of the pathophysiologies of fetal growth restriction and preeclampsia. American journal of obstetrics and gynecology. 2006;195(1):40–9. doi: 10.1016/j.ajog.2005.07.049. [DOI] [PubMed] [Google Scholar]
- Nicola C, Timoshenko AV, Dixon SJ, Lala PK, Chakraborty C. Ep1 receptor-mediated migration of the first trimester human extravillous trophoblast: The role of intracellular calcium and calpain. J Clin Endocrinol Metab. 2005a;90(8):4736–46. doi: 10.1210/jc.2005-0413. [DOI] [PubMed] [Google Scholar]
- Nicola C, Timoshenko AV, Dixon SJ, Lala PK, Chakraborty C. Ep1 receptor-mediated migration of the first trimester human extravillous trophoblast: The role of intracellular calcium and calpain. The Journal of clinical endocrinology and metabolism. 2005b;90(8):4736–46. doi: 10.1210/jc.2005-0413. [DOI] [PubMed] [Google Scholar]
- Novakovic B, Gordon L, Wong NC, Moffett A, Manuelpillai U, Craig JM, Sharkey A, Saffery R. Wide-ranging DNA methylation differences of primary trophoblast cell populations and derived cell lines: Implications and opportunities for understanding trophoblast function. Molecular human reproduction. 2011;17(6):344–53. doi: 10.1093/molehr/gar005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsi NM, Tribe RM. Cytokine networks and the regulation of uterine function in pregnancy and parturition. J Neuroendocrinol. 2008;20(4):462–9. doi: 10.1111/j.1365-2826.2008.01668.x. [DOI] [PubMed] [Google Scholar]
- Osburn WO, Wakabayashi N, Misra V, Nilles T, Biswal S, Trush MA, Kensler TW. Nrf2 regulates an adaptive response protecting against oxidative damage following diquat-mediated formation of superoxide anion. Archives of biochemistry and biophysics. 2006;454(1):7–15. doi: 10.1016/j.abb.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HR, Kamau PW, Loch-Caruso R. Involvement of reactive oxygen species in brominated diphenyl ether-47-induced inflammatory cytokine release from human extravillous trophoblasts in vitro. Toxicology and applied pharmacology. 2014;274(2):283–92. doi: 10.1016/j.taap.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltier MR, Klimova NG, Arita Y, Gurzenda EM, Murthy A, Chawala K, Lerner V, Richardson J, Hanna N. Polybrominated diphenyl ethers enhance the production of proinflammatory cytokines by the placenta. Placenta. 2012;33(9):745–9. doi: 10.1016/j.placenta.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijnenborg R, Bland JM, Robertson WB, Brosens I. Uteroplacental arterial changes related to interstitial trophoblast migration in early human pregnancy. Placenta. 1983;4(4):397–413. doi: 10.1016/s0143-4004(83)80043-5. [DOI] [PubMed] [Google Scholar]
- Pijnenborg R, Dixon G, Robertson WB, Brosens I. Trophoblastic invasion of human decidua from 8 to 18 weeks of pregnancy. Placenta. 1980;1(1):3–19. doi: 10.1016/s0143-4004(80)80012-9. [DOI] [PubMed] [Google Scholar]
- Prokopenko VM, Partsalis GK, Pavlova NG, Burmistrov SO, Arutyunyan AV. Glutathione-dependent system of antioxidant defense in the placenta in preterm delivery. Bulletin of experimental biology and medicine. 2002;133(5):442–3. doi: 10.1023/a:1019845217485. [DOI] [PubMed] [Google Scholar]
- Rangasamy T, Cho CY, Thimmulappa RK, Zhen L, Srisuma SS, Kensler TW, Yamamoto M, Petrache I, Tuder RM, Biswal S. Genetic ablation of nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. The Journal of clinical investigation. 2004;114(9):1248–59. doi: 10.1172/JCI21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangasamy T, Guo J, Mitzner WA, Roman J, Singh A, Fryer AD, Yamamoto M, Kensler TW, Tuder RM, Georas SN, Biswal S. Disruption of nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. The Journal of experimental medicine. 2005;202(1):47–59. doi: 10.1084/jem.20050538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remacle J, Raes M, Toussaint O, Renard P, Rao G. Low levels of reactive oxygen species as modulators of cell function. Mutat Res. 1995;316(3):103–22. doi: 10.1016/0921-8734(95)90004-7. [DOI] [PubMed] [Google Scholar]
- Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic Biol Med. 2010;49(11):1603–16. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riewe SD, Mans JJ, Hirano T, Katz J, Shiverick KT, Brown TA, Lamont RJ. Human trophoblast responses to porphyromonas gingivalis infection. Molecular oral microbiology. 2010;25(4):252–9. doi: 10.1111/j.2041-1014.2010.00573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Espinoza J, Chaiworapongsa T, Kalache K. Infection and prematurity and the role of preventive strategies. Semin Neonatol. 2002;7(4):259–74. doi: 10.1016/s1084-2756(02)90121-1. [DOI] [PubMed] [Google Scholar]
- Rushmore TH, Morton MR, Pickett CB. The antioxidant responsive element. Activation by oxidative stress and identification of the DNA consensus sequence required for functional activity. The Journal of biological chemistry. 1991;266(18):11632–9. [PubMed] [Google Scholar]
- Shanmugam N, Todorov IT, Nair I, Omori K, Reddy MA, Natarajan R. Increased expression of cyclooxygenase-2 in human pancreatic islets treated with high glucose or ligands of the advanced glycation endproduct-specific receptor (ager), and in islets from diabetic mice. Diabetologia. 2006;49(1):100–7. doi: 10.1007/s00125-005-0065-7. [DOI] [PubMed] [Google Scholar]
- Sian J, Dexter DT, Lees AJ, Daniel S, Agid Y, Javoy-Agid F, Jenner P, Marsden CD. Alterations in glutathione levels in parkinson’s disease and other neurodegenerative disorders affecting basal ganglia. Annals of neurology. 1994;36(3):348–55. doi: 10.1002/ana.410360305. [DOI] [PubMed] [Google Scholar]
- Sjodin A, Wong LY, Jones RS, Park A, Zhang Y, Hodge C, Dipietro E, McClure C, Turner W, Needham LL, Patterson DG., Jr Serum concentrations of polybrominated diphenyl ethers (pbdes) and polybrominated biphenyl (pbb) in the united states population: 2003–2004. Environmental science & technology. 2008;42(4):1377–84. doi: 10.1021/es702451p. [DOI] [PubMed] [Google Scholar]
- Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3:Article3. doi: 10.2202/1544-6115.1027. [DOI] [PubMed] [Google Scholar]
- Taylor RC, Acquaah-Mensah G, Singhal M, Malhotra D, Biswal S. Network inference algorithms elucidate nrf2 regulation of mouse lung oxidative stress. PLoS computational biology. 2008;4(8):e1000166. doi: 10.1371/journal.pcbi.1000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimmulappa RK, Lee H, Rangasamy T, Reddy SP, Yamamoto M, Kensler TW, Biswal S. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. The Journal of clinical investigation. 2006;116(4):984–95. doi: 10.1172/JCI25790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjoa ML, Oudejans CB, van Vugt JM, Blankenstein MA, van Wijk IJ. Markers for presymptomatic prediction of preeclampsia and intrauterine growth restriction. Hypertens Pregnancy. 2004;23(2):171–89. doi: 10.1081/PRG-120028292. [DOI] [PubMed] [Google Scholar]
- Tjoa ML, van Vugt JM, Go AT, Blankenstein MA, Oudejans CB, van Wijk IJ. Elevated c-reactive protein levels during first trimester of pregnancy are indicative of preeclampsia and intrauterine growth restriction. J Reprod Immunol. 2003;59(1):29–37. doi: 10.1016/s0165-0378(02)00085-2. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency; Office of Pollution Prevention & Toxics, editor Polybrominated diphenyl ethers (pbdes) project plan. 2006. [Google Scholar]
- Vince GS, Starkey PM, Austgulen R, Kwiatkowski D, Redman CW. Interleukin-6, tumour necrosis factor and soluble tumour necrosis factor receptors in women with pre-eclampsia. Br J Obstet Gynaecol. 1995;102(1):20–5. doi: 10.1111/j.1471-0528.1995.tb09020.x. [DOI] [PubMed] [Google Scholar]
- Wakabayashi N, Slocum R, Skoko J, Shin S, Kensler TW. When nrf2 talks, who’s listening? Antioxidants and redox signaling. 2010;13(11):1669–1963. doi: 10.1089/ars.2010.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt NT, Taylor DR, Gillott A, Thomas DA, Perera WS, Hooper NM. Reactive oxygen species-mediated beta-cleavage of the prion protein in the cellular response to oxidative stress. The Journal of biological chemistry. 2005;280(43):35914–21. doi: 10.1074/jbc.M507327200. [DOI] [PubMed] [Google Scholar]
- Wenstrom KD, Andrews WW, Tamura T, DuBard MB, Johnston KE, Hemstreet GP. Elevated amniotic fluid interleukin-6 levels at genetic amniocentesis predict subsequent pregnancy loss. Am J Obstet Gynecol. 1996;175(4 Pt 1):830–3. doi: 10.1016/s0002-9378(96)80007-x. [DOI] [PubMed] [Google Scholar]
- Wruck CJ, Huppertz B, Bose P, Brandenburg LO, Pufe T, Kadyrov M. Role of a fetal defence mechanism against oxidative stress in the aetiology of preeclampsia. Histopathology. 2009;55(1):102–6. doi: 10.1111/j.1365-2559.2009.03339.x. [DOI] [PubMed] [Google Scholar]
- Wu K, Xu X, Liu J, Guo Y, Li Y, Huo X. Polybrominated diphenyl ethers in umbilical cord blood and relevant factors in neonates from guiyu, china. Environmental science & technology. 2010;44(2):813–9. doi: 10.1021/es9024518. [DOI] [PubMed] [Google Scholar]
- Zenclussen AC, Lim E, Knoeller S, Knackstedt M, Hertwig K, Hagen E, Klapp BF, Arck PC. Heme oxygenases in pregnancy ii: Ho-2 is downregulated in human pathologic pregnancies. American journal of reproductive immunology. 2003;50(1):66–76. doi: 10.1034/j.1600-0897.2003.00047.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.