Abstract
Increased bone marrow adiposity is a common feature of advanced age, obesity and associated metabolic pathologies. Augmented numbers of marrow adipocytes positively correlate with dysregulated bone remodeling, also a well-established complication of metastatic disease. We have shown previously that marrow adiposity accelerates prostate tumor progression in the skeleton and promotes extensive destruction of the bone; however, the factors behind adipocyte-driven osteolysis in the skeletal tumor microenvironment are not currently known. In this study, utilizing in vivo diet-induced models of bone marrow adiposity, we reveal evidence for positive correlation between increased marrow fat content, bone degradation by ARCaP(M) and PC3 prostate tumors, and augmented levels of host-derived CXCL1 and CXCL2, ligands of CXCR2 receptor. We show by in vitro osteoclastogenesis assays that media conditioned by bone marrow adipocytes is a significant source of CXCL1 and CXCL2 proteins. We also demonstrate that both the adipocyte-conditioned media and the recombinant CXCL1 and CXCL2 ligands efficiently accelerate osteoclast maturation, a process that can be blocked by neutralizing antibodies to each of the chemokines. We further confirm the contribution of CXCR2 signaling axis to adiposity-driven osteoclastogenesis by blocking fat cell-induced osteoclast differentiation with CXCR2 antagonist or neutralizing antibodies. Together, our results link CXCL1 and CXCL2 chemokines with bone marrow adiposity and implicate CXCR2 signaling in promoting effects of marrow fat on progression of skeletal tumors in bone.
Keywords: bone marrow adipocytes, CXCL1, CXCL2, CXCR2, bone metastasis, prostate cancer
Introduction
Bone is a complex and dynamic organ that plays critical roles in hematopoiesis, inflammation, metabolism, and structural support [1, 2]. Multiple cell types, factors, and events within bone marrow niche participate in maintaining and protecting normal bone homeostasis [1], a state which is often disturbed by diseases associated with inflammation and enhanced bone remodeling, such as osteoporosis, diabetes [3-5], rheumatoid arthritis [6], and metastatic cancers of the breast and prostate [7]. One important component of the bone marrow is the adipocyte, a cell type with capabilities of storing and secreting fatty acids, adipokines, inflammatory factors and performing specialized, metabolically important functions [3, 4, 8]. It is well established that adipocyte numbers in bone marrow niche increase greatly with advanced age, obesity, and associated metabolic pathologies [1, 3, 4]. There is also growing laboratory and clinical evidence that their numbers are inversely correlated with bone mineral density (BMD) [1, 3, 9-12].
Localization of marrow fat to the trabecular area of the bone, the site of active remodeling, is an indication of its involvement in bone degradation [3]. Several different factors have been proposed as links between increased marrow adiposity and dysregulated bone health. They include a shift in mesenchymal cell lineage commitment from osteoblast to adipocyte resulting in reduced bone mass acquisition [8, 10, 13], fatty acid-driven acceleration of osteoclast differentiation and prolonged survival [14], enhancement of PPARγ2 expression [15, 16], and increased levels of pro-osteoclastic cytokines: macrophage colony-stimulating factor (M-CSF) and receptor activator of nuclear factor κB ligand (RANKL) [3, 10]. Whether adipocytes can supply additional factors to support and enhance normal M-CSF/RANKL-driven osteoclastogenesis is not well understood.
Studies in mouse models of high fat diet (HFD)-induced marrow adiposity have linked decreases in trabecular bone volume and reduced BMD with upregulation of bone-degrading proteases such as cathepsin K, escalated levels of proinflammatory cytokines, particularly interleukin 6 (IL-6) and tumor necrosis factor α (TNF-α), and decreases in levels of IL-10, a negative regulator of osteoclastogenesis [11, 12, 17-19]. We have shown recently that levels of two additional host-derived inflammatory factors, CCL2 and COX-2, are highly escalated in mice on HFD [1]. We have also reported that an increase in marrow fat content has promoting effects on growth and progression of skeletal prostate tumors [20], a finding in line with previous studies indicating that fatty marrow is a depot of host-derived factors that create a favorable environment for tumor colonization and progression in bone [21, 22].
One class of chemotactic factors known to promote inflammation and support tumor growth is the CXC chemokine family, specifically CXCL1 and CXCL2 [23]. Both chemokines bind to the G-protein coupled receptor CXCR2 (IL-8RB) expressed on macrophages, neutrophils, and epithelial cells [24-26] and their classical function is to act as chemotactic factors attracting neutrophils to sites of injury [23]. Little is currently known about the potential role(s) of CXCR2 and its ligands in osteoclast formation and maturation. Limited studies to date link CXCR2 signaling with osteoclastogenesis through another CXCR2 ligand, interleukin 8 (IL-8) in human monocytes [27], and only recently begin to point to a possible involvement of CXCL1 and CXCL2 in osteoclast precursor migration and/or differentiation [28, 29]. The activation of CXCR2 and overexpression of its ligands have been noted as one of the key regulatory mechanisms in melanoma, pancreatic cancer, and non-small cell lung carcinoma (reviewed in [30]); however, the function of CXCR2 signaling in adipocyte-driven dysregulation of bone homeostasis, particularly in a context of tumor-induced osteolysis of the bone, is not known.
The objective of the present study was to examine the role of CXCL1/CXCL2 chemokines and their receptor CXCR2 in adiposity-induced osteoclastogenesis and prostate tumor-driven osteolysis of the bone. Using an in vivo diet-induced obesity (DIO) model, a documented approach to induce significant marrow adiposity [1, 4, 11, 18, 20] we demonstrate positive correlation between increased marrow fat content, augmented levels of host-derived CXCL1 and CXCL2, and bone degradation by ARCaP(M) and PC3 prostate tumors. Via in vitro osteoclastogenesis assays, we also show that media conditioned by bone marrow adipocytes accelerates osteoclast differentiation and increases expression of proteolytic genes critical for osteoclast formation and function. We further reveal that bone marrow adipocytes are a significant source of CXCL1 and CXCL2 chemokines, secretion of which is potentiated by adipocyte-tumor cell interactions. We directly confirm the contribution of CXCR2 signaling axis to adipocyte-driven osteoclastogenesis by neutralizing the activity of either the CXCL1/CXCL2 ligands or their receptor. Collectively, our results reveal a new mechanism of bone marrow adipocyte involvement in tumor-driven osteolysis of the bone.
MATERIALS AND METHODS
Materials
Dulbecco’s modified Eagle’s medium (DMEM), Minimum Essential Medium (MEMα), tartrate resistant acid phosphatase (TRAcP) staining kit, and other chemicals, unless otherwise stated, were obtained from Sigma (St. Louis, MO). Fetal bovine serum (FBS) was from Invitrogen (Carlsbad, CA). Rabbit anti-human/mouse Cathepsin K antibody was from Abcam (Cambridge, UK). Rabbit anti-human/mouse α-actin antibody was from Novus Biologicals (Littleton, CO). Monoclonal mouse anti human CXCR2 antibody, recombinant mouse CXCL1 and CXCL2, goat anti-mouse CXCL1 and CXCL2 neutralizing antibodies, macrophage-colony stimulating factor (M-CSF), Receptor Activator of NFκB ligand proteins (RANKL) and Quantikine mouse CXCL1 and CXCL2 ELISA kits were from R&D Systems (Minneapolis, MN). Immunoblotting “Western Lightning ECL Plus” and “Luminata Forte Western HRP Substrate” detection kits were from Perkin Elmer LLC (Waltham, MA) and Millipore (Billerica,MA), respectively. RNeasy Mini Kits were from Qiagen (Valencia, CA).
Animals
All experiments involving mice were performed in accordance with the protocol approved by the institutional Animal Investigational Committee of Wayne State University and NIH guidelines. In vivo xenograft studies were performed in 8- to 10-week old male mice in the FVB/N background with homozygous null mutations in the Rag-1 gene (FVB/N/N5, Rag-1−/−, Cat K+/+). Mice were bred in-house.
Diets
At 5 weeks of age, mice caged in the groups of 4 were started on either a low-fat (LFD; N=9) diet (10% calories from fat; Research Diets no. D12450Bi) or a high-fat (HFD; N=11) diet (60% calories from fat; Research Diets no. D12492i) as previously described [20]. Mice were maintained on respective diets for 8 weeks prior to and 6 to 8 weeks following the tumor implantation into bone (total of 16 weeks).
Cell Lines
PC3, an androgen-independent osteolytic cell line derived from a bone metastasis of a high-grade adenocarcinoma were purchased from American Type Culture Collection (Manassas, VA, USA). ARCaP(M), an androgen-repressed metastatic prostate cancer cells M (‘Mesenchymal’ Clone) were purchased from Novicure Biotechnology (Birmingham, AL, USA). The ARCaP(M)-DsRed cell line was established by stable transfection with pDsRed2-N1 as previously described [31]. L929 cells (source of M-CSF for osteoclast precursors) were cultured in DMEM containing 10% FBS until confluent and conditioned media was collected, centrifuged, and stored at −80 °C until ready for use. Cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% FBS. All cells were maintained in a 37 °C humidified incubator ventilated with 5% CO2.
Intratibial Injections of Tumor Cells
Intratibial tumor injections were performed under isoflurane inhalational anesthesia according to previously published procedures [31]. Briefly, a cell preparation containing 5 × 105 of PC3 or ARCaP(M) cells in PBS (20 μl, right tibia), or PBS alone (control, 20 μl, left tibia) was injected into the bone marrow. Six or eight weeks post-injection (for PC3 and ARCaP(M) cells, respectively) mice were euthanized, and control and tumor-bearing tibiae were removed and imaged ex vivo. X-ray images were obtained using a Carestream XVivo Multimodal Animal Imager. Half of the intratibial tumor samples from each group were then fixed in Z-fix, bone tumors were decalcified, and all samples were embedded in paraffin. The 5μm longitudinal sections from tibiae were deparaffinized, and stained with tartrate resistant acid phosphatase (Sigma) according to the manufacturer’s instructions. Digital images were captured under 5× and 10× magnification using a Zeiss Scope A.1 conventional light microscope with CCD camera. Remaining tissues were snap-frozen in liquid nitrogen, powderized using a tissue pulverizer and RNA was isolated using Trizol and RNeasy Mini Kit.
Bone Marrow Adipocyte-Conditioned Media
Primary mouse bone marrow stromal cells (mBMSC) were isolated from femurs and tibiae of 6- to 8-week old FVB/N mice and induced to become bone marrow adipocytes as previously described [20]. Briefly, mBMSC cells were plated in 3D collagen I gels, grown to confluency for 48-72 hours and treated with adipogenic cocktail (30% StemXVivo Adipogenic Supplement, 1 μM insulin, 2 μM Rosiglitazone; DMEM and 10% FBS) for 8-10 days. Differentiated bone marrow adipocyte cultures were cultured in serum-free DMEM for 12-16 hours and media was collected, centrifuged, and stored at −80°C. Prior to use, serum-free medium collected from adipocyte cultures was diluted 1:1 with MEMα appropriate for osteoclast treatment and designated ‘Adipo CM’. Cells were maintained in a 37°C humidified incubator ventilated with 5% CO2.
Isolation of osteoclast precursor cells
Bone marrow macrophages (BMMs) were differentiated from primary murine bone marrow cells. Bone marrow was flushed from femurs and tibiae of 10 to 12-week-old FVB/N male mice with BMM growth medium (MEMα containing 20% FBS and 30% L929 conditioned media as the source of M-CSF [31]). The cell suspension was plated on Petri dishes and incubated for 4-5 days to obtain differentiated bone marrow macrophages (BMMs).
Osteoclast Formation for Tartrate Resistant Acid Phosphatase Staining
BMMs were seeded at a density of 250,000 cells per well in a 24-well dish on glass coverslips in BMM growth medium as described above and allowed to attach overnight. For osteoclastogenesis assays, cells were cultured with 1:1 ratio of MEMα and DMEM or Adipo CM containing 10% FBS, 20 ng/mL M-CSF and 10 ng/mL RANKL. When indicated, assays were performed in the absence or presence of recombinant proteins to CXCL1 (0.5ug/mL) and CXCL2 (0.25ug/mL), neutralizing CXCL1/CXCL2 antibodies (3μg/mL), CXCR2 neutralizing antibodies (5ug/mL) or CXCR2 antagonist SB225002 (2.5μM; from Cayman Chemical, Ann Arbor, MI). Every 48 hours, half of the media was removed and replenished with fresh media supplemented with M-CSF, RANKL and appropriate treatment reagents. Data were collected from at least 3 independent experiments performed in duplicate. Osteoclasts were formed within 4 to 6 days and TRAcP staining was performed according to manufacturer’s instructions.
Quantification of TRAcP-positive cells
TRAcP-positive cells that contained three or more nuclei were considered osteoclasts and counted. Six representative images per coverslip were captured using a Zeiss Scope A.1 conventional light microscope at 5× magnification to evaluate the number and surface area of osteoclasts per field. For each experimental condition, the total number and cell surface area of osteoclasts were manually counted using ImageJ software.
RNA and Lysate Isolation of Osteoclasts
BMMs were seeded at a density of 500,000 cells per well in a 12-well dish for 24 hours as described above. BMMs were then treated with RANKL and M-CSF in the presence or absence of Adipo CM. When indicated, cells were treated with recombinant CXCL1 and CXCL2 proteins, CXCL1/CXCL2 neutralizing antibodies, CXCR2 blocking antibody, or CXCR2 antagonist SB225002 as described above. Following formation of mature osteoclasts, cells were washed three times with PBS, collected in SME lysis buffer (250mM sucrose, 25mM 2-(N-morpholino) ethanesulfonic acid (MES), 1mM EDTA, 0.1% Triton-X 100 pH 6.5) and stored at −80°C for future use. For RNA isolation, cells were washed with PBS cells and collected into RLT buffer and RNA purified according to the RNeasy Mini Kit instructions.
Adipocyte co-culture with PC3 tumor cells
For indirect adipocyte-tumor cell co-cultures, mBMSC cells were seeded in collagen I-coated 6-well plates, differentiated into adipocytes, and tumor cells were seeded on top of a Transwell filter (0.2 μm pore size) to allow sharing of soluble factors between the two cell types. Cells were cultured as described for 48 hours and serum-starved for additional 12-16 hours prior to sample collection for analyses. For ELISA analyses, media were concentrated through 3K Millipore centrifugal filters, and all samples were stored at −80°C for future use.
Taqman RT-PCR analyses
The cDNA from cells and in vivo samples was prepared from 1-2 μg of total RNA using High-Capacity cDNA Reverse Transcription kit (Applied Biosystems) as previously described [31]. The analyses of genes associated with bone remodeling and inflammation were performed using mouse-specific TaqMan® Individual Gene Expression assays for cathepsin K (Mm00484039), matrix metalloproteinase-9 (Mm00442991), calcineurin (Ms00432282), CXCL1 (Mm01354329), CXCL2 (Mm00436450), and DC-STAMP (Mm04209236). Assays were done on three biological replicates using TaqMan® Fast Universal PCR Master Mix and 25 ng of cDNA/well for RNA isolated from cells and 50ng cDNA/well for in vivo samples. All reactions were run on an Applied Biosystems StepOnePlus™ system. In vivo data were normalized to HPRT1 (Mm00446968) and in vitro data were normalized to 18S (Mm03928990). DataAssist™ Software (Applied Biosystems) was used for all analyses.
Immunoblot and ELISA analyses
Lysates were equally loaded based on DNA concentrations as previously described. Proteins were electrophoresed on 12% SDS-PAGE gels, transferred to PVDF membranes and immunoblotted for cathepsin K (1:500) and β-actin (1:5,000). Secondary antibodies labeled with horseradish peroxidase were used at 1:10,000. Quantification and analyses of bands were performed using a Luminescent Image Analyzer LAS-1000 Plus (Fujifilm, Stamford, CT) and expressed as arbitrary units (AU) per square millimeter. For ELISA assays, media from each condition were diluted based on DNA concentrations in cell lysates and were run in duplicate according to manufacturer’s instructions (R&D Systems). Optical density of each well was determined at 450nm with correction wavelength set to 540 using TECAN-Infinite M200 PRO plate reader (Männedorf, Switzerland). The data were analyzed based on the standard curve values using a four parameter logistic (4-PL) curve-fit.
Cathepsin K Activity Assay
Enzymatic activity of cathepsin K was measured in cell lysates utilizing the fluorescent substrate Z-Glycine-Proline-Arginine-7-amido-4-methylcoumarin-HCl (Z-Gly-Pro-Arg-AMC) from Bachem Chemical (100μM; Torrance, CA). The reaction was performed in the presence of the selective inhibitor to Cathepsin B, CA074 (1 μM) to eliminate the activity due to cathepsin B-mediated cleavage of Z-Gly-Pro-Arg-AMC [32]. The progress of the reaction was monitored every minute for a period of 30 minutes on a Tecan SpectraFluor Plus plate reader. Results of activity assays are expressed as maximum fluorescence units formed per minute. Equal amounts of cell lysate were used based on DNA concentrations in cell lysates.
MTT Assay
BMMs were seeded at a density of 20,000 cells per well in a 96-well in MEMα media with 15% L929 conditioned media and 10% FBS. After 24 hours, cells were treated with DMSO (control) or increasing concentrations of SB225002 or CXCR2 neutralizing antibody. Cells were retreated after 48 hours and Vybrant® MTT Cell Proliferation Assay kit (Life Technologies) was performed after 4 days. Conversion of MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) to formazan by viable tumor cells was measured at 540 nm according to manufacturer’s instructions using Infinite® F200 Pro plate reader.
Statistical Analyses
All data analyses were performed using GraphPad Software version 6.05. Data were presented as mean +/− SEM and statistically analyzed using student’s T-test. For three or more groups, one-way analysis of variance was used.
Results
Bone remodeling is increased in tibiae of mice with increased marrow adiposity
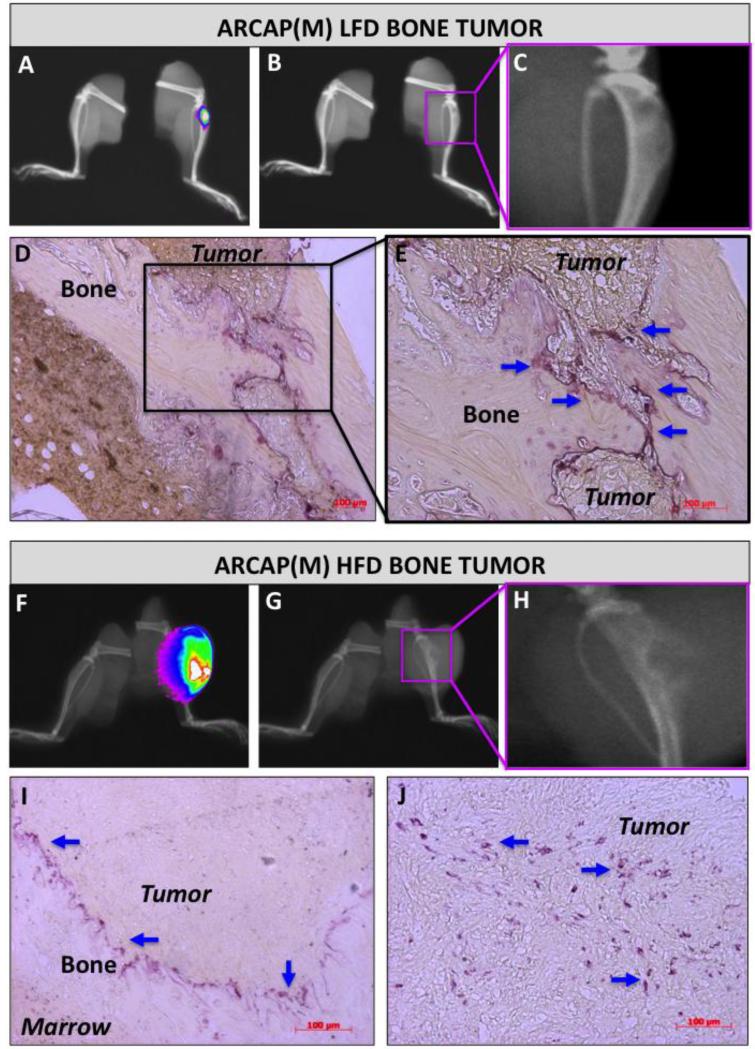
Bone marrow adiposity has been linked to changes in bone density, increased numbers of osteoclasts, fewer numbers of osteoblasts, and accelerated growth of prostate cancer in bone [33, 34]. We have shown previously that mice fed high fat diet (HFD) have more adipocytes in the bone marrow compared to low fat diet (LFD) control mice [20]. We have also demonstrated that the increased marrow adiposity promotes growth and progression of PC3 prostate tumors in bone [20]. Based on these findings we investigated the effects of increased marrow adiposity in another system using intratibially implanted ARCaP(M) cells. These cells are known to exhibit mixed osteoblastic/osteolytic phenotype in vivo, and thus better reflect the bone metastatic phenotype in humans [35, 36]. As expected, under LFD conditions, ARCaP(M) tumor nests remained embedded within the bone matrix with x-ray and histological evidence of both osteolysis and new bone acquisition (Figure 1A-E), whereas HFD tumors exhibited extensive destruction of the bone (Figure 1F-J). For validation of these findings we measured the area of each tibia that is not occupied by the tumor and confirmed significant reduction in intact bone tissue in tumor-bearing HFD mice as compared to LFD mice (Supplementary Figure 1). This is in line with our previous report of adiposity-stimulated bone destruction in mice bearing PC3 tibial tumors [20]. For more in-depth analysis of the effects of marrow adiposity on bone osteolysis we performed TRAcP staining of tibial cross sections from ARCaP(M)- and PC3-bearing mice and determined the numbers and localization of osteoclasts. In both ARCaP(M) and PC3 models established in LFD mice, TRAcP-positive cells localized to the bone-tumor interface and appeared as a string of cells surrounding the tumor (Figure 1D, E and Supplementary Figure 2C, E). In sharp contrast to LFD tumors, a significant presence of osteoclastic clusters throughout the tumor and around the remaining bone fragments was revealed in HFD tumors (Figure 1I, J and Supplementary Figure 2D, F). These findings were in line with x-ray and histology results and they suggest that HFD-induced marrow adiposity may be contributing to tumor-driven osteolysis of the bone.
Figure 1. Diet-induced marrow adiposity correlates with increased osteolysis in ARCaP(M) bone tumors.
FVBN/N/N5 Rag−/− mice were fed a normal (LFD; A-E) or high fat (HFD; F-J) diets for 8 weeks followed by intratibial injections of ARCaP-DsRed cells into the right tibia. Tumors were imaged at 8 weeks post injection (N=9 mice/LFD group and N=11 mice/HFD group). The overlay of x-ray and 600nm RFP fluorescence (A,F). B,G: x-ray images depicting osteolytic changes in the bone occupied by tumor (pink rectangular area magnified in C and H). TRAcP staining (purple) of osteoclasts (blue arrows) in tibial cross sections of tumor-bearing mice on LFD (D,E) and HFD (I,J). TRAcP data are representative of at least 3 individual sections from 3 separate LFD and HFD tumors.
Adipocyte-derived factors enhance osteoclastogenesis and proteolytic activity of osteoclast-derived cathepsin K in vitro
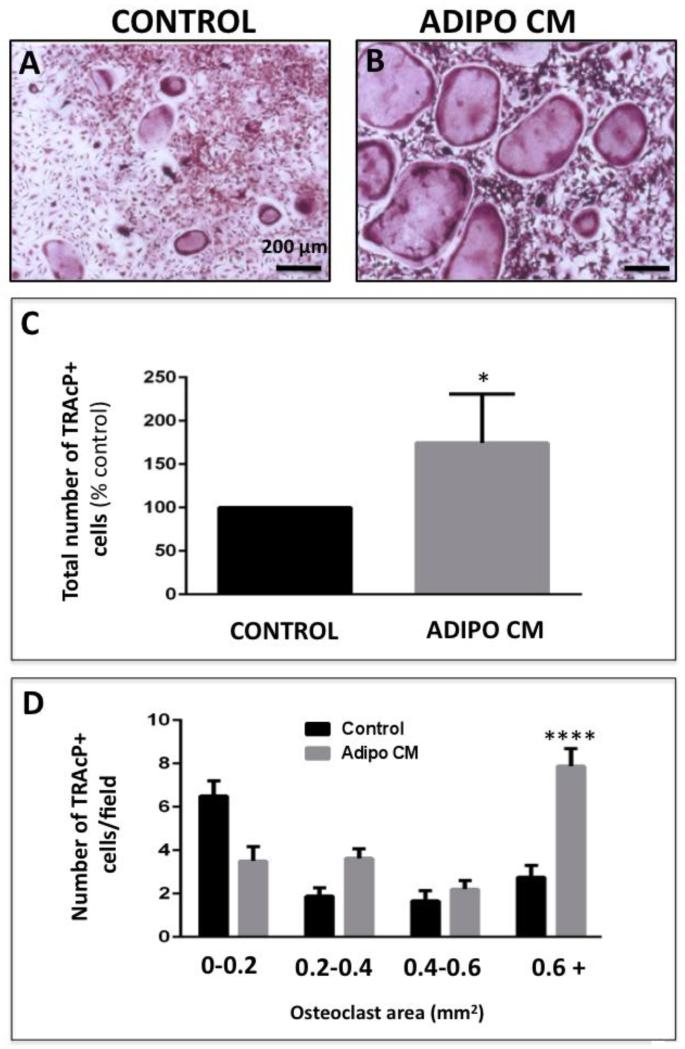
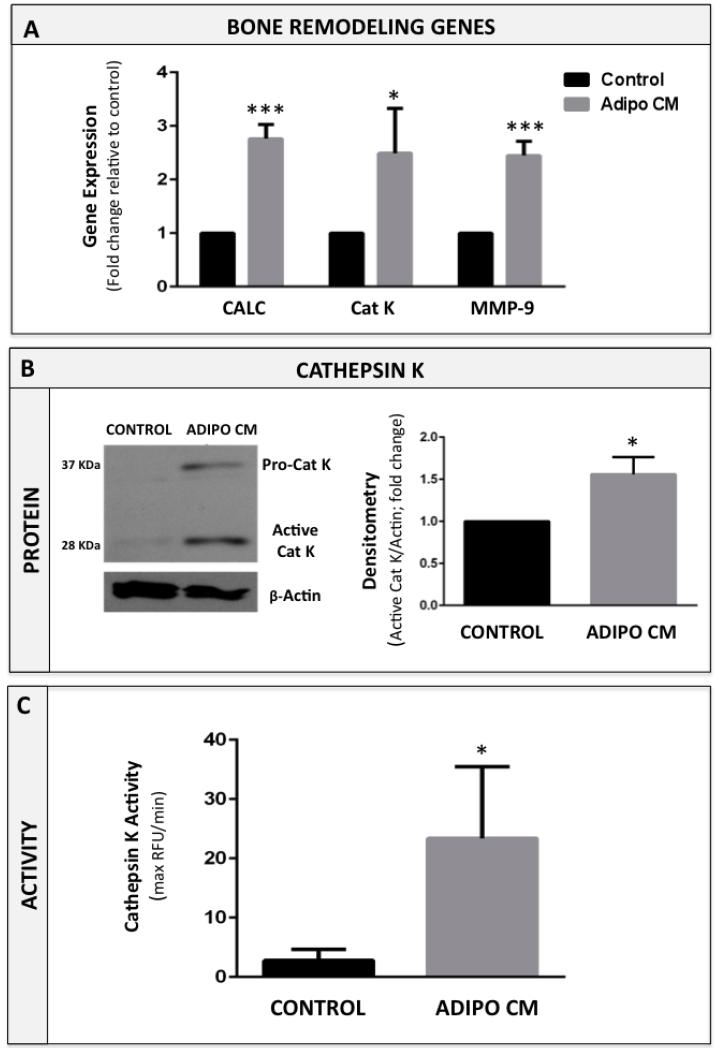
To understand the effects of bone marrow adipocyte-derived factors on osteoclast differentiation, we performed in vitro osteoclastogenesis assays in the absence or presence of media conditioned by marrow adipocytes (Adipo CM). All assays were performed in the presence of RANKL and M-CSF, which are critical factors necessary for initiation of osteoclastic differentiation [37]. However, treatment with Adipo CM resulted in significantly enhanced osteoclast formation in comparison to standard control treatments with RANKL and M-CSF alone (Figure 2A, B). Microscopic evaluation and quantification of TRAcP-positive cells revealed that in the presence of Adipo CM, not only numbers but also the size of the osteoclasts were significantly increased in comparison to control conditions (Figure 2C, D). This was due to the enhanced BMM fusion, as evidenced by the presence of multiple nuclei and the increased expression of osteoclast fusion marker DC-STAMP [38] by the osteoclasts differentiated in the presence of Adipo CM (Supplementary Figure 3). Adipo CM-driven acceleration of osteoclastogenesis was further evidenced by significant overexpression of genes known to be associated with osteoclast differentiation and bone remodeling, such as cathepsin K, calcineurin, and matrix metalloproteinase-9 [39-41] (Figure 3A). Since cathepsin K is a key osteoclast protease responsible for cleaving the collagen and non-collagen components of bone [42], we also examined its protein expression and proteolytic activity in response to Adipo CM treatment. Our results revealed that Adipo CM-treated osteoclasts have more protein expression of both the 37 kDa inactive pro-cathepsin K and 28 kDa mature (active) form of the enzyme (Figure 3B). Importantly, increased protein expression of this protease in osteoclasts differentiated in the presence of Adipo CM correlated with induced proteolytic activity toward its substrate Z-Gly-Pro-Arg-AMC (Figure 3C). Collectively, these results suggest that adipocyte-derived factors enhance RANKL/M-CSF-mediated osteoclastogenesis.
Figure 2. Bone marrow adipocyte-secreted factors promote osteoclastogenesis in vitro.
TRAcP staining of osteoclasts differentiated in the absence (A; Control) or presence of Adipo CM (B). C: Quantification of total number of TRAcP positive cells using ImageJ software shown as percent of control (% control). D: The areas of osteoclasts (in mm2) were measured using ImageJ software and separated based on size from smallest (0-0.2mm2) to largest (0.6mm2+). Graph represents the total number of osteoclasts/field in each size group. Experiments are representative of at least three replicate experiments and shown as mean ± s.e.m; Values indicated by ****(p<0.0001) and * (p<0.05) are considered statistically significant.
Figure 3. Cathepsin K expression and activity are increased in Adipo CM-treated osteoclasts.
A: Taqman RT-PCR analysis of bone remodeling genes: calcineurin (CALC), cathepsin K (CAT K), and matrix metalloproteinase-9 (MMP-9) in osteoclasts differentiated in the absence (CONTROL) or presence of Adipo CM (ADIPO CM). Data are normalized to 18S and are graphed as fold increase relative to control. B: Western blot of cathepsin K in osteoclasts (left panel). Levels of pro-cathepsin K (37 kD) and active cathepsin K (28 kD) are increased in osteoclasts treated with Adipo CM. Densitometric analysis of active cathepsin K levels in osteoclasts (right panel) measured as a ratio of 28 kDa band to β-actin (in AU/mm2) and represented as % control. C: Proteolytic activity of cathepsin K in cell lysates from osteoclasts differentiated in the absence and presence of Adipo CM. Assays were run against fluorescent cathepsin K substrate Z-Gyl-Pro-Arg-7-amido-4-methylcoumarin (Z-Gly-Pro-Arg-AMC) in a presence of 1 μM. cathepsin B inhibitor Ca074. Fluorescence was measured in maximum relative fluorescent units per minute (Max RFU per min). Data are representative of three replicate experiments. All values are shown as mean ± s.e.m. (*** p<0.001, *p<0.05 are considered statistically significant).
Marrow adiposity is associated with increased levels of CXCL1 and CXCL2
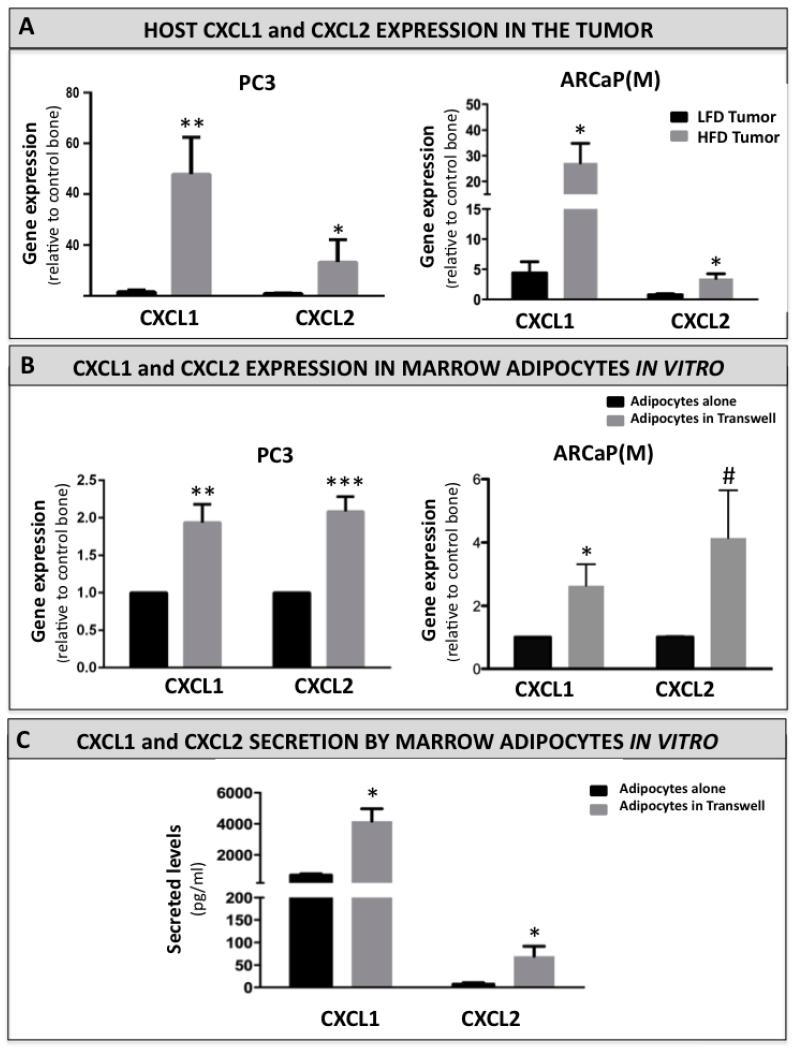
We have shown previously that prostate tumor growth in bone under conditions of increased adiposity is associated with escalated levels of host-derived pro-inflammatory factors, such as CCL2 and COX-2, both of which have been implicated in osteoclastogenesis, regulation of bone metabolism, and tumor growth in bone [1]. This suggested that marrow adiposity is evoking changes in the host marrow microenvironment that are conducive to growth and survival of skeletal tumors, and prompted us to search for additional factors that may be involved in this process. Our Taqman RT PCR analyses revealed that two host-derived chemokines, CXCL1 and CXCL2, are particularly highly upregulated in HFD mice bearing PC3 and ARCaP(M) tumors (Figure 4A). Interestingly, both of these factors have been previously associated with pro-metastatic properties [43, 44], and have been recently suggested to play potential roles in osteoclast precursor migration and/or differentiation [28, 29]. To assure that an observed increase in CXCL1 and CXCL2 gene expression is host-specific we tested the CXCL1 and CXCL2 Taqman probes against human PC3 cells. Neither of the murine chemokines was detectable in PC3 cells (Supplementary Table 1). To mimic the in vivo interaction of bone marrow adipocytes with tumor cells, we grew the marrow-derived fat cells in a transwell co-culture with PC3 or ARCaP(M) cells. We then performed RT PCR analyses to determine the expression of adipocyte-derived CXCL1 and CXCL2. Our results revealed that gene expression of both chemokines is significantly upregulated upon adipocyte exposure to both PC3- and ARCaP(M)- derived factors (Figure 4B). To determine if upregulation of gene expression correlates with increases of secreted proteins we performed ELISA analyses on media conditioned by adipocytes alone as well as in co-culture with PC3 cells (Figure 4C). Both factors were observed to be efficiently secreted by the mature adipocytes (399.2 pg/ml DNA for CXCL1 and 3.111 pg/ml for CXCL2). In line with the RT PCR results, their secretion was further enhanced by the co-culture with tumor cells (7.5-fold and 54-fold increases for CXCL1 and CXCL2, respectively), suggesting the contribution of tumor cell-adipocyte interactions to the CXCL1/2 abundance in the bone microenvironment.
Figure 4. CXCL1 and CXCL2 expression and secretion are increased in adipocytes interacting with tumor cells in vivo and in vitro.
A: Taqman RT-PCR analysis of host CXCL1 and CXCL2 expression in LFD and HFD mice bearing PC3 (left panel) and ARCAP(M) (right panel) tumors. Data are normalized to murine (host) HPRT1 and shown as fold increase relative to control bone. B: Taqman RT-PCR analysis of CXCL1 and CXCL2 expression in bone marrow adipocytes cultured alone (black bar) or in a transwell co-culture (grey bar) with PC3 (left panel) or ARCaP(M) cells (right panel). Data are normalized to HPRT1 and shown as fold increase relative to adipocytes alone. C: ELISA assay results for CXCL1 and CXCL2 secreted by the bone marrow adipocytes grown alone or in a transwell system with PC3 cells. Media samples were diluted based on DNA concentrations in cell lysates. Data are expressed in pg/mL. All data are representative of three replicate experiments. All values are shown as mean ± s.e.m. (*** p<0.001; **p<0.01 and *p<0.05 are considered statistically significant, #p =0.0792).
CXCL1 and CXCL2 chemokines stimulate osteoclast differentiation in vitro
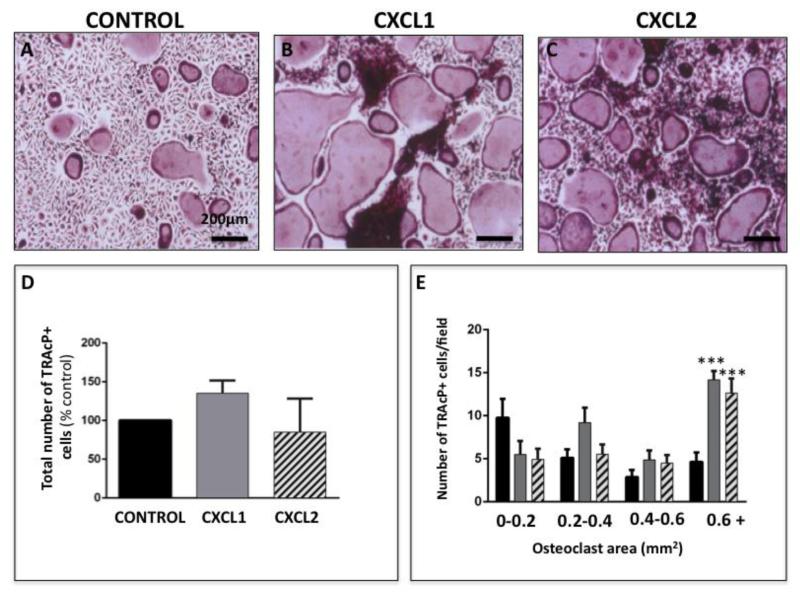
To determine if CXCL1 and CXCL2 are directly involved in accelerating osteoclastogenesis, we differentiated osteoclast precursor cells in the absence and presence of recombinant versions of these chemokines. Results of TRAcP staining demonstrated that treatment with either recombinant CXCL1 or CXCL2 clearly enhanced osteoclast maturation in comparison to control conditions (Figure 5A-C). It is noteworthy that although we did not observe significant increase in the total number of osteoclasts upon CXCL1 and CXCL2 treatment (Figure 5D), the numbers of large cells (i.e., larger than 0.6 mm2) were significantly higher in the presence of CXCL1 and CXCL2 as compared to control conditions (Figure 5E). This observed increase in osteoclast size correlated with augmented expression of cathepsin K (Supplementary Figure 4), a result mirroring changes in expression of this protease upon exposure to Adipo CM (Figure 3)
Figure 5. Recombinant CXCL1 and CXCL2 proteins accelerate osteoclastogenesis.
A-C: TRAcP staining of osteoclasts differentiated in the absence (A; control) or presence of recombinant CXCL1 (B) and CXCL2 (C) proteins. D: Quantification of total number of TRAcP positive cells shown as percent of control (% control). E: Total number of osteoclast/field categorized based on size from the smallest (0-0.2mm2) to largest (0.6mm2+). Significantly larger osteoclasts were formed in the presence of CXCL1 and CXCL2. Values are shown as mean ± s.e.m and are representative of three replicate experiments. (*** p<0.005 is considered statistically significant)
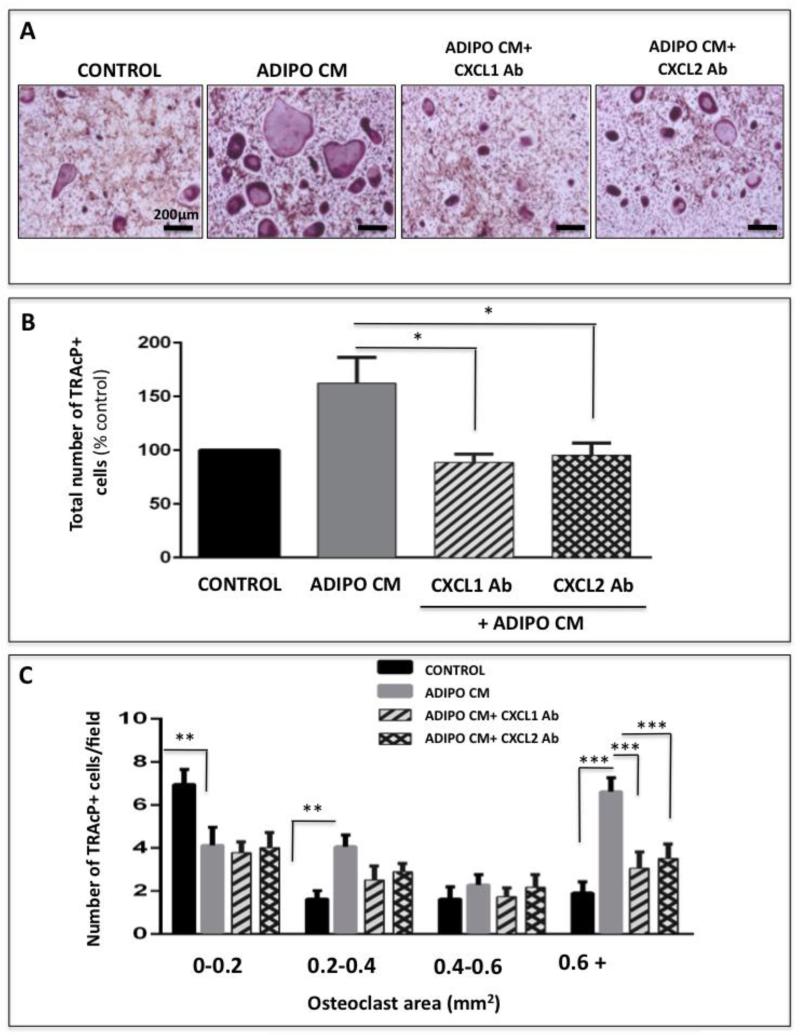
To further demonstrate that adipocyte-derived CXCL1 and CXCL2 have a promoting effect on differentiation of precursor cells into mature osteoclasts we performed osteoclastogenesis assays in the absence or presence of neutralizing antibodies to each of the chemokines (Figure 6A and Supplementary Figure 5). Significant decrease in Adipo CM-stimulated osteoclast differentiation was observed upon antibody treatment as shown by the marked decline in total osteoclast numbers (Figure 6B) as well as size (Figure 6C). This implicates that CXCL1 and CXCL2 are at least partially responsible for the adipocyte-induced acceleration of osteoclastogenesis.
Figure 6. Adipocyte-driven osteoclastogenesis is inhibited by neutralizing CXCL1 and CXCL2.
A: TRAcP staining of osteoclasts differentiated under control conditions (far left panel), with Adipo CM (left middle panel) or with Adipo CM in the presence of neutralizing antibodies to CXCL1 and CXCL2 (right panels). B: Total number of TRAcP positive cells in each experimental condition shown as % control. C: Total number of osteoclasts/field categorized based on size for each experimental condition. Neutralization of CXCL1 and CXCL2 ligands inhibits Adipo CM-stimulated osteoclastogenesis. Data are representative of at least three replicate experiments. ***p<0.001; **p<0 .01 and *p<0.05 are considered statistically significant).
Osteoclastogenesis is partially regulated by the CXCR2 signaling axis
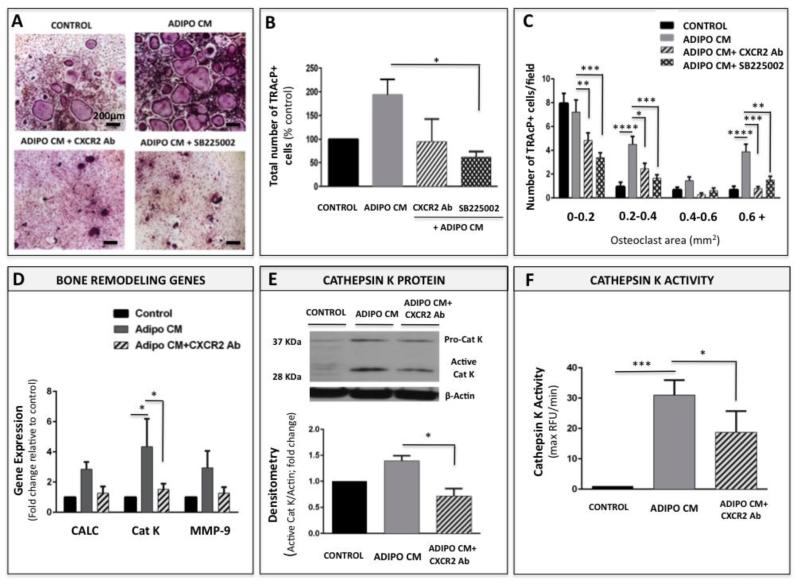
CXCL1 and CXCL2 exert their functions by binding to the G-protein coupled receptor CXCR2 [24]. CXCR2 signaling has been previously shown to play a role in osteoclast formation by binding IL-8 in human monocytes [27], but has not been well-investigated in the context of its other ligands, CXCL1 and CXCL2. Given our above-described findings demonstrating that CXCL1 and CXCL2 are involved in adipocyte-driven osteoclastogenesis, we examined if these effects can be abrogated by blocking their binding capability of CXCR2 receptor. We blocked the CXCR2 signaling using two separate approaches: neutralizing antibody and the CXCR2 antagonist SB225002. Effective doses of the antibody (5 μg/ml) and the antagonist (2.5μM) were determined empirically and viability assays were performed to demonstrate that both compounds are well-tolerated by the osteoclast precursor cells at the concentrations used in osteoclastogenesis assays (Supplementary Fig. 6-7). In line with the results of CXCL1 and CXCL2 blocking, neutralizing the activity of CXCR2 receptor with either the antibody or the antagonist resulted in significant inhibition of osteoclastogenesis (Figure 7A). Both the total osteoclast numbers (Figure 7B) and the osteoclast size (Figure 7C) were reduced significantly upon treatment with each of the compounds. To further validate the importance of CXCR2 signaling in the adipocyte-driven osteoclastogenesis we examined the effects of CXCR2 neutralizing antibody on adipocyte maturation in the presence of Adipo CM. Transcription levels of all 3 bone remodeling genes (calcineurin, cathepsin K and MMP-9) were significantly reduced with antibody treatment (Figure 7D). Similarly, protein expression (Figure 7E) and activity (Figure 7F) of cathepsin K were partially abolished by CXCR2 blocking. Collectively, these data further confirm the involvement of CXCR2 signaling axis in adipocyte-induced osteoclastogenesis.
Figure 7. Blocking the CXCR2 receptor partially inhibits adipocyte-driven osteoclastogenesis.
A: TRAcP staining of osteoclasts differentiated under control conditions (Control), with Adipo CM (Adipo CM) or with Adipo CM in the presence of neutralizing antibodies to CXCR2 (bottom left panel) or CXCR2 antagonist SB225002 (bottom right panel). B: The total number of TRAcP positive cells corresponding to each treatment shown as % control; C: Total number of osteoclasts/field categorized based on size for each experimental condition: Control, Adipo CM, Adipo CM + CXCR2 Ab, Adipo CM + SB225002. D: Taqman RT-PCR analysis of bone remodeling genes: calcineurin, cathepsin K, and MMP-9 in osteoclasts differentiated under control conditions, with Adipo CM, or with Adipo CM in the presence of neutralizing antibodies to CXCR2. Data are normalized to 18S and shown as fold increase relative to control. E: Immunoblot of pro- and active cathepsin K in osteoclasts differentiated under control conditions or with Adipo CM in the absence or presence of CXCR2 neutralizing antibody (top panel); Densitometric analysis of active cathepsin K levels in osteoclasts measured as a ratio of 28 kDa band to β-actin (in AU/mm2) and represented as % control (bottom panel). F: Proteolytic activity of cathepsin K in cell lysates from osteoclasts differentiated in the absence and presence of Adipo CM, or with Adipo CM plus CXCR2 neutralizing antibody. Assays were run against fluorescent cathepsin K substrate Z-Gly-Pro-Arg-AMC in the presence of 1 μM cathepsin B inhibitor Ca074. Fluorescence was measured in Max RFU per min. Data are representative of three replicate experiments (****p<0.0001; ***p<0.001; **p<0 .01 and *p <0.05 are considered statistically significant).
Discussion
Dysregulated bone remodeling is a common complication of bone metastatic disease [1, 34, 45, 46]. In prostate cancer, 75-80% of men with metastatic disease present with skeletal lesions [47, 48] and although most of these lesions appear osteoblastic, there is clear evidence that their formation is associated with an increase in osteoclastic activity [49-52]. Increased marrow adiposity, a common feature of advanced age and metabolic pathologies is a known culprit in accelerated bone resorption [1, 3, 9-12]. Although the age-driven conversion of red marrow to yellow marrow occurs predominantly in the appendicular skeleton [3, 8, 53], even the red marrow contains significant amount (40%) of fat [54]. Notably, fat depots in the bone marrow generally localize to highly resorptive trabecular area of the bone [3], and both the adipocyte-rich red marrow areas of axial skeleton [21, 55] as well as the sites undergoing active bone remodeling are often a preferred loci of colonization for metastatic tumor cells [56, 57]. We have shown previously that increased marrow adiposity due to high fat diet accelerates growth and progression of PC3 prostate tumors in bone [20]. Importantly, and in line with results reported previously by others [21, 55, 58, 59], we specifically demonstrated that interaction and lipid transfer between bone marrow adipocytes and tumor cells has growth and invasion stimulatory effects. This results not only in larger skeletal tumors but also extensive destruction of the bone [20]. Stemming from these findings, studies presented herein focused specifically on the contribution of bone marrow adiposity to tumor-induced osteolysis of the bone. We were able to demonstrate that mice with increased numbers of HFD-induced marrow adipocytes exhibit potentiated osteolysis of the bone implanted with either ARCaP(M) or PC3 tumors. We also showed that gene expression of host-derived CXCL1 and CXCL2 in intratibial tumors from HFD mice is distinctly higher that in tumors from LFD mice and that both CXCL1 and CXCL2 are highly produced and secreted by bone marrow adipocytes in vitro. We further revealed that media conditioned by bone marrow adipocytes significantly accelerates osteoclastogenesis, a process that can be mimicked by the treatments with recombinant CXCL1 and CXCL2 ligands. Notably, our results demonstrating the inhibition of adipocyte-induced osteoclastogenesis by the CXCL1/2 neutralizing antibodies or agents directly blocking the function of CXCR2 receptor offered additional testimony to the importance of CXCR2 signaling in this process.
The role of CXCR2 and specifically its CXCL1 and CXCL2 ligands in osteoclastogenesis is not well understood. Only limited recent reports indicated that these chemokines may be playing important roles in osteoclast precursor migration and/or differentiation [28, 29] and promoting bone loss in periodontal disease [29], rheumatoid arthritis [60], and oral squamous cell carcinoma [61]. Even less is known about the functions of adipocyte-derived CXCL1 and CXCL2. CXCL1 has been reported to be markedly upregulated in 3T3-L1 adipocytes interacting with macrophages [62], and to be associated with inflammatory phenotype of white adipocytes [63], results suggesting its potential role in adipose tissue inflammation. Separate studies noted augmented CXCL2 levels in differentiating adipocytes and in adipocytes and peripheral blood mononuclear cells from obese individuals [64, 65]. This potential association of both chemokines with adipose tissue inflammation may be of high relevance to metastatic bone tumor microenvironment. We have previously demonstrated evidence of bone marrow macrophage-driven inflammation in skeletal prostate tumors that suggested a close interplay between inflammatory, osteolytic and tumor cell-driven events in the bone-tumor microenvironment [31]. We have also shown that increased marrow adiposity results in highly increased levels of host-derived MCP-1 and COX-2 in skeletal prostate tumors [1]. The results of the present study revealed that CXCL1 and CXCL2 are two additional host-derived pro-inflammatory factors significantly increased in prostate bone tumors under conditions of high marrow adiposity. This suggests that this potentiated inflammatory response in tumor skeletal niche may be due to the augmented marrow fat content.
It is noteworthy that in addition to marrow adipocytes, multiple cell types within the bone tumor microenvironment, including macrophages, endothelial cells and tumor cells, can be potential sources of CXCL1 and CXCL2 [23-26, 66], which might explain the observed large induction in the levels of these chemokines under HFD conditions in vivo (Figure 4A). It is well-known that macrophage- and endothelial cell-derived CXCL1 and CXCL2 play important functions in neutrophil recruitment to the tumor site [23, 26, 66]. Tumor cell-specific CXCL1 and CXCL2 appear to be important to metastasis and chemoresistance [25]. Secretion of CXCL1 and CXCL2 by all these cell types in the tumor is likely to be affected by the levels of fat in the marrow. Whether this results in a cumulative effect on osteolysis and tumor growth in bone is an under-investigated concept that warrants further studies. However, regardless of potential contribution from other cell types, our in vitro results utilizing bone marrow-derived adipocytes clearly demonstrate specific roles for adipocyte-supplied CXCL1 and CXCL2 in osteoclastogenesis. This is particularly evident by the results of significant abrogation of adipocyte-induced osteoclast maturation with neutralizing antibodies to each of the chemokines (Figure 6). Similar effects are observed by blocking the activity of the CXCR2 receptor (Figure 7), results that underline the importance of CXCR2 signaling in this process. It is possible that other CXCR2 ligands, specifically CXCL8 (IL-8) and CXCL5, could contribute to the adipocyte-induced effects on osteoclasts. Both chemokines have been shown to stimulate osteoclast differentiation and activity [27, 67, 68] and both are reported to be present in the adipose tissue; however their expression is associated primarily with non-adipose (stromal) component of fat tissue [69-71]. This suggests that marrow adipocyte-induced CXCR2 signaling in osteoclasts is likely driven by CXCL1 and CXCL2 chemokines. Additional studies are, however, needed to understand expression and function of CXCR2 ligands in bone marrow fat, which is distinctively different from white adipose tissue [1, 72].
It is important to mention, that although diet-induced obesity (DIO) model utilized in this study is a well-documented approach to induce significant marrow adiposity [4, 11, 18, 20], we can not exclude potential systemic consequences of the diet itself on both the tumor growth and bone degradation. The roles of dietary lipids in prostate cancer development and progression are a subject of an ongoing debate [73, 74]. Future studies utilizing genetic models of obesity and age-induced models of marrow adiposity will provide more detailed understanding of adipocyte-derived CXCL1 and CXCL2 involvement in tumor-driven osteolysis of the bone. Bone marrow adiposity is emerging as a significant complication of advanced age, obesity and other metabolic pathologies. Although results presented herein did not directly address the dietary effects on bone metastatic niche, they did suggest that marrow adipocytes have potentiating effects on tumor-induced osteolysis of the bone through secretion of CXCL1 and CXCL2 chemokines and activation of CXCR2 receptor on osteoclast precursor cells. To our knowledge, this is the first study linking CXCL1 and CXCL2 with bone marrow adiposity and first to implicate CXCR2 signaling in promoting effects of marrow fat on progression of skeletal tumors in bone.
This work broadens the presently limited understanding of molecular mechanisms behind marrow adipocyte involvement in the regulation of bone homeostasis, and specifically in modulating tumor behavior in bone. Current treatment options for tumor-induced bone disease, including bisphosphonate zoledronic acid and the monoclonal antibody denosumab, although effective in reducing the incidence of skeletal related events (SREs) in prostate cancer, remain palliative [50, 52]. To date, CXCR2 antagonists have only been evaluated in a context of COPD and asthma [75, 76], and it is unclear if direct targeting of this signaling axis in bone would prove to be an advantageous treatment option due to its roles in normal physiological processes. However, based on the results presented herein, and our previous reports on adipocyte and macrophage involvement in metastatic progression in bone [1, 20, 31], it is becoming evident that designing therapies for bone metastatic disease may have to involve targeting multiple components of the skeletal niche. Specifically, combining anti-tumor agents with therapeutics that target adipocyte and osteoclast/macrophage pathways may prove more effective in eliminating debilitating symptoms, prolonging survival and ultimately eradicating this disease.
Supplementary Material
ACKNOWLEDGMENTS
We gratefully acknowledge Dr. Halina Chkourko Gusky (WSU, Karmanos Cancer Institute) and Jonathan Diedrich (WSU, Karmanos Cancer Institute) for critical revisions to the manuscript. We also thank Dr. Karin List for access to her Zeiss Scope A.1 conventional light microscope, and Kamiar Moin, and the Microscopy, Imaging and Cytometry Resources Core (MICR) for assistance with animal imager analyses. Grant support was provided by: NIH/NCI 1 R01 CA181189-01, NIH/NCI 1F31CA165834-01A1, and MICR: P30 CA 22453.
Footnotes
CONFLICTS OF INTEREST:
The authors disclose no potential conflicts of interest
AUTHORS’ CONTRIBUTIONS:
ALH and IP participated in the design of the study; ALH, MKH, and ER carried out experimental data acquisition and performed data analyses. ALH and IP wrote the manuscript. All authors read, critically revised, and approved the final manuscript.
References
- 1.Hardaway AL, Herroon MK, Rajagurubandara E, Podgorski I. Bone marrow fat: linking adipocyte-induced inflammation with skeletal metastases. Cancer Metastasis Rev. 2014;33(2-3):527–43. doi: 10.1007/s10555-013-9484-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raggatt LJ, Partridge NC. Cellular and molecular mechanisms of bone remodeling. J Biol Chem. 2010;285(33):25103–8. doi: 10.1074/jbc.R109.041087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lecka-Czernik B, Rosen CJ, Kawai M. Skeletal aging and the adipocyte program: New insights from an “old” molecule. Cell Cycle. 2010;9(18):3648–54. doi: 10.4161/cc.9.18.13046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosen CJ, Ackert-Bicknell C, Rodriguez JP, Pino AM. Marrow fat and the bone microenvironment: developmental, functional, and pathological implications. Crit Rev Eukaryot Gene Expr. 2009;19(2):109–24. doi: 10.1615/critreveukargeneexpr.v19.i2.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosen CJ, Bouxsein ML. Mechanisms of disease: is osteoporosis the obesity of bone? Nature clinical practice. 2006;2(1):35–43. doi: 10.1038/ncprheum0070. [DOI] [PubMed] [Google Scholar]
- 6.Dimitroulas T, Nikas SN, Trontzas P, Kitas GD. Biologic therapies and systemic bone loss in rheumatoid arthritis. Autoimmun Rev. 2013;12(10):958–66. doi: 10.1016/j.autrev.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 7.Ara T, Declerck YA. Interleukin-6 in bone metastasis and cancer progression. Eur J Cancer. 2010;46(7):1223–31. doi: 10.1016/j.ejca.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lecka-Czernik B. Marrow fat metabolism is linked to the systemic energy metabolism. Bone. 2011 doi: 10.1016/j.bone.2011.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gimble JM, Nuttall ME. Bone and fat: old questions, new insights. Endocrine. 2004;23(2-3):183–8. doi: 10.1385/ENDO:23:2-3:183. [DOI] [PubMed] [Google Scholar]
- 10.Kawai M, de Paula FJ, Rosen CJ. New insights into osteoporosis: the bone-fat connection. J Intern Med. 2012 doi: 10.1111/j.1365-2796.2012.02564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao JJ, Sun L, Gao H. Diet-induced obesity alters bone remodeling leading to decreased femoral trabecular bone mass in mice. Ann N Y Acad Sci. 2010;1192:292–7. doi: 10.1111/j.1749-6632.2009.05252.x. [DOI] [PubMed] [Google Scholar]
- 12.Halade GV, Rahman MM, Williams PJ, Fernandes G. High fat diet-induced animal model of age-associated obesity and osteoporosis. J Nutr Biochem. 2010;21(12):1162–9. doi: 10.1016/j.jnutbio.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jilka RL. Osteoblast progenitor fate and age-related bone loss. Journal of musculoskeletal & neuronal interactions. 2002;2(6):581–3. [PubMed] [Google Scholar]
- 14.Oh SR, Sul OJ, Kim YY, Kim HJ, Yu R, Suh JH, Choi HS. Saturated fatty acids enhance osteoclast survival. Journal of lipid research. 2010;51(5):892–9. doi: 10.1194/jlr.M800626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lecka-Czernik B. PPARs in bone: the role in bone cell differentiation and regulation of energy metabolism. Curr Osteoporos Rep. 2010;8(2):84–90. doi: 10.1007/s11914-010-0016-1. [DOI] [PubMed] [Google Scholar]
- 16.Moerman EJ, Teng K, Lipschitz DA, Lecka-Czernik B. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of PPAR-gamma2 transcription factor and TGF-beta/BMP signaling pathways. Aging cell. 2004;3(6):379–89. doi: 10.1111/j.1474-9728.2004.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao JJ, Gregoire BR, Gao H. High-fat diet decreases cancellous bone mass but has no effect on cortical bone mass in the tibia in mice. Bone. 2009;44(6):1097–104. doi: 10.1016/j.bone.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 18.Halade GV, El Jamali A, Williams PJ, Fajardo RJ, Fernandes G. Obesity-mediated inflammatory microenvironment stimulates osteoclastogenesis and bone loss in mice. Exp Gerontol. 2011;46(1):43–52. doi: 10.1016/j.exger.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kyung TW, Lee JE, Phan TV, Yu R, Choi HS. Osteoclastogenesis by bone marrow-derived macrophages is enhanced in obese mice. The Journal of nutrition. 2009;139(3):502–6. doi: 10.3945/jn.108.100032. [DOI] [PubMed] [Google Scholar]
- 20.Herroon MK, Rajagurubandara E, Hardaway AL, Powell K, Turchick A, Feldmann D, Podgorski I. Bone marrow adipocytes promote tumor growth in bone via FABP4-dependent mechanisms. Oncotarget. 2013;4(11):2108–23. doi: 10.18632/oncotarget.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown MD, Hart CA, Gazi E, Bagley S, Clarke NW. Promotion of prostatic metastatic migration towards human bone marrow stoma by Omega 6 and its inhibition by Omega 3 PUFAs. British journal of cancer. 2006;94(6):842–53. doi: 10.1038/sj.bjc.6603030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown MD, Hart C, Gazi E, Gardner P, Lockyer N, Clarke N. Influence of omega-6 PUFA arachidonic acid and bone marrow adipocytes on metastatic spread from prostate cancer. British journal of cancer. 2010;102(2):403–13. doi: 10.1038/sj.bjc.6605481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu Q, Han X, Peng J, Qin H, Wang Y. The role of CXC chemokines and their receptors in the progression and treatment of tumors. J Mol Histol. 2012;43(6):699–713. doi: 10.1007/s10735-012-9435-x. [DOI] [PubMed] [Google Scholar]
- 24.Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14(21):6735–41. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- 25.Acharyya S, Oskarsson T, Vanharanta S, Malladi S, Kim J, Morris PG, Manova-Todorova K, Leversha M, Hogg N, Seshan VE, Norton L, Brogi E, Massague J. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell. 2012;150(1):165–78. doi: 10.1016/j.cell.2012.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Filippo K, Dudeck A, Hasenberg M, Nye E, van Rooijen N, Hartmann K, Gunzer M, Roers A, Hogg N. Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood. 2013;121(24):4930–7. doi: 10.1182/blood-2013-02-486217. [DOI] [PubMed] [Google Scholar]
- 27.Kopesky P, Tiedemann K, Alkekhia D, Zechner C, Millard B, Schoeberl B, Komarova SV. Autocrine signaling is a key regulatory element during osteoclastogenesis. Biol Open. 2014;3(8):767–76. doi: 10.1242/bio.20148128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Onan D, Allan EH, Quinn JM, Gooi JH, Pompolo S, Sims NA, Gillespie MT, Martin TJ. The chemokine Cxcl1 is a novel target gene of parathyroid hormone (PTH)/PTH-related protein in committed osteoblasts. Endocrinology. 2009;150(5):2244–53. doi: 10.1210/en.2008-1597. [DOI] [PubMed] [Google Scholar]
- 29.Valerio MS, Herbert BA, Basilakos DS, Browne C, Yu H, Kirkwood KL. Critical role of MKP-1 in lipopolysaccharide-induced osteoclast formation through CXCL1 and CXCL2. Cytokine. 2014;71(1):71–80. doi: 10.1016/j.cyto.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 2013;339(6117):286–91. doi: 10.1126/science.1232227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herroon MK, Rajagurubandara E, Rudy DL, Chalasani A, Hardaway AL, Podgorski I. Macrophage cathepsin K promotes prostate tumor progression in bone. Oncogene. 2013;32(12):1580–93. doi: 10.1038/onc.2012.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Podgorski I, Linebaugh BE, Koblinski JE, Rudy DL, Herroon MK, Olive MB, Sloane BF. Bone marrow-derived cathepsin K cleaves SPARC in bone metastasis. Am J Pathol. 2009;175(3):1255–69. doi: 10.2353/ajpath.2009.080906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sadie-Van Gijsen H, Hough FS, Ferris WF. Determinants of bone marrow adiposity: the modulation of peroxisome proliferator-activated receptor-gamma2 activity as a central mechanism. Bone. 2013;56(2):255–65. doi: 10.1016/j.bone.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 34.Weilbaecher KN, Guise TA, McCauley LK. Cancer to bone: a fatal attraction. Nature reviews. 2011;11(6):411–25. doi: 10.1038/nrc3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Josson S, Nomura T, Lin JT, Huang WC, Wu D, Zhau HE, Zayzafoon M, Weizmann MN, Gururajan M, Chung LW. beta2-microglobulin induces epithelial to mesenchymal transition and confers cancer lethality and bone metastasis in human cancer cells. Cancer research. 2011;71(7):2600–10. doi: 10.1158/0008-5472.CAN-10-3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Odero-Marah VA, Wang R, Chu G, Zayzafoon M, Xu J, Shi C, Marshall FF, Zhau HE, Chung LW. Receptor activator of NF-kappaB Ligand (RANKL) expression is associated with epithelial to mesenchymal transition in human prostate cancer cells. Cell research. 2008;18(8):858–70. doi: 10.1038/cr.2008.84. [DOI] [PubMed] [Google Scholar]
- 37.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ. Osteoprotegerin Ligand Is a Cytokine that Regulates Osteoclast Differentiation and Activation. Cell. 1998;93(2):165–76. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 38.Yagi M, Miyamoto T, Sawatani Y, Iwamoto K, Hosogane N, Fujita N, Morita K, Ninomiya K, Suzuki T, Miyamoto K, Oike Y, Takeya M, Toyama Y, Suda T. DC-STAMP is essential for cell-cell fusion in osteoclasts and foreign body giant cells. The Journal of experimental medicine. 2005;202(3):345–51. doi: 10.1084/jem.20050645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okada Y, Naka K, Kawamura K, Matsumoto T, Nakanishi I, Fujimoto N, Sato H, Seiki M. Localization of matrix metalloproteinase 9 (92-kilodalton gelatinase/type IV collagenase = gelatinase B) in osteoclasts: implications for bone resorption. Lab Invest. 1995;72(3):311–22. [PubMed] [Google Scholar]
- 40.Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, Wagner EF, Mak TW, Kodama T, Taniguchi T. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002;3(6):889–901. doi: 10.1016/s1534-5807(02)00369-6. [DOI] [PubMed] [Google Scholar]
- 41.Inaoka T, Bilbe G, Ishibashi O, Tezuka K, Kumegawa M, Kokubo T. Molecular cloning of human cDNA for cathepsin K: novel cysteine proteinase predominantly expressed in bone. Biochem Biophys Res Commun. 1995;206(1):89–96. doi: 10.1006/bbrc.1995.1013. [DOI] [PubMed] [Google Scholar]
- 42.Zhao Q, Jia Y, Xiao Y. Cathepsin K: a therapeutic target for bone diseases. Biochem Biophys Res Commun. 2009;380(4):721–3. doi: 10.1016/j.bbrc.2009.01.139. [DOI] [PubMed] [Google Scholar]
- 43.Kavandi L, Collier MA, Nguyen H, Syed V. Progesterone and calcitriol attenuate inflammatory cytokines CXCL1 and CXCL2 in ovarian and endometrial cancer cells. J Cell Biochem. 2012;113(10):3143–52. doi: 10.1002/jcb.24191. [DOI] [PubMed] [Google Scholar]
- 44.Killian PH, Kronski E, Michalik KM, Barbieri O, Astigiano S, Sommerhoff CP, Pfeffer U, Nerlich AG, Bachmeier BE. Curcumin inhibits prostate cancer metastasis in vivo by targeting the inflammatory cytokines CXCL1 and -2. Carcinogenesis. 2012;33(12):2507–19. doi: 10.1093/carcin/bgs312. [DOI] [PubMed] [Google Scholar]
- 45.Roodman GD. High bone turnover markers predict poor outcome in patients with bone metastasis. J Clin Oncol. 2005;23(22):4821–2. doi: 10.1200/JCO.2005.02.911. [DOI] [PubMed] [Google Scholar]
- 46.Roodman GD. Genes associate with abnormal bone cell activity in bone metastasis. Cancer Metastasis Rev. 2012;31(3-4):569–78. doi: 10.1007/s10555-012-9372-x. [DOI] [PubMed] [Google Scholar]
- 47.Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001;27(3):165–76. doi: 10.1053/ctrv.2000.0210. [DOI] [PubMed] [Google Scholar]
- 48.Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350(16):1655–64. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 49.Kingsley LA, Fournier PG, Chirgwin JM, Guise TA. Molecular biology of bone metastasis. Mol Cancer Ther. 2007;6(10):2609–17. doi: 10.1158/1535-7163.MCT-07-0234. [DOI] [PubMed] [Google Scholar]
- 50.Lee RJ, Saylor PJ, Smith MR. Treatment and prevention of bone complications from prostate cancer. Bone. 2011;48(1):88–95. doi: 10.1016/j.bone.2010.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roato I, D’Amelio P, Gorassini E, Grimaldi A, Bonello L, Fiori C, Delsedime L, Tizzani A, De Libero A, Isaia G, Ferracini R. Osteoclasts are active in bone forming metastases of prostate cancer patients. PLoS ONE. 2008;3(11):e3627. doi: 10.1371/journal.pone.0003627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gartrell BA, Saad F. Managing bone metastases and reducing skeletal related events in prostate cancer. Nature reviews Clinical oncology. 2014;11(6):335–45. doi: 10.1038/nrclinonc.2014.70. [DOI] [PubMed] [Google Scholar]
- 53.Hardaway AL, Herroon MK, Rajagurubandara E, Podgorski I. Bone marrow fat: linking adipocyte-induced inflammation with skeletal metastases. Cancer Metastasis Rev. 2014 doi: 10.1007/s10555-013-9484-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang D,T. Magnetic Resonance Imaging of Bone Marrow: A Review – Part I. J Am Osteopath Coll Radiol. 2012;1(2):1–12. [Google Scholar]
- 55.Gazi E, Gardner P, Lockyer NP, Hart CA, Brown MD, Clarke NW. Direct evidence of lipid translocation between adipocytes and prostate cancer cells with imaging FTIR microspectroscopy. Journal of lipid research. 2007;48(8):1846–56. doi: 10.1194/jlr.M700131-JLR200. [DOI] [PubMed] [Google Scholar]
- 56.Guise TA, Mohammad KS, Clines G, Stebbins EG, Wong DH, Higgins LS, Vessella R, Corey E, Padalecki S, Suva L, Chirgwin JM. Basic mechanisms responsible for osteolytic and osteoblastic bone metastases. Clin Cancer Res. 2006;12(20 Pt 2):6213s–6s. doi: 10.1158/1078-0432.CCR-06-1007. [DOI] [PubMed] [Google Scholar]
- 57.Schneider A, Kalikin LM, Mattos AC, Keller ET, Allen MJ, Pienta KJ, McCauley LK. Bone turnover mediates preferential localization of prostate cancer in the skeleton. Endocrinology. 2005;146(4):1727–36. doi: 10.1210/en.2004-1211. [DOI] [PubMed] [Google Scholar]
- 58.Tokuda Y, Satoh Y, Fujiyama C, Toda S, Sugihara H, Masaki Z. Prostate cancer cell growth is modulated by adipocyte-cancer cell interaction. BJU Int. 2003;91(7):716–20. doi: 10.1046/j.1464-410x.2003.04218.x. [DOI] [PubMed] [Google Scholar]
- 59.Clarke NW, Hart CA, Brown MD. Molecular mechanisms of metastasis in prostate cancer. Asian J Androl. 2009;11(1):57–67. doi: 10.1038/aja.2008.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ha J, Choi HS, Lee Y, Kwon HJ, Song YW, Kim HH. CXC chemokine ligand 2 induced by receptor activator of NF-kappa B ligand enhances osteoclastogenesis. J Immunol. 2010;184(9):4717–24. doi: 10.4049/jimmunol.0902444. [DOI] [PubMed] [Google Scholar]
- 61.Oue E, Lee JW, Sakamoto K, Iimura T, Aoki K, Kayamori K, Michi Y, Yamashiro M, Harada K, Amagasa T, Yamaguchi A. CXCL2 synthesized by oral squamous cell carcinoma is involved in cancer-associated bone destruction. Biochem Biophys Res Commun. 2012;424(3):456–61. doi: 10.1016/j.bbrc.2012.06.132. [DOI] [PubMed] [Google Scholar]
- 62.Yamashita A, Soga Y, Iwamoto Y, Asano T, Li Y, Abiko Y, Nishimura F. DNA microarray analyses of genes expressed differentially in 3T3-L1 adipocytes co-cultured with murine macrophage cell line RAW264.7 in the presence of the toll-like receptor 4 ligand bacterial endotoxin. International journal of obesity. 2008;32(11):1725–9. doi: 10.1038/ijo.2008.153. [DOI] [PubMed] [Google Scholar]
- 63.Dermitzaki E, Liapakis G, Androulidaki A, Venihaki M, Melissas J, Tsatsanis C, Margioris AN. Corticotrophin-Releasing Factor (CRF) and the urocortins are potent regulators of the inflammatory phenotype of human and mouse white adipocytes and the differentiation of mouse 3T3L1 pre-adipocytes. PloS one. 2014;9(5):e97060. doi: 10.1371/journal.pone.0097060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim HS, Han SY, Sung HY, Park SH, Kang MK, Han SJ, Kang YH. Blockade of visfatin induction by oleanolic acid via disturbing IL-6-TRAF6-NF-kappaB signaling of adipocytes. Experimental biology and medicine. 2014;239(3):284–92. doi: 10.1177/1535370213514511. [DOI] [PubMed] [Google Scholar]
- 65.Munoz A, Costa M. Nutritionally mediated oxidative stress and inflammation. Oxidative medicine and cellular longevity. 20132013:610950. doi: 10.1155/2013/610950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hol J, Wilhelmsen L, Haraldsen G. The murine IL-8 homologues KC, MIP-2, and LIX are found in endothelial cytoplasmic granules but not in Weibel-Palade bodies. Journal of leukocyte biology. 2010;87(3):501–8. doi: 10.1189/jlb.0809532. [DOI] [PubMed] [Google Scholar]
- 67.Bendre MS, Montague DC, Peery T, Akel NS, Gaddy D, Suva LJ. Interleukin-8 stimulation of osteoclastogenesis and bone resorption is a mechanism for the increased osteolysis of metastatic bone disease. Bone. 2003;33(1):28–37. doi: 10.1016/s8756-3282(03)00086-3. [DOI] [PubMed] [Google Scholar]
- 68.Sundaram K, Rao DS, Ries WL, Reddy SV. CXCL5 stimulation of RANK ligand expression in Paget’s disease of bone. Laboratory investigation; a journal of technical methods and pathology. 2013;93(4):472–9. doi: 10.1038/labinvest.2013.5. [DOI] [PubMed] [Google Scholar]
- 69.Chavey C, Lazennec G, Lagarrigue S, Clape C, Iankova I, Teyssier J, Annicotte JS, Schmidt J, Mataki C, Yamamoto H, Sanches R, Guma A, Stich V, Vitkova M, Jardin-Watelet B, Renard E, Strieter R, Tuthill A, Hotamisligil GS, Vidal-Puig A, Zorzano A, Langin D, Fajas L. CXC ligand 5 is an adipose-tissue derived factor that links obesity to insulin resistance. Cell metabolism. 2009;9(4):339–49. doi: 10.1016/j.cmet.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bruun JM, Lihn AS, Madan AK, Pedersen SB, Schiott KM, Fain JN, Richelsen B. Higher production of IL-8 in visceral vs. subcutaneous adipose tissue. Implication of nonadipose cells in adipose tissue. American journal of physiology Endocrinology and metabolism. 2004;286(1):E8–13. doi: 10.1152/ajpendo.00269.2003. [DOI] [PubMed] [Google Scholar]
- 71.Fain JN. Release of inflammatory mediators by human adipose tissue is enhanced in obesity and primarily by the nonfat cells: a review. Mediators of inflammation. 2010;2010:513948. doi: 10.1155/2010/513948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Krings A, Rahman S, Huang S, Lu Y, Czernik PJ, Lecka-Czernik B. Bone marrow fat has brown adipose tissue characteristics, which are attenuated with aging and diabetes. Bone. 2012;50(2):546–52. doi: 10.1016/j.bone.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shankar E, Vykhovanets EV, Vykhovanets OV, Maclennan GT, Singh R, Bhaskaran N, Shukla S, Gupta S. High-fat diet activates pro-inflammatory response in the prostate through association of Stat-3 and NF-kappaB. Prostate. 2012;72(3):233–43. doi: 10.1002/pros.21425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Suburu J, Chen YQ. Lipids and prostate cancer. Prostaglandins Other Lipid Mediat. 2012;98(1-2):1–10. doi: 10.1016/j.prostaglandins.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Leaker BR, Barnes PJ, O’Connor B. Inhibition of LPS-induced airway neutrophilic inflammation in healthy volunteers with an oral CXCR2 antagonist. Respir Res. 2013;14:137. doi: 10.1186/1465-9921-14-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nair P, Gaga M, Zervas E, Alagha K, Hargreave FE, O’Byrne PM, Stryszak P, Gann L, Sadeh J, Chanez P. Safety and efficacy of a CXCR2 antagonist in patients with severe asthma and sputum neutrophils: a randomized, placebo-controlled clinical trial. Clin Exp Allergy. 2012;42(7):1097–103. doi: 10.1111/j.1365-2222.2012.04014.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.