Abstract
Background
There is paucity of studies on socioeconomic inequalities in cancer mortality in developing countries. We examined trends in inequalities in cancer mortality by educational attainment in Colombia during a period of epidemiological transition and a rapid expansion of health insurance coverage.
Methods
Population mortality data (1998–2007) were linked to census data to obtain age-standardised cancer mortality rates by educational attainment at ages 25–64 years for stomach, cervical, prostate, lung, colorectal, breast and other cancers. We used Poisson regression to model mortality by educational attainment and estimated the contribution of specific cancers to the Slope Index of Inequality in cancer mortality.
Results
We observed large educational inequalities in cancer mortality, particularly for cancer of the cervix (RR primary versus tertiary groups=5.75, contributing 51% of cancer inequalities), stomach (RR=2.56 for males, contributing 49% of total cancer inequalities, and RR=1.98 for females, contributing 14% to total cancer inequalities), and lung (RR=1.64 for males contributing 17% of total cancer inequalities, and 1.32 for females contributing 5% to total cancer inequalities). Total cancer mortality rates declined faster among those with higher education, with the exception of mortality from cervical cancer, which declined more rapidly in the lower educational groups.
Conclusion
There are large socioeconomic inequalities in preventable cancer mortality in Colombia, which underscore the need for intensifying prevention efforts. Reducing cervical cancer through reducing HPV infection, early detection and improved access to treatment of preneoplasic lesions. Reinforcing anti-tobacco measures may be particularly important to curb inequalities in cancer mortality.
Keywords: cancer, mortality, education, inequalities, developing countries
Introduction
Cancer is among the top three of causes of death in Colombia (1). The distribution of cancer types reflects the dual situation in many middle-income countries, with a relatively high burden of infection-related cancers (primarily cervical and stomach cancer) combined with a growing burden of cancers associated with lifestyle and other risk factors of non-infectious character (primarily prostate, lung, colorectal and breast cancer). Recent analysis shows that cancer mortality is stabilizing or decreasing (1), but no studies have examined how cancer mortality trends differ by socioeconomic status (SES).
Colombia is a middle-income country with large social and economic inequalities. Despite extensive health care reforms leading to almost universal health insurance coverage, large differences in all-cause mortality by SES, including cancer, remain (2). We hypothesize that the association between SES and cancer differs by cancer type, with the poor suffering disproportionately from mortality from infection-related cancers due to their higher risk of infection. In contrast, the higher SES-groups may experience higher mortality from cancers associated with non-communicable risk factors, reflecting their earlier adoption of unhealthy behaviours and longer life expectancy.
In this study, we use a unique administrative dataset to examine trends in cancer mortality by educational level from 1998 to 2007 in Colombia. This is a period of important changes, including a major healthcare reform that resulted in a rapid increase in health insurance coverage from 59.8% in 1998 to 92.5% in 2007 (2). Earlier studies documented large socioeconomic differences in access to screening and treatment in specific sub-populations (3–6), but how these disparities influence inequalities in cancer mortality has not been assessed. We evaluate differences in cancer mortality by educational level and assess time trends in mortality from the most important cancer sites distinguishing infection-related cancers and frequently occurring cancer types associated with other risk factors.
Materials and Methods
Data
National mortality data for the years 1998–2007 were obtained from the National Administrative Department of Statistics (DANE), with causes of death coded according to the 10th revision of the International Classification of Diseases (ICD-10). Information on sex, date of death and educational level are routinely registered on death certificates. Our data comprise 117,597 deaths from invasive malignant neoplasms (ICD-10 C00-C97). Data were analysed for all cancers combined, but also separately for the following groups: Infection-related cancers (represented by stomach cancer (C16) and cervical cancer (C53)); cancers related to other risk factors (represented by prostate (C61), lung (C33–34), colorectum (C18–C21) and breast cancer (C50)); and the group of ‘other cancers’. Deaths due to unspecified uterus cancer (C55) were reassigned to deaths due to cancers of the cervix uteri (C53) or corpus uteri (C54) according to their reported proportions (7). In a similar way cases without information on age, those with a death certificate issued by a non-medical doctor, and causes based on symptoms were redistributed, based on relative frequencies.
Data on age and sex were available for >99% of all cancer deaths, while data on educational level were missing for 16.7% of cancer deaths (varying from 13.0% for breast to 18.9% for lung cancer). The SAS procedure IMPUTE was used to impute educational level for these cases (8), to reduce bias due to the potentially higher rates of missing education for lower educated individuals and to minimize the potential for numerator/denominator bias (9). This procedure fits a sequence of regression models and draws values from the corresponding predictive distributions. The sequential regression procedure was applied based on a model that included sex, region, rural/urban residential area, age and marital status as covariates. Details of this procedure are described elsewhere (8). The imputation procedure was successful in 98% of cases resulting in a total of 115,410 cancer deaths left for analysis (Table 1).
Table 1.
Description of the study population, 1998–2007
| Absolute number of deaths | Percentage of cancer deaths | Age Standardised Mortality Rates (ASR)* | ||||
|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Men | Women | |
| Cancer type | ||||||
| All cancers | 49,809 | 65,601 | 100% | 100% | 76.24 | 93.68 |
| Stomach | 10,075 | 5,966 | 20.2% | 9.1% | 15.61 | 8.34 |
| Cervix | - | 10,455 | - | 15.9% | - | 16.55 |
| Prostate | 2,268 | - | 4.6% | - | 3.87 | - |
| Lung | 6,786 | 4,345 | 13.6% | 6.6% | 10.95 | 6.35 |
| Colorectal | 3,260 | 3,825 | 6.5% | 5.8% | 4.91 | 5.33 |
| Breast | - | 11,005 | - | 16.8% | - | 14.89 |
| Other | 27,420 | 30,005 | 55.1% | 45.7% | 40.90 | 42.36 |
|
| ||||||
| Educational attainment | ||||||
| Primary | 32,111 | 42,755 | 64.5% | 65.2% | 83.01 | 104.76 |
| Secondary | 13,533 | 18,535 | 27.2% | 28.3% | 72.83 | 85.13 |
| Tertiary | 4,164 | 4,311 | 8.4% | 6.6% | 57.23 | 72.80 |
| ALL cases after imputation | 49,809 | 65,601 | 100% | 100% | ||
| ALL cases before imputation | 50,881 | 66,716 | n.a. | n.a. | ||
| Cases not imputed (missing after imputation) | 1072 | 1115 | 2.1% | 1.7% | ||
| Absolute numbers | % of population | |||||
| Population size | Men | Women | Men | Women | ||
|
| ||||||
| Primary | 40,773,078 | 42,981,451 | 46.7% | 45.9% | ||
| Secondary | 33,768,114 | 37,506,347 | 38.6% | 40.1% | ||
| Tertiary | 12,849,341 | 13,079,674 | 14.7% | 14.0% | ||
| Total population | 87,390,533 | 93,567,472 | 100% | 100% | ||
Standardised mortality rates age-standardized to the Segi world population, for ages 25–64
We excluded individuals aged ≤25, because many would not have completed their education before this age. We focused on adult premature mortality (mortality below age 65), an indicator of population health strongly influenced by social, economic and environmental factors (10), and a common indicator of health system performance (11). In addition, information on educational level from death registries has been shown to be unreliable at ages ≥65 (9).
Education was reclassified into three categories based on the highest educational level attained by the deceased: (a) primary (elementary/primary school) education or less, (b) secondary (high school), and (c) tertiary education (post-secondary education after high-school including college and university).
To obtain mid-year population counts we first extracted data on the proportion of individuals in each educational level from the IIASA/VID database (12), which contains information on the distribution of education for every 5-year age group, sex and year combinations for the period 1970 to 2000 obtained from census, national surveys and demographic projections (12). We performed demographic projections to obtain population counts for years in-between every lustrum using the software PASEX (13). We then multiplied the proportion of individuals in each educational category by population counts from national census and statistical projections obtained from DANE (14) to estimate the annual population size of each educational group.
Statistical Analysis
We calculated annual age-standardized mortality rates (ASR, expressed per 100,000 person-years) by educational level and sex using the Doll World Standard Population (15). Annual trends in ASR by sex and educational level were quantified by calculating the estimated annual percentage change (EAPC) in mortality. To test whether an apparent change in mortality trends was statistically significant, we used joinpoint regression, which fits a series of joined straight lines to age-adjusted rates and uses a Monte Carlo Permutation method to identify the best-fitting point (called joinpoint), where the rate of increase or decrease changes significantly (16). EAPC and joinpoints (year in which a significant change in the mortality trend occurred) were determined based on the log-transformed ASRs and their standard errors. We specified a maximum of 2 joinpoints with at least 4 observation points to either extreme of the data (16).
We implemented separate Poisson regression models with number of deaths as dependent variable and the natural log of person-years as offset variable, incorporating age and educational level as independent variables. We first calculated Rate Ratios (RRs) to compare mortality between educational groups. However, changes in RR are difficult to interpret because of rising levels of education over the study period, e.g., the proportion of people with no or only primary education decreased from 55% to 38%. To ‘control’ for these changes in the composition of educational groups, we estimated the slope and relative index of inequality (SII and RII respectively) by regressing mortality on the mid-point of the cumulative distribution of education (17, 18). The RII can be interpreted as the ratio of the mortality rate between a hypothetical person whose relative rank in the distribution of education is zero and a person whose relative rank in the cumulative distribution of education is 100% (19). A value of RII higher than 1 indicates educational inequalities favouring the higher educated (19, 20). To evaluate whether the RII significantly changed over time, an interaction term with calendar year was added to the regression models.
We calculated the contribution of each cancer site to the absolute differences in cancer mortality measured by the slope index of inequality (SII). The SII measures the absolute difference in rates between the population at the top and the bottom of the educational distribution.
Regression analyses were conducted in each of the five multiple databases generated by the multiple imputation process, using standard techniques of the PROC MIANALYZE procedure in SAS to combine estimates from all databases and adjust standard errors to account for uncertainty in the imputation (21). This procedure reads the parameter estimates and associated covariance matrix for each imputed data set, and then derives valid multivariate inferences for these parameters. This allows for valid statistical inference that appropriately reflects uncertainty due to missing values (21). All analyses were conducted in SAS® version 9.2.
Results
The most common causes of cancer deaths were cancers of the cervix (ASR 16.55) and breast (ASR 14.89) among women, and stomach (ASR 15.61) and lung cancer (ASR 10.95) among men (Table 1).
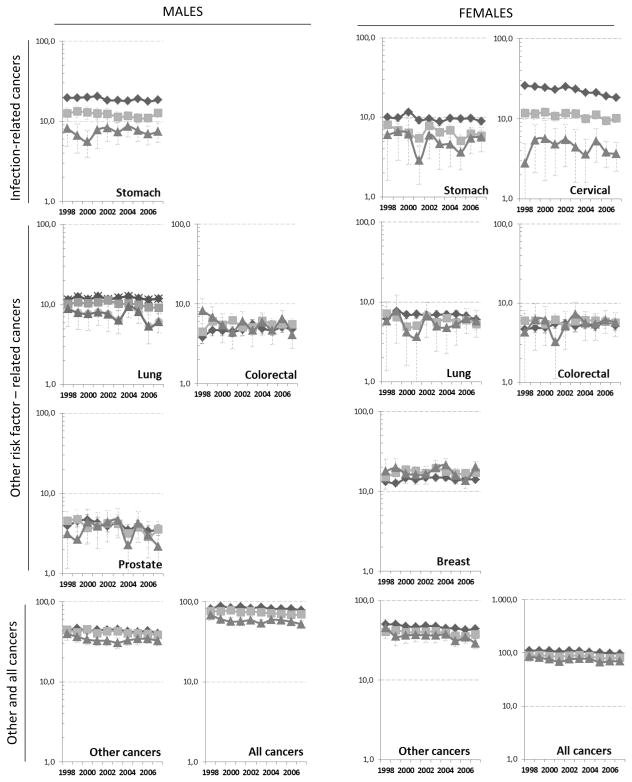
All-cancer mortality decreased significantly among both sexes in all educational groups, with a gradient towards stronger declines amongst the higher educated (Figure 1). Mortality for ‘other cancers’ decreased in all groups over the entire time period, with a joinpoint observed only amongst males with tertiary education in 2001, after which initial strong declines stabilised (Table 2). Stomach cancer mortality rates tended to decrease, but these trends failed to reach statistical significance with the exception of males with primary education or less (EAPC −1.13%). Particularly strong, and statistically significant declines of 2–3% annually were observed for cervical cancer mortality, with strongest declines (EAPC −3.53%) amongst the lowest educated women. Strong declines of about 3% annually were also observed for prostate, lung and colorectal cancer amongst the highest educated males, but these trends failed to reach statistical significance. Mortality of lung, colorectal and female breast cancer did not change significantly between 1998 and 2007 in either educational group or sex, with the exception of males with secondary education, amongst whom lung cancer mortality sharply declined (EAPC −3.70%) after 2002.
Figure 1.
time trends in age-standardised cancer mortality by type of cancer and educational level, 1998–2007, ages 25–64, men and women. Dark lines, diamonds= primary education, light lines, squares=secondary education, dark grey lines, triangles= tertiary education
Table 2.
Joinpoints with corresponding estimated annual percent change (EAPC) and rate ratios (RR) for cancer mortality by educational level, 1998–2007
| Group | Cancer Type |
Educational level |
Men | Women | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| JP | EAPC1 (95% CI) | EAPC2 (95% CI) | RR | 95%CI | JP | EAPC1 (95% CI) | EAPC2 (95% CI) | RR | 95%CI | |||
| TOTAL CANCER | Primary | No | −0.77 (−1.39; −0.16) | 1.54 | (1.48; 1.59) | No | −1.49 (−1.98; −1.01) | 1.62 | (1.57; 1.68) | |||
| Secondary | No | −1.35 (−1.81; −0.89) | 1.34 | (1.29; 1.39) | No | −1.26 (−2.07; −0.45) | 1.35 | (1.30; 1.40) | ||||
| Tertiary | No | −1.43 (−2.79; −0.05) | 1 | No | −1.59 (−3.16; −0.01) | 1 | ||||||
|
| ||||||||||||
| Infection related cancers | Stomach | Primary | No | −1.13 (−2.05; −0.19) | 2.56 | (2.29; 2.86) | No | −1.28 (−3.07; 0.54) | 1.98 | (1.75; 2.24) | ||
| Secondary | No | −1.17 (−2.74; 0.43) | 1.65 | (1.47; 1.85) | No | −2.68 (−5.75; 0.50) | 1.38 | (1.21; 1.59) | ||||
| Tertiary | No | 0.63 (−2.63; 4.01) | 1 | No | −0.33 (−7.07; 6.90) | 1 | ||||||
| Cervix | Primary | n.a. | No | −3.53 (−4.65; −2.40) | 5.75 | (5.05; 6.54) | ||||||
| Secondary | n.a. | No | −2.08 (−3.54; −0.59) | 2.82 | (2.47; 3.22) | |||||||
| Tertiary | n.a. | No | −2.33 (−7.43; 3.06) | 1 | ||||||||
|
| ||||||||||||
| Cancers related to other risk factors | Prostate | Primary | No | −2.45 (−4.57; −0.28) | 1.04 | (0.92; 1.19) | n.a. | |||||
| Secondary | No | −3.74 (−6.38; −1.03) | 1.01 | (0.87; 1.16) | n.a. | |||||||
| Tertiary | No | −3.37 (−10.51; 4.33) | 1 | n.a. | ||||||||
| Lung | Primary | No | −0.15 (−1.16; 0.88) | 1.64 | (1.47; 1.82) | No | −0.84 (−2.51; 0.86) | 1.32 | (1.16; 1.50) | |||
| Secondary | 2002 | 1.69 (−1.59; 5.09) | −3.70 (−5.55; −1.81) | 1.38 | (1.22; 1.55) | No | −1.42 (−4.16; 1.41) | 1.12 | (0.98; 1.28) | |||
| Tertiary | No | −3.01 (−7.09: 1.25) | 1 | No | 0.49 (−4.47; 5.72) | 1 | ||||||
| Colorectal | Primary | No | 1.67 (−0.44: 3.82) | 0.91 | (0.82; 1.01) | No | 1.38 (−0.07; 2.85) | 1.01 | (0.90; 1.13) | |||
| Secondary | No | 0.31 (−2.12; 2.81) | 1.05 | (0.93; 1.19) | No | −0.11 (−1.64; 1.45) | 1.13 | (0.99; 1.28) | ||||
| Tertiary | No | −3.36 (−8.08; 1.61) | 1 | No | 1.31 (−3.59; 6.45) | 1 | ||||||
| Breast | Primary | n.a. | No | 0.71 (−0.61; 2.05) | 0.93 | (0.87; 0.99) | ||||||
| Secondary | n.a. | No | −0.20 (−1.96; 1.58) | 1.13 | (1.06; 1.21) | |||||||
| Tertiary | n.a. | No | 0.03 (−4.18; 4.43) | 1 | ||||||||
|
| ||||||||||||
| OTHER CANCERS | Primary | No | −0.88 (−1.67; −0.08) | 1.39 | (1.33; 1.45) | No | −1.64 (−2.16; −1.12) | 1.50 | (1.43; 1.57) | |||
| Secondary | No | −1.34 (−2.21; −0.47) | 1.30 | (1.24; 1.37) | No | −1.60 (−2.54; −0.65) | 1.27 | (1.21; 1.34) | ||||
| Tertiary | 2001 | −6.48 (−13.14; 0.69) | 0.87 (−1.30; 3.09) | 1 | No | −3.10 (−5.02; −1.13) | 1 | |||||
JP = joinpoint: was there a joinpoint and if so, in which year; EAPC = Estimated Annual Percent Change (%), EAPC1: EAPC during period from 1998 until joinpoint (in case of a joinpoint) or during the whole period, EAPC2: EAPC during the period from joinpoint until 2007; RR = Rate Ratio, apply to period 1998–2007; n.a. = not applicable
Rate ratios (RR) of cancer mortality by educational attainment for the entire period were generally negatively associated with educational attainment (Table 2), with higher rates in those with only primary education as compared to those with tertiary education (RRmen=1.54, 95% Confidence Interval[CI]=1.48, 1.59; RRwomen=1.62, 95%CI 1.57, 1.68), and a clear gradient towards decreased overall cancer mortality with increasing educational level.
The most pronounced educational gradients were observed for cervical (RR 5.75) and stomach cancer (RRmen 2.56, RRwomen 1.98); prostate, lung and other cancers also showed substantial educational gradients. There was no significant educational gradient for colorectal and breast cancer mortality. Breast cancer mortality was highest in women with secondary education (RR=1.13, 95%CI 1.06, 1.21), and lowest in women with primary education (RR=0.93, 95%CI 0.87, 0.99).
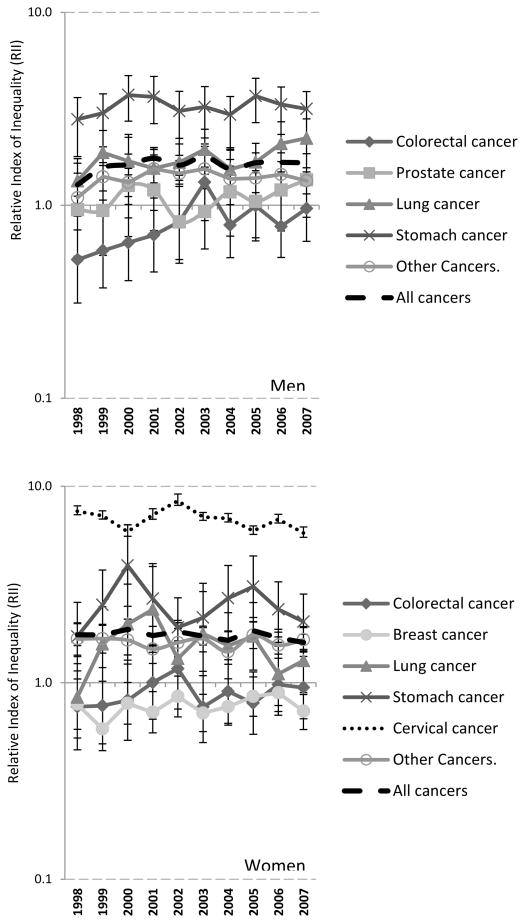
Inequalities in cancer mortality by educational attainmented were statistically signicificant for all cancers except for colorectal, prostate and breast cancer. Until 2002, the mortality of the colorectal, prostate and breast cancer was highest in the highest educated groups (RII significantly smaller than 1) (Figure 2). RII changed little over time, except for increases in RII for male total cancer mortality and male colorectal cancer, which were driven by increased inequalities from 1998–2003 only. None of the other cancer types showed any significant change over time (suppl table 1).
Figure 2.
Time trends in Relative Index of Inequality of cancer mortality by sex and cancer type
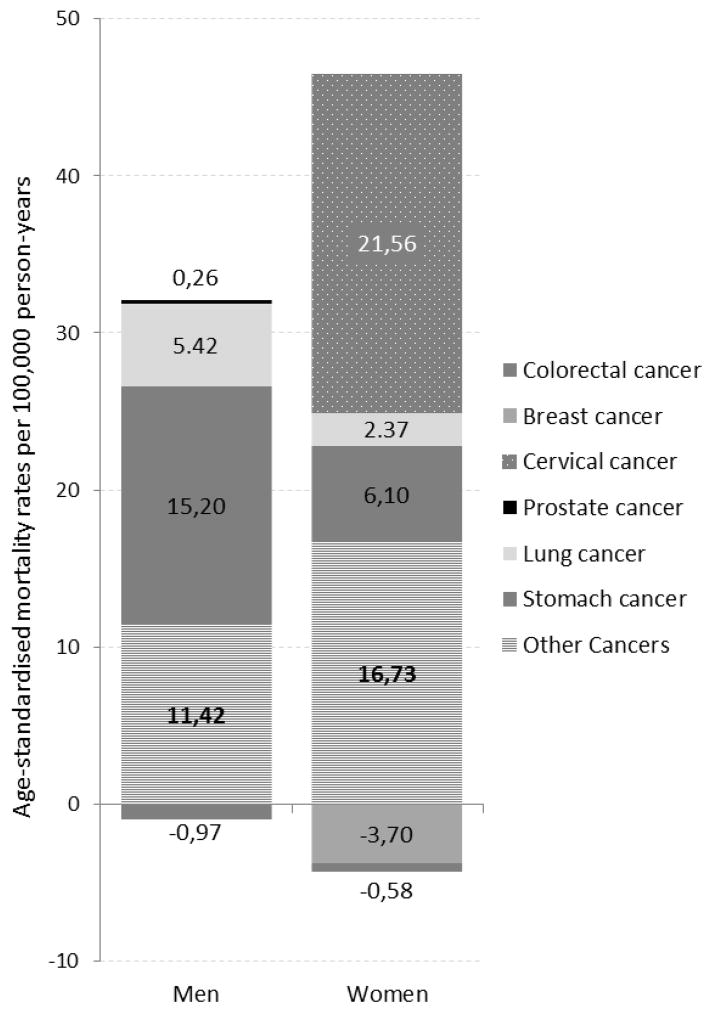
Absolute differences in ASR by educational level expressed by the SII were larger for women than for men. This was almost entirely attributable to the large share of inequalities attributable to cervical cancer mortality, which accounted for 51% of inequalities in total female cancer mortality, while 14% of female inequalities was due to stomach cancer (Figure 3). Among men, the main contributors to inequalities in cancer mortality by education were stomach cancer (49%) and lung cancer (17%). Breast (−9%) and colorectal cancer (−3% for males and −1% for females) contributed inversely to differences in mortality rates, with highest rates amongst the highest educated.
Figure 3.
Slope Index of Inequality (SII) in cancer mortality 1998–2002 versus 2003–2007 by sex.
Discussion
We found large inequalities in cancer mortality by educational level, although associations differed by cancer type. Among women, we observed large inequalities in cervical and stomach cancer, while there were no clear inequalities in colorectal and breast cancer (associated with non-communicable risk factors). Among men, there were inequalities in all but colorectal and prostate cancers, with stomach and lung cancer having the largest inequalities.
Interpretation of results
Several explanations of cancer disparities should be considered, including disparities in preventable risk factors, insurance coverage and health care utilization. We found striking differences in mortality from cervical cancer among women in Colombia. Risk of cervical cancer is related to the mechanisms of transmission of the Human Papilloma Virus (HPV) and reproductive factors such as parity; however, the strongest determinant of cervical cancer mortality is access to regular health care in order to detect and treat preneoplastic lesions (22). HPV vaccination has been advised in many countries to prevent cervical cancer (23), but participation and access to good quality early detection activities remains limited in the lower socioeconomic groups (24).
Although coverage by cytology is relatively high in Colombia, coverage is substantially lower among women with only primary education (74.9% of women aged 25–69 had a cytology in the past 3 years) compared to women with university education (85.4%); differences are also large between low-income (64.5%) vs. high-income (85.8%) women (24). This suggests that there are persistent barriers to access to medical services in the lower-educated groups, which are precisely the groups at highest risks of developing cervical cancer (24).
On the other hand, cervical cancer rates declined faster among lower educated women, which may reflect improved access and adherence to cytology and subsequent treatment for the poorest segments of the population, potentially as a result of the rapidly increasing health insurance coverage since 2002/2002 (2). In 2005/2006, 27% of women with an abnormal pap smear had no access to any of the diagnostic or therapeutic services(25). Although colposcopies and biopsies are by law part of the obligatory health plan since 2004, the gynaecologic consultations previous to the colposcopy were not until 2011 (26). These developments, combined with the introduction of HPV vaccination, may result in narrowing inequalities in cervical cancer mortality in the near future.
Stomach cancer is a very aggressive disease, indicating that stomach cancer mortality is probably more strongly related to risk factor exposure than to health services interventions, and so do the large educational inequalities observed in our study. Despite the presence of other risk factors, such as methods of food preservation, cigarette smoking and overweight, infections, mainly H. Pylori, are believed to be a particularly important risk factor for stomach cancer in Colombia (27). A large proportion of gastric cancers are located in the antrum (28), of which 89% is believed to be related to H. Pylori, and risk factors like smoking and high salt intake show interactions with H. Pylori (27, 29). Early diagnosis may significantly improve prognosis of stomach cancer, but cost-effective early detection programs for a middle-income country setting are unavailable (27).
Cancer types associated with non-communicable risk factors were generally stable over time, with the exception of prostate cancer, which showed declines amongst the primary and secondary educated groups. Despite the discussion regarding potential overdiagnosis of prostate and breast cancer with the currently available screening methods (prostate specific antigen (PSA) testing and mammography respectively), improved access to health care may play a major role in reducing disparities among educational and socioeconomic groups in Colombia. Access to mammographic screening varies by eductional level (17% among women with no education to 59% among university trained women), but generally is low (5). Although we have no data on incidence, we expect breast cancer incidence to be highest amongst the higher educated women because of their higher prevalence of reproductive risk factors for breast cancer such as low parity, high age at first childbirth and short breast feeding periods (30).
The most important risk factors for prostate cancer are old age and access to PSA testing. We did not observe differences in prostate cancer mortality by educational level, which may be due to the low incidence of prostate cancer in our relatively young sample (25–64 years). Most prostate cancer cases are diagnosed after age 65, at which age disparities by education may emerge due to inequities in the use of PSA tests.
Colorectal cancer screening has shown effective in reducing mortality; yet, no major interventions on the subject have been implemented in Colombia and increasing mortality rates and mortality:incidence ratios cause concern (1)(31). Overweight and obesity are implicated in the etiology of colorectal cancer (as well as in postmenopausal breast and potentially prostate cancer) (32, 33), and have increased in Latin America. Although Colombians with primary education have higher body mass index than their higher educated counterparts, inequalities in overweight and obesity by educational level have been stable since since at least the early 1990’s (34), a pattern consistent with the stable trends we observed for cancers associated with these risk factors.
Educational inequalities in ASRs for lung cancer likely reflect differences in smoking prevalence in Colombia. Overall smoking prevalence decreased from 21.4% to 12.8% between 1993 and 2007. In 2007, 14.3% of those with primary education smoked versus 11% and 9.7% of those with university or postgraduate education, respectively (35). Lung cancer mortality rates during 1998–2007, however, reflect smoking patterns in the 1970s, for which data are not available.
Limitations of the study
Some limitations of our study should be considered: Mortaliy data were obtained from official mortality statistics, while data on the population distribution by education were obtained from censuses and demographic projections. This may have led to the so-called numerator/denominator bias, which may have led to overestimation of disparities (9). Additionally, for some years, data on population size were obtained from demographic projections combined with distributions of education from surveys. To assess the impact of this potential bias, we experimented with different education distributions from multiple data sources (12, 36, 37). Although distributions and absolute rates sometimes differed, the overall level and trends observed in our study were robust to different assumptions regarding the distribution of education. As shown in supplemental figure 2, the distribution of education in our dataset mirrors very well data from other sources.
Another limitation is the under-registration of deaths in some regions (38). Previous studies (39, 40) suggest that underregistration was particularly important in the poorest regions in the early years of our study. For example, the estimated proportion of registered deaths in the poorest region was only 25%, compared to ≥90% in the most affluent regions (39). As lower educated persons are more likely to live in areas with more under-registration, our estimates of inequalities are likely underestimated. The extent to which inequalities have increased is probably also underestimated, because underregistration substantially decreased over the study period (39, 40). Our results are therefore probably a conservative estimate, indicative of potentially larger inequalities in mortality by education.
Information on education was missing for 16.7% of cancer deaths, potentially leading to an underestimation of disparities, as missing values are usually more common in the least educated (39). We imputed values for educational level for individuals with missing educational information based on a information on age, sex marital status, region and urban/rural place of residence, thereby limiting the potential impact of this source of bias. Future studies should examine how results from our ‘unlinked’ study compare to more precise linkages based on individual identifiers.
Conclusion and policy implications
We found large educational inequalities in total cancer mortality in Colombia. Several explanations should be considered, including disparities in avoidable risk factors, early detection and treatment. Inequalities are not declining, despite improvements in health insurance coverage. On the contrary, with the exception of infection-related cancers, for which mortality declined faster in the lower educated groups, inequalities in mortality from several cancer sites grew during our study period. We document persistent and large inequalities in cervical cancer, which highlight the need for extending prevention efforts to reduce infection by HPV with a focus on the lower socioeconomic groups. Prevention of HPV infection by sex education and vacciation programmes may prove necessary to reduce inequalities in cervical cancer mortality, accompanied by efforts to improve access to cytology and follow-up care following abnormal pap smears. Large inequalities in stomach cancer highlight the need for identifying effective early detection strategies and public health strategies to eradicate H. pylori. Smoking contibutes importantly to inequalities in cancer mortality particularly among men, highlighting the need to reinforce efforts to reduce tobacco consumption, particularly among lower educated men.
Supplementary Material
Supplementary figure 1: Proportion of Colombian population covered by health insurance according to different schemes
Supplementary figure 2: Comparisons of time trends in educational level in Colombia based on different sources.
Supplementary table 1. Estimates for the RR for the interaction term of RII*Year with corresponding confidence intervals
Thumbnail sketch.
What is already known on this subject?
Earlier studies have documented large and persistent inequalities in mortality from cancer by educational level in high-income countries. However, there is a paucity of studies documenting socioeconomic inequalities in cancer mortality in low- and middle-income countries. Part of this gap in the literature reflects a lack of available data on mortality stratified by meaningful indicators of socioeconomic status.
What this study adds
In this work, we use unique registry-linked data to examine inequalities in mortality by educational level in Colombia. Our results reveal large inequalities by educational level in infection-related cancer mortality, particularly cervical and stomach cancer, which represent a majority share of socioeconomic inequalities in total cancer mortality. Results raise questions on the role of behavioural changes and health insurance coverage in inequalities in avoidable cancer mortality, and the potential role of increased access to early detection and treatment in curbing cancer inequalities.
Acknowledgments
We thank National Administrative Department of Statistics - DANE for the provision of the mortality database. Thanks to Prof. Johan Mackenbach, Dr. Melina Arnold and Dr. Mónica Sierra for their helpful comments. Thanks to Jose Rubio, research assistant at the London School of Economics and Political Science for his help in comparing databases on educational level in Colombia.
Work on this project was partly financed by a Rotterdam Global Health Initiative seed grant. Esther de Vries was supported by an Transfer of Expertise Fellowship from the International Agency for Research on Cancer and the Union for International Cancer Control. Iván Arroyave was supported by the European Union Erasmus Mundus Partnerships Programme Erasmus-Columbus 2013 (Eracol). Mauricio Avendano is supported by a Starting Researcher grant from the European Research Council (ERC grant No 263684), the National Institute on Aging (grants R01AG040248 and R01AG037398), and the McArthur Foundation Research Network on Ageing.
Footnotes
The authors declare that they have no conflicts of interest.
References
- 1.Pineros M, Gamboa O, Hernandez-Suarez G, Pardo C, Bray F. Patterns and trends in cancer mortality in Colombia 1984–2008. Cancer epidemiology. 2013;37(3):233–9. doi: 10.1016/j.canep.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Arroyave I, Cardona D, Burdorf A, Avendano M. The impact of increasing health insurance coverage on disparities in mortality: health care reform in Colombia, 1998–2007. American journal of public health. 2013;103(3):e100–6. doi: 10.2105/AJPH.2012.301143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pineros M, Sanchez R, Perry F, Garcia OA, Ocampo R, Cendales R. Delay for diagnosis and treatment of breast cancer in Bogota, Colombia. Salud publica de Mexico. 2011;53(6):478–85. [PubMed] [Google Scholar]
- 4.Velasquez-De Charry LC, Carrasquilla G, Roca-Garavito S. Equity in access to treatment for breast cancer in Colombia. Salud publica de Mexico. 2009;51(Suppl 2):s246–53. doi: 10.1590/s0036-36342009000800015. [DOI] [PubMed] [Google Scholar]
- 5.Font-Gonzalez A, Pineros M, de Vries E. Self-reported early detection activities for breast cancer in Colombia in 2010: impact of socioeconomic and demographic characteristics. Salud publica de Mexico. 2013;55(4):368–78. doi: 10.21149/spm.v55i4.7220. [DOI] [PubMed] [Google Scholar]
- 6.de Sanjose S, Bosch FX, Muñoz N, Shah K. Social differences in sexual behaviour and cervical cancer. 1997 [PubMed] [Google Scholar]
- 7.Loos AH, Bray F, McCarron P, Weiderpass E, Hakama M, Parkin DM. Sheep and goats: separating cervix and corpus uteri from imprecisely coded uterine cancer deaths, for studies of geographical and temporal variations in mortality. European journal of cancer. 2004;40(18):2794–803. doi: 10.1016/j.ejca.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Ragunathan T, Lepkowski J, Van Hoewyk J, Solenberger P. A Multivariate Technique for Multiply Impyting Missing Values Using a Sequence of Regression Models. Survey Methodology. 2001;27:85–95. [Google Scholar]
- 9.Kunst AE, Groenhof F, Borgan JK, Costa G, Desplanques G, Faggiano F, et al. Socio-economic inequalities in mortality. Methodological problems illustrated with three examples from Europe. Revue d’epidemiologie et de sante publique. 1998;46(6):467–79. [PubMed] [Google Scholar]
- 10.World Health Organization. Final Report of the Commission on Social Determinants of Health. Geneve: World Health Organization; 2008. Closing the gap in a generation: Health equity through action on the social determinants of health. [Google Scholar]
- 11.Smith P, Mossialos E, Papanicolas I, Leatherman S. Performance Measurement for Health System Improvement: Experiences, Challenges and Prospects. Cambridge: Cambridge University Press; 2009. [Google Scholar]
- 12.IIASA/VID. IVEP-1.0 (IIASA/VID education database) 2010 [Google Scholar]
- 13.U.S. Census Bureau. Population Analysis System (PASEX). Population Analysis Spreadsheets. Washington DC: 2011. 2.04g. [Google Scholar]
- 14.DANE. Estadisticas Vitales [ http://www.dane.gov.co]
- 15.Doll R, Smith PG. Comparison between registries: age-standardized rates. In: Waterhouse JH, Muir CS, Shanmugaratnam K, Powell J, Peacham D, Whelan S, editors. Cancer Incidence in Five Continents. VI. Lyon: IARCpress; 1982. pp. 671–5. [Google Scholar]
- 16.Joinpoint Regression Program. Statistical Research and Applications Branch. National Cancer Institute; May, 2013. 4.0.4. [Google Scholar]
- 17.Mackenbach JP, Kunst AE. Measuring the magnitude of socio-economic inequalities in health: An overview of available measures illustrated with two examples from Europe. Social Science & Medicine. 1997;44(6):757–71. doi: 10.1016/s0277-9536(96)00073-1. [DOI] [PubMed] [Google Scholar]
- 18.Harper S, Lynch J, Meersman SC, Breen N, Davis WW, Reichman ME. An overview of methods for monitoring social disparities in cancer with an example using trends in lung cancer incidence by area-socioeconomic position and race-ethnicity, 1992–2004. Am J Epidemiol. 2008;167(8):889–99. doi: 10.1093/aje/kwn016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mackenbach JP, Kunst AE. Measuring the magnitude of socio-economic inequalities in health: an overview of available measures illustrated with two examples from Europe. Social science & medicine. 1997;44(6):757–71. doi: 10.1016/s0277-9536(96)00073-1. [DOI] [PubMed] [Google Scholar]
- 20.Menvielle G, Rey G, Jougla E, Luce D. Diverging trends in educational inequalities in cancer mortality between men and women in the 2000s in France. BMC public health. 2013;13:823. doi: 10.1186/1471-2458-13-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.SAS Institute I; Inc. SI, editor. User’s Guide SAS/STATR 92. Version 8. Cary, NC: SAS Institute Inc; 2008. The MIANALYZE Procedure; pp. 201–33. [Google Scholar]
- 22.Murillo R, Almonte M, Pereira A, Ferrer E, Gamboa OA, Jerónimo J, et al. Cervical cancer screening programs in Latin America and the Caribbean. Vaccine. 2008;26(Suppl 11):L37–48. doi: 10.1016/j.vaccine.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 23.Bosch FX, Broker TR, Forman D, Moscicki AB, Gillison ML, Doorbar J, et al. Comprehensive control of human papillomavirus infections and related diseases. Vaccine. 2013;31(Suppl 7):H1–H31. doi: 10.1016/j.vaccine.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piñeros M, Cendales R, Murillo R, Wiesner C, Tovar S. Pap test coverage and related factors in Colombia, 2005. Rev salud pública. 2007;9(3):327–41. doi: 10.1590/s0124-00642007000300002. [DOI] [PubMed] [Google Scholar]
- 25.Wiesner C, Cendales R, Murillo R, Piñeros M, Tovar S. Following-up females having an abnormal Pap smear in Colombia. Rev salud pública. 2010;12(1):1–13. doi: 10.1590/s0124-00642010000100001. [DOI] [PubMed] [Google Scholar]
- 26.Wiesner C, Tovar S, Cendales R, Vejarano M. Healthcare services arrangement for cervical control in Soacha, Colombia. Rev Colomb Cancerol. 2006;10(2):98–108. [Google Scholar]
- 27.Piazuelo MB, Correa P. Gastric cancer: Overview. Colomb Med (Cali) 2013;44(3):192–201. [PMC free article] [PubMed] [Google Scholar]
- 28.Oliveros R, Navarrera L. Diagnosis, staging and treatment of gastric cancer in Colombia from 2004 to 2008. Rev Col Gastroenterol. 2012;27(4):269–74. [Google Scholar]
- 29.Plummer M, Franceschi S, Vignat J, Forman D, de Martel C. Global burden of gastric cancer attributable to pylori. International journal of cancer Journal international du cancer. 2014 doi: 10.1002/ijc.28999. [DOI] [PubMed] [Google Scholar]
- 30.Olaya-Contreras P, Pierre B, Lazcano-Ponce E, Villamil-Rodriguez J, Posso-Valencia HJ. Reproductive risk factors associated with breast cancer in Colombian women. Revista de saude publica. 1999;33(3):237–45. doi: 10.1590/s0034-89101999000300004. [DOI] [PubMed] [Google Scholar]
- 31. [accessed on 20/march/2014. 2013];GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. [Internet] [Internet]. International Agency for Research on Cancer. Available from: http://globocan.iarc.fr.
- 32.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–78. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 33.De Pergola G, Silvestris F. Obesity as a major risk factor for cancer. J Obes. 2013 doi: 10.1155/2013/291546. Epub 2013 Aug 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lopez-Arana S, Burdorf A, Avendano M. Trends in overweight by educational level in 33 low- and middle-income countries: the role of parity, age at first birth and breastfeeding. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2013;14(10):806–17. doi: 10.1111/obr.12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodríguez J, Ruiz F, Peñaloza E, Eslava J, Gómez L, Sánchez H, et al. Encuesta Nacional de Salud 2007. Resultados Nacionales. Bogotá: Ministerio de la Protección Social; 2009. [Google Scholar]
- 36.World Development Indicators (WDI) http://data.worldbank.org/data-catalog/world-development-indicators.
- 37.Barro R, Lee J-W. NBER Working Paper Series, vol. Working Paper 15902. Cambridge, MA: National Bureau of Economic Research; 2010. A new data set of educational attainment in the world, 1950–2010. [Google Scholar]
- 38.Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet. 1997;349(9061):1269–76. doi: 10.1016/S0140-6736(96)07493-4. [DOI] [PubMed] [Google Scholar]
- 39.Rodríguez-García J. Desigualdades socioeconómicas entre departmentos y su asociación con indicadores de mortalidad en Colombia en 2000. Revista Panamericana de Salud Pública. 2007;21:111–24. doi: 10.1590/s1020-49892007000200006. [DOI] [PubMed] [Google Scholar]
- 40.Florez CE, Méndez R. El Nivel del Subregistro de las Defunciones: Colombia 1990. Colombia: CEDE; 1995. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure 1: Proportion of Colombian population covered by health insurance according to different schemes
Supplementary figure 2: Comparisons of time trends in educational level in Colombia based on different sources.
Supplementary table 1. Estimates for the RR for the interaction term of RII*Year with corresponding confidence intervals