Abstract
This study examined whether chronic kidney disease (CKD) is associated with recurrent falls in older adults in nursing homes (NHs). We used data abstracted over a six month period from 510 NH residents with a history of falls. Thirty-five percent of the NH residents had CKD. In adjusted analyses, the incidence of recurrent falls was similar in those with and without CKD [fall rate ratio (FRR) 1.00, 95% confidence interval (CI) 0.97-1.02]. Orthostatic hypotension (FRR 1.52, 95% CI 1.12-2.05), history of falls during the prior six month period (FRR 1.25, 95% CI 1.05-1.49), cane or walker use (FRR 1.64, 95% CI 1.16-2.33), and ambulatory dysfunction (FRR 1.47, 95% CI 1.23-1.75) were independently associated with increased fall rate. CKD was not an important predictor of falls in this cohort of nursing home residents with prior falls. Instead, traditional fall risk factors were much more strongly associated with recurrent falls.
Keywords: skilled nursing facility, renal insufficiency, frail elderly
Introduction
Nursing home (NH) residents who fall have increased risks of fractures, disability, reduced quality of life, and death.1 Whether injurious or not, NH falls often occur in residents who possess multiple traditional fall risk factors (e.g., cognitive impairment, orthostatic hypotension, low bone mass, visual impairment, and impaired mobility) which are the targets of multifactorial fall prevention strategies.2 Despite fall prevention efforts, NH falls continue to be a significant problem, and the prevalence of kidney disease among NH residents has increased. One of every three NH residents have chronic kidney disease (CKD),3-5 but it is not known whether the sizeable proportion of NH residents with CKD are at increased risk for falls compared to those without CKD.
Elderly community dwellers with either CKD or end stage renal disease (ESRD) have higher fall rates than elderly community dwellers without CKD,6-8 although this association has not been examined in the NH setting. CKD may be associated with falls in NH residents because many fall risk factors such as anemia, vitamin D deficiency, cognitive impairment, and impaired clearance of psychoactive medications are common in patients with CKD.9-14 Also, diabetes and hypertension—the most common etiologies of CKD in older adults--and downstream complications of these conditions (e.g., autonomic insufficiency, visual impairment, and stroke) are themselves associated with falls.15
Understanding whether CKD is independently associated with falls in NHs could help identify NH residents who maintain a higher risk for falls despite management of traditional fall risk factors, and in whom management of CKD-specific fall risk factors (e.g. need for activated vitamin D, management of anemia, addressing blood pressure fluctuations) may be particularly important for fall prevention. We examined the association between CKD and recurrent falls in a population of NH residents who had fallen at least once. According to 2012 Kidney Disease Improving Global Outcomes (KDIGO) guidelines, CKD can be diagnosed in patients with estimated glomerular filtration rate (eGFR) <60 irrespective of presence of markers of kidney damage. An eGFR < 60 indicates a substantial reduction in kidney function.16 As CKD is present when eGFR is low; our hypotheses were that lower eGFR values are associated with higher fall rates and that eGFR is independently associated with fall rates in NH fallers after accounting for traditional fall risk factors.
Materials and Methods
Study Design and Setting
We conducted a retrospective cohort study using NH resident data obtained as part of a larger ongoing randomized controlled trial; CONNECT for Quality, which examines different staff education approaches to fall prevention.17 Although there was no direct resident involvement in the CONNECT for Quality intervention, resident characteristics and fall events were collected by chart review in two six-month periods before and after the intervention. For this observational sub-study, NH resident data were available from both Veterans Affairs (VA) community living centers (CLC) (four CLCs located in North Carolina or Virginia) that participated in the pilot study (2009-2011) and community NHs (located in North Carolina) that participated in the first year of CONNECT for Quality (2011-2012). Institutional Review Boards at Duke University and the Durham Veterans Affairs Medical Center approved the research protocol with waiver of consent.
Participants
By December 2012, 540 unique NH residents had been randomly selected for chart review in the parent study. For each chart review, eligible residents were over age 50 years, sustained at least one fall within the six month observation period and remained in the facility for at least three days after the initial fall. For this sub-study, we excluded residents who did not have a serum creatinine drawn as part of routine clinical care at any point within 6 months before or after the first fall (n = 19) and residents who were under age 50 or were in the facility less than three days (n=11), leaving 510 NH residents for this analysis.
Measures
Data sources for the parent study included NH medical records, the Minimum Data Set (MDS) (a national registry of NH residents), medication administration records, facility fall logs, and incident reports. Trained study staff abstracted data for the parent study from these sources into a database. Data quality was regularly assessed with a second data collection on a random 5% of resident records; inter-rater agreement exceeded 90% for all measures.17
The primary outcome was resident fall rate expressed as falls per 100 patient days. The fall rate was calculated from the number of falls in the fall risk period (excluding the index fall) divided by the resident's fall risk period (i.e., days at risk). This fall risk period was defined as the number of days from the resident's index fall to the first of the following: 1) NH discharge, 2) death, or 3) end of the six month data collection period. The fall risk period (i.e., time at risk) excluded days spent out of the facility, such as for an acute hospitalization. A secondary outcome was injurious fall rate calculated from the number of injurious falls in the fall risk period. An injurious fall was defined as fall resulting in skin tear, hematoma, fracture, or laceration or need for imaging or urgent medical assessment.
NH residents were classified as having CKD if their eGFR was <60 ml/min/1.73m2. We used the Modification of Diet in Renal Disease (MDRD) 4-variable equation to calculate eGFR because it is most widely used by laboratories for eGFR reporting, MDRD eGFR values in this cohort were highly correlated (r=0.95) with eGFR calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, and there is conflicting evidence on the effectiveness of the CKD-EPI equation in frail, older adults.18-20 In estimating GFR we used the serum creatinine measure obtained closest in time and within six months before or after the index fall. Creatinine measurements obtained during an acute hospitalization or emergency room (ER) visit were excluded.
Additional resident data obtained from the parent study included previously established risk factors for falls in NH residents including demographics (race, gender, age), and the following documented medical co-morbidities and medications: 1) history of stroke, 2) peripheral neuropathy, 3) Parkinson's disease, 4) cognitive impairment [history of dementia or Mini-Mental Status Exam score ≤24], 5) visual impairment (history of visual impairment that is not correctable to at least reading ability including, macular degeneration, cataracts that impair vision, or glaucoma that impairs vision), 6) orthostatic hypotension (defined as any documented change in systolic blood pressure ≥15 mm Hg with position change or documented diagnosis of orthostatic hypotension or autonomic insufficiency), 7) history of falls (at least one documented fall event within six months prior to the study period), 8) psychoactive medication use (orders for any of the following classes: benzodiazepine, antidepressant, antipsychotic, sedative-hypnotic agent, or anticholinergic), 9) assistive device use (use of cane, walker, or wheelchair), 10) ambulatory dysfunction [defined as a resident who needs assistance with ambulation (including bedbound residents)], 11) Vitamin D supplement use (defined as presence of a medication order for a Vitamin D supplement of at least 800 international units daily or 50,000 international units every 4 weeks), and 12) anemia (defined as a hemoglobin <13 g/dl for men and <12 g/dl for women within the study period closest to the index fall event).
Statistical Analysis
Our primary hypothesis was that NH resident fall rates are higher among residents with lower eGFRs after accounting for traditional fall risk factors. Our secondary hypothesis was that injurious fall rates would be higher in residents with CKD. We further hypothesized that the association of eGFR with fall rate is modified by specific fall risk factors: age, anemia, cognitive impairment, psychoactive medication use, and vitamin D supplementation.
Resident fall rates, demographics, and fall risk factors were reported as proportions for categorical variables and means with standard deviations or medians with interquartile ranges (IQR) for continuous variables. Bivariate analyses were conducted to compare resident characteristics across three eGFR categories (eGFR≥60 ml/min/1.73m2, eGFR≥45ml/min/1.73m2 and <60 ml/min/1.73m2, and eGFR<45 ml/min/1.73m2). These eGFR categories were derived from the KDIGO CKD stages [eGFR≥60 ml/min/1.73m2 (G1 and G2), eGFR≥45ml/min/1.73m2 and <60 ml/min/1.73m2 (Stage G3a), eGFR<45 ml/min/1.73m2 (Stages G3b, G4, and G5)].16
We used scatterplots and linear regression models to examine the relationship between level of eGFR and fall rate in which eGFR was modeled as both a continuous and categorical variable. Because of significant over-dispersion of the dependent variable (fall count), we used a negative binomial rather than Poisson distribution.21 A test for adjusted deviance confirmed that the negative binomial distribution provided an adequate fit to the data. All bivariate and multivariate models accounted for time at risk (fall risk period) and treated the facility as a random effect to adjust for the effects of different NHs.
Our final multivariable model included eGFR (as a continuous variable), age, race, gender, and all measured variables that were independently associated with falls in bivariate analyses. These included orthostatic hypotension, prior falls, cane use, wheelchair use, and ambulatory dysfunction. Product terms were added to the multivariable model to test for interactions between eGFR and age, anemia, cognitive impairment, psychoactive medication use, and vitamin D supplementation. Because estimates of GFR using the MDRD equation may be less accurate at values >60 ml/min/1.73m2,22,23 we repeated multivariate analyses and tests for interactions using eGFR as a categorical variable. Power calculations confirmed that the sample size was adequate to detect a fall rate ratio of 1.2 for every 10 ml/min change in eGFR (power = 0.8; alpha =0.05, two-tail).24 There was no adjustment for multiple comparisons, and the type-1 error rate was 0.05. All statistical analyses were performed with SAS 9.3 and SAS Enterprise Guide 5.1 (SAS Institute, Inc., Cary, NC).
Results
Cohort Characteristics
In our sample of 510 NH residents, the mean age of cohort members was 77.2 years (SD=11.5), and a majority (63%) resided in VA CLCs. Median eGFR was 71.5 ml/min/1.73m2 [IQR (50.1, 94.6)], and 179 (35%) of cohort members had CKD (an eGFR<60 ml/min/1.73m2). During the six month study period, 162 (32%) of cohort members were hospitalized at least once. Baseline characteristics stratified by eGFR category are presented in Table 1. Residents with eGFR values 45-59 ml/min/1.73m2 were older [80.1 years (SD=10.8)] and included a larger proportion (36%) of females compared with those with eGFR levels both above and below this. There were no statistically significant differences in the distribution of fall risk factors across eGFR categories.
Table 1. Resident Characteristics, Overall, and by Estimated Glomerular Filtration Rate Category.
| Resident Characteristics | Overall N=510 |
eGFR ≥ 60 N=331 |
eGFR 45-60a N=77 |
eGFR < 45 N =102 |
P-value |
|---|---|---|---|---|---|
| eGFR, ml/min/1.73m2, median (IQR) | 71.5(50.1-94.6) | 85.6(72.1-105.2) | 51.2(49.6-55.4) | 33.7(18.6-41.1) | <0.001 |
| Age, y, mean (SD) | 77.2 (11.5) | 76.1 (11.6) | 80.1 (10.8) | 78.7(11.2) | 0.009 |
| Age 50-79 (%) | 254 (49.8) | 175 (52.9) | 32 (41.6) | 47 (46.1) | |
| Age >=80 (%) | 256 (50.2) | 156 (47.1) | 45 (58.4) | 55 (53.9) | |
| Female (%) | 137 (26.9) | 77 (23.3) | 28 (36.4) | 32 (31.4) | 0.034 |
| Race (%) | 0.19 | ||||
| White | 383 (75) | 240 (72.5) | 65 (84.4) | 78 (76.5) | |
| Black | 102 (20) | 73 (22.1) | 8 (10.4) | 21 (20.6) | |
| Other race | 25 (5) | 18 (5.4) | 4 (5.2) | 3 (2.9) | |
| Veterans' Affairs resident (%) | 319 (62.5) | 215 (65.0) | 43 (55.8) | 61 (59.8) | 0.27 |
| Stroke (%) | 162 (31.7) | 113 (34.4) | 25 (32.5) | 24(23.5) | 0.13 |
| Peripheral Neuropathy (%) | 72 (14.1) | 44 (13.3) | 16 (20.8) | 12 (11.8) | 0.18 |
| Parkinson's Disease (%) | 43 (8.4) | 31 (9.4) | 4 (5.2) | 8 (7.8) | 0.48 |
| Orthostatic Hypotension (%) | 44 (8.6) | 28 (8.5) | 8 (10.4) | 8 (7.8) | 0.82 |
| Visual Impairment (%) | 190 (37.2) | 117 (35.4) | 34 (44.2) | 39 (38.2) | 0.35 |
| Cognitive Impairment (%) | 296 (58) | 199 (60.1) | 46 (59.7) | 51 (50.0) | 0.18 |
| Prior Falls in last 180 days (%) | 255 (50) | 162 (48.9) | 43 (55.8) | 50 (49.0) | 0.54 |
| Psychotropic Med Use (%) | 442 (86.7) | 282 (85.2) | 70 (90.9) | 90 (88.2) | 0.36 |
| Assistive Device Use (%) | 0.082 | ||||
| None | 46 (9.0) | 30 (9.1) | 12 (15.6) | 4 (3.9) | |
| Cane or Walker | 244 (47.8) | 159 (48.0) | 37 (48.1) | 48 (47.1) | |
| Wheelchair | 220 (43.1) | 142 (42.9) | 28 (36.4) | 50 (49.0) | |
| Ambulatory Dysfunction (%) | 0.93 | ||||
| Bedbound | 79 (15.4) | 53 (16.0) | 10 (13.0) | 16 (15.7) | |
| Walks without assistance | 203 (39.8) | 128 (38.7) | 32 (41.6) | 43 (42.2) | |
| Walks with assistance | 228 (44.7) | 150 (45.3) | 35 (45.5) | 43 (42.2) | |
| Vitamin D supplement use (%)b | 228 (44.9) | 143 (43.3) | 34 (44.2) | 51 (50.5) | 0.44 |
| Anemia (%)b | 342 (67) | 213 (64.4) | 51 (66.2) | 78 (76.5) | 0.14 |
Abbreviations: eGFR = estimated glomerular filtration rate, SD = standard deviation, IQR = interquartile range
Statistical significance between characteristics of residents by eGFR category detected via t-test for continuous variables, chi-square test for dichotomous variables.
Estimated glomerular filtration rate ≥45 ml/min/1.73m2 and <60 ml/min/1.73m2. A value less than 60ml/min/1.73m2 is considered chronic kidney disease.
Data was not available for all subjects. Number of subjects with missing values: Vitamin D supplement use, n=2; anemia, n=14.
Fall Rates
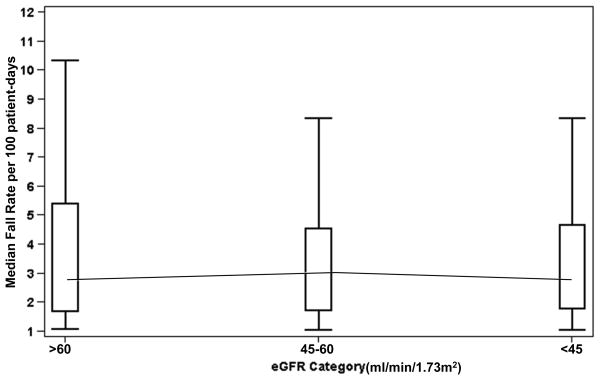
The median fall rate was 2.6 falls per 100 patient days (IQR, 1.3-6.3), measured over an average time at risk of 85.3 days (SD=56.1); neither the median fall rate nor the time at risk differed across eGFR categories (P=0.62) (Figure 1). Nearly half (46%) of falls were associated with injury. Injurious fall rates were similar in residents with CKD and those without CKD (P=0.44).
Figure 1.
Median Fall Rate by Estimated Glomerular Filtration Rate Category
Abbreviations: eGFR = estimated glomerular filtration rate.
The cohort's unadjusted median fall rate per 100 patient-days divided into three categories: eGFR > 60ml/min/1.73m2, eGFR ≥ 45 and <60ml/min/1.73m2 and eGFR <45ml/min/1.73m2.
Unadjusted Analyses
In unadjusted analyses, orthostatic hypotension, history of falls in the 180 days prior to the index fall, assistive device use, and ambulatory dysfunction were each positively associated with fall rate. Level of eGFR was not associated with overall fall risk [(fall rate ratio (FRR) 0.99 per 10 ml/min/1.73 m2, 95% confidence interval (CI) 0.97-1.02)] or with risk of injurious falls (FRR 0.99 per 10 ml/min/1.73 m2, 95% CI 0.95-1.03) in unadjusted analyses (Table 2). Results were similar when eGFR was modeled as a categorical variable.
Table 2. Associations with Fall Rate, Unadjusted and Adjusted Analyses.
| Predictor Variables | Fall Rate Ratio (95% Confidence Interval) |
|
|---|---|---|
| Unadjusted | Adjusted | |
| eGFR (per 10ml/min/1.73m2) | 0.99 (0.97, 1.02) | 1.00 (0.97, 1.02) |
| eGFR Categorya | ||
| eGFR 45-60ml/min/1.73m2 | 0.97 (0.77, 1.23) | 0.97 (0.76, 1.23) |
| eGFR<45ml/min/1.73m2 | 1.05 (0.84, 1.31) | 1.06 (0.85, 1.32) |
| Age | 1.00 (0.99, 1.01) | 1.00 (0.99, 1.01) |
| Femaleb | 0.98 (0.79, 1.21) | 1.01 (0.81, 1.26) |
| Race | ||
| African-American racec | 1.06 (0.85, 1.32) | 1.12 (0.89, 1.39) |
| Other racec | 0.92 (0.61, 1.39) | 0.98 (0.66, 1.46) |
| Stroke | 0.99 (0.82, 1.19) | |
| Peripheral Neuropathy | 1.07 (0.84, 1.37) | |
| Parkinson's Disease | 1.12 (0.82, 1.53) | |
| Orthostatic Hypotension | 1.52 (1.12, 2.07) | 1.52 (1.12, 2.05) |
| Visual Impairment | 1.07 (0.89, 1.29) | |
| Cognitive Impairment | 1.14 (0.95, 1.37) | |
| Prior Falls in last 180 days | 1.25 (1.05, 1.49) | 1.25 (1.05, 1.49) |
| Psychotropic Med use | 1.25 (0.95, 1.65) | |
| Assistive Device Use | ||
| Cane/Walkerd | 1.88 (1.32, 2.67) | 1.64 (1.16, 2.33) |
| Wheelchaird | 1.59 (1.12, 2.26) | 1.38 (0.97, 1.96) |
| Walks with Assistance | 1.48 (1.24, 1.75) | 1.47 (1.23, 1.75) |
| Vitamin D supplement use | 1.01 (0.84, 1.20) | |
| Anemia | 1.17 (0.97, 1.41) | |
Abbreviations: eGFR = estimated glomerular filtration rate.
Compared to eGFR>60ml/min/1.73m2.
Compared to male.
Compared to white race. Other race includes American Indian, Asian, and Hispanic.
Compared to no assistive device use.
Multivariable analyses
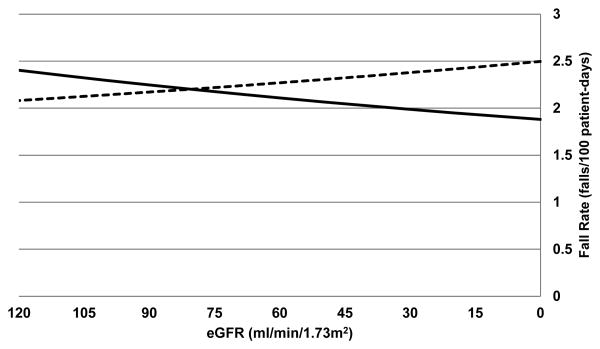
In adjusted analyses, orthostatic hypotension, history of falls, cane or walker use, and ambulatory dysfunction were independently associated with fall rate. Level of eGFR was not associated with falls in adjusted analyses (FRR 1.00 per 10 ml/min/1.73 m2, 95% CI 0.97-1.02) (Table 2). This was also true when eGFR was modeled as a categorical variable. Pre-specified tests for interaction of eGFR with anemia, cognitive impairment, psychoactive medication use, and Vitamin D supplement use did not reach statistical significance (P>0.05); however, we did identify a statistically significant interaction between eGFR and age (P=0.005). For residents under 80 years, each 10 ml/min/1.73m2 decrease in eGFR was associated with 3% higher risk of falling (FRR 1.03 per 10 ml/min/1.73 m2, 95% CI 1.00-1.06), whereas the opposite association was true for residents older than 80 years (FRR 0.96 per 10 ml/min/1.73 m2, 95% CI 0.92-1.00) (Figure 2). In residents under 80 years, median fall rate ranged from 2.4 falls per 100 patient days (IQR, 1.3-6.2) for those with an eGFR ≥60 ml/min/1.73m2 to 3.8 falls per 100 patient days (IQR, 1.9-9.1) for those with an eGFR <45 ml/min/1.73m2 (P=0.048). In contrast, fall rates did not differ significantly across eGFR categories for those aged 80 years and older (P=0.67). The age/eGFR interaction was still of borderline significance when eGFR was modeled as a categorical variable (P=0.07), when the cohort was limited to residents who were ambulatory (n=431) (P=0.06) and residents with an eGFR ≥120 ml/min/1.73m2 were excluded (n=55) (P=0.08).
Figure 2.
Estimated Glomerular Filtration Rate Interaction with Age Threshold of 80 Years
Abbreviations: eGFR = estimated glomerular filtration rate.
Solid line indicates age over 80 years. Dotted line indicates age under 80 years.
Discussion
In this sample of NH residents who had fallen at least once and were thus at high risk for recurrent falls, CKD was common but was not statistically significantly associated with future risk of repeat falls or injurious falls in the overall cohort. This was also the case for other chronic comorbid conditions (e.g., history of stroke, Parkinson's disease) which were also not associated with falls among members of this study cohort. On the other hand, traditional fall risk factors not specific to any one disease process (e.g., orthostatic hypotension, prior history of falls, impaired mobility) were independently associated with recurrent falls among members of this cohort. Our findings generally suggest that NH providers should not expect fall rates to increase because of the increasing prevalence of CKD in NHs.
Fall rates in this study are comparable to national fall statistics for NHs2; however, unlike prior studies among community dwelling older adults, this study did not find that CKD was an independent predictor of falls in NH residents. The two studies that identified CKD as a predictor of falls were subgroup analyses from randomized controlled trials of Vitamin D therapy that enrolled independent older adults (age range = 65-77 years) who had few severe chronic illnesses.7,8 One study enrolled women with osteoporosis (those with severe chronic illnesses were excluded) and, after 36 months of follow-up, 24-hr urine creatinine clearance (CrCl) was a significant predictor of incident falls in the placebo arm.8 In the other study, a relatively healthy cohort of older adults (average albumin was 42.3 g/L) was followed for 36 weeks, and those with a serum CrCl < 65ml/min had at least threefold greater risk of incident falls (Odds Ratio 3.68, 95% CI, 1.38-9.82) than those with CrCl ≥ 65 ml/min.7 These two studies accounted primarily for osteoporosis risk factors,7,8 while we used an expansive list of traditional fall risk factors as covariates. Despite these differences in population and study design, the prevalence of CKD was comparable, if not higher, in our cohort (35%) compared to the community-based cohorts (21-37%).7,8 After consideration of this existing literature, our findings suggest that in an already frail population with a history of prior falls, the presence of CKD does not further increase fall risk, while it does identify those at higher risk in a healthier population. Also, the cohort members already had high fall risk so after accounting for traditional fall risk factors, CKD does not further increase their fall risk.
Our findings in this cohort of NH residents with primarily moderate reductions in eGFR do not rule out an association between lower levels of eGFR with fall risk. Only 8% of our sample had an eGFR < 30ml/min/1.73m2, and because CKD complications are more likely to develop at or below this level of eGFR in older adults, we may not have had enough subjects with advanced CKD to detect an association.25 Patients with end-stage renal disease (ESRD) have higher fall rates than in older adults in the general population6,26; however, because only 4% of the sample had eGFR <15ml/min/1.73m2, we were unable to evaluate for an association of eGFR with falls among NH residents with ESRD. Another consideration is that GFR estimating equations can overestimate kidney function in older adults.22 These equations have limited accuracy at high eGFR values and in individuals with low muscle mass. A large proportion of our cohort had normal eGFR values (and plausibly low muscle mass), so the limited accuracy of eGFR values as a measure of true GFR may have influenced our results.
The association of eGFR with recurrent falls did vary by age among members of this cohort. For residents under age 80, unadjusted median fall rates substantially increased (from 8.8 to 13.9 falls per resident per year) as eGFR values decreased (from ≥60 to <45 ml/min/1.73m2). However, this relationship between eGFR and falls was not present in residents over age 80. An age interaction in the association of eGFR with fall risk seems to support the possibility that the presence of CKD may be a stronger risk factor for falls in younger less frail NH residents, but this finding needs additional research to confirm. These results may also reflect some confounding by activity level as a large proportion of bedbound residents were over the age of 80 in this cohort. Residents who are bedbound have fewer opportunities to experience falls.27
CKD diagnosis in a NH resident who falls could provide clues to evaluate for traditional fall risk factors (e.g., orthostatic hypotension, anemia, and Vitamin D deficiency) that are also complications of CKD. Still, the disease itself is not a significant fall risk factor for NH fallers. This finding supports an argument for greater emphasis on targeting physical and cognitive impairments for fall prevention than targeting disease management.28 Because there are various impairments, existing multifactorial programs remains paramount for fall prevention.
The results of this study should be interpreted with consideration of the following limitations. First, we used single creatinine values obtained during routine clinical care so we were not able to control the timing of eGFR measurement in relation to the index fall. Thus, eGFR values used in our study occurring after a fall might have been a consequence of a fall rather than a potential risk factor for falls. Also, using a single creatinine measure increases the risk of misclassification of kidney function. We attempted to minimize this measurement bias by using eGFR values not associated with hospitalization or ER visits. Second, the accuracy of eGFR by the MDRD equation is limited in populations with age-related muscle wasting.23 GFR estimating equations with greater accuracy at higher eGFR values and in patients with low muscle mass may yield different results (i.e., equations based on serum cystatin-C), but are not widely used in clinical practice.29 Third, we used multiple data sources (e.g., medical records, MDS, fall logs) to identify fall events, but the chart abstraction process is unable to capture undocumented falls. The parent study's chart abstraction process was restricted so we were not able to describe resident functional status or the proportion of residents who required long-term care or short-term rehabilitation. Fourth, this study cohort is not international so it has limited generalizability. Last, we could not account for unmeasured confounders (e.g., blood pressure or medications before each fall event).
Conclusion
Among members of this cohort of nursing home residents were at high risk for falling, the presence of a low eGFR was not a risk factor for recurrent falls in the overall cohort, but was associated with recurrent falls among younger cohort members. In general, traditional fall risk factors that are not tied to a particular underlying disease process (e.g., prior history of falls, impaired mobility, and orthostatic hypotension) were more strongly associated with recurrent falls than individual co-morbid conditions. Although CKD and other chronic conditions are increasing prevalent among NH residents, NHs should continue fall prevention strategies that target traditional fall risk factors.
Acknowledgments
Research reported in this publication was supported by the National Institute on Aging of the National Institutes of Health under Award Number P30AG028716. It was also supported by the National Institute of Nursing Research (R01NR003178). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Dr. Hall was supported in part by a fellowship award from the Office of Academic Affiliations, US Department of Veterans Affairs. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Department of Veterans Affairs or the United States government.
No funding agencies for the authors had a role in the design, execution, analysis, data interpretation, or manuscript preparation for this study.
Abbreviations
- CKD
chronic kidney disease
- eGFR
estimated glomerular filtration rate
- ESRD
end-stage renal disease
- KDIGO
Kidney Disease Improving Global Outcomes
Footnotes
Competing Interests: AMO and CCE receive royalties from UpToDate.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Lawrence R. Landerman, Email: Lawrence.landerman@dm.duke.edu.
Ann M. O'Hare, Email: Ann.OHare@va.gov.
Ruth A. Anderson, Email: Ruth.anderson@dm.duke.edu.
Cathleen S. Colón-Emeric, Email: Cathleen.colonemeric@dm.duke.edu.
References
- 1.Rubenstein LZ. Falls in older people: epidemiology, risk factors and strategies for prevention. Age Ageing. 2006 Sep;(Suppl 2):ii37–ii41. doi: 10.1093/ageing/afl084. [DOI] [PubMed] [Google Scholar]
- 2.Rubenstein LZ, Josephson KR. Falls and their prevention in elderly people: what does the evidence show? Med Clin North Am. 2006 Sep;90(5):807–824. doi: 10.1016/j.mcna.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 3.Cohen JT, Jasimuddin SK, Tommasulo BC, Shapiro EY, Singavarapu A, Vernatter J, Hussain R, Cal C, Dlugacz Y, Mattana J, Wolf-Klein G. Underdiagnosis of chronic kidney disease in the nursing home population. J Am Geriatr Soc. 2009 Jun;57(6):1123–1124. doi: 10.1111/j.1532-5415.2009.02289.x. [DOI] [PubMed] [Google Scholar]
- 4.Garg AX, Papaioannou A, Ferko N, Campbell G, Clarke JA, Ray JG. Estimating the prevalence of renal insufficiency in seniors requiring long-term care. Kidney Int. 2004 Feb;65(2):649–653. doi: 10.1111/j.1523-1755.2004.00412.x. [DOI] [PubMed] [Google Scholar]
- 5.McClellan WM, Resnick B, Lei L, Bradbury BD, Sciarra A, Kewalramani R, Ouslander JG. Prevalence and severity of chronic kidney disease and anemia in the nursing home population. J Am Med Dir Assoc. 2010 Jan;11(1):33–41. doi: 10.1016/j.jamda.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Cook WL, Tomlinson G, Donaldson M, Markowitz SN, Naglie G, Sobolev B, Jassal SV. Falls and fall-related injuries in older dialysis patients. Clin J Am Soc Nephrol. 2006 Nov;1(6):1197–1204. doi: 10.2215/CJN.01650506. [DOI] [PubMed] [Google Scholar]
- 7.Dukas LC, Schacht E, Mazor Z, Stahelin HB. A new significant and independent risk factor for falls in elderly men and women: a low creatinine clearance of less than 65 ml/min. Osteoporos Int. 2005 Mar;16(3):332–338. doi: 10.1007/s00198-004-1690-6. [DOI] [PubMed] [Google Scholar]
- 8.Gallagher JC, Rapuri P, Smith L. Falls are associated with decreased renal function and insufficient calcitriol production by the kidney. J Steroid Biochem Mol Biol. 2007 Mar;103(3-5):610–613. doi: 10.1016/j.jsbmb.2006.12.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Binder EF, White HK, Resnick B, McClellan WM, Lei L, Ouslander JG. A prospective study of outcomes of nursing home residents with chronic kidney disease with and without anemia. J Am Geriatr Soc. 2012 May;60(5):877–883. doi: 10.1111/j.1532-5415.2012.03941.x. [DOI] [PubMed] [Google Scholar]
- 10.Kurella Tamura M, Covinsky KE, Chertow GM, Yaffe K, Landefeld CS, McCulloch CE. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med. 2009 Oct 15;361(16):1539–1547. doi: 10.1056/NEJMoa0904655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olyaei AJ, Bennett WM. Drug dosing in the elderly patients with chronic kidney disease. Clin Geriatr Med. 2009 Aug;25(3):459–527. doi: 10.1016/j.cger.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Robinson B, Artz AS, Culleton B, Critchlow C, Sciarra A, Audhya P. Prevalence of anemia in the nursing home: contribution of chronic kidney disease. J Am Geriatr Soc. 2007 Oct;55(10):1566–1570. doi: 10.1111/j.1532-5415.2007.01389.x. [DOI] [PubMed] [Google Scholar]
- 13.Schnelle J, Osterweil D, Globe D, Sciarra A, Audhya P, Barlev A. Chronic kidney disease, anemia, and the association between chronic kidney disease-related anemia and activities of daily living in older nursing home residents. J Am Med Dir Assoc. 2009 Feb;10(2):120–126. doi: 10.1016/j.jamda.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 14.Shlipak MG, Stehman-Breen C, Fried LF, Song X, Siscovick D, Fried LP, Psaty BM, Newman AB. The presence of frailty in elderly persons with chronic renal insufficiency. Am J Kidney Dis. 2004 May;43(5):861–867. doi: 10.1053/j.ajkd.2003.12.049. [DOI] [PubMed] [Google Scholar]
- 15.Abdel-Rahman EM, Turgut F, Turkmen K, Balogun RA. Falls in elderly hemodialysis patients. QJM. 2011 Oct;104(10):829–838. doi: 10.1093/qjmed/hcr108. [DOI] [PubMed] [Google Scholar]
- 16.Levin A, Stevens PE. Summary of KDIGO 2012 CKD Guideline: behind the scenes, need for guidance, and a framework for moving forward. Kidney Int. 2014 Jan;85(1):49–61. doi: 10.1038/ki.2013.444. [DOI] [PubMed] [Google Scholar]
- 17.Anderson RA, Corazzini K, Porter K, Daily K, McDaniel RR, Jr, Colon-Emeric C. CONNECT for quality: protocol of a cluster randomized controlled trial to improve fall prevention in nursing homes. Implement Sci. 2012;7:11. doi: 10.1186/1748-5908-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willems JM, Vlasveld T, den Elzen WP, Westendorp RG, Rabelink TJ, de Craen AJ, Blauw GJ. Performance of Cockcroft-Gault, MDRD, and CKD-EPI in estimating prevalence of renal function and predicting survival in the oldest old. BMC Geriatr. 2013;13:113. doi: 10.1186/1471-2318-13-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009 May 5;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F Chronic Kidney Disease Epidemiology C. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006 Aug 15;145(4):247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 21.Allison PD SAS Institute. Logistic regression using SAS : theory and application. 2nd. Cary, N.C.: SAS Pub.; 2012. [Google Scholar]
- 22.Dharmarajan TS, Yoo J, Russell RO, Norkus EP. Chronic kidney disease staging in nursing home and community older adults: does the choice of cockcroft-gault, modification of diet in renal disease study, or the chronic kidney disease epidemiology collaboration initiative equations matter? J Am Med Dir Assoc. 2012 Feb;13(2):151–155. doi: 10.1016/j.jamda.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Stevens LA, Coresh J, Greene T, Levey AS. Assessing Kidney Function — Measured and Estimated Glomerular Filtration Rate. New England Journal of Medicine. 2006;354(23):2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 24.Jung SH, Ahn C. Sample size estimation for GEE method for comparing slopes in repeated measurements data. Stat Med. 2003 Apr 30;22(8):1305–1315. doi: 10.1002/sim.1384. [DOI] [PubMed] [Google Scholar]
- 25.Bowling CB, Inker LA, Gutierrez OM, Allman RM, Warnock DG, McClellan W, Muntner P. Age-specific associations of reduced estimated glomerular filtration rate with concurrent chronic kidney disease complications. Clin J Am Soc Nephrol. 2011 Dec;6(12):2822–2828. doi: 10.2215/CJN.06770711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rossier A, Pruijm M, Hannane D, Burnier M, Teta D. Incidence, complications and risk factors for severe falls in patients on maintenance haemodialysis. Nephrol Dial Transplant. 2012 Jan;27(1):352–357. doi: 10.1093/ndt/gfr326. [DOI] [PubMed] [Google Scholar]
- 27.Thapa PB, Brockman KG, Gideon P, Fought RL, Ray WA. Injurious falls in nonambulatory nursing home residents: a comparative study of circumstances, incidence, and risk factors. J Am Geriatr Soc. 1996 Mar;44(3):273–278. doi: 10.1111/j.1532-5415.1996.tb00913.x. [DOI] [PubMed] [Google Scholar]
- 28.Tinetti ME, Fried T. The end of the disease era. Am J Med. 2004 Feb 1;116(3):179–185. doi: 10.1016/j.amjmed.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 29.Lopes MB, Araujo LQ, Passos MT, Nishida SK, Kirsztajn GM, Cendoroglo MS, Sesso RC. Estimation of glomerular filtration rate from serum creatinine and cystatin C in octogenarians and nonagenarians. BMC Nephrol. 2013;14:265. doi: 10.1186/1471-2369-14-265. [DOI] [PMC free article] [PubMed] [Google Scholar]