Abstract
Inhibitors of the PD-1:PD-L1 pathway, a central regulator of T cell exhaustion, have been recently shown to be effective for treatment of different cancers. However, clinical responses are mixed, highlighting the need to better understand the mechanisms of action of PD-1:PD-L1, the role of this pathway in immunity to different tumors, and the molecular and cellular effects of PD-1 blockade. Here we review the molecular regulation of T cell exhaustion, placing recent findings on PD-1 blockade therapies in cancer in the context of the broader understanding of the roles of the PD-1:PD-L1 pathway in T cell exhaustion during chronic infection. We discuss the current understanding of the mechanisms involved in reversal T cell exhaustion, and outline critical areas of focus for future research, both basic and clinical.
Keywords: PD-1, PD-L1, T cell exhaustion, immunotherapy, cancer, chronic infection
A revolution in cancer immunotherapy
Exhaustion was originally identified in CD8+ T cells during chronic lymphocytic choriomeningitis virus (LCMV) infection in mice [1, 2], and was subsequently shown to occur in other mouse models of infection, and in humans afflicted with HIV, HCV, and HBV and cancer [3–10]. A cardinal feature of T cell exhaustion is over-expression of multiple inhibitory receptors, including Programmed Death-1 (PD-1, CD279), cytotoxtic T lymphocyte antigen-4 (CTLA-4, CD152), Lag-3, Tim-3, CD244/2B4, CD160, TIGIT, and others [3]. The discovery that blockade of the PD-1 pathway could partially reverse exhaustion [11] and lead to reduced viral or tumor load [11–13] was a significant breakthrough. These data indicated that TEX were not terminally dysfunctional, but could be reinvigorated, with implications for the treatment of diseases including chronic infections and cancer.
The past decade has witnessed a paradigm shift in cancer treatment, with the advent of approaches that target or manipulate the immune system, collectively termed immunotherapies [14–16]. Although cancer cells can be immunogenic, the immune system often fails to eliminate cancer cells, which are protected by mechanisms that have evolved to prevent recognition of self including central tolerance, ignorance or failure to become activated in the periphery, T cell extrinsic regulation (e.g. regulatory T cells, myeloid-derived suppressor cells, suppressive cytokines such as IL-10, etc), and T cell intrinsic dysfunction upon inappropriate or excessive antigen stimulation (anergy and exhaustion) [15, 17–19]. Antibodies targeting inhibitory pathways including CTLA-4 and PD-1 are paving the way for a new generation of cancer treatment approaches. These “checkpoint blockade” strategies aim to relieve regulatory mechanisms that restrain tumor-infiltrating T cells (TILs) [14–16, 20]. The first of these antibodies to gain FDA approval, ipilimumab in 2011 (anti-CTLA-4, Yervoy, Bristol-Myers Squibb), pembrolizumab in 2014 (anti-PD-1, Keytruda, Merck and Co.), and nivolumab in 2014 (anti-PD-1, Opdivo, Bristol-Myers Squibb), have demonstrated remarkable clinical success [16, 20–24]. However, we have likely only scratched the surface of the potential of immunotherapies for the treatment of cancer and other diseases.
Recent clinical trials have shown that blocking the PD-1:PD-L1 pathway enhances immunity in several cancer types including melanoma, non-small cell lung cancer (NSCLC), renal cell carcinoma (RCC), bladder cancer, and others, leading to objective responses in a number of patients [16, 21–23, 25–32]. However, the majority of patients do not experience complete responses upon anti-PD-1 treatment, and some do not respond at all, highlighting the need to better understand the mechanisms of action of PD-1:PD-L1, the role of this pathway in immunity to different tumors, and the molecular and cellular effects of PD-1 blockade.
Here we review the molecular regulation of T cell exhaustion and the mechanisms involved in reversal of this type of T cell dysfunction, focusing on the PD-1:PD-L1 pathway as a central regulator of T cell exhaustion. We place recent findings on PD-1 blockade therapies in cancer and associated mechanisms in the context of the broader understanding of the roles of the PD-1:PD-L1 pathway in T cell exhaustion gained through studies in mouse models of chronic infection. Finally, we discuss gaps in knowledge and highlight critical areas of focus for future research, both basic and clinical.
Hallmarks of TEX cells
T cell exhaustion is a state of dysfunction that commonly occurs during chronic infections and cancer due to the persistence of antigen and inflammation [3]. Failure to eliminate antigen is associated with a progressive loss of T cell effector functions, altered metabolism, and a unique transcriptional program compared to functional effector (TEF) and memory T cells (TMEM) [3]. Exhaustion is also associated with co-expression of high levels of multiple inhibitory receptors including PD-1, Lag-3, Tim-3, CD160, TIGIT, and others [33–36]. The normal physiological function of PD-1 is thought to be in limiting immunopathology and promoting tolerance to self antigens (Box 1) [37]. Consequently, PD-1 is not a unique phenotypic marker to selectively define TEX cells, but can also be expressed by recently activated TEF cells [11, 33, 34] and T cells rendered tolerant due to encountering autoantigen in the absence of high levels of costimulation and/or inflammation [18, 38–40]. Additionally, recent work showed that PD-1+ CD8+ T cells can be found in healthy adult humans, and these cells did not share the transcriptional program of exhaustion with PD-1high HIV-specific CD8+ T cells in patients afflicted with HIV or TEX cells in chronic LCMV in mice. Instead, these cells were capable of producing effector cytokines following restimulation with anti-CD3/CD28 [41], highlighting the complexity of interpreting PD-1 expression in patients. Hence, use of multiple phenotypic and functional parameters in combination is currently required to identify TEX populations both in mouse models and in patients. Identifying unique phenotypic markers for TEX cells is an important goal since it would enable the clinical monitoring of responses while circumventing the need to know antigen specificity of these T cells.
Box 1. Mechanisms of PD-1-mediated inhibition.
PD-1 is an inhibitory receptor expressed on T cells, B cells, and some myeloid cells, though its functions are best characterized for T cells. PD-1 interacts with two ligands, PD-L1 and PD-L2 [37]. PD-L1 is more widely expressed by hematopoietic and non-hematopoietic cells compared to PD-L2 [37]. However, both PD-L1 and PD-L2 interact with additional receptors: PD-L1 with CD80, delivering a bi-directional inhibitory signal [37], and PD-L2 with RGMb [127]. The physiological consequences of the diversity of these receptor:ligand combinations are not fully understood, but highlight the complexity of this pathway in vivo. The normal physiological function of the PD-1 pathway is thought to be in limiting immunopathology and maintaining tolerance to self-antigens. However, chronic infections and cancer exploit this pathway to evade host immunity, and PD-1 pathway inhibitors aim to reverse this immune suppression [3, 11–13, 20, 37, 76, 96, 117–119].
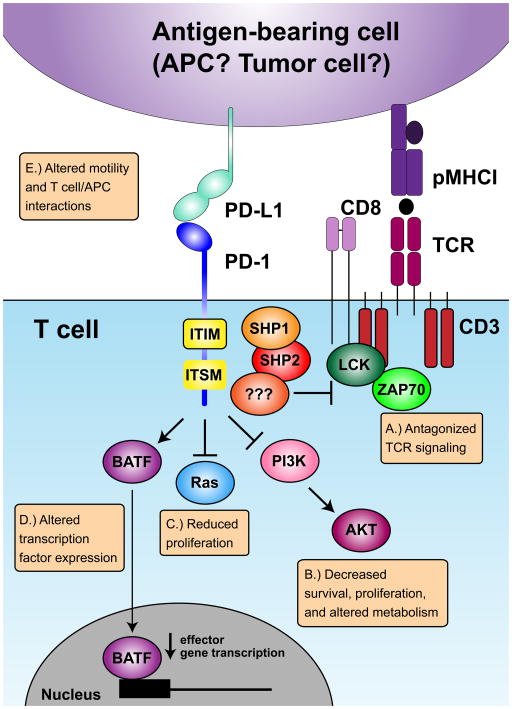
PD-1 is expressed on T cells following T cell receptor (TCR) engagement. Following acutely resolved antigen encounter, PD-1 expression eventually declines, while during chronic antigen exposure, PD-1 expression is sustained. While our understanding of how PD-1 suppresses T cell functions in vivo is incomplete, five mechanisms have been proposed. PD-1 can: (A) antagonize TCR signaling by recruiting phosphatases [107–110], (B) modulate the PI3K/AKT/mTOR pathway, implicating PD-1 in metabolism, nutrient sensing, survival, and cell growth [104, 111, 112], (C) modulate the Ras pathway, linking PD-1 to cell cycle [112], (D) induce expression of BATF, which can repress expression of effector genes [113], and (E) influence T cell motility [114–116] (Figure I). Some of these mechanisms have been described based on work using recently activated T cells (i.e. in vitro or in vivo generated TEF). Therefore, it remains unclear how these mechanisms will apply to chronically stimulated TEX that may have distinct expression of other inhibitory receptors and downstream signaling molecules. While information is beginning to emerge on how PD-1 regulates T cells in vivo, a consensus has not been reached, particularly on how PD-1 regulates T cell motility. Loss of PD-1 induced migratory arrest by CD4+ T cells during delayed-type hypersensitivity responses in the skin [115], and during the breakdown of tolerance in the pancreatic lymph node and islets during Type 1 Diabetes [114], consistent with a model where PD-1 limits the ability of T cells to fully engage with antigen presenting cells. However, during the first week of LCMV infection, blocking PD-1 reversed the migratory T cell arrest signal in the spleen causing more rapid detachment and migration away from antigen presenting cells, suggesting blocking PD-1 reverses exhaustion by relieving or partially interrupting persisting antigen signaling with some changes in motility also reported at day 14 post infection [116]. These studies highlight the complexity of PD-1 modulating T cell functions in vivo.
During the development of exhaustion, CD8+ T cells lose effector functions in a hierarchical manner: Production of IL-2, high proliferative capacity and ex-vivo cytolytic activity are lost first, followed by functional impairments in the production of TNFα, IFNγ, beta chemokines, and degranulation, and at the most terminal stages of exhaustion these cells can be physically deleted, presumably dying due to overstimulation (Figure 1) [3, 42]. However, TEX cells are not functionally inert. TEX cells contribute to the containment of chronic infections, since depleting CD8+ T cells including TEX during SIV infection results in rapid increases in viral titers and progression to AIDS [43, 44] suggesting an important role for the residual function of SIV-specific TEX in maintaining a host-pathogen equilibrium in the case of SIV. Additionally, the selective pressure TEX exert on persisting viruses can drive epitope escape where mutations in T cell epitopes prevent viral recognition by CD8+ and CD4+ T cells [45, 46]. TEX cells often retain the capacity to produce low levels of IFNγ and/or beta chemokines, express high levels of granzyme B, and one subset of TEX discussed further below retains some residual cytotoxicity [3, 33, 35]. High granzyme B expression is an interesting feature of TEX, considering the ex vivo killing capacity of these cells is impaired compared to TEF [3]. However, a role for this serine protease was recently identified in cleaving extracellular matrix components to promote homing, diapedesis, and migration through basement membranes [47], suggesting other potential uses of granzyme B by TEX. It will be important to further elucidate the roles of different effector molecules (including granzyme B) in TEX and determine how these effector pathways might play a role during chronic infection and cancer. Thus, while TEX exhibit impaired effector functions, some residual functionality persists, and this functionality may be important in a host/pathogen or host/tumor stalemate.
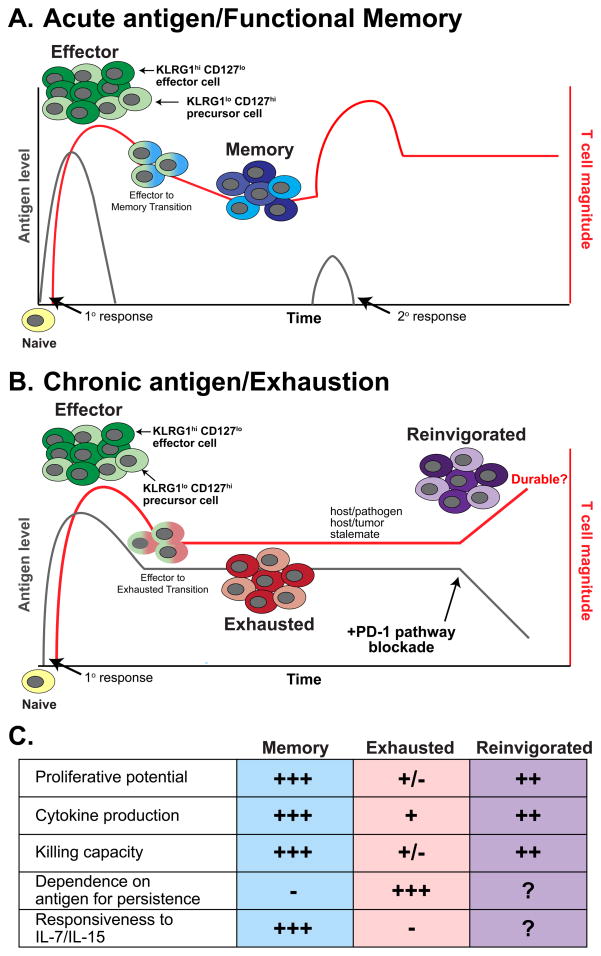
Figure 1. Development and functions of CD8+ T cells responding during acute versus chronic antigen encounter.
(A) Dynamics of CD8+ T cell expansion, contraction, and memory formation following acutely resolved antigen stimulation. Following activation, naïve T cells convert into an effector population consisting of KLRG1hi CD127lo short-lived effector cells and KLRG1lo CD127hi memory precursor cells. Following antigen clearance, memory T cell populations form predominantly from KLRG1lo CD127hi precursor cells. Memory CD8+ T cells retain the ability to re-expand upon secondary antigen encounter, resulting in an anamnestic response that controls antigen more rapidly than during the primary response [61]. (B) Dynamics of CD8+ T cell populations during chronic antigen encounter. Following activation, naïve T cells differentiate into an effector T cell population similarly to that observed following acutely resolved antigen encounter (A). However, the failure to eliminate antigen leads to the progressive development of exhaustion. TEX arise from the KLRG1lo CD127hi subset, a shared feature with memory T cells (A) [55]. These TEX exert pressure on the pathogen or tumor, resulting in a host-pathogen or host-tumor stalemate. Following intervention with immunotherapy including PD-1 pathway blockade, TEX can be reinvigorated, restoring effector functions and increasing cell numbers, resulting in decreased antigen load. However, the durability of this enhancement in the CD8+ T cell response is currently unknown. In (A) and (B), red lines indicate antigen-specific CD8+ T cell magnitude, grey lines indicate antigen level. (C) Comparison of key properties of memory, exhausted, and anti-PD-1:PD-L1-treated “reinvigorated” CD8+ T cells populations [3].
TEX also have altered long-term survival characteristics compared to TMEM. A cardinal feature of functional CD8+ TMEM cells is IL-7- and IL-15-driven, antigen-independent proliferation that allows these cells to persist long after antigen has been eliminated [48]. In contrast, TEX cells cannot undergo antigen-independent proliferation, respond poorly to IL-7 and IL-15, and require continual engagement with antigen to persist long term (Figure 1) [49–51]. For example, removing TEX from mice chronically infected with LCMV (clone 13) and adoptively transferring into antigen free mice results in failure of these cells to persist in an antigen-independent manner. In contrast, similar experiments with TMEM demonstrate efficient long-term persistence via self-renewal [49, 50]. In some settings small numbers of TEX may persist following experimental (transfer from mice infected with chronic LCMV into antigen free mice) or therapeutic (HIV patients following HAART) removal of antigen [49, 50, 52–54], though whether this persistence is due to survival of a small subset of TEX or differentiation of some TEX into a more durable TMEM-like cell is unknown. Further understanding of the pathways and mechanisms controlling TEX persistence in different settings is needed, since these cells could provide a mechanism of durable immunity following therapies that reduce or eliminate chronic infections or cancer.
Development of exhaustion
One fundamental property of exhaustion is that TEX arise from T cells that initially acquired effector functions, but then became dysfunctional during chronic antigenic stimulation [33, 55]. This feature distinguishes exhaustion from other types of T cell dysfunction such as anergy, a state of hyporesponsiveness where cells fail to acquire effector functions because of priming in the absence of adequate costimulation and/or inflammation [56]. Indeed, directly comparing the genome-wide transcriptional profiles of functional TEF and TMEM following acutely resolved LCMV Arm infection to developing TEX during chronic LCMV clone 13 infection demonstrated that it takes several weeks for the transcriptional program of TEX to diverge substantially from that of TEF or TMEM [57, 58]. Furthermore, T cells isolated during the first week of chronic LCMV infection have the potential to form functional memory if adoptively transferred to infection free mice [55]. However, by two to four weeks of chronic LCMV infection this memory development potential is lost [55]. Taken together, these observations indicate that T cell exhaustion is not irreversibly imprinted during priming, but rather develops progressively over time during chronic stimulation.
Heterogeneity within TEX populations
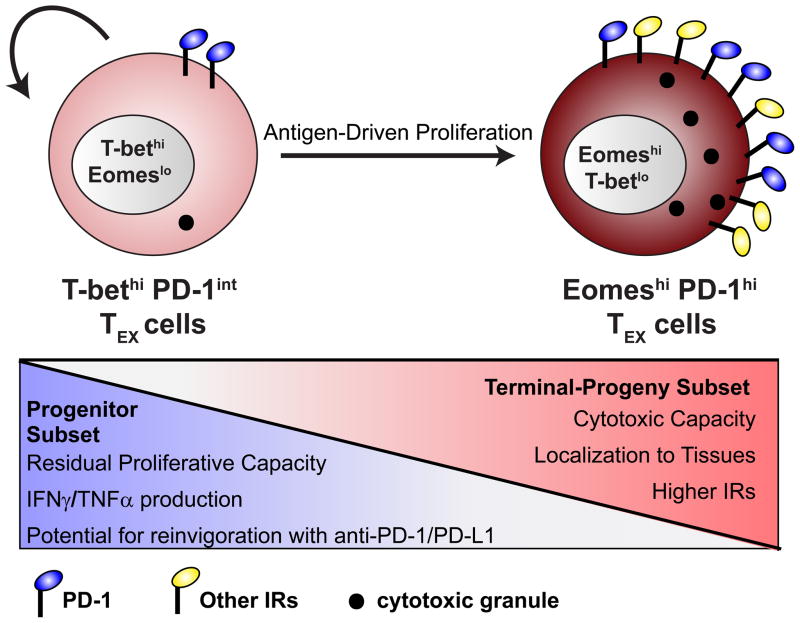
Recent studies have revealed heterogeneity within TEX populations, and have defined TEX subsets that differ in potential for reinvigoration by PD-1 pathway blockade [35, 59]. During chronic LCMV infection, two subsets of TEX can be identified based on expression of the T-box transcription factors T-bet and Eomesodermin (Eomes), in conjunction with PD-1 (Figure 2) [35]. While both TEX subsets exhibit impaired function as compared to TMEM, they retain different residual effector activity. T-betHi EomesLo PD-1int cells retain some potential for future division, produce moderate amounts of IFNγ and TNFα, and are preferentially found in the spleen and blood (Figure 2) [35]. In contrast, EomesHi T-betLo PD-1Hi cells have low potential for future division, produce lower amounts of effector cytokines, and express higher levels of other inhibitory receptors (e.g. Tim-3, Lag-3, CD160), but uniquely maintain cytolytic activity and preferentially accumulate in peripheral tissues [35]. Importantly, there is a lineage relationship between these subsets: T-betHi PD-1int cells serve as a progenitor population that gives rise to EomesHi PD-1Hi cells. This conversion is linked to cell division in the presence of antigen (Figure 2) [35]. This proliferative hierarchy fits with the observation that PD-1 pathway blockade appears to mainly target the T-betHi subset [59], since this population retains some ability to proliferate [35]. Similar subsets of TEX defined by reciprocal patterns of T-bet, Eomes and/or PD-1 expression have also been found in human patients afflicted by HCV and HIV infection [35, 60]. If similar TEX subsets exist during cancer there may be important implications for immunotherapy. For example, the T-betHi subset is more responsive to anti-PD-L1-mediated reinvigoration than the EomesHi subset [59], suggesting that developing strategies to evaluate the presence of this subset or to selectively target these T-betHi TEX may be of considerable interest. Conversely, if combination and/or novel immunotherapies can be identified that can reinvigorate the EomesHi subset, such strategies may be able to engage an even larger population of tumor-reactive TEX by reinvigorating both subsets of TEX.
Figure 2. Heterogeneity in the TEX population.
During chronic infection, two subsets of CD8+ T cells have been identified based on expression of PD-1 and the T-box transcription factors T-bet and Eomes [35]. Cells that express high levels of T-bet, lower levels of Eomes, and intermediate levels of PD-1 retain greater proliferative potential, the ability to produce slightly greater amounts of IFNγ and TNFα, are preferentially found in the spleen and blood [35], and are more responsive to reinvigoration following PD-1 pathway blockade [59]. Cells that express higher Eomes, lower T-bet, and high PD-1 show elevated expression of other inhibitory receptors (IRs) (e.g. Tim-3, CD160, and Lag-3), reduced proliferative potential and reduced co-production of IFNγ and TNFα, but retain a greater capacity for killing and preferentially localize to non-lymphoid tissues [35]. These cells also display reduced potential for reinvigoration by PD-1 pathway blockade [59]. Importantly, there is a lineage relationship between these two subsets. The T-betHi PD-1int subset serves as a progenitor population for both maintaining itself and the EomesHi PD-1Hi terminal-progeny subset [35]. The conversion from T-betHi PD-1int to EomesHi PD-1Hi cells is linked to extensive antigen-driven proliferation. High levels of antigen and/or lack of CD4+ T cell help favor the conversion from T-betHi to EomesHi cells [35].
The roles of T-bet and Eomes in TEX contrast with the roles of these transcription factors in functional effector and memory T cell differentiation. In acute infection, T-bet and Eomes cooperate to promote differentiation of naïve T cells into TEF cells, while during the transition into TMEM, higher amounts of T-bet promote terminal differentiation and higher amounts of Eomes foster the development of TMEM by supporting the quiescent phenotype of these cells as well as their capability for self-renewal [61–66]. The sets of genes or “modules” that T-bet and Eomes associate with in TMEM cells versus TEX cells are largely distinct, especially for Eomes [57]. This observation is consistent with the notion that transcription factors can have context-specific functions [67, 68]. The precise molecular mechanisms controlling these differential associations remains unclear, though possibilities include: Concentration-dependent access to different genomic sites, subcellular localization, transcription co-factors, and/or epigenetic changes influencing binding patterns. Understanding how these transcriptional networks change during immunotherapy and whether the capacity for reinvigoration by PD-1 pathway blockade is linked to context-specific functions of these or other transcription factors are important questions.
In chronic LCMV infection, exhaustion of CD4+ T cells shares many features with CD8+ T cell exhaustion, including over-expression of inhibitory receptors (e.g. PD-1, LAG-3) and impaired production of effector cytokines (e.g. IFNγ, TNFα) [58]. However, there are also some key differences between TEX of each lineage. For example, while there are features of a common transcriptional program of TEX shared between CD4+ and CD8+ T cells during chronic LCMV, there are also many differences between these two cell types [58]. One of the main places of divergence between the transcriptional program of CD4+ and CD8+ TEX is use of transcription factors: CD4+ TEX cells have altered expression patterns of GATA-3, Bcl-6, and Helios, which is not observed in CD8+ TEX cells. Furthermore, Eomes is a key transcription factor expressed by a prominent subset of CD8+ TEX cells, and although Eomes+ CD4 T cells may have relevance in some settings including tumors [69], only a small minority of CD4+ TEX cells expresses this transcription factor [58]). In addition to this altered transcription factor usage, CD4+ TEX cells appear skewed towards a follicular helper phenotype, a feature that may be related to changes in Bcl6 expression [58, 70]. Additionally, CD4+ TEX cells tend to show earlier manifestation of dysfunction than CD8+ TEX, as evidenced by loss of effector cytokine production such as IFNγ, TNFα and IL-2 at earlier times during infection than CD8+ T cells [58, 71–73]. While CD4+ T cells clearly play a critical role in the development of productive immune responses to chronic antigens [74], a more detailed understanding of the molecular mechanisms of CD4+ T cell exhaustion will likely be necessary to specifically target this subset for immunotherapy. Here we will focus on CD8+ T cells because exploiting the ability of these T cells to directly kill malignant cells is a major goal of immunotherapy in patients. Here after, unless otherwise noted, the term “TEX” will refer to the CD8+ subset. However, considering emerging data that CD8+ T cells can profoundly impact local and systemic immune responses independently of simply killing target cells [75], it will be important to continually evaluate/re-evaluate the correlates of immune protection during immunotherapy.
T cell exhaustion in cancer
Although T cell exhaustion was originally defined in chronic infection, a similar dysfunctional state has been observed in cancer [10, 19, 76–80]. In chronic viral infection, hallmarks of T cell exhaustion include: (1) Progressive loss of T cell functions after acquisition of an effector program, (2) elevated expression of multiple inhibitory receptors, (3) impaired effector cytokine production (e.g. IFNγ, TNFα, IL-2), (4) impaired ex vivo cytotoxicity compared to TEF cells, (5) poor responsiveness to IL-7 and IL-15 and requirement for continual antigen engagement for long term survival in the periphery, (6) altered metabolism, (7) distinct transcriptional program, and (8) altered use of key transcription factors compared to TMEM including T-bet and Eomes. There are clear examples of T cells in cancer sharing features with exhaustion in chronic infection [77–86]. For example, PD-1+ tumor infiltrating lymphocytes (TIL) have been observed in several human cancers, including melanoma, breast, prostate, ovarian, RCC, hepatocellular carcinoma, and NSCLC [82, 84, 86–89] and these cells often have reduced effector function distinguishing them from functional PD-1+ TEF. This dysfunction of TIL can often be linked to another key feature of TEX, co-expression of multiple inhibitory receptors. In human cancer, PD-1+ Tim-3+ and PD-1+ Lag-3+ tumor specific CD8+ T cells in melanoma and ovarian cancer showed more severe signs of dysfunction in terms of effector cytokine production than cells expressing only PD-1 or neither receptor [77, 86]. Many similar observations have been described for animal models of cancer [19]. Impaired effector cytokine production is often described for TEX in tumors including for melanoma, CLL, ovarian, and NSCLC [79, 80, 82, 86, 89]. Cytotoxic activity can also be impaired in cancer [85], though exactly how the hierarchy of dysfunction evolves for tumor-specific TEX is less clear than for chronic infections as comparisons temporally and across different tumor types are challenging. Lastly, transcriptional profiling comparing Melan-A/MART-1 tumor-specific CD8+ T cells in metastases from melanoma patients to gp33 virus-specific TEX cells in chronic LCMV infection showed substantial overlap in the molecular program of these populations [78].
Importantly, like in chronic infection, “exhausted” TILs are likely not functionally inert. For example, in melanoma, tumors that contained T cells showed the highest levels of PD-L1 and indoleamine-2,3-dioxygenase (IDO), both of which can be upregulated by IFN-γ [90]. Indeed, in a corresponding mouse model of melanoma this expression of PD-L1 and IDO within the tumor was due to CD8+ T cell production of IFNγ [90]. Hence, while the presence of immune regulatory molecules including PD-1 and PD-L1 may be a positive prognostic indicator for immunotherapy since these pathways reflect an ongoing immune response [28, 29, 91], the steady state function of TEX and TIL is typically not sufficient to control cancer [14, 17]. This failed immune control may reflect poor effector function, upregulation of immunoregulatory pathways or both.
While TEX from cancer and chronic infection can share many features including impaired cytokine production, impaired cytotoxicity, and elevated levels of multiple inhibitory receptors, it remains unclear whether other hallmarks of exhaustion found in chronic infection apply to cancer. T cell dysfunction during cancer may be distinct from that observed during chronic infection for several reasons. In cancer, central and/or peripheral tolerance could shape T cell responses to favor mainly lower affinity clones. Moreover, priming to tumor antigen is more likely to occur in the absence of inflammation, but the presence of immunosuppressive mechanisms such as regulatory T cells [92]. Moreover, one of the defining features of exhaustion in chronic infections is that these T cells acquire effector functions, and then progressively lose those functions during persisting infection. These features may differ during cancer. First, naïve tumor-specific T cells may fail to become properly activated during priming and never differentiate into TEF cells because of low inflammatory and costimulatory priming environments during cancer. Second, in some transplantable tumor models, tumor progression is quite rapid, making it unclear if immune regulatory pathways are controlling exhaustion or simply T cell priming. Genetically engineered mouse models (GEMMs) may have some advantages for studying exhaustion, such as a slower course of progression. However, in many cases, tumors have a low mutational burden [92], exposing T cells to a more narrow breadth of tumor antigens than T cells in patients may encounter. There is also limited data on long-term maintenance or survival of TEX cells in cancer. A hallmark of exhaustion in chronic infection is poor responsiveness to IL-7 and IL-15, contributing to the inability of TEX to survive long-term in the absence of antigen. Melan-A/MART-1-specific CD8+ T cells in metastatic melanoma lesions isolated from patients expressed low levels of the IL-7 receptor (CD127) [82] similar to that observed in chronic infection [49, 50], suggesting that tumor-specific CD8+ T cells may have defects in homeostatic proliferation. However, whether TEX in cancer have the same degree of impairment in TMEM-like antigen-independent maintenance as observed for TEX in chronic infection remains unclear. If tumor-specific TEX fail to persist without antigen, successful immunotherapy resulting in tumor elimination might also result in loss of tumor-specific T cell populations. While an initial clinical success, if tumor relapses, such patients may not have a durable population of T cells present to eliminate re-emerging cancer. Thus, while there are many similarities between TEX that can be identified in cancer and chronic infection, additional work is needed to investigate how key hallmarks of exhaustion compare between these two disease settings.
Despite differences in the way immune responses develop to cancers and infections, studies with preclinical chronic infection models including LCMV have been useful guides to identify pathways and new immunoregulatory targets that have subsequently shown efficacy in cancer, including PD-1 monotherapy as well as co-blockade with Lag-3, Tim-3, and others [3, 11, 14, 16, 34, 93–96], (see additional discussion of this topic below). The LCMV clone 13 model has also been useful in identifying other potential immunotherapeutic targets and molecular pathways, including IL-10, IL-21, and Type I IFN [33, 35, 97–102]. Preclinical models such as LCMV will likely continue to yield additional new pathways that could represent future targets in cancer. The remainder of this review will focus on recent insights into anti-PD-1:PD-L1-mediated reinvigoration of TEX cells because of the emergence of the PD-1 pathway as a central regulator of TEX in chronic infections and cancer in preclinical models and patients, and the promising clinical responses observed targeting this pathway in diverse types of cancer. We will discuss recent advances from both preclinical mouse models and clinical data, and will highlight critical gaps in knowledge posing potential barriers to the success of immunotherapy.
Reinvigoration of TEX cells following PD-1 pathway blockade: Insights from preclinical models
Studies in preclinical models have greatly contributed to our understanding of the PD-1 pathway in the regulation of T cell immunity [3, 37, 96]. The normal physiological function of the PD-1 pathway is thought to be limiting immunopathology and autoimmunity (Box 1) [37]. PD-1 expression is induced on T cells following activation, and is regulated by the transcription factors NFAT, T-bet, Blimp-1, and FoxO1 (Box 2) [103–106]. Chronic antigen stimulation sustains PD-1 expression. Mechanistically, PD-1 ligation can antagonize T cell receptor signaling, promote cell cycle arrest, modulate the P13K/AKT/mTOR pathway, regulate expression of transcription factors including BATF, and influence motility (Box 1) [104, 107–116]. Importantly, PD-1 actively regulates virus-and tumor-specific TEX, since blocking this pathway using antibodies against either PD-1 or PD-L1 can restore effector functions to these T cells and lead to reduced viral load and tumor burden [11–13, 76, 117–119]. In chronic LCMV infection, administering anti-PD-L1 or anti-PD-1 blocking antibodies following the development of exhaustion (3–4 weeks post-infection) leads to increased CD8+ T cell proliferation, cytokine production and cytolytic activity, and reduced viral load [11]. These studies were important because they first established the concept that TEX were not terminal but could be re-invigorated to achieve improved function and enhanced immunity.
Box 2. Regulation of PD-1 expression.
Recent work has provided insight into the mechanisms controlling PD-1 expression, identifying roles for the transcription factors NFAT, FoxO1, Blimp-1, and T-bet [103–106]. NFAT nuclear translocation following T cell receptor (TCR) signaling induces transcription of PD-1, linking antigen recognition to PD-1 expression [106]. Moreover, NFAT binding in the absence of AP-1 appears to promote expression of some features of an exhausted transcriptional program [128]. FoxO1 is also a positive regulator of PD-1 by directly binding the PD-1 locus [104], providing a potential connection between PD-1 and AKT/mTOR, implicating PD-1 regulation in nutrient and metabolic sensing, growth, cell cycle, and survival [129]. In TEX, Blimp-1 has been associated with higher PD-1 expression [105], though how this transcriptional repressor promotes PD-1 is unclear, particularly since in acutely activated T cells Blimp-1 appears to repress PD-1 at least partially via epigenetic mechanisms [130]. T-bet also directly represses PD-1 expression via binding to the Pdcd1 enhancer region [103]. Interestingly, T-bet can repress levels of PD-1 in TEX from “high” to “intermediate,” but cannot completely shut down PD-1 expression, indicating multiple layers of PD-1 transcriptional control [35, 103]. Finally, Eomes is associated with high expression of PD-1 in TEX [35], but whether Eomes directly controls PD-1 transcription remains to be determined.
For T cells, TCR engagement by cognate antigen:MHC is important to drive and sustain PD-1 expression. However, in some settings, PD-1 expression may be independent of ongoing TCR stimulation. For example, TEX from chronic LCMV infection can maintain PD-1 expression following transfer to antigen-free mice [55], and can also sustain PD-1 following rechallenge and expansion in this setting [54]. Mechanistically, this sustained expression may be due to epigenetic modifications in the PD-1 gene. During acute LCMV infection, the Pdcd1 promoter becomes demethylated during the effector phase, and then fully remethylated in TMEM [131]. In contrast, during chronic LCMV infection, the Pdcd1 promoter does not become remethylated, even when levels of persisting virus and PD-1 protein decreased [131]. This methylation pattern was also observed in HIV patients with well-controlled viral load [132]. Such an alteration in the epigenetic environment of the Pdcd1 gene may also explain the distinct effects of Blimp-1 on PD-1 expression in TEX versus acutely activated TEF discussed above. Importantly, recent work showed that TEX in chronic LCMV displayed downregulated diacetylated histone H3, and found that treating with histone deacetylase inhibitors could improve effector functions and memory formation [133]. These data highlight the importance of epigenetic modifications in regulating the exhausted state, though more work is needed to understand the potential for manipulating epigenetic modifications for therapeutic benefit in patients.
In preclinical cancer models, treatment with PD-1:PD-L1 blocking antibodies can also boost T cell effector functions, leading to enhanced tumor control [12, 13, 37, 76, 96, 117–119]. Early studies used over-expression of PD-L1 on P815 mastocytoma tumor cells to show that PD-L1 could suppress CD8+ T cell cytolytic activity. Following transfer of PD-L1-expressing P815 tumor cells into mice, treatment with anti-PD-1 or anti-PD-L1 blocking antibodies suppressed tumor growth implicating the PD-1 pathway in tumor immunity, a finding also confirmed in PD-1 KO mice [12, 13]. Furthermore, anti-PD-1 was able to prevent hematogenous spread of B16 melanoma tumor cells to the liver in B6 mice and CT26 colon cancer cells to the lung in BALB/c mice [117]. Several observations highlight the role for T cells in control of transplantable cancers following PD-1 pathway blockade. When human squamous cell carcinoma of head and neck (SCCHN) cancers were transfected with PD-L1 and transplanted into mice, all of the mice succumbed even when T cells were added. Anti-PD-L1 treatment in this setting could elicit tumor control, but only in the presence of T cells [118]. Similarly, in a xenograft model with human ovarian cancer cells transplanted into mice, T cells activated by tumor antigen-pulsed dendritic cells provided minimal tumor control compared to the effects of combining T cells with anti-PD-L1 [76]. Additionally, depletion of CD8+ T cells can abolish the protective effects of immunotherapy both during CTLA-4 or PD-1 pathway blockade as well as co-blockades with other immunoregulatory pathways [36, 120, 121]. One critical question regarding PD-1:PD-L1 blockade in mouse tumor models has been whether targeting this pathway is truly reversing exhaustion, or if manipulating the PD-1 pathway is impacting priming and/or preventing the development of exhaustion. In many mouse tumor systems, PD-1 pathway blockade is often administered early at time points when full exhaustion may not yet have developed [12, 13, 36, 117, 120]. In contrast to most animal models, PD-1 pathway blockade is typically employed clinically at late stages of disease when prolonged T cell stimulation is likely to have occurred. Additional studies dissecting the roles of the PD-1:PD-L1 pathway at different temporal stages of tumor progression in animal models may help guide expectations and define biomarkers for clinical use of PD-1 pathway inhibitors in humans.
Interestingly, in some models where complete responses are observed, mice can be protected from rechallenge with the homologous tumor. Examples of such responses include protection from rechallenge with MCA-induced sarcoma cell lines F244 and d42m1-T3 following PD-1 blockade, colon carcinoma (CT26) after PD-L1 and TIGIT co-blockade, and a B16-F10 derived cell line called Res 499 for PD-L1 and CTLA-4 co-blockade in combination with radiation therapy [36, 120, 121]. These data suggest that immunotherapy can elicit a durable adaptive anti-tumor response, a scenario that has also been observed in human cancer following treatment with anti-PD-1 [26, 30]. In both preclinical models and cancer patients, the mechanisms involved in complete remission of cancers after immunotherapy is currently unclear, but defining these mechanisms is an important future goal. If TEX cells in cancer share the same dependence on antigen for long term survival as TEX cells in chronic infection [49, 50], changes in tumor burden and availability of tumor antigens following immunotherapy could impact the long-term survival of these reinvigorated T cells. However, how the antigen-independent persistence of TEX is impacted by re-invigoration by PD-1 pathway blockade remains poorly understood. Defining how checkpoint blockade impacts the durability and memory properties of CD8+ T cells in cancer will be critical for understanding the potential for these cells to provide a durable mechanism of immunity in settings of cancer relapse.
Lastly, it will be critical to better define the cellular and molecular changes occurring following PD-1 pathway blockade. A recent study performed transcriptional profiling on TILs in mouse MCA-induced sarcoma cell tumors that were either specific for a major immunodominant neoantigen (mLama4) or bulk TIL, and compared TIL profiles after anti-CTLA-4, anti-PD-1, or co-blockade of both pathways [120]. Here, anti-PD-1 alone impacted mostly pathways associated with metabolism, anti-CTLA-4 alone affected pathways associated with cell cycle and effector memory, and the combination altered T cell effector pathways [120]. Because PD-1 pathway inhibitors are currently a cornerstone of combination therapies for cancer, understanding the cellular and molecular synergy between PD-1 and other pathways (e.g. other inhibitory receptors, cell-extrinsic immune regulatory pathways, chemotherapy/radiation, vaccination, etc.) will be essential to determine which combinations should be used for different patients.
Anatomical location of anti-PD-1:PD-L1-mediated reinvigoration
Although it is well established that PD-1 pathway blockade can partially reverse exhaustion and improve immunity in chronic infections and cancer, several questions remain regarding the precise cell populations and anatomical locations in which these inhibitors are acting to reinvigorate T cells. PD-1 and its ligand PD-L1 can be expressed by cells in lymphoid and non-lymphoid tissues, positioning this pathway as a critical regulator of immune responses both during priming and during the execution of effector functions in peripheral tissues, a highly sought after feature for boosting immune responses therapeutically. However, it remains unclear whether anti-PD-1:PD-L1 is directly impacting T cells within the non-lymphoid tissue or tumor, or if blockade of this pathway also reinvigorates T cells in the secondary lymphoid organs. At least two factors could limit the ability of PD-1 inhibitors to reinvigorate T cell functions in diverse anatomical locations. First, differential bioavailability of the inhibitor in different tissues could be a confounding variable limiting drug activity. This issue may be particularly problematic for delivering PD-1 pathway inhibitors into dense solid tumors. Second, differential susceptibility of different subsets of cells could substantially influence the efficacy of PD-1 inhibitors. For example, in chronic infection, the pool of TEX in the spleen and peripheral blood contains a higher proportion of “progenitor-like” T-betHi cells that are less terminally differentiated and can be reinvigorated after PD-1 pathway blockade. In contrast, the EomesHi terminally-differentiated subset can be found preferentially in peripheral tissues and is not reinvigorated by PD-1 pathway blockade (Figure 2) [35, 59]. A model by which PD-1 pathway blockade enhances responses in secondary lymphoid organs and promotes subsequent trafficking to non-lymphoid tissue or tumor does not rule out a direct effect of PD-1 blockade on reinvigorating T cells within the tumor microenvironment, but rather provides a complementary mechanism of how checkpoint blockade impacts the anti-tumor response. While inhibitors of the PD-1:PD-L1 pathway may improve immunity by acting in both lymphoid and non-lymphoid tissues, the relative contribution of local and distal reinvigoration may vary for different tumors. Understanding the relative contributions of reinvigoration from different anatomical locations and/or different TEX subsets could have important implications for predicting and monitoring patient responses in cancer.
PD-L1 expression on different cell types has important implications for immunotherapy; however, our understanding of PD-1-mediated regulation when a T cell encounters PD-L1 on an antigen presenting cell compared to a non-hematopoietic cell (such as a tumor cell) is currently limited. In chronic LCMV infection, PD-L1 on hematopoietic cells regulates virus-specific CD8+ T cell numbers and function, while PD-L1 on non-hematopoietic cells limits viral load and immunopathology [122]. Clinically, several reports have investigated how PD-1 and PD-L1 levels correlate with cancer prognosis [24], and, more recently, have begun examining whether PD-L1 expression levels pre-treatment predict responsiveness to PD-1 pathway inhibitors [28, 29, 91]. While PD-L1 expression in the tumor has been associated with poor prognosis in several settings [24], recent clinical data showed that PD-L1 expression on immune infiltrates in the tumor positively correlated with responsiveness to anti-PD-L1 immunotherapy [28, 29]. In addition to PD-L1, PD-1 expression in the tumor may also be an important predictor of immune involvement since PD-1+ cells in the tumor indicate immune (likely lymphoid) infiltration while PD-L1 expression often reflects IFNγ production and potential tumor recognition by immune cells [16, 81]. Therefore, it is critical to continue broadening our understanding of the PD-1 pathway in both lymphoid and non-lymphoid tissues to better inform the interpretation and predictive capacity of measuring PD-1 and PD-L1 expression in patients.
Determining the efficacy of PD-1 inhibitors in cancer
PD-1 pathway inhibitors have shown impressive results in cancer patients, particularly in advanced melanoma, NSCLC, RCC, and, recently, metastatic bladder cancer. However, despite promising patient outcomes with PD-1 pathway inhibitors for different cancer types, the majority of patients still fail to achieve robust objective clinical responses [21-23, 25-32]. Additionally, some tumor types have been almost completely refractory to these inhibitors. Thus, identifying biomarkers as determinants of responsiveness to PD-1 pathway blockade antibodies so as to direct better informed therapeutic decisions and more effective treatments remains a major goal in both basic and clinical research. Recent clinical data with anti-PD-L1 antibody (MPDL3280A) described immunological events associated with tumor regression versus progression in multiple tumor types [28]. Patients with objective responses tended to have increased PD-L1 expression on immune cells in the tumor pre-therapy, a dense immune infiltrate, increases in IFNγ production, and extensive tumor cell necrosis [28]. Another clinical trial also showed PD-L1 expression on immune cells infiltrating the tumor correlated with responsiveness to anti-PD-L1 (MPDL3280A) [29], and, further, higher levels of CD8+, PD-1+, and PD-L1+ cells at the invasive margin of the tumor before treatment correlated with positive response rates to anti-PD-1 (pembrolizumab) [91]. Importantly, patients with progressive disease in one trial displayed one of three immune infiltrate patterns in the tumor bed: (1) little or no infiltrating immune cells, (2) immune cells present but little PD-L1 expression, or (3) presence of an immune infiltrate that was largely excluded from the tumor [28]. Continuing to identify barriers to successful immunotherapy with PD-1 pathway inhibitors is essential since this knowledge will likely inform treatment options. For example, for tumors with little or no infiltrating immune cells, perhaps vaccination will induce activation of the anti-tumor adaptive immune response and trafficking to the tumor [120, 123]. However, once this adaptive immune response has been initiated, the addition of PD-1 blockade may aid in overcoming exhaustion induced in the tumor microenvironment. Indeed, recent evidence suggests that checkpoint blockade synergizes with radiation by acting through distinct mechanisms: Radiation, acting as a type of vaccination (e.g. so called RadVax) releases tumor antigens causing diversification of T cells participating in the response, anti-CTLA-4 acts on extrinsic regulation by increasing the CD8+ T cell to regulatory T cell ratio, and anti-PD-L1 dramatically amplifies responses by overcoming CD8+ T cell exhaustion in the tumor [121]. Additional studies showing synergy between PD-1 pathway blockade and adoptive T cell therapies support the use of checkpoint blockade to prevent exhaustion in the tumor microenvironment [124, 125]. With the list of immunotherapy targets growing rapidly, additional work is needed to clarify which factors will correlate with tumor regression versus disease progression to determine how to better predict patient responses to diverse immunotherapies.
Concluding Remarks
FDA approval of pembrolizumab and nivolumab in 2014 marked a significant advance for using checkpoint inhibitors in cancer patients. Though both show substantial clinical benefit, significant challenges for successful immunotherapy in cancer patients remain. For example, while some patients have shown durable responses following immunotherapy [26, 30], others have failed to respond or have subsequently relapsed [21–25, 27]. It is currently unknown if continual treatment or subsequent rounds of discontinuous treatment with immunotherapeutic agents will enhance the anti-tumor response, or if these approaches might lead to the development of resistance similar to what has been observed for targeted small molecule therapies (e.g. tyrosine kinase inhibitors targeted at BCR-ABL) [126]. Indeed, recent work has demonstrated that up-regulation of PD-L1 represents a major mechanism of tumor resistance to anti-CTLA-4 (ipilimumab) treatment in melanoma [121]. Other immune and non-immune tumor escape mechanisms are likely to exist for immune monotherapy in cancer. Therefore, the next generation of cancer immunotherapy will likely rely on combining therapies to improve patient outcomes (Box 3). PD-1 pathway blockade is now at the center of many of these combinatorial immunotherapy approaches, including: Blockade of other inhibitory receptors (e.g. CTLA-4, Lag-3, Tim-3, etc), inhibition of soluble factors such as immunoregulatory (IL-10, TGF-β, IL-35) or inflammatory cytokines (Type I IFN), vaccination (either endogenous through radiation or exogenous by delivery tumor antigens, DNA vaccines, or dendritic cell-based vaccines), or adoptive immune cell therapy (e.g. chimeric antigen receptor (CAR) T cells, adoptive cancer-specific T cell therapy) [14, 15]. Since chemotherapy remains the foundation of cancer therapy, it will also be essential to understand how chemotherapy regimens (contemporaneous or temporally separated) influence checkpoint blockade and vice versa. The abundance of immunotherapeutic options for cancer highlights the need to develop better biomarkers to determine who will benefit from which therapies (Box 3). Addressing these issues will be critical for continuing to improve the success of these immunotherapies.
Box 3. Outstanding Questions.
What factors correlate with potential for responsiveness to PD-1 inhibitors in human cancer? What is the optimal anatomical location for sampling for biomarker assessment? Are there factors in the peripheral blood that will correlate with success of immunotherapy, or will assessment of tumor tissue be optimal?
How durable is enhanced immunity following treatment with PD-1 pathway inhibitors?
What are the mechanisms by which PD-1 modulates T cell functions in vivo? Do these mechanisms change for different types of T cells such as exhausted versus effector T cells?
Does the PD-1 signal delivered from PD-L1 on a professional antigen presenting cell differ from PD-L1 on a non-hematopoietic cell (e.g. tumor cell)?
What are the cellular and molecular mechanisms underlying synergy observed between PD-1 pathway inhibitors and other forms of immunotherapy? What combinations of therapies will be effective for different cancer settings?
The collaboration of basic science and clinical medicine will be essential moving forward as more patients receive these inhibitors. The field will need to further define the mechanisms contributing to immune dysfunction in cancer, the events associated with improvement of immune responses following immunotherapy, and what combination therapies will be most effective (Box 3). Further, patients that have initially responded well to checkpoint blockade will provide a critical opportunity to determine the durability of immunity (Box 3). Cancer immunotherapy is ushering in new ways to approach treatment of many diseases by exploiting the power of selectively targeting the immune system, rather than using chemotherapeutic agents, to destroy diseased cells.
Figure I. Mechanisms of PD-1-mediated inhibition in T cells.
Five main mechanisms have been proposed for how PD-1 modulates T cell functions. PD-1 can: (A) directly antagonize T cell receptor (TCR) signaling by recruiting phosphatases to tyrosine-containing motifs in the PD-1 tail [108], which can prevent LCK-mediated phosphorylation of ZAP70 [110], (B) inhibit CD28-induced activation of PI3K, leading to reduced AKT and mTOR activation [111], (C) inhibit the Ras pathway [112], (D) induce increased expression of transcription factors including BATF, which can directly suppress transcription of various effector genes [113], and (E) impact T cell motility and the stability/duration of T cell/APC interactions [114–116].
Highlights.
T cell exhaustion is present in both chronic infections and cancer.
PD-1 regulates T cell exhaustion and PD-1 blockade enhances tumor and viral immunity
Molecular pathways of exhaustion may reveal biomarkers and immunotherapy targets
Acknowledgments
This work was supported by National Institutes of Health grants AI082630, AI05343, and AI112521 to EJW and the Robertson Foundation/Cancer Research Institute Irvington Fellowship to KEP. We would like to thank Drs. Alexander Huang, Makoto Kurachi, Pamela Odorizzi, and Jason Schenkel for meaningful comments and discussions. EJW has a patent licensing agreement on therapeutic targeting of the PD-1 pathway (US patent application no 20070122378).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zajac AJ, et al. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188(12):2205–13. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gallimore A, et al. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J Exp Med. 1998;187(9):1383–93. doi: 10.1084/jem.187.9.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12(6):492–9. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 4.Goepfert PA, et al. A significant number of human immunodeficiency virus epitope-specific cytotoxic T lymphocytes detected by tetramer binding do not produce gamma interferon. J Virol. 2000;74(21):10249–55. doi: 10.1128/jvi.74.21.10249-10255.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kostense S, et al. High viral burden in the presence of major HIV-specific CD8(+) T cell expansions: evidence for impaired CTL effector function. Eur J Immunol. 2001;31(3):677–86. doi: 10.1002/1521-4141(200103)31:3<677::aid-immu677>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 6.Shankar P, et al. Impaired function of circulating HIV-specific CD8(+) T cells in chronic human immunodeficiency virus infection. Blood. 2000;96(9):3094–101. [PubMed] [Google Scholar]
- 7.Gruener NH, et al. Sustained dysfunction of antiviral CD8+ T lymphocytes after infection with hepatitis C virus. J Virol. 2001;75(12):5550–8. doi: 10.1128/JVI.75.12.5550-5558.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lechner F, et al. Analysis of successful immune responses in persons infected with hepatitis C virus. J Exp Med. 2000;191(9):1499–512. doi: 10.1084/jem.191.9.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reignat S, et al. Escaping high viral load exhaustion: CD8 cells with altered tetramer binding in chronic hepatitis B virus infection. J Exp Med. 2002;195(9):1089–101. doi: 10.1084/jem.20011723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee PP, et al. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat Med. 1999;5(6):677–85. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]
- 11.Barber DL, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439(7077):682–7. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 12.Iwai Y, et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99(19):12293–7. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirano F, et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005;65(3):1089–96. [PubMed] [Google Scholar]
- 14.Sharma P, et al. Novel cancer immunotherapy agents with survival benefit: recent successes and next steps. Nat Rev Cancer. 2011;11(11):805–12. doi: 10.1038/nrc3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480(7378):480–9. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Page DB, et al. Immune modulation in cancer with antibodies. Annu Rev Med. 2014;65:185–202. doi: 10.1146/annurev-med-092012-112807. [DOI] [PubMed] [Google Scholar]
- 17.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331(6024):1565–70. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 18.Schietinger A, Greenberg PD. Tolerance and exhaustion: defining mechanisms of T cell dysfunction. Trends Immunol. 2014;35(2):51–60. doi: 10.1016/j.it.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim PS, Ahmed R. Features of responding T cells in cancer and chronic infection. Curr Opin Immunol. 2010;22(2):223–30. doi: 10.1016/j.coi.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Callahan MK, Wolchok JD. At the bedside: CTLA-4- and PD-1-blocking antibodies in cancer immunotherapy. J Leukoc Biol. 2013;94(1):41–53. doi: 10.1189/jlb.1212631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamid O, et al. Safety and Tumor Responses with Lambrolizumab (Anti-PD-1) in Melanoma. N Engl J Med. 2013 doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Topalian SL, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brahmer JR, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28(19):3167–75. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohaegbulam KC, et al. Human cancer immunotherapy with antibodies to the PD-1 and PD-L1 pathway. Trends Mol Med. 2015;21(1):24–33. doi: 10.1016/j.molmed.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brahmer JR, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lipson EJ, et al. Durable cancer regression off-treatment and effective reinduction therapy with an anti-PD-1 antibody. Clin Cancer Res. 2013;19(2):462–8. doi: 10.1158/1078-0432.CCR-12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolchok JD, et al. Nivolumab plus Ipilimumab in Advanced Melanoma. N Engl J Med. 2013 doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herbst RS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–7. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Powles T, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515(7528):558–62. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 30.Topalian SL, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32(10):1020–30. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robert C, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–30. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 32.Ansell SM, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372(4):311–9. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wherry EJ, et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27(4):670–84. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 34.Blackburn SD, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10(1):29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paley MA, et al. Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science. 2012;338(6111):1220–5. doi: 10.1126/science.1229620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnston RJ, et al. The Immunoreceptor TIGIT Regulates Antitumor and Antiviral CD8(+) T Cell Effector Function. Cancer Cell. 2014;26(6):923–37. doi: 10.1016/j.ccell.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 37.Keir ME, et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fife BT, Pauken KE. The role of the PD-1 pathway in autoimmunity and peripheral tolerance. Ann N Y Acad Sci. 2011;1217:45–59. doi: 10.1111/j.1749-6632.2010.05919.x. [DOI] [PubMed] [Google Scholar]
- 39.Pauken KE, et al. PD-1, but not PD-L1, expressed by islet-reactive CD4+ T cells suppresses infiltration of the pancreas during type 1 diabetes. Diabetes. 2013;62(8):2859–69. doi: 10.2337/db12-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schietinger A, et al. Rescued tolerant CD8 T cells are preprogrammed to reestablish the tolerant state. Science. 2012;335(6069):723–7. doi: 10.1126/science.1214277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duraiswamy J, et al. Phenotype, function, and gene expression profiles of programmed death-1(hi) CD8 T cells in healthy human adults. J Immunol. 2011;186(7):4200–12. doi: 10.4049/jimmunol.1001783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wherry EJ, et al. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77(8):4911–27. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jin X, et al. Dramatic rise in plasma viremia after CD8(+) T cell depletion in simian immunodeficiency virus-infected macaques. J Exp Med. 1999;189(6):991–8. doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmitz JE, et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283(5403):857–60. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 45.Blattman JN, et al. Impact of epitope escape on PD-1 expression and CD8 T-cell exhaustion during chronic infection. J Virol. 2009;83(9):4386–94. doi: 10.1128/JVI.02524-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jamieson BD, et al. Epitope escape mutation and decay of human immunodeficiency virus type 1-specific CTL responses. J Immunol. 2003;171(10):5372–9. doi: 10.4049/jimmunol.171.10.5372. [DOI] [PubMed] [Google Scholar]
- 47.Prakash MD, et al. Granzyme B Promotes Cytotoxic Lymphocyte Transmigration via Basement Membrane Remodeling. Immunity. 2014;41(6):960–72. doi: 10.1016/j.immuni.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 48.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29(6):848–62. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 49.Wherry EJ, et al. Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc Natl Acad Sci U S A. 2004;101(45):16004–9. doi: 10.1073/pnas.0407192101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shin H, et al. Viral antigen and extensive division maintain virus-specific CD8 T cells during chronic infection. J Exp Med. 2007;204(4):941–9. doi: 10.1084/jem.20061937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Obar JJ, et al. Gammaherpesvirus persistence alters key CD8 T-cell memory characteristics and enhances antiviral protection. J Virol. 2006;80(17):8303–15. doi: 10.1128/JVI.00237-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alter G, et al. Longitudinal assessment of changes in HIV-specific effector activity in HIV-infected patients starting highly active antiretroviral therapy in primary infection. J Immunol. 2003;171(1):477–88. doi: 10.4049/jimmunol.171.1.477. [DOI] [PubMed] [Google Scholar]
- 53.Oxenius A, et al. Stimulation of HIV-specific cellular immunity by structured treatment interruption fails to enhance viral control in chronic HIV infection. Proc Natl Acad Sci U S A. 2002;99(21):13747–52. doi: 10.1073/pnas.202372199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Utzschneider DT, et al. T cells maintain an exhausted phenotype after antigen withdrawal and population reexpansion. Nat Immunol. 2013;14(6):603–10. doi: 10.1038/ni.2606. [DOI] [PubMed] [Google Scholar]
- 55.Angelosanto JM, et al. Progressive loss of memory T cell potential and commitment to exhaustion during chronic viral infection. J Virol. 2012;86(15):8161–70. doi: 10.1128/JVI.00889-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–34. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 57.Doering TA, et al. Network analysis reveals centrally connected genes and pathways involved in CD8+ T cell exhaustion versus memory. Immunity. 2012;37(6):1130–44. doi: 10.1016/j.immuni.2012.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Crawford A, et al. Molecular and transcriptional basis of CD4(+) T cell dysfunction during chronic infection. Immunity. 2014;40(2):289–302. doi: 10.1016/j.immuni.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blackburn SD, et al. Selective expansion of a subset of exhausted CD8 T cells by alphaPD-L1 blockade. Proc Natl Acad Sci U S A. 2008;105(39):15016–21. doi: 10.1073/pnas.0801497105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Buggert M, et al. T-bet and Eomes are differentially linked to the exhausted phenotype of CD8+ T cells in HIV infection. PLoS Pathog. 2014;10(7):e1004251. doi: 10.1371/journal.ppat.1004251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kaech SM, Cui W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat Rev Immunol. 2012;12(11):749–61. doi: 10.1038/nri3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Paley MA, et al. Technical Advance: Fluorescent reporter reveals insights into eomesodermin biology in cytotoxic lymphocytes. J Leukoc Biol. 2013;93(2):307–15. doi: 10.1189/jlb.0812400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Intlekofer AM, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6(12):1236–44. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 64.Zhou X, et al. Differentiation and persistence of memory CD8(+) T cells depend on T cell factor 1. Immunity. 2010;33(2):229–40. doi: 10.1016/j.immuni.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Banerjee A, et al. Cutting edge: The transcription factor eomesodermin enables CD8+ T cells to compete for the memory cell niche. J Immunol. 2010;185(9):4988–92. doi: 10.4049/jimmunol.1002042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Intlekofer AM, et al. Requirement for T-bet in the aberrant differentiation of unhelped memory CD8+ T cells. J Exp Med. 2007;204(9):2015–21. doi: 10.1084/jem.20070841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mullen AC, et al. Master transcription factors determine cell-type-specific responses to TGF-beta signaling. Cell. 2011;147(3):565–76. doi: 10.1016/j.cell.2011.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Trompouki E, et al. Lineage regulators direct BMP and Wnt pathways to cell-specific programs during differentiation and regeneration. Cell. 2011;147(3):577–89. doi: 10.1016/j.cell.2011.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Curran MA, et al. Systemic 4–1BB activation induces a novel T cell phenotype driven by high expression of Eomesodermin. J Exp Med. 2013;210(4):743–55. doi: 10.1084/jem.20121190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fahey LM, et al. Viral persistence redirects CD4 T cell differentiation toward T follicular helper cells. J Exp Med. 2011;208(5):987–99. doi: 10.1084/jem.20101773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brooks DG, et al. Intrinsic functional dysregulation of CD4 T cells occurs rapidly following persistent viral infection. J Virol. 2005;79(16):10514–27. doi: 10.1128/JVI.79.16.10514-10527.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fuller MJ, Zajac AJ. Ablation of CD8 and CD4 T cell responses by high viral loads. J Immunol. 2003;170(1):477–86. doi: 10.4049/jimmunol.170.1.477. [DOI] [PubMed] [Google Scholar]
- 73.Oxenius A, Zinkernagel RM, Hengartner H. Comparison of activation versus induction of unresponsiveness of virus-specific CD4+ and CD8+ T cells upon acute versus persistent viral infection. Immunity. 1998;9(4):449–57. doi: 10.1016/s1074-7613(00)80628-7. [DOI] [PubMed] [Google Scholar]
- 74.Matloubian M, Concepcion RJ, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J Virol. 1994;68(12):8056–63. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schenkel JM, et al. T cell memory. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science. 2014;346(6205):98–101. doi: 10.1126/science.1254536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Curiel TJ, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9(5):562–7. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 77.Fourcade J, et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010;207(10):2175–86. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Baitsch L, et al. Exhaustion of tumor-specific CD8(+) T cells in metastases from melanoma patients. J Clin Invest. 2011;121(6):2350–60. doi: 10.1172/JCI46102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Riches JC, et al. T cells from CLL patients exhibit features of T-cell exhaustion but retain capacity for cytokine production. Blood. 2013;121(9):1612–21. doi: 10.1182/blood-2012-09-457531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zenz T. Exhausting T cells in CLL. Blood. 2013;121(9):1485–6. doi: 10.1182/blood-2013-01-475939. [DOI] [PubMed] [Google Scholar]
- 81.Gros A, et al. PD-1 identifies the patient-specific CD8(+) tumor-reactive repertoire infiltrating human tumors. J Clin Invest. 2014;124(5):2246–59. doi: 10.1172/JCI73639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ahmadzadeh M, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114(8):1537–44. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mumprecht S, et al. Programmed death 1 signaling on chronic myeloid leukemia-specific T cells results in T-cell exhaustion and disease progression. Blood. 2009;114(8):1528–36. doi: 10.1182/blood-2008-09-179697. [DOI] [PubMed] [Google Scholar]
- 84.Fourcade J, et al. PD-1 is a regulator of NY-ESO-1-specific CD8+ T cell expansion in melanoma patients. J Immunol. 2009;182(9):5240–9. doi: 10.4049/jimmunol.0803245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Radoja S, et al. CD8(+) tumor-infiltrating T cells are deficient in perforin-mediated cytolytic activity due to defective microtubule-organizing center mobilization and lytic granule exocytosis. J Immunol. 2001;167(9):5042–51. doi: 10.4049/jimmunol.167.9.5042. [DOI] [PubMed] [Google Scholar]
- 86.Matsuzaki J, et al. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci U S A. 2010;107(17):7875–80. doi: 10.1073/pnas.1003345107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sfanos KS, et al. Human prostate-infiltrating CD8+ T lymphocytes are oligoclonal and PD-1+ Prostate. 2009;69(15):1694–703. doi: 10.1002/pros.21020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Muenst S, et al. The presence of programmed death 1 (PD-1)-positive tumor-infiltrating lymphocytes is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2013;139(3):667–76. doi: 10.1007/s10549-013-2581-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang Y, et al. Programmed death-1 upregulation is correlated with dysfunction of tumor-infiltrating CD8+ T lymphocytes in human non-small cell lung cancer. Cell Mol Immunol. 2010;7(5):389–95. doi: 10.1038/cmi.2010.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Spranger S, et al. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med. 2013;5(200):200ra116. doi: 10.1126/scitranslmed.3006504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tumeh PC, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–71. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Savage PA, Leventhal DS, Malchow S. Shaping the repertoire of tumor-infiltrating effector and regulatory T cells. Immunol Rev. 2014;259(1):245–58. doi: 10.1111/imr.12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jin HT, et al. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc Natl Acad Sci U S A. 2010;107(33):14733–8. doi: 10.1073/pnas.1009731107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McMahan RH, et al. Tim-3 expression on PD-1+ HCV-specific human CTLs is associated with viral persistence, and its blockade restores hepatocyte-directed in vitro cytotoxicity. J Clin Invest. 2010;120(12):4546–57. doi: 10.1172/JCI43127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nakamoto N, et al. Synergistic reversal of intrahepatic HCV-specific CD8 T cell exhaustion by combined PD-1/CTLA-4 blockade. PLoS Pathog. 2009;5(2):e1000313. doi: 10.1371/journal.ppat.1000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24(2):207–12. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wilson EB, et al. Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science. 2013;340(6129):202–7. doi: 10.1126/science.1235208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Teijaro JR, et al. Persistent LCMV infection is controlled by blockade of type I interferon signaling. Science. 2013;340(6129):207–11. doi: 10.1126/science.1235214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yi JS, Du M, Zajac AJ. A vital role for interleukin-21 in the control of a chronic viral infection. Science. 2009;324(5934):1572–6. doi: 10.1126/science.1175194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Frohlich A, et al. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science. 2009;324(5934):1576–80. doi: 10.1126/science.1172815. [DOI] [PubMed] [Google Scholar]
- 101.Elsaesser H, Sauer K, Brooks DG. IL-21 is required to control chronic viral infection. Science. 2009;324(5934):1569–72. doi: 10.1126/science.1174182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brooks DG, et al. Interleukin-10 determines viral clearance or persistence in vivo. Nat Med. 2006;12(11):1301–9. doi: 10.1038/nm1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kao C, et al. Transcription factor T-bet represses expression of the inhibitory receptor PD-1 and sustains virus-specific CD8+ T cell responses during chronic infection. Nat Immunol. 2011;12(7):663–71. doi: 10.1038/ni.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Staron MM, et al. The Transcription Factor FoxO1 Sustains Expression of the Inhibitory Receptor PD-1 and Survival of Antiviral CD8(+) T Cells during Chronic Infection. Immunity. 2014;41(5):802–14. doi: 10.1016/j.immuni.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shin H, et al. A role for the transcriptional repressor Blimp-1 in CD8(+) T cell exhaustion during chronic viral infection. Immunity. 2009;31(2):309–20. doi: 10.1016/j.immuni.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Oestreich KJ, et al. NFATc1 regulates PD-1 expression upon T cell activation. J Immunol. 2008;181(7):4832–9. doi: 10.4049/jimmunol.181.7.4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yokosuka T, et al. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J Exp Med. 2012;209(6):1201–17. doi: 10.1084/jem.20112741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chemnitz JM, et al. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol. 2004;173(2):945–54. doi: 10.4049/jimmunol.173.2.945. [DOI] [PubMed] [Google Scholar]
- 109.Riley JL. PD-1 signaling in primary T cells. Immunol Rev. 2009;229(1):114–25. doi: 10.1111/j.1600-065X.2009.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sheppard KA, et al. PD-1 inhibits T-cell receptor induced phosphorylation of the ZAP70/CD3zeta signalosome and downstream signaling to PKCtheta. FEBS Lett. 2004;574(1–3):37–41. doi: 10.1016/j.febslet.2004.07.083. [DOI] [PubMed] [Google Scholar]
- 111.Parry RV, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25(21):9543–53. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Patsoukis N, et al. Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci Signal. 2012;5(230):ra46. doi: 10.1126/scisignal.2002796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Quigley M, et al. Transcriptional analysis of HIV-specific CD8+ T cells shows that PD-1 inhibits T cell function by upregulating BATF. Nat Med. 2010;16(10):1147–51. doi: 10.1038/nm.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fife BT, et al. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nat Immunol. 2009;10(11):1185–92. doi: 10.1038/ni.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Honda T, et al. Tuning of antigen sensitivity by T cell receptor-dependent negative feedback controls T cell effector function in inflamed tissues. Immunity. 2014;40(2):235–47. doi: 10.1016/j.immuni.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zinselmeyer BH, et al. PD-1 promotes immune exhaustion by inducing antiviral T cell motility paralysis. J Exp Med. 2013;210(4):757–74. doi: 10.1084/jem.20121416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Iwai Y, Terawaki S, Honjo T. PD-1 blockade inhibits hematogenous spread of poorly immunogenic tumor cells by enhanced recruitment of effector T cells. Int Immunol. 2005;17(2):133–44. doi: 10.1093/intimm/dxh194. [DOI] [PubMed] [Google Scholar]
- 118.Strome SE, et al. B7-H1 blockade augments adoptive T-cell immunotherapy for squamous cell carcinoma. Cancer Res. 2003;63(19):6501–5. [PubMed] [Google Scholar]
- 119.Blank C, et al. PD-L1/B7H-1 inhibits the effector phase of tumor rejection by T cell receptor (TCR) transgenic CD8+ T cells. Cancer Res. 2004;64(3):1140–5. doi: 10.1158/0008-5472.can-03-3259. [DOI] [PubMed] [Google Scholar]
- 120.Gubin MM, et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515(7528):577–81. doi: 10.1038/nature13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Twyman-Saint Victor C, RAJ, Maity A, Rengan R, Pauken KE, Stelekati E, Benci JL, Xu B, Dada H, Odorizzi PM, Herati RS, Mansfield KD, Patsch D, Amaravadi RK, Schuchter LM, Ishwaran H, Mick R, Pryma DA, Xu X, Feldman MD, Gangadhar TC, Hahn SM, Wherry EJ, Vonderheide RH, Minn AJ. Radiation and dual checkpoint blockade activates non-redundant immune mechanisms in cancer. Nature. doi: 10.1038/nature14292. p. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mueller SN, et al. PD-L1 has distinct functions in hematopoietic and nonhematopoietic cells in regulating T cell responses during chronic infection in mice. J Clin Invest. 2010;120(7):2508–15. doi: 10.1172/JCI40040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yadav M, et al. Predicting immunogenic tumour mutations by combining mass spectrometry and exome sequencing. Nature. 2014;515(7528):572–6. doi: 10.1038/nature14001. [DOI] [PubMed] [Google Scholar]
- 124.Peng W, et al. PD-1 blockade enhances T-cell migration to tumors by elevating IFN-gamma inducible chemokines. Cancer Res. 2012;72(20):5209–18. doi: 10.1158/0008-5472.CAN-12-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pilon-Thomas S, et al. Blockade of programmed death ligand 1 enhances the therapeutic efficacy of combination immunotherapy against melanoma. J Immunol. 2010;184(7):3442–9. doi: 10.4049/jimmunol.0904114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yang K, Fu LW. Mechanisms of resistance to BCR-ABL TKIs and the therapeutic strategies: A review. Crit Rev Oncol Hematol. 2015;93(3):277–292. doi: 10.1016/j.critrevonc.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 127.Xiao Y, et al. RGMb is a novel binding partner for PD-L2 and its engagement with PD-L2 promotes respiratory tolerance. J Exp Med. 2014;211(5):943–59. doi: 10.1084/jem.20130790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Martinez GJ, et al. The Transcription Factor NFAT Promotes Exhaustion of Activated CD8 T Cells. Immunity. 2015;42:265–278. doi: 10.1016/j.immuni.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pollizzi KN, Powell JD. Integrating canonical and metabolic signalling programmes in the regulation of T cell responses. Nat Rev Immunol. 2014;14(7):435–46. doi: 10.1038/nri3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lu P, et al. Blimp-1 represses CD8 T cell expression of PD-1 using a feed-forward transcriptional circuit during acute viral infection. J Exp Med. 2014;211(3):515–27. doi: 10.1084/jem.20130208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Youngblood B, et al. Chronic virus infection enforces demethylation of the locus that encodes PD-1 in antigen-specific CD8(+) T cells. Immunity. 2011;35(3):400–12. doi: 10.1016/j.immuni.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Youngblood B, et al. Cutting edge: Prolonged exposure to HIV reinforces a poised epigenetic program for PD-1 expression in virus-specific CD8 T cells. J Immunol. 2013;191(2):540–4. doi: 10.4049/jimmunol.1203161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang F, et al. Epigenetic manipulation restores functions of defective CD8(+) T cells from chronic viral infection. Mol Ther. 2014;22(9):1698–706. doi: 10.1038/mt.2014.91. [DOI] [PMC free article] [PubMed] [Google Scholar]