Abstract
High adherence is critical for achieving clinical benefits of HIV antiretroviral therapy (ART) and particularly challenging for children. We conducted 35 qualitative interviews with caregivers of HIV-infected Ugandan children who were followed in a longitudinal study of real-time ART adherence monitoring; 18 participants had undetectable HIV RNA, while 17 had detectable virus. Interviews blinded to viral suppression status elicited information on adherence experiences, barriers and facilitators to adherence, and social support. Using an inductive content analytic approach, we identified ‘lack of resources,’ ‘Lazarus effect,’ ‘caregiver's sense of obligation and commitment,’ and ‘child's personal responsibility’ as categories of influence on adherence, and defined types of caregiver social support. Among children with viral suppression, high hopes for the child's future and ready access to private instrumental support appeared particularly important. These findings suggest clinical counseling should explore caregivers' views of their children's futures and ability to access support in overcoming adherence barriers.
Keywords: Antiretroviral therapy, Human immunodeficiency virus, Adherence, Children, Uganda
Introduction
In 2012, 3.3 million people under age 15 were living with HIV, with approximately 260,000 new infections yearly. Ninety-one percent—or 2.9 million—of these children live in sub-Saharan Africa [1]. Access to antiretroviral therapy (ART) throughout the world has increased dramatically, with roughly 630,000 children younger than 15 years receiving treatment worldwide in 2012. Approximately 544,000 (or 86 %) of these children were in sub-Saharan Africa [2]. Reports of pediatric adherence to antiretroviral medication (ARVs) have largely been high in sub-Saharan Africa [3, 4]. However, adherence challenges are evident in the suboptimal rates of viral suppression and increasing drug resistance that have been observed [4–8]. These challenges may in part reflect periodic gaps in otherwise high average levels of adherence [3, 9].
Adhering to treatment is a particularly complex task for children, and numerous barriers to adherence have been identified [10–12]. Influencing factors include the medication regimen (e.g., side-effects, use of liquid formulations, changing regimens and doses over time, disruptions in medication supply), the characteristics of the child (e.g., developmental stage, health, knowledge of HIV status) [13–15], the characteristics of the caregiver and family (e.g., permanence in the child's life, understanding of ARV regimen, relationship to the child), and the child's environment (e.g., cultural norms, relationship with providers, distance to clinic, access to resources, stigma) [13, 16–23]. Moreover, ARV adherence must be sustained over a lifetime [24], and children face potentially more years of dose-taking than adults. A significant amount of this time may be spent during important years in their brain development [25–27], especially in the case of vertical transmission, which has important long-term consequences reaching into adulthood [28]. Therefore early childhood intervention has been characterized as one of the most strategic means for averting poor health, psychosocial, cognitive, and economic outcomes in adulthood [29, 30].
For adults, high levels of adherence in resource-limited settings appear to be strongly influenced by social support [21, 31]. Social support is typically conceptualized as the range of mechanisms through which interpersonal relationships provide a buffer against a stressful environment [32]; the specific nature of the support is characterized as instrumental, emotional, or informational [33]. Social support is thought to be one of the mechanisms through which interpersonal relationships can influence health and health behaviors [34, 35], as well as help overcome barriers to adherence [21, 36, 37]. These issues are also likely relevant for pediatric adherence, although data are not available to our knowledge. The caregiver plays a central role in supporting the adherence of most young children, and caregivers in turn may depend on their own social support systems.
In this study, we conducted in-depth interviews with the caregivers of HIV-infected children in Uganda who had been followed in a longitudinal study of real-time adherence monitoring [38]. Our goal was to understand the caregiver's and child's experiences with ARV adherence, barriers and facilitators of adherence, and specifically the role of social support for the caregiver. We also aimed to understand differences in these factors based on the child's level of viral suppression.
Methods
Study Population
Children for this study were identified from a longitudinal, observational cohort study involving real-time adherence monitoring that was conducted in 2011 and described elsewhere [38, 39]. The original cohort included 46 children, who were aged 2–10 years old and who were recruited from the Children's HIV/AIDS Care Clinic in Mbarara, Uganda, which is located approximately 300 km southwest of Kampala. Participants included children initiating ART and those established on ART (some of whom started in infancy). The clinic provides ARVs free of charge and has a current caseload of approximately 700. HIV RNA levels were not routinely obtained in clinical care, and use of second line regimens was low due to evolving guidelines and limited ARV access at the time of the study. Adherence in the cohort study was monitored over a 6-month period with a medication container (called Wisepill) that transmits a cellular signal with each opening, as a proxy for medication dosing [40, 41]. Adherence was calculated as (the number of events/the number of expected events over a 6-month period) multiplied by 100. There were two versions of the wireless monitor: a pill container was used for older children, and a larger bag that held medication bottles was used for young children taking liquid formulations. HIV RNA levels were assessed in the cohort study at baseline and 6 months (Roche Amplicor HIV-1 Monitor Test, New Jersey, USA).
Sampling and Recruitment
We created two groups of pediatric participants from the original cohort study—those who had viral suppression and those who did not have viral suppression at the 6-month study visit. Viral suppression was defined as HIV RNA <1,000 copies/ml in order to limit misclassification due to potential transient low-level viremia [42]. The author conducting the interviews was blinded as to which group had detectable or undetectable HIV RNA, and created convenience samples for the interviews by drawing evenly from the two groups until a total of thirty-five participants were enrolled in the qualitative study.
Data Collection
One in-depth, in-person qualitative interview was completed with each caregiver within 6 months of completing the cohort study. The goals of the interviews were to explore: (1) the caregiver's and child's experiences with ARV adherence, (2) barriers and facilitators of adherence, and (3) the role of social support for the caregiver. Interviews were conducted in private locations using the local language (i.e., Runyankole) and following a pre-specified interview guide (see Supplementary Materials). Interviews were audio-recorded with permission, and averaged about an hour and a half in length. After each interview was completed, interviewers produced a complete transcript in English that was reviewed for quality and accuracy of translation.
Data Analysis
An inductive, content analytic approach was used to identify factors influencing adherence behavior [43]. First, interview transcripts were reviewed for content about factors influencing adherence. Concepts suggesting influences on adherence were identified and grouped in general categories representing “types of influences on adherence.” Because we specifically asked about social support during the interviews, we also used an a priori approach in which we created categories involving social support that were guided both by the questions we asked as well as the text data. Descriptive categories of factors that influenced adherence were also constructed to characterize caregivers' feelings about adherence and approaches to overcoming barriers to adherence. For these categories, we used a conventional analytic approach, pulling themes directly from the text data using systematic review and interpretation. Interview quotes were used to illustrate and provide evidence for each category. We required that all quotes show direct impact of social support on adherence to strengthen validity and transparency of the a priori approach. Two of the researchers (P.K.O., J.E.H.) independently performed the initial analytic step to strengthen validity. We then used the descriptive categories to construct matrices of influences on adherence that were then analyzed for recurring patterns in the data. We assembled the themes from this analysis into an explanatory logic to understand pediatric adherence to ARVs.
After this analysis, researchers were un-blinded to the viral suppression status of the children to allow for comparison between the two groups. Categories and themes were sorted by groups and responses from caregivers in the two groups were compared. Findings were then further considered in the context of the above-noted explanatory logic.
Ethics Statement
The study was approved by the Mbarara University of Science and Technology Faculty of Medicine Research Ethics Committee, Mbarara, Uganda; the Harvard University Faculty of Medicine Committee on Human Studies, Boston, MA; and the Partners Health Care Human Research Committee, Boston, MA. We additionally received clearance from the Uganda National Council on Science and Technology, Kampala, Uganda and from the Research Secretariat in the Office of the President. Written informed consent was obtained from all caregivers, and verbal assent was obtained from children when possible based on their developmental stage and cognitive abilities (typically those 7 years of age and older).
Results
Participant Characteristics
A total of 35 caregivers of HIV-infected children were interviewed. Their median age was 41 years (interquartile range [IQR] 33–48), 91 % were female, 62 % were biological relatives, 60 % were infected with HIV, and 34 % were receiving ARVs. Their children were a median age of 7 years (IQR 5–8), and 40 % were female. Their median duration of ARV therapy was 5 years (IQR 5–6) and 97 % were on first line ARV regimens.
Overview of Qualitative Interview Results
We identified four broad categories representing types of influence on adherence: (1) lack of resources, (2) the ‘Lazarus Effect,’ (3) caregiver's sense of obligation and commitment, and (4) the child's personal responsibility. We also specified types of social support available to the caregiver for achieving medication adherence for the child. These categories are presented in turn below.
Category 1: Lack of Resources
Caregivers described barriers to adherence that revolved around a lack of resources, especially money. Salaried positions were rare, with the majority of caregivers working day laborer jobs and often going long stretches without employment. ARVs were available free of charge; however, money was necessary for transportation to clinic appointments and the pharmacy to pick up ARVs. Because money was also needed for other aspects of childcare, such as food and school fees, adherence could not always be given priority.
Yes I am in need, but everything comes back to money. [My child] can be sent away [from school] because of [lack of] school fees if I don't have money in the house. … I have to borrow money so that [he] has something to eat. … Even if it's sugar or transport to clinic, it all revolves around money.
I failed to pay for [school fees]. I had to be going to the hospital [for clinic appointments for the child and myself]. So instead of school fees I decided to use the money for the hospital.
Other times you don't go on the review date [to get medication] … because that time you don't have [money for] transportation.
Category 2: The ‘Lazarus Effect’
For nearly all caregivers, motivation to ensure the child's adherence began when he or she first started taking ARVs. Most of the children were diagnosed with HIV after they presented tomedical care for acute and severe illness, but their health dramatically improved with the initiation of ARVs. Caregivers described this phenomenon, also called the ‘Lazarus effect’ [44], as a major event that impressed upon them the importance of ARVs and the necessity of adherence.
From her past I suffered a lot with her because she was doing very badly, so it happened to stick in the back of my mind that at 8:00 she has to take medication, and I don't want her to go back as how she was before.
Because when I tested him in 2005, he had not reached the level of taking ARVs, and then he started taking them and has lasted this long. What if he had not been taking them? I don't think he would be still alive.
Category 3: Caregiver's Sense of Obligation and Commitment
Caregivers described the types of efforts they made on the behalf of their child's ARV adherence. Oftentimes, they made sacrifices, such as going without food themselves so the child could eat. Proper nutrition is critical for ARV success: children on ARVs have increased appetite and often need food to avoid side effects of ARVs [45] and good nutrition is critical for proper immune function and reconstitution [46]. Other caregivers signed up for social programs or research studies to ensure the child received good medical care. These efforts were often taxing on caregivers, but were done purposefully because of a sense of obligation and/or commitment to the child and reflected a strong child-caregiver relationship.
If [the food we have] is not enough then the child has to eat first, then me I will see what to do.
It's an obligation [to care for the child], and I have to fulfill it. That's why I'm laboring to do all possible to make sure I get his tablets. I make myself available for [research] studies, and all that is part of the obligation.
[Giving medications] is really hard because you can get tired along the way, but to do what I have done for all this time without missing a dose needs love and commitment, so I bear it as my cross.
Category 4: The Child's Personal Responsibility
Among children who were cognitively and developmentally mature, several assumed partial responsibility for their own medical care. They typically reminded the caregiver of dosing times, but also took medication by themselves, or even went to the clinic by themselves. These actions supported the caregiver in shifting some of the onus of care onto the child.
Even if I haven't yet remembered to give him the medicine, for him he knows he is supposed to take the medication at that time. He calls me, ‘Mummy surprise’ then I know he wants to take his medication.
No, she has no problem taking the medication, she takes it at 7:30 in the morning and in the evening. And even when I am not there, she knows the time she is supposed to take it and takes it.
Social Support for the Caregiver
Caregivers described a collection of social support they relied upon to manage the child's care and overcome barriers to adherence. This support included private and institutional forms of instrumental support, as well as emotional and informational support. Each form of support is described below.
Private and Institutional Instrumental Social Support
In overcoming barriers to caring for the child, caregivers described accessing instrumental support, or tangible aid and services [47], which was either private or institutional. Private instrumental support consisted of help through money, food, transportation, or childcare from individuals in the community—family, friends, and/or neighbors. This form of instrumental support was typically based on prior relationships and required varying levels of disclosure of the child's HIV status and coordination of actions. Assistance came from both outside and inside the home. It was both fluid, allowing caregivers to access help and support at last minute notice, as well as formal with assigned roles.
My neighbors, I told them [the child's problem … so that when I am away and … if [the child] gets a problem, they could rush him to the hospital, tell the doctors the mother is not around and that he is on tablets.
I was late in a meeting, and I called [my neighbor] to give [the child] medication.
All the family knows because I did not want to hide it from them since that would make them not give him his medication. Even when I have gone somewhere, I call and inquire whether he has taken the medication.
When [the other children] are on holiday they make a timetable; everyone knows their day. … If you know Wednesday is your [day] and I have gone that day then you are in charge [of the child's care].
In contrast to private instrumental support, institutional instrumental support consisted of help—mostly loans— from formal (i.e. banks) and informal (i.e. shops) institutions in the community. This support was primarily derived from banks, microfinance lenders, ‘loan clubs’ (in which a group of individuals creates a fund from which they can borrow on a revolving basis), or microloans from individual shops. Some support could also be obtained from nongovernmental, non-profit, or religious organizations, or HIV support groups. Their support was used to achieve adherence, as well as help with other necessities like food, transportation, and school fees.
[The child] has to take food first before she takes the medicine. [If there is no food] at least I get a debt … at a grocery shop.
Of course [taking loans out when I don't have money] makes me feel bad, but there is nothing I can do because I have to make sure I take the child to the hospital to see the doctor and get the medicine.
Emotional and Informational Social Support
Caregivers also received emotional and informational support through relationships with people with whom they could discuss the child's care. Often, these relationships were with other caregivers at the Children's HIV/AIDS Care Clinic. Emotional social support is defined as expressions of empathy, love, trust, and caring, and informational support provides advice, suggestions, and information [47]. Accessing emotional and informational support from others required disclosure of the child's status, and some caregivers noted that fear of stigma limited their ability to discuss the child's illness with others. Informational support reinforced the importance of adherence and provided strategies for caregivers to optimize adherence.
You know Africans are very tough, especially Ugandans. When they know [the child's status] … they begin discriminating the [child], they don't respect him. That's why I don't want [to disclose].
We are like six with my friends. You advise each other, ask and consult each other. Yeah, we talk to each other as friends and because we all have the same goal of looking after our children until they grow.
[Other caregivers I've met at the clinic] talk about our children's welfare and all that; side effects of the medicine. And since we started going to the clinic, only three children have died in our whole lot, so we have some hope.
Conceptual Model of Adherence
Based on these categories and definitions, we created a conceptual model of ARV adherence (Fig. 1) that begins with the relationship between the caregiver and the child. In the model, the “Lazarus effect” in the child initially motivates the caregiver to support the child's adherence to ARVs. The caregiver's sense of obligation and commitment promotes adherence over time. Caregivers face a number of barriers to adherence, primarily related to a lack of resources. To overcome these barriers, caregivers may access several forms of social support, including private and institutional instrumental support, as well as emotional and informational support. When possible, the child also may assume a role in supporting his or her own adherence.
Fig. 1.
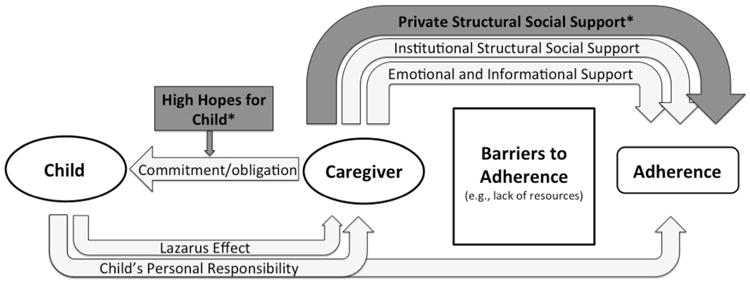
Conceptual model of ARV adherence. Initial motivation for ARV adherence appears to come from the ‘Lazarus effect.’ The caregiver's sense of obligation and commitment, bolstered by a caregiver's high hopes for the child's future, acts as further motivation for adherence and for organizing several forms of social support to help in maintaining high adherence. Caregivers are able to access different forms of social support to overcome barriers to adherence, especially private instrumental social support from friends, family, and neighbors, which allowed caregivers to overcome unforeseen adherence barriers. The child also may assume a role in supporting his or her own adherence. Asterisks indicate themes that emerged from the comparative analysis
Group Differences in Viral Suppression
With the above understanding of pediatric adherence to ARVs, we looked at unblinded data regarding the virologic status of the children. Seventeen participants had HIV RNA ≥1,000 copies/ml, and 18 participants had HIV RNA < 1,000 copies/ml. Adherence was high in both groups, with a median adherence of 93 % (IQR 89–95 %) and 93 % (IQR 89–96 %), respectively.
We then re-examined the categories of adherence and formsofsocial support separately for the two groups. Two key differences in themes between the two groups emerged. The first difference was related to the caregivers' views of the child's future and was evidenced by statements that they held the child in high regard. While nearly all participants reported caring for the child, greater emphasis on the child' potential and on hope for the future was apparent in the group with viral suppression. Participants in the group without viral suppression often emphasized that they worried about the child's future, whereas caregivers in the group with viral suppression often mentioned that the child excelled beyond her peers and commented that the child would have a bright future.
Group with Viral Suppression
The only prayer I have is that I could change him to a better school with higher standards so that he can be someone of importance in future. The good thing he is very bright.
You know that [my] child is gifted… He even goes in [my neighbors’] homes, plays with the children … and teaches them, gives them exams. So my neighbors see he is gifted and talented compared to their own children.
Group Without Viral Suppression
[I worry] maybe if difficulty comes in the future, … but I cannot know what the future holds.
[In thinking about the future, I think about] nothing really. Even if everything else went wrong, I would know my children are satisfied and have slept, that is enough for me.
The second difference was related to private instrumental support, which appeared to be more important for caregivers in the group with viral suppression. While caregivers in both groups accessed social support as described above, the levels and availability of private instrumental support were greater among the caregivers of children in the group with viral suppression and featured more prominently in our discussions. Caregivers in the group with viral suppression also appeared to have organized more options for private instrumental support both within and outside of the household.
Group with viral suppression
It's very often that [my mother] sends food. As long as I call she sends the food. I can't count the times because they are many times she has helped.
Group Without Viral Suppression
When I was still in town, the mosque used to help me with money for transport. But now that I am far from them, no one helps so I do not have transport [to the clinic].
With respect to the conceptual model of adherence outlined above, high hopes for the child's future appear to moderate the caregiver's sense of obligation and commitment. Although the caregivers' comments did not specifically link their hopes for the child's future to ARV adherence, the nature and depth of the hopes spoke to their underlying motivations in providing care. Additionally, the comparison between these two groups of children emphasized the importance of fluid and readily available private instrumental social support, which seemed critical for responding to the many predicted and unpredicted barriers to the child's ARV adherence.
Discussion
In this qualitative study, we inductively derived four descriptive categories to shed light on adherence to ARVs in rural Uganda: lack of resources, the ‘Lazarus effect,’ the caregiver's sense of obligation and commitment, and the child's personal responsibility. Additionally, we elucidated different forms of social support available to caregivers to overcome adherence barriers, including private and institutional instrumental support, as well as emotional and informational support. Achievement of viral suppression appeared to be related to parental aspirations for the child and access to private instrumental support.
Our finding that the caregiver's sense of obligation and commitment has an important effect on ARV adherence is consistent with prior studies showing that a strong care-giver-child relationship can facilitate adherence [15, 16, 48]. While our study was not designed to fully understand the dynamics within the child-caregiver relationships, the conceptual model derived from our interviews posits that caregiver sacrifice and personal motivation to provide care are salient features for supporting the child's adherence. A strong bond between the caregiver and child may be the foundation upon which solutions to adherence barriers are established. Parental hopes for their child's future, as expressed by the caregivers of children with viral suppression, further emphasized the importance of this relationship. In addition to the child-caregiver relationship, caregivers nearly universally mentioned the ‘Lazarus effect’ as an initial motivator of adherence. The ‘Lazarus effect’ strongly affected both how the caregivers viewed the efficacy of the medications and how they viewed their own role in keeping the child healthy.
The specific barriers we identified were also similar in many ways to those already identified in the literature [10–12], with the primary barrier being lack of resources like money, food, and transportation. Caregivers then relied on various forms of social support—instrumental, informational, and emotional—to help them overcome these barriers. Our qualitative findings are consistent with the general thrust of the literature on the “buffering” hypothesis about the moderating effects of social support against life strains [34, 49], as well as with epidemiological studies conducted in the region [50]. Likewise, prior studies have also shown the importance of social support in relation to adherence [21], and specifically the role of caregiver support [16, 36]. In our study, access to private instrumental support seemed particularly important for treatment success, as caregivers could readily obtain help to manage adherence barriers, especially those that were unanticipated. Additionally, multiple sources of support allowed different options for help with different types of problems. In earlier work in Uganda and Zambia, adherence challenges were found to be associated with disruptions in routine [3, 14]. Having strong private instrumental social support allowed caregivers to preempt and/or overcome such disruptions and ensure the child received his or her medication. Moreover, we found personal responsibility on the part of the child, even at a young age, can also be critical component of this network of support, assuming the child has the necessary cognitive abilities and knowledge.
Other significant forms of support caregivers received included emotional and informational support. Emotional support appeared to help caregivers through non-structural difficulties, such as providing a space for them to discuss their worries about the child's future. Additionally, although caregivers did not specifically emphasize fatigue in caring for their children, it has been shown to significantly compromise adherence in children with cancer [51] and most likely plays a role in ARV adherence as well [10]. We hypothesize that a strong emotional support network may lessen this fatigue, as caregivers often described emotional support as ‘encouragement.’ Informational support allowed caregivers to better handle specific issues of the child's care and, importantly, to learn from caregivers who had success and whose children were thriving on ARVs.
To access certain types of social support, it was necessary for the caregiver to disclose the child's HIV status to others. While we did not set out to study disclosure specifically in this study, it emerged as an important intermediate step in accessing support, particularly when organizing a network of private instrumental support. Studies of pediatric ARV adherence both in the United States and sub-Saharan Africa have shown that disclosure likely plays a significant role in adherence [3, 16, 52, 53], and it is strongly encouraged by the World Health Organization and many national guidelines [54, 55]. The limited data available, however, suggests that rates of disclosure in sub-Saharan Africa are low [53] and are negatively affected by the stigma of HIV [56]. Challenges include anticipated stigma, guilt in the case of vertical transmission, and emotional damage to the child, among others. The findings in this study, however, suggest that disclosure may play an important role in activating social networks [57] and achieving treatment success. Further work in understanding how to successfully encourage disclosure is needed [58].
Strengths of this study include the availability of objective adherence monitoring data and HIV RNA levels, and our ability to leverage this data in advancing a qualitative understanding of adherence behavior. Mixed methods approaches to understanding pediatric ARV adherence have rarely been adopted, with the exception of one study in India that featured fully structured interviews that were more focused on eliciting barriers to adherence [9]. There were also several weakness that limit interpretation of our findings. First, the sample size of 35 caregivers was relatively small. However, sufficient data was obtained to produce four robust categories characterizing types of influence on adherence and to distinguish and describe two different types of social support for caregivers. Second, our sample did not include any adolescents, who face numerous adherence challenges [59]. Given the numerous developmental and social differences in young children and adolescents, a separate study focusing on this specific population is warranted. Third, we used convenience samples in enrolling participants. We may therefore have not obtained a complete picture of adherence in this population, although saturation of themes was largely achieved. Finally, bias could have entered the interpretation of the findings. We attempted to minimize this possibility, however, through a rigorous review process that included researchers blinded to viral suppression and having two researchers review the data independently.
The conceptual framework developed in this study creates potential opportunities for adherence interventions in future research. Counseling interventions, for example, could be directed toward strengthening the caregiver-child relationship, as well as quality and accessibility of various forms of social support systems. Additionally, future studies could assess the ability of mobile technology to facilitate coordinating these complex social support networks, especially as mobile phones become increasingly pervasive in sub-Saharan Africa [60].
Conclusions
Pediatric ART adherence is complex, especially in the setting of limited resources. This study highlights the importance of both the caregiver-child relationship and the various types of social support systems available to the caregiver. The child-caregiver relationship may help motivate adherence in the face of daily barriers to adherence, and social support systems may enable caregivers to overcome these barriers. This study supports consideration of caregiver feelings and attitudes in adherence counseling, as well as strengthening social support for caregivers as an adherence intervention. The lessons learned from those who have achieved treatment success provide fertile areas upon which further research and interventions may be built.
Supplementary Material
Acknowledgments
All authors contributed to the conception and design of the study, data analysis and interpretation, and/or development of the manuscript. The manuscript was drafted by P.K.O. and J.E.H. All authors critically reviewed the manuscript, suggested revisions and editorial changes, and approved the final version. The authors would like to thank the study participants, as well as the following study staff: Flavia Ninsiima, Ambrose Mugyenyi, Sarah Namwanje, Constance Katabazi, and Teddy Komuhangi. Financial support for the qualitative research presented here was provided by U.S. National Institutes Health K23MH087228 (J.E.H.) and K24MH090894 (N.C.W.), and the Harvard Medical School Class of 1984 Scholarship (P.K.O.). The authors also acknowledge salary support from NIH K23MH096620 (A.C.T.). A preliminary version of this work have been presented by P.K.O. at the 7th International Conference on HIV Treatment and Prevention Adherence, Miami, FL, June 3–5, 2012 (Abs. Number 80075).
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10461-014-0924-7) contains supplementary material, which is available to authorized users.
Conflict of interest There are no conflicts of interest.
Contributor Information
Peter K. Olds, Harvard Medical School, Boston, MA, USA
Julius P. Kiwanuka, Department of Paediatrics, Mbarara University of Science and Technology, Mbarara, Uganda
Norma C. Ware, Department of Global Health and Social Medicine, Department of Psychiatry, Harvard Medical School, Boston, MA, USA
Alexander C. Tsai, Department of Psychiatry and Center for Global Health, Massachusetts General Hospital, Boston, MA, USA; Department of Medicine, Harvard Medical School, Boston, MA, USA
Jessica E. Haberer, Email: jhaberer@partners.org, Department of Medicine, Harvard Medical School, Boston, MA, USA;Department of Medicine and Center for Global Health, MGH Center for Global Health, Massachusetts General Hospital, 100 Cambridge St., 15th Floor, Boston, MA 02114, USA.
References
- 1.UNAIDS. Global Report: UNAIDS report on the global AIDS epidemic 2013. Geneva: UNAIDS; 2013. [Google Scholar]
- 2.WHO. Global update on HIV treatment 2013: Results, impact and opportunities. 2013 [Google Scholar]
- 3.Haberer JE, Cook A, Walker AS, et al. Excellent adherence to antiretrovirals in HIV + Zambian children is compromised by disrupted routine, HIV nondisclosure, and paradoxical income effects. PLoS ONE. 2011;6(4):e18505. doi: 10.1371/journal.pone.0018505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barro M, Some J, Foulongne V, et al. Short-term virological efficacy, immune reconstitution, tolerance, and adherence of once-daily dosing of didanosine, lamivudine, and efavirenz in HIV-1-infected African children: ANRS 12103 Burkiname. J Acquir Immune Defic Syndr. 2011;57(Suppl 1):S44–9. doi: 10.1097/QAI.0b013e31821fd64f. [DOI] [PubMed] [Google Scholar]
- 5.Orrell C, Levison J, Ciaranello A, et al. Resistance in pediatric patients experiencing virologic failure with first-line and second-line antiretroviral therapy. Pediatr Infect Dis J. 2013;32(6):644–7. doi: 10.1097/INF.0b013e3182829092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahoua L, Guenther G, Rouzioux C, et al. Immunovirological response to combined antiretroviral therapy and drug resistance patterns in children: 1- and 2-year outcomes in rural Uganda. BMC Pediatr. 2011;11:67. doi: 10.1186/1471-2431-11-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dehority W, Deville JG, Lujan-Zilbermann J, Spector SA, Viani RM. Effect of HIV genotypic drug resistance testing on the management and clinical course of HIV-infected children and adolescents. Int J STD AIDS. 2013;24(7):549–53. doi: 10.1177/0956462412473958. [DOI] [PubMed] [Google Scholar]
- 8.Barth RE, Tempelman HA, Smelt E, Wensing AM, Hoepelman AI, Geelen SP. Long-term outcome of children receiving anti-retroviral treatment in rural South Africa: substantial virologic failure on first-line treatment. Pediatr Infect Dis J. 2011;30(1):52–6. doi: 10.1097/INF.0b013e3181ed2af3. [DOI] [PubMed] [Google Scholar]
- 9.Shet A, DeCosta A, Heylen E, Shastri S, Chandy S, Ekstrand M. High rates of adherence and treatment success in a public and public-private HIV clinic in India: potential benefits of standardized national care delivery systems. BMC Health Serv Res. 2011;11:277. doi: 10.1186/1472-6963-11-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haberer J, Mellins C. Pediatric adherence to HIV antiretroviral therapy. Curr HIV/AIDS Rep. 2009;6(4):194–200. doi: 10.1007/s11904-009-0026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vreeman RC, Wiehe SE, Pearce EC, Nyandiko WM. A systematic review of pediatric adherence to antiretroviral therapy in low- and middle-income countries. Pediatr Infect Dis J. 2008;27(8):686–91. doi: 10.1097/INF.0b013e31816dd325. [DOI] [PubMed] [Google Scholar]
- 12.Scanlon ML, Vreeman RC. Current strategies for improving access and adherence to antiretroviral therapies in resource-limited settings. HIV AIDS (Auckl) 2013;5:1–17. doi: 10.2147/HIV.S28912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiser SD, Palar K, Frongillo EA, et al. Longitudinal assessment of associations between food insecurity, antiretroviral adherence and HIV treatment outcomes in rural Uganda. AIDS. 2013;28(1):115–20. doi: 10.1097/01.aids.0000433238.93986.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haberer JE, Kiwanuka J, Nansera D, Ragland K, Mellins C, Bangsberg DR. Multiple measures reveal antiretroviral adherence successes and challenges in HIV-infected Ugandan children. PLoS ONE. 2012;7(5):e36737. doi: 10.1371/journal.pone.0036737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buchanan AL, Montepiedra G, Sirois PA, et al. Barriers to medication adherence in HIV-infected children and youth based on self- and caregiver report. Pediatrics. 2012;129(5):e1244–51. doi: 10.1542/peds.2011-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fetzer BC, Mupenda B, Lusiama J, Kitetele F, Golin C, Behets F. Barriers to and facilitators of adherence to pediatric antiretroviral therapy in a sub-Saharan setting: insights from a qualitative study. AIDS Patient Care STDS. 2011;25(10):611–21. doi: 10.1089/apc.2011.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coetzee B, Kagee A, Vermeulen N. Structural barriers to adherence to antiretroviral therapy in a resource-constrained setting: the perspectives of health care providers. AIDS Care. 2011;23(2):146–51. doi: 10.1080/09540121.2010.498874. [DOI] [PubMed] [Google Scholar]
- 18.Tuller DM, Bangsberg DR, Senkungu J, Ware NC, Emenyonu N, Weiser SD. Transportation costs impede sustained adherence and access to HAART in a clinic population in southwestern Uganda: a qualitative study. AIDS Behav. 2010;14(4):778–84. doi: 10.1007/s10461-009-9533-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mutwa PR, Van Nuil JI, Asiimwe-Kateera B, et al. Living situation affects adherence to combination antiretroviral therapy in HIV-infected adolescents in Rwanda: a qualitative study. PLoS ONE. 2013;8(4):e60073. doi: 10.1371/journal.pone.0060073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malee K, Williams PL, Montepiedra G, et al. The role of cognitive functioning in medication adherence of children and adolescents with HIV infection. J Pediatr Psychol. 2009;34(2):164–75. doi: 10.1093/jpepsy/jsn068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katz IT, Ryu AE, Onuegbu AG, et al. Impact of HIV-related stigma on treatment adherence: systematic review and meta-synthesis. J Int AIDS Soc. 2013;16(3 Suppl 2):18640. doi: 10.7448/IAS.16.3.18640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lankowski AJ, Siedner MJ, Bangsberg DR, Tsai AC. Impact of geographic and transportation-related barriers on HIV outcomes in sub-Saharan Africa: a systematic review. AIDS Behav. 2014;18(7):1199–223. doi: 10.1007/s10461-014-0729-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siedner MJ, Lankowski A, Tsai AC, et al. GPS-measured distance to clinic, but not self-reported transportation factors, are associated with missed HIV clinic visits in rural Uganda. AIDS. 2013;27(9):1503–8. doi: 10.1097/QAD.0b013e32835fd873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsai AC, Bangsberg DR. The importance of social ties in sustaining medication adherence in resource-limited settings. J Gen Intern Med. 2011;26(12):1391–3. doi: 10.1007/s11606-011-1841-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boivin MJ, Bangirana P, Nakasujja N, et al. A year-long care-giver training program improves cognition in preschool Ugandan children with human immunodeficiency virus. J Pediatr. 2013;163(5):1409–19. doi: 10.1016/j.jpeds.2013.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boivin MJ, Bangirana P, Nakasujja N, et al. A year-long care-giver training program to improve neurocognition in preschool Ugandan HIV-exposed children. J Dev Behav Pediatr. 2013;34(4):269–78. doi: 10.1097/DBP.0b013e318285fba9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nelson CA, 3rd, Zeanah CH, Fox NA, Marshall PJ, Smyke AT, Guthrie D. Cognitive recovery in socially deprived young children: the Bucharest Early Intervention project. Science. 2007;318(5858):1937–40. doi: 10.1126/science.1143921. [DOI] [PubMed] [Google Scholar]
- 28.Venkataramani AS. Early life exposure to malaria and cognition in adulthood: evidence from Mexico. J Health Econ. 2012;31(5):767–80. doi: 10.1016/j.jhealeco.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 29.Heckman JJ. Skill formation and the economics of investing in disadvantaged children. Science. 2006;312(5782):1900–2. doi: 10.1126/science.1128898. [DOI] [PubMed] [Google Scholar]
- 30.Heckman JJ, Masterov DV. The productivity argument for investing in young children. Rev Agric Econ. 2007;29(3):446–93. [Google Scholar]
- 31.Talisuna-Alamo S, Colebunders R, Ouma J, et al. Socioeconomic support reduces nonretention in a comprehensive, community-based antiretroviral therapy program in Uganda. J Acquir Immune Defic Syndr. 2012;59(4):e52–9. doi: 10.1097/QAI.0b013e318246e2aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen S, McKay G. Social support, stress and the buffering hypothesis: a theoretical analysis. In: Baum A, Taylor SE, Singer JE, editors. Handbook of psychology and health: social psychological aspects of health. Hillsdale: N.J.: L. Erlbaum Associates; 1984. pp. 253–267. [Google Scholar]
- 33.Cohen S, Wills TA. Stress, social support, and the buffering hypothesis. Psychol Bull. 1985;98(2):310–57. [PubMed] [Google Scholar]
- 34.Cassel J. The contribution of the social environment to host resistance: the Fourth Wade Hampton Frost Lecture. Am J Epidemiol. 1976;104(2):107–23. doi: 10.1093/oxfordjournals.aje.a112281. [DOI] [PubMed] [Google Scholar]
- 35.Dean A, Lin N. The stress-buffering role of social support. Problems and prospects for systematic investigation. J Nerv Ment Dis. 1977;165(6):403–17. doi: 10.1097/00005053-197712000-00006. [DOI] [PubMed] [Google Scholar]
- 36.Russell S, Seeley J, Ezati E, Wamai N, Were W, Bunnell R. Coming back from the dead: living with HIV as a chronic condition in rural Africa. Health Policy Plan. 2007;22(5):344–7. doi: 10.1093/heapol/czm023. [DOI] [PubMed] [Google Scholar]
- 37.Mills EJ, Nachega JB, Buchan I, et al. Adherence to antiretroviral therapy in sub-Saharan Africa and North America: a meta-analysis. JAMA. 2006;296(6):679–90. doi: 10.1001/jama.296.6.679. [DOI] [PubMed] [Google Scholar]
- 38.Haberer JE, Kiwanuka J, Nansera D, et al. Realtime adherence monitoring of antiretroviral therapy among HIV-infected adults and children in rural Uganda. AIDS. 2013;27(13):2166–8. doi: 10.1097/QAD.0b013e328363b53f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haberer J. Novel measures and theory of pediatric antiretroviral therapy adherence in Uganda. Mbarara: Massachusetts General Hospital; 2010. [Google Scholar]
- 40.Haberer JE, Kiwanuka J, Nansera D, Wilson IB, Bangsberg DR. Challenges in using mobile phones for collection of antiretroviral therapy adherence data in a resource-limited setting. AIDS Behav. 2010;14(6):1294–301. doi: 10.1007/s10461-010-9720-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haberer JE, Kahane J, Kigozi I, et al. Real-time adherence monitoring for HIV antiretroviral therapy. AIDS Behav. 2010;14(6):1340–6. doi: 10.1007/s10461-010-9799-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Havlir DV, Bassett R, Levitan D, et al. Prevalence and predictive value of intermittent viremia with combination hiv therapy. JAMA. 2001;286(2):171–9. doi: 10.1001/jama.286.2.171. [DOI] [PubMed] [Google Scholar]
- 43.Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277–88. doi: 10.1177/1049732305276687. [DOI] [PubMed] [Google Scholar]
- 44.Leland J. The end of AIDS? Newsweek. 1996;128(23):64–68. 71, 73. [PubMed] [Google Scholar]
- 45.Nagata JM, Magerenge RO, Young SL, Oguta JO, Weiser SD, Cohen CR. Social determinants, lived experiences, and consequences of household food insecurity among persons living with HIV/AIDS on the shore of Lake Victoria, Kenya. AIDS Care. 2012;24(6):728–36. doi: 10.1080/09540121.2011.630358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anabwani G, Navario P. Nutrition and HIV/AIDS in sub-Saharan Africa: an overview. Nutrition. 2005;21(1):96–9. doi: 10.1016/j.nut.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 47.Glanz K, Rimer BK, Viswanath K. Health behavior and health education : theory, research, and practice. 4th. San Francisco: Jossey-Bass; 2008. [Google Scholar]
- 48.Mellins CA, Brackis-Cott E, Dolezal C, Abrams EJ. The role of psychosocial and family factors in adherence to antiretroviral treatment in human immunodeficiency virus-infected children. Pediatr Infect Dis J. 2004;23(11):1035–41. doi: 10.1097/01.inf.0000143646.15240.ac. [DOI] [PubMed] [Google Scholar]
- 49.Cobb S. Social support as a moderator of life stress. Psychosom Med. 1976;38(5):300–14. doi: 10.1097/00006842-197609000-00003. [DOI] [PubMed] [Google Scholar]
- 50.Tsai AC, Bangsberg DR, Frongillo EA, et al. Food insecurity, depression and the modifying role of social support among people living with HIV/AIDS in rural Uganda. Soc Sci Med. 2012;74(12):2012–9. doi: 10.1016/j.socscimed.2012.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Davies B, Whitsett SF, Bruce A, McCarthy P. Atypology of fatigue in children with cancer. J Pediatr Oncol Nurs. 2002;19(1):12–21. doi: 10.1053/jpon.2002.30012. [DOI] [PubMed] [Google Scholar]
- 52.Foster SD, Nakamanya S, Kyomuhangi R, et al. The experience of “medicine companions” to support adherence to antiretroviral therapy: quantitative and qualitative data from a trial population in Uganda. AIDS Care. 2010;22(Suppl 1):35–43. doi: 10.1080/09540120903500027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vreeman RC, Gramelspacher AM, Gisore PO, Scanlon ML, Nyan-diko WM. Disclosure of HIV status to children in resource-limited settings: a systematic review. J Int AIDS Soc. 2013;16:18466. doi: 10.7448/IAS.16.1.18466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.WHO. Guidline on HIV Disclosure Counselling for Children up to 12 Years of Age. WHO; 2011. [PubMed] [Google Scholar]
- 55.Institute NYSDoHA. Disclosure of HIV to Perinatally Infected Children and Adolescents. [Accessed January 10, 2014.];2009 http://www.hivguidelines.org/clinical-guidelines/infants-children/disclosure-of-hiv-to-perinatally-infected-children-and-adolescents/
- 56.Tsai AC, Bangsberg DR, Kegeles SM, et al. Internalized stigma, social distance, and disclosure of HIV seropositivity in rural Uganda. Ann Behav Med. 2013;46(3):285–94. doi: 10.1007/s12160-013-9514-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perry BL, Pescosolido BA. Social network activation: the role of health discussion partners in recovery from mental illness. Soc Sci Med. 2014 doi: 10.1016/j.socscimed.2013.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kalichman SC, et al. The harms of internalized AIDS stigma: a comment on Tsai.et.al. Ann Behav Med. 2013;46(3):256–7. doi: 10.1007/s12160-013-9529-z. [DOI] [PubMed] [Google Scholar]
- 59.MacDonell K, Naar-King S, Huszti H, Belzer M. Barriers to medication adherence in behaviorally and perinatally infected youth living with HIV. AIDS Behav. 2013;17(1):86–93. doi: 10.1007/s10461-012-0364-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zurovac D, Otieno G, Kigen S, et al. Ownership and use of mobile phones among health workers, caregivers of sick children and adult patients in Kenya: cross-sectional national survey. Global Health. 2013;9:20. doi: 10.1186/1744-8603-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


