Abstract
Ambient temperature reduction (ATR) can extend the lifespan of organisms, but the underlying mechanism is poorly understood. In this study, cellular degradation activity was evaluated in the muscle of an annual fish (Nothobranchius rachovii) reared under high (30 °C), moderate (25 °C), and low (20 °C) ambient temperatures. The results showed the following: (i) the activity of the 20S proteasome and the expression of polyubiquitin aggregates increased with ATR, whereas 20S proteasome expression did not change; (ii) the expression of microtubule-associated protein 1 light chain 3-II (LC3-II) increased with ATR; (iii) the expression of lysosome-associated membrane protein type 2a (Lamp 2a) increased with ATR, whereas the expression of the 70-kD heat shock cognate protein (Hsc 70) decreased with ATR; (iv) lysosome activity increased with ATR, whereas the expression of lysosome-associated membrane protein type 1 (Lamp 1) did not change with ATR; and (v) the expression of molecular target of rapamycin (mTOR) and phosphorylated mTOR (p-mTOR) as well as the p-mTOR/mTOR ratio did not change with ATR. These findings indicate that ATR activates cellular degradation activity, constituting part of the mechanism underlying the longevity-promoting effects of ATR in N. rachovii.
Keywords: Aging, Temperature, Degradation, Autophagy, Fish
Introduction
Sustained ambient temperature reduction (ATR) has been shown to successfully extend the lifespan of organisms, including Caenorhabditis elegans, Drosophila melanogaster, and fishes (Cynolebias adloffi, N. furzeri, N. rachovii, and N. guentheri) (Wilson et al. 2006; Galbadage and Hartman 2008; Xiao et al. 2013; Zheng et al. 2005; Liu and Walford 1966; Valenzano et al. 2006; Hsu and Chiu 2009; Wang et al. 2014). In general, low ambient temperatures prolong the lifespan of these organisms, and high ambient temperatures shortened their lifespan. This observation is supported by evidence collected under the following conditions: (i) C. elegans cultured at 25, 20, and 15 °C as well as at 25, 18.5, and 12 °C (Wilson et al. 2006; Galbadage and Hartman 2008; Xiao et al. 2013); (ii) D. melanogaster maintained at 29, 25, and 18 °C (Zheng et al. 2005); (iii) N. furzeri bred at 25 and 22 °C (Valenzano et al. 2006); (iv) N. rachovii reared at 30, 25, and 20 °C (Hsu and Chiu 2009); and (v) N. guentheri reared at 26 and 22 °C (Wang et al. 2014).
In addition, ATR has been shown to delay lipid oxidative damage in D. melanogaster, N. rachovii, and N. guentheri (Zheng et al. 2005; Hsu and Chiu 2009; Wang et al. 2014), reduce the accumulation of lipofuscin granules in N. furzeri, N. rachovii, and N. guentheri (Valenzano et al. 2006; Hsu and Chiu 2009; Wang et al. 2014), reduce reactive oxygen species (ROS) production and protein oxidation in N. rachovii (Hsu and Chiu 2009), increase catalase (CAT), glutathione peroxidase (GPx), and manganese-superoxide dismutase (Mn-SOD) activity in N. rachovii and N. guentheri (Hsu and Chiu 2009; Wang et al. 2014), and increase mitochondrial function (Hsu and Chiu 2009).
Two major cellular degradation pathways remove cytoplasmic materials and organelles through lysosomal degradation: (i) the ubiquitin-proteasome pathway, which is responsible for the degradation of most short-lived proteins (Ciechanover 2005), and (ii) the autophagy-lysosomal pathway, which is responsible for the degradation of most long-lived proteins and some organelles (Cuervo et al. 2005). The autophagy-lysosomal pathway can be further divided into three different types: macroautophagy, microautophagy, and chaperone-mediated autophagy (CMA), depending on the route for the delivery of intracellular cargos to the lysosomal lumen (Levine and Klionsky 2004; Cuervo et al. 2005). Microtubule-associated protein 1 light chain 3 (LC3) (Atg8) serves as a regulatory protein for macroautophagy. Specifically, microtubule-associated protein 1 light chain 3-II (LC3-II) is a membrane-bound form of LC3 that localizes to autophagosomes and can be used as a biomarker of macroautophagic activity (Kabeya et al. 2000; Hsu et al. 2014). 70-kD heat shock cognate protein (Hsc 70) recognizes substrate proteins containing the KFERQ pentapeptide motif and subsequently binds to the lysosomal membrane receptor Lamp 2a. The substrate proteins are then transported into the lysosomal matrix via lysosomal Hsc 70 and are rapidly degraded by the lysosomal proteases (Majeski and Dice 2004). Hsc 70 and Lamp 2a are used as biomarkers of CMA activity (Cuervo and Dice 2000a; Majeski and Dice 2004). Autophagy can be inhibited by molecular target of rapamycin (mTOR) or its phosphorylated, activated form phosphorylated mTOR (p-mTOR) (Ser2448) (Dennis et al. 2001; Díaz-Troya et al. 2008).
The current study sought to clarify the relationship between cellular degradation activity and lifespan extension under ATR by evaluating cellular degradation biomarkers including the 20S proteasome, polyubiquitin aggregates, LC3-II, Hsc 70, Lamp 2a, acid phosphatase, Lamp 1, mTOR, and p-mTOR in the muscle of an annual fish (N. rachovii) reared at high (30 °C), moderate (25 °C), and low (20 °C) ambient temperatures.
Materials and methods
Fish breeding and maintenance
An in-house tank (120 × 45 × 30 cm3) was evenly divided into four sectors. A gap was created at the bottom between two sectors to allow water circulation. Sequentially, the first sector was used to filter circulating water and pump air, and the remaining sectors were used for fish breeding. The circulating water was sucked at the last sector and poured into the first sector. Each fish-breeding sector was subdivided into 12 cages, and each cage contained only one male for breeding. Each cage was 10 × 10 × 30 cm3 and was enclosed with a 200-mesh plastic network to prevent the escape of brine shrimp and water fleas to assure that all of the supplied food was consumed by the fish. The temperatures of the three tanks were set at 30, 25, or 20 °C. A total of 108 4-month-old male N. rachovii were randomly assigned to the three tanks. Thus, each fish-breeding sector contained 12 males, with one male per cage. The male fish were maintained on a 14-h light/10-h dark cycle and were fed live brine shrimp and water fleas twice daily. Each male was fed approximately 1200 live brine shrimp and 270 water fleas at each feeding time. The fish were reared at 30, 25, or 20 °C for 45 days for the following analyses.
20S proteasome activity
Cellular supernatant preparation was carried out as described previously (Hsu et al. 2008; Hsu and Chiu 2009). Briefly, 1 g of muscle tissue consisting of both red and white muscle was homogenized in phosphate-buffered saline (PBS) containing 0.5 % Triton X-100 without protease inhibitors, followed by centrifugation at 5000g for 10 min at 4 °C. The protein concentration was determined using a protein assay reagent (500-0006; Bio-Rad Laboratories, Hercules, CA, USA). The activity of the 20S proteasome was quantified using a 20S Proteasome Activity Assay Kit (APT280; Chemicon International, Temecula, CA, USA) according to the manufacturer’s instructions, as described previously (Hsu et al. 2014). The specific activity was expressed as 7-amino-4-methylcoumarin (AMC) pmol min−1 mg−1 of protein. This experiment was biologically replicated ten times for each of the three temperature settings.
Lysosomal activity
Acid phosphatase is one of the acid hydrolases present in the lysosome. Thus, we evaluated lysosomal activity by measuring acid phosphatase activity with an Acid Phosphatase Assay Kit (CS0740; Sigma, Saint Louis, MO, USA) according to the manufacturer’s instructions, as described previously (Hsu et al. 2014). The specific activity was expressed as milliunits (mUnits) mg−1. This experiment was biologically replicated ten times for each of the three temperature settings.
Western blotting
Muscle was isolated from each individual of N. rachovii. Approximately 0.2 g of muscle tissue was homogenized in 1 ml of radioimmunoprecipitation assay (RIPA) buffer containing protease inhibitors (11697498001; Roche Applied Science, Indianapolis, IN, USA), followed by centrifugation at 5000g for 10 min at 4 °C. The protein concentration in the resulting supernatant was determined using a protein assay reagent (500-0006; Bio-Rad Laboratories). A total of 30 μg of protein from the supernatant of each sample was electrophoresed in 10–15 % acrylamide SDS/PAGE gels and transferred to polyvinylidene fluoride (PVDF) membranes. After blocking by 5 % skim milk for 1 h at 25 °C, the membranes were incubated with antibodies against the 20S proteasome (1:1000) (sc-58412; Santa Cruz Biotechnology, Santa Cruz, CA, USA), LC3 (1:1000) (ap1801b; Abgent, San Diego, CA, USA), Hsc 70 (1:1000) (sc-24; Santa Cruz Biotechnology), Lamp 2a (1:1000) (ab18528; Abcam, Cambridge, MA, USA), Lamp 1 (1:1000) (AP1823b; Abgent), mTOR (cs-8319; Santa Cruz Biotechnology), p-mTOR (GTX79009; GeneTex, Irvine, CA, USA), ubiquitin (1:1000) (3936; Cell Signaling Technology, Danvers, MA, USA), or tubulin (1:10,000) (ab6046; Abcam). The membranes were then probed with corresponding secondary antibodies labeled with horseradish peroxidase (1:10,000) (Klionsky et al. 2012). The immunolabeled proteins were detected using a chemiluminescence method (PerkinElmer, Covina, CA, USA) and analyzed with the Image J software (NIH, Bethesda, MA, USA). Protein expression levels were normalized to that of tubulin. This experiment was biologically replicated six times for each of the three temperature settings.
Statistical analysis
The differences between the mean values obtained in the three temperature treatment groups were analyzed via one-way ANOVA and using Tukey’s HSD for pairwise comparisons. Statistical significance was set at 0.05.
Results
20S proteasome
To examine the activity of the ubiquitin-proteasome pathway, we assessed the activity and expression of the 20S proteasome in the muscle of N. rachovii. The mean 20S proteasome activities were 47.83 ± 1.80, 40.42 ± 3.52, and 35.35 ± 3.25 pmol of 7-amino-4-methylcoumarin (AMC) min−1 mg−1 of the protein in the muscle of N. rachovii reared at 20, 25, and 30 °C, respectively, indicating that ATR increased 20S proteasome activity (n = 10, P < 0.05; Fig. 1a). However, the expression of the 20S proteasome did not show a statistically significant change at different temperatures (n = 6, P > 0.05; Fig. 1b, c), indicating that ATR did not affect 20S proteasome expression.
Fig. 1.
The activity and expression of 20S proteasome and the expression of polyubiquitin aggregates in the muscle of N. rachovii reared at 20, 25, and 30 °C. a 20S proteasome activity. The bars represent the mean ± the standard error of the mean (SEM). b The expression of the 20S proteasome was analyzed through western blotting. Tubulin served as the loading control. c The levels of 20S proteasome expression were calculated from the densities of the low bands and normalized to that of the 20 °C sample; the results are shown as percentages and represent the mean ± SEM. Asterisk indicates statistical significance determined via one-way ANOVA (*P < 0.05). d The expression of polyubiquitin aggregates was analyzed through western blotting. Tubulin served as the loading control
To confirm the activity of the ubiquitin-proteasome pathway, we examined the expression of polyubiquitin aggregates in the muscle of N. rachovii. The expression of polyubiquitin aggregates decreased with ATR (n = 6, Fig. 1d), indicating that ATR activated the ubiquitin-proteasome pathway.
Autophagy
To investigate the activity of the autophagy-lysosomal pathway, we assessed the expression of LC3, Hsc 70, and Lamp 2a in the muscle of N. rachovii. The expression of LC3-II was high at 20 °C, moderate at 25 °C, and low at 30 °C (Fig. 2a). The observed differences in LC3-II expression were statistically significant (n = 6, P < 0.01; Fig. 2b), suggesting that LC3-II expression is inversely correlated with temperature in N. rachovii. This finding suggests that ATR increases macroautophagic activity in N. rachovii.
Fig. 2.
LC3-II expression in the muscle of N. rachovii reared at 20, 25, and 30 °C. a LC3-II expression was analyzed through western blotting. Tubulin served as the loading control. b LC3-II expression levels were normalized to that of the 20 °C sample. The results are shown as percentages and represent the mean ± SEM. Asterisks indicate statistical significance determined via one-way ANOVA (**P < 0.01)
In contrast, the expression of Hsc 70 was low at 20 °C, moderate at 25 °C, and high at 30 °C (Fig. 3a). This positive correlation between Hsc 70 expression and temperature was also statistically significant in N. rachovii (n = 6, P < 0.01; Fig. 3b). In addition, a significant correlation between Lamp 2a expression and temperature was observed, where Lamp 2a expression was high at 20 °C, moderate at 25 °C, and low at 30 °C (n = 6, P < 0.05; Fig. 3c, d). These findings suggest that ATR increases CMA activity in N. rachovii.
Fig. 3.
Hsc 70 and Lamp 2a expression in the muscle of N. rachovii reared at 20, 25, and 30 °C. Protein expression was determined by western blot. Tubulin served as the loading control. Band intensity was used to quantify protein level and was normalized to that of the 20 °C sample. a Hsc 70 western blot. b Normalized Hsc 70 protein levels. c Lamp 2a western blot. d Normalized Lamp 2a protein levels. The results are shown as percentages and represent the mean ± SEM. Asterisks indicate statistical significance determined via one-way ANOVA (*P < 0.05, **P < 0.01)
Lysosome activity and density
Lysosomes play important roles in cellular degradation. Acid phosphatase is one of the acid hydrolases found in lysosomes that has been used as a classical marker of lysosome activity (Hsu et al. 2014). Therefore, we measured acid phosphatase activity to evaluate lysosome function. The mean acid phosphatase activities were 15.25 ± 1.17, 13.43 ± 0.73, and 11.91 ± 0.78 milliunits (mUnits) mg−1 in the muscle of N. rachovii reared at 20, 25, and 30 °C, respectively, indicating that ATR increases lysosome activity (n = 10, P < 0.05; Fig. 4a).
Fig. 4.
Lysosomal activity and Lamp 1 expression in the muscle of N. rachovii reared at 20, 25, and 30 °C. a Lysosomal activity. The bars represent the mean ± SEM. b Lamp 1 expression was analyzed through western blotting. Tubulin served as the loading control. c Lamp 1 expression levels were normalized to that of the 20 °C samples. The results are shown as percentages and represent the mean ± SEM. Asterisk indicates statistical significance determined via one-way ANOVA (*P < 0.05)
Given that acid phosphatase is located inside the lysosome, we then evaluated whether the increase in acid phosphatase activity was due to an increase in the amount of lysosomes. Lysosome density was assessed based on Lamp 1 expression. Lamp 1 expression did not show a statistically significant change at different rearing temperatures (20, 25, and 30 °C) (n = 6, P > 0.05; Fig. 4b, c), suggesting that lysosome density is independent of temperature in N. rachovii.
mTOR activity
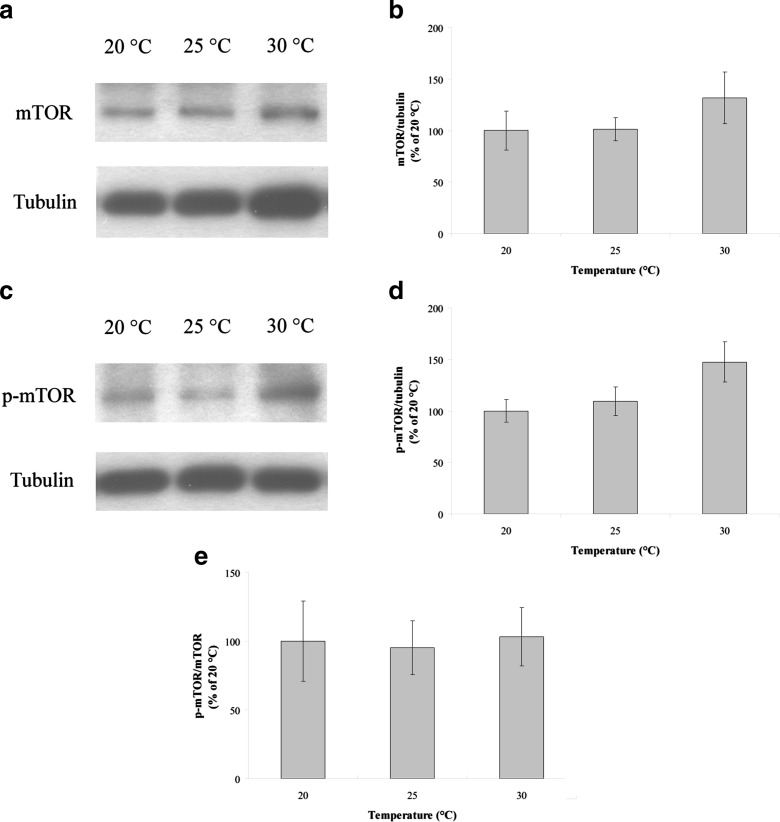
To further evaluate the cellular degradation activity, we assessed mTOR activity because mTOR is known to inhibit autophagy (Dennis et al. 2001). The expression of neither mTOR (n = 6, P > 0.05; Fig. 5a, b) nor p-mTOR (n = 6, P > 0.05; Fig. 5c, d), which is the activated form, was significantly altered under ATR. Furthermore, the p-mTOR/mTOR ratio, which was used to represent mTOR activity, also showed no statistically significant difference (n = 6, P > 0.05; Fig. 5e), suggesting that ATR-induced autophagy might not be regulated by mTOR.
Fig. 5.
mTOR expression and activity in the muscle of N. rachovii reared at 20, 25, and 30 °C. Protein expression was determined by western blot. Tubulin served as the loading control. Band intensity was used to quantify protein level and was normalized to that of the 20 °C sample. a mTOR western blot. b Normalized mTOR protein levels. c p-mTOR western blot. d Normalized p-mTOR protein levels. e The p-mTOR/mTOR ratio. The results are shown as percentages and represent the mean ± SEM
Discussion
In this study, we evaluated cellular degradation activity in the muscle of N. rachovii reared at 20, 25, and 30 °C. The activity of the 20S proteasome, the expression of polyubiquitin aggregates, LC3-II and Lamp 2a, and lysosomal activity were all increased under ATR. However, Hsc 70 expression was decreased with ATR. In contrast, the expression of the 20S proteasome, Lamp 1, mTOR, and p-mTOR as well as the p-mTOR/mTOR ratio did not change with ATR. These results demonstrate that ATR activates cellular degradation activity, which may be the underlying mechanism for the longevity-promoting effects of ATR.
20S proteasome
The proteasome is responsible for the degradation of normal proteins and proteins that have been damaged by oxidation or misfolding. Previous studies have shown that the activity of the proteasome system declines with aging (Bulteau et al. 2000, 2002; Ferrington et al. 2005; Hsu et al. 2014). In this study, 20S proteasome activity was found to increase under ATR in the muscle of N. rachovii reared at 20, 25, and 30 °C, indicating that low ambient temperature induces high 20S proteasome activity, whereas high ambient temperature inhibits 20S proteasome activity. Low ambient temperature has been shown to extend the lifespan of organisms and appears to exhibit cellular rejuvenation potential, whereas high ambient temperature has been demonstrated to shorten the lifespan of organisms and appears to display cellular senescence potential (Wilson et al. 2006; Galbadage and Hartman 2008; Xiao et al. 2013; Zheng et al. 2005; Liu and Walford 1966; Valenzano et al. 2006; Hsu and Chiu 2009). Compared with the cellular consequences of aging, low ambient temperature was observed to activate 20S proteasome activity, hence inducing cellular rejuvenation; in contrast, high ambient temperature was found to inhibit 20S proteasome activity, leading to cellular senescence. This inference is supported by previous studies showing that young worker bees (Apis mellifera) tend to present a higher level of 20S proteasome activity, which results in trophocytes with clear homogeneous cytoplasms; distinct, smooth organelle morphologies; and clear organelle inclusions. In contrast, old workers tend to exhibit a lower level of the 20S proteasome activity, which leads to trophocytes with dark cytoplasm; vague, rough organelle morphologies; dark organelle inclusions; and a crowded distribution of organelles (Hsieh and Hsu 2011; Hsu et al. 2014).
Proteasome activity decreases with aging as a result of protein modifications, including oxidation, ubiquitination, glycation, glycoxidation, and conjugation with lipid peroxidation products (Bulteau et al. 2000). In this study, the decrease in 20S proteasome activity associated with the increase in ambient temperature may have had similar causes. This speculation is supported by previous studies showing that high ambient temperature increases protein oxidation and lipid peroxidation (Zheng et al. 2005; Hsu and Chiu 2009). In addition, earlier studies have indicated that proteasome activity can be inhibited by lipofuscin/ceroid and that an increase in lipofuscin/ceroid accumulation leads to a decrease in proteasome activity in human lung fibroblasts (Sitte et al. 2000) as well as in the trophocytes and fat cells of worker bees (Hsu et al. 2014). In this study, 20S proteasome activity was found to increase under ATR, which, combined with the fact that lipofuscin accumulation decreases with ATR (Hsu and Chiu 2009), indicates that lipofuscin might inhibit 20S proteasome activity at high ambient temperatures in N. rachovii.
The expression level of the 20S proteasome has been shown to increase with age (Ferrington et al. 2005; Hsu et al. 2014). Increased expression of the 20S proteasome has also been hypothesized to compensate for the decline in the 20S proteasome function (Ferrington et al. 2005; Hsu et al. 2014). The gene expression of proteasome subunits and ubiquitin-conjugated enzymes increases with ATR (from 30 to 23, 17, or 10 °C) in the common carp (Cyprinus carpio) for 21–19 days (Gracey et al. 2004). In addition, the gene expression of 26S proteasome increases with ATR (37, 26, and 20 °C) in an annual killifish (Austrofundulus limnaeus) for 14 days (Podrabsky and Somero 2004). In this study, the expression of the 20S proteasome was not found to change with ATR in the muscle of N. rachovii reared at 20, 25, and 30 °C. This finding is not consistent with the cellular consequences of aging and ATR in the common carp and killifish (Ferrington et al. 2005; Hsu et al. 2014; Gracey et al. 2004; Podrabsky and Somero 2004). The most likely reason for this result is that ambient temperatures did not influence the accumulation of 20S proteasome, that the duration of high-temperature-induced cellular senescence was not sufficiently long for 20S proteasome accumulation to occur, or that post-transcriptional, translational, and degradation regulation influenced the synthesis of 20S proteasome under ATR (Vogel and Marcotte 2012).
Autophagy
Macroautophagy sequesters damaged organelles and proteins in a double-membrane autophagosome that then fuses with the lysosome to form the autophagolysosome, the contents of which are degraded by acidic lysosomal hydrolases (Klionsky et al. 2012). LC3 participates in the formation of the autophagosome, suggesting that a decrease in LC3-II expression may indicate a decrease in autophagosome formation. Previous studies showed that macroautophagic activity declines with aging (Del Roso et al. 2003; Taneike et al. 2010; Caramés et al. 2010; Hsu and Chan 2013; Hsu et al. 2014). In this study, LC3-II expression increased with ATR in the muscle of N. rachovii reared at 20, 25, and 30 °C, indicating that low ambient temperature activated macroautophagy activity, whereas high ambient temperature inhibited macroautophagy activity. This observation is similar to our findings regarding 20S proteasome activity.
CMA uses Hsc 70 to select soluble cytosolic proteins that are directly translocated across the lysosome membrane for degradation via Lamp 2a (Klionsky et al. 2012). Previous studies demonstrated that the expression level of Hsc 70 decreases with age (Bernstein et al. 2000; Bonelli et al. 2008; Unterluggauer et al. 2009; Hsu et al. 2014). In the present study, Hsc 70 expression decreased with ATR in the muscle of N. rachovii reared at 20, 25, and 30 °C, a phenomenon that is not consistent with the cellular consequences of aging. A possible explanation for this discrepancy is that high temperature induces Hsc 70 expression to response heat stress (Liu et al. 2012).
The expression levels of Hsc 70 and Lamp 2a are known to correlate with the extent of CMA activity (Cuervo and Dice 2000a; Majeski and Dice 2004). The binding of substrates to Lamp 2a is the limiting step for their degradation via CMA (Cuervo and Dice 1996). Previous studies showed that the expression levels of Lamp 2a decrease with age (Cuervo and Dice 2000b; Kiffin et al. 2007; Zhang and Cuervo 2008), indicating that CMA activity declines with age. In this study, Lamp 2a expression increased with ATR in the muscle of N. rachovii reared at 20, 25, and 30 °C, indicating that low ambient temperature activated CMA activity whereas high ambient temperature inhibited CMA activity. This finding is consistent with those of the 20S proteasome activity and macroautophagy activity.
Similar to 20S proteasome, low ambient temperature may activate CMA activity to induce cellular rejuvenation and high ambient temperature may inhibit CMA activity to induce senescence. This postulation is supported by previous studies showing that both old rodents and senescent cells in culture exhibit reduced rates of substrate protein translocation into lysosomes through CMA (Cuervo and Dice 2000b; Martinez-Vicente et al. 2005) and that the age-dependent decrease in CMA activity contributes to the intracellular accumulation of oxidized proteins in aged organisms (Martinez-Vicente et al. 2005; Zhang and Cuervo 2008).
In addition, previous studies have indicated that lipofuscin accumulation reduces CMA activity, leading to the accumulation of oxidized proteins in worker bees (Hsu et al. 2014; Hsieh and Hsu 2011). Likewise, it has been shown that lysosomes with higher CMA activity rarely accumulate lipofuscin in their lumens (Kiffin et al. 2006). In this study, CMA activity was observed to increase with ATR, while lipofuscin accumulation decreased with ATR (Hsu and Chiu 2009), suggesting that lipofuscin might inhibit CMA activity at high ambient temperatures in N. rachovii.
Lysosome activity and density
Lysosomes are single-membrane organelles that contain acid hydrolase enzymes for degrading cellular macromolecules and organelles. Previous studies showed that lysosomal activity increases with age (Cuervo and Dice 2000b; Yoon et al. 2010; Hsu et al. 2014). Lysosomal activity is increased to scavenge accumulated intracellular waste in aged individuals. In the present study, we observed increased lysosome activity under ATR, indicating that low ambient temperature activates lysosome activity, while high ambient temperature inhibits lysosome activity. This result is consistent with the findings regarding 20S proteasome activity, macroautophagy, and CMA, suggesting that ATR induces “housekeeping” activities in cells.
Lamp 1 is a lysosomal membrane protein that may serve as a biomarker of lysosomes (Banno et al. 2012). Previous studies revealed that the density of lysosomes increases with age (Cuervo and Dice 2000b; Yoon et al. 2010; Hsu et al. 2014). In this study, Lamp 1 expression did not change with ATR, indicating that the lysosomal density was not affected by ATR, a phenomenon that is not consistent with the phenotypes of aging cells. The most likely reason for this discrepancy is that ambient temperatures have no effect on lysosomal accumulation or that the duration of high-temperature-induced senescence was not sufficiently long for lysosomal accumulation to take place.
mTOR activity
Autophagy can be inhibited by the mTOR protein, which is activated by phosphorylation at serine-2448 (p-mTOR) (Dennis et al. 2001; Díaz-Troya et al. 2008). Previous studies showed that mTOR expression increases with age (Zhou et al. 2009; Hsu et al. 2014). Earlier studies also demonstrated that macroautophagy could be inhibited by mTOR (Dennis et al. 2001; Hsu et al. 2014). In the present study, neither the expression of mTOR and p-mTOR nor the p-mTOR/mTOR ratio was altered with ATR, suggesting that mTOR does not regulate ATR-induced autophagy.
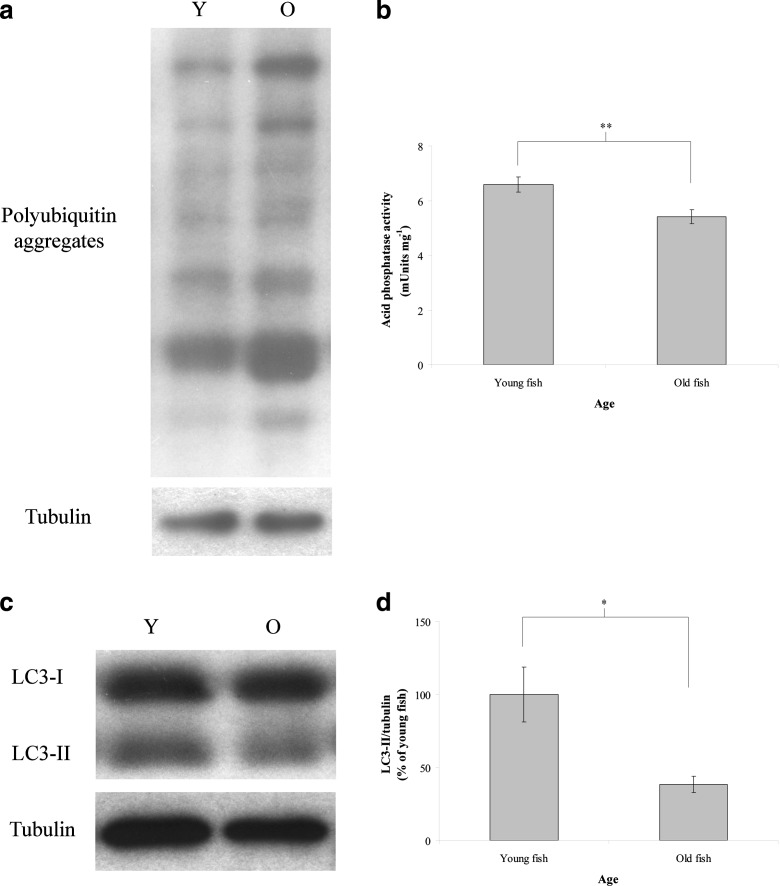
In this study, we demonstrated that ATR activates cellular degradation activity in N. rachovii. Previously, we showed that ATR could extend the lifespan of N. rachovii (Hsu and Chiu 2009). Together, these results suggest that ATR may activate cellular degradation activity to promote cellular rejuvenation, thus leading to lifespan extension. This inference is supported by the results of polyubiquitin aggregates, lysosomal activity, and LC3-II expression in the muscle of young (2-month-old) and old (5-month-old) N. rachovii. Young N. rachovii express lower polyubiquitin aggregates (Fig. 6a), higher lysosomal activity (n = 6, P <0.01; Fig. 6b), and higher LC3-II expression (n = 6, P < 0.05; Fig. 6c, d) than old N. rachovii. The results of ATR were reversely proportion to those of aging. This inference is also supported by previous studies showing that young worker bees display higher levels of cellular degradation activity, which leads to trophocytes with clear, homogeneous cytoplasm; distinct, smooth organelle morphologies; and clear organelle inclusions. In contrast, old worker bees exhibit lower levels of cellular degradation activity, resulting in trophocytes with dark cytoplasm; vague, rough organelle morphologies; dark organelle inclusions; and a crowded distribution of organelles (Hsieh and Hsu 2011; Hsu et al. 2014). This inference is also supported by previous studies showing that CMA, macroautophagy, and proteolysis are all decreased during aging (Cuervo and Dice 2000a; Del Roso et al. 2003; Tavernarakis and Driscoll 2002; Cui et al. 2012) and that reduced autophagy accelerates aging, while stimulation of autophagy promotes anti-aging effects (Madeo et al. 2010).
Fig. 6.
Polyubiquitin aggregates, lysosomal activity, and LC3-II expression in the muscle of young (2-month-old) and old (5-month-old) N. rachovii. a The expression of polyubiquitin aggregates in the muscle of young and old N. rachovii was analyzed through western blotting. Tubulin served as the loading control. b Lysosomal activity. The bars represent the mean ± SEM. c LC3-II expression was analyzed through western blotting. Tubulin served as the loading control. d LC3-II expression levels were normalized to that of young N. rachovii. The results are shown as percentages and represent the mean ± SEM. Asterisks indicate statistical significance determined by two-sample t tests (*P ≤ 0.05, **P ≤ 0.01)
Acknowledgments
This work was supported by grants NSC 99-2311-B-182-002-MY3, CMRPD1C0081, and CMRPD180353 from the Ministry of Science and Technology, Taiwan, and the Chang Gung Memorial Hospital, Linkou, Taiwan.
Conflict of interest
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Author contributions
CH conceived and designed the experiments, analyzed the data, and prepared the manuscript; CH and CL performed the experiments.
Abbreviations
- ATR
Ambient temperature reduction
- LC3-II
Microtubule-associated protein 1 light chain 3-II
- Lamp 2a
Lysosome-associated membrane protein type 2a
- Hsc 70
70-kD heat shock cognate protein
- Lamp 1
Lysosome-associated membrane protein type 1
- mTOR
Molecular target of rapamycin
- p-mTOR
Phosphorylated mTOR
- ROS
Reactive oxygen species
- CAT
Catalase
- GPx
Glutathione peroxidase
- Mn-SOD
Manganese-superoxide dismutase
- CMA
Chaperone-mediated autophagy
References
- Banno A, Goult BT, Lee H, Bate N, Critchley DR, Ginsberg MH. Subcellular localization of talin is regulated by inter-domain interactions. J Biol Chem. 2012;287:13799–13812. doi: 10.1074/jbc.M112.341214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein SL, Liu AMH, Hansen BC, Somiari RI. Heat shock cognate-70 gene expression declines during normal aging of the primate retina. Invest Ophthalmol Vis Sci. 2000;41:2857–2862. [PubMed] [Google Scholar]
- Bonelli MA, Desenzani S, Cavallini G, Donati A, Romani AA, Bergamini E, Borghetti AF. Low-level caloric restriction rescues proteasome activity and Hsc70 level in liver of aged rats. Biogerontology. 2008;9:1–10. doi: 10.1007/s10522-007-9111-9. [DOI] [PubMed] [Google Scholar]
- Bulteau AL, Petropoulos I, Friguet B. Age-related alterations of proteasome structure and function in aging epidermis. Exp Gerontol. 2000;35:767–777. doi: 10.1016/S0531-5565(00)00136-4. [DOI] [PubMed] [Google Scholar]
- Bulteau AL, Szweda LI, Friguet B. Age-dependent decline in proteasome activity in the heart. Arch Biochem Biophys. 2002;397:298–304. doi: 10.1006/abbi.2001.2663. [DOI] [PubMed] [Google Scholar]
- Caramés B, Taniguchi N, Otsuki S, Blanco FJ, Lotz M. Autophagy is a protective mechanism in normal cartilage and its aging-related loss is linked with cell death and osteoarthritis. Arthritis Rheum. 2010;62:791–801. doi: 10.1002/art.27305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciechanover A. Proteolysis: from the lysosome to ubiquitin and the proteasome. Nat Rev Mol Cell Biol. 2005;6:79–87. doi: 10.1038/nrm1552. [DOI] [PubMed] [Google Scholar]
- Cuervo AM, Dice JF. A receptor for the selective uptake and degradation of proteins by lysosomes. Science. 1996;273:501–503. doi: 10.1126/science.273.5274.501. [DOI] [PubMed] [Google Scholar]
- Cuervo AM, Dice JF. Regulation of Lamp2a levels in the lysosomal membrane. Traffic. 2000;1:570–583. doi: 10.1034/j.1600-0854.2000.010707.x. [DOI] [PubMed] [Google Scholar]
- Cuervo AM, Dice JF. Age-related decline in chaperone-mediated autophagy. J Biol Chem. 2000;275:31505–31513. doi: 10.1074/jbc.M002102200. [DOI] [PubMed] [Google Scholar]
- Cuervo AM, Bergamini E, Brunk UT, Dröge W, Ffrench M, Terman A. Autophagy and aging: the importance of maintaining “clean” cells. Autophagy. 2005;1:131–140. doi: 10.4161/auto.1.3.2017. [DOI] [PubMed] [Google Scholar]
- Cui J, Bai XY, Shi S, Cui S, Hong Q, Cai G, Chen X. Age-related changes in the function of autophagy in rat kidneys. Age. 2012;34:329–339. doi: 10.1007/s11357-011-9237-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Roso A, Vittorini S, Cavallini G, Donati A, Gori Z, Masini M, Pollera M, Bergamini E. Ageing-related changes in the in vivo function of rat liver macroautophagy and proteolysis. Exp Gerontol. 2003;38:519–527. doi: 10.1016/S0531-5565(03)00002-0. [DOI] [PubMed] [Google Scholar]
- Dennis PB, Jaeschke A, Saitoh M, Fowler B, Kozma SC, Thomas G. Mammalian TOR: a homeostatic ATP sensor. Science. 2001;294:1102–1105. doi: 10.1126/science.1063518. [DOI] [PubMed] [Google Scholar]
- Díaz-Troya S, Pérez-Pérez MS, Florencio FJ, Crespo JL. The role of TOR in autophagy regulation from yeast to plants and mammals. Autophagy. 2008;4:1–15. doi: 10.4161/auto.6555. [DOI] [PubMed] [Google Scholar]
- Ferrington DA, Husom AD, Thompson LV. Altered proteasome structure, function and oxidation in aged muscle. FASEB J. 2005;19:644–646. doi: 10.1096/fj.04-2578fje. [DOI] [PubMed] [Google Scholar]
- Galbadage T, Hartman PS. Repeated temperature fluctuation extends the life span of Caenorhabditis elegans in a daf-16-dependent fashion. Mech Ageing Dev. 2008;129:507–514. doi: 10.1016/j.mad.2008.04.012. [DOI] [PubMed] [Google Scholar]
- Gracey AY, Fraser EJ, Li W, Fang Y, Taylor RR, Rogers J, Brass A, Cossins AR. Coping with cold: an integrative, multitissue analysis of the transcriptome of a poikilothermic vertebrate. Proc Natl Acad Sci U S A. 2004;101:16970–16975. doi: 10.1073/pnas.0403627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh YS, Hsu CY. Honeybee trophocytes and fat cells as target cells for cellular senescence studies. Exp Gerontol. 2011;46:233–240. doi: 10.1016/j.exger.2010.10.007. [DOI] [PubMed] [Google Scholar]
- Hsu CY, Chan YP. The use of honeybees reared in a thermostatic chamber for aging studies. Age. 2013;35:149–158. doi: 10.1007/s11357-011-9344-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu CY, Chiu YC. Ambient temperature influences aging in an annual fish (Nothobranchius rachovii) Aging Cell. 2009;8:726–737. doi: 10.1111/j.1474-9726.2009.00525.x. [DOI] [PubMed] [Google Scholar]
- Hsu CY, Chiu YC, Hsu WL, Chan YP. Age-related markers assayed at different developmental stages of the annual fish Nothobranchius rachovii. J Gerontol A Biol Sci Med Sci. 2008;63:1267–1276. doi: 10.1093/gerona/63.12.1267. [DOI] [PubMed] [Google Scholar]
- Hsu CY, Chuang YL, Chan YP. Changes in cellular degradation activity in young and old worker honeybees (Apis mellifera) Exp Gerontol. 2014;50:128–136. doi: 10.1016/j.exger.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiffin R, Bandyopadhyay U, Cuervo AM. Oxidative stress and autophagy. Antioxid Redox Signal. 2006;8:152–162. doi: 10.1089/ars.2006.8.152. [DOI] [PubMed] [Google Scholar]
- Kiffin R, Kaushik S, Zeng M, Bandyopadhyay U, Zhang C, Massey AC, Martinez-Vicente M, Cuervo AM. Altered dynamics of the lysosomal receptor for chaperone-mediated autophagy with age. J Cell Sci. 2007;120:782–791. doi: 10.1242/jcs.001073. [DOI] [PubMed] [Google Scholar]
- Klionsky DJ, Abdalla FC, Abeliovich H, Abraham RT, Acevedo-Arozena A, et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8:445–544. doi: 10.4161/auto.19496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6:463–477. doi: 10.1016/S1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- Liu RK, Walford RL. Increased growth and life-span with lowered ambient temperature in the annual fish, Cynolebia adloffi. Nature. 1966;212:1277–1278. doi: 10.1038/2121277a0. [DOI] [Google Scholar]
- Liu T, Daniels CK, Cao S. Comprehensive review on the HSC70 functions, interactions with related molecules and involvement in clinical diseases and therapeutic potential. Pharmacol Ther. 2012;136:354–374. doi: 10.1016/j.pharmthera.2012.08.014. [DOI] [PubMed] [Google Scholar]
- Madeo F, Tavernarakis N, Kroemer G. Can autophagy promote longevity? Nat Cell Biol. 2010;12:842–846. doi: 10.1038/ncb0910-842. [DOI] [PubMed] [Google Scholar]
- Majeski AE, Dice JF. Mechanisms of chaperone-mediated autophagy. Int J Biochem Cell Biol. 2004;36:2435–2444. doi: 10.1016/j.biocel.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Martinez-Vicente M, Sovak G, Cuervo AM. Protein degradation and aging. Exp Gerontol. 2005;40:622–633. doi: 10.1016/j.exger.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Podrabsky JE, Somero GN. Changes in gene expression associated with acclimation to constant temperatures and fluctuating daily temperatures in an annual killifish Austrofundulus limnaeus. J Exp Biol. 2004;207:2237–2254. doi: 10.1242/jeb.01016. [DOI] [PubMed] [Google Scholar]
- Sitte N, Huber M, Grune T, Ladhoff A, Doecke WD, Von Zglinicki T, Davies KJ. Proteasome inhibition by lipofuscin/ceroid during post-mitotic aging of fibroblasts. FASEB J. 2000;14:1490–1498. doi: 10.1096/fj.14.11.1490. [DOI] [PubMed] [Google Scholar]
- Taneike M, Yamaguchi O, Nakai A, Hikoso S, Takeda T, Mizote I, Oka T, Tamai T, Oyabu J, Murakawa T, Nishida K, Shimizu T, Hori M, Komuro I, Shirasawa T, Mizushima N, Otsu K. Inhibition of autophagy in the heart induces age-related cardiomyopathy. Autophagy. 2010;6:600–606. doi: 10.4161/auto.6.5.11947. [DOI] [PubMed] [Google Scholar]
- Tavernarakis N, Driscoll M. Caloric restriction and lifespan: a role for protein turnover? Mech Aging Dev. 2002;123:215–229. doi: 10.1016/S0047-6374(01)00341-4. [DOI] [PubMed] [Google Scholar]
- Unterluggauer H, Micutkova L, Lindner H, Sarg B, Hernebring M, Nystrom T, Jansen-Dürr P. Identification of Hsc70 as target for AGE modification in senescent human fibroblasts. Biogerontology. 2009;10:299–309. doi: 10.1007/s10522-008-9193-z. [DOI] [PubMed] [Google Scholar]
- Valenzano DR, Terzibasi E, Cattaneo A, Domenici L, Cellerino A. Temperature affects longevity and age-related locomotor and cognitive decay in the short-lived fish Nothobranchius furzeri. Aging Cell. 2006;5:275–278. doi: 10.1111/j.1474-9726.2006.00212.x. [DOI] [PubMed] [Google Scholar]
- Vogel C, Marcotte EM. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat Rev Genet. 2012;13:227–232. doi: 10.1038/nrg3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Chang Q, Wang Y, Su F, Zhang S. Late-onset temperature reduction can retard the aging process in aged fish via a combined action of an anti-oxidant system and the insulin/insulin-like growth factor 1 signaling pathway. Rejuvenation Res. 2014;17:507–517. doi: 10.1089/rej.2014.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, Shukitt-Hale B, Kalt W, Ingram DK, Joseph JA, Wolkow CA. Blueberry polyphenols increase lifespan and thermotolerance in Caenorhabditis elegans. Aging Cell. 2006;5:59–68. doi: 10.1111/j.1474-9726.2006.00192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao R, Zhang B, Dong Y, Gong J, Xu T, Liu J, Xu XZS. A genetic program promotes C. elegans longevity at cold temperatures via a thermosensitive TRP channel. Cell. 2013;152:806–817. doi: 10.1016/j.cell.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J, Kim KJ, Choi YW, Shin HS, Kim YH, Min J. The dependence of enhanced lysosomal activity on the cellular aging of bovine aortic endothelial cells. Mol Cell Biochem. 2010;340:175–178. doi: 10.1007/s11010-010-0415-8. [DOI] [PubMed] [Google Scholar]
- Zhang C, Cuervo AM. Restoration of chaperone-mediated autophagy in aging liver improves cellular maintenance and hepatic function. Nat Med. 2008;14:959–965. doi: 10.1038/nm.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Mutcherson R, Helfand SL. Calorie restriction delays lipid oxidative damage in Drosophila melanogaster. Aging Cell. 2005;4:209–216. doi: 10.1111/j.1474-9726.2005.00159.x. [DOI] [PubMed] [Google Scholar]
- Zhou L, Cai G, Liu F, Fu B, Liu W, Hong Q, Ma Q, Peng Y, Wang J, Chen X. Expression and mechanism of mammalian target of rapamycin in age-related renal cell senescence and organ aging. Mech Aging Dev. 2009;130:700–708. doi: 10.1016/j.mad.2009.08.005. [DOI] [PubMed] [Google Scholar]