Abstract
We performed an association analysis of fragile X mental retardation 1 (FMR1) CGG repeats in 321 essential tremor (ET) cases and 296 controls at Columbia University. In addition to analyzing the allele distribution (10–49 CGG repeats) in the entire sample, we also performed a screen for ET cases with the FMR1 premutation allele (55–200 CGG repeats), and evaluated an association between ET and FMR1 alleles that included gray zone alleles (41–54 CGG repeats). CGG premutation alleles and gray zone alleles were rare in ET cases, and we found no evidence for association of premutation or gray zone alleles with ET. These data suggest that FMR1 CGG repeats are not a genetic risk factor for ET.
Keywords: Essential Tremor, FMR1, Fragile X, gray zone alleles, premutation alleles, genetics
Introduction
Essential tremor (ET) is a chronic, progressive neurologic disease [1]. The hallmark feature of ET is a 4–12 Hz kinetic tremor (i.e., tremor occurring during voluntary movements) of the arms, which may also eventually spread to involve the neck, voice, and jaw [2]. ET is among the most prevalent neurological diseases [3]. It shares a number of clinical features with Fragile X associated tremor/ataxia syndrome (FXTAS), which is caused by a premutation of the fragile X mental retardation 1 (FMR1) gene, especially among male carriers [4]. Three previous studies have screened 71 ET cases [5], 81 ET cases [6], and 196 ET cases [7] for the FMR1 premutation allele. Although ET cases carrying the permutation allele (55–200 CGG repeats) were not identified, ET cases with CGG repeats falling within the “gray zone” (41–54 CGG repeats) were observed in 0.0% [5], 1.6% [6], and 1.5% [7] of ET cases. Recently, there has been increased interest in performing genotype-phenotype correlations of gray zone alleles in neurological diseases [8]. In the current study, we expand the sample size considerably to 321 ET cases and 296 controls; hence, the number of ET cases is similar magnitude to that of all prior studies combined. As a departure from prior studies, our enlarged sample size also allowed us to stratify ET cases based on important clinical features. In addition to analyzing the allele distribution (10–49 CGG repeats) in the entire sample, we also performed a screen for ET cases with the FMR1 premutation allele (55–200 CGG repeats), and evaluated an association between ET and FMR1 alleles that included gray zone alleles (41–54 CGG repeats).
Patients and Methods
Subjects
As described [9], 321 ET cases and 296 controls were enrolled in a clinical-epidemiological study at the Neurological Institute of New York, Columbia University, New York (2000 – 2007). All participants underwent a demographic and medical history questionnaire, a family history questionnaire (any first- or second-degree relative with ET), and a videotaped neurological examination. ET diagnoses were assigned using research criteria [9]. The study was approved by the Institutional Review Board at Columbia University.
Genotyping
PCR amplification of genomic DNA was performed using the expand long template PCR system (Roche Applied Science) and fragment analysis was performed using an automated DNA sequencer (ABI prism 3100). Allele sizes were determined using GeneScan (Applied Biosystems). Primers and PCR conditions have been described previously [10]. Female subjects with single peaks (apparently homozygous) corresponding to ≤ or ≥ 40 repeats were not further evaluated by Southern blot analysis to determine the presence of a second expanded allele in the heterozygous state (>100 repeats) undetectable by fragment analysis. Fragments were detectable and allele sizes were determined in all male subjects included in the analysis indicating the absence of expanded alleles (>100 repeats) in the premutation or full mutation range.
Statistical Analysis
Allele frequencies were calculated from observed genotypes. CLUMP analysis, used for association testing when markers produce sparse contingency tables, was used to test differences in allele distribution between ET cases and controls. Linear regression and Pearson’s correlation was used to calculate the relationship between CGG repeat size and age at onset of ET and the correlation coefficient (r) was determined.
Results
Demographic and Clinical Characteristics of ET Cases and Controls
The demographic and clinical characteristics of subjects included in the study are shown in Table 1. The mean age at tremor onset was 44.2 (±22.0) years and 29.3% of ET cases reported a family history of ET. The ethnicity included non-hispanic whites (93.8% ET cases and 86.1% controls), non-hispanic blacks (2.2% ET cases and 5.7% controls) and hispanics (2.5% ET cases and 4.1% controls). A subset of the sample reported Ashkenazi Jewish (AJ) ancestry (28.8%).
Table 1.
Demographic and Clinical Characteristics of Genotyped Subjects
| ET Cases (N=321) |
Controls (N=296) |
Statistical test |
p-value | |
|---|---|---|---|---|
| % Male (n) | 48.0 (154) | 41.65 (123) | χ2=2.31 | 0.1285 |
| Mean age at tremor onset (years) (SD) | 44.2 (22.0) | NA | NA | NA |
| % with family history of ET (n) | 29.3 (94) | NA | NA | NA |
| % Ashkenazi Jewish ancestry (n)* | 38.6 (124) | 19.6 (58) | χ2=25.92 | <.0001 |
| % Age at onset • 40 yr (n)** | 39.9 (128) | NA | NA | NA |
| % Age at onset > 40 yr (n)** | 56.1 (180) | NA | NA | NA |
| % Age at onset ≤ 55 yr (n)** | 60.4 (194) | NA | NA | NA |
| % Age at onset > 55 yr (n)** | 35.5 (114) | NA | NA | NA |
| % Non-hispanic White (n)*** | 93.8 (301) | 86.1(255) | χ2=9.2 | 0.0024 |
| % Non-hispanic Black (n)*** | 2.2(7) | 5.7(17) | χ2=4.32 | 0.0377 |
| % Hispanic (n)*** | 2.5(8) | 4.1(12) | χ2=0.75 | 0.3865 |
| % Non-hispanic White-AJ (n)* | 38.0(122) | 18.9(56) | χ2=26.41 | <.0001 |
P<.0001;
Age at onset data not available for 13 ET cases.
Ethnicity data not available on 5 ET cases and 12 controls. NA, Not applicable;
Analysis of Premutation and Gray Zone alleles
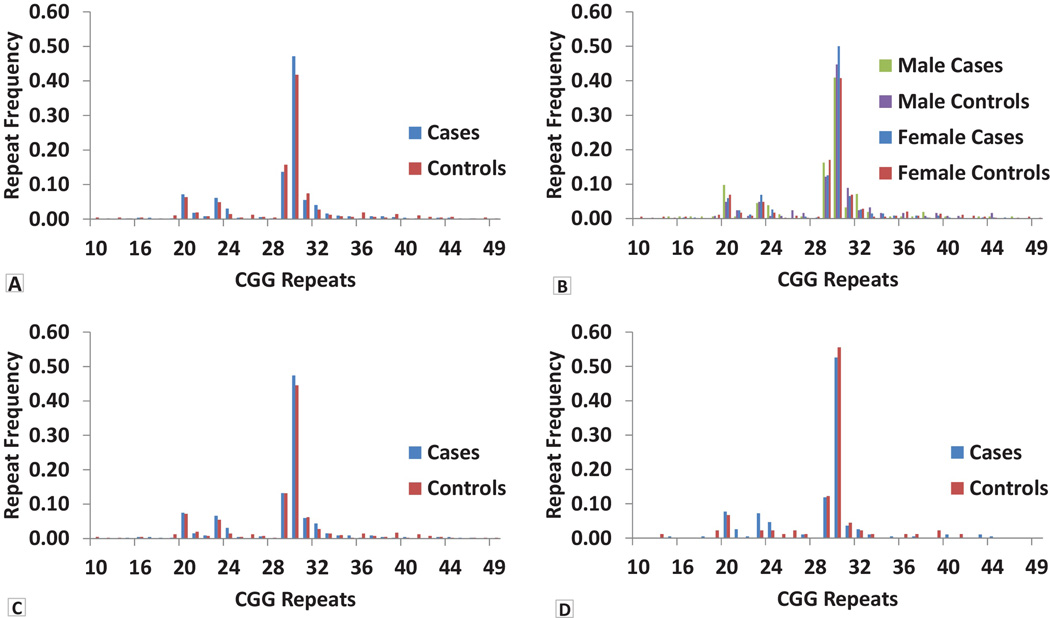
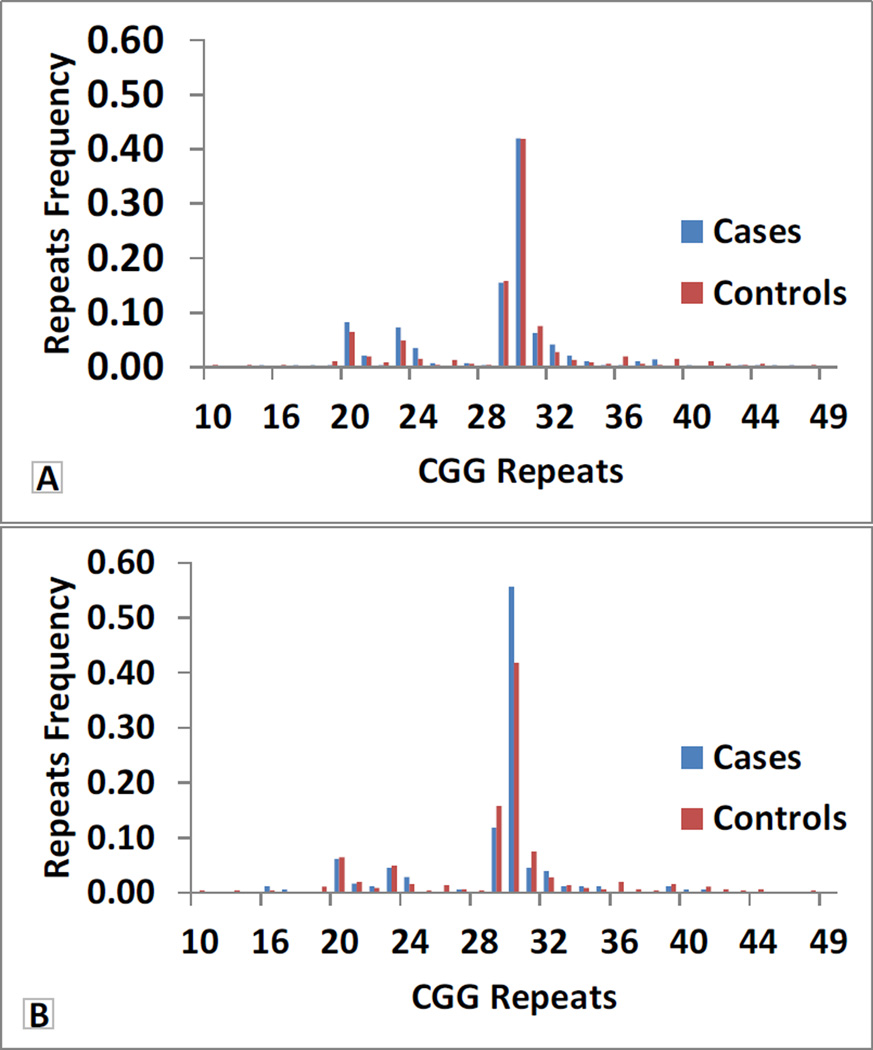
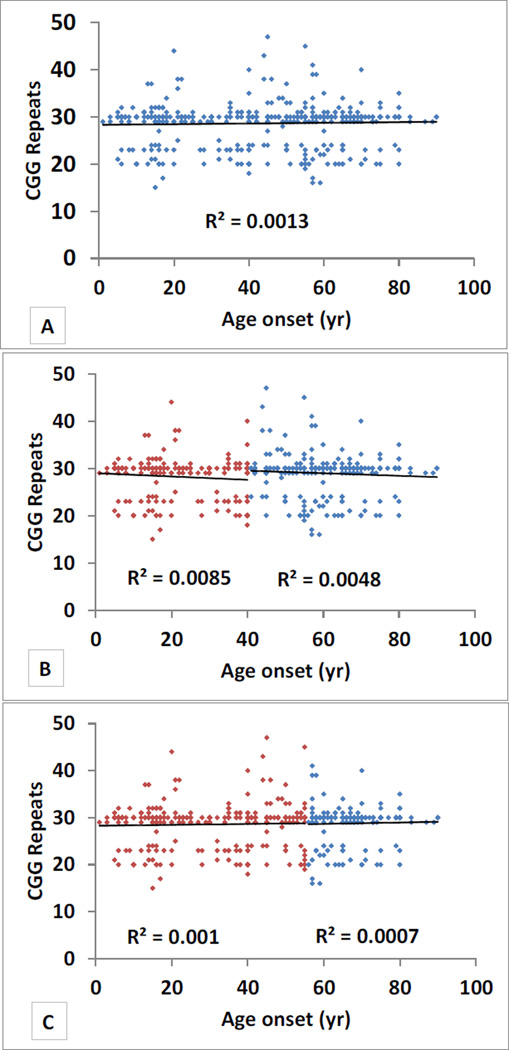
We did not detect CGG premutation alleles (55–200 CGG repeats) in any of the 488 × chromosomes of ET cases (154 males and 167 females) or 469 × chromosomes of controls (123 males and 173 females). There were 37 alleles ranging in size from 10–49 CGG repeats. A total of 7/321 (2.2%) ET cases and 16/296 (5.4%) controls carried gray zone alleles (41–54 CGG repeats); this difference was significant (Chi square=4.69, p=0.03). The largest repeat size observed in 617 subjects was 49 (1 female control). We also stratified the analysis by clinical and demographic features, including age at ET onset (≤40 years vs. > 40 years and ≤50 years vs. > 50 years), family history of ET, gender, ethnicity (non-hispanic black, nonhispanic white, hispanic) and ancestry (Non hispanic white AJ vs. others) (Figure 1, Figure 2 and Figure 3). In non-hispanic blacks, we observed a significant association of alleles in the size range 29–30 CGG repeats (Chi square=20.87, p=0.04). However, the sample size of non-hispanic blacks included in this study is small (7 cases and 17 controls) and, therefore, the result should be treated with caution. There was no evidence of association with FMR1 CGG repeat alleles or premutation or gray zone alleles in any of the other stratified analyses and there was an absence of correlation between repeat size and age at onset of ET. (Data not shown for stratified analysis of family history of ET).
Figure 1. Distribution of CGG allele frequency in ET cases and controls.
A: All cases and controls; B: all cases and controls, stratified by gender; C: restricted to cases and controls of non-hispanic white ethnicity; D: restricted to cases and controls of non-hispanic white Ashkenazi Jewish ancestry.
Figure 2. Distribution of CGG repeat allele frequency stratified by age at onset ≤55 years vs. >55 years in ET cases.
A: ET cases and all controls stratified by age at onset ≤55 years in ET cases; B: ET cases and all controls stratified by age at onset >55 years in ET cases
Figure 3. Correlations Between Age of Onset and Repeat Size.
A) Correlation between age at onset and CGG repeat size in all ET cases.
B) Correlation between age at onset and CGG repeat size. ET cases are stratified into those with age of onset •40 years (red) and age onset >40 years (blue).
C) Correlation between age at onset and CGG repeat size. ET cases are stratified into those with age of onset •55 years (red) and age onset >55 years (blue).
Discussion
We performed an association analysis of the FMR1 CGG repeat in a large case-control study of ET. Our results, and those of prior studies, suggest that CGG premutation alleles or gray zone alleles are rare in ET cases. We found no evidence for a positive association of premutation or gray zone alleles with ET. When we stratified the analysis with age at onset that is consistent with FXTAS (≤50 years vs. > 50 years) we still did not observe an association of CGG premutation alleles with ET. Our enlarged sample size also allowed us to stratify ET cases based on important clinical features. These analyses similarly did not detect evidence of an association of FMRI CGG repeat alleles with ET. In the AJ ET cases and non-AJ ET cases that we analyzed in the current study we did not observe a higher proportion of premutations. It is possible that had we enriched for ET cases with a family history of offspring with intellectual disabilities we may have observed an association; however, information about family history of intellectual disability was not ascertained in our cohort. A subset of the sample in our study is AJ. A recent study that evaluated 4,344 AJ and 4,985 non-AJ subjects reported a higher rate of premutations in the 55–59 repeat range (1:114 vs. 1:277) among the AJ women and concluded that AJ women have a high fragile X syndrome carrier rate and mostly low-range premutations [11]. In the AJ ET cases (including AJ ET females) and non-AJ ET cases that we analyzed in the current study we did not observe a higher proportion of premutations.
In conclusion, these data suggest that FMR1 CGG repeats are not a genetic risk factor for ET.
Highlights.
ET shares clinical features with Fragile X associated tremor/ataxia syndrome.
Analyzed FMR1 CGG repeats in 321 ET cases and 296 controls.
Analysis included overall allele distribution, premutation and gray zone alleles.
We found no evidence for a positive association of FMR1 CGG repeat alleles with ET.
Acknowledgements
Dr. Louis has received research support from the National Institutes of Health (NIH): NINDS #R01 NS042859 (principal investigator), NINDS #R01 NS39422 (principal investigator), NINDS #R01 NS086736 (principal investigator), NINDS #R01 NS073872 (principal investigator), NINDS #R01 NS085136 (principal investigator), NINDS #T32 NS07153-24 (principal investigator), NINDS #R21 NS077094 (co-Investigator), and NINDS #R01 NS36630 (co-Investigator).
Dr. Clark is funded by NIH grants R21NS050487 (PI), R01NS060113 (PI), R01NS0738072 (CoPI), P50AG008702 (CoI), P50 NS038370 (CoI), the Parkinson’s Disease foundation (PI) and the Michael J Fox foundation (CoI).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Louis ED. Clinical practice. Essential tremor. N Engl J Med. 2001;345:887–891. doi: 10.1056/NEJMcp010928. [DOI] [PubMed] [Google Scholar]
- 2.Louis ED, Gerbin M, Galecki M. Essential tremor 10, 20, 30, 40: clinical snapshots of the disease by decade of duration. Eur J Neurol. 2013;20:949–954. doi: 10.1111/ene.12123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Louis ED, Ferreira JJ. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov Disord. 2010;25:534–541. doi: 10.1002/mds.22838. [DOI] [PubMed] [Google Scholar]
- 4.Apartis E, Blancher A, Meissner WG, Guyant-Marechal L, Maltete D, et al. FXTAS: new insights and the need for revised diagnostic criteria. Neurology. 2012;79:1898–1907. doi: 10.1212/WNL.0b013e318271f7ff. [DOI] [PubMed] [Google Scholar]
- 5.Tan EK, Zhao Y, Puong KY, Law HY, Chan LL, et al. Fragile X premutation alleles in SCA, ET, and parkinsonism in an Asian cohort. Neurology. 2004;63:362–363. doi: 10.1212/01.wnl.0000130199.57181.7b. [DOI] [PubMed] [Google Scholar]
- 6.Garcia Arocena D, Louis ED, Tassone F, Gilliam TC, Ottman R, et al. Screen for expanded FMR1 alleles in patients with essential tremor. Mov Disord. 2004;19:930–933. doi: 10.1002/mds.20043. [DOI] [PubMed] [Google Scholar]
- 7.Deng H, Le W, Jankovic J. Premutation alleles associated with Parkinson disease and essential tremor. JAMA. 2004;292:1685–1686. doi: 10.1001/jama.292.14.1685-b. [DOI] [PubMed] [Google Scholar]
- 8.Hall DA. In the Gray Zone in the Fragile X Gene: What are the Key Unanswered Clinical and Biological Questions? Tremor Other Hyperkinet Mov (N Y) 2014;4:208. doi: 10.7916/D8NG4NP3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark LN, Park N, Kisselev S, Rios E, Lee JH, et al. Replication of the LINGO1 gene association with essential tremor in a North American population. European journal of human genetics : EJHG. 2010;18:838–843. doi: 10.1038/ejhg.2010.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tassone F, Pan R, Amiri K, Taylor AK, Hagerman PJ. A rapid polymerase chain reaction-based screening method for identification of all expanded alleles of the fragile X (FMR1) gene in newborn and high-risk populations. J Mol Diagn. 2008;10:43–49. doi: 10.2353/jmoldx.2008.070073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weiss K, Orr-Urtreger A, Kaplan Ber I, Naiman T, Bardugu E, Yaron Y, Ben-Shachar S. Ethnic effect on FMR1 carrier rate and AGG repeat interruptions among Ashkenazi women. Genet Med. 2014;16:940–944. doi: 10.1038/gim.2014.64. [DOI] [PubMed] [Google Scholar]