Abstract
Adherence to antiretroviral (ART) medication is vital to reducing morbidity and mortality among HIV positive persons. People who inject drugs (PWID) are at high risk for HIV infection in transitional/low/middle income countries (TLMIC). We conducted a systematic review of studies reporting adherence to ARTs among persons with active injection drug use and/or histories of injection drug use in TLMIC. Meta-regression was performed to examine relationships between location, adherence measurements, and follow-up period. Fifteen studies were included from seven countries. Adherence levels ranged from 33% to 97%; mean weighted adherence was 72%. ART adherence was associated with different methods of measuring adherence and studies conducted in Eastern Europe and East Asia. The great heterogeneity observed precludes generalization to TLMIC as a whole. Given the critical importance of ART adherence more research is needed on ART adherence among PWID in TLMIC, including the use of standardized methods for reporting adherence to ARTs.
Keywords: Persons who inject drugs, antiretroviral therapy, HIV, developing countries, medication adherence
INTRODUCTION
The first cases of acquired immune deficiency syndrome (AIDS) among people who inject drugs (PWID) were reported in 1981 (1). By the mid-1980s, human immunodeficiency virus (HIV), the virus that causes AIDS, had been discovered (2). HIV has infected millions of individuals, with current estimates reporting over 38 million current infections worldwide (3). In many countries HIV prevalence among the general population is less than 1%, but among people who inject drugs (PWID) HIV prevalence has reached levels of 20% or greater in Eastern Europe, Asia and Africa (4). Outside of African countries, PWID make up over 33% of new HIV infections (5).
Antiretroviral (ART) therapy, which was started in the late 1980’s with AZT and further expanded to combination highly active antiretroviral therapy (HAART) in the mid 1990’s (6), led to significant reductions in mortality among HIV infected individuals (7). However, these medications were often only available in high-income countries due to the cost limited healthcare staff.
Strategies to seek, test, and treat individuals at high risk for HIV infection have been increasingly prioritized in settings of high HIV prevalence (8), but questions still arise from clinicians as to the best way to treat HIV infected individuals who continue to use drugs. The main concerns that clinicians have in treating PWID is that suboptimal ART adherence (below 90% of medication taken) among individuals could lead to increased ART resistance (9).
There are a number of reasons why adherence to ART medications among PWID may be lower among transitional/low/middle-income countries (TLMIC) compared to high-income countries. First, TLMIC may not have the financial resources or availability of ART medication for HIV infected individuals, leading to supply shortages and gaps in medication administration (10). Second, PWID in TLMIC may face greater stigmatization and discrimination, leading to less utilization of ART medication and fewer follow-up visits (11). Third, there may be greater law enforcement interference with PWID visiting ART therapy locations in TLMIC, particularly in locations where PWID may be incarcerated in “detention” centers or in locations where they may suffer extortion or brutality from police (12). Finally, ART therapy in high-income settings may be implemented in the context of other large scale evidence-based HIV/HCV-related prevention programs including needle exchange programs and opiate substitution treatment/medication-assisted treatment (13). As a result, ART therapy in high-income countries may benefit from the synergistic effects of “combined” prevention programming (14, 15). In contrast, many ART therapy centers in TLMIC are implemented as stand-alone clinics/centers or in hospital settings where complementary harm reduction programs traditionally do not exist (16).
In this review, we examine adherence to ART therapy in TLMIC among active PWID and persons with a history of injection drug use, and compare the results found in TLMIC to ART adherence among active PWID and persons with a history of injection drug use in high-income countries. The goal is to determine if TLMIC, given more limited resources and funding, are able to attain the same level of ART adherence that has been found in studies of active PWID and persons with a history of injection drug use in high-income countries.
METHODS
Search Strategies
The literature search conducted for this review utilized strict PRISMA guidelines (17). Studies were selected from several sources including PubMed, EMBASE, NLM Gateway, and abstracts from International AIDS Society (IAS) 2000–2012 and International Harm Reduction Association (IHRA) 2000–2012 conferences. Systematic literature searches were conducted to identify potentially eligible articles from journals and conference presentations. We also searched references from review articles assessing drug-using populations for any country designated as a TLMIC. Figure 1 presents the search terminology used to locate potentially eligible studies.
Figure 1.

Search Terms Used for Systematic Review
Study Selection and Eligibility Criteria
In order for a study to be included, there had to be documentation of ongoing ART therapy in a sample of opiate users with current and/or history of injection and with measurements of ART adherence. In all of the primary samples, at least 90% of the sample identified as current or previous injection drug users (a percentage that is traditionally used as a minimum for a PWID sample). Only one study contained only active PWID (Shaboltas et al); all other studies had a combination of current PWID or persons with a history of injection drug use. Adherence was measured in a number of ways, including recall of adherence by participants (throughout the entire treatment period, or in the last 2–7 days), review of medical records, and recall of medication taken over varying periods of time. We excluded any study from prison or institutionalized settings due to the distinct structural nature of these programs. Locations were restricted to countries that fit the TLMIC designation defined by the World Bank (18); all high-income location studies were excluded (unless they included a separate analysis of a TLMIC as well).
Data extraction and analysis
A standardized coding form was developed to document pertinent information for each study. Information collected included demographics of the drug using population, study design characteristics of the ART program including location and services offered, type of ART therapy used, and information related to changes in ART adherence for each participant over time. Each study was assigned a reference ID number after completion of coding. Data was extracted from each coding form and entered into a database in order to assess ART adherence over time for participants in each study.
Weighted ART adherence was calculated for each study, with study categorization done based on measurement of ART adherence by the primary authors. Adherence was defined in the primary studies using three different measurements (Table 1):
Table 1.
Adherence Measurements used in Primary Studies
| Antiretroviral Adherence Type | Description of Adherence Measurement |
|---|---|
| 1. Still on ART at end of follow-up period (based on chart documentation or patient data) | Participants still on ART medication, measured medical record review at the end of the ART adherence assessment period, with at least 90% of medication taken during the assessment period (through review of patient treatment data; data was not from direct questioning of patients). In these studies, participants were under ART therapy at the start of adherence assessment and participants who left treatment were considered non-adherent. |
| 2. Participant recall of adherence during (percentage of medications taken) during 2–7 day period prior to interview (based upon self-report of percentage of medication taken during period of ART adherence measurement) | Participant recall (first person) of medications taken in the 2–7 days prior to interview. Adherence was defined as reporting having taken at least 90% of prescribed doses of ART medication during this 2–7 day assessment period. Patients self-reported number of pills taken during the 2–7 day period. Patients who had left treatment were not included |
| 3. Participant recall of percentage of ART medication taken from start to end of period of ART adherence evaluation (based upon self-report of percentage of medication taken during period of ART adherence measurement) | Participant recall of adherence to ART medications over the course of the ART adherence assessment period. Participants were considered ART adherent if they reported at least 90% adherence to medications during the ART adherence assessment period. Participants who left treatment were not included. |
Meta-regression analysis was conducted to examine region, year of study, number of months of follow-up, and type of adherence measurement used in primary studies in relationship to ART adherence levels. Heterogeneity tests were conducted to assess variability among the individual studies in the review.
Assessment of Risk of Bias in Studies
A quality check was performed to document the strengths and weaknesses of each study; items in the quality checklist included recruitment method, comparability in loss to follow-up and retained participants, and assessment of follow-up period. This checklist was modified from quality checklists for primary studies by the Cochrane Collaboration of systematic reviews (19).
RESULTS
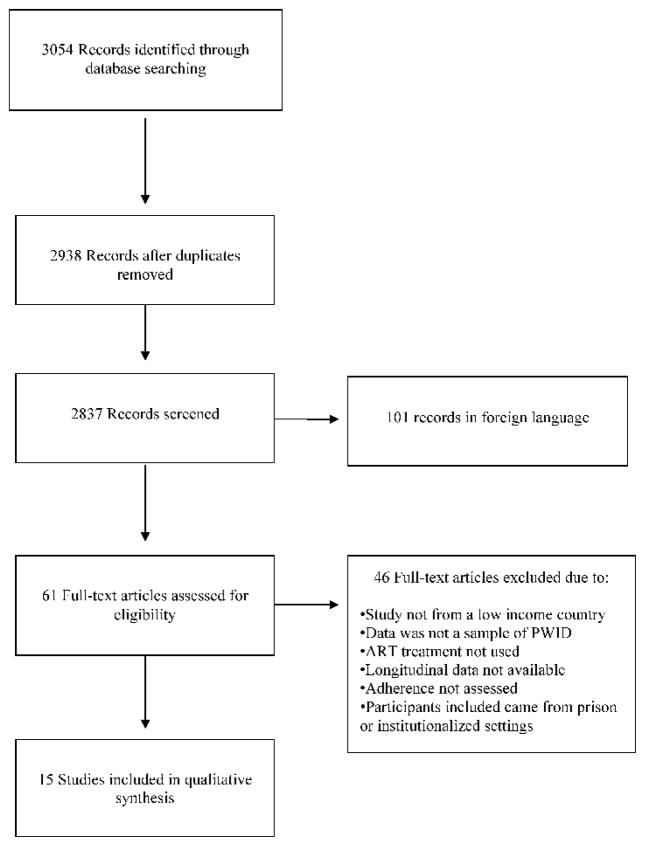
Figure 2 shows the PRISMA diagram for the search that led to the final number of studies included in this review. The search included all published studies from January 1 1994 (when ART medication began to be distributed on a wider basis to HIV positive persons) through December 13 2013. Searching identified 3054 article titles. After removal of 116 duplicate papers, we screened 2938 abstracts against the inclusion criteria and retrieved 61 full text articles after excluding a total of 101 foreign language citations that could not be accessed. Of the articles and reports retrieved, 15 met all criteria for inclusion and were coded for our review. These 15 studies described 16 different samples of persons with current or a history of injection drug use from 7 different countries. The included studies contained a total of 21,258 program participants of which 60 were identified as current PWID and 21,198 were identified as being current or former PWID. The 21,258 participants were from studies conducted in the following countries: Brazil, China, Estonia, India, Indonesia, Russia, and Vietnam.
Figure 2.
Flow chart of literature search review for review
The studies included in this review were conducted at ART therapy sites located at stand-alone locations (HIV specialty clinics) or within hospitals. Different ART medications were used, including Efavirenz, Lamivudine, Nevirapine, Ritronavir, Stavudine, and Zidovudine (using monotherapy or cocktail therapies). The studies in the review included follow-up data from 1996 through 2012. Follow-up (defined as the period for assessing ART adherence among participants in each primary study) times varied among included studies, ranging from one month to as high as 148 months, with an average follow-up of 27 months. In primary studies, locations that involved ART medications did not offer additional harm reduction services (such as opiate substitution therapy, needle exchange, mental health services, or other substance treatment).
Medication Adherence
ART adherence measurements were grouped by type of end point measurement by the researchers in the individual studies (see Table 1). Category 1 reported a mean weighted adherence of 60.2% (95% CI: 45.2%–75.1%); category 2 mean weighted adherence was 87.4% (95% CI: 84.8%–90%); category 3 mean weighted adherence was 94% (95% CI: 91.2%–96.8%). The overall mean weighted adherence among all studies in the review was 71.9% (95% CI: 58.8%–85.1%). Table 2 gives ART adherence measurements for the individual studies in addition to information on study location, years of data collection, type of ART regimen used in each program (if available), and length of follow-up measuring ART adherence among participants in each primary study.
Table 2.
Adherence levels among PWID in LMIC ART programs
| Study | Country | Study Year | Adherence Category (1–3)* | Months of Follow-up | Country | ART Regimen | PWID (n) | Adherence (%) |
|---|---|---|---|---|---|---|---|---|
| Campos 2010 (25) | Brazil | 2001–2002 | 2 | 12 | Brazil | NRTI Protease Inhibitor Ritonavir |
17 | 35.3 |
| Malta 2009 (49) | Brazil | 2000–2006 | 1 | 72 | Brazil | [HAART]: Not specified | 12231 | 33.1 |
| Melo 2006 (20) | Brazil | 1996–2002 | 1 | 84 | Brazil | [HAART]: Not specified | 67 | 38.9 |
| DeSilva 2013 (21) | China | 2012 | 3 | 1 | China | [HAART]: Not specified | 10 | 97.2 |
| Ma 2010 (22) | China | 2007–2008 | 3 | 12 | China | [HAART]: Not specified | 180 | 70.6 |
| Wang 2008 (26) | China | 2006 | 2 | 4 | China | Zidovudine, Stavudine Didanosine, Lamivudine Nevirapine, Efavirenz |
25 | 56 |
| Zhao 2013 (27) | China | 2011 | 3 | 6 | China | Zidovudine, Lamivudine Nevirapine, Efavirenz |
3642 | 67.5 |
| Zhao 2013 (27) | China | 2011 | 3 | 6 | China | Zidovudine, Lamivudine Nevirapine, Efavirenz |
932 | 72.3 |
| Uuskula 2012 (23) | Estonia | 2010 | 2 | 6 | Estonia | [HAART]: Not specified | 144 | 87.5 |
| Sharma 2007 (50) | India | 2004–2005 | 3 | 5 | India | Stavudine, Lamivudine Zidovudine, Nevirapine Efavirenz |
226 | 67.2 |
| Wisaksana 2010 (28) | Indonesia | 1996–2008 | 2 | 148 | Indonesia | Neviprapine, Efavirenz Zidovudine, Stavudine Lamivudine |
530 | 87.2 |
| Shaboltas 2013 (29) | Russia | 2007–2008 | 3 | 8 | Russia | Didanosine, Lamivudine Zidovudine, Nevirapine Lopinavir |
60 | 88.3 |
| Amirkhanian 2011 (51) | Russia | 2008–2009 | 2 | 12 | Russia | [HAART]: Not specified | 312 | 94.1 |
| Burdon 2006 (24) | Vietnam | 2005 | 2 | 2 | Vietnam | [HAART]: Not specified | 300 | 92.3 |
| Jordan 2009 (52) | Vietnam | 2006 | 3 | 1 | Vietnam | Didanosine, Lamivudine Zidovudine, Nevirapine Lopinavir, Nelfinavir Abacavir, Stavudine |
91 | 96.3 |
| Nguyen 2013 (53) | Vietnam | 2008–2009 | 1 | 48 | Vietnam | Tenofovir Lamivudine Efavirenz |
2491 | 63.3 |
See Table 1 for breakdown of the different ART adherence measurement categories
After examining the adherence rates of the individual studies, we performed meta-regression to determine factors associated with ART adherence. Variables entered into the meta-regression model included adherence measurement type (categories 1–3, defined in Table 1), months of follow-up in primary studies, the year of follow-up of the study, and region of data collection. The meta-regression results showed method of measuring adherence (p<0.001) and studies conducted in Eastern Europe and East Asia (p=0.016) were associated with higher levels of ART adherence. Table 3 gives the results of the meta-regression. Overall heterogeneity among studies included was very high (I2 = 99.7).
Table 3.
Meta-Regression of Factors Associated with Adherence among Primary Studies
| Variable | Coefficient. | Standard. Error. | t | P>|t| | 95% Confidence Interval |
|---|---|---|---|---|---|
| Adherence type | −18.46 | 3.91 | −4.72 | 0.001 | −27.07, −9.85 |
| Months of follow-up | −0.02 | 0.08 | −0.22 | 0.827 | −0.19, 0.13 |
| End Year of Study | 0.79 | 1.25 | 0.63 | 0.539 | −1.96, 3.54 |
| Region | −8.79 | 3.09 | −2.84 | 0.016 | −15.59, −1.99 |
Assessment of Risk of Bias
There were five studies that did not use systematic sampling to recruit participants into the study (20–24). There were 10 studies that did not control for confounders in their analysis of follow-up adherence among ART participants (20–22, 24–29). While there was clearly bias introduced among included primary studies, we did not note any systematic bias that would lead to removal from the analysis. As a result, all of these studies were included in the final measurements of ART adherence.
DISCUSSION
Previous research into ART adherence has led to guidelines specifying that an individual patient should take at least 90% of ART medication in order to reach viral suppression (30). There was considerable variation in ART adherence outcomes according to how adherence was measured in the studies reviewed here, but there were many studies in which substantial minorities of patients were not meeting the 90% adherence standard.
Our findings of sub-optimal adherence levels among PWID participant samples with current and histories of injection drug use in TLMIC are comparable to several studies conducted among PWID in high-income settings which also found sub-optimal adherence among large numbers of PWID participants with current and histories of injection drug use. A study conducted among PWID with current and histories of injection drug use in Denmark found ART adherence of less than 90% in 55% of PWID (defined by satisfying the 90% medication adherence and remaining in treatment to the end of the study evaluation) (31). A long term follow-up study among PWID with current and histories of injection drug use in Canada from 1996 through 2008 recruited over 400 HIV positive PWID, and only 63% remained adherent over the entire adherence analysis period (defined by satisfying the 90% medication adherence and remaining in treatment to the end of the study evaluation) (32). Among 350 PWID with current and histories of injection drug use that were analyzed as part of the ALIVE study in Baltimore (33), adherence was measured over a five year period of HIV treatment, and the results showed that only 26.7% of participants were compliant with ART therapy (defined by 90% of medication taken during follow-up periods) (34). As part of the MANIF2000 cohort study in France, adherence was measured among HIV infected PWID with current and histories of injection drug use over a two and half year period, with 65.2% of participants classified as ART adherent at the end of the study (defined by satisfying the 90% medication adherence and remaining in treatment to the end of the study evaluation) (35).
All of the studies included in this review included a small proportion of individuals who discontinued treatment due to the side effects of the ART medications given. Maintaining adherence among persons with adverse reactions to medications may require adjusting, or changing the ART regimen. Unfortunately, most of the studies included here did not address medication adjustments with respect to those who were non-adherent to the original ART therapy, and did not examine drop-out participants in detail. As many participants who discontinued treatment did not return for follow-up, there was no opportunity for the program to offer alternative treatments to these “drop outs” that may carry fewer side effects than the first ART regimen given.
The results of the meta-regression showed that adherence measurement type and the regions of Eastern Europe and East Asia were associated with higher rates of ART adherence. Recent research into coverage of HIV positive PWID reported higher levels of coverage in Eastern Europe and East/Central Asia compared to Southeast Asia. Among PWID in developing countries, approximately 8% of PWID are covered by ART. However, among PWID located in Eastern Europe and East/Central Asia, 14% of those receiving ART medication are identified as PWID, compared to Southeast Asia where only 1% of people on ART medication were PWID with current and histories of injection drug use (36). The higher coverage rates in Eastern Europe and Eastern/Central Asia suggest that there may be more experience among clinicians related to opiate addiction and ART medication administration, which in turn increases ART adherence levels among PWID with current and histories of injection drug use.
For drug users who seek treatment for HIV infection, particularly PWID with current and histories of injection drug use, there are often barriers that prevent high adherence to medications or treatment. Among many drug using populations, poverty can affect adherence to care as financial resources may prevent drug users from being able to travel to centers for medication. Unemployment, inadequate housing, or lack of funding for medications in locations where it is not free can also serve as obstacles to high ART adherence (37).
Structural factors could affect adherence rates among drug users who participated in the primary studies (38). Factors including logistical barriers to accessing treatment (including long wait times and inconvenient clinic hours), inadequate training of medical staff for treating HIV infected drug users, and lack of treatment for mental health disorders, have been associated with compromised levels of ART adherence among drug users (39).
Discrimination exists against PWID in many countries, and the stigma attached to being HIV positive and a drug injector may cause PWID to under-utilize available services (40, 41). In certain countries, however, government officials are addressing these discriminations; in China, recent government initiatives including the CHARE HIV program (42) have highlighted their pledge to providing treatment to all HIV infected individuals while combating stigma of HIV and drug use through public education (26).
Several programs have now begun to integrate treatment for opiate addiction into current ART programs for PWID. There is evidence that PWID on opiate substitution treatment (OST) reduce drug use, making it easier for them to adhere to medication schedules while providing an opportunity for directly observed therapy. Additionally, HIV positive OST participants have documented ART adherence that is comparable to HIV infected persons on ART medication in the general population (35, 43). In addition to OST, needle and syringe exchange programs for PWID may also provide adherence support services for ART therapy. Although none of the studies collected in this review utilized opiate substitution among participants, future programs that address HIV infected opiate users should build upon the synergistic relationship of OST and ART to promote increased adherence to both treatment regimens.
Perhaps one of the most important factors in ART adherence is having adequate support services for PWID in treatment. Primary studies have shown the importance of stable primary care and adequate support services to ensure continued adherence. Improving the overall environment in which PWID receive treatment has been shown to be independently associated with adherence to ART (44).
Limitations
There are several limitations for this review that should be noted. Some of the studies did not report the specific type of ART medication used, but did adhere to ART guidelines used in HIV treatment programs (45); in the studies that did report ART medication type, we found wide variation in the type of therapies that were given to the participants in each study, ranging from monotherapy to triple drug combinations. While we attempted to contact authors to get ART medication information, we were not able to acquire this information for several of the studies, especially those in Brazil, China, Russia, and Vietnam. The way ART adherence was measured varied among studies; while some studies defined ART adherence by continued participation in treatment (retention), other studies measured adherence with more precise indicators including recall of the number of pills taken over a short time period. Adherence in many cases was also based on self-report responses from participants; this could lead to recall bias, especially in studies with long follow-up times. In studies with long recall periods, subjects may have generalized from recent experience and failed to report adherence problems that occurred early in treatment.
Given the wide range of treatment regimens given to participants, adherence could have been influenced by side effects to particular regimens. As some ART medications cause more severe side effects than others, the type of ART therapy used could have influenced ART adherence rates among participants. However, examination of the medications given in the studies included did not show any particular regimen being associated with lower rates of medication adherence, and the number of participants who were not adherent to ART in the primary studies due to adverse events was relatively small.
In most of the primary studies included, the authors did not distinguish between PWID with current versus histories of injection drug use; in only one study was the sample described as only current PWID. Due to the small number of participants in this one study (n=60) compared to the entire sample (21,258), we were not able to conduct a sub-analysis of ART adherence by injection status.
CONCLUSION
The ART adherence levels among PWID with current and histories of injection drug use in TLMIC are sub-optimal and are probably well below the levels needed to have both positive treatment outcomes for individuals and a population-level treatment as prevention effect on HIV transmission. Given the critical importance of adherence to HIV treatment to prevent ART resistance, more research is needed to help elucidate the interactions between injection drug use and adherence to ART medications (46). In addition, standardizing outcome measures of ART adherence among studies should be implemented, including the use of multiple methods for assessing adherence, separating retention in the program from medication adherence among those continuing to participate in the program, conducting analysis for current PWID versus those with a history of injection drug use, providing complete descriptions of the program policies and procedures, and transparent reporting among researchers. Further integration of ART interventions into current services, including needle exchange and opiate substitution, is recommended, so PWID with current and histories of injection drug use can benefit from the synergistic effects of different services and treatment approaches (47, 48).
Acknowledgments
This study was funded by the National Institutes of Health (R01 AI 083035). The views expressed in this publication do not necessarily represent the position of the National Institutes of Health, Beth Israel Medical Center, or the University of Tartu
Footnotes
Financial Disclosure: The authors disclose no financial conflicts of interest
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the organizations and/or agencies the authors are affiliated with
References
- 1.CDC. First Report of AIDS. Atlanta: Centers for Disease Control; 1981. Contract No. 21. [Google Scholar]
- 2.Gallo RC, Montagnier L. The discovery of HIV as the cause of AIDS. N Engl J Med. 2003 Dec 11;349(24):2283–5. doi: 10.1056/NEJMp038194. [DOI] [PubMed] [Google Scholar]
- 3.UNAIDS. UNAIDS Report on the Global AIDS Epidemic. UNAIDS; 2012. [Google Scholar]
- 4.Mathers B, Degenhardt L, Phillips B, Wiessing L, Hickman M, Strathdee S, et al. Global epidemiology of injecting drug use and HIV among people who inject drugs: A systematic review. Lancet. 2008;372(9651):1733–45. doi: 10.1016/S0140-6736(08)61311-2. [DOI] [PubMed] [Google Scholar]
- 5.Malinowska-Sempruch K, Wolfe D. Illicit drug policies and the global HIV epidemic: Effects of UN and national government approaches. 2004. [Google Scholar]
- 6.Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338(13):853–60. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 7.New York City Department of Health and Mental Hygiene. HIV Surveillance & Epidemiology Program - HIV/AIDS Annual Surveillance Statistics. New York City: NYCDOHMH; 1982–2013. [Google Scholar]
- 8.Volkow ND, Montaner J. Enhanced HIV testing, treatment, and support for HIV-infected substance users. JAMA. 2010;303(14):1423–4. doi: 10.1001/jama.2010.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vervoort SC, Borleffs JC, Hoepelman AI, Grypdonck MH. Adherence in antiretroviral therapy: a review of qualitative studies. AIDS. 2007;21(3):271–81. doi: 10.1097/QAD.0b013e328011cb20. [DOI] [PubMed] [Google Scholar]
- 10.Mills EJ, Nachega JB, Bangsberg DR, Singh S, Rachlis B, Wu P, et al. Adherence to HAART: a systematic review of developed and developing nation patient-reported barriers and facilitators. PLoS Med. 2006;3(11):e438. doi: 10.1371/journal.pmed.0030438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salter ML, Go VF, Minh NL, Gregowski A, Ha TV, Rudolph A, et al. Influence of Perceived Secondary Stigma and Family on the Response to HIV Infection Among Injection Drug Users in Vietnam. AIDS Educ Prev. 2010 Dec;22(6):558–70. doi: 10.1521/aeap.2010.22.6.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Booth RE, Kennedy J, Brewster T, Semerik O. Drug injectors and dealers in Odessa, Ukraine. J Psychoactive Drugs. 2003 Oct-Dec;35(4):419–26. doi: 10.1080/02791072.2003.10400488. [DOI] [PubMed] [Google Scholar]
- 13.Turner KM, Hutchinson S, Vickerman P, Hope V, Craine N, Palmateer N, et al. The impact of needle and syringe provision and opiate substitution therapy on the incidence of hepatitis C virus in injecting drug users: pooling of UK evidence. Addiction. 2011 Nov;106(11):1978–88. doi: 10.1111/j.1360-0443.2011.03515.x. [DOI] [PubMed] [Google Scholar]
- 14.Des Jarlais DC, Arasteh K, McKnight C, Hagan H, Perlman DC, Torian LV, et al. HIV infection during limited versus combined HIV prevention programs for IDUs in New York City: the importance of transmission behaviors. Drug Alcohol Depend. 2010 Jun 1;109(1–3):154–60. doi: 10.1016/j.drugalcdep.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.UNAIDS. Combination HIV Prevention: Tailoring and Coordinating Biomedical, Bheavioural and Structural Strategies to Reduce New HIV Infections. Geneva: UNAIDS; 2010. [Google Scholar]
- 16.Vlahov D, Robertson A, Strathdee S. Prevention of HIV infection among injection drug users in resource-limited settings. Clin Infect Dis. 2010;50(Suppl 3):S114–21. doi: 10.1086/651482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009 Oct;62(10):e1–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 18.World Bank. Country and Lending Groups by Income-World Bank Country and Lending Groups. Washington DC: World Bank; Jul 18, 2011. [Google Scholar]
- 19.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons; 2011. [Google Scholar]
- 20.Melo AC, Caiaffa WT, Cesar CC, Dantas RV, Couttolenc BF. Utilization of HIV/AIDS treatment services: comparing injecting drug users and other clients. Cad Saude Publica. 2006 Apr;22(4):803–13. doi: 10.1590/s0102-311x2006000400019. [DOI] [PubMed] [Google Scholar]
- 21.DeSilva MB, Gifford AL, Keyi X, Li Z, Feng C, Brooks M. Feasibility and acceptability of a real-time adherence device among HIV-positive IDU patients in. J of Clin Nutr. 2001;10(1):31–8. doi: 10.1155/2013/957862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma YLJ, Huang ZJ, Liu Z, Zhang F. AIDS. Vienna Austria: 2010. Antiretroviral treatment outcome and adherence among 180 IDU AIDS patients in China. [Google Scholar]
- 23.Uuskula A, Laisaar KT, Raag M, Smidt J, Semjonova S, Kogan J, et al. Antiretroviral therapy (ART) adherence and correlates to nonadherence among people on ART in Estonia. AIDS Care. 2012 Dec;24(12):1470–9. doi: 10.1080/09540121.2012.672724. [DOI] [PubMed] [Google Scholar]
- 24.Budon RPV, Cuong DD. Supporting ART adherence in a predominantly IDU driven epidemic: the Vietnam Experience. International Conference on AIDS; 2006; Toronto. [Google Scholar]
- 25.Campos LN, Guimaraes MD, Remien RH. Anxiety and depression symptoms as risk factors for non-adherence to antiretroviral therapy in Brazil. AIDS Behav. 2010 Apr;14(2):289–99. doi: 10.1007/s10461-008-9435-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang H, He G, Li X, Yang A, Chen X, Fennie KP, et al. Self-reported adherence to antiretroviral treatment among HIV-infected people in Central China. AIDS Patient Care STDS. 2008;22(1):71–80. doi: 10.1089/apc.2007.0047. [DOI] [PubMed] [Google Scholar]
- 27.Zhao YSXC, McGoogan JM, Rou K, Zhang F, Wu Z. Methadone maintenance treatment and mortality in HIV-positive people who inject opioids in China. Bulletin World Health Organization. 2013;91:93–101. doi: 10.2471/BLT.12.108944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wisaksana R, Indrati AK, Fibriani A, Rogayah E, Sudjana P, Djajakusumah TS, et al. Response to first-line antiretroviral treatment among human immunodeficiency virus-infected patients with and without a history of injecting drug use in Indonesia. Addiction. 2010;105(6):1055–61. doi: 10.1111/j.1360-0443.2010.02898.x. [DOI] [PubMed] [Google Scholar]
- 29.Shaboltas AV, Skochilov RV, Brown LB, Elharrar VN, Kozlov AP, Hoffman IF. The feasibility of an intensive case management program for injection drug users on antiretroviral therapy in St. Petersburg, Russia. Harm Reduct J. 2013;10(1):15. doi: 10.1186/1477-7517-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sethi AK, Celentano DD, Gange SJ, Moore RD, Gallant JE. Association between adherence to antiretroviral therapy and human immunodeficiency virus drug resistance. Clin Infect Dis. 2003;37(8):1112–8. doi: 10.1086/378301. [DOI] [PubMed] [Google Scholar]
- 31.Larsen MV, Omland LH, Gerstoft J, Roge BT, Larsen CS, Pedersen G, et al. Impact of injecting drug use on response to highly active antiretroviral treatment in HIV-1-infected patients: a nationwide population-based cohort study. Scand J Infect Dis. 2010 Dec;42(11–12):917–23. doi: 10.3109/00365548.2010.511258. [DOI] [PubMed] [Google Scholar]
- 32.Werb D, Milloy MJ, Kerr T, Zhang R, Montaner J, Wood E. Injection drug use and HIV antiretroviral therapy discontinuation in a Canadian setting. AIDS Behav. 2013 Jan;17(1):68–73. doi: 10.1007/s10461-012-0136-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vlahov D, Anthony J, Muiioz A. The ALIVE Study. A longitudinal study of HIV-1 infection in intravenous drug users: description of methods and characteristics of participants NIDA. Res Monogr. 1991;109:75–100. [PubMed] [Google Scholar]
- 34.Fu TC, Westergaard RP, Lau B, Celentano DD, Vlahov D, Mehta SH, et al. Changes in sexual and drug-related risk behavior following antiretroviral therapy initiation among HIV-infected injection drug users. AIDS. 2012 Nov 28;26(18):2383–91. doi: 10.1097/QAD.0b013e32835ad438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moatti JP, Carrieri MP, Spire B, Gastaut JA, Cassuto JP, Moreau J. Adherence to HAART in French HIV-infected injecting drug users: the contribution of buprenorphine drug maintenance treatment. The Manif 2000 study group. AIDS. 2000 Jan 28;14(2):151–5. doi: 10.1097/00002030-200001280-00010. [DOI] [PubMed] [Google Scholar]
- 36.Aceijas C, Oppenheimer E, Stimson G. Antiretroviral treatment for injecting drug users in 174. developing and transitional countries one year before the end of the “Treating 3 million by 2005. Making it happen. The WHO strategy” (3by5) Addiction. 2006 doi: 10.1111/j.1360-0443.2006.01509.x. [DOI] [PubMed] [Google Scholar]
- 37.Kagee A, Remien R, Berkman A, Hoffman S, Campos L, Swartz L. Structural barriers to ART adherence in Southern Africa: challenges and potential ways forward. Global Public Health. 2011;6(1):83–97. doi: 10.1080/17441691003796387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Breen A, Swartz L, Joska J, Flisher AJ, Corrigall J. Adherence to treatment in poorer countries: a new research direction? Psychiatric services (Washington, DC) 2007 Apr;58(4):567–8. doi: 10.1176/ps.2007.58.4.467. [DOI] [PubMed] [Google Scholar]
- 39.Antelman G, Kaaya S, Wei R, Mbwambo J, Msamanga GI, Fawzi WW, et al. Depressive symptoms increase risk of HIV disease progression and mortality among women in Tanzania. J Acquir Immune Defic Syndr. 2007;44(4):470–7. doi: 10.1097/QAI.0b013e31802f1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krüsi A, Wood E, Montaner J, Kerr T. Social and structural determinants of HAART access and adherence among injection drug users. Int J of Drug Policy. 2010;21(1):4–9. doi: 10.1016/j.drugpo.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 41.Wolfe D, Carrieri MP, Shepard D. Treatment and care for injecting drug users with HIV infection: a review of barriers and ways forward. Lancet. 2010;376(9738):355–66. doi: 10.1016/S0140-6736(10)60832-X. [DOI] [PubMed] [Google Scholar]
- 42.Zhang FJ, Jennifer P, Lan Y, Yi W, Yan Z. Current progress of China’s free ART program. Cell Res. 2005;15(11):877–82. doi: 10.1038/sj.cr.7290362. [DOI] [PubMed] [Google Scholar]
- 43.Weber R, Huber M, Rickenbach M, Furrer H, Elzi L, Hirschel B, et al. Uptake of and virological response to antiretroviral therapy among HIV-infected former and current injecting drug users and persons in an opiate substitution treatment programme: the Swiss HIV Cohort Study. HIV Med. 2009;10(7):407–16. doi: 10.1111/j.1468-1293.2009.00701.x. [DOI] [PubMed] [Google Scholar]
- 44.Chaisson RE, Keruly JC, Moore RD. Race, sex, drug use, and progression of human immunodeficiency virus disease. N Engl J Med. 1995;333(12):751–6. doi: 10.1056/NEJM199509213331202. [DOI] [PubMed] [Google Scholar]
- 45.Panel on Antiretroviral Guidelines for Adults Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; 2009. pp. 1–161. [Google Scholar]
- 46.Castro A. Adherence to antiretroviral therapy: merging the clinical and social course of AIDS. PLoS Med. 2005;2(12):e338. doi: 10.1371/journal.pmed.0020338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kurth AE, Celum C, Baeten JM, Vermund SH, Wasserheit JN. Combination HIV prevention: significance, challenges, and opportunities. Curr HIV/AIDS Rep. 2011;8(1):62–72. doi: 10.1007/s11904-010-0063-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Des Jarlais DC, Arasteh K, McKnight C, Hagan H, Perlman DC, Torian LV, et al. HIV infection during limited versus combined HIV prevention programs for IDUs in New York City: the importance of transmission behaviors. Drug and Alc Depend. 2010;109(1):154–60. doi: 10.1016/j.drugalcdep.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Malta M, Bastos FI, da Silva CM, Pereira GF, Lucena FF, Fonseca MG, et al. Differential survival benefit of universal HAART access in Brazil: a nation-wide comparison of injecting drug users versus men who have sex with men. J Acquir Immune Defic Syndr. 2009 Dec;52(5):629–35. doi: 10.1097/QAI.0b013e3181b31b8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sharma M, Singh RR, Laishram P, Kumar B, Nanao H, Sharma C, et al. Access, adherence, quality and impact of ARV provision to current and ex-injecting drug users in Manipur (India): an initial assessment. Int J of Drug Policy. 2007;18(4):319–25. doi: 10.1016/j.drugpo.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 51.Amirkhanian YA, Kelly JA, Kuznetsova AV, DiFranceisco WJ, Musatov VB, Pirogov DG. People with HIV in HAART-era Russia: transmission risk behavior prevalence, antiretroviral medication-taking, and psychosocial distress. AIDS and Behav. 2011;15(4):767–77. doi: 10.1007/s10461-010-9793-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jordan MR, La H, Nguyen HD, Sheehan H, Lien TTM, Duong D, et al. Correlates of HIV-1 viral suppression in a cohort of HIV-positive drug users receiving antiretroviral therapy in Hanoi, Vietnam. Int J of STD & AIDS. 2009;20(6):418–22. doi: 10.1258/ijsa.2008.008389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nguyen DB, Do NT, Shiraishi RW, Le YN, Tran QH, Nguyen HH, et al. Outcomes of Antiretroviral Therapy in Vietnam: Results from a National Evaluation. PloS One. 2013;8(2):e55750. doi: 10.1371/journal.pone.0055750. [DOI] [PMC free article] [PubMed] [Google Scholar]