Abstract
A major subset of patients with advanced solid tumors shows a spontaneous T cell-inflamed tumor microenvironment, which has prognostic import and is associated with clinical response to immunotherapies. As such, understanding the mechanisms governing the generation of spontaneous T cell responses in only a subset of patients is critical for advancing immunotherapeutic approaches further. Here, we discuss characteristics of T cell-inflamed versus non-inflamed tumors, including a type I IFN signature associated with T cell priming against tumor antigens. We review recent findings that have pointed towards the STING pathway of cytosolic DNA sensing as an important innate immune sensing mechanism driving type I IFN production in the tumor context. Knowledge of this pathway is guiding the further development of novel immunotherapeutic strategies.
Introduction
The molecular identification of tumor antigens has transformed the field of tumor immunology and cancer immunotherapy. While many initial antigens were defined that were shared between patients, such as those encoded by MAGE family genes [1], more recent work has revealed unique antigens that arise from point mutations in normal genes generated during the genomic instability that is part of the process of carcinogenesis [2-5]. Whole exome sequencing of human cancers of various histologies has revealed that many tumors, especially those induced by carcinogens such as UV light or tobacco, contain hundreds or even thousands of non-synonymous mutations [6]. Therefore, the current thinking is that most tumors express some level of antigens that could theoretically be recognized by T cells of the immune system. Knowledge of these antigens combined with high-throughput genomics technologies has provided tools for analyzing patient tumor, blood, and lymphoid tissues for antigen-specific T cell populations and features of the host anti-tumor immune response, either spontaneous or in response to therapeutic interventions. Much of this work has culminated with the notion that features of the tumor microenvironment are critical determinants of patient outcome. Therefore, an expanded effort in studying the immune phenotype of the tumor microenvironment has emerged.
In early stage colorectal cancer, the presence of activated CD8+ T cells within the tumor and in the peri-tumoral stroma has been shown to have significant positive prognostic import [7-9]. A subset of patients with other solid tumor histologies also appears to have a spontaneous T cell infiltrate that may have similar positive prognostic value. This includes breast cancer, renal cell carcinoma, melanoma, ovarian cancer, and gastrointestinal stromal tumors (GIST) [10-14]. A presumption is that a component of this T cell infiltrate includes tumor antigen-specific T cells that have been activated spontaneously in response to the growing tumor, perhaps through immune surveillance mechanisms [15]. This attempted host immune response, even if it does not eliminate the tumor completely, is thought to delay tumor progression and thus yield improved clinical outcome.
The fact that a subset of patients appears to generate a spontaneous anti-tumor immune response while another major subset does not has generated several important biologic questions that have implications for further refinement of cancer immunotherapies. One central mystery has been identifying the innate immune mechanisms that give rise to a spontaneous adaptive T cell response against tumor antigens in the absence of exogenous infection. Clues have been gleaned from human cancer gene expression profiling studies revealing an association between a type I IFN signature, T cell infiltration, and clinical outcome. This has allowed focus on innate immune sensing pathways known to trigger type I IFN production that might represent critical intermediate mechanistic steps. Before discussing these pathways in detail, the T cell-inflamed tumor microenvironment will be put into context of our current understanding of the therapeutic efficacy of contemporary cancer immunotherapy approaches.
The T cell-inflamed versus non-T cell-inflamed tumor microenvironment in metastatic disease
The motivation for analyzing the tumor microenvironment in metastatic melanoma was initially derived from the hypothesis that resistance mechanisms downstream from T cell priming following vaccination against tumor antigens might be dominant and enable tumor escape [16,17]. To explore this question in patients, baseline biopsies of melanoma metastases were evaluated by gene expression profiling. It became clear that two major subsets of tumor microenvironment could be identified that were largely characterized by the presence or absence of a transcriptional profile indicative of a pre-existing T cell infiltrate. The T cell-inflamed subset of tumors was dominated by T cell markers and chemokines that likely mediate effector T cell recruitment [18-20]. Immunohistochemistry confirmed the presence of CD8+ T cells, macrophages, as well as some B cells and plasma cells in these lesions [18]. This T cell-inflamed subset of melanoma metastases is remarkably similar to the phenotype described in early stage colon cancer and other tumors in which activated T cells have been associated with favorable prognosis [7-9]. In several small studies of HLA-A2+ patients, CD8+ T cells specific for melanoma differentiation antigens were identified from tumor sites using peptide-HLA-A2 tetramer analysis [21-23]. Therefore, at least a subset of T cells specific for tumor antigens is present among these infiltrates. In fact, this is arguably the starting point for adoptive T cell approaches utilizing tumor-infiltrating lymphocytes (TIL), which has meaningful clinical activity in metastatic patients [24]. However, functional analysis has indicated various degrees of dysfunction of these tumor antigen-specific T cells when analyzed directly ex vivo [21-23]. Together, these results suggest that the reason for tumor progression despite the presence of specific adaptive immunity in this subset of patients is likely secondary to immune suppressive mechanisms acting at the level of the tumor microenvironment. Interestingly, in some cases the presence of memory virus-specific CD8+ T cells also has been observed in these T cell-inflamed melanomas. However, their function seems to be intact [21,25], and these probably represent non-specifically recruited activated T cells migrating along chemokine gradients but not participating in tumor recognition. These observations suggest that at least a component of T cell dysfunction in the tumor microenvironment is antigen-specific and restricted to tumor-reactive T cells.
The T cell-inflamed subset of tumors was therefore probed for candidate immune-inhibitory mechanisms that might contribute to T cell dysfunction in situ. Gene expression profiling data revealed the presence of transcripts encoding indoleamine-2,3-dioxygenase (IDO) in these tumors, a factor that had already been demonstrated to contribute to peripheral tolerance [26]. Interrogation for additional candidates revealed that these tumors additionally expressed PD-L1 and Foxp3 transcripts [27,28]. Quantitative analysis of individual tumors revealed that the expression level of each of these three transcripts was significantly correlated, and that the degree of expression was also associated with T cell markers. IHC confirmed that PD-L1 and IDO protein expression, and also nuclear Foxp3+CD4+ cells, were found within T cell-inflamed tumors in the same region as CD8+ T cells. However, non-T cell-inflamed melanomas generally lacked these factors. These observations suggested that these immune suppressive mechanisms might not be a property of the tumor cells themselves but rather immune-intrinsic negative feedback processes that follow the recruitment of activated CD8+ T cells. Indeed, mouse mechanistic studies confirmed that CD8+ T cells were required for the upregulation of all of these three factors within the tumor microenvironment. For PD-L1 and IDO induction, the requisite factor produced by the CD8+ T cells was IFN-γ. For FoxP3+ Tregs, production of the chemokine CCL22 was identified, which mediated Treg recruitment into tumor sites [28]. Using laser capture microdissection, a correlation between IFN-γ production by TILs and local PD-L1 expression also was observed by Taube and colleagues in human tumors [29], supporting the notion that infiltrating T cells become activated by specific antigen and consequently produce IFN-γ and upregulate PD-L1 expression. The fact that these immune evasion mechanisms are part of the host response implies that targeting these pathways therapeutically should have an increased likelihood of efficacy because they are less dependent on tumor cell properties and the associated mutability that can frequently lead to therapeutic resistance.
In contrast to the rich set of immune genes expressed in the T cell-inflamed tumor microenvironment phenotype, the non-T cell-inflamed tumors lacked this broad signature. In particular, there is a lack of T cell markers and of chemokines that can mediate T cell recruitment [18]. These tumors still contain macrophages and vascular endothelial cells, and work from others has indicated the presence of fibroblasts and extracellular matrix, and in some cases immature dendritic cells [30-34]}. It is not yet certain whether tumors that lack spontaneous T cell infiltration are defective only at the level of initial T cell priming against tumor antigens or whether there are additional mechanisms that exclude activated T cells from migrating into the tumor microenvironment, but is seems plausible that both processes may be operational.
Baseline T cell infiltration and therapeutic efficacy of checkpoint blockade
The original hypothesis in the context of melanoma vaccine studies was that the patients showing clinical benefit might have low expression of immune inhibitory mechanisms in the tumor microenvironment whereas the resistant patients might show the highest expression. However, the opposite pattern was paradoxically observed. A baseline T cell-inflamed tumor microenvironment (that includes the presence of PD-L1, IDO, and Treg cells) was positively associated with clinical benefit from these vaccines [19,20,35-37]. Thus, the ability of a melanoma tumor microenvironment to support chemokine production and to recruit activated T cells appears to be instrumental for clinical benefit when it does occur. Viewed from another perspective, the presence of a smoldering immune response that is held in check by negative regulation provides at least a minimal host immune response that can be manipulated to restore an immunologic advantage through therapeutic intervention. Still, not all patients with the T cell-inflamed tumor microenvironment phenotype respond to vaccines, arguing that the defined immune suppressive mechanisms still constitute a functional barrier in many of those cases.
Similar to results obtained with vaccine trials, a baseline T cell-inflamed tumor microenvironment phenotype has been reported to be associated with clinical response to the anti-CTLA-4 mAb ipilimumab [38]. This includes a positive correlation between Foxp3 and IDO expression and clinical benefit [39], which initially seemed paradoxical as mentioned above. However, these data support the notion that these immune suppressive factors are markers of ongoing inflammation and are indicative of the capability of activated T cells to home into tumor sites. The early analyses of patients treated with anti-PD-1 mAb indicated that clinical responses were preferentially seen in patients with high baseline expression of PD-L1 within the tumor microenvironment [29,40], which as mentioned above is indicative of local T cell infiltration and IFN-γ production. More recently, clinical response with anti-PD-1 in melanoma was found more directly to be associated with pre-existing T cell infiltrates in the region of PD-L1 upregulation [41]. Following anti-PD-1 administration, these CD8+ T cells seemed to proliferate and expand to penetrate throughout the tumor, which was correlated with tumor regression [41]. Consistent with these clinical observations, mouse model data have indicated that tumor regression upon checkpoint blockade was almost completely mediated by re-activation of CD8+ T cells directly within the tumor site, which was dominated by restoration of IL-2 production and proliferation [42]. In these studies, the therapeutic activity of checkpoint blockade was largely preserved even when blockade of new T cell exit from lymph nodes was achieved using the S1P receptor inhibitor FTY720 [42]. These results indicate that the majority of tumor regression resulting from checkpoint blockade can be accounted for by reactivation of T cells that are already present within the tumor site. Taken together, these observations point toward the critical role for a baseline spontaneous anti-tumor CD8+ T cell response to provide a substrate for restoration of immune-mediated tumor control following several distinct immunotherapeutic interventions in patients. A corollary to this notion is that patients who lack any T cell infiltrate at baseline are unlikely to respond clinically to these therapies.
Mechanism of innate immune sensing driving spontaneous adaptive immunity: role of type I IFNs
Given the functional importance of spontaneous T cell priming against tumor-associated antigens as a predictive biomarker for clinical response to immunotherapies, understanding the mechanism by which this occurs has become paramount. In general, in order for productive T cell responses to be induced against specific antigens, dendritic cells (DCs) or other antigen-presenting cells (APCs) need to be activated by additional molecular entities. In the setting of pathogen infections, this is often mediated through stimulation of Toll-like receptors (TLRs) by pathogen-associated molecular patterns (PAMPs), such as endotoxin that engages TLR4 [43]. However, which factors might provide innate immune signaling in the context of sterile tumors in the absence of infection had been poorly understood. Clues regarding innate immune pathways that might be involved with spontaneous induction of an adaptive T cell response against tumors were gleaned from gene expression profiling studies of tumor biopsies. Melanoma metastases containing evidence for T cell infiltration also showed expression of a type I IFN gene signature [18,44]. A similar composite gene expression profile also was predictive of favorable clinical outcome with therapeutic cancer vaccines [19,20]. A gene expression profile including a type I IFN signature has been found to have positive prognostic value in breast cancer [45-47], and also has been identified in patients undergoing the evolution of vitiligo [48]. Together, these correlative data suggested that type I IFN production might be integrally involved with adaptive T cell responses against tumor antigens. Mechanistic experiments in mouse models demonstrated an increased incidence of tumors induced by the carcinogen methylcholanthrene in type I IFNR−/− mice [49]. In transplantable tumor systems, type I IFNR−/− mice, or mice deficient in the downstream transcription factor Stat1, showed markedly reduced T cell responses against tumor antigens in vivo [44,50]. The requirement for type I IFN signaling was mapped to the level of APCs, and in particular to the Batf3-driven lineage DC subset, which encompasses DCs expressing CD8α or CD103 [44,50,51]. Together, these data suggest that early innate immune recognition of cancer cells in vivo involves activation of a pathway that leads to IFN-β production by DCs, which in turn is necessary for productive cross-priming of CD8+ T cells via the subset of Batf3-lineage DCs [52].
STING pathway as innate immune sensing to drive type I IFN production and adaptive immunity
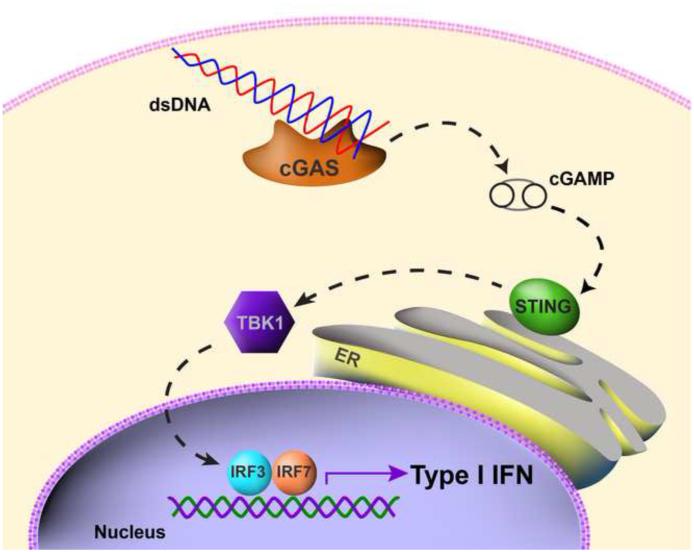
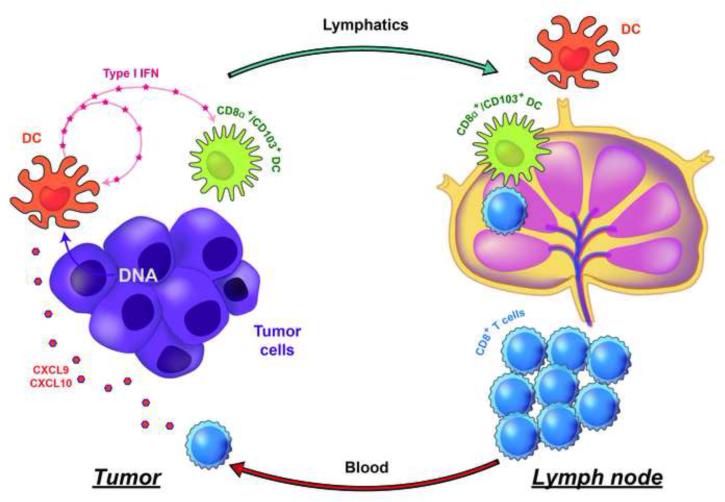
Given the evidence indicating that I IFN production was necessary for optimal T cell priming against tumor antigens, a next critical mechanistic question has been to identify the receptor system and putative ligands that trigger IFN-β production by host DCs in response to a growing tumor in vivo. Recent evidence has pointed toward a critical role for the STING pathway in this process. STING is an adapter that is activated by cyclic dinucelotides generated by cGAS, which in turn is directly activated by cytosolic DNA [53-55]. Activated STING forms aggregates in a peri-nuclear region and leads to activation of the kinase TBK1, which in turn phosphorylates IRF3 that directly contributes to type I IFN gene transcription (Figure 1). This pathway has been implicated in the sensing of DNA viruses, but also in selected autoimmune models [56,57]. Moreover, activating mutations of STING have recently been identified in human patients with a vasculitis/pulmonary inflammation syndrome that is characterized by increased type I IFN production [58]. Mechanistic studies using mouse transplantable tumor models revealed that STING−/− mice and IRF3−/− mice showed defective spontaneous T cell priming against tumor antigens in vivo, and rejection of immunogenic tumors was ablated [59]. Tumor-derived DNA was found within the cytosol of a major population of tumor-infiltrating DCs, which was associated with STING pathway activation and IFN-β production. Therefore, the host STING pathway appears to be a major innate immune sensing pathway that detects the presence of a tumor to drive DC activation and subsequent T cell priming against tumor-associated antigens in vivo. A summary of these processes is illustrated in Figure 2.
Figure 1. Model for STING pathway activation by cytosolic DNA.
The appearance of DNA in the cytosol engages cGAS, which generates intracellular cyclic dinucleotides as a second messenger. This results in aggregation of STING, which leads to TBK1 phosphorylation and activation, which in turn phosphorylates the transcription factor IRF3. The latter contributes directly to transcription of type I IFN genes.
Figure 2. Working model for innate immune sensing leading to spontaneous anti-tumor T cell responses in vivo.
Tumor-derived DNA, presumably generated during tumor cell stress or death, can be found within the cytosol of intratumoral DCs. This is associated with STING pathway activation and IFN-β production. The use of gene-targeted mice has revealed a critical role for STING, IRF3, type I IFN production and sensing, and the Batf3-lineage of DCs for spontaneous anti-tumor T cell responses in vivo. STING pathway activation also leads to chemokine production, which likely contributes to effector T cell recruitment into the inflamed tumor microenvironment.
A functional role for the STING pathway in vivo has also been reported in other mouse tumor systems. An inducible glioma model utilizing a sleeping beauty transposon system was shown to result in induction of a type I IFN gene signature as part of the host response. This induction was substantially reduced in STING−/− mice, and tumors grew more aggressively leading to shorter mouse survival. Exogenous delivery of cyclic dinucleotides as STING agonists exerted a therapeutic effect in vivo [60]. A critical role for host type I IFNs and the host STING pathway was confirmed in the B16.OVA and EL4.OVA models in response to cryoablation. Interestingly, the mechanisms involved paralleled what was observed in the Bm12 mouse model of lupus, as host STING was also required for maximal production of anti-DNA Abs [61]. Thus, the anti-tumor immune response triggered in part by tumor DNA has overlap with the mechanisms involved in autoimmunity driven by extracellular DNA. A role for STING also has been explored in an inducible colon cancer model. Using an AOM/DSS-induced colitis model that gives rise to intestinal tumors, STING-deficient hosts were shown to display markedly increased tumor formation with accelerated kinetics [62]. In this study, STING−/− mice were found to show decreased IL-18 production but increased IL-6 levels, the latter which has been shown to promote Stat3 activation within intestinal epithelial cells. . This study also performed intestinal microbiome sequencing and failed to find differences in commensal bacteria that could account for different levels of inflammation. Thus, STING-dependent innate immunity appears to control tumor development in this model. However, in other models of inflammation-induced cancer, type I IFNs have been shown to contribute to carcinogenesis [63]. As such, using a system in which DMBA is applied to the skin of mice, induction of STING pathway activation was observed to occur early during carcinogenesis. Tumor development was ablated in STING−/− hosts, arguing that activation of this pathway is a necessary component of inflammation-induced carcinogenesis in some settings [64].
Therapeutic implications of the STING pathway in cancer
There are two major clinical implications of the findings that the host STING pathway is critical for innate immune sensing of tumors. First, it seems likely that the ability of a cancer in an individual patient to support STING pathway activation is linked to the spontaneous generation of a T cell-inflamed tumor microenvironment. Because this phenotype is associated with improved prognosis of early stage cancer patients, and also with clinical response to immunotherapies in the metastatic setting, then failed STING activation may represent an early functional block, and therefore itself may have prognostic/predictive value as a biomarker. Such studies could proceed, for example, by enumerating the frequency of Batf3-lineage DCs, or the fraction of tumor-infiltrating DCs expressing phosphorylated TBK1/IRF3. Second, strategies that activate or mimic the output of the host STING pathway should have immunotherapeutic potential in the clinic. Inasmuch as non-T cell-inflamed tumors appear to lack evidence of a type I IFN transcriptional signature, strategies to promote robust innate signaling via APCs in the tumor microenvironment might facilitate improved cross-priming of tumor antigen-specific CD8+ T cells and also augment chemokine production for subsequent effector T cell trafficking. Recent studies have pursued intratumoral injection of STING agonists to engage this pathway directly. 5,6-Dimethylxanthenone-4-acetic acid (DMXAA) is a flavonoid compound that had previously been shown to have anti-tumor activity in mouse models [65]. This drug ultimately failed in humans when combined with chemotherapy in a Phase 3 trial in non-small cell lung cancer [66]. Structure-function studies of mouse and human STING demonstrated that DMXAA directly binds mouse STING but not human STING [67,68], explaining the lack of clinical activity of this compound. Preliminary findings from our group have confirmed that DMXAA is a strong agonist of the mouse STING pathway in vitro and in vivo, and intratumoral injection of DMXAA augmented endogenous priming of tumor antigen-specific CD8+ T cells and had dramatic anti-tumor activity via a mechanism that was dependent on host STING. Development of new STING agonists that stimulate all known human STING polymorphic variants should be considered for clinical translation
A second approach for promoting innate immune activation directly within the tumor microenvironment has been to provide an increased local concentration of type I IFNs. A major challenge is developing strategies for systemic delivery of type I IFNs that give rise to local accumulation at tumor sites. One consideration is by employing tumor-targeting monoclonal antibodies (mAbs) coupled to IFN-β as a payload. Indeed, either anti-Her2 or anti-EGFR mAbs coupled to IFN-β led to tumor regression in tumor models expressing the corresponding receptors, through a mechanism that was dependent on host T cells [69]. Conditional type I IFNR−/− mice lacking type I IFN signaling specifically on CD11c+ cells lost the therapeutic effect with this strategy, arguing that host immune priming was an essential component [69]. Thus, transient expression of low doses of IFN-β within the tumor microenvironment appears to facilitate adaptive immunity to tumors. Interestingly, the mechanism of anti-tumor activity of type I IFNs may vary depending on the delivery strategy utilized, which likely is related to the dose and duration of type I IFN presence within the tumor microenvironment. Transfection of B16 melanoma cells to express high levels of IFN-β led to tumor regression that was largely independent of host adaptive immunity [70]. Rather, this approach led to an elimination of the tumor vasculature, consistent with a potent anti-angiogenic effect. Most of the therapeutic effect was preserved in RAG−/− and NK cell-depleted mice, and conditional type I IFNR−/− mice lacking type I IFN signaling exclusively on vascular endothelial cells lost the therapeutic effect of high-dose IFN-β [70]. Optimal combinations with type I IFNs and T cell-directed immunotherapies in the future may depend on a careful consideration of the dose and schedule of IFN-α or IFN-β being used.
An additional option for promoting appropriate innate immune activation in the tumor microenvironment is through targeted radiation. Directed radiation to the tumor site also appears to induce type I IFN production, to augment specific T cell priming, and to support T cell-mediated tumor control [71]. Recent work has indicated that the mechanism by which radiation therapy induces type I IFN production and adaptive T cell responses against tumor antigens also depends on the host STING pathway [72]. Thus, radiation may facilitate the proper acquisition of tumor-derived DNA by host DCs in the tumor microenvironment, thereby leading to improved T cell priming as well as coordination of the effector phase of the anti-tumor immune response. The mechanism by which DCs uptake and sort tumor-derived material in this setting are not clear, but could involve the recently characterized receptors Clec9A or Dectin-1 [73,74].
Concluding Remarks
As a relatively new area of investigation, numerous unanswered questions remain regarding the role of the STING pathway in anti-tumor immune response in vivo (see Box 1). First, the mechanism by which DNA can be derived from dying tumor cells and gain access to the cytosol of host APCs is not yet understood. Free DNA alone does not activate DCs in vitro but requires the addition of Lipofectamine or another transfection reagent [59]. In principle, extracellular DNA would be degraded by DNAse I in the serum, and also by DNAse II within lysosomes of APCs. Therefore, it seems likely that tumor-derived DNA might need to be protected in order to escape this degradation. A plausible mechanism could be through packaging in a lipid-bound vesicle, such as a type of exosome. Future work will be required to identify this mechanism, which could ultimately shed light on the process by which cross-presentation of protein antigens to generate class I MHC-presented peptides occurs, which also requires access to the cytosol and remains poorly understood from the cell biology perspective. Understanding the mechanism by which this occurs in T cell-inflamed tumors should also highlight possible blocks in the non-T cell-inflamed subset of tumors. Second, while work to date has focused on the role of the STING pathway within APCs, it is conceivable that the STING pathway could be functionally relevant in other cell types. In the tumor microenvironment, it is critical to assess whether vascular endothelial cells, fibroblasts, or even tumor cells themselves might be capable of activating the pathway, in particular in response to STING agonists. Third, while it appears that radiation therapy of tumors can initiate host immune response via STING pathway activation and type I IFNs, it is not known to what extent this occurs with other conventional cancer therapeutics. Thus, investigating the functional role of the host STING pathway and type I IFNs in the setting of administration of specific chemotherapeutic agents or targeted inhibitors of mutated kinases will be of paramount importance, and the mechanisms might be distinct [75]. These considerations are especially important for planning future combinations between immunotherapies and conventional cancer therapies. Fourth, additional aspects of the biology of the STING pathway in humans need to be better understood. The human STING gene is polymorphic [76], and it is not known whether these polymorphisms might be associated with clinical outcome from cancer immunotherapies such as anti-PD-1 mAb. In addition, there is limited knowledge regarding negative regulators of the STING pathway, which could be envisioned as part of a negative feedback loop controlling the duration of innate immune activation. A greater understanding of potential feedback regulation could point towards additional opportunities for therapeutic targeting.
Box 1. Outstanding Questions.
What is the mechanism by which tumor-derived DNA gains access to the cytosol of DCs?
Is the STING pathway activated in other cell types in the tumor microenvironment besides APCs?
Do conventional cancer therapies indirectly activate the STING pathway?
Are human STING polymorphisms associated with clinical outcome?
Is STING pathway activation within tumor-infiltrating DCs associated with favorable clinical outcome?
Are there negative regulatory factors that control the STING pathway that might be pharmacologically manipulated?
Highlights.
A T cell-inflamed tumor microenvironment is associated with benefit to immunotherapies
The mechanism of endogenous T cell priming against tumor antigens had been elusive
Mechanistic data indicate a critical role for type I IFNs as a bridge to adaptive immunity
Recent data implicate the host STING pathway in innate immune sensing of tumors
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van den Eynde B, Knuth A, Boon T. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643–1647. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 2.Robbins PF, Lu YC, El-Gamil M, Li YF, Gross C, Gartner J, Lin JC, Teer JK, Cliften P, Tycksen E, et al. Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nat Med. 2013 doi: 10.1038/nm.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Rooij N, van Buuren MM, Philips D, Velds A, Toebes M, Heemskerk B, van Dijk LJ, Behjati S, Hilkmann H, El Atmioui D, et al. Tumor exome analysis reveals neoantigen-specific T-cell reactivity in an ipilimumab-responsive melanoma. J Clin Oncol. 2013;31:e439–442. doi: 10.1200/JCO.2012.47.7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gubin MM, Zhang X, Schuster H, Caron E, Ward JP, Noguchi T, Ivanova Y, Hundal J, Arthur CD, Krebber WJ, et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515:577–581. doi: 10.1038/nature13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Linnemann C, van Buuren MM, Bies L, Verdegaal EM, Schotte R, Calis JJ, Behjati S, Velds A, Hilkmann H, Atmioui DE, et al. High-throughput epitope discovery reveals frequent recognition of neo-antigens by CD4+ T cells in human melanoma. Nat Med. 2015;21:81–85. doi: 10.1038/nm.3773. [DOI] [PubMed] [Google Scholar]
- 6.Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Jr., Kinzler KW. Cancer genome landscapes. Science. 2013;339:1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 8.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 9.Mlecnik B, Tosolini M, Kirilovsky A, Berger A, Bindea G, Meatchi T, Bruneval P, Trajanoski Z, Fridman WH, Pages F, et al. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol. 2011;29:610–618. doi: 10.1200/JCO.2010.30.5425. [DOI] [PubMed] [Google Scholar]
- 10.Azimi F, Scolyer RA, Rumcheva P, Moncrieff M, Murali R, McCarthy SW, Saw RP, Thompson JF. Tumor-infiltrating lymphocyte grade is an independent predictor of sentinel lymph node status and survival in patients with cutaneous melanoma. J Clin Oncol. 2012;30:2678–2683. doi: 10.1200/JCO.2011.37.8539. [DOI] [PubMed] [Google Scholar]
- 11.Kreike B, van Kouwenhove M, Horlings H, Weigelt B, Peterse H, Bartelink H, van de Vijver MJ. Gene expression profiling and histopathological characterization of triple-negative/basal-like breast carcinomas. Breast Cancer Res. 2007;9:R65. doi: 10.1186/bcr1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, Ellis IO, Green AR. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol. 2011;29:1949–1955. doi: 10.1200/JCO.2010.30.5037. [DOI] [PubMed] [Google Scholar]
- 13.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 14.Rusakiewicz S, Semeraro M, Sarabi M, Desbois M, Locher C, Mendez R, Vimond N, Concha A, Garrido F, Isambert N, et al. Immune Infiltrates Are Prognostic Factors in Localized Gastrointestinal Stromal Tumors. Cancer Res. 2013;73:3499–3510. doi: 10.1158/0008-5472.CAN-13-0371. [DOI] [PubMed] [Google Scholar]
- 15.Bui JD, Schreiber RD. Cancer immunosurveillance, immunoediting and inflammation: independent or interdependent processes? Curr Opin Immunol. 2007;19:203–208. doi: 10.1016/j.coi.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Peterson AC, Harlin H, Gajewski TF. Immunization with Melan-A peptide-pulsed peripheral blood mononuclear cells plus recombinant human interleukin-12 induces clinical activity and T-cell responses in advanced melanoma. J Clin Oncol. 2003;21:2342–2348. doi: 10.1200/JCO.2003.12.144. [DOI] [PubMed] [Google Scholar]
- 17.Gajewski TF, Meng Y, Blank C, Brown I, Kacha A, Kline J, Harlin H. Immune resistance orchestrated by the tumor microenvironment. Immunol Rev. 2006;213:131–145. doi: 10.1111/j.1600-065X.2006.00442.x. [DOI] [PubMed] [Google Scholar]
- 18.Harlin H, Meng Y, Peterson AC, Zha Y, Tretiakova M, Slingluff C, McKee M, Gajewski TF. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res. 2009;69:3077–3085. doi: 10.1158/0008-5472.CAN-08-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gajewski TF, Louahed J, Brichard VG. Gene signature in melanoma associated with clinical activity: a potential clue to unlock cancer immunotherapy. Cancer J. 2010;16:399–403. doi: 10.1097/PPO.0b013e3181eacbd8. [DOI] [PubMed] [Google Scholar]
- 20.Ulloa-Montoya F, Louahed J, Dizier B, Gruselle O, Spiessens B, Lehmann FF, Suciu S, Kruit WH, Eggermont AM, Vansteenkiste J, et al. Predictive gene signature in MAGE-A3 antigen-specific cancer immunotherapy. J Clin Oncol. 2013;31:2388–2395. doi: 10.1200/JCO.2012.44.3762. [DOI] [PubMed] [Google Scholar]
- 21.Harlin H, Kuna TV, Peterson AC, Meng Y, Gajewski TF. Tumor progression despite massive influx of activated CD8(+) T cells in a patient with malignant melanoma ascites. Cancer Immunol Immunother. 2006;55:1185–1197. doi: 10.1007/s00262-005-0118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mortarini R, Piris A, Maurichi A, Molla A, Bersani I, Bono A, Bartoli C, Santinami M, Lombardo C, Ravagnani F, et al. Lack of terminally differentiated tumor-specific CD8+ T cells at tumor site in spite of antitumor immunity to self-antigens in human metastatic melanoma. Cancer Res. 2003;63:2535–2545. [PubMed] [Google Scholar]
- 23.Zippelius A, Batard P, Rubio-Godoy V, Bioley G, Lienard D, Lejeune F, Rimoldi D, Guillaume P, Meidenbauer N, Mackensen A, et al. Effector function of human tumor-specific CD8 T cells in melanoma lesions: a state of local functional tolerance. Cancer Res. 2004;64:2865–2873. doi: 10.1158/0008-5472.can-03-3066. [DOI] [PubMed] [Google Scholar]
- 24.Rosenberg SA, Dudley ME. Cancer regression in patients with metastatic melanoma after the transfer of autologous antitumor lymphocytes. Proc Natl Acad Sci U S A. 2004;101 Suppl 2:14639–14645. doi: 10.1073/pnas.0405730101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaufman HL, Kim DW, DeRaffele G, Mitcham J, Coffin RS, Kim-Schulze S. Local and distant immunity induced by intralesional vaccination with an oncolytic herpes virus encoding GM-CSF in patients with stage IIIc and IV melanoma. Ann Surg Oncol. 2010;17:718–730. doi: 10.1245/s10434-009-0809-6. [DOI] [PubMed] [Google Scholar]
- 26.Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Brown C, Mellor AL. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 27.Gajewski TF. Failure at the effector phase: immune barriers at the level of the melanoma tumor microenvironment. Clin Cancer Res. 2007;13:5256–5261. doi: 10.1158/1078-0432.CCR-07-0892. [DOI] [PubMed] [Google Scholar]
- 28.Spranger S, Spaapen R, Zha Y, Williams J, Meng Y, Ha TT, Gajewski TF. Upregulation of PD-L1, IDO and Tregs in the melanoma tumor microenvironment is driven by CD8+ T cells. Science Translational Medicine. 2013 doi: 10.1126/scitranslmed.3006504. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4:127ra137. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kraman M, Bambrough PJ, Arnold JN, Roberts EW, Magiera L, Jones JO, Gopinathan A, Tuveson DA, Fearon DT. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science. 2010;330:827–830. doi: 10.1126/science.1195300. [DOI] [PubMed] [Google Scholar]
- 31.Salmon H, Franciszkiewicz K, Damotte D, Dieu-Nosjean MC, Validire P, Trautmann A, Mami-Chouaib F, Donnadieu E. Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J Clin Invest. 2012;122:899–910. doi: 10.1172/JCI45817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Demoulin S, Herfs M, Delvenne P, Hubert P. Tumor microenvironment converts plasmacytoid dendritic cells into immunosuppressive/tolerogenic cells: insight into the molecular mechanisms. J Leukoc Biol. 2013;93:343–352. doi: 10.1189/jlb.0812397. [DOI] [PubMed] [Google Scholar]
- 33.Watkins SK, Zhu Z, Riboldi E, Shafer-Weaver KA, Stagliano KE, Sklavos MM, Ambs S, Yagita H, Hurwitz AA. FOXO3 programs tumor-associated DCs to become tolerogenic in human and murine prostate cancer. J Clin Invest. 2011;121:1361–1372. doi: 10.1172/JCI44325. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Buckanovich RJ, Facciabene A, Kim S, Benencia F, Sasaroli D, Balint K, Katsaros D, O'Brien-Jenkins A, Gimotty PA, Coukos G. Endothelin B receptor mediates the endothelial barrier to T cell homing to tumors and disables immune therapy. Nat Med. 2008;14:28–36. doi: 10.1038/nm1699. [DOI] [PubMed] [Google Scholar]
- 35.Gajewski TF, Meng Y, Harlin H. Chemokines expressed in melanoma metastases associated with T cell infiltration. Journal of Clinical Oncology, 2007 ASCO Annual Meeting Proceedings Part I. 2007;Vol 25:8501. [Google Scholar]
- 36.Gajewski TF, Zha Y, Thurner B, Schuler G. Association of gene expression profile in melanoma and survival to a dendritic cell-based vaccine. J. Clin. Oncol. 2009;27:9002. [Google Scholar]
- 37.Vansteenkiste JF, Zielinski M, Dahabreh IJ, Linder A, Lehmann F, Gruselle O, Therasse P, Louahed J, Brichard VG. Association of gene expression signature and clinical efficacy of MAGE-A3 antigen-specific cancer immunotherapeutic (ASCI) as adjuvant therapy in resected stage IB/II non-small cell lung cancer (NSCLC). J Clin Oncol. 2008;26 Abstract 7501. [Google Scholar]
- 38.Ji RR, Chasalow SD, Wang L, Hamid O, Schmidt H, Cogswell J, Alaparthy S, Berman D, Jure-Kunkel M, Siemers NO, et al. An immune-active tumor microenvironment favors clinical response to ipilimumab. Cancer Immunol Immunother. 2011 doi: 10.1007/s00262-011-1172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hamid O, Chasalow SD, Tsuchihashi Z, Alaparthy S, Galbraith S, Berman D. Association of baseline and on-study tumor biopsy markers with clinical activity in patients with advanced melanoma treated with ipilimumab. J. Clin. Oncol. 2009;27 Abstract 9008. [Google Scholar]
- 40.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, Activity, and Immune Correlates of Anti-PD-1 Antibody in Cancer. N Engl J Med. 2012 doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spranger S, Koblish HK, Horton B, Scherle PA, Newton R, Gajewski TF. Mechanism of tumor rejection with doublets of CTLA-4, PD-1/PD-L1, or IDO blockade involves restored IL-2 production and proliferation of CD8(+) T cells directly within the tumor microenvironment. J Immunother Cancer. 2014;2:3. doi: 10.1186/2051-1426-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 44.Fuertes MB, Kacha AK, Kline J, Woo SR, Kranz DM, Murphy KM, Gajewski TF. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. J Exp Med. 2011 doi: 10.1084/jem.20101159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Callari M, Musella V, Di Buduo E, Sensi M, Miodini P, Dugo M, Orlandi R, Agresti R, Paolini B, Carcangiu ML, et al. Subtype-dependent prognostic relevance of an interferon-induced pathway metagene in node-negative breast cancer. Mol Oncol. 2014;8:1278–1289. doi: 10.1016/j.molonc.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bertucci F, Ueno NT, Finetti P, Vermeulen P, Lucci A, Robertson FM, Marsan M, Iwamoto T, Krishnamurthy S, Masuda H, et al. Gene expression profiles of inflammatory breast cancer: correlation with response to neoadjuvant chemotherapy and metastasis-free survival. Ann Oncol. 2014;25:358–365. doi: 10.1093/annonc/mdt496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ascierto ML, Kmieciak M, Idowu MO, Manjili R, Zhao Y, Grimes M, Dumur C, Wang E, Ramakrishnan V, Wang XY, et al. A signature of immune function genes associated with recurrence-free survival in breast cancer patients. Breast Cancer Res Treat. 2012;131:871–880. doi: 10.1007/s10549-011-1470-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bertolotti A, Boniface K, Vergier B, Mossalayi D, Taieb A, Ezzedine K, Seneschal J. Type I interferon signature in the initiation of the immune response in vitiligo. Pigment Cell Melanoma Res. 2014;27:398–407. doi: 10.1111/pcmr.12219. [DOI] [PubMed] [Google Scholar]
- 49.Dunn GP, Bruce AT, Sheehan KC, Shankaran V, Uppaluri R, Bui JD, Diamond MS, Koebel CM, Arthur C, White JM, et al. A critical function for type I interferons in cancer immunoediting. Nat Immunol. 2005;6:722–729. doi: 10.1038/ni1213. [DOI] [PubMed] [Google Scholar]
- 50.Diamond MS, Kinder M, Matsushita H, Mashayekhi M, Dunn GP, Archambault JM, Lee H, Arthur CD, White JM, Kalinke U, et al. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med. 2011;208:1989–2003. doi: 10.1084/jem.20101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M, Calderon B, Schraml BU, Unanue ER, Diamond MS, et al. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322:1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fuertes MB, Woo SR, Burnett B, Fu YX, Gajewski TF. Type I interferon response and innate immune sensing of cancer. Trends Immunol. 2013;34:67–73. doi: 10.1016/j.it.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455:674–678. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339:786–791. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang X, Shi H, Wu J, Sun L, Chen C, Chen ZJ. Cyclic GMP-AMP containing mixed phosphodiester linkages is an endogenous high-affinity ligand for STING. Mol Cell. 2013;51:226–235. doi: 10.1016/j.molcel.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ahn J, Gutman D, Saijo S, Barber GN. STING manifests self DNA-dependent inflammatory disease. Proc Natl Acad Sci U S A. 2012;109:19386–19391. doi: 10.1073/pnas.1215006109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ahn J, Ruiz P, Barber GN. Intrinsic Self-DNA Triggers Inflammatory Disease Dependent on STING. J Immunol. 2014;193:4634–4642. doi: 10.4049/jimmunol.1401337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu Y, Jesus AA, Marrero B, Yang D, Ramsey SE, Montealegre Sanchez GA, Tenbrock K, Wittkowski H, Jones OY, Kuehn HS, et al. Activated STING in a vascular and pulmonary syndrome. N Engl J Med. 2014;371:507–518. doi: 10.1056/NEJMoa1312625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Woo S-R, Fuertes Mercedes B, Corrales L, Spranger S, Furdyna Michael J, Leung Michael YK, Duggan R, Wang Y, Barber Glen N, Fitzgerald Katherine A, et al. STING-Dependent Cytosolic DNA Sensing Mediates Innate Immune Recognition of Immunogenic Tumors. Immunity. 2014;41:830–842. doi: 10.1016/j.immuni.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ohkuri T, Ghosh A, Kosaka A, Zhu J, Ikeura M, David M, Watkins SC, Sarkar SN, Okada H. STING Contributes to Antiglioma Immunity via Triggering Type I IFN Signals in the Tumor Microenvironment. Cancer Immunol Res. 2014 doi: 10.1158/2326-6066.CIR-14-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Klarquist J, Hennies CM, Lehn MA, Reboulet RA, Feau S, Janssen EM. STING-Mediated DNA Sensing Promotes Antitumor and Autoimmune Responses to Dying Cells. J Immunol. 2014;193:6124–6134. doi: 10.4049/jimmunol.1401869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhu Q, Man SM, Gurung P, Liu Z, Vogel P, Lamkanfi M, Kanneganti TD. Cutting Edge: STING Mediates Protection against Colorectal Tumorigenesis by Governing the Magnitude of Intestinal Inflammation. J Immunol. 2014;193:4779–4782. doi: 10.4049/jimmunol.1402051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Trinchieri G. Type I interferon: friend or foe? J Exp Med. 2010;207:2053–2063. doi: 10.1084/jem.20101664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ahn J, Xia T, Konno H, Konno K, Ruiz P, Barber GN. Inflammation-driven carcinogenesis is mediated through STING. Nat Commun. 2014;5:5166. doi: 10.1038/ncomms6166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baguley BC, Ching LM. Immunomodulatory actions of xanthenone anticancer agents. BioDrugs. 1997;8:119–127. doi: 10.2165/00063030-199708020-00005. [DOI] [PubMed] [Google Scholar]
- 66.Lara PN, Jr., Douillard JY, Nakagawa K, von Pawel J, McKeage MJ, Albert I, Losonczy G, Reck M, Heo DS, Fan X, et al. Randomized phase III placebo-controlled trial of carboplatin and paclitaxel with or without the vascular disrupting agent vadimezan (ASA404) in advanced non-small-cell lung cancer. J Clin Oncol. 2011;29:2965–2971. doi: 10.1200/JCO.2011.35.0660. [DOI] [PubMed] [Google Scholar]
- 67.Conlon J, Burdette DL, Sharma S, Bhat N, Thompson M, Jiang Z, Rathinam VA, Monks B, Jin T, Xiao TS, et al. Mouse, but not human STING, binds and signals in response to the vascular disrupting agent 5,6-dimethylxanthenone-4-acetic acid. J Immunol. 2013;190:5216–5225. doi: 10.4049/jimmunol.1300097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gao P, Ascano M, Zillinger T, Wang W, Dai P, Serganov AA, Gaffney BL, Shuman S, Jones RA, Deng L, et al. Structure-function analysis of STING activation by c[G(2',5')pA(3',5')p] and targeting by antiviral DMXAA. Cell. 2013;154:748–762. doi: 10.1016/j.cell.2013.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang X, Zhang X, Fu ML, Weichselbaum RR, Gajewski TF, Guo Y, Fu YX. Targeting the tumor microenvironment with interferon-beta bridges innate and adaptive immune responses. Cancer Cell. 2014;25:37–48. doi: 10.1016/j.ccr.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Spaapen RM, Leung MY, Fuertes MB, Kline JP, Zhang L, Zheng Y, Fu YX, Luo X, Cohen KS, Gajewski TF. Therapeutic Activity of High-Dose Intratumoral IFN-beta Requires Direct Effect on the Tumor Vasculature. J Immunol. 2014;193:4254–4260. doi: 10.4049/jimmunol.1401109. [DOI] [PubMed] [Google Scholar]
- 71.Burnette BC, Liang H, Lee Y, Chlewicki L, Khodarev NN, Weichselbaum RR, Fu YX, Auh SL. The efficacy of radiotherapy relies upon induction of type i interferon-dependent innate and adaptive immunity. Cancer Res. 2011;71:2488–2496. doi: 10.1158/0008-5472.CAN-10-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, Li X-D, Mauceri H, Beckett M, Darga T, et al. STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity. 2014;41:843–852. doi: 10.1016/j.immuni.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ahrens S, Zelenay S, Sancho D, Hanc P, Kjaer S, Feest C, Fletcher G, Durkin C, Postigo A, Skehel M, et al. F-actin is an evolutionarily conserved damage-associated molecular pattern recognized by DNGR-1, a receptor for dead cells. Immunity. 2012;36:635–645. doi: 10.1016/j.immuni.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 74.Chiba S, Ikushima H, Ueki H, Yanai H, Kimura Y, Hangai S, Nishio J, Negishi H, Tamura T, Saijo S, et al. Recognition of tumor cells by Dectin-1 orchestrates innate immune cells for anti-tumor responses. Elife. 2014;3:e04177. doi: 10.7554/eLife.04177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sistigu A, Yamazaki T, Vacchelli E, Chaba K, Enot DP, Adam J, Vitale I, Goubar A, Baracco EE, Remedios C, et al. Cancer cell-autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat Med. 2014;20:1301–1309. doi: 10.1038/nm.3708. [DOI] [PubMed] [Google Scholar]
- 76.Yi G, Brendel VP, Shu C, Li P, Palanathan S, Cheng Kao C. Single nucleotide polymorphisms of human STING can affect innate immune response to cyclic dinucleotides. PLoS One. 2013;8:e77846. doi: 10.1371/journal.pone.0077846. [DOI] [PMC free article] [PubMed] [Google Scholar]