Abstract
Objective
To develop a comprehensive set of common data elements (CDEs), data definitions, case report forms and guidelines for use in spinal cord injury (SCI) clinical research, as part of the CDE project at the National Institute of Neurological Disorders and Stroke (NINDS) of the USA National Institutes of Health.
Setting
International Working Groups
Methods
Nine working groups composed of international experts reviewed existing CDEs and instruments, created new elements when needed, and provided recommendations for SCI clinical research. The project was carried out in collaboration with and cross-referenced to development of the International Spinal Cord Society (ISCoS) International SCI Data Sets. The recommendations were compiled, subjected to internal review, and posted online for external public comment. The final version was reviewed by all working groups and the NINDS CDE team prior to release.
Results
The NINDS SCI CDEs and supporting documents are publically available on the NINDS CDE website and the ISCoS website. The CDEs span the continuum of SCI care and the full range of domains of the International Classification of Functioning, Disability and Health.
Conclusions
Widespread use of common data elements can facilitate SCI clinical research and trial design, data sharing, and retrospective analyses. Continued international collaboration will enable consistent data collection and reporting, and will help ensure that the data elements are updated, reviewed and broadcast as additional evidence is obtained.
Keywords: Common Data Elements, Data Sets, Clinical Research, Clinical Outcomes, assessments, spinal cord injury
Introduction
There has been increasing interest in developing common data elements (CDEs) to facilitate start-up of clinical studies and to enable improved coordination, sharing and analyses of research data 1-4. Across the neuroscience community, this endeavor has been guided in large part by the National Institute of Neurological Disorders and Stroke (NINDS), of the National Institutes of Health (NIH)5. The NINDS CDE Project began in 2006 and has resulted in development of General CDEs that can be used across neurological diseases, and disease-specific CDEs, corresponding case report forms (CRFs) and guidance documents for fourteen neurologic diseases and conditions to date, including traumatic brain injury6,7, stroke8, epilepsy9, amyotrophic lateral sclerosis10, Friedreich's ataxia11 and others. Central to this effort is the creation of meaningful common definitions to facilitate the organization, analysis, sharing, and dissemination of data captured and recorded across studies. The stated goals of the NINDS CDE project5,8,9 are:
To disseminate standards for the collection of data from participants enrolled in studies of neurological diseases;
To create easily accessible tools for investigators to collect study data;
To encourage focused and simplified data collection to reduce the burden on investigators and practice-based clinicians to facilitate their participation in clinical research; and
To improve data quality while controlling cost by providing uniform data descriptions and tools across NINDS funded clinical studies.
The use of standardized CDEs can provide a number of benefits for investigators and the research community, including 1) rapid and efficient study start-up by enabling access to defined data elements and case report form templates; 2) improved patient safety by facilitating creation of common report templates for Data and Safety Monitoring Boards; 3) enriched data sharing and data aggregation using standard definitions and forms; and 4) adoption of common outcome measures that may be relevant across neurological diseases. A centralized web site including the data standards and accompanying tools is maintained by the NINDS at (http://www.CommonDataElements.ninds.nih.gov).
CDEs have been developed for each of the neurological disease areas using a common iterative approach. An initial organizing committee of disease experts is convened to define the specific domains for data collection across the disease area. Within each domain, this group then identifies a team of experts to review the state of the field and choose and define the instruments and data elements used for clinical studies. NINDS maintains a hands-off role, enabling the experts to identify the key topics and instruments while providing administrative support and guidance as needed5.
Development of the CDEs for SCI was somewhat unique compared to that of other disease sets with regard to the working process and the breadth of content. From the start, the SCI CDE process included an active collaboration with an existing international effort to create clinically-directed International SCI Data Sets by expert working groups12,13. The International SCI Data Set project began in 2002 to provide “a common language among SCI centers worldwide”. There are currently 21 completed International SCI Data Sets, which provide a working resource of guidelines and data collection forms for widespread use by the international SCI clinical community. The International SCI Data Sets are updated regularly by a process that includes review and approval by the International Spinal Cord Society (ISCoS) and the American Spinal Injury Association (ASIA), which represent the two major international professional societies of SCI clinicians and scientists. The NINDS CDE and ISCoS International SCI Data Set projects have overlapping but distinct goals. Yet, due to the active collaboration approach, they use standard variable names and a common database structure14. To continue to provide open access to both initiatives, the approved International SCI Data Sets, organized by topic and listed by date of approval, are publically available both on the ISCoS website (http://www.iscos.org.uk/international-sci-data-sets) and the NINDS SCI CDE website (http://www.commondataelements.ninds.nih.gov/SCI.aspx).
In addition to the cooperative international process, the SCI CDEs are also somewhat unique because they encompass an exceptionally wide breadth of content. SCI can disrupt long ascending and descending spinal pathways as well as segmental and autonomic neural circuitry. Therefore, the consequences and relevant outcome measures in SCI research can involve biological functions extending below, at, and above the neurological level of the injury. In addition, while the spinal cord level and severity of injury can determine the degree of sensory and motor impairment, SCI also impacts other activities of daily living as a result of impaired function of many body systems (e.g., bladder and urinary, bowel and gastrointestinal, sexual, respiratory, cardiovascular, and thermoregulatory systems). Some other health and quality of life consequences and complications common following SCI include the development of pressure ulcers, infections, altered bone and muscle composition, impaired mobility and participation, psychosocial distress, and persistent pain. The expert teams also recognized that neurological and functional assessments are sometimes insufficient and can be enhanced by established electrodiagnostics, and that modern imaging techniques which visualize the damaged spinal cord parenchyma now play a critical decision support role in the early assessment and management of SCI. Thus, the working groups agreed that a comprehensive range of outcomes and a correspondingly large number of clinical research CDEs were needed in this field.
The overarching goal of the participants in the SCI CDE project was to provide recommendations to assess all domains of the World Health Organization International Classification of Functioning, Disability and Health (ICF), including personal factors and body structures and functions, as well as activity, participation, and environmental factors (WHO, 2001)15. The selection and recommendation of data elements and instruments were also designed to consider wide variation in the severity of injury, the time of encounter along the SCI continuum (acute vs. chronic injuries) as well as the time of onset and etiology of non-traumatic SCI.
Methods
Development of NINDS CDEs for Spinal Cord Injury
The SCI CDE project began in 2012 as part of the larger NINDS CDE effort. An organizing committee with representatives from ISCoS, ASIA, and the NINDS was convened at an international meeting. The committee invited SCI experts to form working groups (WGs), each composed of five to seven members with knowledge and experience relevant to the primary clinical SCI domains. These experts included clinicians, clinical researchers, clinical trial experts, and representatives from industry as well as private and public funding organizations. The WGs were initially organized into the following domains: 1) Demographics; 2) Care; 3) Neurological outcomes; 4) Functional outcomes; 5) Participation and Quality of Life; 6) Electrodiagnostics; and 7) Imaging. These domains were chosen by discussion and consensus of the initial organizing committee, with the understanding that the process would likely unveil important areas that would require further consideration. Indeed, soon after starting, an additional WG was created to address Pain outcomes, and after several meetings, a subgroup was recruited to specifically address Psychological outcome measures, bringing the total to nine WGs (see Acknowledgements). The Chair of each WG, together with the organizing committee, constituted an advisory team that communicated throughout the development phase to coordinate goals and to identify shared solutions. Each WG was tasked with identifying existing data elements and/or assessment instruments in the assigned domain and to provide guidelines and recommendations for their use in SCI clinical studies. The WGs developed CRFs by selecting the most relevant items from existing CDEs and instruments, or identified and recommended the use of copyrighted instruments, or, when it was necessary, they developed new CDEs, instruments and recommendations de novo. Brief details of how this was done in the individual groups are described below.
Terminology of the NINDS CDEs
Consistent with guidance across the NINDS CDE project, the WGs were also charged with classifying each of the recommended SCI CDEs and instruments as “Core”, “Supplemental” or “Exploratory” according to the following definitions:
Core CDE: A data element identified for use by all SCI studies, and strongly encouraged for use by any SCI study. These are few in number, and they are used to provide consistent data items across all studies, especially regarding basic participant information. The Core SCI CDEs also include those CDEs defined by the NINDS as “Core for All Neurological Diseases”.
Supplemental CDE: A data element which is recommended for collection and use for a significant proportion of SCI clinical research studies, but the relevance for each study depends upon either the study design (e.g., clinical trial, cohort study, acute or chronic, Phase I/II or III, etc.) or the type of research or intervention (e.g. inpatient vs. community or survey, epidemiology vs. rehabilitation). Supplemental CDEs constitute the majority of the recommendations of the NINDS project as a whole and the majority of SCI CDEs. Within the Supplemental CDEs, the WGs categorized those that are most highly recommended for specific types of studies as “Supplemental/Highly Recommended”. This designation was used for CDEs of exceptionally high relevance, with strong validity and psychometric properties and wide support from the international SCI clinical community.
Exploratory CDE: A data element that may fill a current gap in the CDE panel, but which requires further validation before reaching a consensus recommendation. For the SCI WGs, CDEs were classified as “Exploratory” either because they lacked validity testing, robust psychometrics, or, despite widespread use for other conditions, they lacked evidence of validity in SCI research studies at the time of the initial CDE development.
A Conceptual Framework
Managing the depth and breadth of SCI data required an organizational framework to help visualize the essential data categories and to allow WG autonomy while minimizing gaps and overlapping efforts. The goal of the approach was to maintain the commitment to include the full continuum and severity of SCI and encompass the complete range of ICF domains. The Care WG thus built on the experience of the NINDS traumatic brain injury CDE project6, and conceived a working map to address the CDE concept organization (Table 1, and see Care WG below). Each stage along the SCI care continuum (left column) was associated with the relevant ICF domains. The main categories were defined and then the concept domains were organized, using descriptive terms consistent across the NINDS CDEs http://www.commondataelements.ninds.nih.gov/SCI.aspx#tab=Data_Standards. Finally, SCI-specific subdomains were identified and the resulting topics were distributed among the WGs for review and recommendations.
Table 1. Data Element Structural Map: Data Concepts Spanning the Care Continuum and WHO-ICF Domains.
| SCI Continuum | ICF Domain | Main Category | Concept Domain | Subdomains |
|---|---|---|---|---|
|
| ||||
| Participant and Family History | Personal Factors | Participant Characteristics | Demographics | Demographics Socioeconomics |
|
| ||||
| Personal Factors | General Health History | Participant History Family Medical History | Medical history Medication history Behavioral history |
|
| Family Medical History | Medical conditions and diagnoses | |||
|
| ||||
| History of Injury and Pre-hospital Care | Environmental Factors | History of Injury | Injury Onset Date/Etiology | Traumatic SCI |
| Non-traumatic SCI | ||||
|
| ||||
| Body Functions and Structures | Pre-hospital Care | Pre-hospital Assessments | SCI assessments | |
| Other medical assessments and treatments | ||||
|
| ||||
| Hospital Management | Environmental Factors | Care History | Acute Management | Admissions/discharges Medications, Adverse Events |
| Rehabilitation Management | Admissions/discharges Medications, Adverse Events | |||
|
| ||||
| Clinical Assessments and Examinations | Body Functions and Structures | Outcomes and Assessments | Neurological Assessments | Neurological exam |
| Spinal cord imaging; injury measurements, features | ||||
| Electrodiagnostics | ||||
|
| ||||
| Body Functions and Structures | Outcomes and Assessments | Whole Body Assessments | Physical exam and vital signs | |
| Spinal column diagnosis | ||||
| Pain assessment | ||||
| Other body systems assessments | ||||
| Laboratory tests | ||||
|
| ||||
| Body Functions and Structures | Outcomes and Assessments | Functional Assessments | Skeletomuscular function | |
| Activity | Functional Assessments | Overall function and independence | ||
| Participation | Psychological Assessments | Participation and quality of life | ||
| Psychological outcomes | ||||
|
| ||||
| Treatments and Interventions | Body Functions and Structures | Surgical Interventions and Procedures | Spinal Surgery | Spinal surgery level, approach, decompression, open reduction |
| Spinal Interventions | Other spinal interventions including experimental spinal surgical, drug, biologics, devices and other spinal therapies | |||
| Body Functions and Structures | Other Interventions | Other Surgeries and Procedures | Surgeries and procedures of other organ systems | |
| Systemic Interventions | Systemic treatments including experimental drug, biologics, devices and other systemic therapies | |||
|
| ||||
| Therapies | Activity | Rehabilitation | Musculoskeletal System | Bed rest, external mobilization Physical rehabilitation |
| Activity | Other Organ Systems | Other medical rehabilitation | ||
| Participation | Mental Health | Other rehabilitation | ||
|
| ||||
| Community Living | Body Functions and Structures | Outcomes and Assessments | Neurological Assessments | Neurological exam |
| Other Clinical Assessments | Spinal Cord Imaging, injury measurements, features | |||
| Other body systems assessments | ||||
| Pain assessments | ||||
|
| ||||
| Body Functions and Structures | Outcomes and Assessments | Functional Assessments | Skeletomuscular function | |
| Activity | Overall function and independence | |||
| Participation | Psychological Assessments | Participation and quality of life | ||
| Psychological assessments | ||||
|
| ||||
| Environmental Factors | Community Interactions | Access and limitations Caregiver burden | ||
Abbreviations: WHO-ICF, World Health Organization International Classification of Functioning, Disability and Health; SCI, Spinal cord injury
Review Process
After compiling the CDEs and definition tables, recommendations, CRFs, and guidelines from each of the WGs, the draft documents were disseminated for internal review by the full panel of WG experts and the NINDS CDE project team. Changes and/or clarifications were made as appropriate. The internally approved documents were then introduced and released to the public in May 2014 as Version 0.0, and were made widely available for external review by downloading from the NINDS CDE website, as well through a link from the ISCoS website. To encourage public input, directed notices of the review period and instructions were sent to SCI clinicians and researchers, industry representatives, research centre directors and SCI organizations, including research foundation leaders and consumers. Members of ISCoS were informed via a newsletter, and a formal announcement was made at the 2014 ASIA Annual Scientific Meeting. Interested parties provided comments that were then compiled and discussed by each of the WGs and further revisions were made. Version 1.0 of the NINDS SCI CDEs was released on June 30 and revised on August 30, 2014.
Results
WG Process
Each of the WGs proceeded with slightly different approaches, which were largely dependent on the status of existing data standards and elements. For example, the Demographics and Care WGs covered domains with the most cross-over to other clinical conditions and neurological diseases, and thus, they started with and selected data elements from many existing individual CDEs and added a few available instruments that were most appropriate for SCI studies. The Neurological and Functional outcomes WGs both were also required to critically evaluate the current reliability and validity of historically accepted and newer SCI clinical outcome measures to make recommendations for future studies. The Participation and Quality of Life WG, the Pain WG and the Psychological outcomes WG each covered domains that are less well established for SCI studies, but instead draw from a wide array of current and validated assessment tools outside of the SCI clinical area. Finally, the Electrodiagnostics and Imaging WGs developed entirely new CDEs, CRFs and clinical instruments, as there was very little in the way of established outcome measures or guidelines for use in SCI studies.
To select the CDEs for consideration, each WG had a chairperson who, in cooperation with the NINIDS CDE project staff initially collected potential CDEs, measures, and tools for discussion in the group. In monthly WG meetings individuals in each group were typically assigned subdomains in their area of expertise, and researched existing outcome measures and tools for evidence of validity, reliability and acceptance in the community. All items were discussed and the decisions of which items to include and how they should be classified were made in subsequent teleconference calls by consensus of all WG members. All procedural or cross-WG questions or concerns were brought up for review by the full organizing committee.
WG Recommendations
The SCI CDE Version 1.0 release includes over 1150 distinct CDEs, many of which are compiled into template CRFs. As an example, the CRF used to collect data elements on the history of injury is shown in Figure 1. The final CDEs include seven Core elements that are required for all NINDS CDE sets, as well as two SCI-specific Core CDEs, the date and etiology of injury, and one Core instrument, the International Standards for Neurological Classification of Injury (ISNCSCI). There are eight Supplemental instruments classified as Highly Recommended for use in clinical SCI research whenever appropriate (each instrument includes several individual CDEs). The remainder of the CDEs and instruments are classified as either Supplemental or Exploratory. The detailed list and breakdown of all of the recommended CDEs and instruments by WG domain and classification is provided in Table 2 (see refs 16-37). This table will be available on the main NINDS SCI CDE website page and updated as indicated by future review recommendations. Specific considerations of each of the WGs are described briefly in the sections below.
Figure 1.
Example of a case report form, in this case a form to record elements related to the history of injury. The forms directly support the underlying common data structure, and each form includes specific user instructions to enable permissible and valid data entry. Asterisks * are used to designate those elements that are considered Core, to be collected in all spinal cord injury studies.
Table 2. Summary of National Institute for Neurological Disorders and Stroke (NINDS) Spinal Cord Injury Common Data Elements (CDEs) and Instruments: Listed by Working Group Assigned Domains and Subdomains.
The categories and concept domains from Table 1 were divided among the WGs for detailed recommendations, with attention paid to minimizing overlap and gaps as described in the text. This table lists each of the elements and instruments that were recommended by the expert WGs as well as the case report forms (CRF) that were created by the WG from non-copyrighted sources.
| WG Domain | Core | Supp./Highly Recommended | Supplemental | Exploratory |
|---|---|---|---|---|
|
| ||||
| Demographics WG | ||||
| Demographics and Socioeconomics | * Gender * Birthdate * Race (extended) * Ethnicity * Education years |
Age Marital status Number in household Area of residence (city, town size) Primary occupation Secondary occupation Type of occupation |
Citizenship Birth country Family income range Income and basic needs status |
|
|
| ||||
| Care WG | ||||
| Participant and Family History | * Medical Condition (SNOMED CT Code) * Medical HistoryTerm |
Medical History CRFa Prior and Concomitant Medications CRFb Alcohol and Tobacco Use CRFc Substance Use CRF |
Family History CRF Medical history, additional questionsa Alcohol Use Disorders Identification Test (AUDIT)© Food Frequency Questionnaire (FFQ) © |
|
| History of Injury and Pre-hospital Care | Date of visit Date of injury Injury etiology |
History of Injury CRFd Pre-Hospital Assessment CRFe(including the Glasgow Coma Scale, Abbreviated Injury Scale, and Injury Severity Scale) |
History of Injury CRF (Non-traumatic etiology)d | |
| Hospital Management | Acute Admission/Discharge CRFf Adverse Events CRF |
|||
| Clinical Assessments and Examinations (Including non‐ neurological body systems and sleep assessments) | Rehabilitation Admission/Discharge CRFf Physical Exam CRF Clinical Assessment CRFg Vital Signs and Tests CRFh Laboratory Tests; serum lipid profile from the Internat. SCI Endocrine and Metabolic Basic Data Set19 The American Academy of Sleep Medicine (AASM) International Classification of Sleep Disorders (ICSD) Criteria © |
SCI Pressure Ulcer Scale (SCIPUS) © Clinical Assessment, additional questionsg Braden Scale © Swallowing Disturbance Questionnaire (SwDQ)© NIH Laboratory Tests CRF Berlin questionnaire © Epworth Sleepiness Score (ESS)© Functional Outcomes of Sleep Questionnaire (FOSQ) © Pittsburgh Sleep Quality Index (PSQI) © |
||
| Treatments, Interventions & Therapies | Surgical and Procedural Interventions CRFi Other Investigational Treatments or Clinical Trials CRF |
Rehabilitation Therapies CRF (New)j | ||
|
| ||||
| Neurological WG | ||||
| Sensory & Motor Impairment | ISNCSCI35(International Standards for the Neurological Classification of Spinal Cord Injury) | Change in ISNCSCI Motor Score or Motor Level | ||
| Reflexes & Spasticity | Modified Ashworth Scale for Grading Spasticity © | NINDS Myotatic Reflex Scale © Pathological Reflex Tests © Tardieu Scale © Spinal Cord Assessment Tool for Spastic Reflexes (SCATS) © Penn Spasm Frequency Scale © Pendulum (Wartenberg) Test © |
||
|
| ||||
| Functional WG | ||||
| Gait & Balance | 10 Meter Timed Walk © 6 Minute Walk Test © Berg Balance Scale (BBS) © | 2 Minute Walk Test © Five Times to Sit and Stand Test © Spinal Cord Injury Functional Ambulation Inventory (SCI-FAI) © Stair Climb © Stride Analysis and Gait Variability © Timed Up and Go (TUG) © Walking Index for Spinal Cord Injury (WISC II) © |
The Activities-based Balance Level Evaluation (ABLE) Scale © | |
| Upper Extremity | International SCI Upper Extremity Basic Data Set33 | Capabilities of Upper Extremity Questionnaire © Jebsen-Taylor Hand Function Test © |
Capabilities of Upper Extremity Test (CUE-T) © Graded Redefined Assessment of Strength, Sensibility, and Prehension (GRASSP) © Grasp and Release Test © Nine-Hole Peg Test © Quadriplegia Index of Function (QIF) © Sollerman Hand Function Test © Toronto Rehabilitation Instit Hand Function Test © Tetraplegia Hand Activity Questionnaire © |
|
| Overall Function | Spinal Cord Injury Independence Measure (SCIM) III © | Canadian Occupational Performance Measure (COPM) © Wheelchair Circuit © | Borg Rating of Perceived Exertion (RPE)Scale © Neuromuscular Recovery Scale © Spinal Cord Injury-Functional Index (SCI-FI) © Wheelchair Skills Test (WST 2.4) © |
|
|
| ||||
| Participation & Quality of Life WG | ||||
| Health Related QoL | Short Form Health Survey-36 (SF-36) Walk Wheel Modification for SCI © EuroQol-5 Dimension Questionnaire (EQ-5D) © Qualiveen © World Health Organization Quality of Life Assessment (WHOQOL-BREF) © |
Spinal Cord Injury-Quality of Life (SCI-QOL) © | ||
| Life Satisfaction | Life Satisfaction Questionnaire (LISAT-9) © Satisfaction with Life Scale © |
Quality of Life Index (QLI) – SCI Version © International SCI Quality of Life Basic Data Set36 |
||
| Participation | Craig Handicap and Assessment Reporting Technique (CHART-SF) © | |||
|
| ||||
| Pain & Psychological Outcomes WG | ||||
| Pain | International SCI Pain Basic Data Set Version 2.037 | Multidimensional Pain inventory Pain Severity Subscale (MPI-PS) © | Douleur Neuropathique 4 (DN4) © painDETECT © Neuropathic Pain Questionnaire (NPQ) © Neuropathic Pain Symptom Inventory (NPSI) © Pain Quality Assessment Scale (PQAS)© |
|
| Psychological | * Columbia Suicide Severity Rating Scale | Hospital Anxiety and Depression Scale (HADS)© Patient Health Questionnaire 9 (PHQ-9) © |
Generalized Anxiety Disorder 7-item (GAD-7) Scale © Impact of Events Scale (IES) © Moorong Self-Efficacy Scale (MSES) © Multidimensional Scale of Perceived Social Support © Perceived Manageability Scale (PMnac) Positive Affect and Well-Being Scale of the Neurology-Quality of Life (Neuro-QOL) Measure © |
|
|
| ||||
| Electrodiagnostics WGk | ||||
| Motor tests | Motor evoked potentials (MEPs) CRF (New) | Brain Motor Control Assessment CRF (New) | ||
| Nerve and muscle | Peripheral Nerve Studies CRF (New) | |||
| Sensory tests | Quantitative Sensory Testing (QST) CRF (New) Sympathetic Skin Response CRF (New) Sensory Evoked Potentials CRF (New) |
Electrical Perceptual Threshold CRF (New) | ||
|
| ||||
| Imaging WG | CT angiography (CTA), MR angiography (MRA), magnetization transfer (MT), functional MR (fMRI), perfusion, spectroscopy (MRS), myelin water fraction, diffusion kurtosis imaging (DKI) | |||
| Magnetic Resonance Imaging CRF (New)l Diffusion Tensor imaging (DTI) CRF (New) |
||||
Symbols are defined as follows:
* - Required as NINDS Core Elements for use in All Neurological Diseases and Conditions
© - One or more recommended CDEs come from copyrighted instruments. Copyright information is included on the NINDS-CDE website.
(New)- CRFs and recommendations were created de novo by the WG
Abbreviations:SCI, Spinal cord injury; WG, Working Group; CDE, Common Data Element; CRF, Case Report Form
Footnotes:Many of the CRFs prepared from recommendations of the Care WG included individual CDEs selected from the ISCoS International SCI Data Sets. Footnotes a-j document the source of CDEs derived from the ISCoS International SCI Data Sets where space in the Table was not sufficient.
The Medical History CRF contains Supplemental and Exploratory CDEs, including date (from the International SCI Core Data Set16) and cause(s) of death and body systems function questions (from the International SCI Cardiovascular Function Basic Data Set17, the International SCI Pulmonary Basic Data Set18, the International SCI Endocrine and Metabolic Function Basic Data Set19, the International SCI Musculoskeletal Basic Data Set20, the International SCI Lower Urinary Tract Function Basic Data Set21 and the International SCI Bowel Function Basic Data Set22.
The Prior and Concomitant Medicine CRF contains CDEs selected from the International SCI Lower Urinary Tract Function Basic Data Set21; the International SCI Bowel Function Basic Data Set22;the International SCI Cardiovascular Function Basic Data Set17; and the International SCI Musculoskeletal Basic Data Set20
The Alcohol and Tobacco Use CRF contains a tobacco use CDE selected from the International SCI Pulmonary Basic Data Set18.
The History of Injury CRF contains Supplemental and Exploratory CDEs from the International SCI Core Data Set16, the International Non-Traumatic SCI Basic and Extended Data Sets23 and the International SCI Spinal Column Injury Basic Data Set24.
The Pre-hospital Assessment CRF contains CDEs selected from the International SCI Core Data Set16.
The Acute Admission/Discharge CRF and Rehabilitation Admission/Discharge CRF both contain items from the International SCI Core Data Set16.
The Clinical Assessment CRF contains CDEs selected from the following International SCI Data Sets: Supplementary CDEs were selected from the International SCI Spinal Column Injury Basic Data Set24; the International SCI Pulmonary Function Basic Data Set18; the International SCI Cardiovascular Function Basic Data Set17; the International Lower Urinary Tract Basic Data Set21; All CDEs from the International SCI Urinary Tract Infection Basic Data Set25; and selected CDEs from the International SCI Bowel Basic Data Set22, the International SCI Skin and Thermoregulation Function Basic Data Set26, the International SCI Musculoskeletal Basic Data Set20, the International SCI Endocrine and Metabolic Basic Data Set19, the International SCI Male Sexual Function Basic Data Set27, and the International SCI Female Sexual and Reproductive Function Basic Data Set28; Exploratory CDEs were selected from questions from the International SCI Bowel Function Extended Data Set29.
The Vital Signs and Tests CRF, includes all CDEs from the International SCI Urinary Tract Imaging Data Set30, and selected CDEs from the International SCI Endocrine and Metabolic Basic Data Set 19 (height and weight), the International Cardiovascular Function Basic Data Set 17, the International Skin and Thermoregulation Basic Data Set 26 (temperature), the International SCI Pulmonary Function Basic Data Set 18(pulmonary function tests), and all CDEs from the International SCI Urodynamic Basic Data Set31.
Surgical and Procedural Interventions CRF, includes all CDEs from the International SCI Spinal Interventions and Surgical Procedures Basic Data Set32 and selected CDEs from the International Lower Urinary Tract Function Basic Data Set –surgical procedures21, the International SCI Skin and Thermoregulation Basic Data Set –surgical procedures26, the International SCI Musculoskeletal Basic Data Set –surgical procedure20, the International SCI Bowel Basic Data Set- surgical procedures22; and the International SCI Upper Extremity Basic Data Set – surgical procedures33.
Rehabilitation Therapies This CRF was based largely on the SCIRehab Project34.
Electrodiagnostics WG Recommendations: Detailed recommendations for these CDEs are provided in Table 3.
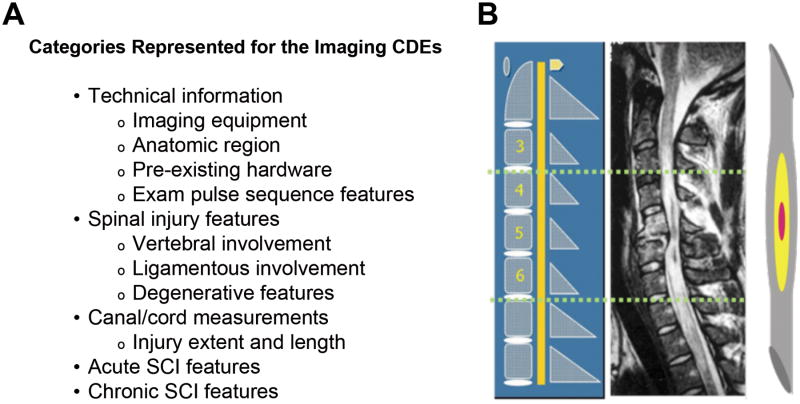
Imaging WG Recommendations: Recommended MRI values are listed in Figure 2A, including technical specifications, spinal injury type, spinal canal and cord measurements, SCI features and locations, and chronic SCI features.
Demographics WG
Demographic and socioeconomic CDEs have been studied extensively and are cross-referenced across many disease areas. This WG reviewed the General NINDS CDEs and then focused on the common variables or measures included in existing SCI registries and clinical studies regarding demographics, socioeconomic status, injury etiology, and vital status. Particular emphasis was placed on the existing elements in the International Spinal Cord Injury Core Data Set16, the Spinal Cord Injury Model Systems (SCIMS) Database38,39, and new elements that are in consideration for inclusion in the International Spinal Cord Injury Socio-Demographic Data Set, which is currently in development. Criteria for inclusion of the demographics CDEs for SCI included utility and perceived acceptability to both United States and international research studies. The NINDS Core Demographics CDEs for all diseases were also included in the resulting CRF.
Care WG
Given the wide range of possible SCI studies and varied care history of potential participants, this WG had the most complex and largest number of subdomains and existing CDEs to consider. The overarching structure they adapted for categorizing the CDEs into subdomains was modeled largely after work conducted to develop data elements for traumatic brain injury6. The Concept Domains covered were participant and family history, history of injury and pre-hospital care, hospital management, clinical assessments and examinations, and treatments, interventions and therapies (Table 1). To prevent overlap of efforts, the Care WG focused on non-neurological body systems, and did not make recommendations for the key clinical assessments and subdomains covered by the other WGs, such as the neurological and functional assessments, pain or psychological assessments, electrodiagnostics or imaging. They did review and provide recommendations regarding instruments to assess sleep.
For the Care Domain, the CDEs found within the International SCI Data Sets were aptly used as the foundation for many of the recommendations, as these were already developed and approved by International SCI experts and represent clinical data sets deemed most relevant to Clinical Care and Assessment factors across the SCI spectrum (refs 16-37). These CDEs were supplemented by items from SCI registries such as the Rick Hansen SCI Registry40 and the North American Clinical Trial Network SCI Registry41, as well as other NIH CDEs and published outcome measures. The NINDS General Core CDEs for Medical History, using the SNOMED CT Code and/or a text based Medical History Term were included in the Medical History CRF to provide consistent reporting across all diseases.
Neurological outcomes WG
This WG emphasized that accurate characterization of the spinal cord level and severity of SCI are fundamental to the prognosis and progress of any recovery associated with a standard of care or therapeutic intervention, especially over the first few months after SCI. The International Standards for the Neurological Classification of Spinal Cord Injury (ISNCSCI) is recognized as the current worldwide standard instrument for the examination and classification of neurological sensorimotor impairment after SCI an essential assessment tool35. The ISNCSCI was thus designated as a Core instrument, required for all SCI clinical studies or intervention trials.
Notably, this WG did not recommend the use of grades (A-E) to describe sensory and motor preservation according to the ASIA Impairment Scale (AIS). They noted that in prior studies, improvement of two AIS grades (e.g. AIS-A to AIS-C) has been recommended and/or used as a clinical trial endpoint or response criterion42-45. However, no experimental treatment to date has been shown to be effective based on this endpoint 42. Furthermore, they noted that in some situations, a two-grade AIS change can represent an improvement that may not be functionally meaningful.
Neurological reflex tests are also valuable for the accurate diagnosis and classification of neurological impairment and can be used to distinguish physiological loss of supraspinal drive from similar loss of function that is due to segmental injury to motor efferents. The NINDS Myotatic Reflex Scale46 was recommended for evaluating reflexes in SCI studies. In addition, the Modified Ashworth Scale, Tardieu Scale, Spinal Cord Assessment Tool for Spastic Reflexes (SCATS), Penn Spasm Frequency Scale, and/or the Pendulum Test were each recommended for use in quantifying spasticity. Additional guidelines for selection and use of these neurological outcome measures and further references and copyright information can be found on the NINDS CDE website.
Functional outcomes WG
The Functional outcomes WG reviewed many sources, including existing SCI functional instruments, NINDS CDEs from other diseases, and the International SCI Data Sets. Those determined to not be measuring true function or deemed inappropriate for SCI clinical research were eliminated. The selection of measures was then narrowed down to include only those with existing psychometric data in SCI. The selected measures were categorized into three subdomains, 1) Gait and Balance, 2) Upper Extremity, and 3) Overall Function (Table 2). In addition to reviewing these sources and the existing literature for each measure, current functional outcome measure recommendations from key organizations were adopted and referenced as appropriate. These included the Spinal Cord Injury Rehabilitation Evidence (SCIRE) website (http://www.scireproject.com/), the SCI EDGE task force website (Evidence Database to Guide Effectiveness; a project of the of the American Physical Therapy Association, APTA, Neurology Section; http://www.neuropt.org/professional-resources/neurology-section-outcome-measures-recommendations/spinal-cord-injury), and the Rehabilitation Measures Database website (http://www.rehabmeasures.org/rehabweb/rhaboutus.aspx).
The time post-injury and level/severity of SCI are necessary qualifiers for selection of the recommended functional outcome measures. For this reason, there are no functional measures that are appropriate for all SCI studies, and thus, no Core elements or instruments were recommended by this WG. Based on validation and widespread clinical acceptance, the 10 Meter Walk Test and 6 Minute Walk Test were designated as Supplemental/Highly Recommended instruments for all studies evaluating gait; the Berge Balance Scale for those evaluating balance; the International SCI Upper Extremity Basic Data Set33 for descriptive characterization of upper extremity function and the Spinal Cord Independence Measure III for overall function. The remaining validated instruments and tools were designated as Supplemental. There were also a number of newer outcome measures designed specifically for SCI functional assessment; these were categorized as Exploratory as there is still a significant amount of psychometric testing required for validation. References for the copyrighted instruments are available on standard forms found on the NINDS SCI CDE website.
Participation and Quality of Life WG
This WG focused on identifying potential instruments from the literature and clinical expertise that address three principle areas relevant to SCI outcome: health related quality of life (HRQOL), life satisfaction, and participation. Specific preference was given to those measures that 1) have been extensively used in SCI, 2) could be applicable to both the acute and chronic situations, or 3) were in an extensive phase of development specific to SCI that encouraged inclusion. In each instance, timing of the assessments was deemed critical. Specifically, during the acute rehabilitation stage, perceptions of HRQOL and life satisfaction are likely to be influenced by adjustment to injury, acute hospitalization, recovery from concomitant injuries, and other factors. In contrast, participation is difficult to assess in the acute stage and is better conceptualized once an individual with SCI has returned to the community and begins to re-integrate into life activities. Seven copyrighted questionnaire instruments were recommended as Supplemental, while three, which require further validation, were listed as Exploratory. In all cases, an emphasis was placed on those instruments and tools that are widely used for health assessment internationally, including the EuroQOL, WHOQoL-BREF, and SF-36® QualityMetrics instruments. One notable instrument that is Recommended for SCI studies is the SCI-QOL©, or spinal cord injury multidimensional quality of life instrument. This was developed using item response theory and computer adaptive testing concepts from the NIH PROMIS47 and Neuro-QOL48 tools, but SCI-QOL49 is adapted specifically for use in the SCI population.
Pain WG
The Pain WG selected evidence-based instruments using both pre-existing frameworks and specific pain-relevant domains. Determination of utility was made based on availability of published psychometric data in the SCI population. Issues unique to SCI, e.g., multiple consequences of SCI and the presence of several concomitant pains, were considered in the development of the SCI pain assessment instruments.
The Pain WG recommended use of the International SCI Pain Basic Dataset (ISCIPBDS37) and the International SCI Pain Classification (also part of the ISCIPBDS50,51) for assessing pain. All domains included in the ISCIPBDS were classified as “Supplemental/Highly Recommended.” The ISCIPBDS is intended to be used in its entirety and was endorsed as such by major SCI (ASIA, ISCoS, Academy of Spinal Cord Injury Professionals) and pain organizations (American Pain Society and the International Association for the Study of Pain) and by individual reviewers. Some domains include several recommended measures, so the choice of specific measure will be dependent on the purpose of the study, similar to the principles of PROMIS47. Notably, the ISCIPBDS is designed to address the presence of multiple pains by evaluating each individual pain problem separately.
Psychological Outcomes WG
Late into the development process, a recognized gap in the list of assement and outcomes led to the development of the ninth WG, focused on Psychological status or Psychological outcomes. After reviewing the available instruments, this WG recommended the Hospital Anxiety and Depression Scale52 and the Patient Health Questionnaire53,54 as Supplemental/Highly Recommended for assessing SCI-related psychological status. All other recommended measures reviewed by the group were classified as Supplemental or Exploratory, where Exploratory measures were those that need further studies to establish their psychometric properties and thus their respective utility for SCI.
Electrodiagnostics WG
The Electrodiagnostics WG had the task of defining sensitive and reliable tests for physiological assessments, with no pre-existing lists or resources for commonly used tests to choose from. The WG thus developed de novo test names and descriptions of the purpose of each, providing guidelines for the level or duration of SCI for the test, creating recommendations for required test equipment and cost as well as needed training, defining the parameters to be measured or calculated, making suggestions for data analysis or interpretation, and defining potential pitfalls, and any relationship between the test and other outcomes. The committee members divided up the tasks for description and then associated CRFs were developed or edited from other CDE efforts. An essential component of the online posted Electrodiagnostics documents is a general introduction to the set of tests, written to explain the rationale for which tests to use and for what purposes. This introduction emphasizes the difference between tests that measure the conduction of electrical signals across the level of SCI (relatively more established) and those that assess processing of those signals by neural circuitry below the level of injury (less established) as well as describes other tests of signal processing that are currently under development. Table 3 gives an overview of the electrodiagnostics recommendations.
Table 3. Electrodiagnostics tests.
| Test | Assessment | Injury Level | Injury Severity | Injury stage (Acute-Chronic) | Comments |
|---|---|---|---|---|---|
| Nerve Conduction Studies (NCS) | Peripheral nerve or root involvement | C2-S3 | AIS A-E | All | Perform across level of potential lower motor neuron (LMN) damage |
|
| |||||
| Electromyography (EMG) | Lower motor neuron, peripheral nerve, muscle function | C2-S3 | AIS A-E | All | Perform across (and below) level of potential lower motor neuron (LMN) damage |
|
| |||||
| Quantitative Sensory Testing (QST) | Sensitivity of clinically impaired sensation | C2-S3 | AIS A-E | All, qualified: Participant must report stimulus intensity. | Multiple measures to ensure validity with day-to-day variation. Not an electrodiagnostic test |
|
| |||||
| Electrical Perceptual Thresholds (EPTs) | Cutaneous sensory perception | C2-S3 | AIS A-E | All, qualified: Participant must report sensitivity to stimulus. | Exploratory, corresponds well with SSEP, but not yet used widely |
|
| |||||
| Somatosensory evoked potentials (SSEPs), can be done by dermatome (dSSEPs) | Large diameter fibers, spinal dorsal column conduction | C2-S5 | AIS A-E: Likely absent in A before intervention | All | Widely used. Amplitude varies, latency is more useful measure. Sensitive to functional deterioration. |
|
| |||||
| Contact Heat/Laser Evoked Potentials (CHEPs, LEPs) | Small diameter fibers, spinothalamic tract conduction | C2-S5 | AIS A-E: Likely absent in A before intervention | All, qualified: Participant must be alert and attentive. | Beginning use in clinical applications in SCI |
|
| |||||
| Motor Evoked Potentials (MEPs) using transcutaneous magnetic stimulation (TMS) | Conduction of cortico-spinal innervation to motor neurons of targeted muscle(s) | C2-T12 | AIS A-E: Likely absent in A/B before intervention | All | False negatives are possible. Consider facilitation techniques if indicated. |
|
| |||||
| Brain Motor Control Assessment (BMCA) (arm, trunk, leg EMG to supraspinal influence) | Motor control, across muscles coordination, “discompleteness”, processing of signals | C2-S3 | AIS A-E | All, qualified: Participant must cognitively cooperate. | Medications can diminish responsiveness. |
|
| |||||
| Sympathetic Skin Responses (SSRs) | autonomic sympathetic outflow | C2-S3 | AIS A-E | All | Further tests required to assess autonomic function of systems other than sweat production. |
Abbreviations: AIS, American Spinal Injury Association Impairment Scale; SCI, Spinal cord injury
Imaging WG
The Imaging WG also developed recommendations in an area where no previous guidelines existed. The WG evaluated a wide range of existing imaging approaches and techniques in common clinical use for spinal trauma and SCI, including radiography, computed tomography (CT) and magnetic resonance imaging (MRI). The CRFs began as a working document that was derived from an amalgamation of anatomic MRI features that have been used successfully in the published literature, and some of the technical information was adapted directly from the traumatic brain injury imaging CDEs. Key factors that can have a direct effect on the imaging features of SCI were taken into consideration including: injury to imaging time interval, the use of methylprednisolone in the acute period, injury acuity or chronicity and if instrumentation was placed before imaging. In addition, instrumentation is an obstacle to overall image quality; even non-ferrous instrumentation can significantly hamper visualization of the SCI.
Because MRI possesses the unique capability to non-invasively depict the damaged substructure of the spinal cord, the WG advocated that Imaging CDEs for SCI studies going forward be derived only from MRI datasets. In contrast, radiography and CT are used primarily to visualize the extent of bony injury. The resulting MRI CRF was developed to represent anatomic findings that are routinely discernible on commercial MRI platforms at 1.5 Tesla and above (Figure 2) 55,56. In addition, the WG included diffusion tensor imaging (DTI) data elements, with the rationale that this technology has matured sufficiently that it is available and feasible with most modern clinical systems 57. The CDEs were divided into discrete sections which included: technical parameters, spinal injury characteristics, spinal canal and cord measurements, SCI features and locations and chronic SCI features. Technical parameters (protocols) for obtaining generic DTI of the spinal cord were included as well as a standardized reporting system for DTI.
Figure 2.
General categories represented with the Imaging common data elements (CDEs). A. The case report form (CRF) developed for the MRI CDEs includes elements associated with technical and anatomical information. B. A diagram illustrating the methodology for anatomic localization of spinal cord injury features is included in the CRF. This provides a reproducible method for mapping the location of spinal cord injury features relative to the anatomic spinal level (modified from 55).
Additional imaging techniques, including some that were considered experimental or under development (e.g. computed tomography angiography (CTA), magnetic resonance angiogram (MRA), magnetization transfer (MT), functional MRI (fMRI), perfusion imaging, MR spectroscopy (MRS), myelin water fraction and advanced diffusion methods such as diffusion kurtosis imaging (DKI)), were also discussed. These are listed as Exploratory instruments in Table 2, but CRFs were not created for these. Further guideline documents will be posted on the NINDS CDE website as these methods become more widely used.
Discussion
Implications and Use of the NINDS CDEs for SCI
The NINDS CDEs for SCI (Version 1.0) include over 1150 unique Data Elements. While some of these were created de novo, many of the SCI CDEs are also used across other domains and diseases. Importantly, the widespread and common use and identification of these data elements with their unique IDs and nomenclature will facilitate sharing of data across a wide range of study types. Furthermore, sharing of IDs and cross referencing with the ISCoS International SCI Data Sets will enable a common language across the full spectrum of clinical research studies worldwide.
As with all of the NINDS disease areas, the SCI CDEs are intended to be a resource to facilitate developing, designing and writing protocols for any clinical studies related to SCI. The CRFs and copyrighted instruments are listed on the NINDS CDE website, and the guidelines and recommendations provided with each of the domains should be consulted to help select and apply the relevant items for a particular project. CRFs from noncopyrighted instruments may be downloaded and used without any charge, while links and contact information to obtain necessary permissions or licenses required for copyrighted instruments are provided as needed. Individual CDEs and the SCI specific CRFs can also be located using a CRF Search tool on the NINDS CDE website. These may be downloaded and assembled to accommodate a wide range of study designs using Form Builder tools and can be easily incorporated into computer entry forms for any study sites with established data collection systems. Note, users are advised to keep the selected format, permissible values and nomenclature for each unique element intact and consistent to enable useful data sharing. Copyrighted instruments may not be altered without consultation with the copyright holders.
While realizing the great advantage of a publically available CDE resource, it is important to caution that the NINDS CDEs are recommendations, but are not intended as definitive requirements for study protocols. The selection of CDEs and reading the associated guidelines cannot substitute for the researchers' own judgment and/or collaborative input from experts with experience designing clinical studies and those that are familiar with each of the outcome instruments and tools. Specific recommendations for designing clinical trials have been developed by the combined efforts of a number of organizations as part of the International Campaign for Cures of Spinal Cord Injury Paralysis (ICCP) 58-60.
While the benefits of using the working group consensus approach to develop CDE recommendations are clear, there are also potential limitations of both the process and outcome. The recommendations are clearly based on the current knowledge, experience, and perceptions related to SCI and developed by a subset of all SCI clinical research experts. Some disparate opinions of the strength of evidence, the classifications, or even of the overall ICF framework should be anticipated 61 and the oversight group is designed to consider open discussion and changes in community perception over time. In addition, the NINDS SCI CDEs are intended to be incorporated and relevant across many neurological disease and conditions, so some SCI-specific concerns must be considered in relation to consistency and general use across the larger CDE project. These issues should be minimized through public input as part of the planned ongoing review process described below.
Navigating the NINDS CDE SCI Website
An introduction to the SCI CDE project can be found on the main screen of the SCI CDE webpage cited above. New users should begin with the resources in the “Learn” tab, which provides a project overview, instructions, glossary, references and more. The WG recommended CRFs and corresponding guidelines are listed in alphabetical order in each section, and the underlying data element information (“CDE Details”, containing the CDE IDs, definitions, permissible values, etc.) or copyright instrument information can be downloaded from the adjacent location. Finally, tabs at the top of the main page can be used to search the CDE or CRF data base and to build custom forms for specific study use.
Future Developments and Gaps
The NINDS CDEs are intended to provide a stable resource, while enabling incorporation of new instruments and recognizing validation efforts and changes in impact in the field. Continuing review and further modifications of the CDEs or their classifications will occur in time based on user feedback, new developments, and validation studies. Thus, researchers and the community must be proactive and provide feedback to the NINDS CDE Project team regarding items that are particularly useful, and should be considered for more widespread use, as well as those that should be refined or removed. Those who are developing data archives and sharing platforms should also be aware that modest changes may be made in time, although the goal is to minimize the frequency of extensive revisions in order to maintain the integrity of previously coded data.
During the internal review process, the WG Chairs discussed issues that crossed WG domains as well as gaps in the spectrum of selected CDEs. For example, the use of electrodiagnostics or imaging may be especially informative when applied in combination with particular functional and/or neurological outcomes to reveal information that is lost or cannot be measured using a single approach. An additional issue that has been raised is the need to increase consumer awareness and add relevant input into the CDEs in the ongoing review process. The WGs included non-profit organization representatives and an active consumer and consumer liaison, but with the online resource now widely available, greater engagement with SCI consumers and advocates is encouraged and quite feasible. With regard to content gaps, a review of the final recommendations has revealed a gap in the identification of CDEs to assess community interactions and caregiver activities and burden. This is also an area where we encourage greater engagement and community feedback. There is also clear agreement that, as in traumatic brain injury, many of the recommendations that are appropriate for use with adults with SCI are not valid or easily translated for use with pediatric SCI study participants62. To address this latter concern, the NINDS CDE team has assembled a new WG to review and develop recommendations for Pediatric SCI clinical studies and will release guidelines for these CDEs within the next year. Finally, the oversight committee is also in the process of reaching out to other SCI data registry sources, many of which had representatives on the WG teams, in order to further define and continue collaborative and shared efforts.
Similar to the reliability testing occurring with the International SCI Data Sets 63-65 as outlined by Biering-Sørensen et al.66, changes to the NINDS CDEs will be based on evidence and reviewed first by experts in the field. In keeping with the established collaborative effort, recommendations for additions or revisions to the NINDS CDEs will be subsequently discussed by the NINDS Oversight Committee in collaboration with the International SCI Data Set Committee to ensure continued alignment. Following the first level of review, any significant proposed changes or additions to the CDEs will then be available for public comment, followed by revision prior to posting on the NINDS CDE website. The NINDS CDE project has committed to review the CDEs at approximately 6 month intervals, to ensure these are relevant and up to date. As a stable resource, major changes will be considered only after allowing sufficient time (e.g. 3 years) for the community to use and test the CDEs in a research environment. All suggestions and recommendations can be submitted directly to the Project Officer or the website project managers by using the CONTACT link at the top of all NINDS CDE web pages.
Conclusion
The NINDS CDEs for SCI clinical research provide a wide-ranging resource for investigators, including common standards and tools, variable names, range checks, permissible values, and standard definitions for use across SCI studies. The SCI CDE WGs have volunteered their expertise and time to identify a catalogue of CDEs, including informed guidance documents and recommendations for their use, and have assembled and included relevant references that can be used when designing a broad range clinical studies and trials for SCI. NIH encourages use of the CDEs for all clinical research, patient registries, and other human studies. The use of CDEs is not, at present, a requirement for studies; however, researchers receiving funding from NINDS are advised when preparing grant applications to use these CDEs in CRFs and data management systems whenever possible and to incorporate the CDEs into their required data sharing plans for all clinical research studies and clinical trials.
Acknowledgments
The views expressed here are those of the authors and do not represent those of the National Institutes of Health (NIH), the National Institute of Neurological Disorders and Stroke (NINDS), or the US Government.
Logistics support for this project was provided in part through NIH contract HHSN271201200034C.
The development of the NINDS SCI CDEs was made possible thanks to the great investment of time and effort of all organizing committee and WG members and members of the NINDS CDE Project team, participating from 2007-2014.
Contributors
SCI CDE Organizing Committee
Joanne Odenkirchen, MPH (NINDS CDE Project Officer) - National Institutes of Health/National Institute of Neurological Disorders and Stroke (NIH/NINDS), Bethesda, Maryland, USA
Naomi Kleitman, PhD – NIH/NINDS, Bethesda, Maryland, USA (2008-2012) and Craig H. Neilsen Foundation, Encino, California, USA (2012-present)
Fin Biering-Sørensen, MD, DMSc - Department for Spinal Cord Injuries, Rigshospitalet and Glostrup Hospital, University of Copenhagen, Denmark
Michael DeVivo, DrPH - Department of Physical Medicine and Rehabilitation, University of Alabama at Birmingham, Birmingham, AL, USA
Vanessa Noonan, PhD, PT - Rick Hansen Institute, Vancouver, British Columbia, Canada
Susan Charlifue, PhD - Craig Hospital, Englewood, CO, USA
Linda Jones, PT, MS - Craig H. Neilsen Foundation, Encino, California, USA (2012 - present)
Lyn Jakeman, PhD – NIH/NINDS, Bethesda, Maryland, USA (2013-present)
Working Group Members
Demographics WG
Yuying Chen, MD, PhD (Chair) - University of Alabama at Birmingham, Birmingham, Alabama, USA
Fin Biering-Sørensen, MD, DMSc - Department for Spinal Cord Injuries, Rigshospitalet and Glostrup Hospital, University of Copenhagen, Denmark
Susan Charlifue, PhD - Craig Hospital, Englewood, Colorado, USA
Vanessa Noonan, PhD, PT - Rick Hansen Institute, Vancouver, British Columbia, Canada
Peter New, MBBS, FAFRM - Caulfield Hospital, Alfred Health, Caulfield, Victoria, Australia
Vanessa Noonan, PhD, PT - Rick Hansen Institute, Vancouver, British Columbia, Canada
Marcel Post, PhD - University Medical Center Utrecht and De Hoogstraat, The Netherlands
Care WG
Vanessa Noonan, PhD, PT (Chair) - Rick Hansen Institute, Vancouver, British Columbia, Canada
David Berlowitz, PhD - Victorian Respiratory Support Service Department of Respiratory and Sleep Medicine, Melbourne, Australia
Fin Biering-Sørensen, MD, DMSc - Department for Spinal Cord Injuries, Rigshospitalet and Glostrup Hospital, University of Copenhagen, Denmark
James S. Harrop, MD - Jefferson Medical College, Philadelphia, Pennsylvania, USA
Michael Fehlings, MD, FACS, FRCSC, PhD - Toronto Western Hospital, Toronto, Ontario, Canada
David W. Wright, MD, FACEP - Emory University School of Medicine, Atlanta, GA, USA
Neurological outcomes WG
John Steeves, Professor, ICORD (Chair) - University of British Columbia and Vancouver Coastal Health, Vancouver, BC, Canada
Andrew R. Blight, PhD - Acorda Therapeutics, Inc., Ardsley, New York, USA
Armin Curt, MD FRCPC - University of Zurich, University Hospital Balgrist, Zurich, Switzerland
Linda Jones, PT, MS - Craig H. Neilsen Foundation, Encino, California, USA
Daniel P. Lammertse, MD - Craig Hospital, Englewood, Colorado, USA
Keith Tansey, MD, PhD - Emory University and VA Medical Center, Atlanta, Georgia, USA
Functional outcomes WG
Kim Anderson-Erisman, PhD (Chair) - The Miami Project to Cure Paralysis, Miami, Florida, USA
Andrea L. Behrman, PT, PhD, FAPTA - Kentucky Spinal Cord Injury Research Center, University of Louisville, Louisville, Kentucky, USA
Edelle Field-Fote, PhD, PT, FAPTA - University of Miami Miller School of Medicine, Miami, Florida, USA
Linda Jones, PT, MS - Craig H. Neilsen Foundation, Encino, California, USA
MJ Mulcahey, PhD, OTR\L - Thomas Jefferson University, Philadelphia, Pennsylvania, USA
Participation/Quality of Life WG
Susan Charlifue, PhD (Chair) - Craig Hospital, Englewood, Colorado, USA
Allen W. Heinemann, PhD, ABPP (RP), FACRM - Northwestern University, Chicago, Illinois, USA
Alan M Jette, PT, PhD - Health & Disability Research Institute, Boston University, Boston, Massachusetts, USA
Marcel Post, PhD - University Medical Center Utrecht and De Hoogstraat, The Netherlands
Denise G Tate PhD, ABPP, FACRM - University of Michigan Medical School, Ann Arbor, Michigan, USA
David S. Tulsky, PhD - University of Michigan Medical School, Ann Arbor, Michigan, USA
Gale Whiteneck, PhD, FACRM - Craig Hospital, Englewood, Colorado, USA
Electrodiagnostics WG
Keith Tansey, MD, PhD (Chair) - Emory University and VA Medical Center, Atlanta, Georgia, USA
Peter Ellaway, PhD - Imperial College of London, London, England, United Kingdom
Keith C. Hayes, PhD - The University of Western Ontario, London, Ontario, Canada
John Kramer, PhD - Shepherd Center, Atlanta, Georgia, USA
Barry McKay, BS - Shepherd Center, Atlanta, Georgia, USA
Martin Schubert, MD - Balgrist University Hospital, Zurich, Switzerland
Arthur M. Sherwood, PE, PhD - Baylor College of Medicine, Houston, Texas, USA
Imaging WG
Adam E. Flanders, MD (Chair) - Thomas Jefferson University Hospital, Philadelphia, Pennsylvania, USA
David Dungan, MD - Radiology Imaging, Associates, P.C., Englewood, Colorado, USA
Ralph F. Frankowski, BSc, MS, MPH, PhD - University of Texas Health Science Center at Houston, School of Public Health,Houston, Texas, USA
Daniel P. Lammertse, MD - Craig Hospital, Englewood, Colorado, USA
Edward D. Wirth III, MD, PhD - Asterias Biotherapeutics, Menlo Park, California, USA
Pain WG and Psychological outcomes WG
Eva Widerström-Noga, DDS, PhD (Chair) - University of Miami, Miller School of Medicine, Miami, Florida, USA
Charles Bombardier, PhD - University of Washington School of Medicine, Seattle, Washington, USA
Thomas N. Bryce, MD - University of Miami Miller School of Medicine, New York, NY, USA
Nanna Finnerup, MD, DMSc - Aarhus University Hospital, Aarhus, Denmark Paul Kennedy, PhD - The Oxford Institute of Clinical Psychology Training, Oxford, United Kingdom
Mark Jensen, PhD - University of Washington, Seattle, Washington, USA John Kramer, PhD - Shepherd Center, Atlanta, GA, USA
Philip Siddall, MD, PhD - Kolling Institute of Medical Research, The University of Sydney, Sydney, Australia
NINDS CDE Team
Joanne Odenkirchen, MPH - NINDS CDE Project Officer, National Institutes of Health/National Institute of Neurological Disorders and Stroke (NIH/NINDS), Bethesda, Maryland, USA
Courtney Ashton, MBA - KAI Research, Inc. (An Altarum Company), Rockville, Maryland, USA (2007 - 2012)
Stacie Grinnon, MS - KAI Research, Inc. (An Altarum Company), Rockville, Maryland, USA (2007 - 2012)
Christina You, MSPH - KAI Research, Inc. (An Altarum Company), Rockville, Maryland, USA (2007 - 2012)
Yun Lu, PhD - KAI Research, Inc. (An Altarum Company), Rockville, Maryland, USA (2007 - 2012)
Aria Lans, MPH - The EMMES Corporation, Rockville, Maryland, USA (2012 - present)
Sherita Ala'i, MS - The EMMES Corporation, Rockville, Maryland, USA (2012 - present)
Naomi Kleitman, PhD -- Program Director, National Institutes of Health/National Institute of Neurological Disorders and Stroke (NIH/NINDS), Bethesda, Maryland, USA (2007-2012)
Lyn Jakeman, PhD – Program Director, National Institutes of Health/National Institute of Neurological Disorders and Stroke (NIH/NINDS), Bethesda, Maryland, USA (2013-present)
Footnotes
Conflict of Interest:All authors declare no conflict of interest.
References
- 1.Groft SC, Rubinstein YR. New and evolving rare diseases research programs at the National Institutes of Health. Public Health Genomics. 2013;16:259–67. doi: 10.1159/000355929. [DOI] [PubMed] [Google Scholar]
- 2.Nadkarni PM, Brandt CA. The Common Data Elements for cancer research: remarks on functions and structure Methods Inf Med. 2006;45:594–601. [PMC free article] [PubMed] [Google Scholar]
- 3.Pathak J, Wang J, Kashyap S, Basford M, Li R, Masys DR, Chute CG. Mapping clinical phenotype data elements to standardized metadata repositories and controlled terminologies: the eMERGE Network experience. J Am Med Inform Assoc. 2011;18:376–386. doi: 10.1136/amiajnl-2010-000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warzel DB, Andonaydis C, McCurry B, Chilukuri R, Ishmukhamedov S, Covitz P. Common data element (CDE) management and deployment in clinical trials. AMIA. Annu Symp Proc. 2003:1048. [PMC free article] [PubMed] [Google Scholar]
- 5.Grinnon ST, Miller K, Marler JR, Lu Y, Stout A, Odenkirchen J, Kunitz S. National Institute of Neurological Disorders and Stroke Common Data Element project - approach and methods. Clin Trials. 2012;9:322–329. doi: 10.1177/1740774512438980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maas AI, Harrison-Felix CL, Menon D, Adelson PD, Balkin T, Bullock R, Engel DC, Gordon W, Langlois-Orman J, Lew HL, Robertson C, Temkin N, Valadka A, Verfaellie M, Wainwright M, Wright DW, Schwab K. Standardizing data collection in traumatic brain injury. J Neurotrauma. 2011;28:177–187. doi: 10.1089/neu.2010.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hicks R, Giacino J, Harrison-Felix CL, Manley GT, Valadka A, Wilde EA. Progress in developing common data elements for traumatic brain injury research: version two – The end of the beginning. J Neurotrauma. 2013;30:1852–1861. doi: 10.1089/neu.2013.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saver JL, Warach S, Janis S, Odenkirchen J, Becker K, Benavente O, Broderick J, Dromerick A, Duncan P, Elkind MSV, Johnston K, Kidwell CS, Meschia JF, Schwamm L for the NINDS Common Data Element Working Group. Standardizing the structure of stroke clinical and epidemiological research data: The NINDS Stroke Common Data Element (CDE) Project. Stroke. 2012;43:967–973. doi: 10.1161/STROKEAHA.111.634352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loring D, Lowenstein D, Barbaro N, Fureman B, Odenkirchen J, Jacobs M, Austin J, Dlugos D, French J, Gaillard W, Hermann B, Hesdorffer D, Roper S, VanCott A, Grinnon S, Stout A. Common Data Elements in Epilepsy Research: Development and Implementation of the NINDS epilepsy CDE Project. Epilepsia. 2011;52:1186–1191. doi: 10.1111/j.1528-1167.2011.03018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sherman AV, Gubitz AK, Al-Chalabi A, Bedlack R, Berry J, Conwit R, Harris BT, Horton DK, Kaufmann P, Leitner ML, Miller R, Shefner J, Vonsattel JP, Mitsumoto H. Infrastructure resources for clinical research in amyotrophic lateral sclerosis Amyotroph Lateral Scler Frontotemporal Degener. 2013;14 Suppl 1:53–61. doi: 10.3109/21678421.2013.779058. 2013. [DOI] [PubMed] [Google Scholar]
- 11.Lynch DR, Pandolfo M, Schulz JB, Perlman S, Delatycki MB, Payne RM, Shaddy R, Fischbeck KH, Farmer J, Kantor P, Raman SV, Hunegs L, Odenkirchen J, Miller K, Kaufmann P. Common data elements for clinical research in Friedreich's ataxia. Mov Disord. 2013;28:1–5. doi: 10.1002/mds.25201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biering-Sørensen F, Charlifue S, DeVivo M, Noonan V, Post M, Stripling T, Wing P. International Spinal Cord Injury Data Sets. Spinal Cord. 2006;44:530–534. doi: 10.1038/sj.sc.3101930. [DOI] [PubMed] [Google Scholar]
- 13.Biering-Sørensen F, Charlifue S, DeVivo MJ, Grinnon ST, Kleitman N, Lu Y, Odenkirchen J. Using the spinal cord injury Common Data Elements. Topics in Spinal Cord Inj Rehabil. 2012;18:23–27. doi: 10.1310/sci1801-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biering-Sørensen F, Charlifue S, Devivo MJ, Grinnon ST, Kleitman N, Lu Y, Odenkirchen J. Incorporation of the International Spinal Cord Injury Data Set elements into the National Institute of Neurological Disorders and Stroke Common Data Elements. J Spinal Cord. 2011;49:60–64. doi: 10.1038/sc.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kostanjsek N. Use of The International Classification of Functioning, Disability and Health (ICF) as a conceptual framework and common language for disability statistics and health information systems. BMC Public Health. 2011;11 Suppl 4:S3. doi: 10.1186/1471-2458-11-S4-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeVivo M, Biering-Sørensen F, Charlifue S, Noonan V, Post M, Stripling T, Wing P. International Spinal Cord Injury Core Data Set. Spinal Cord. 2006;44:535–540. doi: 10.1038/sj.sc.3101958. [DOI] [PubMed] [Google Scholar]
- 17.Krassioukov A, Alexander MS, Karlsson AK, Donovan W, Mathias CJ, Biering-Sørensen F. International spinal cord injury cardiovascular function basic data set. Spinal Cord. 2010;48:586–590. doi: 10.1038/sc.2009.190. [DOI] [PubMed] [Google Scholar]
- 18.Biering-Sørensen F, Krassioukov A, Alexander MS, Donovan W, Karlsson AK, Mueller G, Perkash I, William Sheel A, Wecht J, Schilero GJ. International Spinal Cord Injury Pulmonary Function Basic Data Set. Spinal Cord. 2012;50:418–421. doi: 10.1038/sc.2011.183. [DOI] [PubMed] [Google Scholar]
- 19.Bauman WA, Biering-Sørensen F, Krassioukov A. International Spinal Cord Injury Endocrine and Metabolic Basic Data Set (version 1.2) Spinal Cord. 2012;50:567. doi: 10.1038/sc.2012.37. [DOI] [PubMed] [Google Scholar]
- 20.Biering-Sørensen F, Burns AS, Curt A, Harvey LA, Jane Mulcahey M, Nance PW, Sherwood AM, Sisto SA. International Spinal Cord Injury Musculoskeletal Basic Data Set. Spinal Cord. 2012;50:797–802. doi: 10.1038/sc.2012.102. [DOI] [PubMed] [Google Scholar]
- 21.Biering-Sørensen F, Craggs M, Kennelly M, Schick E, Wyndaele JJ. International Spinal Cord Injury Lower Urinary Tract Function Basic Data Set. Spinal Cord. 2008;46:325–330. doi: 10.1038/sj.sc.3102145. [DOI] [PubMed] [Google Scholar]
- 22.Krogh K, Perkash I, Stiens SA, Biering-Sørensen F. International Spinal Cord Injury Bowel Function Basic Data Set. Spinal Cord. 2009;47:230–234. doi: 10.1038/sc.2008.102. [DOI] [PubMed] [Google Scholar]
- 23.New PW, Marshall R. International Spinal Cord Injury Data Sets for Non-Traumatic Spinal Cord Injury. Spinal Cord. 2014;52:123–132. doi: 10.1038/sc.2012.160. [DOI] [PubMed] [Google Scholar]
- 24.Dvorak MF, Wing PC, Fehlings MG, Vaccaro AR, Itshayek E, Biering-Sorensen F, Noonan VK. International Spinal Cord Injury Spinal Column Injury Basic Data Set. Spinal Cord. 2012;50:817–821. doi: 10.1038/sc.2012.60. [DOI] [PubMed] [Google Scholar]
- 25.Goetz LL, Cardenas DD, Kennelly M, Bonne Lee BS, Linsenmeyer T, Moser C, Pannek J, Wyndaele JJ, Biering-Sorensen F. International Spinal Cord Injury Urinary Tract Infection Basic Data Set. Spinal Cord. 2013;51:700–704. doi: 10.1038/sc.2013.72. [DOI] [PubMed] [Google Scholar]
- 26.Karlsson AK, Krassioukov A, Alexander MS, Donovan W, Biering-Sørensen F. International Spinal Cord Injury Skin and Thermoregulation Function Basic Data Set. Spinal Cord. 2012;50:512–516. doi: 10.1038/sc.2011.167. [DOI] [PubMed] [Google Scholar]
- 27.Alexander MS, Biering-Sørensen F, Elliott S, Kreuter M, Sønksen J. International Spinal Cord Injury Male Sexual Function Basic Data Set. Spinal Cord. 2011;49:795–798. doi: 10.1038/sc.2010.192. [DOI] [PubMed] [Google Scholar]
- 28.Alexander MS, Biering-Sørensen F, Elliott S, Kreuter M, Sønksen J. International Spinal Cord Injury Female Sexual and Reproductive Function Basic Data Set. Spinal Cord. 2011;49:787–790. doi: 10.1038/sc.2011.7. [DOI] [PubMed] [Google Scholar]
- 29.Krogh K, Perkash I, Stiens SA, Biering-Sørensen F. International Bowel Function Extended Data Set. Spinal Cord. 2009;47:235–241. doi: 10.1038/sc.2008.103. [DOI] [PubMed] [Google Scholar]
- 30.Biering-Sørensen F, Craggs M, Kennelly M, Schick E, Wyndaele JJ. International Urinary Tract Imaging Basic Spinal Cord Injury Data Set. Spinal Cord. 2009;47:379–383. doi: 10.1038/sc.2008.149. [DOI] [PubMed] [Google Scholar]
- 31.Biering-Sørensen F, Craggs M, Kennelly M, Schick E, Wyndaele JJ. International Spinal Cord Injury Urodynamic Basic Data Set. Spinal Cord. 2008;46:513–516. doi: 10.1038/sj.sc.3102174. [DOI] [PubMed] [Google Scholar]
- 32.Dvorak MF, Itshayek E, Fehlings MG, Vaccaro AR, Wing PC, Biering-Sorensen F, Noonan VK. International Spinal Cord Injury Spinal Interventions and Surgical Procedures Basic Data Set. 2014 doi: 10.1038/sc.2014.182. submitted. [DOI] [PubMed] [Google Scholar]
- 33.Biering-Sørensen F, Bryden A, Curt A, Friden J, Harvey LA, Mulcahey MJ, Popovic MR, Prochazka A, Sinnott KA, Snoek G. International Spinal Cord Injury Upper Extremity Basic Data Set. Spinal Cord. 2014;52:652–657. doi: 10.1038/sc.2014.87. [DOI] [PubMed] [Google Scholar]
- 34.Gassaway J, Whiteneck G, Dijkers M. Clinical taxonomy development and application in spinal cord injury research: the SCIRehab Project. J Spinal Cord Med. 2009;32:260–269. doi: 10.1080/10790268.2009.11760780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kirshblum SC1, Waring W, Biering-Sorensen F, Burns SP, Johansen M, Schmidt-Read M, Donovan W, Graves D, Jha A, Jones L, Mulcahey MJ, Krassioukov A. Reference for the 2011 revision of the International Standards for Neurological Classification of Spinal Cord Injury. J Spinal Cord Med. 2011;34:547–554. doi: 10.1179/107902611X13186000420242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Charlifue S, Post MW, Biering-Sørensen F, Catz A, Dijkers M, Geyh S, Horsewell J, Noonan V, Noreau L, Tate D, Sinnott KA. International Spinal Cord Injury Quality of Life Basic Data Set. Spinal Cord. 2012;50:672–675. doi: 10.1038/sc.2012.27. [DOI] [PubMed] [Google Scholar]
- 37.Widerström-Noga E, Biering-Sørensen F, Bryce TN, Cardenas DD, Finnerup NB, Jensen MP, Richards JS, Siddall PJ. The International Spinal Cord Injury Pain Basic Data Set (version 2.0) Spinal Cord. 2014;52:282–286. doi: 10.1038/sc.2014.4. [DOI] [PubMed] [Google Scholar]
- 38.DeVivo MJ, Go BK, Jackson AB. Overview of the national spinal cord injury statistical center database. J Spinal Cord Med. 2002;25:335–338. doi: 10.1080/10790268.2002.11753637. [DOI] [PubMed] [Google Scholar]
- 39.Devivo MJ. Epidemiology of traumatic spinal cord injury: trends and future implications. Spinal Cord. 2012;50:365–72. doi: 10.1038/sc.2011.178. [DOI] [PubMed] [Google Scholar]
- 40.Noonan VK, Kwon BK, Soril L, Fehlings MG, Hurlbert RJ, Townson A, Johnson M, Dvorak MF RHSCIR Network. The Rick Hansen Spinal Cord Injury Registry (RHSCIR): a national patient-registry. Spinal Cord. 2012;50:22–27. doi: 10.1038/sc.2011.109. [DOI] [PubMed] [Google Scholar]
- 41.Grossman RG, Toups EG, Frankowski RF, Burau KD, Howley S. North American Clinical Trials Network for the Treatment of Spinal Cord Injury: goals and progress. J Neurosurg Spine. 2012;17(1 Suppl):6–10. doi: 10.3171/2012.4.AOSPINE1294. [DOI] [PubMed] [Google Scholar]
- 42.Steeves JD, Lammertse D, Curt A, Fawcett JW, Tuszynski MH, Ditunno JF, Ellaway PH, Fehlings MG, Guest JD, Kleitman N, Bartlett PF, Blight AR, Dietz V, Dobkin BH, Grossman R, Short D, Nakamura M, Coleman WP, Gaviria M, Privat A. Guidelines for the conduct of clinical trials for spinal cord injury (SCI) as developed by the ICCP panel: clinical trial outcome measures. Spinal Cord. 2007;45:206–221. doi: 10.1038/sj.sc.3102008. [DOI] [PubMed] [Google Scholar]
- 43.Steeves JD, Lammertse DP, Kramer JL, Kleitman N, Kalsi-Ryan S, Jones L, Curt A, Blight AR, Anderson KD. Outcome Measures for Acute/Subacute Cervical Sensorimotor Complete (AIS-A) Spinal Cord Injury During a Phase 2 Clinical Trial. Top Spinal Cord Inj Rehabi. 2012;18:1–14. doi: 10.1310/sci1801-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kramer JKL, Lammertse DP, Schubert M, Curt A, Steeves JD. Relationship between motor recovery and independence after sensorimotor complete cervical spinal cord injury. Neurorehabil Neural Repair. 2012;26:1064–1071. doi: 10.1177/1545968312447306. [DOI] [PubMed] [Google Scholar]
- 45.Lammertse DP, Jones LAT, Charlifue SB, et al. Autologous incubated macrophage therapy in acute, complete spinal cord injury: results of the phase 2 randomized controlled multicenter trial. Spinal Cord. 2012;50:661–671. doi: 10.1038/sc.2012.39. [DOI] [PubMed] [Google Scholar]
- 46.Hallett M. NINDS myotatic reflex scale. Neurology. 1993;43:2723. doi: 10.1212/wnl.43.12.2723. [DOI] [PubMed] [Google Scholar]
- 47.Gershon RC, Rothrock N, Hanrahan R, Bass M, Cella D. The use of PROMIS and assessment center to deliver patient-reported outcome measures in clinical research. J Appl Meas. 2010;1:304–314. [PMC free article] [PubMed] [Google Scholar]
- 48.Cella D, Nowinski C, Peterman A, Victorson D, Miller D, Lai JS, Moy C. The Neurology Quality of Life measurement initiative. Arch Phys Med Rehabil. 2011;92(10 Suppl):S28–36. doi: 10.1016/j.apmr.2011.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tulsky DS, Kisala PA, Victorson D, Tate D, Heinemann AW, Amtmann D, Cella D. Developing a contemporary patient-reported outcomes measure for spinal cord injury. Arch Phys Med Rehabil. 2011;92(10 Suppl):S44–51. doi: 10.1016/j.apmr.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bryce TN, Biering-Sorensen F, Finnerup NB, Cardenas DD, Defrin R, Lundeberg T, Norrbrink C, Richards JS, Siddall PJ, Stripling T, Treede RD, Waxman SG, Widerström-Noga E, Yezierski RP, Dijkers M. International Spinal Cord Injury Pain (ISCIP) classification: part I. background and description Spinal Cord. 2012;50:413–417. doi: 10.1038/sc.2011.156. [DOI] [PubMed] [Google Scholar]
- 51.Bryce TN, Biering-Sorensen F, Finnerup NB, Cardenas DD, Defrin R, Ivan E, Lundeberg T, Norrbrink C, Richards JS, Siddall PJ, Stripling T, Treede RD, Waxman SG, Widerström-Noga E, Yezierski RP, Dijkers M. International Spinal Cord Injury Pain (ISCIP) classification: part 2. initial validation using vignettes Spinal Cord. 2012;50:404–412. doi: 10.1038/sc.2012.2. [DOI] [PubMed] [Google Scholar]
- 52.Kennedy P, Lude P, Elfström ML, Smithson E. Cognitive appraisals, coping and quality of life outcomes: A multi-centre study of spinal cord injury rehabilitation. Spinal Cord. 2010;48:762–769. doi: 10.1038/sc.2010.20. [DOI] [PubMed] [Google Scholar]
- 53.Bombardier CH, Richard JS, Krause JS, Tulsky D, Tate DG. Symptoms of major depression in people with spinal cord injury: implications for screening. Arch Phys Med Rehabil. 2004;95:1749–1756. doi: 10.1016/j.apmr.2004.07.348. [DOI] [PubMed] [Google Scholar]
- 54.Bombardier CH, Kalpakjan CZ, Graves DE, Dyer JR, Tate DG, Fann JR. Validity of the Patient Health Questionnaire - 9 in assessing major depressive disorder during inpatient spinal cord injury rehabilitation. Arch Phys Med Rehabil. 2012;93:1838–1845. doi: 10.1016/j.apmr.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 55.Flanders AE, Spettell CM, Tartaglino LM, Friedman DP, Herbison GJ. Forecasting motor recovery after cervical spinal cord injury: value of MR imaging. Radiology. 1996;201:649–655. doi: 10.1148/radiology.201.3.8939210. [DOI] [PubMed] [Google Scholar]
- 56.Furlan JC, Fehlings MG, Massicotte EM, Aarabi B, Vaccaro AR, Bono CM, Madrazo I, Villanueva C, Grauer JN, Mikulis D. A quantitative and reproducible method to assess cord compression and canal stenosis after cervical spine truama: a study of interrater and intrarater reliability. Spine. 2007;32:2083–2091. doi: 10.1097/BRS.0b013e318145a91c. [DOI] [PubMed] [Google Scholar]
- 57.Shanmuganathan K, Gullapalli, Zhuo J, Mirvis SE. Diffusion tensor MR imaging in cervical spine trauma. AJNR. 2008;29:655–659. doi: 10.3174/ajnr.A0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lammertse D, Tuszynski MH, Steeves JD, Curt A, Fawcett JW, Rask C, Ditunno JF, Fehlings MG, Guest JD, Ellaway PH, Kleitman N, Blight AR, Dobkin BH, Grossman R, Katoh H, Privat A, Kalichman M. Guidelines for the conduct of clinical trials for spinal cord injury (SCI) as developed by the ICCP panel: clinical trial design. Spinal Cord. 2007;45:232–242. doi: 10.1038/sj.sc.3102010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tuszynski MH, Steeves JD, Fawcett JW, Lammertse D, Kalichman M, Rask C, Curt A, Ditunno JF, Fehlings MG, Guest JD, Ellaway PH, Kleitman N, Bartlett PF, Blight AR, Dietz V, Dobkin BH, Grossman R, Privat A. Guidelines for the conduct of clinical trials for spinal cord injury (SCI) as developed by the ICCP panel: clinical trial inclusion/exclusion criteria and ethics. Spinal Cord. 2007;45:222–231. doi: 10.1038/sj.sc.3102009. [DOI] [PubMed] [Google Scholar]
- 60.Fawcett JW, Curt A, Steeves JD, et al. Guidelines for the conduct of clinical trials for spinal cord injury (SCI) as developed by the ICCP panel: Spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord. 2007;45:190–205. doi: 10.1038/sj.sc.3102007. [DOI] [PubMed] [Google Scholar]
- 61.Ptyushkin P1, Vidmar G, Burger H, Marincek C. Use of the International Classification of Functioning, Disability, and Health in traumatic brain injury rehabilitation: linking issues and general perspectives. Am J Phys Med Rehabil. 2012;91:S48–S54. doi: 10.1097/PHM.0b013e31823d4e99. [DOI] [PubMed] [Google Scholar]
- 62.Miller AC, Odenkirchen J, Duhaime AC, Hicks R. Common data elements for research on traumatic brain injury: pediatric considerations. J Neurotrauma. 2012;29:621–628. doi: 10.1089/neu.2011.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jensen MP, Widerström-Noga E, Richards JS, Finnerup NB, Biering-Sørensen F, Cardenas DD. Reliability and validity of the International Spinal Cord Injury Basic Pain Data Set items as self-report measures. Spinal Cord. 2010;48:230–238. doi: 10.1038/sc.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Juul T, Bazzocchi G, Coggrave M, Johannesen IL, Hansen RB, Thiyagarajan C, Poletti E, Krogh K, Christensen P. Reliability of the International Spinal Cord Injury bowel function basic and extended data sets. Spinal Cord. 2011;49:886–891. doi: 10.1038/sc.2011.23. [DOI] [PubMed] [Google Scholar]
- 65.Post MWM, Adriaansen JJE, Charlifue S, Biering-Sørensen F, van Asbeck FWA. Good validity of the International Spinal Cord Injury basic quality of life data set. submitted, Spinal Cord. 2014 doi: 10.1038/sc.2015.99. [DOI] [PubMed] [Google Scholar]
- 66.Biering-Sørensen F, Alexander MS, Burns S, Charlifue S, Devivo M, Dietz V, Krassioukov A, Marino R, Noonan V, Post MW, Stripling T, Vogel L, Wing P. Recommendations for translation and reliability testing of international spinal cord injury data sets. Spinal Cord. 2011;49:357–360. doi: 10.1038/sc.2010.153. [DOI] [PubMed] [Google Scholar]