Abstract
Asthma is frequently caused and/or exacerbated by sensitization to allergens, which are ubiquitous in many indoor and outdoor environments. Severe asthma is characterized by airway hyperresponsiveness and bronchial constriction in response to an inhaled allergen, leading to a disease course that is often very difficult to treat with standard asthma therapies. As a result of interactions among inflammatory cells, structural cells, and the intercellular matrix of the allergic lung, patients with sensitization to allergens may experience a greater degree of tissue injury followed by airway wall remodeling and progressive, accumulated pulmonary dysfunction as part of the disease sequela. In addition, turnover of extracellular matrix (ECM) components is a hallmark of tissue injury and repair. This review focuses on the role of the glycosaminoglycan hyaluronan (HA), a component of the ECM, in pulmonary injury and repair with an emphasis on allergic asthma. Both the synthesis and degradation of the ECM are critical contributors to tissue repair and remodeling. Fragmented HA accumulates during tissue injury and functions in ways distinct from the larger native polymer. There is gathering evidence that HA degradation products are active participants in stimulating the expression of inflammatory genes in a variety of immune cells at the injury site. In this review, we will consider recent advances in the understanding of the mechanisms that are associated with HA accumulation and inflammatory cell recruitment in the asthmatic lung.
Keywords: Asthma, extracellular matrix, hyaluronan
INTRODUCTION
Allergic airway diseases such as rhinitis and bronchial asthma are complex disorders that are thought to arise as a result of aberrant immune cell responses to non-infectious environmental antigens (Nassenstein et al., 2005; Umetsu and DeKruyff, 2006). Recent statistics (2011) show that asthma afflicts 8.2% of adults and children in the United States, nearly 26 million people (Knutsen et al., 2012). In patients with moderate or severe persistent asthma, there is increased morbidity and significantly increased use of health care support and costs (Knutsen et al., 2012). Epidemiological studies in the U.S. and Europe have associated mold sensitivity, particularly to Alternaria alternate and Cladosporium herbarum, with the development, persistence, and severity of asthma (O’Driscoll et al., 2005; Knutsen et al., 2012). In addition, sensitivity to Aspergillus fumigatus has been associated with severe persistent asthma in adults (O’Driscoll et al., 2005; Chaudhary and Marr, 2011; Knutsen et al., 2012).
Sensitization to fungus is a particular risk factor for severe asthma that is difficult to treat with standard therapies and, as a result, can lead to considerable morbidity that may require multiple hospitalizations (O’Driscoll et al., 2005). The airway hyperresponsiveness (AHR) and peribronchial inflammation that result from the inhalation of conidia narrows the caliber of the airway lumen, limiting air exchange. Fungal spores (conidia) are ubiquitous in the atmosphere, and the persistent perturbation of the lung that results from repeated inhalations of conidia causes chronic remodeling of the allergic airway (Hogaboam et al., 2000; Hernandez et al., 2004; Hoselton et al., 2010; Ghosh et al., 2012b), consisting of peribronchial fibrosis with excessive accumulation of ECM (Murdock et al., 2012; Ghosh et al., 2014b), smooth muscle hypertrophy (Murdock et al., 2012; Shreiner et al., 2012), and goblet cell metaplasia that further restrict airflow (Hogaboam et al., 2000; Hoselton et al., 2010; Ghosh et al., 2012a; Ghosh et al., 2014a). As a consequence of repeated and persistent inflammation due to constant exposure to fungus, lung morphology eventually becomes altered even in mild forms of the disease, causing chronic dysfunction of the lung (Dagenais and Keller, 2009; Murdock et al., 2012; Pandey et al., 2013).
In the lung, the ECM was once considered to be inert scaffolding with a mechanical role in supporting and maintaining tissue structure. However, recent findings indicate that the role of ECM components is much broader than previously thought (Jiang et al., 2007; 2011). It is now known that the ECM has roles in cell attachment (Oharazawa et al., 1999), movement (Noble and Jiang, 2006), activation (McKee et al., 1996), tissue growth and repair (Noble, 2002), proliferation (Paez-Pereda et al., 2005), and differentiation (Lu et al., 2011), and thereby can play a role in mediating inflammation. Clearly, it functions in processes of both health and disease (Noble and Jiang, 2006; Lennon and Singleton, 2011), and understanding the contribution of ECM components to asthma pathogenesis may lead to new therapeutics for patients with asthma.
The ECM comprises a diverse group of proteins and glycoproteins, which can be divided into three groups: i) structural proteins, such as collagen and elastin, ii) specialized adhesion proteins, such as fibronectin, fibrilin, tenascin, and laminin, iii) glycosaminoglycans (GAGs) and proteoglycans (Olczyk et al., 2014). All of these components of the ECM provide structural integrity and mechanical support for tissues and provides a meshwork for cell adhesion and motility (Derya et al., 2014; Olczyk et al., 2014). Collagen (type I, III, and V on the airway wall and type IV and VIII under the basement membrane) and elastin account for approximately 2/3 of the dry weight of the ECM, while the remainder is made up of glycoproteins (fibronectin, tenascin, laminin) and other matrix components (heparin sulfate, HA) (Liang et al., 2011; Derya et al., 2014). HA is a major component of the ECM that undergoes dynamic regulation under conditions of inflammation.
HA is widely distributed, from lower organisms such as bacteria to complex eukaryotes (Lowther and Rogers, 1955). HA was first isolated from vitreous humor of bovine eyes in 1934 by Meyer and Palmer (Meyer and Palmer, 1934). It is a non-sulfated GAG polymer consisting of repeating disaccharide units of D-glucuronic acid and D-N-acetylglucosamine that is synthesized by a variety of cell types, including stromal cells (Laurent and Fraser, 1992), fibroblasts (Smith and Ghosh, 1986), epithelial cells (Lauer et al., 2008), and smooth muscle cells (Lauer et al., 2009). The total normal systemic kinetics of HA are well established in several species including humans. The average 70 kg (154 lb.) person has roughly 15 grams of HA in the body and the normal turnover of HA is 10–100 mg/day in the adult (Laurent and Fraser, 1992; Stern, 2004). Similarly, the removal of HA from the circulation is very efficient, with a half-life of 2–6 min (Laurent and Fraser, 1992).
The association of increased HA deposition into the ECM after tissue injury and during inflammatory disease has been recognized for over 25 years (Hallgren et al., 1989). In the lung, HA expression increases after ozone damage and in the progression of a number of pulmonary diseases, including chronic obstructive pulmonary disease (COPD) (Sahu and Lynn, 1978; Dentener et al., 2005; Li et al., 2011), idiopathic arterial pulmonary hypertension (Papakonstantinou et al., 2008), acute respiratory distress syndrome (ARDS) (Hallgren et al., 1989), and allergic asthma (Sahu and Lynn, 1978; Cheng et al., 2011; 2013; Ghosh et al., 2014b), suggesting a role in pulmonary disease mediation. The successful repair of tissue injury requires a well-coordinated host response to limit the extent of tissue damage. The physical, mechanical, and cellular innate immune responses are the first line of defense against the insult caused by inhaled organisms and inform the adaptive inflammatory response. The host must also minimize the extent of structural cell damage. The ultimate outcome depends on the balance between the containment of the perceived pathogen (defense) and the cellular and molecular mechanisms that are associated with the breakdown or turnover of ECM components to maintain homeostasis (tissue integrity). This review focuses on the current understanding of molecular mechanisms that are associated with HA synthesis/accumulation/degradation and its role in mediating inflammation in allergic pulmonary disease.
HYALURONAN METABOLISM IN THE LUNG
HA is a normal component of the basement membrane and makes approximately 10% of the proteoglycan content of the lung (Noble and Jiang, 2006; Liang et al., 2011). In the healthy lung, HA exists as a high molecular mass hyaluronan (HMM HA) polymer, in excess of 106 Da, found in the peribronchial and perialveolar spaces where it assists in structural integrity, cushioning and cell movement (Noble, 2002; Jiang et al., 2011).. However, during inflammation, HA undergoes dynamic regulation and can be broken down into low molecular mass hyaluronan (LMM HA) fragments by the activity of hyaluronidases and reactive oxygen species (ROS) (Noble, 2002; Jiang et al., 2010; Monzon et al., 2010; Lennon and Singleton, 2011). HA differentially promote or inhibit lung pathology based on its molecular mass and accessibility to various HA binding proteins (Lennon and Singleton, 2011; Singleton and Lennon, 2011). Several studies have shown that LMM HA exhibits pronounced biological effects on cells and tissues (Noble and Jiang, 2006; Papakonstantinou and Karakiulakis, 2009; Jiang et al., 2011; Maharjan et al., 2011). Most importantly, LMM HA has multiple pro-inflammatory effects not observed for HMM HA (Noble, 2002; Yang et al., 2012). In fact, HMM HA can block the pro-inflammatory effects of LMM HA and help support tissue integrity (Jiang et al., 2011; Yang et al., 2012).
As HA regulates lung pathology, it is important to understand how cells regulate its metabolism. HA is synthesized by three different isozymes (HAS1-3) located in the plasma membrane, whereas HA degradation is mediated by a series of hyaluronidases (HYAL1-3) that produce HA fragments that can modulate inflammation and immune responses (Laurent, 1989; Lennon and Singleton, 2011; Vigetti et al., 2012). Therefore, regulation of HASes, together with HYALs are main control points to determine tissue HA content. Increased amounts of HA and its degradation products are observed in many allergy models of asthma (Cheng et al., 2011; Lennon and Singleton, 2011; Liang et al., 2011; Cheng et al., 2013; Ghosh et al., 2014b; Petrey and de la Motte, 2014a). Further, increased HA levels are observed in bronchoalveolar lavage (BAL) fluid and/or plasma from patients with lung disorders such as asthma (Soderberg et al., 1989; Lennon and Singleton, 2011; Liang et al., 2011; Eszes et al., 2014)
HYALURONAN SYNTHESIS IN THE LUNG
HA is synthesized by membrane-bound synthases on the inner surface of the plasma membrane, and the chains are extruded through pore-like structures into the extracellular space (Lowther and Rogers, 1955; Laurent, 1989; Noble and Jiang, 2006; Liang et al., 2011). This mode of HA synthesis is unusual, as it is made at the inner face of the plasma membrane and not inside the Golgi like other GAGs (Vigetti et al., 2012). The growing HA chain is extended at the reducing rather than the non-reducing terminus and, as the polymer grows, it is extruded into the extracellular space via the membrane spanning domains of the HASes (Toole, 2000; Singleton and Lennon, 2011). There are three mammalian hyaluronan synthases (HAS1, HAS2, and HAS3) that catalyze the same reaction (Laurent and Fraser, 1992; Watanabe and Yamaguchi, 1996; Lee and Spicer, 2000). The genomic location and structure of these HA synthases have been determined. HAS1, HAS2, and HAS3 are located on different chromosomes, suggesting that the HAS gene family may have arisen early in vertebrate evolution by sequential duplication of an ancestral HAS gene (O’Regan et al., 1994; Watanabe and Yamaguchi, 1996; Toole, 2004; Weigel and DeAngelis, 2007). All of the HAS isozymes are highly homologous in their amino acid sequences and have similar hydropathic features, suggesting that they are similarly organized within the membrane. Two types of membrane domains are present: transmembrane domains that span the membrane and membrane-associated domains that do not go all the way through the membrane (O’Regan et al., 1994; DeAngelis, 1999; Weigel and DeAngelis, 2007). There are 6–8 transmembrane domains and two membrane-associated domains. Over 60% of the protein (including the amino and carboxyl termini) is inside the cell (DeAngelis, 2002). Only 5% of the protein is exposed to the outside of the cell (O’Regan et al., 1994; DeAngelis, 1999).
The HAS enzymes use the two activated sugar precursors for HA biosynthesis: Uridine diphospho-glucuronic acid (UDP-GlcA) and Uridine diphospho-N-acetylglucosamine (UDP-GlcNAc) as substrates (Figure 1) (Sugahara et al., 1979; Vigetti et al., 2012). In order to make a disaccharide unit and extend the growing HA chain, hyaluronan synthases utilizes at least six distinct steps (Figure 1). These activities include two different sugar nucleotide binding sites, two different glycosyltransferase activities, one or more binding sites for the growing hyaluronan-UDP chain and the ability to transfer the HA chain, within the enzyme, to set up the next round of saccharide addition. The UDP-sugar substrates are produced and used by the hyaluronan synthase inside the cell, and the HA chain is continuously transferred (translocated) across the membrane so that it is extruded to the cell exterior (Sugahara et al., 1979; Toole, 2000). In the case of bacteria, this HA forms a capsule (Laurent and Fraser, 1992), whereas for many eukaryotic cells, the result is either that HA forms a pericellular coat surrounding the cell or is released into the surrounding ECM (Figure 1).
Figure 1. Schematic diagram illustrating hyaluronan biosynthesis.
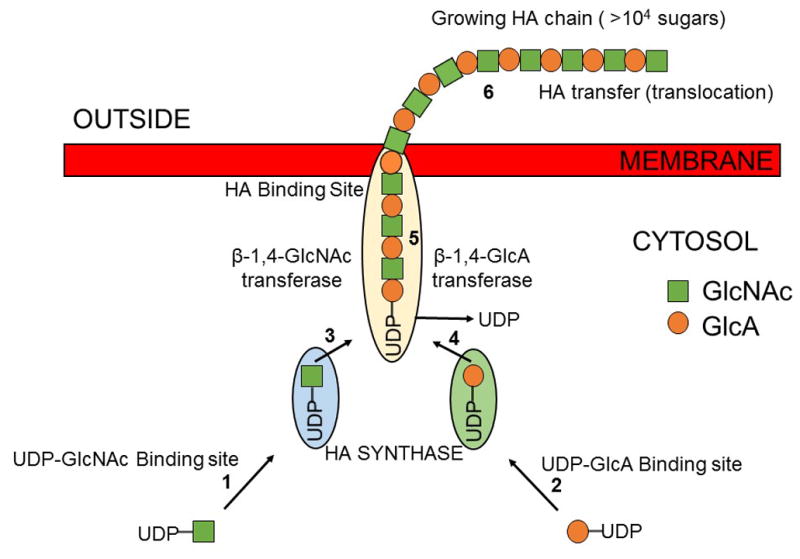
A membrane-bound hyaluronan synthase utilizes uridine diphospho-glucuronic acid (UDP-GlcA) and uridine diphospho-N-acetylglucosamine (UDP-GlcNAc) as substrates for hyaluronan biosynthesis. The UDP-sugar substrates are used by the hyaluronan synthase inside the cell and the hyaluronan chain is continuously translocated across the membrane at the reducing end. Two enzymes are shown to illustrate how one enzyme could alternately make each of the two types of glycoside bonds in hyaluronan.
Of the three HA synthases, the most research has been done on HAS2 (Laurent and Fraser, 1992; Toole, 2004). Targeted deletion of HAS2 has yielded important insights into the function of HA (Lennon and Singleton, 2011), as the deletion of HAS2 causes major abnormalities in heart and blood vessel development (Jiang et al., 2007), which results in an embryonic lethal phenotype (Lennon and Singleton, 2011; Liang et al., 2011). HAS2 null embryos completely lack endocardial cushions, showing a critical role of the HA-ECM interaction in cardiac development (Camenisch et al., 2000; Liang et al., 2011). However, elucidating the role of HAS2 in lung injury/disease has been difficult because of the lethal phenotype in the HAS2 deficient mice. HAS1 and HAS3 have been reported to generate HA with broad size distributions (molecular masses of 2 × 105 to approximately 2 × 106 Da), whereas HAS2 generates HA with broad but extremely large sizes (average molecular mass of > 2 × 106 Da) (Krasinski and Tchorzewski, 2007; Liang et al., 2011).
Dysregulation of HA synthases and their activities has been reported during tissue injury, consistent with the findings that HA accumulates during a number of injuries (Adamia et al., 2005; Jiang et al., 2011; Aya and Stern, 2014). Increased accumulation of HA and increased HAS expression have been reported in allergic asthma (Engstrom-Laurent, 1989; Soderberg et al., 1989; Cheng et al., 2011; 2013; Ghosh et al., 2014b). Although HA synthesis and total HA concentration are important in regulating lung function, we must also consider HA degradation products as they may directly or indirectly alter downstream signaling pathways.
HYALURONAN DEGRADATION IN THE LUNG
Enzymatic degradation of HA is initiated by hyaluronidases (also called hyaluronoglucosaminidases or HYALs), a family of endoglycosidases that hydrolyze the hexosaminidic β (1-4) linkages between N-acetyl-D-glucosamine and D-glucuronic acid residues of HA and release HA fragments (Csoka et al., 2001; Chowdhury et al., 2013). Complete digestion of HA with hyaluronidase releases disaccharide D-glucuronic acid-N-acetyl-D-glucosamine. These enzymes also hydrolyze β (1-4) glycosidic linkages between N-acetyl-galactosamine or N-acetylgalactosamine sulfate and glucuronic acid in chondroitin, chondroitin 4-sulfate, chondroitin 6-sulfate, and dermatan (Lepperdinger et al., 2001; Gushulak et al., 2012). In humans, genes for six hyaluronidase family members have been identified to date: HYAL-1-4, PH-20, and HYALP1 (Lathrop et al., 1990; Csoka et al., 2001; Noble and Jiang, 2006). With the exception of the psuedogene HYALP1, each member encodes for protein products, and all but HYAL-3 have been shown to participate in HA catabolism (Laurent, 1989; Noble, 2002; Lennon and Singleton, 2011).
The hyaluronidase 1 (HYAL1) gene encodes a hyaluronidase found in the major parenchymal organs such as the liver, kidney, spleen, lung, and heart (Csoka et al., 2001; Stern, 2003). HYAL1 is also present in the human serum and urine (Ikegami-Kawai et al., 2004). The enzyme degrades HA within the cell, is active at an acidic pH, and is the major hyaluronidase in plasma (Ikegami-Kawai et al., 2004). HYAL2 is a glycosylphosphatidylinositol-anchored cell-surface receptor in all tissue types except the brain (Noble and Jiang, 2006; Singleton and Lennon, 2011). HYAL2 has very low hyaluronidase activity in comparison to serum hyaluronidase HYAL1 (Lepperdinger et al., 2001). Furthermore, unlike HYAL1, the HYAL2 enzyme hydrolyzes only HA of high molecular mass, yielding intermediate-sized HA fragments of approximately 20 kDa (Lepperdinger et al., 1998; Lepperdinger et al., 2001). HYAL3 transcripts show the strongest expression of this enzyme in testis and bone marrow with relatively weak expression in other organs (Reitinger et al., 2007; Atmuri et al., 2008). The role of HYAL3 in the degradation of HA is not clear, and there are currently no studies that demonstrate HYAL3 activity (Csoka et al., 2001; Noble and Jiang, 2006). PH-20 is a testicular enzyme encoded by the sperm adhesion molecule 1 (SPAM1) gene, which plays a role in fertilization (Lathrop et al., 1990).
Expression and activity of hyaluronidases have long been observed in diseases such as rheumatoid arthritis and periodontal disease (Soltes et al., 2006). Increased accumulation of HA and increased HYAL expression have also been reported in mouse models of allergic asthma (Jiang et al., 2011; Cheng et al., 2013; Ghosh et al., 2014b). However, hyaluronidases are not the only HA-degrading moiety in the lung, and other factors like ROS that accumulate at sites of tissue injury also provide a mechanism for generating HA fragments (Liang et al., 2011; Petrey and de la Motte, 2014b). ROS have been reported to degrade ECM components such as collagen, laminin, and HA (Papakonstantinou and Karakiulakis, 2009; Petrey and de la Motte, 2014b). Accumulation of ROS in chronic inflammatory conditions has been noted, and direct depolymerization of HA by ROS has been illustrated largely in vitro (Agren et al., 1997). In an epithelial airway culture system, xanthine/xanthine oxidase generation of ROS led to HA degradation and tissue kallikrein-mediated epidermal growth factor (EGF) receptor activation (Casalino-Matsuda et al., 2004). ROS species are also capable of stimulating HYAL2 gene expression via p39 mitogen-activated protein kinases (p39 MAPK) (Monzon et al., 2010). However, whether HA fragments generated directly by ROS species are functionally equivalent to enzymatic products is currently not known in allergic pulmonary disease.
THE HYALADHERINS - HYALURONAN BINDING PROTEINS
HA exists in the ECM in soluble form and can covalently bind to a variety of proteins to influence their functions (Jiang et al., 2011). These binding proteins have been grouped together and are called hyaladherins (Day and Prestwich, 2002). The differential activities of HA are regulated in the lung, in part, through interactions with HA binding proteins (hyaladherins) including CD44, receptor for hyaluronan mediated motility (RHAMM), tumor necrosis factor-α-stimulated glycoprotein-6 (TSG-6), lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1), and many other proteins (Lesley et al., 1993; Hall et al., 1994; Milner and Day, 2003; Toole, 2004). The role of these proteins in lung injury is discussed below (also see Table 1). Some HA binding proteins are associated with cell membranes, whereas others are found in the ECM. Structurally, the link module and the B(X7) B motif (where B is arginine and X is any nonacidic amino acid) are thought to constitute the HA-binding region (Toole, 1990; 2004).
Table 1. Hyaluronan binding proteins and their associated lung pathologies.
Summary of the HA binding proteins discussed in this review, their association with various associated lung pathologies and corresponding references.
| HA Binding Protein | Associated Lung Pathology | References |
|---|---|---|
| CD44 | LPS-induced lung injury Non-infectious lung injury COPD Asthma |
(Do et al., 2004; Krasinski and Tchorzewski, 2007; Li et al., 2009; Jiang et al., 2010; 2011) |
| RHAMM | Asthma LPS-induced lung injury COPD ARDS |
(Tolg et al., 2006; Jiang et al., 2010; 2011; Foley et al., 2012) |
| TSG-6 | Asthma Bleomycin-induced lung injury |
(Swaidani et al., 2013; Foskett et al., 2014) |
| TLR2/TLR4 | Asthma LPS-induced lung injury COPD Non-infectious lung injury |
(Chaudhuri et al., 2005; Garantziotis et al., 2010; Jiang et al., 2010; Bezemer et al., 2012; Foley et al., 2012) |
CD44
CD44 is the best characterized cell-surface HA binding protein. It is a structurally variable and multifunctional glycoprotein whose expression may be induced on most cell types (Lesley et al., 1993). It participates in many cellular processes including regulation of cell division (Lesley et al., 1993), survival (Baaten et al., 2010b), migration (Peck and Isacke, 1998), and adhesion (Kawakami et al., 1999) through the binding of its major ligand, HA. The human CD44 gene is located on chromosome 11p13 and consists of 19 coding exons of which 9, residing between constitutive exons 5 and 6, can be alternatively spliced into many different isoforms (Ponta et al., 2003; Weidle et al., 2011). The CD44 family consists of a standard form (CD44s) and variant isoforms (CD44v) (Entwistle et al., 1996). The standard form of CD44 (CD44s) contains none of the 9 variable exons, whereas the variant isoforms (CD44v2 - v10) includes them all (exon v1 is not expressed in humans) (Entwistle et al., 1996; Ponta et al., 2003). The CD44v3 - v10 isoform has one less exon and the CD44v8-10 isoform includes only the last three of the variable exons (Entwistle et al., 1996; Naor et al., 1997). Additional isoforms formed by alternative splicing, and various posttranslational modifications further increase the heterogeneity of the CD44 protein products. The standard form (CD44s) is ubiquitously expressed in vertebrates, mostly in quiescent cells (Entwistle et al., 1996; Naor et al., 1997). On the other hand the CD44 variants (CD44v) are expressed in a tissue-specific and cycle-specific manner (Entwistle et al., 1996). The different expression of CD44s and CD44v is probably correlated to the fact that variant isoforms have specific function, most likely different from that of CD44s (Entwistle et al., 1996; Naor et al., 1997).
CD44 binds HA via the link domain encoded by exon 2 (Teriete et al., 2004). This link domain, which is well conserved and similar to the link domains found in other hyaladherins, is ~100 amino acids long, has two antiparallel β-sheets crossed over by two α-helices, and is held together by two sulfide bridges (Teriete et al., 2004). The receptor on resting leukocytes does not bind HA until it is “activated” by proinflammatory cytokines, which results in the modification of the N-glycans on CD44 (Gee et al., 2003). It is thought that tumor necrosis factor-alpha (TNF-α) induces sialidase activity to remove sialic acid from the N-glycans, which then allows HA-binding to the receptor (Gee et al., 2003).
The most significant feature that distinguishes CD44 from other HA binding proteins is that CD44 binding to HA takes place at the cell surface where multiple, closely arrayed CD44-receptor molecules interact with the highly multivalent repeating disaccharide chain of HA (Lesley et al., 2000). Although glycosaminoglycan side chains associated with some CD44 isoforms can bind a subset of heparin-binding growth factors, cytokines, and ECM proteins such as fibronectin, most of the function ascribed to CD44 thus far can be attributed to its ability to bind and internalize HA (Rothenberg, 2003; Banerji et al., 2007). N-glycosylation regulates CD44 structure by altering both the affinity and avidity of CD44-HA binding (Skelton et al., 1998). Most cells express the standard isoform, which is approximately 85 kDa protein that undergoes posttranslational modification (Skelton et al., 1998; Banerji et al., 2007). Some examples of signaling pathways and molecules activated by HA binding of CD44 include Rac activation, ERM and merlin proteins, SRC, and ROCK (Do et al., 2004; Banerji et al., 2007).
A growing body of evidence indicates that HA/CD44 binding regulates both lymphoid and myeloid cells (Rafi et al., 1997; Do et al., 2004; Katoh et al., 2007; Ruffell and Johnson, 2008; Jiang et al., 2011; Cheng et al., 2013). HA-CD44 interactions play an important role in development (Graham et al., 2007), inflammation (Pure and Cuff, 2001), T cell recruitment and activation (Kawakami et al., 1999), and tumor growth and metastasis (Baaten et al., 2010a). More recently, CD44 has been shown to play a role in inflammatory cell recruitment in the allergic airway (Pure and Cuff, 2001; Rothenberg, 2003; Li et al., 2009) and CD44 expression is increased in the lungs of rats with experimental asthma (Girodet et al., 2010). CD44v5 and CD44v6 expression has also been shown to positively correlate with IgE levels in asthmatic patients and CD44v6 is up regulated in bronchial smooth muscle of asthmatic patients (Li et al., 2009; Girodet et al., 2010; Lennon and Singleton, 2011). Furthermore, antibody blockage of CD44 decreases mast cell adhesion to human bronchial smooth muscle cells in vitro, a process associated with AHR and remodeling (Girodet et al., 2010; Lennon and Singleton, 2011). While CD44 is regarded as the primary cell-surface receptor for HA in many cell types, investigation into the mechanisms underlying HA fragment signaling has demonstrated that HA fragments are capable of signaling independently of CD44 (Jiang et al., 2005; Hill et al., 2012).
RHAMM
RHAMM is present as both a plasma membrane GPI-anchored HA receptor and intracellular HA-binding protein lacking both transmembrane and cytoplasmic signal sequences (Turley et al., 1987; Entwistle et al., 1995). Consequently, it is also called intracellular HA binding protein (IHABP) or CD168 (Underhill, 1989). The human gene encoding RHAMM is located on chromosome 5q33.3 and contains 18 exons, which therefore makes it a likely candidate for alternative splicing like CD44 (Turley et al., 1987; Underhill, 1989; Entwistle et al., 1995). RHAMM was originally identified as a hyaladherin derived from chick embryonic heart fibroblasts, while its expression was later found on the surface of chick heart fibroblasts in the presence of HA (Turley, 1982; Turley et al., 1985). Following this initial discovery, the masses reported for RHAMM have been quite variable, ranging from 58 kDa on B cells to 120 kDa in fibroblasts from src null mice, based on immunoreactivity against anti-RHAMM antibodies (Turley et al., 1987; Underhill, 1989). The wide variety of molecular masses could be due to proteolysis, different glycosylation states of the receptor, or the presence of splice variants. The full-length human RHAMM, which is about 85% homologous to murine RHAMM, is identified as a 725 amino acid protein that migrates at ~84 kDa by sodium dodecyl sulfate polyacrylamide gel electrophoresis (Yang et al., 1994).
Although RHAMM lacks both a signal sequence and transmembrane domain, it is thought to be exported via an unconventional transport pathway (Turley, 1982; Turley et al., 1985). Unconventionally, exported RHAMM can exhibit extracellular function by coupling with integral cell surface receptor proteins such as CD44 or platelet-derived growth factor (Vigetti et al., 2014). Interestingly, RHAMM is not only located on the cell surface, but also released from the surface, and soluble forms have been shown to attenuate cell cycle progression n (Turley et al., 2002). RHAMM is a functional receptor in many cell types including endothelial cells (Savani et al., 2001), smooth muscle cells (Evanko et al., 2004), fibroblasts (Tolg et al., 2006), neuronal cells (Nagy et al., 1995), and thymocytes (Pilarski et al., 1994).
RHAMM is believed to be a HA receptor involved in cell locomotion. RHAMM is alternatively spliced like CD44 and it activates extracellular signal-regulated kinases (ERKs) and regulates mitotic-spindle integrity (Lokeshwar and Selzer, 2000). RHAMM binds to HA and has been shown to promote inflammatory cell recruitment and cell growth by a complex network of signal transduction events and interaction with the cytoskeleton (Jiang et al., 2011; Solis et al., 2012). Transforming growth factor-β1 (TGF-β1), which is a potent stimulator of cell motility, elicits the synthesis and expression of RHAMM and HA, and thus initiates locomotion (Samuel et al., 1993). RHAMM expression is increased on macrophages after bleomycin injury and HA-stimulated macrophage chemotaxis is inhibited by anti-RHAMM antibody (Zaman et al., 2005). Furthermore, daily administration of anti-RHAMM antibody to injured animals resulted in a 40% decrease in macrophage accumulation and lung architecture was improved suggesting a role of RHAMM in tissue injury and repair (Zaman et al., 2005). However, there are no studies regarding RHAMM-mediated inflammation in the allergic airways.
TSG-6
TSG-6, the gene product of tumor necrosis factor (TNF)-stimulated gene-6, is a ~35 kDa secreted protein comprised almost entirely of a Link module and a CUB module (Wisniewski and Vilcek, 1997; Milner and Day, 2003). Its amino acid sequence is very highly conserved between species, with the mouse and human proteins being >94% identical (Milner and Day, 2003). TSG-6 is not constitutively produced in healthy adult tissues, but is rapidly induced in a wide variety of cell types (e.g. monocytes (Bayliss et al., 2001), fibroblasts (Klampfer et al., 1994), vascular smooth muscle cells (Ye et al., 1997), cervical smooth muscle cells (Fujimoto et al., 2002), synoviocytes (Bayliss et al., 2001), chondrocytes (Maier et al., 1996), and epithelial cells (Janssen et al., 2001)) under conditions of inflammation (Milner and Day, 2003). It has been found to be associated with inflammatory conditions such as arthritis and bacterial sepsis (Dias et al., 2001; Getting et al., 2002; Kehlen et al., 2003; Milner and Day, 2003). Additionally, it is also produced during certain normal physiological processes that can be defined as ‘inflammation-like’ such as ovulation and cervical ripening (Mukhopadhyay et al., 2001; Fujimoto et al., 2002; Wisniewski et al., 2005). Recent studies have revealed that TSG-6 protects against cartilage matrix destruction and has potent anti-inflammatory effects in mouse models of arthritis (Getting et al., 2002; Glant et al., 2002). These studies suggest that TSG-6 is part of a negative feedback loop capable of down-regulating the inflammatory response and initiating tissue repair process. Similarly, Swaidani and colleagues have implicated a role of TSG-6 in an experimental model of allergic asthma (Swaidani et al., 2013). They demonstrated that endogenous TSG-6 is dispensable for the induction of Th2 immunity, robust increase in pulmonary HA deposition, eosinophilic inflammation, and AHR (Swaidani et al., 2013). This suggests that TSG-6 may have an important role in the initiation and maintenance of allergic asthma and that future studies are warranted to delineate the downstream signaling pathways activated by TSG-6-HA interaction.
LYVE-1
LYVE-1 is a type I, single-pass ~60 kDa plasma membrane glycoprotein containing 322 amino acids (Banerji et al., 1999; Prevo et al., 2001). A link domain, which comprises the bulk of the receptor ectodomain, binds only HA, not other GAGs. The cysteine’s (Cys) of this link domain are highly conserved, but only three other residues (Lys46, Try87, and Asn109) are conserved in the link family and known to be involved in HA binding (Banerji et al., 1999; Prevo et al., 2001). LYVE-1 has an overall homology of 43% with CD44 and binds both soluble and immobilized HA (Jiang et al., 2011; Wu et al., 2014). Expression of mouse LYVE1 remains restricted to the lymphatic system, and it has been implicated in the trafficking of cells within lymphatic vessels and lymph nodes (Prevo et al., 2001). However, to date, no study has reported a role for this receptor in allergen-induced asthma.
TOLL-LIKE RECEPTORS
HA is a component of the cell coat of groups A and C Streptococcus and Pasteurella multocida (DeAngelis, 2002; Noble, 2002; Boeriu et al., 2013). The repeating disaccharide structure of HA has features characteristic of pathogen-associated molecular patterns (PAMPs) (Jiang et al., 2006; Lafferty et al., 2010; Jiang et al., 2011; Black et al., 2013; Ebid et al., 2014). Many PAMPs on pathogens trigger innate immune responses via toll-like receptors (TLRs). Similarly, HA that has been modified by the inflammatory milieu attains functions similar to those of a PAMP. While an increasing number of studies have shown that TLRs are involved in HA signaling, the underlying mechanisms remain unclear. TLR4, the primary signaling receptor for lipopolysaccharides, and TLR2, a recognition receptor for mycoplasma and gram-positive bacteria, are both involved in the recognition of fragmented HA (Chaudhuri et al., 2005; Jiang et al., 2011; Bezemer et al., 2012). In dendritic cells, TLR4 is required for recognizing LMM HA, and this recognition is independent of CD44, TLR2, and RHAMM (Termeer et al., 2000; Termeer et al., 2002). However, studies by Noble and colleagues in a non-infectious lung injury model have shown that macrophages isolated from either TLR2 or TLR4 knockout mice are still capable of chemokine gene expression induced by HA fragments, while macrophages from TLR2/4 double knockout mice are not (Jiang et al., 2005; Jiang et al., 2011). They also demonstrated that HA-TLR signaling was abolished in myeloid differentiation primary response gene (MyD88)-deficient macrophages (Jiang et al., 2005). Similarly, in an ozone-induced lung injury model, Hollingsworth and colleagues have shown a role of the HA-TLR4-MyD88-TIRAP signaling pathway in mediating AHR and cytokine production (Li et al., 2011). However, a recent study indicates that HA fragments of ~200 kDa are capable of inducing type 1 interferons by a TLR4 MyD88-independent pathway (Black et al., 2013). These differences observed in signaling complex requirements may depend upon the size of HA used (135 vs 200 kDa) or the receptors present at the cell surface during experiments. Although data support CD44-independent TLR signaling by HA fragments in certain cell types, it is not clear if there is competition between these two receptors for HA or if they work in a cooperative fashion. These findings offer a new target for the diagnosis and treatment of asthma and may also provide insights into the mechanisms of asthma development. Future studies are needed to define the signaling pathways activated by different sizes of HA fragments and the receptors utilized.
HYALURONAN FRAGMENTS AS SIGNALING MOLECULES
The trafficking of inflammatory cells in allergic asthma is dependent on integrins, selectins, and cytokine/chemokine gradients. However, HA-based ECM defines where the problem is locally and subsequently promotes adhesion and likely subsequent activation of inflammatory cells (Figure 2). In its native state, HA exists as HMM HA, usually in excess of 106 Da (Laurent, 1989). However, under conditions of inflammation and tissue injury, HA is more polydisperse and contains a variety of HA polymers with overlapping lengths and functions, with a preponderance of low-molecular mass forms (Figure 2) (Laurent, 1989). A number of studies have shown that low molecular mass forms of HA (< 500 kDa, but not the native form >1000 kDa), induce inflammatory responses in various cell types, supporting the concept that HA fragments generated at sites of inflammation, but not native HMM HA, can stimulate the production of inflammatory mediators by many cell types (Ohkawara et al., 2000; Jiang et al., 2007; Krasinski and Tchorzewski, 2007). One possible explanation for these different functions of HA is that LMM HA may bind more firmly to cells to induce receptor cross-linking than the HMM HA, although this possibility needs further investigation. Additionally, the indicated molecular mass of HA used in different studies, represent the average molecular mass of HA of polydisperse distribution, and the preparations of HA used are not homogenous with respect to size. As such, care must be taken when considering the effects of different polymer sizes. A summary of the genes induced by LMM HA in different cell types is shown in Table 2.
Figure 2. Hyaluronan metabolism in the allergic lung.
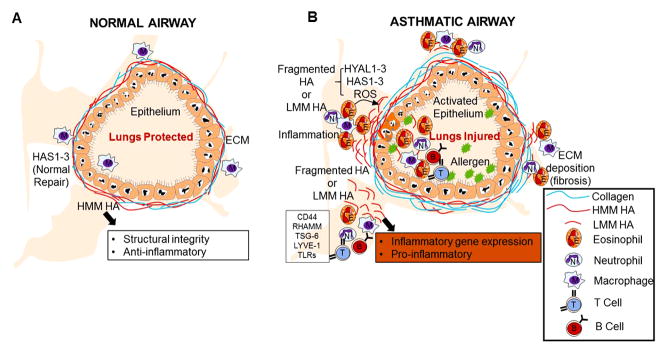
(A) Under physiological conditions high molecular mass HA (HMM HA) protects the lung and helps in the repair process. (B) Under conditions of chronic inflammation or tissue injury hyaluronan synthases (HAS1, HAS2, and HAS3) and hyaluronidases (HYAL1-3) play a role in the generation of fragmented HA (LMM HA). If hyaluronan fragments are generated, the balance shifts from HMM HA to LMM HA. This LMM HA is pro-inflammatory and helps in cellular recruitment and inflammatory gene expression. The differential activities of LMM HA in the lung are regulated through interactions with HA binding proteins including CD44, RHAMM, TSG-6, LYVE-1, and TLRs. Altering the balance of LMM HA and HMM HA may have therapeutic utility in the treatment of lung inflammation.
Table 2. Selected genes induced by HA fragments.
Summary of the various genes that are induced by HA binding in different cell types discussed in this review, and corresponding references.
| Category | Gene | Cell type | References |
|---|---|---|---|
| Cytokines | IL-12 | Macrophages | (Hodge-Dufour et al., 1997) |
| TNF-α | Macrophages | (Jiang et al., 2006) | |
| IL-1β | Macrophages | (Jiang et al., 2006; Feng et al., 2012) | |
| IL-6 | Fibroblasts, Macrophages, B Cells | (Iwata et al., 2009; Yamawaki et al., 2009; Vistejnova et al., 2014) | |
| IL-8 | Fibroblasts, Airway Epithelial Cells, Macrophages, Endothelial Cells | (McKee et al., 1996; Taylor et al., 2004; Boodoo et al., 2006; Vistejnova et al., 2014) | |
| IL-10 | Regulatory T Cells, B Cells | (Bollyky et al., 2009; Iwata et al., 2009; Bollyky et al., 2011) | |
| IFNβ | Macrophages | (Black et al., 2013) | |
| Chemokines | MIP-α | Macrophages | (Hodge-Dufour et al., 1997) |
| KC | Macrophages | (Horton et al., 1998) | |
| MCP-1 | Macrophages | (Yamawaki et al., 2009) | |
| CXCL2 | Macrophages | (Jiang et al., 2005) | |
| CCL5 | Macrophages | (McKee et al., 1996) | |
| CXCL9 | Macrophages | (Horton et al., 1998) | |
| CXCL10 | Macrophages | (McKee et al., 1996; Horton et al., 1998) | |
| CXCL1 | Endothelial Cells | (Takahashi et al., 2005) | |
| CCL3 | Macrophages | (McKee et al., 1996) | |
| CCL4 | Macrophages | (McKee et al., 1996) | |
| ECM | MMP-9 | Fibroblasts, Macrophages, Dendritic Cells | (Fieber et al., 2004; Stern et al., 2006) |
| MMP-13 | Fibroblasts | (Fieber et al., 2004; Stern et al., 2006) | |
| MMP-10 | Endothelial Cells | (Stern et al., 2006) | |
| PAI-1 | Macrophages | (Horton et al., 2000) | |
| uPA | Macrophages | (Horton et al., 2000) | |
| MME | Macrophages | (Horton et al., 1999) | |
| Transcription factors | NF-kappa B | Dendritic Cells | (Fieber et al., 2004) |
| IκBα | Macrophages | (Noble et al., 1996) | |
| AP-1 | Endothelial Cells | (Deed et al., 1997) | |
| Growth factors | TGF-β1 | Eosinophils, Macrophages, B cells | (Ohkawara et al., 2000; Iwata et al., 2009; Tolg et al., 2014) |
| TGF-β2 | Monocytes | (Taylor et al., 2007) | |
| IGF-1 | Macrophages | (Noble et al., 1993) | |
| Others | cPLA2α | Monocytes, Macrophages | (Sokolowska et al., 2014) |
| c-jun | Endothelial Cells | (Wang et al., 2011) | |
| c-fos | Endothelial Cells | (Wang et al., 2011) | |
| Cdc2 (Cdk1) | Endothelial Cells | (Matou-Nasri et al., 2009) | |
| Src | Endothelial Cells | (Wang et al., 2011) | |
| iNOS | Hepatocytes, Endothelial Cells | (Rockey et al., 1998) | |
| MDR-1 | Lymphocytes | (Tsujimura et al., 2006) |
HYALURONAN AS AN IMMUNE REGULATOR IN THE ALLERGIC LUNG
HA is present in low concentrations in the bronchoalveolar lavage (BAL) fluid from healthy individuals, while increased concentrations have been reported in the BAL fluid from patients with allergic asthma (Noble, 2002; Jiang et al., 2011; Eszes et al., 2014). Furthermore, HA levels in BAL fluids are significantly elevated in patients with chronic persistent asthma, in comparison with patients with intermittent asthma (Liang et al., 2011). The increase in the concentrations of HA in the BAL fluid of asthma patients is a result of either increased HA synthesis or HA breakdown by inflammatory cells (Figure 2B). While it is unclear whether the generation of HA fragments is the result of HA catabolic enzymes (HYAL1-4), reactive oxygen species (ROS), a truncated product of the HA biosynthetic enzymes (HAS1-3), or a combination of these mechanisms, it is clear that HA polymers of specific sizes contain distinct biological properties (Figure 2). Similarly, an increase in TGF-β levels in asthma has been associated with the increase in GAGs by smooth muscle cells (Papakonstantinou and Karakiulakis, 2009). IL-11β and TNF-α have a synergistic effect on LMM HA accumulation in lung fibroblasts (Ellis et al., 1992). Fibroblasts from subjects with the most hyperresponsive airways in asthma produced significantly more total proteoglycans, such as HA, than cells from subjects with less reactive or normal airways (Todorova et al., 2010). Some of the cell types that are known to interact with HA are T cells, eosinophils, macrophages, fibroblasts, smooth muscle cells, and B lymphocytes.
T CELLS
CD44, one of HA’s major receptors, undergoes dynamic regulation on T lymphocytes (Do et al., 2004; Ruffell and Johnson, 2008; Jiang et al., 2011). The interaction of cell-surface HA and CD44 on T cells is manifested by polarization, spreading, and co-localization of cell-surface CD44 with a rearranged actin cytoskeleton (Ariel et al., 2000). Thus, cytokines and chemokines present in the vicinities of blood vessels or present intravascularly in tissues where immune reactions take place can rapidly activate the CD44 molecules expressed on T cells (Liang et al., 2011; Maeshima et al., 2011). HA binding requires the activation of T cells, and their activation is associated with the increased surface levels of CD44. In addition, binding of CD44 on activated T lymphocytes to endothelial HA can promote extravasation and egress of T lymphocytes on inflamed vascular beds by mediating a primary adhesive interaction under shear stress (Katoh et al., 2010; Winkler et al., 2012). CD4+ T cells expressing high levels of CD44 are recruited to the allergic lung after antigen administration (Kennedy et al., 1995). Recently, Cheng and colleagues reported a correlation between HA deposition and lymphocytes in a cockroach-induced model of asthma (Cheng et al., 2011; 2013).
HA binding to CD44 on T cells has also been correlated to superior suppressor activity, suggesting that CD44 is more than a cell surface marker and plays a role in regulating regulatory T cell (Treg) function (Bollyky et al., 2007; Bollyky et al., 2009). HMM HA has been shown to enhance human CD4+CD25+ regulatory T cell functional suppression of responder cell proliferation, whereas LMM HA does not (Bollyky et al., 2007; Bollyky et al., 2009). In addition, HMM HA has been reported to up-regulate the transcription factor FOXP3 and CD4+CD25+ regulatory T cells (Bollyky et al., 2007; Bollyky et al., 2009).
EOSINOPHILS
The recruitment, activation, and degranulation of eosinophils in the lung is a characteristic hallmark of allergic asthma (Schmekel and Venge, 1993; Wardlaw et al., 2000; Venge, 2010; Ghosh et al., 2013; Acharya and Ackerman, 2014). HA stimulates the growth of CD34+ progenitor cells into specifically differentiated, mature eosinophils (Hamann et al., 1995). Both TNF-α and HA contribute to the long-term survival of eosinophils in vivo by enhancing the production of granulocyte macrophage colony stimulating factor (GM-CSF) and TGF-β in asthma (Esnault and Malter, 2001; 2003). Ohkawara and colleagues have reported that the LMM HA has a pronounced effect on eosinophil survival in both patients with asthma and healthy subjects in a dose-dependent fashion (Ohkawara et al., 2000; Esnault and Malter, 2001). The HMM HA had a smaller effect on eosinophil survival than did the LMM HA (Ohkawara et al., 2000). Furthermore, they demonstrated that the LMM HA results in morphologic changes in eosinophils such as transforming from a round to a spindle shape and in homotypic aggregation, up regulates intercellular adhesion molecule-1 expression, and increases TGF-β mRNA expression and protein secretion by eosinophils (Ohkawara et al., 2000). We and other research groups have also reported a correlation between LMM HA deposition and eosinophils in allergic asthma (Cheng et al., 2011; 2013; Ghosh et al., 2014b). Other groups have suggested a role of CD44 in eosinophil migration in the allergic lung as administration of anti-CD44 monoclonal Ab prevents eosinophil accumulation in the lung and blocked elevation of Th2 cytokines/chemokines (Katoh et al., 2003). These observations suggest previously unforeseen interactions between eosinophils and LMM HA, and thus, novel pathways by which eosinophils may contribute to the regulation of airway inflammation and airway remodeling in allergic asthma.
MACROPHAGES
Elevated concentrations of HA are associated with the accumulation of macrophages in the lung after injury (Savani et al., 2000; Noble and Jiang, 2006). Similarly, HA has been reported in macrophage migration through interactions with RHAMM, TLR2, and CD44 (Noble, 2002; Turley et al., 2002; Shi et al., 2006; Foley et al., 2012). CD44-deficient mice succumb to unremitting inflammation following lung injury, characterized by persistent accumulation of HA fragments at the site of tissue injury, and impaired activation of TGF-β1 (Liang et al., 2007). This phenotype was partially reversed by reconstitution with CD44+ macrophages, thus demonstrating a critical role for this receptor and macrophages in resolving lung inflammation (Liang et al., 2007). In a LPS-induced lung injury model, alveolar macrophages have been shown to promote HA synthesis in a TLR4 dependent manner to further modulate the inflammatory response (Chang et al., 2014). LMM HA has been reported to activate cytosolic Phospholipase A2α and promote eicosanoid production in a TLR4 dependent pathway in macrophages (Sokolowska et al., 2014). Similarly, LMM HA is able to polarize human macrophages to a M1 phenotype, and CD44 has been shown to regulate phagocytosis of apoptotic neutrophils by human macrophages (McCutcheon et al., 1998; Sokolowska et al., 2014). A recent study also demonstrates that Interferon-β (IFN-β) is induced in murine macrophages by LMM HA fragments by a novel pathway independent of MyD88 but dependent on TLR4 via TIR-domain-containing adapter-inducing interferon-β (TRIF) and Interferon Regulatory Factor-3 (IRF-3) (Black et al., 2013). Additionally, Feng and colleagues have demonstrated a role of HA in cleavage of macrophage-derived caspase1 and IL-1β, suggesting a role for alveolar macrophages in Nlrp3-dependent AHR (Feng et al., 2012).
FIBROBLASTS
Fibroblasts are the main cell that synthesizes HA (Smith and Ghosh, 1987; Noble, 2002; Liang et al., 2011; Maharjan et al., 2011). Fibroblasts express all three isoforms of HA synthases and release HA upon tissue injury when stimulated by inflammatory factors such as IL-1β and TNF-α. LMM HA stimulates fibroblasts to release cytokines and chemokines and regulate inflammatory responses (Prosdocimi and Bevilacqua, 2012; Tolg et al., 2014). Moreover, HA modulates TGF-β dependent myofibroblast differentiation (Thannickal et al., 2003).
HA-binding proteins regulate fibroblast functions through their interactions with HA. CD44 has been shown to play a role in fibroblast migration and injury in acute lung injury (Acharya et al., 2008). RHAMM deficient fibroblasts are defective in CD44-mediated ERK signaling, demonstrating a role of RHAMM in CD44 signaling and fibroblast migration (Tolg et al., 2006). A recent study also reported a role of HMM HA in promoting fibroblast invasion by increasing snail2 expression (Craig et al., 2009).
SMOOTH MUSCLE CELLS
GAGs, especially HA, regulate tissue flexibility, cell motility, and inflammation. Airway smooth muscle cells (ASMCs) of patients with asthma exhibit abnormal HA metabolism, which contributes to inflammation and remodeling (Papakonstantinou et al., 2012). Growth factors and cytokines at sites of inflammation or tissue injury regulate HA production by smooth muscle cells (Lauer et al., 2009). For example, Platelet-derived growth factor (PDGF) stimulates HA production in vascular smooth muscle cells, while IL-15 inhibits HA production and smooth muscle cell migration (Goueffic et al., 2006; Iwasaki et al., 2007; Lauer et al., 2009). Furthermore, HA binding proteins regulate smooth muscle proliferation and migration during lung injury (Boudreau et al., 1991). For example, RHAMM is necessary for the migration of smooth muscle cells, which is mediated by the PI3K-dependent Rac activation pathway (Goueffic et al., 2006). Other studies have reported a decrease in secretion of HA by ASMCs from patients with asthma or COPD (Klagas et al., 2009). This decrease was associated with a significant reduction in the expression of HAS1 and HAS2 and a significant increase of HYAL1. Furthermore, the expression of the HA receptor, CD44, was significantly decreased, whereas RHAMM was not expressed in asthma or COPD. This suggests that a decreased expression of HA by ASMC in asthma and COPD is associated with a synergistic regulation of HA metabolizing enzymes that may regulate the pathological airway remodeling in these lung diseases. It is also possible that reduced CD44 and RHAMM expression are responsible for impaired clearance of LMM HA from the lung, resulting in persistent inflammation.
A recent report suggests a role of glucocorticoids and long-acting beta agonists (LABAs) in inhibiting the increased secretion and deposition of total GAGs by asthmatic ASMCs (Papakonstantinou et al., 2012). However, these ASMCs secreted and deposited HA of high molecular mass (Papakonstantinou et al., 2012). This effect was attributed to increased mRNA and protein expression of HAS1 and to the reduced expression of HYAL1. Furthermore, drug treatment stimulated the expression of CD44 receptors in asthmatic ASMCs indicating that the combination of glucocorticoids with long-acting beta agonists (LABAs) counteracts the pathologic degradation of HA, and may reduce the proinflammatory potential of asthmatic ASMCs (Papakonstantinou et al., 2012).
B LYMPHOCYTES
The interaction of CD44 with its ligand, HA, plays a vital role in lymphopoiesis and lymphocyte homing, and there is growing evidence that HA/CD44 binding may play a role in the regulation of both lymphoid and myeloid cells (Rafi et al., 1997; Kryworuchko et al., 1999; Do et al., 2004; Katoh et al., 2007; Ruffell and Johnson, 2008; Jiang et al., 2011). In vitro, HA promotes survival, differentiation, and IgM production by B lymphocytes (Rafi et al., 1997). A recent report by Cheng and colleagues shows HA co-localization with B lymphocytes in the allergic lung (Cheng et al., 2013). Although CD44 helps to facilitate B lymphocyte adhesion to HA and proliferation in the spleen (Rafi et al., 1997; Vasconcellos et al., 2010), the contribution of HA/CD44 to B lymphocyte migration and activation in the context of allergic asthma has not been investigated and remains a focus for future studies.
THERAPEUTIC POTENTIAL OF HYALURONAN
Although HMM HA (≥1 million Da) is produced endogenously and is an integral component of the ECM, synovial fluid and vitreous humor, recent attention has been focused on the use of exogenously administered HMM HA in a variety of diseases including lung diseases (Noble and Jiang, 2006; Bollyky et al., 2007; Gaffney et al., 2010). In vitro, exogenous administration of HMM HA inhibits ROS, nitrotyrosine and inflammatory cytokine production as well as promotes immune tolerance (Garantziotis et al., 2010; Miki et al., 2010). Excess production of endogenous HMM HA in mice overexpressing HAS2 in airway epithelia protects against bleomycin-induced lung injury and ozone-induced AHR (McKee et al., 1996; Liang et al., 2011). Singleton and colleagues have demonstrated that intravenous administration of HMM HA (~1 million Da) four hours after intratracheal administration of LPS provides protection against lung injury in mice (Singleton et al., 2010). Nadkarni also demonstrated that pretreatment of hamsters with aerosolized HMM HA protects against endotoxin-induced lung injury (Nadkarni et al., 2005). Interestingly, treatment with aerosolized HMM HA after endotoxin treatment actually enhanced lung inflammation, indicating the timing and route of administration are important determinants of HMM HA’s effectiveness (Cantor, 2007; Singleton and Lennon, 2011).
Smoking is a well-recognized cause of lung injury that can lead to the development of emphysema and COPD (Cantor et al., 1999; Cantor et al., 2005). Cigarette smoke is believed to induce an imbalance in the protease-antiprotease levels in the lung (Shen et al., 2011). This imbalance, which develops due to increased inflammatory cell recruitment, activation and release of protease enzymes (including elastase), leads to the proteolytic breakdown of the ECM and the elastin fibers. Breakdown of the elastin fibers can lead to alveolar distention and rupture, a prominent feature of emphysema and COPD (Cantor et al., 2011; Shen et al., 2011). Although HA itself does not inhibit protease activity, studies by Cantor and Turino have shown that it may be protective against elastin fiber breakdown (Cantor et al., 1997; Cantor et al., 2011). They have shown that aerosolized LMM HA (150kDa) binds or closely associates with the elastin fibers and may physically protect them from degradation by proteases (Cantor et al., 2011; Shen et al., 2011). A clinical trial is currently underway to determine the use of HA as a treatment in COPD.
In relation to asthma, very few studies have examined the role of exogenous HMM HA on airway hyperreactivity. Aerosolized HMM HA has been shown to reduce neutrophil elastase induced bronchoconstriction in sheep (Cantor et al., 1998; Scuri and Abraham, 2003). In addition, oropharyngeal administration of exogenous HMM HA either before or after ozone exposure significantly attenuates airway hyperreactivity in mice (Garantziotis et al., 2009). Further, pre-treatment of aerosolized HMM HA protects asthmatic patients from exercise-induced bronchoconstriction (Petrigni and Allegra, 2006).
HA may be a double-edged sword with regard to its therapeutic efficacy. In the absence of existing inflammation, HMM HA may exert a beneficial effect, as both an anti-inflammatory agent and a shielding barrier against the degradative activities of various inflammatory products. Conversely, the presence of a pre-existing inflammatory milieu has the potential to convert HMM HA into a LMM HA proinflammatory mediator and thereby counteract its therapeutic activity. However, all these studies suggest a possible therapeutic role of HA in mediating lung injury and similar studies in mouse models of allergic asthma are needed to fully understand the specific molecular interactions of HA fragments with inflammatory cells.
CONCLUSIONS
The synthesis and degradation of the ECM are fundamental components of lung injury and repair. HA appears to have important functions in this biological process. Observations from experimental animals and asthmatic patients suggest a direct participation of HA in mediating the pathophysiology associated with allergic pulmonary disease, although the mechanisms by which HA contributes to the pathogenesis are rather complicated. Emerging evidence suggests that HA has a broad range of functions beyond that of a basic structural support in allergic asthma. The initiation and maintenance of allergic asthma relies on the balance between the low and high molecular mass HA accumulation. More extensive studies are needed to fully understand the specific molecular interactions between HA and HA-binding proteins in the initiation and resolution of inflammatory response in allergic asthma. Future studies should focus on the various molecular mass products of HA and their role in regulating the allergic phenotype in response to allergens. Selective interference with HA production or interaction with receptors may present a therapeutic target that can be exploited to minimize long-term damage associated with excessive collagen deposition in severe asthma. Additionally, studies involving the recognition of epigenetic factors in regulating the HA turnover in allergic disease may lead to new therapeutic approaches in the future.
Acknowledgments
The authors gratefully acknowledge financial support from the National Institutes of health (NIH) (Grant 1R15HL117254-01) to JMS. We also thank Jessica Ebert for critical reading of the manuscript.
Footnotes
CONFLICTS OF INTEREST
The authors report no conflicts of interest regarding the publication of this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acharya KR, Ackerman SJ. Eosinophil granule proteins: form and function. J Biol Chem. 2014;289:17406–17415. doi: 10.1074/jbc.R113.546218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acharya PS, Majumdar S, Jacob M, Hayden J, Mrass P, Weninger W, Assoian RK, Pure E. Fibroblast migration is mediated by CD44-dependent TGF beta activation. J Cell Sci. 2008;121:1393–1402. doi: 10.1242/jcs.021683. [DOI] [PubMed] [Google Scholar]
- Adamia S, Maxwell CA, Pilarski LM. Hyaluronan and hyaluronan synthases: potential therapeutic targets in cancer. Curr Drug Targets Cardiovasc Haematol Disord. 2005;5:3–14. doi: 10.2174/1568006053005056. [DOI] [PubMed] [Google Scholar]
- Agren UM, Tammi RH, Tammi MI. Reactive oxygen species contribute to epidermal hyaluronan catabolism in human skin organ culture. Free Radic Biol Med. 1997;23:996–1001. doi: 10.1016/s0891-5849(97)00098-1. [DOI] [PubMed] [Google Scholar]
- Ariel A, Lider O, Brill A, Cahalon L, Savion N, Varon D, Hershkoviz R. Induction of interactions between CD44 and hyaluronic acid by a short exposure of human T cells to diverse pro-inflammatory mediators. Immunology. 2000;100:345–351. doi: 10.1046/j.1365-2567.2000.00059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atmuri V, Martin DC, Hemming R, Gutsol A, Byers S, Sahebjam S, Thliveris JA, Mort JS, Carmona E, Anderson JE, Dakshinamurti S, Triggs-Raine B. Hyaluronidase 3 (HYAL3) knockout mice do not display evidence of hyaluronan accumulation. Matrix Biol. 2008;27:653–660. doi: 10.1016/j.matbio.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Aya KL, Stern R. Hyaluronan in wound healing: Rediscovering a major player. Wound Repair Regen. 2014 doi: 10.1111/wrr.12214. [DOI] [PubMed] [Google Scholar]
- Baaten BJ, Li CR, Bradley LM. Multifaceted regulation of T cells by CD44. Commun Integr Biol. 2010a;3:508–512. doi: 10.4161/cib.3.6.13495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baaten BJ, Li CR, Deiro MF, Lin MM, Linton PJ, Bradley LM. CD44 regulates survival and memory development in Th1 cells. Immunity. 2010b;32:104–115. doi: 10.1016/j.immuni.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerji S, Ni J, Wang SX, Clasper S, Su J, Tammi R, Jones M, Jackson DG. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol. 1999;144:789–801. doi: 10.1083/jcb.144.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerji S, Wright AJ, Noble M, Mahoney DJ, Campbell ID, Day AJ, Jackson DG. Structures of the Cd44-hyaluronan complex provide insight into a fundamental carbohydrate-protein interaction. Nat Struct Mol Biol. 2007;14:234–239. doi: 10.1038/nsmb1201. [DOI] [PubMed] [Google Scholar]
- Bayliss MT, Howat SL, Dudhia J, Murphy JM, Barry FP, Edwards JC, Day AJ. Up-regulation and differential expression of the hyaluronan-binding protein TSG-6 in cartilage and synovium in rheumatoid arthritis and osteoarthritis. Osteoarthritis Cartilage. 2001;9:42–48. doi: 10.1053/joca.2000.0348. [DOI] [PubMed] [Google Scholar]
- Bezemer GF, Sagar S, Van Bergenhenegouwen J, Georgiou NA, Garssen J, Kraneveld AD, Folkerts G. Dual role of Toll-like receptors in asthma and chronic obstructive pulmonary disease. Pharmacol Rev. 2012;64:337–358. doi: 10.1124/pr.111.004622. [DOI] [PubMed] [Google Scholar]
- Black KE, Collins SL, Hagan RS, Hamblin MJ, Chan-Li Y, Hallowell RW, Powell JD, Horton MR. Hyaluronan fragments induce IFNbeta via a novel TLR4-TRIF-TBK1-IRF3-dependent pathway. J Inflamm (Lond) 2013;10:23. doi: 10.1186/1476-9255-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeriu CG, Springer J, Kooy FK, Van Den Broek LaM, Eggink G. Production Methods for Hyaluronan. International Journal of Carbohydrate Chemistry. 2013;2013:14. [Google Scholar]
- Bollyky PL, Falk BA, Long SA, Preisinger A, Braun KR, Wu RP, Evanko SP, Buckner JH, Wight TN, Nepom GT. CD44 costimulation promotes FoxP3+ regulatory T cell persistence and function via production of IL-2, IL-10, and TGF-beta. J Immunol. 2009;183:2232–2241. doi: 10.4049/jimmunol.0900191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollyky PL, Lord JD, Masewicz SA, Evanko SP, Buckner JH, Wight TN, Nepom GT. Cutting edge: high molecular weight hyaluronan promotes the suppressive effects of CD4+CD25+ regulatory T cells. J Immunol. 2007;179:744–747. doi: 10.4049/jimmunol.179.2.744. [DOI] [PubMed] [Google Scholar]
- Bollyky PL, Wu RP, Falk BA, Lord JD, Long SA, Preisinger A, Teng B, Holt GE, Standifer NE, Braun KR, Xie CF, Samuels PL, Vernon RB, Gebe JA, Wight TN, Nepom GT. ECM components guide IL-10 producing regulatory T-cell (TR1) induction from effector memory T-cell precursors. Proc Natl Acad Sci U S A. 2011;108:7938–7943. doi: 10.1073/pnas.1017360108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boodoo S, Spannhake EW, Powell JD, Horton MR. Differential regulation of hyaluronan-induced IL-8 and IP-10 in airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2006;291:L479–486. doi: 10.1152/ajplung.00518.2005. [DOI] [PubMed] [Google Scholar]
- Boudreau N, Turley E, Rabinovitch M. Fibronectin, hyaluronan, and a hyaluronan binding protein contribute to increased ductus arteriosus smooth muscle cell migration. Dev Biol. 1991;143:235–247. doi: 10.1016/0012-1606(91)90074-d. [DOI] [PubMed] [Google Scholar]
- Camenisch TD, Spicer AP, Brehm-Gibson T, Biesterfeldt J, Augustine ML, Calabro A, Jr, Kubalak S, Klewer SE, Mcdonald JA. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J Clin Invest. 2000;106:349–360. doi: 10.1172/JCI10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor JO. Potential therapeutic applications of hyaluronan in the lung. Int J Chron Obstruct Pulmon Dis. 2007;2:283–288. [PMC free article] [PubMed] [Google Scholar]
- Cantor JO, Cerreta JM, Armand G, Osman M, Turino GM. The pulmonary matrix, glycosaminoglycans and pulmonary emphysema. Connect Tissue Res. 1999;40:97–104. doi: 10.3109/03008209909029105. [DOI] [PubMed] [Google Scholar]
- Cantor JO, Cerreta JM, Armand G, Turino GM. Further investigation of the use of intratracheally administered hyaluronic acid to ameliorate elastase-induced emphysema. Exp Lung Res. 1997;23:229–244. doi: 10.3109/01902149709087369. [DOI] [PubMed] [Google Scholar]
- Cantor JO, Cerreta JM, Armand G, Turino GM. Aerosolized hyaluronic acid decreases alveolar injury induced by human neutrophil elastase. Proc Soc Exp Biol Med. 1998;217:471–475. doi: 10.3181/00379727-217-44260. [DOI] [PubMed] [Google Scholar]
- Cantor JO, Cerreta JM, Ochoa M, Ma S, Chow T, Grunig G, Turino GM. Aerosolized hyaluronan limits airspace enlargement in a mouse model of cigarette smoke-induced pulmonary emphysema. Exp Lung Res. 2005;31:417–430. doi: 10.1080/01902140590918669. [DOI] [PubMed] [Google Scholar]
- Cantor JO, Cerreta JM, Ochoa M, Ma S, Liu M, Turino GM. Therapeutic effects of hyaluronan on smoke-induced elastic fiber injury: does delayed treatment affect efficacy? Lung. 2011;189:51–56. doi: 10.1007/s00408-010-9271-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casalino-Matsuda SM, Monzon ME, Conner GE, Salathe M, Forteza RM. Role of hyaluronan and reactive oxygen species in tissue kallikrein-mediated epidermal growth factor receptor activation in human airways. J Biol Chem. 2004;279:21606–21616. doi: 10.1074/jbc.M309950200. [DOI] [PubMed] [Google Scholar]
- Chang MY, Tanino Y, Vidova V, Kinsella MG, Chan CK, Johnson PY, Wight TN, Frevert CW. A rapid increase in macrophage-derived versican and hyaluronan in infectious lung disease. Matrix Biol. 2014;34:1–12. doi: 10.1016/j.matbio.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary N, Marr KA. Impact of Aspergillus fumigatus in allergic airway diseases. Clin Transl Allergy. 2011;1:4. doi: 10.1186/2045-7022-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri N, Dower SK, Whyte MK, Sabroe I. Toll-like receptors and chronic lung disease. Clin Sci (Lond) 2005;109:125–133. doi: 10.1042/CS20050044. [DOI] [PubMed] [Google Scholar]
- Cheng G, Swaidani S, Sharma M, Lauer ME, Hascall VC, Aronica MA. Hyaluronan deposition and correlation with inflammation in a murine ovalbumin model of asthma. Matrix Biol. 2011;30:126–134. doi: 10.1016/j.matbio.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G, Swaidani S, Sharma M, Lauer ME, Hascall VC, Aronica MA. Correlation of hyaluronan deposition with infiltration of eosinophils and lymphocytes in a cockroach-induced murine model of asthma. Glycobiology. 2013;23:43–58. doi: 10.1093/glycob/cws122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury B, Hemming R, Hombach-Klonisch S, Flamion B, Triggs-Raine B. Murine hyaluronidase 2 deficiency results in extracellular hyaluronan accumulation and severe cardiopulmonary dysfunction. J Biol Chem. 2013;288:520–528. doi: 10.1074/jbc.M112.393629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig EA, Parker P, Camenisch TD. Size-dependent regulation of Snail2 by hyaluronan: its role in cellular invasion. Glycobiology. 2009;19:890–898. doi: 10.1093/glycob/cwp064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csoka AB, Frost GI, Stern R. The six hyaluronidase-like genes in the human and mouse genomes. Matrix Biol. 2001;20:499–508. doi: 10.1016/s0945-053x(01)00172-x. [DOI] [PubMed] [Google Scholar]
- Dagenais TR, Keller NP. Pathogenesis of Aspergillus fumigatus in Invasive Aspergillosis. Clin Microbiol Rev. 2009;22:447–465. doi: 10.1128/CMR.00055-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day AJ, Prestwich GD. Hyaluronan-binding proteins: tying up the giant. J Biol Chem. 2002;277:4585–4588. doi: 10.1074/jbc.R100036200. [DOI] [PubMed] [Google Scholar]
- Deangelis PL. Hyaluronan synthases: fascinating glycosyltransferases from vertebrates, bacterial pathogens, and algal viruses. Cell Mol Life Sci. 1999;56:670–682. doi: 10.1007/s000180050461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deangelis PL. Microbial glycosaminoglycan glycosyltransferases. Glycobiology. 2002;12:9R–16R. doi: 10.1093/glycob/12.1.9r. [DOI] [PubMed] [Google Scholar]
- Deed R, Rooney P, Kumar P, Norton JD, Smith J, Freemont AJ, Kumar S. Early-response gene signalling is induced by angiogenic oligosaccharides of hyaluronan in endothelial cells. Inhibition by non-angiogenic, high-molecular-weight hyaluronan. Int J Cancer. 1997;71:251–256. doi: 10.1002/(sici)1097-0215(19970410)71:2<251::aid-ijc21>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Dentener MA, Vernooy JH, Hendriks S, Wouters EF. Enhanced levels of hyaluronan in lungs of patients with COPD: relationship with lung function and local inflammation. Thorax. 2005;60:114–119. doi: 10.1136/thx.2003.020842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derya M, Yulmaz I, Aytekin M. The Role of Extracellular Matrix in Lung Diseases. Biology and medicine. 2014;6:1–8. [Google Scholar]
- Dias AA, Goodman AR, Dos Santos JL, Gomes RN, Altmeyer A, Bozza PT, Horta MF, Vilcek J, Reis LF. TSG-14 transgenic mice have improved survival to endotoxemia and to CLP-induced sepsis. J Leukoc Biol. 2001;69:928–936. [PubMed] [Google Scholar]
- Do Y, Nagarkatti PS, Nagarkatti M. Role of CD44 and hyaluronic acid (HA) in activation of alloreactive and antigen-specific T cells by bone marrow-derived dendritic cells. J Immunother. 2004;27:1–12. doi: 10.1097/00002371-200401000-00001. [DOI] [PubMed] [Google Scholar]
- Ebid R, Lichtnekert J, Anders HJ. Hyaluronan is not a ligand but a regulator of toll-like receptor signaling in mesangial cells: role of extracellular matrix in innate immunity. ISRN Nephrol. 2014;2014:714081. doi: 10.1155/2014/714081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis I, Grey AM, Schor AM, Schor SL. Antagonistic effects of TGF-beta 1 and MSF on fibroblast migration and hyaluronic acid synthesis. Possible implications for dermal wound healing. J Cell Sci. 1992;102 (Pt 3):447–456. doi: 10.1242/jcs.102.3.447. [DOI] [PubMed] [Google Scholar]
- Engstrom-Laurent A. Changes in hyaluronan concentration in tissues and body fluids in disease states. Ciba Found Symp. 1989;143:233–240. doi: 10.1002/9780470513774.ch14. discussion 240-237, 281-235. [DOI] [PubMed] [Google Scholar]
- Entwistle J, Hall CL, Turley EA. HA receptors: regulators of signalling to the cytoskeleton. J Cell Biochem. 1996;61:569–577. doi: 10.1002/(sici)1097-4644(19960616)61:4<569::aid-jcb10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Entwistle J, Zhang S, Yang B, Wong C, Li Q, Hall CL, AJ, Mowat M, Greenberg AH, Turley EA. Characterization of the murine gene encoding the hyaluronan receptor RHAMM. Gene. 1995;163:233–238. doi: 10.1016/0378-1119(95)00398-p. [DOI] [PubMed] [Google Scholar]
- Esnault S, Malter JS. Granulocyte macrophage-colony-stimulating factor mRNA is stabilized in airway eosinophils and peripheral blood eosinophils activated by TNF-alpha plus fibronectin. J Immunol. 2001;166:4658–4663. doi: 10.4049/jimmunol.166.7.4658. [DOI] [PubMed] [Google Scholar]
- Esnault S, Malter JS. Hyaluronic acid or TNF-alpha plus fibronectin triggers granulocyte macrophage-colony-stimulating factor mRNA stabilization in eosinophils yet engages differential intracellular pathways and mRNA binding proteins. J Immunol. 2003;171:6780–6787. doi: 10.4049/jimmunol.171.12.6780. [DOI] [PubMed] [Google Scholar]
- Eszes N, Toldi G, Bohacs A, Ivancso I, Muller V, Rigo J, Jr, Losonczy G, Vasarhelyi B, Tamasi L. Relationship of circulating hyaluronic acid levels to disease control in asthma and asthmatic pregnancy. PLoS One. 2014;9:e94678. doi: 10.1371/journal.pone.0094678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evanko SP, Parks WT, Wight TN. Intracellular hyaluronan in arterial smooth muscle cells: association with microtubules, RHAMM, and the mitotic spindle. J Histochem Cytochem. 2004;52:1525–1535. doi: 10.1369/jhc.4A6356.2004. [DOI] [PubMed] [Google Scholar]
- Feng F, Li Z, Potts-Kant EN, Wu Y, Foster WM, Williams KL, Hollingsworth JW. Hyaluronan activation of the Nlrp3 inflammasome contributes to the development of airway hyperresponsiveness. Environ Health Perspect. 2012;120:1692–1698. doi: 10.1289/ehp.1205188. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Fieber C, Baumann P, Vallon R, Termeer C, Simon JC, Hofmann M, Angel P, Herrlich P, Sleeman JP. Hyaluronan-oligosaccharide-induced transcription of metalloproteases. J Cell Sci. 2004;117:359–367. doi: 10.1242/jcs.00831. [DOI] [PubMed] [Google Scholar]
- Foley JP, Lam D, Jiang H, Liao J, Cheong N, Mcdevitt TM, Zaman A, Wright JR, Savani RC. Toll-like receptor 2 (TLR2), transforming growth factor-beta, hyaluronan (HA), and receptor for HA-mediated motility (RHAMM) are required for surfactant protein A-stimulated macrophage chemotaxis. J Biol Chem. 2012;287:37406–37419. doi: 10.1074/jbc.M112.360982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foskett AM, Bazhanov N, Ti X, Tiblow A, Bartosh TJ, Prockop DJ. Phase-directed therapy: TSG-6 targeted to early inflammation improves bleomycin-injured lungs. Am J Physiol Lung Cell Mol Physiol. 2014;306:L120–131. doi: 10.1152/ajplung.00240.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto T, Savani RC, Watari M, Day AJ, Strauss JF., 3rd Induction of the hyaluronic acid-binding protein, tumor necrosis factor-stimulated gene-6, in cervical smooth muscle cells by tumor necrosis factor-alpha and prostaglandin E(2) Am J Pathol. 2002;160:1495–1502. doi: 10.1016/s0002-9440(10)62575-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffney J, Matou-Nasri S, Grau-Olivares M, Slevin M. Therapeutic applications of hyaluronan. Mol Biosyst. 2010;6:437–443. doi: 10.1039/b910552m. [DOI] [PubMed] [Google Scholar]
- Garantziotis S, Li Z, Potts EN, Kimata K, Zhuo L, Morgan DL, Savani RC, Noble PW, Foster WM, Schwartz DA, Hollingsworth JW. Hyaluronan mediates ozone-induced airway hyperresponsiveness in mice. J Biol Chem. 2009;284:11309–11317. doi: 10.1074/jbc.M802400200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- Garantziotis S, Li Z, Potts EN, Lindsey JY, Stober VP, Polosukhin VV, Blackwell TS, Schwartz DA, Foster WM, Hollingsworth JW. TLR4 is necessary for hyaluronan-mediated airway hyperresponsiveness after ozone inhalation. Am J Respir Crit Care Med. 2010;181:666–675. doi: 10.1164/rccm.200903-0381OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- Gee K, Kozlowski M, Kumar A. Tumor necrosis factor-alpha induces functionally active hyaluronan-adhesive CD44 by activating sialidase through p38 mitogen-activated protein kinase in lipopolysaccharide-stimulated human monocytic cells. J Biol Chem. 2003;278:37275–37287. doi: 10.1074/jbc.M302309200. [DOI] [PubMed] [Google Scholar]
- Getting SJ, Mahoney DJ, Cao T, Rugg MS, Fries E, Milner CM, Perretti M, Day AJ. The link module from human TSG-6 inhibits neutrophil migration in a hyaluronan- and inter-alpha-inhibitor-independent manner. J Biol Chem. 2002;277:51068–51076. doi: 10.1074/jbc.M205121200. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Hoselton SA, Asbach SV, Steffan BN, Wanjara SB, Dorsam GP, Schuh JM. B lymphocytes regulate airway granulocytic inflammation and cytokine production in a murine model of fungal allergic asthma. Cell Mol Immunol. 2014a doi: 10.1038/cmi.2014.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Hoselton SA, Dorsam GP, Schuh JM. Eosinophils in fungus-associated allergic pulmonary disease. Front Pharmacol. 2013;4:8. doi: 10.3389/fphar.2013.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Hoselton SA, Schuh JM. Characterization of CD19(+)CD23(+)B2 lymphocytes in the allergic airways of BALB/c mice in response to the inhalation of Aspergillus fumigatus conidia. Open Immunol J. 2012a;5:46–54. doi: 10.2174/1874226201205010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Hoselton SA, Schuh JM. mu-chain-deficient mice possess B-1 cells and produce IgG and IgE, but not IgA, following systemic sensitization and inhalational challenge in a fungal asthma model. J Immunol. 2012b;189:1322–1329. doi: 10.4049/jimmunol.1200138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Samarasinghe AE, Hoselton SA, Dorsam GP, Schuh JM. Hyaluronan deposition and co-localization with inflammatory cells and collagen in a murine model of fungal allergic asthma. Inflamm Res. 2014b;63:475–484. doi: 10.1007/s00011-014-0719-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girodet PO, Ozier A, Trian T, Begueret H, Ousova O, Vernejoux JM, Chanez P, Marthan R, Berger P, Tunon De Lara JM. Mast cell adhesion to bronchial smooth muscle in asthma specifically depends on CD51 and CD44 variant 6. Allergy. 2010;65:1004–1012. doi: 10.1111/j.1398-9995.2009.02308.x. [DOI] [PubMed] [Google Scholar]
- Glant TT, Kamath RV, Bardos T, Gal I, Szanto S, Murad YM, Sandy JD, Mort JS, Roughley PJ, Mikecz K. Cartilage-specific constitutive expression of TSG-6 protein (product of tumor necrosis factor alpha-stimulated gene 6) provides a chondroprotective, but not antiinflammatory, effect in antigen-induced arthritis. Arthritis Rheum. 2002;46:2207–2218. doi: 10.1002/art.10555. [DOI] [PubMed] [Google Scholar]
- Goueffic Y, Guilluy C, Guerin P, Patra P, Pacaud P, Loirand G. Hyaluronan induces vascular smooth muscle cell migration through RHAMM-mediated PI3K-dependent Rac activation. Cardiovasc Res. 2006;72:339–348. doi: 10.1016/j.cardiores.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Graham VA, Marzo AL, Tough DF. A role for CD44 in T cell development and function during direct competition between CD44+ and CD44− cells. Eur J Immunol. 2007;37:925–934. doi: 10.1002/eji.200635882. [DOI] [PubMed] [Google Scholar]
- Gushulak L, Hemming R, Martin D, Seyrantepe V, Pshezhetsky A, Triggs-Raine B. Hyaluronidase 1 and beta-hexosaminidase have redundant functions in hyaluronan and chondroitin sulfate degradation. J Biol Chem. 2012;287:16689–16697. doi: 10.1074/jbc.M112.350447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall CL, Wang C, Lange LA, Turley EA. Hyaluronan and the hyaluronan receptor RHAMM promote focal adhesion turnover and transient tyrosine kinase activity. J Cell Biol. 1994;126:575–588. doi: 10.1083/jcb.126.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallgren R, Samuelsson T, Laurent TC, Modig J. Accumulation of hyaluronan (hyaluronic acid) in the lung in adult respiratory distress syndrome. Am Rev Respir Dis. 1989;139:682–687. doi: 10.1164/ajrccm/139.3.682. [DOI] [PubMed] [Google Scholar]
- Hamann KJ, Dowling TL, Neeley SP, Grant JA, Leff AR. Hyaluronic acid enhances cell proliferation during eosinopoiesis through the CD44 surface antigen. J Immunol. 1995;154:4073–4080. [PubMed] [Google Scholar]
- Hernandez Y, Herring AC, Huffnagle GB. Pulmonary defenses against fungi. Semin Respir Crit Care Med. 2004;25:63–71. doi: 10.1055/s-2004-822306. [DOI] [PubMed] [Google Scholar]
- Hill DR, Kessler SP, Rho HK, Cowman MK, De La Motte CA. Specific-sized hyaluronan fragments promote expression of human beta-defensin 2 in intestinal epithelium. J Biol Chem. 2012;287:30610–30624. doi: 10.1074/jbc.M112.356238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge-Dufour J, Noble PW, Horton MR, Bao C, Wysoka M, Burdick MD, Strieter RM, Trinchieri G, Pure E. Induction of IL-12 and chemokines by hyaluronan requires adhesion-dependent priming of resident but not elicited macrophages. J Immunol. 1997;159:2492–2500. [PubMed] [Google Scholar]
- Hogaboam CM, Blease K, Mehrad B, Steinhauser ML, Standiford TJ, Kunkel SL, Lukacs NW. Chronic airway hyperreactivity, goblet cell hyperplasia, and peribronchial fibrosis during allergic airway disease induced by Aspergillus fumigatus. Am J Pathol. 2000;156:723–732. doi: 10.1016/S0002-9440(10)64775-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton MR, Burdick MD, Strieter RM, Bao C, Noble PW. Regulation of hyaluronan-induced chemokine gene expression by IL-10 and IFN-gamma in mouse macrophages. J Immunol. 1998;160:3023–3030. [PubMed] [Google Scholar]
- Horton MR, Olman MA, Bao C, White KE, Choi AM, Chin BY, Noble PW, Lowenstein CJ. Regulation of plasminogen activator inhibitor-1 and urokinase by hyaluronan fragments in mouse macrophages. Am J Physiol Lung Cell Mol Physiol. 2000;279:L707–715. doi: 10.1152/ajplung.2000.279.4.L707. [DOI] [PubMed] [Google Scholar]
- Horton MR, Olman MA, Noble PW. Hyaluronan fragments induce plasminogen activator inhibitor-1 and inhibit urokinase activity in mouse alveolar macrophages: a potential mechanism for impaired fibrinolytic activity in acute lung injury. Chest. 1999;116:17S. [PubMed] [Google Scholar]
- Hoselton SA, Samarasinghe AE, Seydel JM, Schuh JM. An inhalation model of airway allergic response to inhalation of environmental Aspergillus fumigatus conidia in sensitized BALB/c mice. Med Mycol. 2010;48:1056–1065. doi: 10.3109/13693786.2010.485582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami-Kawai M, Okuda R, Nemoto T, Inada N, Takahashi T. Enhanced activity of serum and urinary hyaluronidases in streptozotocin-induced diabetic Wistar and GK rats. Glycobiology. 2004;14:65–72. doi: 10.1093/glycob/cwh011. [DOI] [PubMed] [Google Scholar]
- Iwasaki S, Minamisawa S, Yokoyama U, Akaike T, Quan H, Nagashima Y, Nishimaki S, Ishikawa Y, Yokota S. Interleukin-15 inhibits smooth muscle cell proliferation and hyaluronan production in rat ductus arteriosus. Pediatr Res. 2007;62:392–398. doi: 10.1203/PDR.0b013e31813c9339. [DOI] [PubMed] [Google Scholar]
- Iwata Y, Yoshizaki A, Komura K, Shimizu K, Ogawa F, Hara T, Muroi E, Bae S, Takenaka M, Yukami T, Hasegawa M, Fujimoto M, Tomita Y, Tedder TF, Sato S. CD19, a response regulator of B lymphocytes, regulates wound healing through hyaluronan-induced TLR4 signaling. Am J Pathol. 2009;175:649–660. doi: 10.2353/ajpath.2009.080355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen U, Thomas G, Glant T, Phillips A. Expression of inter-alpha-trypsin inhibitor and tumor necrosis factor-stimulated gene 6 in renal proximal tubular epithelial cells. Kidney Int. 2001;60:126–136. doi: 10.1046/j.1523-1755.2001.00779.x. [DOI] [PubMed] [Google Scholar]
- Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA, Homer RJ, Goldstein DR, Bucala R, Lee PJ, Medzhitov R, Noble PW. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11:1173–1179. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- Jiang D, Liang J, Li Y, Noble PW. The role of Toll-like receptors in non-infectious lung injury. Cell Res. 2006;16:693–701. doi: 10.1038/sj.cr.7310085. [DOI] [PubMed] [Google Scholar]
- Jiang D, Liang J, Noble PW. Hyaluronan in tissue injury and repair. Annu Rev Cell Dev Biol. 2007;23:435–461. doi: 10.1146/annurev.cellbio.23.090506.123337. [DOI] [PubMed] [Google Scholar]
- Jiang D, Liang J, Noble PW. Regulation of non-infectious lung injury, inflammation, and repair by the extracellular matrix glycosaminoglycan hyaluronan. Anat Rec (Hoboken) 2010;293:982–985. doi: 10.1002/ar.21102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D, Liang J, Noble PW. Hyaluronan as an immune regulator in human diseases. Physiol Rev. 2011;91:221–264. doi: 10.1152/physrev.00052.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh S, Ishii N, Nobumoto A, Takeshita K, Dai SY, Shinonaga R, Niki T, Nishi N, Tominaga A, Yamauchi A, Hirashima M. Galectin-9 inhibits CD44-hyaluronan interaction and suppresses a murine model of allergic asthma. Am J Respir Crit Care Med. 2007;176:27–35. doi: 10.1164/rccm.200608-1243OC. [DOI] [PubMed] [Google Scholar]
- Katoh S, Maeda S, Fukuoka H, Wada T, Moriya S, Mori A, Yamaguchi K, Senda S, Miyagi T. A crucial role of sialidase Neu1 in hyaluronan receptor function of CD44 in T helper type 2-mediated airway inflammation of murine acute asthmatic model. Clin Exp Immunol. 2010;161:233–241. doi: 10.1111/j.1365-2249.2010.04165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh S, Matsumoto N, Kawakita K, Tominaga A, Kincade PW, Matsukura S. A role for CD44 in an antigen-induced murine model of pulmonary eosinophilia. J Clin Invest. 2003;111:1563–1570. doi: 10.1172/JCI16583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami N, Nishizawa F, Sakane N, Iwao M, Tsujikawa K, Ikawa M, Okabe M, Yamamoto H. Roles of integrins and CD44 on the adhesion and migration of fetal liver cells to the fetal thymus. J Immunol. 1999;163:3211–3216. [PubMed] [Google Scholar]
- Kehlen A, Pachnio A, Thiele K, Langner J. Gene expression induced by interleukin-17 in fibroblast-like synoviocytes of patients with rheumatoid arthritis: upregulation of hyaluronan-binding protein TSG-6. Arthritis Res Ther. 2003;5:R186–192. doi: 10.1186/ar762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy JD, Hatfield CA, Fidler SF, Winterrowd GE, Haas JV, Chin JE, Richards IM. Phenotypic characterization of T lymphocytes emigrating into lung tissue and the airway lumen after antigen inhalation in sensitized mice. Am J Respir Cell Mol Biol. 1995;12:613–623. doi: 10.1165/ajrcmb.12.6.7766426. [DOI] [PubMed] [Google Scholar]
- Klagas I, Goulet S, Karakiulakis G, Zhong J, Baraket M, Black JL, Papakonstantinou E, Roth M. Decreased hyaluronan in airway smooth muscle cells from patients with asthma and COPD. Eur Respir J. 2009;34:616–628. doi: 10.1183/09031936.00070808. [DOI] [PubMed] [Google Scholar]
- Klampfer L, Lee TH, Hsu W, Vilcek J, Chen-Kiang S. NF-IL6 and AP-1 cooperatively modulate the activation of the TSG-6 gene by tumor necrosis factor alpha and interleukin-1. Mol Cell Biol. 1994;14:6561–6569. doi: 10.1128/mcb.14.10.6561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutsen AP, Bush RK, Demain JG, Denning DW, Dixit A, Fairs A, Greenberger PA, Kariuki B, Kita H, Kurup VP, Moss RB, Niven RM, Pashley CH, Slavin RG, Vijay HM, Wardlaw AJ. Fungi and allergic lower respiratory tract diseases. J Allergy Clin Immunol. 2012;129:280–291. doi: 10.1016/j.jaci.2011.12.970. quiz 292-283. [DOI] [PubMed] [Google Scholar]
- Krasinski R, Tchorzewski H. Hyaluronan-mediated regulation of inflammation. Postepy Hig Med Dosw (Online) 2007;61:683–689. [PubMed] [Google Scholar]
- Kryworuchko M, Diaz-Mitoma F, Kumar A. Interferon-gamma inhibits CD44-hyaluronan interactions in normal human B lymphocytes. Exp Cell Res. 1999;250:241–252. doi: 10.1006/excr.1999.4524. [DOI] [PubMed] [Google Scholar]
- Lafferty EI, Qureshi ST, Schnare M. The role of toll-like receptors in acute and chronic lung inflammation. J Inflamm (Lond) 2010;7:57. doi: 10.1186/1476-9255-7-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathrop WF, Carmichael EP, Myles DG, Primakoff P. cDNA cloning reveals the molecular structure of a sperm surface protein, PH-20, involved in sperm-egg adhesion and the wide distribution of its gene among mammals. J Cell Biol. 1990;111:2939–2949. doi: 10.1083/jcb.111.6.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer ME, Erzurum SC, Mukhopadhyay D, Vasanji A, Drazba J, Wang A, Fulop C, Hascall VC. Differentiated murine airway epithelial cells synthesize a leukocyte-adhesive hyaluronan matrix in response to endoplasmic reticulum stress. J Biol Chem. 2008;283:26283–26296. doi: 10.1074/jbc.M803350200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer ME, Mukhopadhyay D, Fulop C, De La Motte CA, Majors AK, Hascall VC. Primary murine airway smooth muscle cells exposed to poly(I,C) or tunicamycin synthesize a leukocyte-adhesive hyaluronan matrix. J Biol Chem. 2009;284:5299–5312. doi: 10.1074/jbc.M807965200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent T. The biology of hyaluronan. Introduction. Ciba Found Symp. 1989;143:1–20. [PubMed] [Google Scholar]
- Laurent TC, Fraser JR. Hyaluronan. FASEB J. 1992;6:2397–2404. [PubMed] [Google Scholar]
- Lee JY, Spicer AP. Hyaluronan: a multifunctional, megaDalton, stealth molecule. Curr Opin Cell Biol. 2000;12:581–586. doi: 10.1016/s0955-0674(00)00135-6. [DOI] [PubMed] [Google Scholar]
- Lennon FE, Singleton PA. Role of hyaluronan and hyaluronan-binding proteins in lung pathobiology. Am J Physiol Lung Cell Mol Physiol. 2011;301:L137–147. doi: 10.1152/ajplung.00071.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepperdinger G, Mullegger J, Kreil G. Hyal2--less active, but more versatile? Matrix Biol. 2001;20:509–514. doi: 10.1016/s0945-053x(01)00170-6. [DOI] [PubMed] [Google Scholar]
- Lepperdinger G, Strobl B, Kreil G. HYAL2, a human gene expressed in many cells, encodes a lysosomal hyaluronidase with a novel type of specificity. J Biol Chem. 1998;273:22466–22470. doi: 10.1074/jbc.273.35.22466. [DOI] [PubMed] [Google Scholar]
- Lesley J, Hascall VC, Tammi M, Hyman R. Hyaluronan binding by cell surface CD44. J Biol Chem. 2000;275:26967–26975. doi: 10.1074/jbc.M002527200. [DOI] [PubMed] [Google Scholar]
- Lesley J, Hyman R, Kincade PW. CD44 and its interaction with extracellular matrix. Adv Immunol. 1993;54:271–335. doi: 10.1016/s0065-2776(08)60537-4. [DOI] [PubMed] [Google Scholar]
- Li L, Yang L, Tang H, Jin R. Role of CD44 on airway inflammatory response in rats with asthma. Zhongguo Dang Dai Er Ke Za Zhi. 2009;11:142–145. [PubMed] [Google Scholar]
- Li Z, Potts-Kant EN, Garantziotis S, Foster WM, Hollingsworth JW. Hyaluronan signaling during ozone-induced lung injury requires TLR4, MyD88, and TIRAP. PLoS One. 2011;6:e27137. doi: 10.1371/journal.pone.0027137. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- Liang J, Jiang D, Griffith J, Yu S, Fan J, Zhao X, Bucala R, Noble PW. CD44 is a negative regulator of acute pulmonary inflammation and lipopolysaccharide-TLR signaling in mouse macrophages. J Immunol. 2007;178:2469–2475. doi: 10.4049/jimmunol.178.4.2469. [DOI] [PubMed] [Google Scholar]
- Liang J, Jiang D, Jung Y, Xie T, Ingram J, Church T, Degan S, Leonard M, Kraft M, Noble PW. Role of hyaluronan and hyaluronan-binding proteins in human asthma. J Allergy Clin Immunol. 2011;128:403–411. e403. doi: 10.1016/j.jaci.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokeshwar VB, Selzer MG. Differences in hyaluronic acid-mediated functions and signaling in arterial, microvessel, and vein-derived human endothelial cells. J Biol Chem. 2000;275:27641–27649. doi: 10.1074/jbc.M003084200. [DOI] [PubMed] [Google Scholar]
- Lowther DA, Rogers HJ. Biosynthesis of hyaluronate. Nature. 1955;175:435. doi: 10.1038/175435a0. [DOI] [PubMed] [Google Scholar]
- Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol. 2011:3. doi: 10.1101/cshperspect.a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeshima N, Poon GF, Dosanjh M, Felberg J, Lee SS, Cross JL, Birkenhead D, Johnson P. Hyaluronan binding identifies the most proliferative activated and memory T cells. Eur J Immunol. 2011;41:1108–1119. doi: 10.1002/eji.201040870. [DOI] [PubMed] [Google Scholar]
- Maharjan AS, Pilling D, Gomer RH. High and low molecular weight hyaluronic acid differentially regulate human fibrocyte differentiation. PLoS One. 2011;6:e26078. doi: 10.1371/journal.pone.0026078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier R, Wisniewski HG, Vilcek J, Lotz M. TSG-6 expression in human articular chondrocytes. Possible implications in joint inflammation and cartilage degradation. Arthritis Rheum. 1996;39:552–559. doi: 10.1002/art.1780390403. [DOI] [PubMed] [Google Scholar]
- Matou-Nasri S, Gaffney J, Kumar S, Slevin M. Oligosaccharides of hyaluronan induce angiogenesis through distinct CD44 and RHAMM-mediated signalling pathways involving Cdc2 and gamma-adducin. Int J Oncol. 2009;35:761–773. doi: 10.3892/ijo_00000389. [DOI] [PubMed] [Google Scholar]
- Mccutcheon JC, Hart SP, Canning M, Ross K, Humphries MJ, Dransfield I. Regulation of macrophage phagocytosis of apoptotic neutrophils by adhesion to fibronectin. J Leukoc Biol. 1998;64:600–607. doi: 10.1002/jlb.64.5.600. [DOI] [PubMed] [Google Scholar]
- Mckee CM, Penno MB, Cowman M, Burdick MD, Strieter RM, Bao C, Noble PW. Hyaluronan (HA) fragments induce chemokine gene expression in alveolar macrophages. The role of HA size and CD44. J Clin Invest. 1996;98:2403–2413. doi: 10.1172/JCI119054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer K, Palmer JW. The polysaccharide of the Vitreous humor. J Biol Chem. 1934:629–634. [Google Scholar]
- Miki Y, Teramura T, Tomiyama T, Onodera Y, Matsuoka T, Fukuda K, Hamanishi C. Hyaluronan reversed proteoglycan synthesis inhibited by mechanical stress: possible involvement of antioxidant effect. Inflamm Res. 2010;59:471–477. doi: 10.1007/s00011-009-0147-y. [DOI] [PubMed] [Google Scholar]
- Milner CM, Day AJ. TSG-6: a multifunctional protein associated with inflammation. J Cell Sci. 2003;116:1863–1873. doi: 10.1242/jcs.00407. [DOI] [PubMed] [Google Scholar]
- Monzon ME, Fregien N, Schmid N, Falcon NS, Campos M, Casalino-Matsuda SM, Forteza RM. Reactive oxygen species and hyaluronidase 2 regulate airway epithelial hyaluronan fragmentation. J Biol Chem. 2010;285:26126–26134. doi: 10.1074/jbc.M110.135194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay D, Hascall VC, Day AJ, Salustri A, Fulop C. Two distinct populations of tumor necrosis factor-stimulated gene-6 protein in the extracellular matrix of expanded mouse cumulus cell-oocyte complexes. Arch Biochem Biophys. 2001;394:173–181. doi: 10.1006/abbi.2001.2552. [DOI] [PubMed] [Google Scholar]
- Murdock BJ, Falkowski NR, Shreiner AB, Sadighi Akha AA, Mcdonald RA, White ES, Toews GB, Huffnagle GB. Interleukin-17 drives pulmonary eosinophilia following repeated exposure to Aspergillus fumigatus conidia. Infect Immun. 2012;80:1424–1436. doi: 10.1128/IAI.05529-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadkarni PP, Kulkarni GS, Cerreta JM, Ma S, Cantor JO. Dichotomous effect of aerosolized hyaluronan in a hamster model of endotoxin-induced lung injury. Exp Lung Res. 2005;31:807–818. doi: 10.1080/01902140600574942. [DOI] [PubMed] [Google Scholar]
- Nagy JI, Hacking J, Frankenstein UN, Turley EA. Requirement of the hyaluronan receptor RHAMM in neurite extension and motility as demonstrated in primary neurons and neuronal cell lines. J Neurosci. 1995;15:241–252. doi: 10.1523/JNEUROSCI.15-01-00241.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naor D, Sionov RV, Ish-Shalom D. CD44: structure, function, and association with the malignant process. Adv Cancer Res. 1997;71:241–319. doi: 10.1016/s0065-230x(08)60101-3. [DOI] [PubMed] [Google Scholar]
- Nassenstein C, Braun A, Nockher WA, Renz H. Neurotrophin effects on eosinophils in allergic inflammation. Curr Allergy Asthma Rep. 2005;5:204–211. doi: 10.1007/s11882-005-0039-3. [DOI] [PubMed] [Google Scholar]
- Noble PW. Hyaluronan and its catabolic products in tissue injury and repair. Matrix Biol. 2002;21:25–29. doi: 10.1016/s0945-053x(01)00184-6. [DOI] [PubMed] [Google Scholar]
- Noble PW, Jiang D. Matrix regulation of lung injury, inflammation, and repair: the role of innate immunity. Proc Am Thorac Soc. 2006;3:401–404. doi: 10.1513/pats.200604-097AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble PW, Lake FR, Henson PM, Riches DW. Hyaluronate activation of CD44 induces insulin-like growth factor-1 expression by a tumor necrosis factor-alpha-dependent mechanism in murine macrophages. J Clin Invest. 1993;91:2368–2377. doi: 10.1172/JCI116469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble PW, Mckee CM, Cowman M, Shin HS. Hyaluronan fragments activate an NF-kappa B/I-kappa B alpha autoregulatory loop in murine macrophages. J Exp Med. 1996;183:2373–2378. doi: 10.1084/jem.183.5.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’driscoll BR, Hopkinson LC, Denning DW. Mold sensitization is common amongst patients with severe asthma requiring multiple hospital admissions. BMC Pulm Med. 2005;5:4. doi: 10.1186/1471-2466-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’regan M, Martini I, Crescenzi F, De Luca C, Lansing M. Molecular mechanisms and genetics of hyaluronan biosynthesis. Int J Biol Macromol. 1994;16:283–286. doi: 10.1016/0141-8130(94)90056-6. [DOI] [PubMed] [Google Scholar]
- Oharazawa H, Ibaraki N, Lin LR, Reddy VN. The effects of extracellular matrix on cell attachment, proliferation and migration in a human lens epithelial cell line. Exp Eye Res. 1999;69:603–610. doi: 10.1006/exer.1999.0723. [DOI] [PubMed] [Google Scholar]
- Ohkawara Y, Tamura G, Iwasaki T, Tanaka A, Kikuchi T, Shirato K. Activation and transforming growth factor-beta production in eosinophils by hyaluronan. Am J Respir Cell Mol Biol. 2000;23:444–451. doi: 10.1165/ajrcmb.23.4.3875. [DOI] [PubMed] [Google Scholar]
- Olczyk P, Mencner L, Komosinska-Vassev K. The role of the extracellular matrix components in cutaneous wound healing. Biomed Res Int. 2014;2014:747584. doi: 10.1155/2014/747584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paez-Pereda M, Kuchenbauer F, Arzt E, Stalla GK. Regulation of pituitary hormones and cell proliferation by components of the extracellular matrix. Braz J Med Biol Res. 2005;38:1487–1494. doi: 10.1590/s0100-879x2005001000005. [DOI] [PubMed] [Google Scholar]
- Pandey S, Hoselton SA, Schuh JM. The impact of Aspergillus fumigatus viability and sensitization to its allergens on the murine allergic asthma phenotype. Biomed Res Int. 2013;2013:619614. doi: 10.1155/2013/619614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papakonstantinou E, Karakiulakis G. The ‘sweet’ and ‘bitter’ involvement of glycosaminoglycans in lung diseases: pharmacotherapeutic relevance. Br J Pharmacol. 2009;157:1111–1127. doi: 10.1111/j.1476-5381.2009.00279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papakonstantinou E, Klagas I, Karakiulakis G, Hostettler K, S’ng CT, Kotoula V, Savic S, Tamm M, Roth M. Steroids and beta2-agonists regulate hyaluronan metabolism in asthmatic airway smooth muscle cells. Am J Respir Cell Mol Biol. 2012;47:759–767. doi: 10.1165/rcmb.2012-0101OC. [DOI] [PubMed] [Google Scholar]
- Papakonstantinou E, Kouri FM, Karakiulakis G, Klagas I, Eickelberg O. Increased hyaluronic acid content in idiopathic pulmonary arterial hypertension. Eur Respir J. 2008;32:1504–1512. doi: 10.1183/09031936.00159507. [DOI] [PubMed] [Google Scholar]
- Peck D, Isacke CM. Hyaluronan-dependent cell migration can be blocked by a CD44 cytoplasmic domain peptide containing a phosphoserine at position 325. J Cell Sci. 1998;111 ( Pt 11):1595–1601. doi: 10.1242/jcs.111.11.1595. [DOI] [PubMed] [Google Scholar]
- Petrey AC, De La Motte CA. Hyaluronan, a Crucial Regulator of Inflammation. Front Immunol. 2014a:5. doi: 10.3389/fimmu.2014.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrey AC, De La Motte CA. Hyaluronan, a crucial regulator of inflammation. Front Immunol. 2014b;5:101. doi: 10.3389/fimmu.2014.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrigni G, Allegra L. Aerosolised hyaluronic acid prevents exercise-induced bronchoconstriction, suggesting novel hypotheses on the correction of matrix defects in asthma. Pulm Pharmacol Ther. 2006;19:166–171. doi: 10.1016/j.pupt.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Pilarski LM, Masellis-Smith A, Belch AR, Yang B, Savani RC, Turley EA. RHAMM, a receptor for hyaluronan-mediated motility, on normal human lymphocytes, thymocytes and malignant B cells: a mediator in B cell malignancy? Leuk Lymphoma. 1994;14:363–374. doi: 10.3109/10428199409049691. [DOI] [PubMed] [Google Scholar]
- Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol. 2003;4:33–45. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- Prevo R, Banerji S, Ferguson DJ, Clasper S, Jackson DG. Mouse LYVE-1 is an endocytic receptor for hyaluronan in lymphatic endothelium. J Biol Chem. 2001;276:19420–19430. doi: 10.1074/jbc.M011004200. [DOI] [PubMed] [Google Scholar]
- Prosdocimi M, Bevilacqua C. Exogenous hyaluronic acid and wound healing: an updated vision. Panminerva Med. 2012;54:129–135. [PubMed] [Google Scholar]
- Pure E, Cuff CA. A crucial role for CD44 in inflammation. Trends Mol Med. 2001;7:213–221. doi: 10.1016/s1471-4914(01)01963-3. [DOI] [PubMed] [Google Scholar]
- Rafi A, Nagarkatti M, Nagarkatti PS. Hyaluronate-CD44 interactions can induce murine B-cell activation. Blood. 1997;89:2901–2908. [PubMed] [Google Scholar]
- Reitinger S, Laschober GT, Fehrer C, Greiderer B, Lepperdinger G. Mouse testicular hyaluronidase-like proteins SPAM1 and HYAL5 but not HYALP1 degrade hyaluronan. Biochem J. 2007;401:79–85. doi: 10.1042/BJ20060598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockey DC, Chung JJ, Mckee CM, Noble PW. Stimulation of inducible nitric oxide synthase in rat liver by hyaluronan fragments. Hepatology. 1998;27:86–92. doi: 10.1002/hep.510270115. [DOI] [PubMed] [Google Scholar]
- Rothenberg ME. CD44--a sticky target for asthma. J Clin Invest. 2003;111:1460–1462. doi: 10.1172/JCI18392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffell B, Johnson P. Hyaluronan induces cell death in activated T cells through CD44. J Immunol. 2008;181:7044–7054. doi: 10.4049/jimmunol.181.10.7044. [DOI] [PubMed] [Google Scholar]
- Sahu S, Lynn WS. Hyaluronic acid in the pulmonary secretions of patients with asthma. Biochem J. 1978;173:565–568. doi: 10.1042/bj1730565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel SK, Hurta RA, Spearman MA, Wright JA, Turley EA, Greenberg AH. TGF-beta 1 stimulation of cell locomotion utilizes the hyaluronan receptor RHAMM and hyaluronan. J Cell Biol. 1993;123:749–758. doi: 10.1083/jcb.123.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savani RC, Cao G, Pooler PM, Zaman A, Zhou Z, Delisser HM. Differential involvement of the hyaluronan (HA) receptors CD44 and receptor for HA-mediated motility in endothelial cell function and angiogenesis. J Biol Chem. 2001;276:36770–36778. doi: 10.1074/jbc.M102273200. [DOI] [PubMed] [Google Scholar]
- Savani RC, Hou G, Liu P, Wang C, Simons E, Grimm PC, Stern R, Greenberg AH, Delisser HM, Khalil N. A role for hyaluronan in macrophage accumulation and collagen deposition after bleomycin-induced lung injury. Am J Respir Cell Mol Biol. 2000;23:475–484. doi: 10.1165/ajrcmb.23.4.3944. [DOI] [PubMed] [Google Scholar]
- Schmekel B, Venge P. Markers for eosinophils and T-lymphocytes as predictors of late asthmatic response. Allergy. 1993;48:94–97. doi: 10.1111/j.1398-9995.1993.tb04708.x. discussion 110–111. [DOI] [PubMed] [Google Scholar]
- Scuri M, Abraham WM. Hyaluronan blocks human neutrophil elastase (HNE)-induced airway responses in sheep. Pulm Pharmacol Ther. 2003;16:335–340. doi: 10.1016/S1094-5539(03)00089-0. [DOI] [PubMed] [Google Scholar]
- Shen N, Gong T, Wang JD, Meng FL, Qiao L, Yang RL, Xue B, Pan FY, Zhou XJ, Chen HQ, Ning W, Li CJ. Cigarette smoke-induced pulmonary inflammatory responses are mediated by EGR-1/GGPPS/MAPK signaling. Am J Pathol. 2011;178:110–118. doi: 10.1016/j.ajpath.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Leng L, Wang T, Wang W, Du X, Li J, Mcdonald C, Chen Z, Murphy JW, Lolis E, Noble P, Knudson W, Bucala R. CD44 is the signaling component of the macrophage migration inhibitory factor-CD74 receptor complex. Immunity. 2006;25:595–606. doi: 10.1016/j.immuni.2006.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shreiner AB, Murdock BJ, Sadighi Akha AA, Falkowski NR, Christensen PJ, White ES, Hogaboam CM, Huffnagle GB. Repeated exposure to Aspergillus fumigatus conidia results in CD4+ T cell-dependent and -independent pulmonary arterial remodeling in a mixed Th1/Th2/Th17 microenvironment that requires interleukin-4 (IL-4) and IL-10. Infect Immun. 2012;80:388–397. doi: 10.1128/IAI.05530-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton PA, Lennon FE. Acute Lung Injury Regulation by Hyaluronan. J Allergy Ther. 2011;(Suppl 4) doi: 10.4172/2155-6121.S4-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton PA, Mirzapoiazova T, Guo Y, Sammani S, Mambetsariev N, Lennon FE, Moreno-Vinasco L, Garcia JG. High-molecular-weight hyaluronan is a novel inhibitor of pulmonary vascular leakiness. Am J Physiol Lung Cell Mol Physiol. 2010;299:L639–651. doi: 10.1152/ajplung.00405.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelton TP, Zeng C, Nocks A, Stamenkovic I. Glycosylation provides both stimulatory and inhibitory effects on cell surface and soluble CD44 binding to hyaluronan. J Cell Biol. 1998;140:431–446. doi: 10.1083/jcb.140.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MM, Ghosh P. The effects of some polysulphated polysaccharides on hyaluronate (HA) synthesis by human synovial fibroblasts. Agents Actions Suppl. 1986;18:55–62. doi: 10.1007/978-3-0348-7684-1_8. [DOI] [PubMed] [Google Scholar]
- Smith MM, Ghosh P. The synthesis of hyaluronic acid by human synovial fibroblasts is influenced by the nature of the hyaluronate in the extracellular environment. Rheumatol Int. 1987;7:113–122. doi: 10.1007/BF00270463. [DOI] [PubMed] [Google Scholar]
- Soderberg M, Bjermer L, Hallgren R, Lundgren R. Increased hyaluronan (hyaluronic acid) levels in bronchoalveolar lavage fluid after histamine inhalation. Int Arch Allergy Appl Immunol. 1989;88:373–376. doi: 10.1159/000234719. [DOI] [PubMed] [Google Scholar]
- Sokolowska M, Chen LY, Eberlein M, Martinez-Anton A, Liu Y, Alsaaty S, Qi HY, Logun C, Horton M, Shelhamer JH. Low molecular weight hyaluronan activates cytosolic phospholipase A2alpha and eicosanoid production in monocytes and macrophages. J Biol Chem. 2014;289:4470–4488. doi: 10.1074/jbc.M113.515106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solis MA, Chen YH, Wong TY, Bittencourt VZ, Lin YC, Huang LLH. Hyaluronan Regulates Cell Behavior: A Potential Niche Matrix for Stem Cells. Biochemistry Research International. 2012;2012:11. doi: 10.1155/2012/346972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltes L, Mendichi R, Kogan G, Schiller J, Stankovska M, Arnhold J. Degradative action of reactive oxygen species on hyaluronan. Biomacromolecules. 2006;7:659–668. doi: 10.1021/bm050867v. [DOI] [PubMed] [Google Scholar]
- Stern R. Devising a pathway for hyaluronan catabolism: are we there yet? Glycobiology. 2003;13:105R–115R. doi: 10.1093/glycob/cwg112. [DOI] [PubMed] [Google Scholar]
- Stern R. Hyaluronan catabolism: a new metabolic pathway. Eur J Cell Biol. 2004;83:317–325. doi: 10.1078/0171-9335-00392. [DOI] [PubMed] [Google Scholar]
- Stern R, Asari AA, Sugahara KN. Hyaluronan fragments: an information-rich system. Eur J Cell Biol. 2006;85:699–715. doi: 10.1016/j.ejcb.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Sugahara K, Schwartz NB, Dorfman A. Biosynthesis of hyaluronic acid by Streptococcus. J Biol Chem. 1979;254:6252–6261. [PubMed] [Google Scholar]
- Swaidani S, Cheng G, Lauer ME, Sharma M, Mikecz K, Hascall VC, Aronica MA. TSG-6 protein is crucial for the development of pulmonary hyaluronan deposition, eosinophilia, and airway hyperresponsiveness in a murine model of asthma. J Biol Chem. 2013;288:412–422. doi: 10.1074/jbc.M112.389874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Li L, Kamiryo M, Asteriou T, Moustakas A, Yamashita H, Heldin P. Hyaluronan fragments induce endothelial cell differentiation in a CD44- and CXCL1/GRO1-dependent manner. J Biol Chem. 2005;280:24195–24204. doi: 10.1074/jbc.M411913200. [DOI] [PubMed] [Google Scholar]
- Taylor KR, Trowbridge JM, Rudisill JA, Termeer CC, Simon JC, Gallo RL. Hyaluronan fragments stimulate endothelial recognition of injury through TLR4. J Biol Chem. 2004;279:17079–17084. doi: 10.1074/jbc.M310859200. [DOI] [PubMed] [Google Scholar]
- Taylor KR, Yamasaki K, Radek KA, Di Nardo A, Goodarzi H, Golenbock D, Beutler B, Gallo RL. Recognition of hyaluronan released in sterile injury involves a unique receptor complex dependent on Toll-like receptor 4, CD44, and MD-2. J Biol Chem. 2007;282:18265–18275. doi: 10.1074/jbc.M606352200. [DOI] [PubMed] [Google Scholar]
- Teriete P, Banerji S, Noble M, Blundell CD, Wright AJ, Pickford AR, Lowe E, Mahoney DJ, Tammi MI, Kahmann JD, Campbell ID, Day AJ, Jackson DG. Structure of the regulatory hyaluronan binding domain in the inflammatory leukocyte homing receptor CD44. Mol Cell. 2004;13:483–496. doi: 10.1016/s1097-2765(04)00080-2. [DOI] [PubMed] [Google Scholar]
- Termeer C, Benedix F, Sleeman J, Fieber C, Voith U, Ahrens T, Miyake K, Freudenberg M, Galanos C, Simon JC. Oligosaccharides of Hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med. 2002;195:99–111. doi: 10.1084/jem.20001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Termeer CC, Hennies J, Voith U, Ahrens T, Weiss JM, Prehm P, Simon JC. Oligosaccharides of hyaluronan are potent activators of dendritic cells. J Immunol. 2000;165:1863–1870. doi: 10.4049/jimmunol.165.4.1863. [DOI] [PubMed] [Google Scholar]
- Thannickal VJ, Lee DY, White ES, Cui Z, Larios JM, Chacon R, Horowitz JC, Day RM, Thomas PE. Myofibroblast differentiation by transforming growth factor-beta1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. J Biol Chem. 2003;278:12384–12389. doi: 10.1074/jbc.M208544200. [DOI] [PubMed] [Google Scholar]
- Todorova L, Bjermer L, Miller-Larsson A, Westergren-Thorsson G. Relationship between matrix production by bronchial fibroblasts and lung function and AHR in asthma. Respir Med. 2010;104:1799–1808. doi: 10.1016/j.rmed.2010.06.015. [DOI] [PubMed] [Google Scholar]
- Tolg C, Hamilton SR, Nakrieko KA, Kooshesh F, Walton P, Mccarthy JB, Bissell MJ, Turley EA. Rhamm−/− fibroblasts are defective in CD44-mediated ERK1,2 motogenic signaling, leading to defective skin wound repair. J Cell Biol. 2006;175:1017–1028. doi: 10.1083/jcb.200511027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolg C, Telmer P, Turley E. Specific sizes of hyaluronan oligosaccharides stimulate fibroblast migration and excisional wound repair. PLoS One. 2014;9:e88479. doi: 10.1371/journal.pone.0088479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toole BP. Hyaluronan and its binding proteins, the hyaladherins. Curr Opin Cell Biol. 1990;2:839–844. doi: 10.1016/0955-0674(90)90081-o. [DOI] [PubMed] [Google Scholar]
- Toole BP. Hyaluronan is not just a goo! J Clin Invest. 2000;106:335–336. doi: 10.1172/JCI10706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. 2004;4:528–539. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- Tsujimura S, Saito K, Kohno K, Tanaka Y. Fragmented hyaluronan induces transcriptional up-regulation of the multidrug resistance-1 gene in CD4+ T cells. J Biol Chem. 2006;281:38089–38097. doi: 10.1074/jbc.M601030200. [DOI] [PubMed] [Google Scholar]
- Turley EA. Purification of a hyaluronate-binding protein fraction that modifies cell social behavior. Biochem Biophys Res Commun. 1982;108:1016–1024. doi: 10.1016/0006-291x(82)92101-5. [DOI] [PubMed] [Google Scholar]
- Turley EA, Bowman P, Kytryk MA. Effects of hyaluronate and hyaluronate binding proteins on cell motile and contact behaviour. J Cell Sci. 1985;78:133–145. doi: 10.1242/jcs.78.1.133. [DOI] [PubMed] [Google Scholar]
- Turley EA, Moore D, Hayden LJ. Characterization of hyaluronate binding proteins isolated from 3T3 and murine sarcoma virus transformed 3T3 cells. Biochemistry. 1987;26:2997–3005. doi: 10.1021/bi00385a007. [DOI] [PubMed] [Google Scholar]
- Turley EA, Noble PW, Bourguignon LY. Signaling properties of hyaluronan receptors. J Biol Chem. 2002;277:4589–4592. doi: 10.1074/jbc.R100038200. [DOI] [PubMed] [Google Scholar]
- Umetsu DT, Dekruyff RH. The regulation of allergy and asthma. Immunol Rev. 2006;212:238–255. doi: 10.1111/j.0105-2896.2006.00413.x. [DOI] [PubMed] [Google Scholar]
- Underhill CB. The interaction of hyaluronate with the cell surface: the hyaluronate receptor and the core protein. Ciba Found Symp. 1989;143:87–99. doi: 10.1002/9780470513774.ch6. discussion 100–106, 281-105. [DOI] [PubMed] [Google Scholar]
- Vasconcellos FC, Swiston AJ, Beppu MM, Cohen RE, Rubner MF. Bioactive polyelectrolyte multilayers: hyaluronic acid mediated B lymphocyte adhesion. Biomacromolecules. 2010;11:2407–2414. doi: 10.1021/bm100570r. [DOI] [PubMed] [Google Scholar]
- Venge P. The eosinophil and airway remodelling in asthma. Clin Respir J. 2010;4(Suppl 1):15–19. doi: 10.1111/j.1752-699X.2010.00192.x. [DOI] [PubMed] [Google Scholar]
- Vigetti D, Deleonibus S, Moretto P, Karousou E, Viola M, Bartolini B, Hascall VC, Tammi M, De Luca G, Passi A. Role of UDP-N-acetylglucosamine (GlcNAc) and O-GlcNAcylation of hyaluronan synthase 2 in the control of chondroitin sulfate and hyaluronan synthesis. J Biol Chem. 2012;287:35544–35555. doi: 10.1074/jbc.M112.402347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigetti D, Karousou E, Viola M, Deleonibus S, De Luca G, Passi A. Hyaluronan: biosynthesis and signaling. Biochim Biophys Acta. 2014;1840:2452–2459. doi: 10.1016/j.bbagen.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Vistejnova L, Safrankova B, Nesporova K, Slavkovsky R, Hermannova M, Hosek P, Velebny V, Kubala L. Low molecular weight hyaluronan mediated CD44 dependent induction of IL-6 and chemokines in human dermal fibroblasts potentiates innate immune response. Cytokine. 2014 doi: 10.1016/j.cyto.2014.07.006. [DOI] [PubMed] [Google Scholar]
- Wang YZ, Cao ML, Liu YW, He YQ, Yang CX, Gao F. CD44 mediates oligosaccharides of hyaluronan-induced proliferation, tube formation and signal transduction in endothelial cells. Exp Biol Med (Maywood) 2011;236:84–90. doi: 10.1258/ebm.2010.010206. [DOI] [PubMed] [Google Scholar]
- Wardlaw AJ, Brightling C, Green R, Woltmann G, Pavord I. Eosinophils in asthma and other allergic diseases. Br Med Bull. 2000;56:985–1003. doi: 10.1258/0007142001903490. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Yamaguchi Y. Molecular identification of a putative human hyaluronan synthase. J Biol Chem. 1996;271:22945–22948. doi: 10.1074/jbc.271.38.22945. [DOI] [PubMed] [Google Scholar]
- Weidle UH, Maisel D, Klostermann S, Weiss EH, Schmitt M. Differential splicing generates new transmembrane receptor and extracellular matrix-related targets for antibody-based therapy of cancer. Cancer Genomics Proteomics. 2011;8:211–226. [PubMed] [Google Scholar]
- Weigel PH, Deangelis PL. Hyaluronan synthases: a decade-plus of novel glycosyltransferases. J Biol Chem. 2007;282:36777–36781. doi: 10.1074/jbc.R700036200. [DOI] [PubMed] [Google Scholar]
- Winkler CW, Foster SC, Matsumoto SG, Preston MA, Xing R, Bebo BF, Banine F, Berny-Lang MA, Itakura A, Mccarty OJ, Sherman LS. Hyaluronan anchored to activated CD44 on central nervous system vascular endothelial cells promotes lymphocyte extravasation in experimental autoimmune encephalomyelitis. J Biol Chem. 2012;287:33237–33251. doi: 10.1074/jbc.M112.356287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski HG, Snitkin ES, Mindrescu C, Sweet MH, Vilcek J. TSG-6 protein binding to glycosaminoglycans: formation of stable complexes with hyaluronan and binding to chondroitin sulfates. J Biol Chem. 2005;280:14476–14484. doi: 10.1074/jbc.M411734200. [DOI] [PubMed] [Google Scholar]
- Wisniewski HG, Vilcek J. TSG-6: an IL-1/TNF-inducible protein with anti-inflammatory activity. Cytokine Growth Factor Rev. 1997;8:143–156. doi: 10.1016/s1359-6101(97)00008-7. [DOI] [PubMed] [Google Scholar]
- Wu M, Du Y, Liu Y, He Y, Yang C, Wang W, Gao F. Low molecular weight hyaluronan induces lymphangiogenesis through LYVE-1-mediated signaling pathways. PLoS One. 2014;9:e92857. doi: 10.1371/journal.pone.0092857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamawaki H, Hirohata S, Miyoshi T, Takahashi K, Ogawa H, Shinohata R, Demircan K, Kusachi S, Yamamoto K, Ninomiya Y. Hyaluronan receptors involved in cytokine induction in monocytes. Glycobiology. 2009;19:83–92. doi: 10.1093/glycob/cwn109. [DOI] [PubMed] [Google Scholar]
- Yang B, Yang BL, Savani RC, Turley EA. Identification of a common hyaluronan binding motif in the hyaluronan binding proteins RHAMM, CD44 and link protein. EMBO J. 1994;13:286–296. doi: 10.1002/j.1460-2075.1994.tb06261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Cao M, Liu H, He Y, Xu J, Du Y, Liu Y, Wang W, Cui L, Hu J, Gao F. The high and low molecular weight forms of hyaluronan have distinct effects on CD44 clustering. J Biol Chem. 2012;287:43094–43107. doi: 10.1074/jbc.M112.349209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L, Mora R, Akhayani N, Haudenschild CC, Liau G. Growth factor and cytokine-regulated hyaluronan-binding protein TSG-6 is localized to the injury-induced rat neointima and confers enhanced growth in vascular smooth muscle cells. Circ Res. 1997;81:289–296. doi: 10.1161/01.res.81.3.289. [DOI] [PubMed] [Google Scholar]
- Zaman A, Cui Z, Foley JP, Zhao H, Grimm PC, Delisser HM, Savani RC. Expression and role of the hyaluronan receptor RHAMM in inflammation after bleomycin injury. Am J Respir Cell Mol Biol. 2005;33:447–454. doi: 10.1165/rcmb.2004-0333OC. [DOI] [PMC free article] [PubMed] [Google Scholar]


