Abstract
The need to achieve ≥95% adherence to HAART for treatment effectiveness may be a barrier for universal initiation at early stages of HIV. Using longitudinal data collected from 2006-2011 from cohort studies of MSM (MACS) and IDUs (ALIVE study), we estimated the minimum adherence needed to achieve HIV RNA suppression (<50 copies/mL), defined as the level at which at least 80% were virally suppressed, and the odds of suppression was not significantly different than that observed with ≥95% adherence. In the MACS, ≥80% suppression was observed with 80-84% adherence and the odds ratio for suppression (vs.≥95% adherence) was 1.43 (0.61, 3.33). In the ALIVE study where <35% were on newer drugs, only 71.4% were suppressed among those who reported ≥95% adherence. Although IDUs on older HAART regimens may need to be ≥95% adherent, concerns related to non-adherence may be less of a barrier to initiation of modern HAART regimens.
Keywords: Adherence, HAART, HIV RNA suppression, men who have sex with men, injection drug users
INTRODUCTION
Approximately 34 million people were living with HIV at the end of 2011 (1). With theintroduction of highly active antiretroviral therapy (HAART) in 1996, there was a significant decline in AIDS-related mortality, and a longer life expectancy among HIV-infected persons treated with HAART (2-6). The treatment of HIV has evolved over the past two decades from highly toxic, complex regimens, to newer formulations with improved pharmacokinetics that are easier to administer (7). Effective treatment is defined by achieving low, undetectable plasma HIV RNA levels (copies/mL) (7). HAART needs to be administered daily over the course of a patient's lifetime in order to keep HIV RNA levels suppressed, decrease rates of resistance, and prevent progression to AIDS and HIV-related death.
Researchers investigating the effectiveness of HAART found that 95% adherence or better was necessary for approximately 80% of the population to achieve viral load suppression (8,9). This high level of adherence has been challenging for many individuals due to barriers to adherence (7) that include treatment complexity (10,11), patient-related high-risk behaviors such as use of non-prescription drugs, lack of social support, and sociodemographic factors such as age and comorbidities (12). Individual responses to treatment such as tolerability, drug-resistance, durability of virologic and immunologic responses, and pharmacokinetic factors vary, and sometimes untreated symptoms due to suboptimal viral load suppression may impair subsequent adherence to HAART (13). There are negative consequences of requiring high levels of adherence. First, physicians may be reluctant to prescribe HAART universally to patients early in the infection due to concerns about the ability to maintain adherence to HAART over time (14). Second, for patients on HAART, a significant amount of resources have been invested in improving adherence to HAART (7). Resource-intensive strategies to improve HAART adherence have included electronic reminders, administration of medications under supervised settings, self-monitoring, counseling (15), and adherence improvement strategies such as Directly Administered Antiretroviral Therapy (DAART) (16) for drug users, and Sharing Medical Adherence Responsibilities Together (SMART for couples) (16). In addition to the costs, these strategies are often administered only for fixed periods of time, and adherence to HAART and viral suppression may not be sustained after the strategies are withdrawn (7,17).
Given the improved pharmacokinetics of newer HAART regimens over therapies administered in the earlier treatment era, and concerns about the need to maintain high adherence, empirical data are needed to know whether viral load suppression is possible at lower levels of adherence at the population level. A lower level of adherence required for effective treatment may alleviate the concerns noted above, result in earlier initiation of treatment in patients, and also enable physicians to determine which patients require in-depth counseling for adherence. Improving access to, and consistent use of medicines by HIV-infected individuals would decrease their risk of transmitting the virus to others, according to a recent report by the Institute of Medicine (IOM) on HIV treatment and quality of care (18).
This study aimed to determine whether the association between adherence and HIV RNA suppression has changed over time, and to estimate the minimum optimal cutoff of adherence for HIV RNA suppression. The hypothesis was that the effectiveness of currently available HAART, measured by the suppression of HIV RNA to <50 copies/mL, does not require the near perfect levels of adherence (≥95%) as was required with earlier regimens.
METHODS
We used longitudinal data collected prospectively between March 2001 and December 2011 from the participants in the Multicenter AIDS Cohort Study (MACS), and the AIDS Linked to the Intravenous Experience (ALIVE) study who reported using HAART between 2001 and 2011 for at least one visit.
The MACS is an ongoing prospective study of HIV-1 infection among men who have sex with men (MSM) in the United States (19). A total of 6,992 men have been recruited since 1984 in 3 waves of recruitment: 5,622 men before 1991, 1,350 men in 2001-03, and 20 men since 2010, in Baltimore, MD, Chicago, IL, Los Angeles, CA, and Pittsburgh, PA (19). Eligible persons had to be sexually active, 18 years or older, and free of an acquired immunodeficiency syndrome (AIDS)-defining illness, i.e., opportunistic infection or malignancy (20). Every six months, the study visits entail physical examinations, collection of blood for concomitant laboratory testing and storage, and standardized interviews to collect information on demographics, medical history, and behaviors. MACS study protocols were approved by institutional review boards at each study center, and informed consent was obtained from all participants.
The ALIVE study is a prospective community-based cohort study of injection drug users (IDUs) in Baltimore, MD (21). A total of 2,946 IDUs were recruited initially through community outreach in 1988-1989 (21). This was followed by three waves of recruitment in 1994-1995, 1998, and 2005-08, with a total of 1,067 participants being followed over time since 1996 (17). Eligible persons had to be 18 years or older, free of AIDS during initial recruitment waves, and have a history of injection drug use. Similar to the MACS, at each 6-month study visit, researchers collect information on sociodemographic characteristics, medical history, HIV risk behaviors (sexual and drug-related), drug abuse treatment, and collection of blood for concomitant laboratory testing and storage. ALIVE study procedures were reviewed and approved by the institutional review board at the Johns Hopkins Bloomberg School of Public Health, and all participants provided written informed consent.
In both cohorts, HIV RNA levels were determined using the Roche Ultrasensitive RNA PCR assay (Hoffman-LaRoche, Nutley, NJ, U.S.A.) with a detection limit of 50 copies/ml, and CD4+ levels were quantified using standardized flow cytometry (19, 21). The Baltimore MACS site and the ALIVE study use the same laboratory for flow cytometry and HIV RNA quantification.
Definition of HAART
HAART was defined using the DHHS guidelines as ‘a combination antiretroviral treatment regimen containing at least 3 antiretroviral drugs - 2 nucleoside reverse-transcriptase inhibitor (NRTI) medications plus a protease inhibitor (PI), a non-nucleoside reverse-transcriptase inhibitor (NNRTI), or an integrase strand transfer inhibitor (INSTI)’(22).
Based on the type of HAART regimen used in the general population, we classified the treatment of HIV into 3 eras: early HAART, 1996 through 2000; mid- HAART, 2001 through 2005; and current HAART, 2006 through 2011. Date of HAART initiation was set as the visit date of the first HAART report.
Study population
Specifically, our study population was restricted to HIV-positive men and women who were on HAART from March 1, 2001 to December 31, 2011 (mid- and current eras). In addition, to examine trends in adherence and viral load over time, we further restricted the population to those contributing data from January 1, 2009 onwards. This latter restriction was implemented to avoid bias in temporal trends due to earlier attrition as a result of worse outcomes; for example, examining temporal trends by including those who developed AIDS and died before 2009, would result in different persons comprising calendar periods under study and bias the results. Only visits at which participants reported using HAART were included in the analysis.
Outcomes and exposures
Adherence to HAART was defined using self-reported information collected at the study visits. In the MACS, the participant was asked about his actual use of each antiretroviral medication over the four days prior to the study visit. These responses were compared to the prescribed usage to determine adherence:
As an exposure, adherence to HAART was treated as a categorical variable based on the distribution of adherence in the study population, and was also dichotomized as ≥95% or less. As an outcome, adherence was treated as a continuous variable. In the ALIVE study, self-reported adherence data were collected and the adherence percent was calculated similar to the MACS, except that usage over a 3-day period was ascertained.
Potential predictors of viral load suppression (<50 copies/mL) and adherence were sociodemographic and behavioral characteristics reported for the 6 months prior to when adherence and HIV RNA were measured. These included age, race, annual income (<$10,000 versus ≥$10,000), insurance status (private, public, none), current injection drug use, non-injection drug use (including cocaine, crystal methamphetamine, marijuana, heroin, poppers), current smoking, and moderate-heavy alcohol intake (defined as 3-4 drinks/day or more for more than once a month or ≥ 5 drinks/day for less than once a month) compared to lower quantities. Treatment and disease characteristics included number of antiretrovirals, CD4 cell count, and self-reported depressive symptoms (measured using the Centers for Epidemiologic Studies Depression Scale (CES-D)) (23). Persons with scores greater than 16 on the CES-D were classified as having symptoms of depression (23). Both CD4 cell count and CES-D scores were lagged to the previous visit. In the ALIVE study, additional variables included homelessness, incarceration (≥1 week), and the length of the visit interval. We also controlled for the type of HAART regimen (NNRTI-based, PI-based, INSTI-based, and single pill) in both cohorts. Gaps in treatment were calculated for both cohorts since they were likely to impact the association between adherence and viral load suppression. Gaps in treatment were defined as not being on HAART for at least one visit since treatment was initiated. In addition to being included as a confounder in the analysis, an interaction term between adherence and having one or more gaps in treatment was included to check for potential effect modification.
Statistical methods
The 2 cohorts were analyzed separately since they represented two distinct risk groups (MSM and IDU), which could modify associations, and also because they presented with very different distributions of adherence. We restricted the analysis to person-visits with non-missing covariates, representing about 95% of the sample. Exclusion of these person-visits did not alter any trends or results from univariate analysis that used the full population. In both cohorts, for those with first HAART visits after 2006 with missing values in lagged CD4 cell counts, we used the CD4 count at that visit (MACS: 0.1%, ALIVE: 6.9%). The average change in adherence over time was determined at the population and individual levels. Linear mixed effects models with random intercept and slope, adjusted for confounders were used to study the effect of time on adherence. Adherence was modeled as a continuous outcome and two models were fit. In the first model, time was modeled as a dichotomous variable (<2006 and ≥2006), and in the second model, time was modeled as a discrete variable, using 2-year intervals. The fixed components of the model, the β coefficients, were used to determine the average change in adherence accounting for individual correlation between observations. The variance of the random slope, σ 22, estimated using maximum likelihood, was used to determine between-person changes over time. A likelihood ratio test was used to test if the random effects were significant.
To initially examine whether the proportion suppressing HIV RNA changed over time among those not fully adherent, we graphically depicted the proportion suppressed from 2001-2011 among those with <95% adherence. The best fit for the relationship between proportion suppressed and time was determined based on the Akaike Information Criterion (AIC) statistic. To define the minimum optimal adherence cutoff in the population, two criteria had to be met: 1) since historically, 80% of treated HIV-infected persons with ≥95% adherence had suppressed viral load (8, 9), this level had to be achieved; and 2) the odds of viral load suppression at the cutoff could not be statistically different from that observed in the population with ≥95% adherence. Since we were interested in defining this cutoff for adherence to current HAART regimens, we restricted this analysis to data from 2006 onwards. The proportion suppressed was plotted according to categories of adherence based on the observed distribution by cohort. Logistic regression models with viral load suppression as the outcome, and adherence percent as the primary exposure controlling for repeated measures over time and adjusting for confounders were used to compare the odds of suppression at the adherence category to that observed in the reference category (≥95% adherence).
All analyses were performed using SAS 9.2 (Cary, North Carolina, USA) and STATA 12.1 (College Station, Texas, USA). A p-value threshold of 0.05 was used to define statistical significance.
RESULTS
Study population
A total of 1,215 MACS participants contributed 12,310 person-visits, and 337 ALIVE participants contributed 2,188 person-visits in 2001-2011, of whom 1,026 and 197, respectively, contributed data since 2009. After excluding 5% of the person-visits due to missing covariates in the MACS, the study population consisted of 11,678 person-visits contributed by 1,194 participants, of which 1,006 were seen since 2009. The missing data by covariates were: alcohol use (1.8%), smoking (1.6%), non-injection drug use (1.9%), and depression (2.3%). Characteristics of the study populations seen since 2009 according to adherence are described in Table 1. Supplemental Table 1 shows the characteristics of all participants, compared to those seen since 2009. In the MACS, adherence was significantly associated with higher income, having private insurance, abstinence from injection and non-injection drug use, a higher CD4 count, and virologic suppression. In the ALIVE study, those with ≥95% adherence had a significantly lower proportion of alcohol users, and current injection and non-injection drug users. The overall proportion suppressed was also significantly higher for visits with ≥95% adherence compared to visits with <95% adherence.
Table 1.
Study population characteristics (2001-2011)δ
| Characteristics | MACS (N person-visits*=10,971, N=1,006) | ALIVE (N person-visits*=1,745, N=197) | ||||
|---|---|---|---|---|---|---|
| Adherence <95% (N person-visits=1,275, N=516) (%) | Adherence ≥95% (N person-visits=9,696, N=998) (%) | P-value | Adherence <95% (N person-visits=197, N=95) (%) | Adherence ≥95% (N person-visits=1,548, N=193) (%) | P-value | |
| Age, mean (SD) | 47.6 (8.4) | 49.4 (8.7) | <0.01 | 48.9 (6.1) | 49.9 (6.6) | 0.06 |
| Race | ||||||
| Black | 33.5 | 24. | <0.01 | 96.5 | 95.9 | 0.49 |
| Sex | ||||||
| Male | 100 | - | 69.0 | 67.8 | 0.78 | |
| Income | ||||||
| ≤$10,000 | 24.7 | 20.0 | <0.01 | 93.4 | 90.5 | 0.19 |
| >$10,000 | 75.3 | 80.0 | 6.6 | 9.5 | ||
| Insurance status¶ | ||||||
| Private | 61.1 | 66.1 | <0.05 | 6.6 | 8.1 | 0.47 |
| Public | 46.3 | 42.9 | <0.05 | 82.3 | 77.6 | 0.14 |
| None | 9.6 | 6.6 | <0.01 | 4.1 | 5.3 | 0.45 |
| Current smoking# | 32.0 | 30.3 | 0.20 | 79.2 | 75.6 | 0.35 |
| Alcohol consumption& | 15.0 | 13.5 | 0.20 | 46.7 | 32.4 | <0.01 |
| Current injection drug use | 2.8 | 1.6 | 0.01 | 32.0 | 17.4 | <0.01 |
| Non-injecting recreational drug use | 54.1 | 47.0 | <0.01 | 26.4 | 17.8 | <0.01 |
| CD4 count at visit (cells/mm3), mean (SD) | 570.2 (284.2) | 593.3 (275.9) | <0.05 | 399.7 (311.4) | 372.5 (231.0) | 0.14 |
| Viral load suppression | 70.1 | 81.2 | <0.01 | 52.8 | 67.2 | <0.01 |
| Depression status^ | 23.3 | 20.6 | 0.03 | 37.8* | 29.1* | 0.04 |
Population restricted to participants seen since 2009
public & private not mutually exclusive
Current smoking (including occasional smoking)
MACS: moderate-to-heavy consumption, ALIVE: drank >1 day/week
CES-D (≥16)
2006-2011
In the MACS, the use of multiple pill regimens which were PI-based and NNRTI-based declined from 51% and 39%, respectively in 2006, to 36% and 16%, respectively in 2011. Concomitantly, the use of newer regimens –single pill, and INSTI-based has increased steeply over time from 5.7% and 0% in 2006 to 27% and 19% in 2011, respectively. In the ALIVE study, the use of PI-based and NNRTI-based regimens also declined from 70% and 23%, respectively in 2006, to 60% and 5%, respectively in 2011. The use of single pill and INSTI-based regimens rose from 0% to the current use of 21% and 12% in 2011.
Adherence over time
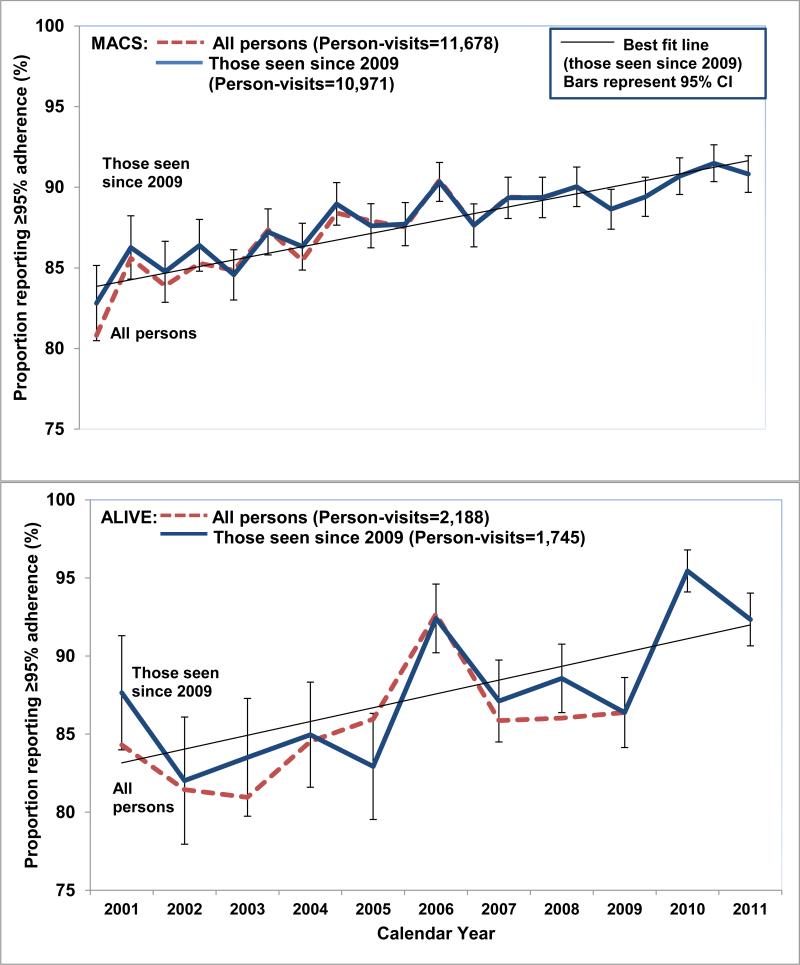
As shown in Figure 1, the proportion reporting 100% adherence increased in both cohorts from 2001 through 2011. Restricting the population to those seen from 2009-2011, the increases in reporting 100% adherence from 2001-2011 was 84% to 90%, and 87% to 92% in the MACS and ALIVE study, respectively. Table 2 shows the results from linear mixed models used to examine the change in adherence over time. There was an increase in the average adherence over time in the MACS after accounting for within-person changes - an 11% increase in average adherence every two years, and a 33% increase in average adherence in the latter era compared to the earlier era. Adjusting for confounders attenuated the change in adherence over time in the population. There was significant variability (σ 22=3.3 (2.8, 3.9)) in the change of adherence over time. In the ALIVE study, there was a 14% increase in the average adherence every two years, and a 22% increase in the adherence in the latter era compared to the earlier era using the results from the adjusted model. There was significant variability in the change of adherence over time (σ 22=3.1 (1.3, 7.3)), and high variability in this trajectory in the ALIVE (Root Mean Square error: 11.69) as seen in Figure 1.
Figure 1. Proportion reporting ≥95% adherence over time in the MACS and ALIVE study (2001-2011).
The red dashed and blue solid lines represent the trends in reporting ≥95% adherence among all persons using HAART, and those seen since 2009, respectively. The thin black line represents the best fit line for those seen since 2009. The error bars represent 95% confidence intervals (CI) for observed proportions for those seen since 2009.
Table 2.
Change in adherence over time (2001-2011)
| Model | Time | Unadjusted estimate | Unadjusted estimate | Adjusted estimate | Adjusted estimate |
|---|---|---|---|---|---|
| MACS | ALIVE | MACS& | ALIVE# | ||
| Model 1: Mixed | Per 2- year interval | 0.11 (−0.06, 0.27) | 0.18 (−0.25, 0.61) | −0.03 (−0.22, 0.16) | 0.14 (−0.35, 0.64) |
| Model 2: Mixed | 2006-11 vs. 2001-05 | 0.33 (−0.14, 0.81) | 0.39 (−0.96, 1.74) | 0.05 (−0.47, 0.57) | 0.22 (−1.26, 1.69) |
Adjusted for age, race, alcohol use, smoking, type of HAART, and non-injection drug use
Adjusted for age, alcohol use, non-injection recreational drug use, injection drug use, smoking, and visit interval
Almost 25% of HAART users in the MACS reported at least one gap in treatment and 12.9% reported multiple gaps of HAART such that HAART use was not reported for 7.7% of 13,339 person-visits following initiation. In the ALIVE, 73% of HAART users reported at least one gap in treatment, and 53.4% reported multiple gaps such that HAART use was not reported for 34.6% of 3,426 person-visits following initiation.
HIV RNA suppression
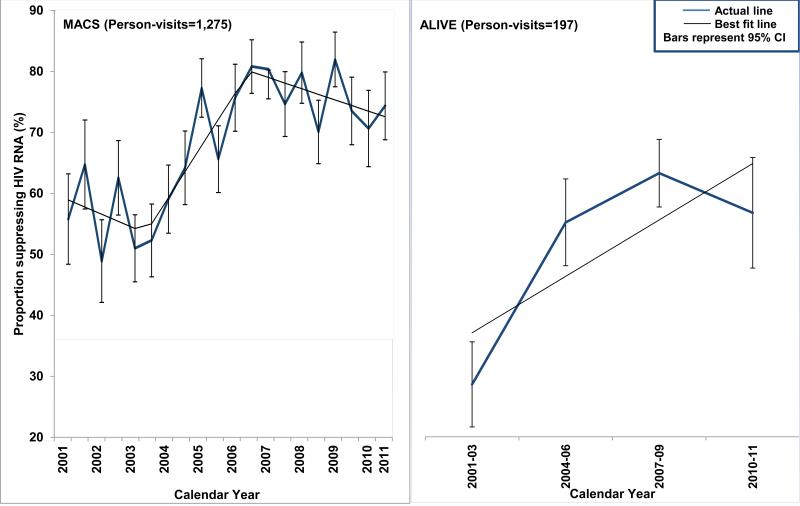
Overall, 79.9% of the MACS person-visits had undetectable HIV RNA since 2001 (Supplemental Table 1), and the proportion suppressing HIV RNA in the MACS participants with <95% adherence increased since 2001, and ranged between 75% and 79% since 2006 (Figure 2). For the MACS, the model with time included as a piecewise linear term (AIC: 130.1) was a better fit than time modeled as a linear term (AIC: 138.2), or as a polynomial term (AIC: 139.2). For the ALIVE study, the model with time included as a linear term (AIC: 32.24) was a better fit than time modeled as a quadratic term (AIC: 33.74).
Figure 2. Proportion suppressing HIV RNA (<50 copies/mL) over time among participants with <95% adherence in the MACS and ALIVE study from 2001-2011.
The blue thick line represents the observed proportion suppressed, and the black thin line represents the best fit line. The error bars represent 95% CI for the observed proportions.
Minimum optimal adherence
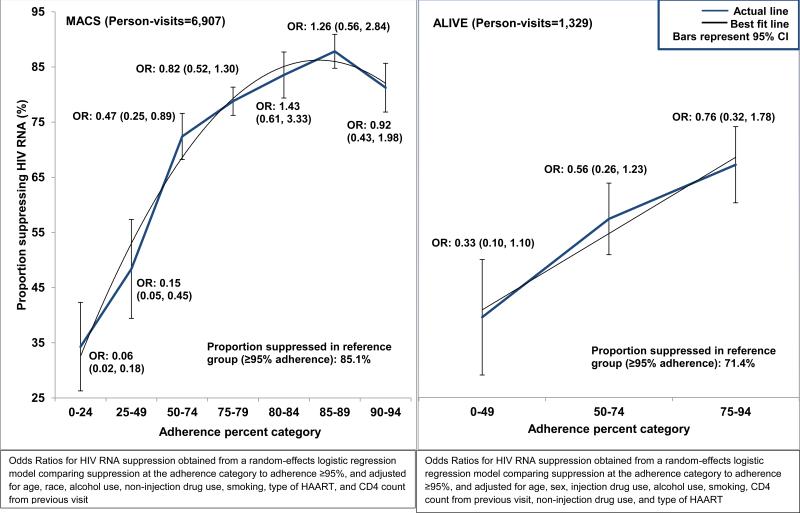
In the current era (2006-11), the proportion suppressing HIV RNA increased with increasing adherence (Figure 3). At adherence levels between 80% and 84%, the proportion suppressing HIV RNA was greater than 80% (83.5%). For those with ≥95% adherence, 85.1% had undetectable HIV RNA levels. Random-effects logistic regression models with viral load suppression as outcome, adjusted for age, number of drugs, recreational non-injection drug use, alcohol use, race, smoking, lagged CD4 cell count, and type of HAART, confirmed that at adherence levels between 80% and 84%, the odds of viral load suppression were not significantly different than that among those with adherence levels ≥95%. Although HIV RNA suppression was significantly less likely among those with a gap in treatment (OR: 0.61 (0.55, 0.67)), there was no statistically significant interaction between adherence and having at least one gap in treatment.
Figure 3. Proportion suppressing HIV RNA (<50 copies/mL) by HAART adherence category (2006-2011).
The blue thick line represents the observed proportions, and the black thin line represents the best fit line. The error bars represent 95% CI for the observed proportions. The odds ratios and 95% CI were obtained from a random-effects logistic regression model comparing the HIV RNA suppression at lower adherence levels to adherence ≥95%, and adjusting for confounders. In the MACS, confounders were age, race, alcohol use, non-injection drug use, smoking, type of HAART, and CD4 count from previous visit. In the ALIVE study, confounders were age, injection drug use, alcohol use, smoking, CD4 count from previous visit, non-injection drug use, and type of HAART.
In the ALIVE study, we did not observe a minimum optimal adherence cutoff below 95% because less than 80% of the population was suppressed among those with ≥95% adherence (71.4%). Further, the adjusted odds of viral load suppression were appreciably lower with levels of adherence <95%, compared to the odds of viral load suppression at ≥95% adherence, although not statistically significant. Among those reporting ≥95% adherence, those who were currently injecting drugs were less likely to suppress HIV RNA than those not injecting drugs (55.4% vs. 74.8%, P<0.001). Similar to that seen in the MACS, the odds of suppression was significantly lower among those with a gap in treatment (OR: 0.50 (0.36, 0.69)), and there was no statistically significant interaction between adherence and having at least one gap in treatment.
DISCUSSION
In these prospective cohorts of HIV-infected MSM and IDUs, there was an observable increase in the proportion reporting ≥95% adherence to HAART between 2001 and 2011. Our data are consistent with the hypothesis that adherence to HAART has become easier over time with newer and simpler HAART formulations. This concurs with previous studies reporting increased ease of adherence to once-daily regimens compared to multi-dose regimens (10, 24, 25). Both cohorts reported an increase in the use of single pill regimens since 2006 (MACS: 5.7% to 27.2%, ALIVE: 0 to 21.4%, in 2011).
Newer drugs have also made viral load suppression possible at adherence levels lower than the 95%. Second-generation PIs (e.g., darunavir and tipranavir), NNRTIs (e.g., rilpivirine, etravirine), and newer classes such as INSTIs (e.g., raltegravir), enable durable viral load suppression with generally easier administration owing to high potency and improved pharmacokinetic profiles (26,27). Importantly, they also have improved tolerability profiles, which may lead to better adherence. While newer HAART formulations may now be easier to administer, they also do not necessarily require consistently high levels of adherence for viral load suppression as suggested in previous studies (26-28).
However, the population-level benefit of these newer formulations may be limited since some marginalized groups are not being prescribed these drugs as often as others. In our study, while more than 50% of MACS participants reported recent use of a newer HAART formulation, fewer than 35% of ALIVE participants were on these HAART regimens in 2011. This finding is consistent with a previous study by Mehta et al which reported that IDUs in Baltimore were initiating care at more advanced disease stages, and were not receiving newer HAART regimens (29). The low proportion of suppressed visits in the ALIVE may thus in part be attributed to drawbacks of using older HAART regimens with shorter half-lives, increased pill burden, poor tolerability and drug resistance. However, it is also likely that the observation of a lower overall viral load suppression rate in the ALIVE study reflected a higher frequency of treatment gaps and greater barriers to consistent HAART use including frequent homelessness, incarceration, ongoing substance use, and more limited insurance. Discontinuous HAART use in this population (21,30) may also have led to the development of drug resistance and subsequently higher rates of treatment failure.
Our data suggest that adherence levels as low as 80% to 84% may be sufficient for viral load suppression in populations using newer HAART formulations. This is consistent with literature suggesting that chronically ill patients using 80% of their medications, are generally categorized as being adherent to their treatment (31). However, this message should be interpreted with caution. While our study points to lower adherence levels for effectiveness than previously established, the goal is not to encourage patients to be less adherent to medications. It is important for HIV providers to continue emphasizing the importance of 100% medication adherence. However, keeping in mind that some patients may not be as adherent to their medications due to specific barriers, they can divert resources for comprehensive counseling sessions towards patients with barriers to adherence.
Important predictors of high adherence to HAART and viral load suppression in the MSM cohort were found to be older age, non-Black race, higher CD4 count, and non-use of alcohol, cigarettes or recreational non-injection drugs, consistent with previous studies evaluating predictors of adherence to HAART in the MACS, and in other populations (8,12,32-34).
Similarly, in the ALIVE study, older age, non-use of alcohol, cigarettes, recreational injection and non-injection drugs, and not being incarcerated, were shown to predict high adherence, consistent with other studies of HIV-infected IDUs (35-37).
As stated earlier, it was not possible for us to confirm a minimum optimal adherence cutoff lower than 95% for IDUs in the ALIVE. Although the lack of statistical significance in ORs could be attributed to the small sample size, only 71.4% were suppressed among those with ≥95% adherence, which is less than optimal, and lower, when compared to that observed in previous literature (8). In addition to use of older regimens and low retention in treatment, this study population consisted of low-income individuals with a high proportion of substance use. When stratified by current injection drug use, significant differences were observed in the proportion of individuals suppressed at ≥95% adherence. Therefore, rate-limiting steps to achieving optimal adherence in this population may be patient-related behaviors, in addition to physician prescribing behaviors.
The use of self-reported adherence is a limitation to the study. Self-reported adherence is associated with recall error and social-desirability bias, which may lead participants to overestimate their actual adherence (34). Additionally, self-reported adherence may be less reliable in a population of IDUs (38). This may have led to the relatively low proportion of suppression among those reporting ≥95% adherence in the ALIVE study. Another possible explanation for the lower level of suppression achieved by adherent ALIVE participants may be drug resistance. However, these data were not available. Another limitation may be misclassification of the antiretroviral medications used by the participants. Although cross-checking with medical records would address the reliability of self-report, it would not assess the validity of the actual use. Given that caveat, an earlier study in the MACS did show high agreement between self-reported and prescribed medication use (39).
There were several strengths associated with this study as well. Both the MACS and the ALIVE are long-standing cohort studies examining the natural and treated histories of HIV in two important risk groups in the United States – MSM and IDU – that use standardized methods for data collection, and have relatively low attrition. Although self-reported data for adherence are associated with biases as described earlier, an important strength of our data is that persons were not reporting their adherence to their providers. This may have decreased the social desirability bias to some extent, since providers are more likely to counsel patients with suboptimal adherence, and make changes to their treatment regimen. Participants reported their adherence before their viral load test, and therefore, in addition to temporality of the relationship, there was no bias in the reporting of adherence due to knowing the HIV RNA test outcome.
CONCLUSION
In summary, in the current era of HIV treatment, in addition to the ease of use of newer formulations which make high levels of adherence easy to achieve, improved formulations have made viral load suppression possible at lower adherence levels, which is consistent with evidence from recent studies (7, 40). While in a population of MSM HAART users on newer HAART regimens, being 80% adherent to treatment may be sufficient for viral load suppression, IDUs on older HAART regimens may need to be more than 95% adherent to HAART. In a population with more limited access and poorer engagement in care, the prescription of newer drugs may potentially help alleviate the barriers to treatment, and improve overall treatment outcomes. Future studies should aim to determine whether adherence and viral load suppression among IDUs may be similar to that observed in the MSM population if given the same opportunity for newer regimens.
HIV providers should therefore not let concerns regarding adherence assume primacy and hinder the appropriate use of modern HAART regimens broadly at earlier stages of HIV disease. In parallel, retention and engagement in care should continue to be a primary objective, and this together with more universal prescribing patterns, will potentially improve individual outcomes and indirectly alleviate disease burden in the population. While HIV providers should continue to urge patients to achieve perfect adherence, comprehensive adherence counseling support may be best targeted to persons with more limited engagement in care and those dealing with substance use.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank Multicenter AIDS Cohort Study (MACS) and AIDS Linked to the Intravenous Experience (ALIVE) study participants for their continued dedication. MACS centers (Principal Investigators) are at: Johns Hopkins University Bloomberg School of Public Health (Joseph Margolick), Northwestern University (Steven Wolinsky), University of California, Los Angeles (Roger Detels), University of Pittsburgh (Charles Rinaldo), and the Center for Analysis and Management of MACS, Johns Hopkins University Bloomberg School of Public Health (Lisa Jacobson). The MACS study is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the National Cancer Institute (NCI), National Heart, Lung, and Blood Institute (NHLBI), and the National Institute on Deafness and Communication Disorders (NIDCD): U01-AI35042, U01-AI35040, U01-AI35039, U01-AI35041, UM1-AI35043, and UL1-TR000424 (JHU CTSA). The ALIVE study is funded by the National Institute on Drug Abuse (NIDA), a part of the National Institutes of Health (NIH): DA04334 and DA12568.
REFERENCES
- 1.Global Health Observatory (GHO) [Jun 5, 2013];World Health Organization. Available at: http://www.who.int/gho/hiv/en/.
- 2.Chu C, Umanski G, Blank A, Meissner P, Grossberg R, Selwyn PA. Comorbidity-Related Treatment Outcomes among HIV-Infected Adults in the Bronx, NY. J Urban Health. 2011;88(3):507–516. doi: 10.1007/s11524-010-9540-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adeyemi OM, Badri SM, Max B, Chinomona N, Barker D. HIV infection in older patients. Clin. Infect Dis. 2003;36:1347. doi: 10.1086/374871. [DOI] [PubMed] [Google Scholar]
- 4.Manfredi R. HIV infection and advanced age emerging epidemiological, clinical, and management issues. Ageing Research Reviews. 2004;3(1):31–54. doi: 10.1016/j.arr.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Deeks SG, Phillips AN. HIV infection, antiretroviral treatment, ageing, and non-AIDS related morbidity. BMJ. 2009;338:288–292. doi: 10.1136/bmj.a3172. [DOI] [PubMed] [Google Scholar]
- 6.Wada N, Jacobson LP, Cohen M, French A, Phair J, Muñoz A. Cause-Specific Life Expectancies After 35 Years of Age for Human Immunodeficiency Syndrome-Infected and Human Immunodeficiency Syndrome-Negative Individuals Followed Simultaneously in Long-term Cohort Studies, 1984–2008. Am J Epidemiol. 2013;177(2):116–125. doi: 10.1093/aje/kws321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kobin BA, Sheth NU. Levels of Adherence Required for Virologic Suppression Among Newer Antiretroviral Medications. Ann Pharmacother. 2011;45:372–9. doi: 10.1345/aph.1P587. [DOI] [PubMed] [Google Scholar]
- 8.Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133:21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 9.Nelson M, Girard PM, DeMasi R, et al. Suboptimal adherence to darunavir/ritonavir has minimal effect on efficacy compared with lopinavir/ritonavir in treatment-naïve HIV-infected patients: 96 week ARTEMIS data. J Antimicrob Chemother. 2010;65:1505–9. doi: 10.1093/jac/dkq150. [DOI] [PubMed] [Google Scholar]
- 10.Cooper V, Horne R, Gellaitry G, et al. The impact of once-nightly versus twice-daily dosing and baseline beliefs about HAART on adherence to efavirenz-based HAART over 48 weeks: the NOCTE study. J Acquir Immune Defic Syndr. 2010;53(3):369–77. doi: 10.1097/QAI.0b013e3181ccb762. [DOI] [PubMed] [Google Scholar]
- 11.Chesney M. Adherence to HAART regimens. AIDS Patient Care STDS. 2003;17(4):169–77. doi: 10.1089/108729103321619773. [DOI] [PubMed] [Google Scholar]
- 12.Silverberg MJ, Leyden W, Horberg MA, DeLorenze GN, Klein D, Quesenberry CP., Jr Older age and the response to and tolerability of Antiretroviral therapy. Arch Intern Med. 2007;267:684–691. doi: 10.1001/archinte.167.7.684. [DOI] [PubMed] [Google Scholar]
- 13.Gulick RM. Adherence to antiretroviral therapy: how much is enough. Clin Infect Dis. 2006;43(7):942–904. doi: 10.1086/507549. [DOI] [PubMed] [Google Scholar]
- 14.Westergaard RP, Ambrose BK, Mehta SH, Kirk GD. Provider and clinic-level correlates of deferring antiretroviral therapy for people who inject drugs: a survey of North American HIV providers. J Int AIDS Soc. 2012;15(1):10. doi: 10.1186/1758-2652-15-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zaric GS, Bayoumi AM, Brandeau ML, Owens DK. The Cost-Effectiveness of Counseling Strategies to Improve Adherence to Highly Active Antiretroviral Therapy among Men Who Have Sex with Men. Med Decis Making. 2008;28:359–376. doi: 10.1177/0272989X07312714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. [July 14, 2013];Good Evidence Medication Adherence Interventions. Available at: http://www.cdc.gov/hiv/topics/research/prs/ma-good-evidence-interventions.htm.
- 17.Westergaard RP, Hess T, Astemborski J, Mehta SH, Kirk GD. Longitudinal changes in engagement in care and viral load suppression for HIV-infected injection drug users. AIDS. 2013;27(16):2559–66. doi: 10.1097/QAD.0b013e328363bff2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. [Jun 20, 2013];Monitoring HIV Care in the United States. Available at: http://www.iom.edu/~/media/Files/Report%20Files/2012/Monitoring-HIV-Care-in-the-United-States/MonitoringHIV_rb.pdf.
- 19.Kaslow RA, Ostrow DG, Detels R, Phair JP, Polk BF, Rinaldo CR., Jr The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol. 1987;126:310–8. doi: 10.1093/aje/126.2.310. [DOI] [PubMed] [Google Scholar]
- 20.CDC 1993 Revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. [Jun 10, 2014];MMWR. 1992 41 [No. RR-17]. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/00018871.htm. [PubMed] [Google Scholar]
- 21.Vlahov D, Anthony JC, Munoz A, et al. The ALIVE study, a longitudinal study of HIV-1 infection in intravenous drug users: description of methods and characteristics of participants. NIDA Res Monogr. 1991;109:75–100. [PubMed] [Google Scholar]
- 22.Panel on Antiretroviral Guidelines for Adults and Adolescents . Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; [Jul 1, 2013]. Available at http://www.aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. [Google Scholar]
- 23.Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 24.Gallant JE, DeJesus E, Arribas JR, et al. Tenofovir DF, emtricitabine, and efavirenz vs. Zidovudine, lamivudine and efavirenz for HIV. N Eng J Med. 2006;354:251–260. doi: 10.1056/NEJMoa051871. [DOI] [PubMed] [Google Scholar]
- 25.Cooper V, Horne R, Moyle G, Fisher M, The SWEET study group Simplification with easier emtricitabine and tenofovir (SWEET): results of a 48 week analysis of patients’ perceptions of treatment and adherence; The XVII International AIDS Conference; Mexico City, Mexico. August 3–8, 2008; [abstract] [Google Scholar]
- 26.Hughes CA, Robinson L, Tseng A, Macarthur RD. New antiretroviral drugs: a review of the efficacy, safety, pharmacokinetics, and resistance profile of tipranavir, darunavir, etravirine, rilpivirine, maraviroc, and raltegravir. Expert Opin. Pharmacother. 2009;10(15):2445–2466. doi: 10.1517/14656560903176446. [DOI] [PubMed] [Google Scholar]
- 27.Shuter J, Sarlo JA, Kanmaz KA, Rode RA, Zingman BS. HIV-infected patients receiving lopinavir/ritonavir-based antiretroviral therapy achieve high rates of virologic suppression despite adherence rates below 95%. J Acquir Immune Defic Syndr. 2007;45(1):4–8. doi: 10.1097/QAI.0b013e318050d8c2. [DOI] [PubMed] [Google Scholar]
- 28.Maggiolo F, Airoldi M, Kleinloog HG, et al. Effect of Adherence to HAART on Virologic Outcome and on the Selection of Resistance-Conferring Mutations in NNRTI- or PI-Treated Patients. HIV Clin Trials. 2007;8(5):282–92. doi: 10.1310/hct0805-282. [DOI] [PubMed] [Google Scholar]
- 29.Mehta SH, Kirk GD, Astemborski J, Galai N, Celentano CD. Temporal Trends in Highly Active Antiretroviral Therapy Initiation among Injection Drug Users in Baltimore, Maryland, 1996–2008. Clinical Infectious Diseases. 2010;50(12):1664–1671. doi: 10.1086/652867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kavasery R, Galai N, Astemborski J, et al. Nonstructured treatment interruptions among injection drug users in Baltimore, MD. J Acquir Immune Defic Syndr. 2009;50(4):360–6. doi: 10.1097/QAI.0b013e318198a800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ho MP, Bryson CL, Rumsfeld JS. Medication adherence: its importance in cardiovascular outcomes. Circulation. 2009;119:3028–3035. doi: 10.1161/CIRCULATIONAHA.108.768986. [DOI] [PubMed] [Google Scholar]
- 32.Kleeberger CA, Phair JP, Strathdee SA, Detels R, Kingsley L, Jacobson LP. Determinants of heterogeneous adherence to HIV-antiretroviral therapies in the Multicenter AIDS Cohort Study. J Acquir Immune Defic Syndr. 2001;26(1):82–92. doi: 10.1097/00126334-200101010-00012. [DOI] [PubMed] [Google Scholar]
- 33.Kleeberger CA, Buechner J, Palella F, et al. Changes in adherence to highly active antiretroviral therapy medications in the Multicenter AIDS Cohort Study. AIDS. 2004;18(4):683–688. doi: 10.1097/00002030-200403050-00013. [DOI] [PubMed] [Google Scholar]
- 34.Lazo M, Gange SJ, Wilson TE, et al. Patterns and predictors of changes in adherence to highly active antiretroviral therapy: longitudinal study of men and women. Clin Infect Dis. 2007;45(10):1377–1385. doi: 10.1086/522762. [DOI] [PubMed] [Google Scholar]
- 35.Vlahov D, Celentano DD. Access to highly active antiretroviral therapy for injection drug users: adherence, resistance, and death. Cad Saude Publica. 2006;22:705–718. doi: 10.1590/s0102-311x2006000400002. [DOI] [PubMed] [Google Scholar]
- 36.Malta M, Magnanini MMF, Strathdee SA, Bastos FI. Adherence to Antiretroviral Therapy Among HIV-Infected Drug Users: A Meta-Analysis. AIDS Behav. 2010;14:731–747. doi: 10.1007/s10461-008-9489-7. [DOI] [PubMed] [Google Scholar]
- 37.Kerr T, Palepu A, Barness G, et al. Psychosocial determinants of adherence to highly active antiretroviral therapy among injection drug users in Vancouver. Antivir Ther. 2004;9(3):407–14. [PubMed] [Google Scholar]
- 38.Kerr T, Hogg RS, Yip B, et al. Validity of Self-Reported Adherence Among Injection Drug Users. J Int Assoc Physicians AIDS Care (Chic) 2008;7(4):157–9. doi: 10.1177/1545109708320686. [DOI] [PubMed] [Google Scholar]
- 39.Cole SR, Jacobson LP, Tien PC, Kingsley L, Chmiel JS, Anastos K. Using Marginal Structural Measurement-Error Models to Estimate the Long-term Effect of Antiretroviral Therapy on Incident AIDS or Death. Am J Epidemiol. 2010;171:113–122. doi: 10.1093/aje/kwp329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bangsberg D. Less Than 95% Adherence to Nonnucleoside Reverse-Transcriptase Inhibitor Therapy Can Lead to Viral Suppression. Clin Infect Dis. 2006;43(7):939–941. doi: 10.1086/507526. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.