Abstract
Angiogenesis is a hallmark of cancer as its induction is indispensable to fuel an expanding tumor. The tumor microenvironment contributes to tumor vessel growth, and distinct myeloid cells recruited by the tumor have been shown to not only support angiogenesis but to foster an immune suppressive environment that supports tumor expansion and progression. Recent findings suggest that the intertwined regulation of angiogenesis and immune modulation can offer therapeutic opportunities for the treatment of cancer. Here we review the mechanisms by which distinct myeloid cell populations contribute to tumor angiogenesis, discuss current approaches in the clinic that are targeting both angiogenic and immune suppressive pathways, and highlight important areas of future research.
Introduction
The onset of tumor neovascularization is a multi-step process that can occur by different mechanisms of which angiogenesis is the most prominent. These are orchestrated by a wealth of activating and inhibiting factors whose balance will dictate whether endothelial cells are in a quiescent or activated state [1, 2]. Pathological tumor angiogenesis differs from physiological angiogenesis, such as during wound-healing, in that the balance between activating and inhibiting factors becomes lost, resulting in an endothelium undergoing continuous sprouting and expansion [3, 4]. Accordingly, tumors have been described by Harold Dvorak as “wounds that never heal” [5]. Recognition of angiogenesis as a hallmark of cancer, together with vascular endothelial growth factor (VEGF) as one of the most important angiogenic drivers, has provided a convincing rationale for the development of VEGF and VEGF receptor inhibitors [6-8]. This has led to FDA approval of bevacizumab (Avastin, Genentech/Roche), a VEGF-trapping monoclonal antibody, as well as sorafenib (Nexavar, Bayer) and sunitinib (Sutent, Pfizer), kinase inhibitors that target the VEGF receptor (VEGFR) tyrosine kinases as well as other RTKs [9, 10]. Despite the encouraging and favorable effects of these inhibitors in some patients, antiangiogenic therapy has ultimately been found to have rather transient beneficial effects, [9-11]. With the short-lived nature of patient response, it has become evident that tumors have the ability to adapt to the pressures of vascular growth restriction, and the uncovering and suppression of such adaptations has become the focus of much research.
One bypass mechanism involves the recruitment of myeloid cells (Figure 1). Similar to wounds, tumors drive the recruitment and infiltration of several innate immune cell populations, which include macrophages, immature monocytic and granulocytic myeloid-derived suppressor cells (M- or G-MDSC, respectively), and neutrophils. Numerous preclinical studies have revealed that innate immune cells can drive angiogenesis during normal tumor progression, primarily through the production or liberation of angiogenic molecules within the tumor microenvironment. For example, macrophage-produced VEGF was shown to facilitate the angiogenic switch in the PyMT model of breast cancer [12, 13], while VEGF released from the tumor extracellular matrix by myeloid cell-derived MMP-9 induced angiogenesis in models of cervical, brain and pancreatic cancer [14-16]. Gr1-expressing cells, which include MDSC and neutrophils, have also been shown to drive angiogenesis in various tumor models at least in part via VEGF and MMP-9 production [17-20]. Myeloid cells recruited to the tumor microenvironment during VEGF-signaling inhibition are thought to evoke resistance via the production of alternative proangiogenic factors, and several pathways facilitating such recruitment have already been identified and include upregulation of the Ang2-Tie2 signaling axis, GCSF production, and the PlGF-VEGFR1 signaling axis [21-23]. Accordingly, dual inhibition of VEGF-Ang2 using the bispecific CrossMab antibody has had promising preclinical results and is currently in a phase I clinical trial as a single agent for patients with locally advanced or metastatic solid tumors (NCT01688206).
Figure 1. Hypoxia mediates recruitment of angiogenic myeloid cells that drive both tumor progression and resistance to antiangiogenic therapy.
Solid tumors eventually reach a size that, due to oxygen and nutrient diffusion limits, cannot be sustained by the existing vasculature. This results in a decrease in oxygen tension within the tumor. Hypoxia positively regulates the expression of a variety of genes in tumor cells, many of which result in the infiltration or accumulation of angiogenic myeloid cells. For example, tumor-derived VEGF, CSF-1, MCP-1, and SDF1α recruit angiogenic monocytes including macrophages and Gr1+ G-MDSC and MMDSC into tumors; CXCL2 recruits angiogenic neutrophils and monocytes; Ang2 recruits angiogenic Tie2-expressing monocytes and macrophages (TEMs); IL-4 and IL-6 induce the differentiation of infiltrating monocytes into angiogenic and immune-suppressive macrophages; also, Sema3A brings Nrp1-expressing TAMs into hypoxic regions where they are reprogrammed to an angiogenic and immune-suppressive phenotype. Tumor-associated MDSC, TAM, TEMS, and neutrophils then secrete or liberate sequestered angiogenic factors, of which VEGF is dominant to facilitate neovascularization. This in turn leads to continued tumor growth and disease progression. Blocking persistent vessel growth can blunt tumor growth; however, this increases hypoxia and hypoxia-induced gene expression. Thus, tumors reinitiate the recruitment of angiogenic MDSC, TAM, TEMS, and neutrophils via the secretion of hypoxia- regulated factors, many of which drive myeloid-cell recruitment during normal tumor progression. These cells then reinstatement tumor angiogenesis via VEGF-independent pathways, thereby conferring tumor resistance to VEGF-blockade. Abbreviations: VEGF, vascular endothelial growth factor; Sema3A, semaphorin-3A; MCP-1, monocyte chemotactic protein-1; CSF-1, colony stimulating factor-1; CXCL2, (C-X-C motif) ligand 2; IL-6, interleukin-6; IL-4, interleukin-4; Ang2, angiopoietin 2; SDF1α, stromal-derived factor 1α; GCSF, granulocyte colony stimulating factor; PlGF, placental growth factor; G- or M- MDSC, granulocytic or monocytic myeloid-derived suppressor cell; TAN, tumor-associated neutrophil; TAM, tumor-associated macrophage; TEM, Tie2+-expressing macrophage.
In contrast to wounds, where innate immune cells are initially recruited to the site to clear microbial cells and debris via Th1-responses and later become immune-suppressive and proangiogenic in the resolution phase where tissues are repaired, myeloid cells infiltrating into tumors become often immediately suppressors of immunity. That myeloid cells drive tumor growth not only by activating angiogenesis, but also by allowing the tumor to escape anti-tumor immune responses suggests a regulatory link between immune-suppression and proangiogenic activity in tumor-associated myeloid cell types. This stems from their lack in cytotoxic activity and their ability to block effector T cell expansion and function primarily via depletion of amino acids, induction of oxidative stress, and production of Th2 cytokines [24, 25].
From this perspective it is conceivable that skewing myeloid cells from an immune-suppressive towards an immune-stimulating phenotype is akin to killing two birds with one stone and could be presumed favorable over cell depletion strategies as these leave intact the innate immune systems pivotal function in generating immunity.
Here, we will summarize the implication of distinct myeloid cell populations in tumor angiogenesis and highlight intratumoral mediators that regulate and likely couple myeloid immune suppression and angiogenesis. We will discuss the advantage of strategies that tackle both phenotypes and propose that simultaneously inhibiting the protumoral activities of myeloid cells may prove more effective than agents targeting single myeloid populations. Several ongoing clinical trials are currently assessing the effects of targeting distinct myeloid populations; therefore understanding such mechanisms is imperative to design powerful antiangiogenic immune therapies.
Tumor angiogenesis
The induction of angiogenesis has been defined by Hanahan & Weinberg as one of the six pivotal hallmarks of cancer [26]. Like normal tissues, tumors require an adequate supply of oxygen and the removal of metabolic waste, although these requirements vary among tumor types and change over the course of tumor progression. Accordingly, solid tumors undergo a context-dependent angiogenic switch in which they induce the formation of new blood vessels once they outgrow the reach of the surrounding preexisting vasculature. Several distinct mechanisms have been described that lead to the formation of a new vasculature within tumors (see Box 1), with angiogenesis being the most prominent and best-understood mechanism. Under normal physiological conditions, the adult vasculature is mostly quiescent and is maintained in this state via the balance of proangiogenic molecules, which include VEGF, fibroblast growth factor (FGF) and angiopoietin (Ang) family members, as well as angiostatic molecules, which include thrombospondin-1, certain endogenous ECM and basement membrane degradation products like endostatin and angiostatin, and some CXCL chemokines [1, 27, 28]. Angiogenesis arises through the production of conditions that break this balance in favor of proangiogenic signals; this results in the activation of endothelial cells and their migration towards a gradient of proangiogenic factors, in which they self-assemble into expanding “sprouts”, with proliferating stalk cells and guiding tips cells at the leading edge. New vessels arise when such sprouts meet and anastamose, and upon cessation of angiogenic signals, vessels stabilize with the formation of a basement membrane and recruitment and embracement of mural cells [29].
Box 1. Mechanisms of tumor neovascularization.
Solid tumors induce the formation of new blood vessels when the existing vasculature is unable to meet oxygen and nutrient demands. Neovascularization can occur as a consequence of either sprouting angiogenesis from the existing vascular network, vasculogenesis via the recruitment and differentiation of vascular progenitor cells, or the partitioning of existing vessels into smaller vessels via intussusception. Sprouting angiogenesis occurs when angiogenic stimuli such as VEGF or FGF activate endothelial cells on preexisting blood vessels [94]. Activated endothelial cells migrate towards the source of the angiogenic cues by forming a sprout that consists of a single endothelial tip cell at the front followed by endothelial stalk cells attached to a preexisting vessel. The tip cell leads the nascent vessel by using filopodia to sense angiogenic cues and guide its migration, while stalk cells proliferate and give the vessel length. The dichotomy between endothelial tip and stalk cells stems from angiogenic factor-induced tip cell expression of the Notch ligand Dll4. This induces Notch activation in adjacent stalk cells which blocks the tip cell phenotype. Recently, metabolic control over tip versus stalk phenotype was revealed, where oxidative phosphorylation activated Notch and blocked the tip cell phenotype, and glycolytic flux was required for Notch repression and acquisition of the tip cell phenotype [95, 96]. Angiogenic cues also recruit endothelial and pericyte progenitors from the bone marrow. Endothelial progenitor cells express CD34 and VEGFR2, and unlike hematopoietic cells that also express these markers, can incorporate into blood vessels and form lumens [97]. Circulating pericyte progenitor cells express the hematopoietic marker Sca1 and PDGFRβ [98]. These extravasate through the nascent vessel and take on a perivascular distribution. Once associated with the vessel, these cells mature into α-SMA and desmin-expressing pericytes, which stabilize the structure and provide survival factors to the underlying layer of endothelium. Intussusception involves the bifurcation of vessels through a process that involves the indentation, attachment, and subsequent protease-driven division of opposing endothelial cells from a single vessel to form two daughter vessels; this mode of neovascularization does not require the generation of new endothelial cells and has only been demonstrated in tumors in response to treatment with the RTKi vatalinib [99]. Abbreviations: VEGF, vascular endothelial growth factor; FGF2, fibroblast growth factor-2; Dll4, delta-like ligand 4; VEGFR2, vascular endothelial growth factor receptor-2; PDGFRβ, platelet-derived growth factor β; CD34, cluster of differentiation-34; Sca1, stem cell antigen-1; αSMA, α-smooth muscle actin; RTKi, receptor tyrosine kinase inhibitor.
Similar to physiological angiogenesis, an excess of proangiogenic signals drives the initiation of tumor angiogenesis; however, unlike its normal counterpart, tumor angiogenesis is unable to reach homeostasis as there remains an overabundance of proangiogenic cues. This results in vessels that are abnormal at every level, with endothelial cells that are poorly interconnected and sometimes multilayered, uneven basement membranes, and fewer and more loosely attached pericytes [29]. As a consequence, the tumor vasculature becomes leaky, easing tumor cell intravasation and dissemination; furthermore, blood flow is irregular and sluggish, which together with an expanding tumor mass increases interstitial pressure and hypoxia further exacerbating the angiogenic response [3, 29, 30]. In addition, tumor angiogenesis, in part through VEGF, also promotes tumor evasion from immune responses, thus reversion of anti-tumor immunity may actually enhance the benefits of antiangiogenic therapy [1].
VEGF links angiogenesis and immune suppression
VEGF was among the first proangiogenic factors identified, and was initially isolated from tumor-related ascites and conditioned medium from cultured tumor cells as a vascular permeability factor [31]. VEGF expression and bioavailability within the tumor is regulated by multiple mechanisms within the tumor milieu, and it has become clear that VEGF is one of the most important angiogenic factors during development and frequently upregulated in many solid cancers [4]. The bulk of VEGF's angiogenic activity stems from its interaction with the receptor tyrosine kinase VEGF receptor 2 (VEGFR2) on endothelial cells, and inhibitors targeting the VEGF/VEGFR-pathway are the most widely used antiangiogenic strategies in the clinic today [32]. In addition to its role in angiogenesis, VEGF has also been shown to inhibit immunity via multiple mechanisms (Figure 2). For example, VEGF binds to VEGF receptor 1 (VEGFR1) on CD34+ hematopoietic progenitors and inhibits their differentiation into mature dendritic cells via suppression of NFkB-mediated transcription, which results in defective antigen presentation within tumors [33]. VEGF also induces PDL1 expression on dendritic cells; PDL1 inhibits T-cell activation and promotes self tolerance through interactions with the PDL1 receptor programmed cell death protein 1 (PD1), or the costimulatory molecule CD80 [34]. Furthermore, VEGF impedes T-cell extravasation by limiting T-cell adhesion to the luminal surfaces of blood vessels, inhibits the proliferation and cytotoxicity of cytotoxic T-cells (CTL), and stimulates the proliferation of T-regulatory cells (Treg) [35-37].
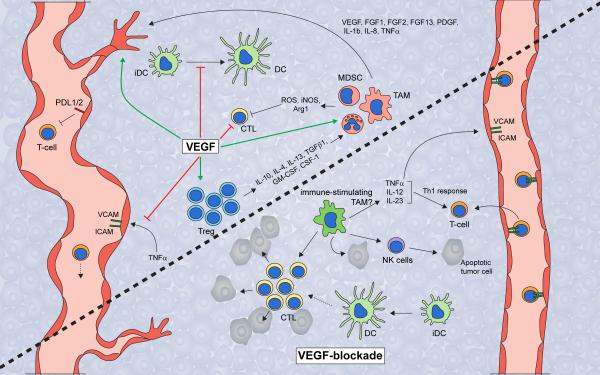
Figure 2. VEGF regulates intratumoral immune response.
VEGF promotes tumor growth by both inducing angiogenesis and suppressing anti-tumor immunity. VEGF inhibits the adhesion of T-cells to the luminal surfaces of blood vessels by blocking TNFα-induced expression of VCAM and ICAM, thereby blocking T-cell extravasation into the tumor. VEGF also blocks dendritic cell function by inhibiting dendritic cell maturation and inducing PDL1 expression on mature dendritic cells. VEGF also inhibits the proliferation and effector function of cytotoxic T-cells, while inducing Treg proliferation. Tregs secrete high levels of cytokines and growth factors, including IL-10, IL-4, IL-13, TGFβ1, GM-CSF, and CSF-1, which, like VEGF itself, drive recruitment and infiltration of angiogenic and immune-suppressive MDSC and macrophages. MDSC and macrophages then produce reactive oxygen species, nitric oxide, and arginase to suppress T-cell proliferation, viability, and activity. In contrast, inhibition of VEGF should restore many of these phenotypes. VEGF inhibition enables dendritic cell maturation and function, which leads to an increase in intratumoral effector T-cell numbers. Furthermore, VEGF-blockade should enable the endothelium to facilitate T-cell infiltration. Presumably, VEGF-blockade also results in an increase in Th1 cytokine-secreting tumoricidal and immune-supporting myeloid cells such as macrophages. Altogether, VEGF-blockade should unleash the anti-tumor immune response and leads to increased tumor cell apoptosis. However, the antiangiogenic effect of VEGF-blockade results in hypoxia, which drives the recruitment and polarization of immune-suppressive and angiogenic myeloid populations. Thus, therapeutic approaches aimed at activating immune response may enhance or prolong the efficacy of antiangiogenic therapy. Abbreviations: VEGF, vascular endothelial growth factor; PDL1/2, programmed death ligand 1/2; VCAM, vascular cell adhesion molecule; ICAM, intercellular adhesion molecule; iDC, immature dendritic cell; DC, dendritic cell; CTL, cytotoxic T-cell; Treg, regulatory T-cell; ROS, reactive oxygen species; NO, nitric oxide; Arg1, arginase-1; IL-10, -4, - 13, -12, -23, -1b, -8, interleukin-10, -4, -13, -12, -23, -1b, -8; TGFβ1, transforming growth factor-β1; GM-CSF, granulocyte/monocyte-colony stimulating factor; CSF-1, colony stimulating factor-1; G- or M- MDSC, granulocytic or monocytic-myeloid derived suppressor cell; TAM, tumor-associated macrophage; FGF-1, -2, -13, fibroblast growth factor-1, -2, -13; PDGF, platelet-derived growth factor; TNFα, tumor necrosis factor-α; NK-cell, natural killer cell; Th1, T-helper 1.
Mechanisms of myeloid-driven angiogenesis
Although historically it has been believed that tumor cells produce proangiogenic factors to induce neovascularization, it has become evident that host cells in the tumor environment significantly contribute to the production of proangiogenic molecules. Specifically, tumors recruit a variety of innate immune cell types that, once within the tumor, secrete angiogenic molecules that drive tumor angiogenesis [12, 14, 16, 38-41]. These factors regulate various aspects of vessel formation and include a number of growth factors and cytokines (VEGF, FGF2, tumor-necrosis-factor α (TNF-α), TGF-β, platelet-derived growth factor, placental growth factor (PIGF)), Neuropilin-1, CXCL chemokines (CXCL-8,-12), semaphorins, as well as various proteases including matrix metalloproteinases (MMP-2,-7,-9, and -14) and cysteine cathepsin proteases [15, 16, 23, 29, 42-49].
One of the most prominent myeloid cell types are tumor associated macrophages (TAM). Historically, they have been defined as either antitumoral M1-skewed, exhibiting features similar to LPS and IFNγ “classically” activated macrophages, or protumoral M2-skewed, having properties similar to IL-4 and IL-13 “alternatively” activated macrophages [50]. In addition to mediating the angiogenic switch in a variety of tumor models, TAM are potent suppressors of anti-tumor immunity, expressing a variety of Th2 cytokines including IL-10, and suppressing T-cell function through several mechanisms including engagement of immune checkpoints via PDL1/2 and suppression of TCR reexpression via arginase secretion [24]. Over the years it has become evident that the M1/M2 polarization model appears too simplistic to appropriately describe the heterogeneous macrophage phenotypes in tumors and therefore it has been recently suggested to rather define myeloid cells by their phenotype, function and context [51, 52].
The significance of TAM in tumor angiogenesis has been confirmed in various preclinical models. In the polyoma middle T-antigen (PyMT) breast tumor model, depletion of intratumoral macrophages or genetic deletion of VEGF in macrophages delayed the angiogenic switch, thus VEGF produced by tumor-infiltrating macrophages facilitates the angiogenic switch and the progression to malignancy in this model [12, 13]. In addition, MMP-9 produced by tumor-infiltrating macrophages and neutrophils has been shown to increase the bioavailability of ECM-sequestered VEGF, thus providing an alternative mechanism of VEGF-induced angiogenesis in tumors [14-16]. TAM express the receptor for colony stimulating factor 1 (CSF1R), and the studies identifying a role for TAM in angiogenesis targeted TAM by using mice harboring a null mutation for the CSF1R ligand CSF1 [12, 13]. More recently, CSF1R-inhibition with the small molecule inhibitor BLZ945 was found to not only reduce vascularity in murine glioma, but also suppress the expression of several immune-tolerant markers including Arg1 and Mrc1, thus skewing TAM towards an immune-stimulating phenotype and presumably activating anti-tumor immunity [53]. Indeed, CSF1R-inhibition was shown to induce the infiltration of CD8+ T-cells, likely CTLs, in models of cervical, breast, and pancreatic cancer [54-56]. TAM conversion towards an immune-stimulating phenotype has also been observed after B-cell depletion, though the contribution of CSF1R here is unclear [57]. Interestingly, the effects of targeting CSF1R seem to be context dependent. While in glioma, there was no decrease in TAM after treatment, yet in the cervical, breast, and pancreatic cancer models CSF1R-inhibition reduced TAM content. This warrants further investigation into the differences in molecular makeup between these cancers; as the retention of tumoricidal TAM is favorable for approaches that activate immunity, uncovering the mechanisms that maintain TAM content is essential. Overall, the abundance of preclinical data has led to the initiation of several clinical trials targeting TAM including a phase II trial with the CSF1R-inhibitor PLX3397 for recurrent glioblastoma (NCT01349036) and a phase I trial with the anti-CSF1R antibody IMC-CS4 for advanced solid tumors (NCT01346358).
Tie2-expressing monocytes/macrophages (TEM) are a highly angiogenic and immune-suppressive TAM subpopulation that express the angiopoietin receptor Tie2 and are often aligned in close juxtaposition to blood vessels through endothelial cell expression of the Tie2 ligand Ang2 [50, 58, 59]. Tie2 was originally described as an endothelial cell receptor that could either bind Ang1 to promote vessel stability, or bind Ang2 to antagonize Tie2-Ang1 effects; thus inhibiting the Ang2-Tie2 axis has, besides targeting TEM, also direct effects on the tumor endothelium [60]. The immunosuppressive nature of TEM is thought to come largely from their ability to produce IL-10, which inhibits T-cell activation and stimulates the expansion of Tregs [61]. The relevance of TEM to tumor angiogenesis and subsequent tumor growth has been demonstrated by either selectively ablating TEM by virtue of Tie2 promoter-driven thymidine kinase expression or by antibody-mediated neutralization Ang2, approaches which led to striking vessel regression in mouse models of mammary, pancreatic neuroendocrine (PNET), and brain tumors [62, 63]. Importantly, though Tie2 was also expressed on the tumor endothelium, knockdown of Tie2 expression in TEM was sufficient to drive vessel regression. Such results have spurred the development of therapeutic approaches that inhibit the Tie2-Ang2 axis [64]. For example, the Ang1/2-neutralizing Fc-peptide fusion AMG-386 is currently in a phase II clinical trial for castration-resistant prostate cancer in combination with abiraterone (NCT01553188), and a phase III clincal trial for ovarian, peritoneal, and fallopian tube cancer in combination with paclitaxel (NCT01204749). Interestingly, as TEM were found to represent a highly angiogenic and immune-suppressive fraction of TAM, it seems possible that the effect of CSF1R-inhibtion on activating immunity is through the selective depletion or conversion of TEM [59, 62], Indeed, CSF1 has been shown to induce Tie2 expression on macrophages, thus linking CSF1R and TEM [65]. This would suggest that CSF1-CSF1R and Ang2-Tie2 act in concert to drive the TAM phenotype.
CD11b+ Gr1-expressing cells are a diverse group of myeloid cells comprised of multiple populations including neutrophils and MDSC [41, 66]. Similar to TAM, tumor-associated neutrophils (TAN) have been described as either N1 or N2 based on their relative level of cytotoxicity and expression of inflammatory factors [67]. MDSC are immature CD11b+ myeloid cells with either monocytic (M-MDSC; Ly6CHigh, Ly6GLow) or granulocytic (G-MDSC; Ly6GHigh Ly6CLow) features [68]. Like TAM and TEM, MDSC suppress anti-tumor immunity by inhibiting T-cell activity and inducing Treg expansion [25]. The angiogenic properties of tumor-associated Gr1+CD11b+ cells have been demonstrated in various tumor models at least in part via VEGF and MMP-9 production [17-20, 69]. However, most studies relating to the proangiogenic activities of Gr1+ cells during tumor progression have not differentiated between neutrophils and MDSC, but solely referred to them as Gr1+CD11b+ cells, thus the precise Gr1-expressing myeloid cell population responsible for such activity is currently unclear. Notably, Gr1+CD11b+ cells appear to play a more prominent role in therapeutic resistance to antiangiogenic therapy, as discussed further below.
In summary, these results highlight the implication of multiple myeloid cell populations to contribute to the modulation of tumor angiogenesis and immunity. It is therefore tempting to speculate that such functional redundancies can result in the compensation of TAM by Gr1+ cells and vice versa. In support of the existence of such a compensatory mechanism, TAM were found to drive angiogenesis in a spontaneous model of cervical cancer through the production of MMP, yet genetic ablation of TAM resulted in the recruitment of MMP9-producing TAN, which then took over the role of promoting blood vessel formation [70].
Regulation of myeloid cell recruitment and function by hypoxia
How do tumors assemble the mobilization and infiltration of protumoral myeloid cells? Although tumors can inherently produce factors involved in myeloid cell recruitment, expansion, and differentiation (including G-CSF, CSF-1, GM-CSF), there is emerging evidence that low-oxygen tension activates many of the molecules and pathways that not only attract myeloid cells but also polarize them to an angiogenic and immune-suppressive phenotype. This is conceivable because hypoxia is a major regulator of angiogenesis and is mediated by the hypoxia-inducible factor (HIF) family of transcription factors that coordinates a transcriptional program that ensures metabolic and vascular adaptation to low-oxygen tension (Figure 1) [71, 72]. HIF stabilization leads to an upregulation of various proangiogenic growth factors and chemokines that, besides directly engaging in vessel growth like VEGF, PlGF and Ang2 [72-75], facilitate the mobilization and recruitment of bone-marrow-derived myeloid cells that support neovascularization to the tumor site [76]. VEGF is one of the most prominent hypoxia-regulated angiogenic factors that, besides affecting endothelial cells, can serve as a mobilizer and chemoattractant for myeloid cells via VEGFR1 on monocytes [77]. Further, CXCL12 (SDF1α), implicated in the retention of myeloid cells, is induced by HIF-1α [16, 78], as is its chemokine receptor CXCR4 [79, 80]. Hif1α is pivotal to mediate SDF1α and VEGF-dependent angiogenesis in a mouse model of glioblastoma multiforme (GBM), an aggressive brain tumor that constitutes one of the most angiogenic and hypoxic tumors [81]. Genetic deletion of Hif1α in tumor cells abrogated vascular remodeling concomitant with a substantial reduction of tumor-infiltrating myeloid cells. Furthermore, blood vessel formation in GBM was found to rely, to a substantial degree, on myeloid-derived MMP9 that released ECM-sequestered VEGF, thus underscoring the concept that Hif1α-mobilized myeloid cells can indeed evoke angiogenesis.
Semaphorin3A (Sema3A) is another hypoxia-induced factor in tumors that is implicated in macrophage recruitment and subsequent angiogenesis. Sema3A interacts with the transmembrane guidance protein neuropilin 1 (Nrp1) which drives signaling of a PlexinA1/PlexinA4/VEGFR1 holoreceptor complex that leads to VEGFR1 activation in TAM and their subsequent migration into hypoxic regions where they secrete various immune suppressive and proangiogenic factors including Arg1, Ccl22, IL-10, VEGF, Sema3a, and Mmp9 [82]. As soon as TAM are positioned in the hypoxic environment, Nrp1 expression is repressed; this terminates the migratory response of TAMs to Sema3A. Interestingly, hypoxia-dependent Nrp1 repression is facilitated by HIF2α-mediated activation of the NF-kB pathway. Loss of Nrp-1 on macrophages prevents TAM infiltration in hypoxic regions and thereby maintains an immune-stimulatory phenotype, resulting in delayed tumor growth, which is in turn characterized by impaired vascularization and improved anti-tumor immunity [82]. As mentioned above, hypoxia-induced factors also activate Tregs. In fact the gene for the Treg transcriptional master regulator FoxP3 contains putative hypoxia response elements within its promoter, rendering its expression sensitive to Hif1α activation [83]. Taken together, hypoxia and hypoxia-inducible factors regulate a wide range of tumor-promoting processes including neovascularization, immune suppression, and the recruitment of protumoral myeloid cells (Figure 1, Box 1).
Innate immune cells regulate reneovascularization during anti-angiogenic therapy
Hypoxia-induced infiltration of myeloid cells also represents a critical escape mechanism for tumors to evade the effects of antiangiogenic therapy, in part by stimulating VEGF-independent pathways (Figure 1) [16, 63]. As mentioned above, Gr1+CD11b+ cells, presumably containing neutrophils, G-MDSC, and M-MDSC, were found to be responsible for the resistance to VEGF-blockade in experimental mouse models of lymphoma and lung carcinoma [22]. These cells expressed a variety of immune-suppressive and angiogenic factors, were recruited to therapy-refractory tumors, and, when transferred to mice harboring tumors that were sensitive to antiangiogenic therapy, rendered non-refractory tumors resistant. Furthermore, Gr1+ cell-depletion with an anti-Gr1 antibody enhanced the response of refractory tumors to VEGF-blockade to a certain extent. Gr1+ cell-depletion also reduced blood vessel density within tumors, further implicating a role for Gr1+ cells in VEGF-independent angiogenesis. Interestingly, Gr1+CD11b+ cells underwent selective expansion in the bone marrow of mice harboring resistant tumors, suggestive of a role for tumor-derived factors in Gr1-cell recruitment. Indeed, later studies revealed Gr1+ cell-mediated resistance was mediated by an IL-17/GCSF/Bv8 axis that drives the expansion and recruitment of these cells to tumors [84]. Although the identification of the precise Gr1+ populations involved with Bv8-mediated resistance are unclear, these studies demonstrate Gr1-expressing can drive resistance to antiangiogenic therapy.
Preclinical studies in mice that develop spontaneous PNET have revealed adaptive upregulation of the Ang2-Tie2 signaling axis during VEGFR2 inhibition concomitant with enhanced infiltration by TEM [21]. Conversely, dual Ang2/VEGFR2 blockade suppressed revascularization and progression in PNET undergoing VEGFR2 inhibition [21]. Another study found that, although the vascular-disrupting agent combretastatin A4 phosphate (CA4P) was able to control the growth of spontaneous MMTV-PyMT mammary tumors, the hypoxia it produced from destroying the tumor vasculature enhanced CXCL12 expression and led to the infiltration of CXCR4+ TEM [85]. These TEM then shielded the residual tumor from the effects of CA4P. Indeed, pharmacological inhibition of CXCR4 impeded TEM infiltration and exacerbated CA4P antitumor effects. These results suggest that vessel regression by VEGF blockade or VDA agents induces expression and secretion of Ang2, which activates Tie2-mediated VEGF-independent angiogenic activity of TEM in a non-redundant fashion.
Ang2 has also been shown to impair the efficacy of VEGF-blockade in numerous other preclinical models [21]. It is worth noting that, though the additive effects of dual Ang2/VEGF-inhibition on tumor vascularity have been described in these various models, still little is known as to what extent this approach impacts anti-tumor immunity. This is a timely question that warrants close attention because there are currently multiple ongoing phase I (NCT01688960, NCT01688206, NCT01248949) and phase II (NCT01664182, NCT01249521) clinical trials assessing the effects of combined Ang2/VEGF pathway inhibition in various advanced cancers. Given the identification of the Tie2-Ang2 axis as an escape mechanism for tumors to reinitiate growth in the face of VEGF inhibition, it will be important to see whether Ang2-inhibition will benefit patients whose tumors have become refractory to anti-VEGF treatment and whether this is due to combined effects on the vasculature and immunity.
While not specifically assessing the role of TEM, another study demonstrated that TAM limit the sensitivity of tumors to multiple antiangiogenic approaches targeting the VEGF pathway [86]. Here, macrophage depletion using clodronate enhanced the effects of VEGF-blockade on blood vessel reduction; therefore the antagonizing activity of TAM on angiogenesis inhibition was due to their proangiogenic functions. Furthermore, antiangiogenic therapy reduced tumor blood vessel density without reducing TAM content, suggesting that these cells undergo a phenotypic switch as opposed to becoming selectively recruited when tumors respond to treatment or become refractory. In line with this notion, VEGF-blockade enhanced the expression of several angiogenic factors including VEGF, Sdf-1, Fgf-1 and 2, Mmp9, Cxcl1, and Plgf. Similarly, targeting the VEGFR1-ligand PlGF was sufficient to reproduce the effect of TAM depletion, and blocked the induction of angiogenic gene expression in response to antiangiogenic therapy [86]; thus PlGF facilitates macrophage recruitment and phenotypic programming. Indeed, macrophage-secreted PlGF was found to act in an autocrine fashion to drive TAM polarization towards an immune-suppressing phenotype, while inhibiting PlGF expression with histidine-rich glycoprotein induced an immune-stimulating TAM phenotype characterized by reductions in Mrc1, Arg1, CCL2, and IL-10 expression, and an increase in CXCL9 [23].
Congruent with this observation, we found that angiogenic inhibitors targeting the VEGF/VEGFR pathway were able to skew distinct intratumoral myeloid cells to an immune-stimulatory and angiostatic phenotype in mouse models of pancreatic and mammary tumors if the myeloid PI3Kδ/γ pathway was non-active (unpublished observations). PI3Kγ is a class IB PI3K isoform that is highly enriched in myeloid cells and facilitates myeloid cell infiltration and inflammation in tumors [19, 87, 88]. In further support with these observations, targeting mTOR, a downstream component of the PI3K-signaling pathway, with the inhibitor rapamycin caused monocytes to differentiate to immune-supporting macrophages while knockdown of the tuberous sclerosis complex 2 (TSC2), which is an upstream negative regulator of mTOR, promoted an immunosuppressive and angiogenic phenotype [89]. Moreover, macrophage depletion was sufficient to block the antiangiogenic effects of rapamycin in murine tumor xenografts [89]. Together, these studies suggest that enhancing macrophage-mediated immune-suppression and angiogenesis can allow for persistent tumor growth in the face of VEGF-blockade.
Given the tie between immune suppression and angiogenesis, specifically targeting the VEGF/VEGFR pathway should exhibit beneficial effects because VEGF not only promotes angiogenesis but also conveys different suppressive effects on the immune response (Figure 2) [35]. In turn, recent data have provided evidence that VEGF inhibition enhances immune-therapeutic approaches by improving overall vessel perfusion and creating a homogeneous distribution of perfused vessels throughout the tumor [90]. This led to decreased hypoxia and polarized TAM to an immune-supporting state that resulted in increased T-cell infiltration. Further, vascular normalization by deletion of Rgs5 in pericytes increased T-cell infiltration into tumors and substantially improved survival after adoptive T-cell transfer in mice [91]. Importantly, VEGF inhibition directly affected myeloid cells because it enabled dendritic cell maturation and function, which lead to an increase in intratumoral effector T-cell numbers.
Concluding Remarks
Recent successes in the clinic underscore the therapeutic benefit of activating the immune system in cancer but the overall response rate of immune therapy has been rather modest. Similarly, therapies to disable vascular growth in tumors have also shown beneficial effects in many cancer patients, but they are transient and followed by fast regrowth. By combining the two strategies, however, anti-angiogenic immunotherapy offers the possibility to more vigorously inhibit tumor angiogenesis and simultaneously impact the immune-inhibitory effects of the proangiogenic tumor milieu. One such approach could entail the reprogramming of intratumoral myeloid cells in combination with antiangiogenic therapy. This is based on the prevailing view that myeloid cells exert immune-stimulating as well as immune-suppressive properties to convey differing functions in the homeostasis repair program. Tumors, in order to grow and progress, produce factors to hijack myeloid cells and induce their immune-suppressive and proangiogenic properties. In lieu of the fact that both angiogenesis and immunosuppression are regulated by myeloid cells and coincide raises the question whether myeloid reprogramming may not only promote immune stimulation but also blunt the angiogenic contribution of myeloid cells which together should substantially extend the efficacy of antiangiogenic therapies. In support of this notion, recent approaches of antiangiogenic immune-therapies that entail blockade of self-tolerance checkpoints to reverse immune suppression, such as the anti-CTLA-4 antibody ipilimumab and PD1-antibody lambrolizumab in combination with bevacizumab have revealed encouraging preliminary results. Among 46 melanoma patients, the combined therapy of ipilimumab and bevacizumab yielded a 19.6% objective response rate and a median survival of 25.1 months—roughly twice expectations for ipilimumab alone in metastatic melanoma [92, 93]. Ongoing and future studies will be instrumental to expose adequate combinations of anti-angiogenic therapies with various immune-modulating strategies to more robustly inhibit tumor angiogenesis and promote an enduring immune-stimulatory milieu that leads to prolonged survival benefits in cancer patients. There are a plethora of questions (Box 3) that such studies could address to understand the link between angiogenesis and immunity and specifically to obtain a more sophisticated and nuanced understanding of the mechanisms of myeloid cell programming and functional plasticity during tumor angiogenesis and anti-angiogenic therapies. The knowledge forthcoming will yield new insights into the nature and function of the heterogeneous myeloid cell populations and provide valuable information as to how these cells can be exploited for therapeutic strategies. These studies may further identify the most efficient strategies to block myeloid-driven angiogenesis and immune suppression, for example by exploiting different approaches to either manipulate myeloid reprogramming or hinder trafficking to tumors.
Box 3. Outstanding Questions.
What are the local mediators and conditions in tumors besides hypoxia that program angiogenic and immune-suppressive features of myeloid cells? Understanding under which circumstances tumors skew innate immune cells from promoting immunity to supporting angiogenesis and suppressing immunity will be instrumental in designing novel therapeutic strategies.
As angiogenesis and immune-suppression appear to be co-regulated in innate immune cells, do immune-stimulatory myeloid cells become angiostatic? Determining the molecular mechanisms responsible for the interregulation of these differing phenotypes may uncover more efficient ways to inhibit the protumoral aspects of innate immune cell behavior.
Does VEGF inhibition besides directly blocking endothelial cell proliferation, foster an immune-stimulating and angiostatic environment in tumors?
Does VEGF inhibition affect myeloid polarization? Myeloid cell phenotypes may change during antiangiogenic therapy, thereby restraining tumor propagation in responding tumors while exacerbating it upon tumor relapse.
To which extent hinges the efficacy of antiangiogenic therapy on fostering an immune-stimulatory environment?
Will the effects of antiangiogenic therapy be enhanced and prolonged with inhibitors that reprogram myeloid cells and create a durable immune-stimulating microenvironment to impede reneovascularization and enhance T-cell mediated cytotoxicity?
Box 2. Escape mechanisms from antiangiogenic therapy.
Antiangiogenic therapies targeting the VEGF-signaling pathway inhibit tumor angiogenesis and generate tumor response; however this response is typically transient and tumors develop resistance by either reinstating the angiogenic cascade or though circumventing the necessity of angiogenesis. Reinstatement of neovascularization can involve the expression of alternative angiogenic growth factors by the tumor and/or the recruitment of myeloid cells that express such factors, which results in the induction of VEGF-independent angiogenesis (see Figure 1). To bypass the need for neovascularization, tumors have been found to alter the manner in which they grow, or to utilize vessels that were able to withstand the deleterious effects of therapy due to increased pericyte coverage. Numerous preclinical studies have demonstrated that targeting VEGF-signaling can result in an invasive or metastatic growth pattern presumably to overcome the anti-tumor effects of VEGF-blockade [100-103]. For example, genetic ablation or pharmacological inhibition of VEGF signaling in Rip1Tag2 pancreatic neuroendocrine tumors resulted in a substantial increase in tumor cell invasion as well as increased metastatic dissemination [101], while targeting VEGF in glioblastoma models resulted in a perivascular pattern of invasive tumor growth [100, 102]. Interestingly, these invasive growth patterns were due to activation of MET [102, 103]. Reneovascularization was also found to be dispensible in tumors whose vasculature had substantial pericyte coverage [104]. Here. antiangiogenic therapy was unable to overcome pericyte-derived endothelial cell survival cues, and tumors exploited such vessels for growth in the absence of reneovascularization. Only after targeting pericytes was antiangiogenic therapy able to induce tumor response.
Highlights.
Tumor infiltrating myeloid cells link angiogenesis and immune tolerance to drive tumor growth
Hypoxia fuels pro-angiogenic and immune-suppressive programming of myeloid cells in tumors
Immune-suppressive myeloid cells can drive tumor resistance to anti-angiogenic therapy
Anti-angiogenic therapy efficacy may partly depend on promoting an immunestimulating environment
Acknowledgements
This work was supported by grants from the NIH (RO1 CA099948, U54CA163155) and the AACR Carcinoid foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Motz GT, Coukos G. Deciphering and reversing tumor immune suppression. Immunity. 2013;39:61–73. doi: 10.1016/j.immuni.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nature reviews. Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 3.Baluk P, et al. Cellular abnormalities of blood vessels as targets in cancer. Current opinion in genetics & development. 2005;15:102–111. doi: 10.1016/j.gde.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 5.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. The New England journal of medicine. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 6.Chi AS, et al. Angiogenesis as a therapeutic target in malignant gliomas. The oncologist. 2009;14:621–636. doi: 10.1634/theoncologist.2008-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerstner ER, et al. VEGF inhibitors in the treatment of cerebral edema in patients with brain cancer. Nature reviews. Clinical oncology. 2009;6:229–236. doi: 10.1038/nrclinonc.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 9.Jain RK, et al. Biomarkers of response and resistance to antiangiogenic therapy. Nature reviews. Clinical oncology. 2009;6:327–338. doi: 10.1038/nrclinonc.2009.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jain RK. Antiangiogenic therapy for cancer: current and emerging concepts. Oncology. 2005;19:7–16. [PubMed] [Google Scholar]
- 11.Ebos JM, Kerbel RS. Antiangiogenic therapy: impact on invasion, disease progression, and metastasis. Nature reviews. Clinical oncology. 2011;8:210–221. doi: 10.1038/nrclinonc.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin EY, et al. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer research. 2006;66:11238–11246. doi: 10.1158/0008-5472.CAN-06-1278. [DOI] [PubMed] [Google Scholar]
- 13.Lin EY, et al. Vascular endothelial growth factor restores delayed tumor progression in tumors depleted of macrophages. Molecular oncology. 2007;1:288–302. doi: 10.1016/j.molonc.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giraudo E, et al. An amino-bisphosphonate targets MMP-9-expressing macrophages and angiogenesis to impair cervical carcinogenesis. The Journal of clinical investigation. 2004;114:623–633. doi: 10.1172/JCI22087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergers G, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nature cell biology. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du R, et al. HIF1alpha Induces the Recruitment of Bone Marrow-Derived Vascular Modulatory Cells to Regulate Tumor Angiogenesis and Invasion. Cancer cell. 2008;13:206–220. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang L, et al. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer cell. 2004;6:409–421. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 18.Pan PY, et al. Reversion of immune tolerance in advanced malignancy: modulation of myeloid-derived suppressor cell development by blockade of stem-cell factor function. Blood. 2008;111:219–228. doi: 10.1182/blood-2007-04-086835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmid MC, et al. Receptor tyrosine kinases and TLR/IL1Rs unexpectedly activate myeloid cell PI3kgamma, a single convergent point promoting tumor inflammation and progression. Cancer cell. 2011;19:715–727. doi: 10.1016/j.ccr.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kujawski M, et al. Stat3 mediates myeloid cell-dependent tumor angiogenesis in mice. The Journal of clinical investigation. 2008;118:3367–3377. doi: 10.1172/JCI35213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rigamonti N, et al. Role of angiopoietin-2 in adaptive tumor resistance to VEGF signaling blockade. Cell reports. 2014;8:696–706. doi: 10.1016/j.celrep.2014.06.059. [DOI] [PubMed] [Google Scholar]
- 22.Shojaei F, et al. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nature biotechnology. 2007;25:911–920. doi: 10.1038/nbt1323. [DOI] [PubMed] [Google Scholar]
- 23.Rolny C, et al. HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through downregulation of PlGF. Cancer cell. 2011;19:31–44. doi: 10.1016/j.ccr.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 24.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar V, Gabrilovich DI. Hypoxia-inducible factors in regulation of immune responses in tumour microenvironment. Immunology. 2014;143:512–519. doi: 10.1111/imm.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 27.Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nature reviews. Cancer. 2003;3:422–433. doi: 10.1038/nrc1094. [DOI] [PubMed] [Google Scholar]
- 28.Ferrara N. The role of VEGF in the regulation of physiological and pathological angiogenesis. Exs. 2005:209–231. doi: 10.1007/3-7643-7311-3_15. [DOI] [PubMed] [Google Scholar]
- 29.Potente M, et al. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–887. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 30.Jain RK. Angiogenesis and lymphangiogenesis in tumors: insights from intravital microscopy. Cold Spring Harb Symp Quant Biol. 2002;67:239–248. doi: 10.1101/sqb.2002.67.239. [DOI] [PubMed] [Google Scholar]
- 31.Senger DR, et al. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 32.Junttila MR, de Sauvage FJ. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature. 2013;501:346–354. doi: 10.1038/nature12626. [DOI] [PubMed] [Google Scholar]
- 33.Oyama T, et al. Vascular endothelial growth factor affects dendritic cell maturation through the inhibition of nuclear factor-kappa B activation in hemopoietic progenitor cells. Journal of immunology. 1998;160:1224–1232. [PubMed] [Google Scholar]
- 34.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nature reviews. Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Motz GT, Coukos G. The parallel lives of angiogenesis and immunosuppression: cancer and other tales. Nature reviews. Immunology. 2011;11:702–711. doi: 10.1038/nri3064. [DOI] [PubMed] [Google Scholar]
- 36.Terme M, et al. Modulation of immunity by antiangiogenic molecules in cancer. Clinical & developmental immunology. 2012;2012:492920. doi: 10.1155/2012/492920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ziogas AC, et al. VEGF directly suppresses activation of T cells from ovarian cancer patients and healthy individuals via VEGF receptor Type 2. International journal of cancer. Journal international du cancer. 2012;130:857–864. doi: 10.1002/ijc.26094. [DOI] [PubMed] [Google Scholar]
- 38.Coussens LM, et al. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell. 2000;103:481–490. doi: 10.1016/s0092-8674(00)00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bingle L, et al. Macrophages promote angiogenesis in human breast tumour spheroids in vivo. British journal of cancer. 2006;94:101–107. doi: 10.1038/sj.bjc.6602901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shojaei F, et al. Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature. 2007;450:825–831. doi: 10.1038/nature06348. [DOI] [PubMed] [Google Scholar]
- 41.Murdoch C, et al. The role of myeloid cells in the promotion of tumour angiogenesis. Nature reviews. Cancer. 2008;8:618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- 42.de Palma M, Coussens LM. Immune Cells and Inflammatory Mediators as Regulators of Tumor Angiogenesis. In: Figg WD, Folkman J, editors. Angiogenesis: An Integrative Approach from Science to Medicine. Springer Science + Business Media; LLC: 2008. [Google Scholar]
- 43.Betsholtz C. Insight into the physiological functions of PDGF through genetic studies in mice. Cytokine Growth Factor Rev. 2004;15:215–228. doi: 10.1016/j.cytogfr.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 44.Joyce JA, et al. Cathepsin cysteine proteases are effectors of invasive growth and angiogenesis during multistage tumorigenesis. Cancer cell. 2004;5:443–453. doi: 10.1016/s1535-6108(04)00111-4. [DOI] [PubMed] [Google Scholar]
- 45.Ferrara N, et al. The biology of VEGF and its receptors. Nature medicine. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 46.Compagni A, et al. Fibroblast growth factors are required for efficient tumor angiogenesis. Cancer research. 2000;60:7163–7169. [PubMed] [Google Scholar]
- 47.De Falco E, et al. SDF-1 involvement in endothelial phenotype and ischemia-induced recruitment of bone marrow progenitor cells. Blood. 2004;104:3472–3482. doi: 10.1182/blood-2003-12-4423. [DOI] [PubMed] [Google Scholar]
- 48.Wang L, et al. Neuropilin-1-mediated vascular permeability factor/vascular endothelial growth factor-dependent endothelial cell migration. The Journal of biological chemistry. 2003;278:48848–48860. doi: 10.1074/jbc.M310047200. [DOI] [PubMed] [Google Scholar]
- 49.Du R, et al. Matrix metalloproteinase-2 regulates vascular patterning and growth affecting tumor cell survival and invasion in GBM. Neuro-oncology. 2008;10:254–264. doi: 10.1215/15228517-2008-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mantovani A, et al. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 51.Murray PJ, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Davies LC, et al. Tissue-resident macrophages. Nature immunology. 2013;14:986–995. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pyonteck SM, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nature medicine. 2013;19:1264–1272. doi: 10.1038/nm.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mitchem JB, et al. Targeting tumor-infiltrating macrophages decreases tumor- initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer research. 2013;73:1128–1141. doi: 10.1158/0008-5472.CAN-12-2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Strachan DC, et al. CSF1R inhibition delays cervical and mammary tumor growth in murine models by attenuating the turnover of tumor-associated macrophages and enhancing infiltration by CD8 T cells. Oncoimmunology. 2013;2:e26968. doi: 10.4161/onci.26968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.DeNardo DG, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer discovery. 2011;1:54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Affara NI, et al. B cells regulate macrophage phenotype and response to chemotherapy in squamous carcinomas. Cancer cell. 2014;25:809–821. doi: 10.1016/j.ccr.2014.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.De Palma M, et al. Tie2-expressing monocytes: regulation of tumor angiogenesis and therapeutic implications. Trends in immunology. 2007;28:519–524. doi: 10.1016/j.it.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 59.Pucci F, et al. A distinguishing gene signature shared by tumor-infiltrating Tie2-expressing monocytes, blood “resident” monocytes, and embryonic macrophages suggests common functions and developmental relationships. Blood. 2009;114:901–914. doi: 10.1182/blood-2009-01-200931. [DOI] [PubMed] [Google Scholar]
- 60.Jeltsch M, et al. Receptor tyrosine kinase-mediated angiogenesis. Cold Spring Harbor perspectives in biology. 2013:5. doi: 10.1101/cshperspect.a009183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Coffelt SB, et al. Angiopoietin 2 stimulates TIE2-expressing monocytes to suppress T cell activation and to promote regulatory T cell expansion. Journal of immunology. 2011;186:4183–4190. doi: 10.4049/jimmunol.1002802. [DOI] [PubMed] [Google Scholar]
- 62.De Palma M, et al. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer cell. 2005;8:211–226. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 63.Mazzieri R, et al. Targeting the ANG2/TIE2 axis inhibits tumor growth and metastasis by impairing angiogenesis and disabling rebounds of proangiogenic myeloid cells. Cancer cell. 2011;19:512–526. doi: 10.1016/j.ccr.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 64.Gerald D, et al. Angiopoietin-2: an attractive target for improved antiangiogenic tumor therapy. Cancer research. 2013;73:1649–1657. doi: 10.1158/0008-5472.CAN-12-4697. [DOI] [PubMed] [Google Scholar]
- 65.Forget MA, et al. Macrophage colony-stimulating factor augments Tie2-expressing monocyte differentiation, angiogenic function, and recruitment in a mouse model of breast cancer. PloS one. 2014;9:e98623. doi: 10.1371/journal.pone.0098623. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 66.Chung AS, et al. Targeting the tumour vasculature: insights from physiological angiogenesis. Nature reviews. Cancer. 2010;10:505–514. doi: 10.1038/nrc2868. [DOI] [PubMed] [Google Scholar]
- 67.Fridlender ZG, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Talmadge JE, Gabrilovich DI. History of myeloid-derived suppressor cells. Nature reviews. Cancer. 2013;13:739–752. doi: 10.1038/nrc3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang L, et al. Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer cell. 2008;13:23–35. doi: 10.1016/j.ccr.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pahler JC, et al. Plasticity in tumor-promoting inflammation: impairment of macrophage recruitment evokes a compensatory neutrophil response. Neoplasia. 2008;10:329–340. doi: 10.1593/neo.07871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liao D, Johnson RS. Hypoxia: A key regulator of angiogenesis in cancer. Cancer metastasis reviews. 2007 doi: 10.1007/s10555-007-9066-y. [DOI] [PubMed] [Google Scholar]
- 72.Keith B, et al. HIF1alpha and HIF2alpha: sibling rivalry in hypoxic tumour growth and progression. Nature reviews. Cancer. 2011;12:9–22. doi: 10.1038/nrc3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Simon MP, et al. The angiopoietin-2 gene of endothelial cells is up-regulated in hypoxia by a HIF binding site located in its first intron and by the central factors GATA-2 and Ets-1. Journal of cellular physiology. 2008;217:809–818. doi: 10.1002/jcp.21558. [DOI] [PubMed] [Google Scholar]
- 74.Forsythe JA, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Molecular and cellular biology. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kelly BD, et al. Cell type-specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia-inducible factor 1. Circulation research. 2003;93:1074–1081. doi: 10.1161/01.RES.0000102937.50486.1B. [DOI] [PubMed] [Google Scholar]
- 76.Murdoch C, et al. Mechanisms regulating the recruitment of macrophages into hypoxic areas of tumors and other ischemic tissues. Blood. 2004;104:2224–2234. doi: 10.1182/blood-2004-03-1109. [DOI] [PubMed] [Google Scholar]
- 77.Avraham-Davidi I, et al. On-site education of VEGF-recruited monocytes improves their performance as angiogenic and arteriogenic accessory cells. The Journal of experimental medicine. 2013;210:2611–2625. doi: 10.1084/jem.20120690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Grunewald M, et al. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006;124:175–189. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 79.Wang X, et al. Hypoxia enhances CXCR4 expression favoring microglia migration via HIF-1alpha activation. Biochemical and biophysical research communications. 2008;371:283–288. doi: 10.1016/j.bbrc.2008.04.055. [DOI] [PubMed] [Google Scholar]
- 80.Ishikawa T, et al. Hypoxia enhances CXCR4 expression by activating HIF-1 in oral squamous cell carcinoma. Oncology reports. 2009;21:707–712. [PubMed] [Google Scholar]
- 81.Blouw B, et al. The hypoxic response of tumors is dependent on their microenvironment. Cancer cell. 2003;4:133–146. doi: 10.1016/s1535-6108(03)00194-6. [DOI] [PubMed] [Google Scholar]
- 82.Casazza A, et al. Impeding macrophage entry into hypoxic tumor areas by Sema3A/Nrp1 signaling blockade inhibits angiogenesis and restores antitumor immunity. Cancer cell. 2013;24:695–709. doi: 10.1016/j.ccr.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 83.Clambey ET, et al. Hypoxia-inducible factor-1 alpha-dependent induction of FoxP3 drives regulatory T-cell abundance and function during inflammatory hypoxia of the mucosa. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E2784–2793. doi: 10.1073/pnas.1202366109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chung AS, et al. An interleukin-17-mediated paracrine network promotes tumor resistance to anti-angiogenic therapy. Nature medicine. 2013;19:1114–1123. doi: 10.1038/nm.3291. [DOI] [PubMed] [Google Scholar]
- 85.Welford AF, et al. TIE2-expressing macrophages limit the therapeutic efficacy of the vascular-disrupting agent combretastatin A4 phosphate in mice. The Journal of clinical investigation. 2011;121:1969–1973. doi: 10.1172/JCI44562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fischer C, et al. Anti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell. 2007;131:463–475. doi: 10.1016/j.cell.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 87.Gonzalez-Garcia A, et al. Phosphatidylinositol 3-kinase gamma inhibition ameliorates inflammation and tumor growth in a model of colitis-associated cancer. Gastroenterology. 2010;138:1374–1383. doi: 10.1053/j.gastro.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 88.Hirsch E, et al. Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science. 2000;287:1049–1053. doi: 10.1126/science.287.5455.1049. [DOI] [PubMed] [Google Scholar]
- 89.Chen W, et al. Macrophage-induced tumor angiogenesis is regulated by the TSC2-mTOR pathway. Cancer research. 2012;72:1363–1372. doi: 10.1158/0008-5472.CAN-11-2684. [DOI] [PubMed] [Google Scholar]
- 90.Huang Y, et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:17561–17566. doi: 10.1073/pnas.1215397109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hamzah J, et al. Vascular normalization in Rgs5-deficient tumours promotes immune destruction. Nature. 2008;453:410–414. doi: 10.1038/nature06868. [DOI] [PubMed] [Google Scholar]
- 92.Schoenfeld JD, Dranoff G. Anti-angiogenesis immunotherapy. Human vaccines. 2011;7:976–981. doi: 10.4161/hv.7.9.16407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Garber K. Promising early results for immunotherapy-antiangiogenesis combination. Journal of the National Cancer Institute. 2014:106. doi: 10.1093/jnci/dju392. [DOI] [PubMed] [Google Scholar]
- 94.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 95.De Bock K, et al. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell. 2013;154:651–663. doi: 10.1016/j.cell.2013.06.037. [DOI] [PubMed] [Google Scholar]
- 96.Sawada N, et al. Endothelial PGC-1alpha mediates vascular dysfunction in diabetes. Cell metabolism. 2014;19:246–258. doi: 10.1016/j.cmet.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lyden D, et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nature medicine. 2001;7:1194–1201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- 98.Song S, et al. PDGFRbeta+ perivascular progenitor cells in tumours regulate pericyte differentiation and vascular survival. Nature cell biology. 2005;7:870–879. doi: 10.1038/ncb1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hlushchuk R, et al. Tumor recovery by angiogenic switch from sprouting to intussusceptive angiogenesis after treatment with PTK787/ZK222584 or ionizing radiation. The American journal of pathology. 2008;173:1173–1185. doi: 10.2353/ajpath.2008.071131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rubenstein JL, et al. Anti-VEGF antibody treatment of glioblastoma prolongs survival but results in increased vascular cooption. Neoplasia. 2000;2:306–314. doi: 10.1038/sj.neo.7900102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Paez-Ribes M, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer cell. 2009;15:220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lu KV, et al. VEGF Inhibits Tumor Cell Invasion and Mesenchymal Transition through a MET/VEGFR2 Complex. Cancer cell. 2012;22:21–35. doi: 10.1016/j.ccr.2012.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sennino B, et al. Suppression of tumor invasion and metastasis by concurrent inhibition of c-Met and VEGF signaling in pancreatic neuroendocrine tumors. Cancer discovery. 2012;2:270–287. doi: 10.1158/2159-8290.CD-11-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bergers G, et al. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. The Journal of clinical investigation. 2003;111:1287–1295. doi: 10.1172/JCI17929. [DOI] [PMC free article] [PubMed] [Google Scholar]