Abstract
The thalassemia syndromes (α- and β-thalassemia) are the most common and frequent disorders associated with ineffective erythropoiesis. Imbalance of α- or β-globin chain production results in impaired red blood cell synthesis, anemia and more erythroid progenitors in the blood stream. While patients affected by these disorders show definitive altered parameters related to erythropoiesis, the relationship between the degree of anemia, altered erythropoiesis and dysfunctional iron metabolism have not been investigated in both α-thalassemia carriers (ATC) and β-thalassemia carriers (BTC). Here we demonstrate that ATC have a significantly reduced hepcidin and increased soluble transferrin receptor levels but relatively normal hematological findings. In contrast, BTC have several hematological parameters significantly different from controls, including increased soluble transferrin receptor and erythropoietin levels. These changings in both groups suggest an altered balance between erythropoiesis and iron metabolism. The index sTfR/log ferrin and (hepcidin/ferritin)/sTfR are respectively increased and reduced relative to controls, proportional to the severity of each thalassemia group. In conclusion, we showed in this study, for the first time in the literature, that thalassemia carriers have altered iron metabolism and erythropoiesis.
Keywords: Thalassemia, hepcidin, erythropoiesis, iron metabolism, GDF15, ferritin, soluble transferrin receptor, erythropoietin
Introduction
Adult hemoglobin is a heterotetramer composed of two α-globin chains and two β-globin chains (α2β2), each of which contains a heme molecule capable of binding oxygen and facilitating oxygen transport. Thalassemias are a group of hereditary blood disorders characterized by anomalies in the synthesis of α- or β-chains of hemoglobin called α-thalassemia and β-thalassemia, respectively. Together with sickle cell anemia, the thalassemias are the most common inherited disorders of hemoglobin (Hb). Thalassemias are characterized by ineffective erythropoiesis due to the imbalance of globin chain production, resulting in increased apoptosis during erythroblast maturation (1–4).
β-thalassemia results from a variety of molecular alterations, including mutations that affect transcription, translation, and mRNA processing as well as gene deletions (5). Mutations that lead to complete absence of β chains synthesis are defined as β° mutations while those that allow some synthesis of adult hemoglobin are defined as β+ mutations. Based on the combination of these mutations and other genetic modifications (such as mutations leading to elevated levels of fetal hemoglobin synthesis), patients may require only periodic and intermittent red blood cell (RBC) transfusion (β-thalassemia intermedia), or chronic RBC transfusion (β-thalassemia major) (5). Individuals with single heterozygous mutations are considered carriers not requiring treatment.
Deletion or conventional-mutations in α-globin genes (α2 and α1) lead to a spectrum of phenotypes, ranging from practically asymptomatic, α-thalassemia carrier, in which one of four α-globin genes is missing, to lethal hydrops fetalis, in which all of the four genes are defective causing death in uterus or shortly after birth (5).
Patients with different thalassemia syndromes live in Mediterranean countries, South-East Asia, Africa, the Middle East and the Indian subcontinent, where thalassemia carriers account for more than 20% of the population (6). Due to the frequency of immigration from these regions in recent decades, the incidence of the thalassemias has increased also in other geographical areas (7–9).
In particular, in Southeastern Brazil, a frequency of 1.3% of β-thalassemia trait carriers (BTC) was reported in the general population (10), while α-thalassemia trait carriers (ATC), determined by a 3.7-kb DNA deletion (−α3.7/αα), varied from 20 to 25% in Brazil’s African descents (11) and 9 to 12% in general population. Previous studies have shown that the −α3.7 is the most frequent α-globin gene deletion in Brazil (12–15).
Iron metabolism and erythropoiesis are intrinsically linked, as erythropoiesis requires the coordinated biosynthesis of heme and globin chains to produce Hb during differentiation of erythroid precursor. Erythropoietin (EPO) is increased under conditions that required increased RBC synthesis (16). The soluble transferrin receptor (sTfR) concentration is increased in patients with expanded erythropoiesis, including those with hemolytic anemias, myelodysplastic syndromes, and those receiving erythropoietic stimulating agents (17). sTfR is also increased in BTC (18).
Although hepcidin concentration is increased in conditions of primary iron overload, diseases of concurrent anemia and iron overload (i.e. increased and ineffective erythropoiesis) are associated with relatively suppressed hepcidin levels (16,19–21). Several recent studies suggest that growth differentiation factor 15 (GDF15) might not be the main factor secreted during increased and ineffective erythropoiesis to inhibit hepcidin synthesis (22–24). However, GDF15 can be utilized to evaluate the degree of ineffective erythropoiesis (23,24).
Concurrent analysis of these parameters can be used to evaluate the relationship between markers of erythropoiesis and iron metabolism. The ratio of serum hepcidin to ferritin (hepcidin/ferritin) can be utilized as an index of hepcidin response to iron load (25), while the ratio of serum sTfR to ferritin (sTfR/log ferritin) can be used to estimate functional iron requirement (26). Moreover, the ratio of all three parameters ((hepcidin/ferritin)/sTfR) can be utilized to explore and quantify the opposing forces (i.e. iron availability and erythropoietic activity) regulating hepcidin synthesis and iron absorption in absence of inflammatory stimuli (27).
In the current study, we investigate the relationship between degree of anemia, increased and ineffective erythropoiesis, and iron sensing in patients with α- and β-thalassemia silent carriers. To accomplish this goal, we evaluate the correlations between several erythroid and iron related factors, including serum hepcidin. The purpose of this inquiry is to provide new insight into the balance between iron metabolism and erythropoiesis in relatively mild anemias and to explore new clinically relevant measures to characterize patients with these and other anemia-related conditions.
Patients and Methods
Ethics approval
The Faculty of Pharmaceutical Sciences of Ribeirao Preto from University of Sao Paulo (FCFRP/USP) Ethics Committee approved the study with the agreement of The Clinical Hospital of Medical School of Ribeirao Preto (HC/FMRP/USP) Ethics Committee. All patients provided written consent in accordance with the current law and Declaration of Helsinki.
Subjects
Adult subjects were recruited as unselected group of both first time and repeat blood donors (RBD). Samples for analysis were collected prior to blood donation at the Clinical Hematology Laboratory (Ribeirao Preto, FCFRP-USP, Brazil).
Control, RBD and ATC groups
The study subjects were first screened for α-thalassemia deletions (28), and of 177 screened, 15 (8.47%) had one α gene deletion (-α3.7/αα). Among those silent carriers for -α3.7, 1 patient was excluded due to a presumed infection, evidenced by elevated C-reactive protein (CRP) and white blood cell (WBC) count. Thus, 14 patients were selected to form the ATC group.
Of the remaining 162 blood donors screened, 51 met inclusion criteria: Hb greater than 13.0 g/dL for men and 12.0 g/dL for women (29); normal RBC count, ferritin, serum iron, % transferrin saturation (%Tsat), total iron binding capacity (TIBC), and sTfR; CRP less than 3.0 mg/L; absent evidence of hemoglobinopathies or other clinical disorders that alter iron metabolism (e.g. inflammatory and acute or chronic infections); and no presence of dysmetabolic syndrome. These 51 subjects were divided in two groups, Control (n=28) and RBD (n=23). The RBD was defined as those donating blood at least twice in the year prior to study enrollment.
BTM and BTC groups
Adult patients with BTM (n=27) and BTC (n=20) were from previously identified and regularly followed patients in the Hemoglobinopathies Clinic (Ribeirao Preto, HC/FMRP-USP, Brazil). In BTM patients, blood was collected immediately prior to RBC transfusion.
Analysis of mutations
Genomic DNA was extracted using AxyPrep™ Blood Genomic DNA Miniprep Kit (nucleic acid Purification kit), Axygen Biosciences. The conventional RT-PCR protocol was used to detect the most common α-thalassemia deletion −α3.7 in Southeast Brazil and identify ATC individuals (28). Moreover, to assess participants for any unexpected hemoglobinopathies, hemoglobin electrophoresis using acetate cellulose paper at alkaline pH (pH 8.6) was performed and gene analysis was carried out for BTC and BTM patients.
Laboratory analysis
Samples were tested for hematological parameters (Micros ABX 60), serum iron, TIBC, and %Tsat by the colorimetric method (Pointe Scientific, Inc., Canton, MI, USA), ferritin and high sensitive CRP by immunoassay (Immulite 1000), sTfR, EPO and GDF15 (R&D Systems) and hepcidin (Intrinsic LifeSciences, La Jolla, CA) by ELISA.
Normal values and definitions
Suominen et al suggested that the normal ferritin serum levels range is 22 – 203 µg/L and sTfR concentration range is 1.15 – 2.75 mg/L (30). For serum iron, TIBC and %Tsat, the manufacturer suggests each study to perform the normal range. For our study, the normal ranges we established for serum iron, TIBC and %Tsat were 60–170 µg/dL, 160–400 µg/dL and 20–60%, respectively. For CRP, the expected upper normal limit for healthy individuals established in the literature was <3mg/L, however a study performed on 100 healthy volunteers yielded a median of 1.4 mg/L and an upper 97.5th percentile of 11 mg/L (31). The reference range for EPO serum assay established by manufacturer in 123 normal individuals from the Minneapolis/St. Paul, Minnesota area was 3.3 – 16.6 mIU/mL, in our study the range in 46 healthy adults from the region of Ribeirao Preto, Brazil area was 0.5 – 13.4 mIU/mL, mean 4.8 mIU/mL. Tanno et al suggested that GDF15 levels in normal individuals were between 200 – 1150 pg/mL (22). A well validated assay that allows hepcidin quantification reported in a previous study indicated that the 5th–95th percentile for serum hepcidin concentrations in normal adult men were 29 – 254 ng/mL (n=65) and 17 – 286 ng/mL in adult women (n=49), with median concentrations 112 ng/mL in men and 65 ng/mL in women (P<0.001) (32).
Statistical considerations
Statistical analyses of the results were performed by GraphPad Prism version 5.02. Due to the skewed distributions, the parameters were log-transformed to approximate a normal distribution and parametric analyses were conducted. For all variables, geometric means, medians, minimum and maximum, standard errors and standard deviations were calculated. Due to gender differences, we conducted stratified analyses. Pearson correlation coefficients were generated to assess relationships between indices. One-way ANOVA and post-test Dunnett's Multiple Comparison tests were used for group comparisons of the biological parameters. Data is presented as mean ± 95% confidence interval (CI) and p<0.05 was considered statistically significant.
Results
Control versus RBD
Controls were derived from the general blood donor pool so first we compared first time blood donors and RBD. We did not observe any statistical difference comparing various parameters between controls and RBD (Suppl. 1). In addition, the expected correlation between hepcidin and ferritin previously reported (27, 33–35) is only maintained in controls (Table 3). Hepcidin/ferritin is a parameter correlates well with iron absorption (36). These observations suggest that multiple blood donations alter the typical relationship between parameters of iron metabolism (37). For this reason, only first time donors were used as controls in this study.
TABLE 3.
Pearson's correlation coefficients and statistical significance between hepcidin and potential factors influencing its production in each group
| Control | ATC | BTC | BTM | RBD | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Correlation | r | p | r | p | r | p | r | p | r | p |
| Hepcidin vs. Iron | −0.150 | 0.446 | −0.100 | 0.733 | −0.027 | 0.909 | −0.387 | 0.046 | 0.280 | 0.195 |
| Hepcidin vs. Ferritin | 0.749 | <0.0001 | 0.711 | 0.004 | 0.771 | <0.0001 | 0.639 | 0.0003 | 0.139 | 0.528 |
| Hepcidin vs. GDF15 | 0.165 | 0.401 | 0.385 | 0.174 | 0.481 | 0.032 | −0.493 | 0.009 | 0.224 | 0.305 |
| Hepcidin vs. EPO | 0.068 | 0.732 | 0.402 | 0.154 | −0.280 | 0.232 | −0.388 | 0.045 | 0.017 | 0.938 |
| Hepcidin vs. sTfR | 0.176 | 0.370 | −0.268 | 0.353 | 0.118 | 0.621 | −0.484 | 0.010 | 0.061 | 0.780 |
Control group (n=28); ATC indicates α-thalassemia carriers (n=14); BTC indicates β-thalassemia carriers (n=20); BTM, β-thalassemia major (n=27); RBD, repeat blood donors (n=23); GDF15, growth differentiation factor 15; EPO, erythropoietin and sTfR, soluble transferrin receptor. The statistical analyses were performed using Pearson’s correlation test, significant p is shown as bold.
Silent carriers of α-thalassemia
Although the hematological changes in the ATC group are considered in the normal range, the mean corpuscular volume (MCV) and the mean corpuscular hemoglobin (MCH) are significant different from controls (Table 1), suggesting that individual with this profile should be investigated to evaluate if carriers of a single α-globin mutation. ATC also showed significant increased in soluble transferrin receptor levels and decreased of hepcidin levels (Table 2). These taking together suggest that even a deletion of one α-gene shows an increased erythropoietic activity reducing hepcidin levels so the iron absorption can be raised to keep the balance between hemoglobin levels and iron status. These results suggest a measurable compensatory increase in erythropoiesis and its consequent suppressive effect on hepcidin synthesis.
TABLE 1.
Hematological Data of the Different Groups
| Parameters | Control Mean [95% CI] |
ATC Mean [95% CI] |
BTC Mean [95% CI] |
BTM Mean [95% CI] |
|---|---|---|---|---|
| RBC (1012/L) | 4.73 [4.59–4.89] | 4.90 [4.63–5.20] | 5.50 [5.25–5.75]*** | 3.22 [3.10–3.35]*** |
| Hb (g/dL) | 14.3 [13.8–14.8] | 13.8 [13.0–14.6] | 11.3 [10.9–11.8]*** | 9.1 [8.8–9.4]*** |
| Hct (%) | 42.3 [40.9–43.8] | 41.3 [39.1–43.8] | 36.0 [34.5–37.6]*** | 26.9 [25.9–27.9]*** |
| MCV (fL) | 89.5 [88.5–90.5] | 84.3 [82.4–86.2] *** | 66.0 [64.5–67.5]*** | 83.2 [82.0–84.3]*** |
| MCH (pg) | 30.2 [29.8–30.7] | 28.0 [27.2–28.9] *** | 20.9 [20.1–21.6]*** | 28.3 [27.7–29.0] *** |
| MCHC (g/dL) | 33.8 [33.5–34.1] | 33.3 [32.8–33.7] | 31.6 [31.1–32.1]*** | 34.0 [33.4–34.6] |
| RDW (%) | 11.2 [10.9–11.4] | 11.3 [10.9–11.8] | 13.1 [12.7–13.5]*** | 13.9 [13.1–14.8]*** |
Control group (n=28); ATC indicates α-thalassemia carriers (n=14); BTC indicates β-thalassemia carriers (n=20); BTM, β-thalassemia major (n=27); RBC, red blood cells count; Hb, hemoglobin; Hct, hematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration and RDW, red cell distribution width. The statistical analyses were performed using oneway ANOVA and Dunnett's Multiple Comparison post test,
p<0.05,
p<0.01,
p<0.001, relative to controls.
TABLE 2.
Biochemical Data of the Different Groups
| Parameters | Control Mean [95% CI] |
ATC Mean [95% CI] |
BTC Mean [95% CI] |
BTM Mean [95% CI] |
|---|---|---|---|---|
| sTfR (mg/L) | 1.4 [1.3–1.5] | 2.1 [1.7–2.5] ** | 4.5 [3.7–5.5]*** | 12.8 [10.3–15.9]*** |
| EPO (mU/mL) | 3.5 [2.5–4.8] | 4.4 [3.6–5.3] | 8.1 [6.1–10.9]*** | 74.7 [59.7–93.4]*** |
| GDF15 (pg/mL) | 358 [296–434] | 414 [261–655] | 543 [376–785] | 10,250 [8,316–12,619]*** |
| Serum Iron (µg/dL) | 96.0 [83.2–110.8] | 110 [91.6–133] | 98.2 [84.9–114] | 224 [203–246]*** |
| TIBC (µg/dL) | 278 [249–310] | 256 [227–289] | 267 [252–284] | 328 [285–377] |
| %Tsat | 34.6 [30.8–38.9] | 43.1 [35.0–53.1] | 36.7 [31.1–43.3] | 68.2 [62.1–74.8]*** |
| Ferritin (ng/mL) | 110 [86.6–139] | 82.5 [45.3–150] | 156 [88.6–274] | 2,230 [1,649–3,020]*** |
| Hepcidin (ng/mL) | 63.2 [52.8–75.8] | 38.3 [24.0–61.0] * | 81.3 [62.5–106] | 51.7 [38.3–69.9] |
| sTfR/log Ferritin | 0.68 [0.61–0.76] | 1.13 [0.88–1.45] ** | 2.12 [1.66–2.72]*** | 3.83 [3.03–4.84]*** |
| (Hepcidin/Ferritin)/ sTfR |
0.41 [0.35–0.49] | 0.22 [0.13–0.36] * | 0.12 [0.08–0.18] *** | 0.002 [0.001–0.003]*** |
Control group (n=28); ATC indicates α-thalassemia carriers (n=14); BTC indicates β-thalassemia carriers (n=20); BTM, β-thalassemia major (n=27); TIBC, total iron-binding capacity; Tsat, transferrin saturation; sTfR, soluble transferrin receptor; GDF15, growth differentiation factor 15 and EPO, erythropoietin. The statistical analyses were performed using oneway ANOVA and Dunnett's Multiple Comparison post test,
p<0.05,
p<0.01,
p<0.001, relative to controls.
β-thalassemia minor
BTC group showed significant differences for all hematological parameters (Table 1). The rise in the RBC count (Table 1) due erythropoiesis expansion suggests an attempt to compensate for reduced Hb concentration due to lower MCV and MCH. The elevated erythropoietic activity is reflected by increased serum levels of sTfR and EPO (Table 2).
β-thalassemia major
In the BTM group, all hematological parameters except mean corpuscular hemoglobin concentration (MCHC) are significantly different. While RDW is increased, all the others are decreased relative to controls (Table 1). In addition, all the biochemical parameters, except for TIBC and hepcidin, are significantly higher relative to controls (Table 2). However, as previously shown, reduced hepcidin/ferritin indicates that hepcidin levels are disproportionally low relative to the degree of iron overload (25,38,39). Furthermore, we demonstrate that hepcidin concentrations in BTM patients are within the reference values but mean (hepcidin/ferritin)/sTfR (Table 2) is markedly reduced in all patients.
sTfR/log ferritin ratio and sTfR levels
The sTfR/log ferritin ratio can distinguish storage iron depletion, iron deficiency erythropoiesis and iron deficiency anemia when ferritin levels are also low (30). However, when ferritin levels are normal or increased, the sTfR levels reflect the erythropoietic activity (27, 36, 39). All study groups showed a significant raise for sTfR levels and index sTfR/log ferritin proportional to the severity of their deletion/mutation ATC<BTC<BTM (Table 2).
Hepcidin/ferritin)/sTfR
The association of hepcidin, ferritin and sTfR in the formula (Hepcidin/ferritin)/sTfR was useful to distinguish the different groups showing a significant reduction of this index proportional to the severity of their deletion/mutation ATC>BTC>BTM (Table 2).
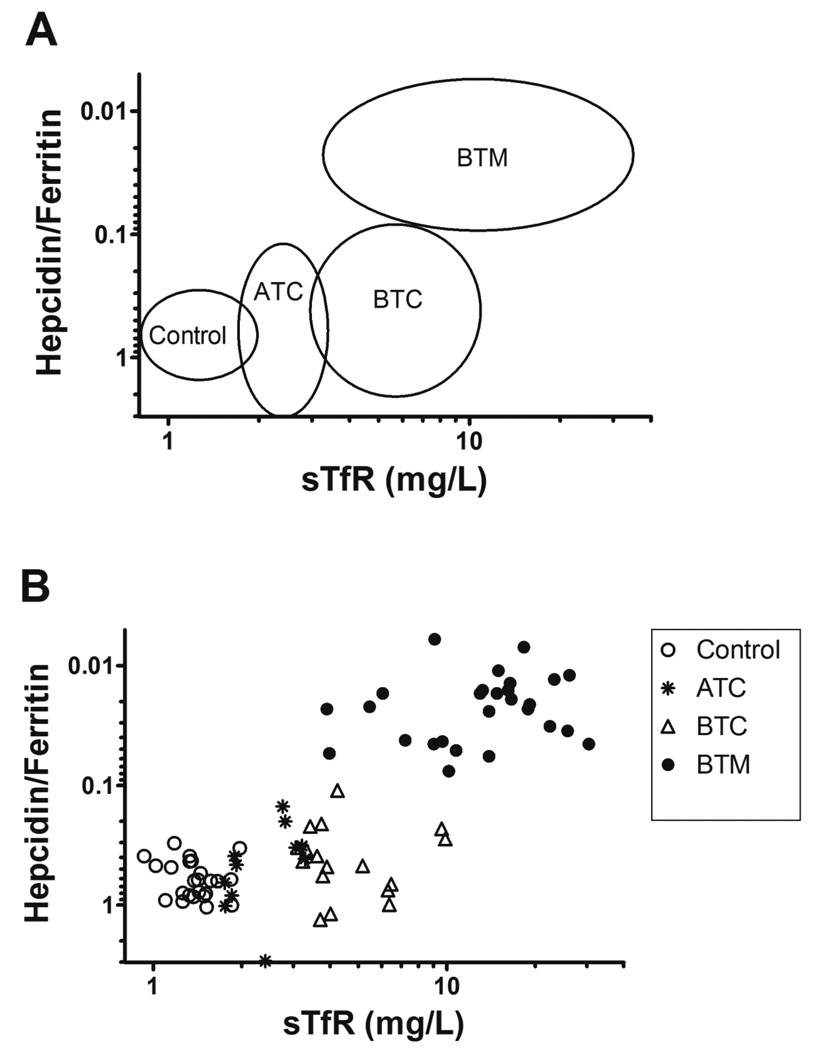
The distribution of the study groups plotting the hepcidin/ferritin ratio against sTfR can be viewed in the Figure 1. While controls are in the lower left corner, thalassemia carriers such ATC and BTC appear dislocated to the right as “normal hepcidin to ferritin ratio-moderately high sTfR” with the sTfR concentration reflecting the raise in the erythropoietic activity. However, BTM patients are in the upper right corner due to “low hepcidin to ferritin ratio-high sTfR” and reflecting a high erythroid demand associated with increased iron absorption, characteristic of iron-overloading anemia.
Figure 1.
Distribution of the groups plotting Hepcidin/Ferritin against sTfR. Plotting hepcidin/ferritin ratios against sTfR levels allowed to distinguish each group. (A) Circles indicate the location of each group. Control (n=28), ATC (n=14), BTC (n=20), and BTM (n=27). (B) Control (open circles); ATC, α-thalassemia carriers (asteriscs); BTC, β-thalassemia carriers (open triangles) and BTM, β-thalassemia major (close circles). Hepcidin/ferritin ratio has been plotted with a reverse y axis because lower hepcidin to ferritin ratios represent higher iron, as can be seen in the ascending line formed by the distribution of the groups.
Discussion
We showed in this study, for the first time in the literature, that thalassemia carriers have altered iron metabolism and erythropoiesis. These aspects were not addressed in any other study with thalassemia carriers, as far as we know. Carriers of α-thalassemia (with 3 function α-genes) known as silent carriers are reported to have no hematological alterations (40–42). Carriers of β-thalassemia with one normal β-gene are reported to have no hematologic abnormalities to borderline asymptomatic anemia with microcytosis and hypochromia (43).
Our study suggests that even a deletion of one α-gene shows an increased erythropoietic activity with reduced hepcidin levels so the iron absorption can be raised to keep the balance between hemoglobin levels and iron status. These results suggest a measurable compensatory increase in erythropoiesis and its consequent suppressive effect on hepcidin synthesis.
In carriers of β-thalassemia, the rise in the RBC count (Table 1) due to erythropoiesis expansion suggests an attempt to compensate for the reduced Hb concentration due to lower MCV and MCH. The elevated erythropoietic activity we found in this population is reflected by increased serum levels of sTfR and EPO (Table 2).
Interestingly, BMT patients show increased sTfR, EPO, sTfR/log ferritin, and GDF15 (Table 2), suggesting that, despite RBC transfusions, expanded erythropoiesis is likely present but ineffective (16).
We also evaluate whether correlations among hepcidin, ferritin and sTfR are useful to estimate the degree of iron absorption as a consequence of the multiple factors controlling hepcidin synthesis in the absence of inflammation, i.e. iron storage and erythropoietic activity. Based on our observations, we combine parameters related to iron metabolism such as hepcidin and ferritin with sTfR, a marker of erythropoietic proliferation, and determine an equation to assist clinicians with the diagnosis of severe thalassemias and to discriminate the thalassemia carriers from controls. The association of hepcidin, ferritin and sTfR in the formula (hepcidin/ferritin)/sTfR was useful to distinguish the study groups showing a significant reduction proportional to the severity of their deletion/mutation ATC>BTC>BTM (Table 2). This could be helpful to monitor the appropriateness of iron absorption and potential issues related to accumulation of iron over time and worsening of erythropoiesis. Several studies have investigated the correlation between erythropoiesis and iron metabolism in BTM patients (16,25,44). However, the molecular mechanism(s) responsible for communicating iron demand for erythropoiesis remain incompletely understood.
Conclusion
There are two main conclusions of this study. First, ATC and to a greater degree BTC showed an altered balance between iron absorption/storage and erythropoiesis. Although a cross-sectional analysis such as this is not designed to determine potential accumulation of ill effects over time, evidence provided here suggests that patient care may benefit from prospective monitoring of ATC and BTC to evaluate whether abnormalities in erythropoiesis and increased iron absorption negatively affect their health over time, such as iron overload. Second, BTM patients demonstrate features consistent with ineffective erythropoiesis (i.e. increased erythroid cell proliferation and cell death as suggested by increased sTfr1 and GDF15 levels), and abnormal iron metabolism (Table 2), despite transfusion to Hb levels of 8 – 10 g/dL, suggesting that additional therapy to complement transfusion and management of iron metabolism are necessary to further benefit patient health. Novel therapeutic agents, such as apotransferrin, Jak2 inhibitors, antisense oligonucleotides to target TMPRSS6, minihepcidins and hypoxia-inducible factor 2-alpha (HIF2α) antagonists to reduce the level of ineffective erythropoiesis and/or decrease iron absorption are currently being developed in this direction (45–49).
Supplementary Material
Acknowledgments
The authors are grateful to Ms. Jennifer M. Chauca Yokoya and Dr. Cristiane F. F. Tavares of the FCFRP from University of Sao Paulo for technical help, to Ms. Elza M. Kimura of the Clinical Pathology Department from University of Campinas for technical help with RT-PCR −α3.7 deletion and to Ms. Edna A. Barizon and Ms. Analuiza S. Costa from SAC of FCFRP from University of Sao Paulo for technical help with Immulite-1 equipment.
Conflict-of-interest disclosure:
This research was supported by grants from CNPq (141608/2011-0) and CAPES (18584-12-8) of Brazil (JSG), and grants from the NIH (1R01DK090554 and 5R01DK095112) of Weill Cornell of Medical College of USA (SR). S. Rivella is a consultant for Novartis, Bayer, Merganser and Isis Pharmaceuticals. Dr. Rivella holds equity in Merganser Biotech. The consulting work and intellectual property of S. Rivella did not affect in any way the design, conduct, or reporting of this research. MW is inventor of the hepcidin assay. MW is the President & CEO and GO is Laboratory Director of Intrinsic LifeSciences (ILS). Intrinsic LifeSciences LLC (La Jolla, CA), holds U.S. patents has pending patents on the hepcidin C-ELISA and related compositions, and MW has equity in ILS and has received honoraria from Centocor-Ortho R&D, Inc. GO is an employee and has equity interests in Intrinsic LifeSciences LLC, and has validated the hepcidin assay for clinical testing.
Footnotes
Authorship contributions:
JSG designed the research, collected, analyzed and interpreted the data, performed experiments and statistical analyses, and wrote the manuscript. JGC collected data and performed experiments. ACSP recruited β-thalassemia patients and apparently healthy subjects and edited the manuscript. GO performed the hepcidin ELISA assays. MW analyzed the hepcidin ELISA results and edited the manuscript. YZG interpreted the data and edited the manuscript. VN provided statistical guidance. SR designed the research, interpreted the data, edited and wrote the manuscript. AMS designed the research, interpreted the data and edited the manuscript. All authors approved the final manuscript.
References
- 1.Olivieri NF. The β-thalassemias. N Eng J Med. 1999;341:99–109. doi: 10.1056/NEJM199907083410207. [DOI] [PubMed] [Google Scholar]
- 2.Rund D, Rachmilewitz E. Medical Progress Beta-thalassemia. N Engl J Med. 2005;353:1135–1146. doi: 10.1056/NEJMra050436. [DOI] [PubMed] [Google Scholar]
- 3.Weatherall DJ. Disorders of globin synthesis: the thalassemias. In: Lichtman MA, et al., editors. Williams Hematology. 7th Edition. New York: McGraw-Hill Medical; 2006. pp. 633–656. [Google Scholar]
- 4.Diego S, Pootrakul P, Sirankapracha P, et al. A correlation of erythrokinetics, ineffective erythropoiesis, and erythroid precursor apoptosis in Thai patients with thalassemia. Blood. 2000;96:2606–2612. [PubMed] [Google Scholar]
- 5.Rivella S, Giardina JP. Thalassemia Syndromes. In: Hoffman R, et al., editors. Hematology: Basic Principles and Practice. Philadelphia: Elsevier; 2013. pp. 505–535. [Google Scholar]
- 6.Modell B, Darlison M. Global epidemiology of hemoglobin disorders and derived service indicators. Bull WHO. 2008;86:480–487. doi: 10.2471/BLT.06.036673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kan YW. Molecular pathology of α-thalassemia. Ann NY Acad Sci. 1985;445:28–36. doi: 10.1111/j.1749-6632.1985.tb17172.x. [DOI] [PubMed] [Google Scholar]
- 8.Vichinsky E. Complexity of alpha thalassemia: growing health problem with new approaches to screening, diagnosis, and therapy. Ann NY Acad Sci. 2010;1202:180–187. doi: 10.1111/j.1749-6632.2010.05572.x. [DOI] [PubMed] [Google Scholar]
- 9.Harteveld C, Higgs D. Alpha-thalassaemia. Orphanet J Rare Dis. 2010;5:13. doi: 10.1186/1750-1172-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramalho AS, Silva RBP, Teixeira RC, Compri MB. Hemoglobin screening: response of a Brazilian community to optional programs. Cad Saude Publica. 1999;15:591–595. doi: 10.1590/s0102-311x1999000300016. [DOI] [PubMed] [Google Scholar]
- 11.Sonati MF, Farah SB, Ramalho AS, Costa FF. High prevalence of alpha-thalassemia in a black population of Brazil. Hemoglobin. 1991;15:309–311. doi: 10.3109/03630269109027884. [DOI] [PubMed] [Google Scholar]
- 12.Tavares CFF, Guimarães JS, Almeida MR, Souza AM. Hemoglobinopatias em escolares de Ribeirão Preto, São Paulo. Congresso Brasileiro de Hematologia e Hemoterapia, 2011, Sao Paulo, Rev Bras Hematol Hemoter. 2011;33:52–52. [Google Scholar]
- 13.Adorno EV, Couto FD, Moura Neto JP, et al. Hemoglobinopathies in newborns from Salvador, Bahia, Northeast Brazil. Rev. Saúde Pública. 2005;21:292–298. doi: 10.1590/s0102-311x2005000100032. [DOI] [PubMed] [Google Scholar]
- 14.Alcoforado GHM, Bezerra CM, Lemos TMAM, et al. Prevalence of α-thalassemia 3.7 kb deletion in the adult population of Rio Grande do Norte, Brazil. Genet Mol Biol. 2012;35:594–598. doi: 10.1590/S1415-47572012005000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Souza AES, Cardoso GL, Takanashi SYL, Guerreiro JF. α-thalassemia (3.7kb deletion) in a population from the Brazilian Amazon region: Santarem, Para State. Genet Mol Res. 2009;8:477–481. doi: 10.4238/vol8-2gmr601. [DOI] [PubMed] [Google Scholar]
- 16.Ginzburg Y, Rivella S. Β-Thalassemia: a Model for Elucidating the Dynamic Regulation of Ineffective Erythropoiesis and Iron Metabolism. Blood. 2011;118:4321–4330. doi: 10.1182/blood-2011-03-283614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Speeckaert MM, Speeckaert R, Delanghe JR. Biological and clinical aspects of soluble transferrin receptor. Crit Rev Clin Lab Sci. 2011;47:213–228. doi: 10.3109/10408363.2010.550461. [DOI] [PubMed] [Google Scholar]
- 18.Demir A, Yarali N, Fisgin T, Duru F, Kara A. Serum transferrin receptor levels in beta-thalassemia trait. J Trop Pediatr. 2004;50:369–371. doi: 10.1093/tropej/50.6.369. [DOI] [PubMed] [Google Scholar]
- 19.Li H, Ginzburg YZ. Crosstalk between Iron Metabolism and Erythropoiesis. Adv Hematol. 2010;2010 doi: 10.1155/2010/605435. 605435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ganz T, Nemeth E. Hepcidin and disorders of iron metabolism. Annu Rev Med. 2011;62:347–360. doi: 10.1146/annurev-med-050109-142444. [DOI] [PubMed] [Google Scholar]
- 21.Gardenghi S, Grady RW, Rivella S. Anemia, ineffective erythropoiesis, and hepcidin: interacting factors in abnormal iron metabolism leading to iron overload in β-thalassemia. Hematol Oncol Clin North Am. 2010;24:1089–1107. doi: 10.1016/j.hoc.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanno T, Bhanu NV, Oneal Pa, et al. High levels of GDF15 in thalassemia suppress expression of the iron regulatory protein hepcidin. Nat Med. 2007;13:1096–1101. doi: 10.1038/nm1629. [DOI] [PubMed] [Google Scholar]
- 23.Casanovas G, Spasic MV, Casu C, et al. The murine growth differentiation factor 15 is not essential for systemic iron homeostasis in phlebotomized mice. Haematologica. 2013;98:444–447. doi: 10.3324/haematol.2012.069807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Musallam KM, Taher AT, Duca L, Cesaretti C, Halawi R, Cappellini MD. Levels of growth differentiation factor-15 are high and correlate with clinical severity in transfusion-independent patients with β thalassemia intermedia. Blood Cells Mol Dis. 2011;47:232–234. doi: 10.1016/j.bcmd.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Origa R, Galanello R, Ganz T, Giagu N, Maccioni L, et al. Liver iron concentrations and urinary hepcidin in beta-thalassemia. Haematologica. 2007;92:583–588. doi: 10.3324/haematol.10842. [DOI] [PubMed] [Google Scholar]
- 26.Skikne BS. Test of the Month Serum transferrin receptor. Am J Hematol. 2008;83:872–875. doi: 10.1002/ajh.21279. [DOI] [PubMed] [Google Scholar]
- 27.Fertrin KY, Lanaro C, Franco-Penteado CF, de Albuquerque DM, de Mello MRB, Pallis FR, et al. Erythropoiesis-driven regulation of hepcidin in human red cell disorders is better reflected through concentrations of soluble transferrin receptor rather than growth differentiation factor 15. Am J Hematol. 2014;89:385–390. doi: 10.1002/ajh.23649. [DOI] [PubMed] [Google Scholar]
- 28.Dodé C, Krishnamoorthy R, Lamb J, Rochette J. Rapid analysis of -α3.7 thalassaemia and αααanti 3.7 triplication by enzymatic amplification analysis. Br J Haematol. 1993;83:105–111. doi: 10.1111/j.1365-2141.1993.tb04639.x. [DOI] [PubMed] [Google Scholar]
- 29.World Health Organization. [Accessed 20 January 2014];WHO information for Iron Deficiency Anaemia. Assessment, prevention, and control. A guide for programme managers. 2001 Available at: http://www.who.int/nutrition/publications/micronutrientsanaemia_iron_deficiency/WHO_NHD_01.3/en/.
- 30.Suominen P, Punnonen K, Rajamäki A, Irjala K. Serum Transferrin Receptor and Transferrin Receptor-Ferritin Index Identify Healthy Subjects With Subclinical Iron Deficits. Blood. 1998;92:2934–2939. [PubMed] [Google Scholar]
- 31.Biasucci LM. CDC/AHA Workshop on Markers of Inflammation and Cardiovascular Disease: Application to Clinical and Public Health Practice: clinical use of inflammatory markers in patients with cardiovascular diseases: a background paper. Circulation. 2004;110:e560–e567. doi: 10.1161/01.CIR.0000148983.88334.80. [DOI] [PubMed] [Google Scholar]
- 32.Ganz T, Olbina G, Girelli D, Nemeth E, Westerman M. Immunoassay for human serum hepcidin. Blood. 2008;112:4292–4297. doi: 10.1182/blood-2008-02-139915. [DOI] [PubMed] [Google Scholar]
- 33.Kuragano T, Shimonaka Y, Kida A, Furuta M, Nanami M, et al. Determinants of hepcidin in patients on maintenance hemodialysis: role of inflammation. Am J Nephrol. 2010;31:534–540. doi: 10.1159/000312381. [DOI] [PubMed] [Google Scholar]
- 34.Karl JP, Lieberman HR, Cable SJ, Williams KW, Young AJ, Mcclung JP. Randomized, double-blind, placebo-controlled trial of an iron-fortifie food product in female soldiers during military training: relations between iron status , serum hepcidin , and inflammation 1–5. Am J Clinial Nutr. 2010;92:93–100. doi: 10.3945/ajcn.2010.29185. [DOI] [PubMed] [Google Scholar]
- 35.Piperno A, Galimberti S, Mariani R, Pelucchi S, Ravasi G, et al. Modulation of hepcidin production during hypoxia-induced erythropoiesis in humans in vivo: data from the HIGHCARE project. Blood. 2011;117:2953–2959. doi: 10.1182/blood-2010-08-299859. [DOI] [PubMed] [Google Scholar]
- 36.Pasricha S, Mcquilten Z, Westerman M, Keller A, Nemeth E, et al. Serum hepcidin as a diagnostic test of iron deficiency in premenopausal female blood donors. Haematologica. 2011;96:1099–1105. doi: 10.3324/haematol.2010.037960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mast AE, Schlumpf KS, Wright DJ, Johnson B, Glynn SA, et al. Hepcidin level predicts hemoglobin concentration in individuals undergoing repeated phlebotomy. Haematologica. 2013;98:1324–1330. doi: 10.3324/haematol.2012.070979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gardenghi S, Marongiu MF, Ramos P, Guy E, Breda L, et al. Ineffective erythropoiesis in beta-thalassemia is characterized by increased iron absorption mediated by down-regulation of hepcidin and up-regulation of ferroportin. Blood. 2007;109:5027–5035. doi: 10.1182/blood-2006-09-048868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kattamis A, Papassotiriou I, Palaiologou D, Apostolakou F, Galani A, et al. The effects of erythropoetic activity and iron burden on hepcidin expression in patients with thalassemia major. Haematologica. 2006;91:809–812. [PubMed] [Google Scholar]
- 40.Vichinsky E. Advances in the treatment of alpha-thalassemia. Blood Reviews. 2012;26(Suppl 1):S31–S34. doi: 10.1016/S0268-960X(12)70010-3. [DOI] [PubMed] [Google Scholar]
- 41.Galanello R. Recent advances in the molecular understanding of non-transfusion-dependent thalassemia. Blood Reviews. 2012;26(Suppl 1):S7–S11. doi: 10.1016/S0268-960X(12)70004-8. [DOI] [PubMed] [Google Scholar]
- 42.Vichinsky EP. Clinical manifestations of α-thalassemia. Cold Spring Harb Perspect Med. 2013;3:a011742. doi: 10.1101/cshperspect.a011742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Musallam KM, Rivella S, Vichinsky E, Rachmilewitz EA. Non-transfusion-dependent thalassemias. Haematologica. 2013;98:833–844. doi: 10.3324/haematol.2012.066845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gardenghi S, Rivella S. New insight on the management of ineffective erythropoiesis and iron overload in β-thalassemia and related hemoglobinopathies. Hematol Educ. 2012;6:331–338. [Google Scholar]
- 45.Li H, Rybicki AC, Suzuka SM, Bonsdorff L, Breuer W, et al. Transferrin therapy ameliorates disease in beta-thalassemic mice. Nat Med. 2010;16:177–182. doi: 10.1038/nm.2073. [DOI] [PubMed] [Google Scholar]
- 46.Gardenghi S, Ramos P, Marongiu MF, Melchiori L, Breda L, et al. Hepcidin as a therapeutic tool to limit iron overload and improve anemia in β-thalassemic mice. J Clin Invest. 2010;120:4466–4477. doi: 10.1172/JCI41717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parrow NL, Gardenghi S, Rivella S. Prospects for a hepcidin mimic to treat β-thalassemia and hemochromatosis. Expert Rev Hematol. 2011;4:233–235. doi: 10.1586/ehm.11.22. [DOI] [PubMed] [Google Scholar]
- 48.Guo S, Casu C, Gardenghi S, Booten S, Aghajan M, et al. Reducing TMPRSS6 ameliorates hemochromatosis and β-thalassemia in mice. J Clin Invest. 2013;123:1531–1541. doi: 10.1172/JCI66969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anderson ER, Taylor M, Xue X, Ramakrishnan SK, Martin A, et al. Intestinal HIF2α promotes tissue-iron accumulation in disorders of iron overload with anemia. Proc Natl Acad Sci USA. 2013;110:E4922–E4930. doi: 10.1073/pnas.1314197110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.