Abstract
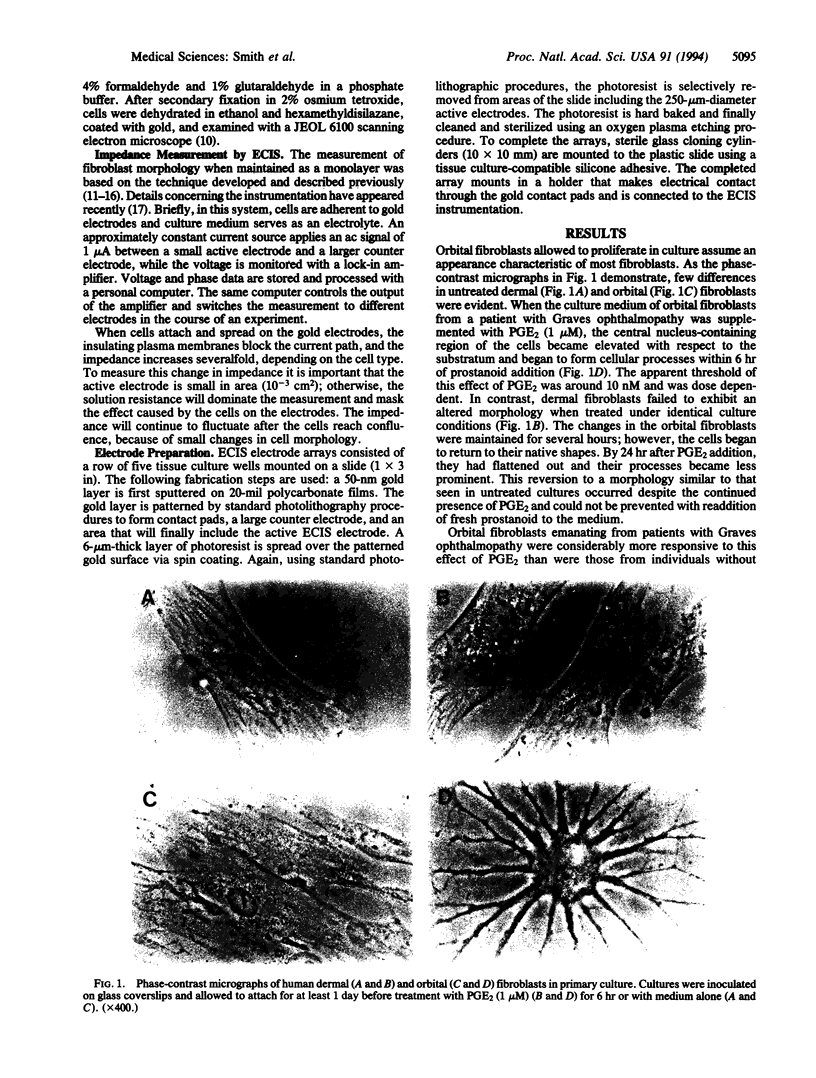
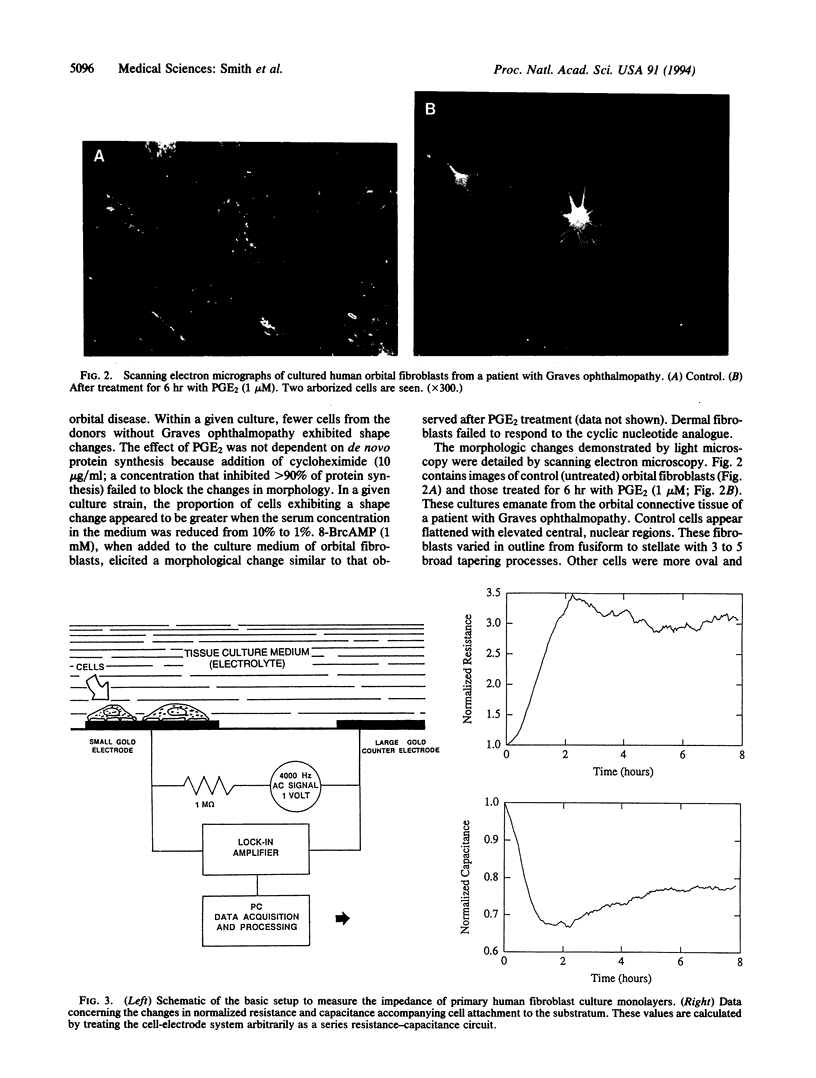
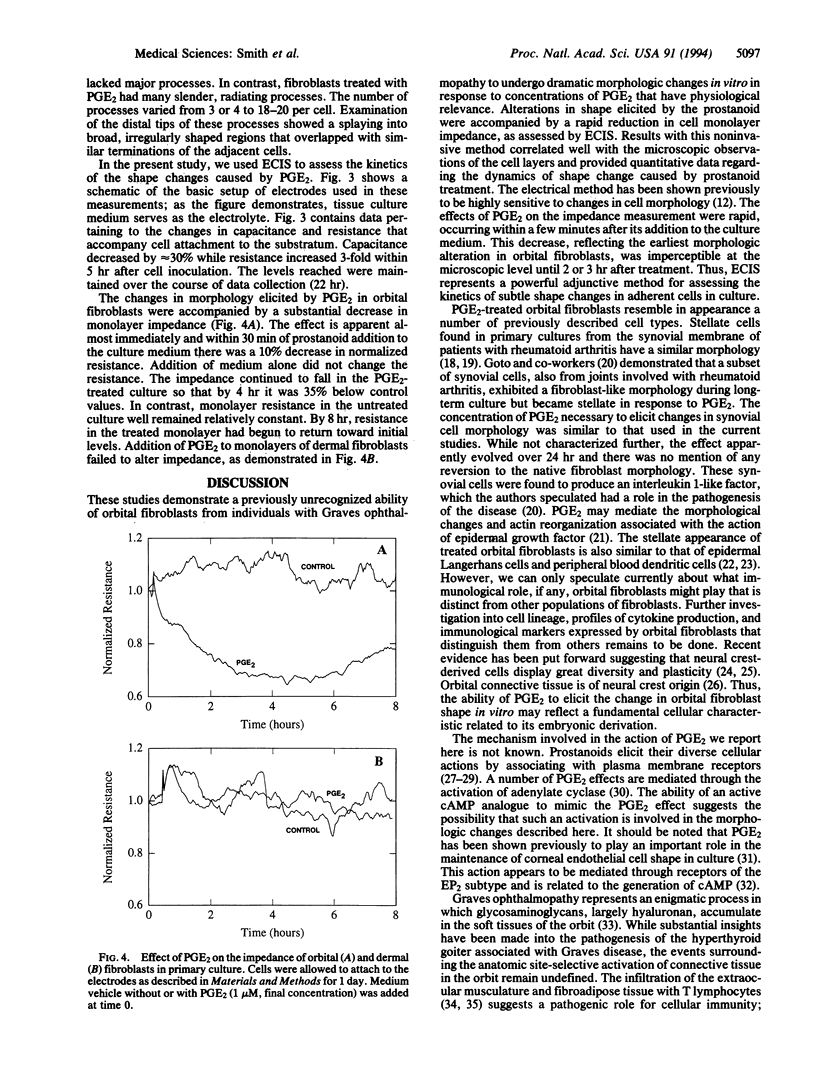
Fibroblasts derived from distinct anatomical regions appear to differ in regard to their behavior in culture. These differences may reflect functions of these cells in vivo that are tissue specific. Moreover, intrinsic differences in fibroblasts may underlie the site-specific connective tissue manifestations associated with systemic disease. We have demonstrated previously that orbital fibroblasts exhibit different cytokine response domains and protein synthetic programs when compared to those emanating from the skin. In the present communication, we demonstrate that prostaglandin E2 (PGE2) elicits in cultured human orbital fibroblasts from patients with Graves ophthalmopathy a rapid and dramatic change in cell morphology in vitro as assessed by phase-contrast and scanning electron microscopy. The central areas of the cells become elevated with respect to the plane of the substratum and are stellate, with long processes that touch neighboring cells. These changes occur within 6 hr of prostanoid addition to culture medium at an apparent concentration threshold of approximately 10 nM. Shape changes are accompanied by marked alterations in monolayer impedance as assessed by electric cell-substrate impedance sensing as described previously. Both morphologic and impedance changes elicited by PGE2 revert over 24 hr toward those found in untreated cells despite the continued presence of the prostanoid in the culture medium. In contrast, dermal fibroblasts fail to respond to PGE2. These observations define a previously unrecognized phenotypic attribute of orbital fibroblasts. Intrinsic differences in these cells may account for the anatomic site-selective vulnerability of the orbit in Graves ophthalmopathy. The culture system described here may be useful for studying the morphogenic actions of prostanoids.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. J., Axel R. Molecular probes for the development and plasticity of neural crest derivatives. Cell. 1985 Sep;42(2):649–662. doi: 10.1016/0092-8674(85)90122-9. [DOI] [PubMed] [Google Scholar]
- Anderson D. J. Molecular control of cell fate in the neural crest: the sympathoadrenal lineage. Annu Rev Neurosci. 1993;16:129–158. doi: 10.1146/annurev.ne.16.030193.001021. [DOI] [PubMed] [Google Scholar]
- Duke O., Panayi G. S., Janossy G., Poulter L. W. An immunohistological analysis of lymphocyte subpopulations and their microenvironment in the synovial membranes of patients with rheumatoid arthritis using monoclonal antibodies. Clin Exp Immunol. 1982 Jul;49(1):22–30. [PMC free article] [PubMed] [Google Scholar]
- Giaever I., Keese C. R. A morphological biosensor for mammalian cells. Nature. 1993 Dec 9;366(6455):591–592. doi: 10.1038/366591a0. [DOI] [PubMed] [Google Scholar]
- Giaever I., Keese C. R. Micromotion of mammalian cells measured electrically. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7896–7900. doi: 10.1073/pnas.88.17.7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaever I., Keese C. R. Monitoring fibroblast behavior in tissue culture with an applied electric field. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3761–3764. doi: 10.1073/pnas.81.12.3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaever I., Keese C. R. Use of electric fields to monitor the dynamical aspect of cell behavior in tissue culture. IEEE Trans Biomed Eng. 1986 Feb;33(2):242–247. doi: 10.1109/TBME.1986.325896. [DOI] [PubMed] [Google Scholar]
- Goto M., Sasano M., Yamanaka H., Miyasaka N., Kamatani N., Inoue K., Nishioka K., Miyamoto T. Spontaneous production of an interleukin 1-like factor by cloned rheumatoid synovial cells in long-term culture. J Clin Invest. 1987 Sep;80(3):786–796. doi: 10.1172/JCI113135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassell T. M., Page R. C., Narayanan A. S., Cooper C. G. Diphenylhydantoin (dilantin) gingival hyperplasia: drug-induced abnormality of connective tissue. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2909–2912. doi: 10.1073/pnas.73.8.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jumblatt M. M., Matkin E. D., Neufeld A. H. Pharmacological regulation of morphology and mitosis in cultured rabbit corneal endothelium. Invest Ophthalmol Vis Sci. 1988 Apr;29(4):586–593. [PubMed] [Google Scholar]
- Jumblatt M. M., Paterson C. A. Prostaglandin E2 effects on corneal endothelial cyclic adenosine monophosphate synthesis and cell shape are mediated by a receptor of the EP2 subtype. Invest Ophthalmol Vis Sci. 1991 Feb;32(2):360–365. [PubMed] [Google Scholar]
- King P. D., Katz D. R. Mechanisms of dendritic cell function. Immunol Today. 1990 Jun;11(6):206–211. doi: 10.1016/0167-5699(90)90084-m. [DOI] [PubMed] [Google Scholar]
- Krane S. M. Heberden Oration 1980: aspects of the cell biology of the rheumatoid synovial lesion. Ann Rheum Dis. 1981 Oct;40(5):433–448. doi: 10.1136/ard.40.5.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra P., Keese C. R., Giaever I. Electric measurements can be used to monitor the attachment and spreading of cells in tissue culture. Biotechniques. 1991 Oct;11(4):504–510. [PubMed] [Google Scholar]
- Negishi M., Sugimoto Y., Irie A., Narumiya S., Ichikawa A. Two isoforms of prostaglandin E receptor EP3 subtype. Different COOH-terminal domains determine sensitivity to agonist-induced desensitization. J Biol Chem. 1993 May 5;268(13):9517–9521. [PubMed] [Google Scholar]
- Peppelenbosch M. P., Tertoolen L. G., Hage W. J., de Laat S. W. Epidermal growth factor-induced actin remodeling is regulated by 5-lipoxygenase and cyclooxygenase products. Cell. 1993 Aug 13;74(3):565–575. doi: 10.1016/0092-8674(93)80057-l. [DOI] [PubMed] [Google Scholar]
- Ransom J. H., Evans C. H., McCabe R. P., Pomato N., Heinbaugh J. A., Chin M., Hanna M. G., Jr Leukoregulin, a direct-acting anticancer immunological hormone that is distinct from lymphotoxin and interferon. Cancer Res. 1985 Feb;45(2):851–862. [PubMed] [Google Scholar]
- Samuelsson B., Goldyne M., Granström E., Hamberg M., Hammarström S., Malmsten C. Prostaglandins and thromboxanes. Annu Rev Biochem. 1978;47:997–1029. doi: 10.1146/annurev.bi.47.070178.005025. [DOI] [PubMed] [Google Scholar]
- Smith T. J., Ahmed A., Hogg M. G., Higgins P. J. Interferon-gamma is an inducer of plasminogen activator inhibitor type 1 in human orbital fibroblasts. Am J Physiol. 1992 Jul;263(1 Pt 1):C24–C29. doi: 10.1152/ajpcell.1992.263.1.C24. [DOI] [PubMed] [Google Scholar]
- Smith T. J., Bahn R. S., Gorman C. A., Cheavens M. Stimulation of glycosaminoglycan accumulation by interferon gamma in cultured human retroocular fibroblasts. J Clin Endocrinol Metab. 1991 May;72(5):1169–1171. doi: 10.1210/jcem-72-5-1169. [DOI] [PubMed] [Google Scholar]
- Smith T. J., Bahn R. S., Gorman C. A. Connective tissue, glycosaminoglycans, and diseases of the thyroid. Endocr Rev. 1989 Aug;10(3):366–391. doi: 10.1210/edrv-10-3-366. [DOI] [PubMed] [Google Scholar]
- Smith T. J., Bahn R. S., Gorman C. A. Hormonal regulation of hyaluronate synthesis in cultured human fibroblasts: evidence for differences between retroocular and dermal fibroblasts. J Clin Endocrinol Metab. 1989 Nov;69(5):1019–1023. doi: 10.1210/jcem-69-5-1019. [DOI] [PubMed] [Google Scholar]
- Smith T. J. Dexamethasone regulation of glycosaminoglycan synthesis in cultured human skin fibroblasts. Similar effects of glucocorticoid and thyroid hormones. J Clin Invest. 1984 Dec;74(6):2157–2163. doi: 10.1172/JCI111642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T. J., Higgins P. J. Interferon gamma regulation of de novo protein synthesis in human dermal fibroblasts in culture is anatomic site dependent. J Invest Dermatol. 1993 Mar;100(3):288–292. doi: 10.1111/1523-1747.ep12469828. [DOI] [PubMed] [Google Scholar]
- Smith T. J., Kottke R. J., Lum H., Andersen T. T. Human orbital fibroblasts in culture bind and respond to endothelin. Am J Physiol. 1993 Jul;265(1 Pt 1):C138–C142. doi: 10.1152/ajpcell.1993.265.1.C138. [DOI] [PubMed] [Google Scholar]
- Smith T. J., Murata Y., Horwitz A. L., Philipson L., Refetoff S. Regulation of glycosaminoglycan synthesis by thyroid hormone in vitro. J Clin Invest. 1982 Nov;70(5):1066–1073. doi: 10.1172/JCI110694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T. J. n-Butyrate inhibition of hyaluronate synthesis in cultured human fibroblasts. J Clin Invest. 1987 May;79(5):1493–1497. doi: 10.1172/JCI112979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- Tiruppathi C., Malik A. B., Del Vecchio P. J., Keese C. R., Giaever I. Electrical method for detection of endothelial cell shape change in real time: assessment of endothelial barrier function. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):7919–7923. doi: 10.1073/pnas.89.17.7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weetman A. P., Cohen S., Gatter K. C., Fells P., Shine B. Immunohistochemical analysis of the retrobulbar tissues in Graves' ophthalmopathy. Clin Exp Immunol. 1989 Feb;75(2):222–227. [PMC free article] [PubMed] [Google Scholar]
- de Carli M., D'Elios M. M., Mariotti S., Marcocci C., Pinchera A., Ricci M., Romagnani S., del Prete G. Cytolytic T cells with Th1-like cytokine profile predominate in retroorbital lymphocytic infiltrates of Graves' ophthalmopathy. J Clin Endocrinol Metab. 1993 Nov;77(5):1120–1124. doi: 10.1210/jcem.77.5.8077301. [DOI] [PubMed] [Google Scholar]




