Abstract
In the present study, effect-directed analysis was used to identify teratogenic compounds in porewater collected from a Superfund site along the Elizabeth River estuary (VA, USA). Zebrafish (Danio rerio) exposed to the porewater displayed acute developmental toxicity and cardiac teratogenesis, presumably because of elevated sediment levels of polycyclic aromatic hydrocarbons (PAHs) from historical creosote use. Pretreatment of porewater with several physical and chemical particle removal methods revealed that colloid-bound chemicals constituted the bulk of the observed toxicity. Size-exclusive chromatography and normal-phase high-performance liquid chromatography were used to fractionate Elizabeth River porewater. Acute toxicity of porewater extracts and extract fractions was assessed as the pericardial area in embryonic zebrafish. The most toxic fraction contained several known aryl hydrocarbon receptor (AhR) agonists (e.g., 1,2-benzofluorene and 1,2-benzanthracene) and cytochrome P450 A1 (CPY1A) inhibitors (e.g., dibenzothiophene and fluoranthene). The second most toxic fraction contained known AhR agonists (e.g., benzo[a]pyrene and indeno[1,2,3-cd]pyrene). Addition of a CYP1A inhibitor, fluoranthene, increased toxicity in all active porewater fractions, suggesting synergism between several contaminants present in porewaters. The results indicate that the observed acute toxicity associated with Elizabeth River porewater results from high concentrations of AhR agonistic PAHs and mixture effects related to interactions between compounds co-occurring at the Elizabeth River site. However, even after extensive fractionation and chemical characterization, it remains plausible that some active compounds in Elizabeth River porewater remain unidentified.
Keywords: Effect-directed analysis, Polycyclic aromatic hydrocarbons (PAHs), Elizabeth River, Porewater, Zebrafish
INTRODUCTION
Effect-directed analysis is a recently developed technique utilizing iterative chemical separation, toxicity evaluation, and qualitative chemical analysis to identify the chemical(s) exerting a specific toxicity in complex mixtures. To date, effect-directed analysis has become a valuable approach to determine the agents responsible for biological effects in various complex environmental samples (reviewed in Brack et al. [1,2]). Although numerous approaches to effect-directed analysis exist in the literature, all methods share several common elements—namely, the extraction, enrichment, and stepwise fractionation of an environmental mixture with manipulations directed by a specific bioassay response. Ultimately, the goal of effect-directed analysis is to facilitate the identification of causative agents, through chemical analysis (e.g., mass spectrometry), responsible for observed toxic response(s) by reducing mixture complexity.
The Elizabeth River estuary (Portsmouth, VA, USA) is a Superfund site contaminated with high levels of polycyclic aromatic hydrocarbons (PAHs), primarily from historic use of creosote at the Atlantic Wood Industries facility [3]. Both estuarine Atlantic killifish (Fundulus heteroclitus) and zebrafish (Danio rerio) embryos exposed to Elizabeth River porewater exhibit adverse developmental outcomes, including cardiac teratogenesis and genotoxicity [4–6]. Phenotypes observed in embryos exposed to Elizabeth River porewater include ethoxyresorufin-O-deethylase (EROD) induction, yolk sac edema, subcutaneous hemorrhaging, reduced growth, and craniofacial malformations. These phenotypic responses are consistent with those observed with early-life exposure to aryl hydrocarbon receptor (AhR) agonists, such as 2,3,7,8-tetrachlorodibenzo-p-dioxin and dioxin-like polychlorinated biphenyls, in small fish species [7]. Acute toxicity observed in embryos exposed to Elizabeth River porewater is presumed to result from high levels of PAHs present in sediments from the historic use of creosote at the Atlantic Wood Industries Superfund site, where total sediment PAH concentrations are commonly measured up to 100 μg/g to 500 μg/g dry sediment [3,8]. Previous studies indicate that the concentrations of 16 commonly monitored priority PAHs inadequately rationalize the observed toxicity for sediments collected at contaminated sites [2,9]. For example, a previous study demonstrated that oxygen and sulfur heterocycles, which are not considered priority PAHs, were major cytochrome P4501A (CYP1A) inducers in contaminated sediments [2]. Recent studies also show that the metabolites and/or degradation products of some PAHs might be more toxic than the parent compounds [9]. Therefore, porewater toxicities at highly contaminated sites, including the Elizabeth River, likely depend on the prevailing environmental conditions of the sampling location. Because the specific chemical or chemical mixtures primarily responsible for this acute toxicity in the Elizabeth River porewater have not been clearly identified, further investigation is warranted.
Adverse developmental outcomes, such as cardiac defects, can be caused by various PAHs acting under compound-specific mechanistic regimes. Previous studies indicate that PAHs may induce developmental cardiotoxicity through both AhR-dependent and AhR-independent pathways depending on PAH ring structure [10–16]. For example, tricyclic PAHs (e.g., phenanthrene) typically acted through AhR-independent pathways [17], whereas several 4-ring to 6-ring PAHs exhibited AhR-dependent toxicity [14,15]. In our previous studies, we found that AHR2 mediates cardiac teratogenesis of PAHs (e.g., benzo[k]fluoranthene) in both Atlantic killifish [11] and zebrafish [16]. Recently, synergism between AhR agonists and CYP1A inhibitors was investigated using simple binary dosing methods [5,10,18,19]. For example, exposure to both an AhR agonist (e.g., benzo[k]fluoranthene) and a CYP1A inhibitor (e.g., fluoranthene) increased the frequency and severity of deformities in embryos relative to embryos exposed to either an AhR agonist or a CYP1A inhibitor [18]. Similarly, embryos coexposed to fluoranthene (500 ng/mL) with Elizabeth River porewater exhibited significantly lower EROD activities but showed much higher levels of deformities when compared with embryos treated with Elizabeth River porewater alone [5]. Mechanisms underlying the enhanced toxicity observed in embryos exposed to CYP1A inhibitors are not well understood but might be attributed to decreased metabolism of AhR agonistic PAHs [9]. To date, most studies investigating the synergistic effects of PAHs have utilized simple coexposures of several well-known AhR agonists (e.g., benzo[k]fluoranthene and benzo[a]pyrene) and CYP1A inhibitors (e.g., fluoranthene). Few studies have investigated the prevalence of this effect in environmentally relevant samples. Therefore, it is of great interest to investigate the potential synergistic effects of pollutants isolated from contaminated sites both before and after chemical fractionation.
Previous effect-directed analysis studies have primarily focused on evaluating the acute toxicity of whole-sediment extracts [2,20,21]. To our knowledge, no study has conducted effect-directed analysis on the toxicity of mixtures present in porewater, a mixture of water and colloidal solids, which represent environmentally relevant exposures for many aquatic invertebrates. One previous study also reported a positive correlation between measured and/or predicted contaminant porewater concentrations and bioavailability of the contaminants to benthic organisms [22]. Therefore, compared with whole-sediment exposure, porewater likely represents a more exposure-relevant matrix with which to conduct effect-directed analysis. However, the processing of porewater for toxicity testing remains a challenge, and various methods have been presented in the literature [22]. In particular, difficulties in separating dissolved and colloid-bound chemicals complicate the evaluation of observed toxicities in porewaters.
In the present study, our goal was to determine the active chemicals causing developmental pericardial edema in zebrafish embryos exposed to Elizabeth River porewater. To this end, we compared filtration, centrifugation, and flocculation of porewater samples to investigate the role of the dissolved and colloid-bound contaminants. Subsequently, effect-directed analysis was used to identify the primary chemical(s) leading to the observed acute toxicity. Lastly, we examined the interactions between CYP1A inhibitors and AhR agonists in different fractions of the porewater. An in vivo zebrafish model was used as the effect-directed analysis end point because of its favorable characteristics as a model for studying developmental toxicity (e.g., high rates of fertilization, transparent embryos, and rapid embryonic development) and for consistency with much of our previous work and that of others [12–17] on PAH-mediated developmental toxicity.
MATERIALS AND METHODS
Chemicals
All PAH standards and isotope-labeled surrogate standards were purchased from AccuStandard. Potassium alum dodeca-hydrate (alum) was purchased from Sigma-Aldrich. All solvents used were high-performance liquid chromatography (HPLC) grade or better.
Sample preparation
Sediment samples containing interstitial water were collected adjacent to the Atlantic Wood Industries Superfund site (36°48′27.2″N, 76°17′38.1″W) in November 2011, stored in precleaned 5-L Homer buckets, transported to the laboratory, and kept at 4 °C prior to porewater preparation. Whole-sediment samples from sites showing acute toxicity in preliminary testing (data not shown) were pooled, homogenized with a scoop, and prepared at room temperature as follows: a 20-mL fraction of the pooled sediment was mixed with 20 mL of deionized water in 50-mL Corning® polypropylene centrifuge tubes, and sediment mixtures were agitated (300 rpm, orbit shaker; LAB-LINE) for 24 h and then allowed to quiesce overnight before decanting the porewater. To obtain sufficient porewater for effect-directed analysis, several batches of sediment were processed, and the resulting porewaters combined, aliquoted, and stored at −80 °C until use. Porewater was processed using several methods to compare the toxicity of particulate-bound and dissolved fractions. In the present study, porewater was processed separately by high-speed centrifugation (287 472 g, 3 h, 4 °C; Thermo Scientific Sorvall RC 6 +, F20S-6 × 100 rotor), low-speed centrifugation (~2000 g, 20 min; Eppendorf 5810R), filtration (0.7-μm glass fiber filter), and alum flocculation. The results indicated that pretreatment by low-speed centrifugation, flocculation, and solvent extraction was most effective at obtaining a representative extract for effect-directed analysis. Supplemental Data, Figure S1, illustrates the sample preparation workflow described below. Eight 40-mL portions of raw porewater (total, 320 mL) were centrifuged at a low speed (200 g; Eppendorf 5810R) to remove large particles. Suspended particulate matter was removed by flocculation with 0.5 mL alum solution (0.05 g/mL) and approximately 100 μL 1 M NaOH to bring the solution to pH 11 for enhanced floc formation. After quiescence for 10 min at room temperature, the porewater became clear and the colloidal material precipitated. After centrifuging at 2500 g, the supernatant was removed and concentrated on solid-phase extraction disks (C18 SPE disk, 47 mm; Whatman) preconditioned with 5 mL methanol and deionized water. After loading and drying, disks were sequentially eluted with 6 mL methanol, dichloromethane, and hexane. Solid-phase extracts were pooled for further analysis. Combined settled solids were ground with anhydrous sodium sulfate and extracted at elevated temperature (250 °C) and high pressure (1500 psi) with dichloromethane:hexane (1:1, v/v) on an accelerated solvent extraction unit (Dionex). Sulfur in the extracts of settled particles was removed by shaking overnight with copper strips activated by submersion in 1 M hydrochloride acid (HCl). Both extracts (supernatant and settled particles) were combined for further toxicity tests and transferred to dimethyl sulfoxide (DMSO). To serve as a procedural blank, 320 mL of HPLC-grade water was simultaneously processed by the same procedure. Procedural blanks and the DMSO vehicle controls showed no apparent toxicity.
Chemical analysis
Thirty-six PAHs, including 16 priority PAHs and several other PAHs known to occur in Elizabeth River porewaters, were analyzed using a gas chromatograph–mass spectrometer (GC 6890N, MS 5975; Agilent) in electron ionization (GC/EI-MS) mode using selected ion monitoring as described in our previous study [3]. A 0.25 mm (inner diameter) × 15 m fused silica capillary column coated with 5% phenyl methylpolysiloxane (0.25 μm film thickness) was used for separation of the analytes. Splitless injections were performed with a programed-temperature vaporization inlet maintained at 250 °C and 10 psi. The nitrogen flow rate was 1.3 mL min−1, and the oven temperature program was as follows: after holding at 40 °C for 0.6 min, the temperature was increased at 10 °C min−1 until reaching 280 °C, at which point isothermal conditions were maintained for 14 min before returning to initial conditions. The transfer line temperature was maintained at 280 °C, and the ion source was held at 230 °C.
Toxicity testing and statistical analyses
The culture of adult zebrafish used in the present study has been described extensively in our previous studies [12,16]. Briefly, adult Ekkwill zebrafish (Ekkwill Waterlife Resources) were maintained at 28 °C in a recirculating AHAB system (Aquatic Habitats) under a 14:10-h light:dark cycle. Adults were fed brine shrimp and a mix of Ziegler’s Adult Zebrafish Complete Diet (Aquatic Habitats). Embryos were collected after spawning of adult zebrafish and maintained in 30% Danieau solution (N-2-hydroxyethylpiperazine-N′-2-ethane-sulfonic acid [HEPES] buffer, pH 7.6, 5 mM sodium chloride, 0.17 mM potassium chloride, 0.33 mM calcium chloride, and 0.33 mM magnesium sulfate) under the same temperature and photoperiod as adults [16]. Adult care and reproductive techniques were noninvasive and approved by the Duke University Institutional Animal Care and Use Committee (A279-08-10).
Within 24 h postfertilization, embryos exhibiting normal development at a similar stage were selected and dosed in 4.5 mL 30% Danieau medium in 10-mL glass scintillation vials, with 10 embryos per vial and 3 vials per treatment. After establishing a concentration–response relationship for the raw porewater, a dosing range that minimized mortality while maintaining overt toxicity was selected for further analysis. Embryos were dosed with raw porewater extracts, fractionated porewater extracts, or controls dissolved in DMSO, which was less than 0.5% in the final medium. To evaluate possible synergistic effect between AhR agonists and CYP inhibitors, changes in toxicity were monitored with and without the addition of fluoranthene (200 ng/mL) to active fractions. Embryonic development was scored daily for mortality, hatching rate, and gross malformation. At 96 h postfertilization, hatched zebrafish embryos were screened by observers (who were blinded to sample treatment conditions) for cardiac deformities via measurement of pericardial effusion, a semiquantitative indicator to describe acute toxicity [7,10,23]. Fish were anesthetized with MS-222 (Sigma-Aldrich), placed in a left lateral orientation in 3% methylcellulose on depression slides, and imaged under 60± magnification (Zeiss Axioskop) with a Nikon camera (digital sight DS-Fi1). The 2-dimensional image of the pericardial area was manually traced and then quantified using Image J (National Institutes of Health). A certified 1-mm scale was used as the scale to convert pixels to millimeters.
Mortality, malformation, and pericardial area were scored and averaged for 10 larvae in each scintillation vial. The average (n = 3) and standard error of each dose were calculated based on the average of each vial. All test statistics were calculated using a 2-tailed model in SigmaPlot 12.0 (Systat Software) at a significance level (α) of 0.05. A one-way analysis of variance model tested differences in toxicity among porewater pretreatments (e.g., filtration vs flocculation) and in porewater concentration–response relationships. The Newman-Keuls post hoc test identified samples that were significantly different from the DMSO control. Paired t tests evaluated differences in toxicity among several active fractions with or without the addition of fluoranthene.
Fractionation procedures
Porewater extracts, along with solvent blank extracts, were fractionated by high-performance gel-permeation chromatography (HP-GPC) and semipreparative normal-phase HPLC (NP-HPLC). Extracts were dried over sodium sulfate, solvent-exchanged to dichloromethane, concentrated to approximately 2 mL under a gentle stream of nitrogen, and accurately weighed prior to removing a 1-mL subsample for HP-GPC fractionation. The subsample was diluted to 2 mL with dichloromethane and separated into 3 fractions under isocratic elution conditions (5 mL min−1, 100% dichloromethane) by an HP-GPC system consisting of a binary pump, 2 EnviroGel GPC cleanup columns connected in series (19 mm × 150 mm and 19 mm × 300 mm, 100-Å pore size, 15 μm particles; Waters), a high-pressure manual injector with a 2-mL sample loop (Rheodyne), and an automated fraction collector (Gilson). The performance of the GPC column was assessed by repeated (n = 3) injection of a standard containing corn oil and sulfur; relative standard deviation for the retention time (minutes) and peak width (measured at the peak base, minutes) were less than 1% and 5%, respectively, for both compounds in all experimental replicates. In the fractionation of porewater extracts, 3 equally timed fractions (6 min each) were collected between the elution volumes of corn oil and sulfur as determined by analysis of standard solutions. A GPC fraction blank was prepared in parallel and showed no difference in toxicity compared with DMSO controls.
Further fractionation was achieved for select extracts on an NP-HPLC system consisting of an Agilent 1100 series HPLC pump equipped with a degasser, an automated injector, a 1-mL injection loop, and a Supelcosil LC-Diol column (250 mm × 10 mm, 5 μm particle size). The GPC extracts were evaporated to near dryness under a gentle stream of nitrogen and reconstituted in hexane to a final volume of 1 mL prior to injection of 0.9 mL and separation by isocratic elution with hexane for 10 min, followed by gradient elution with dichloromethane/methanol (90:10, v/v; 0%–99% over 20 min), at a flow rate of 4 mL min−1. Elution was monitored by ultraviolet (UV)–visible spectroscopy. Fractions were collected with the automated fraction collector every minute throughout the separation. Fractions were split for toxicity testing and chemical analysis. One milliliter of each fraction was evaporated under nitrogen gas and reconstituted in DMSO for toxicity test, and the remaining 3 mL was retained for chemical analysis.
Chemical identification
Fractions with the highest levels of observed toxicity were subjected to qualitative mass spectrometric analysis by GC/EI-MS operated in full-scan mode (m/z 50–1050). Chromatographic conditions were identical to the above-mentioned PAH analysis. Structural elucidation was performed using MS-Chemstation software (Ver E.02.01.1177) from Agilent equipped with the National Institute of Standards and Technology (NIST) mass spectral library (Ver 2005) and NIST Mass Spectral Search Program (Ver 2.0d). The mass spectrum of each nontargeted component was extracted, and its peaks were assigned identities by the automated mass spectral deconvolution and identification system and the NIST-05 library. Peaks with a signal-to-noise ratio above 20 were library-searched and assigned structures based on the presence of a corresponding mass signature with a match factor of at least 80 (out of 100), which was previously reported to show greater confidence in identifications [24]. For isomeric PAHs, further confirmation was achieved by comparing retention times of suspect peaks to those of PAH authentic standards. In all cases, the observed toxicity was recovered by NP-HPLC during isocratic elution with hexane, indicating that the toxicants were nonpolar compounds and therefore amenable to analysis by the GC-MS; thus, no further chemical analysis was conducted. In the raw porewater, PAHs were quantified using our standard method. Full details on the method and limits of quantification can be found in Clark et al. [3].
RESULTS AND DISCUSSION
Comparison of porewater preparation methods
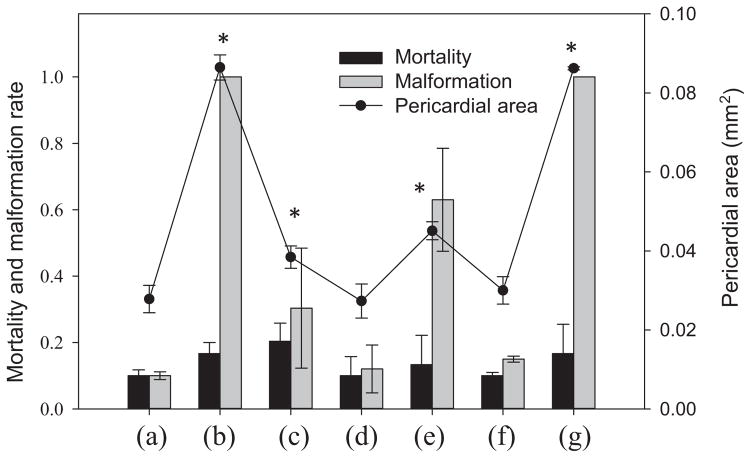
Because raw porewater contains both dissolved and colloid-bound components, we examined the relative toxicity of these 2 fractions. Separation of dissolved and colloidal fractions of porewater may be achieved by both physical (e.g., filtration and ultracentrifugation) and chemical (e.g., flocculation) treatment. Recently, flocculation has been reported to effectively separate dissolved PAHs and colloid-bound PAHs without disturbing their chemical equilibrium [25,26]. A comparison of the toxicity of the different fractions is presented in Figure 1. Treatment by high-speed centrifugation and filtration resulted in significantly lower toxicity, as measured by pericardial area (Figure 1c and d). Furthermore, the malformation rate and the pericardial area decreased by a factor of 2 after filtration. The porewater subjected to ultracentrifugation showed no obvious toxicity (Figure 1d) compared with the control. Low-speed centrifugation recovered only approximately 70% of the original toxicity based on the measure of pericardial area (Figure 1e). No obvious toxicity was observed in porewater after flocculation (Figure 1f), suggesting that soluble toxic compounds (e.g., polar PAH metabolites) contribute little to the overall observed acute toxicity. Most of the toxicity was recovered after overnight resuspension of the flocculated particles (Figure 1g), however, indicating that particulate-bound toxicants contributed to most of the observed toxicity. Therefore, in the present study, the colloidal fraction of Elizabeth River porewater was isolated through flocculation and solvent-extracted as described in Materials and Methods.
Figure 1.
Average mortality, malformation rate, and pericardial area of 96–h postfertilization zebrafish embryos (n = 3, 10 embryos in each vial) dosed with 5% porewater with different pretreatment methods: (a) control with high-performance liquid chromatography (HPLC)–grade water, (b) raw porewater, (c) filtered porewater using a 0.7-μm glass fiber filter, (d) porewater after ultracentrifugation at 287 472 × g, (e) porewater after slow-speed centrifugation at 1000 ×g, (f) porewater after flocculation with alum, (g) porewater from overnight resuspension of settled particles after flocculation by alum. Error bar represents standard error. *Significant difference with the HPLC-grade water control.
Developmental toxicity and targeted analysis of PAHs in the porewater extract
The concentration–response of porewater extracts over a concentration range corresponding to 1% to 10% of untreated porewater in dosing solution determined dosing levels for effect-directed analysis. As shown in Supplemental Data, Figure S2, porewater extracts caused a concentration-dependent increase in pericardial area. Malformations were observed at porewater concentrations as low as 1% in the medium, and more than 80% mortality was observed at a composition of 8% porewater. At 5% raw porewater, a severe malformation was observed with only limited mortality (20%). Based on these observations, we chose a dose corresponding to 5% raw porewater for effect-directed analysis. The results also indicated that pericardial area could be used as a semiquantitative index in the effect-directed analysis. Compared with the EROD index used in previous effect-directed analysis studies [2,20,21], pericardial area offered a more time-effective and representative measure of overt toxicity.
The PAH levels in raw Elizabeth River porewater extracts were measured (Supplemental Data, Table S1). Consistent with previous work at the Elizabeth River Superfund site [3], high levels of PAHs were detected in porewaters. Mean concentrations of 36 selected PAHs were 5073 ± 409 ng/mL porewater (average ± standard deviation of 5 replicate porewater extracts). The most abundant PAHs included naphthalene, acenaphthene, fluorene, carbazole, and fluoranthene. Also, several AhR agonists, such as benzo[a]pyrene, benzo[k]fluoranthene, 1,2-benzanthracene, and pyrene, were detected at concentrations of 40.2 ± 3.1 ng/mL, 26.5 ± 2.2 ng/mL, 84.1 ± 4.7 ng/mL, and 288 ± 21.3 ng/mL, respectively. The cumulative AhR agonistic effect was assessed using fish potency factors for PAHs measured in the porewater. Fish potency factors were calculated as the mean of the potency factors of CYP1A induction and AhR binding reported in 18 in vitro and in vivo studies using fish and mammalian model organisms [27]. As shown in Supplemental Data, Table S1, 1,2-benzofluorene, 1,2-benzanthracene, benzo[-k]fluoranthene, benzo[a]pyrene, indeno[1,2,3-cd]pyrene, and benzo[b]fluoranthene were likely the major contributors to EROD activity in the mixtures.
Fractionation of the Elizabeth River porewater extract and toxicity test
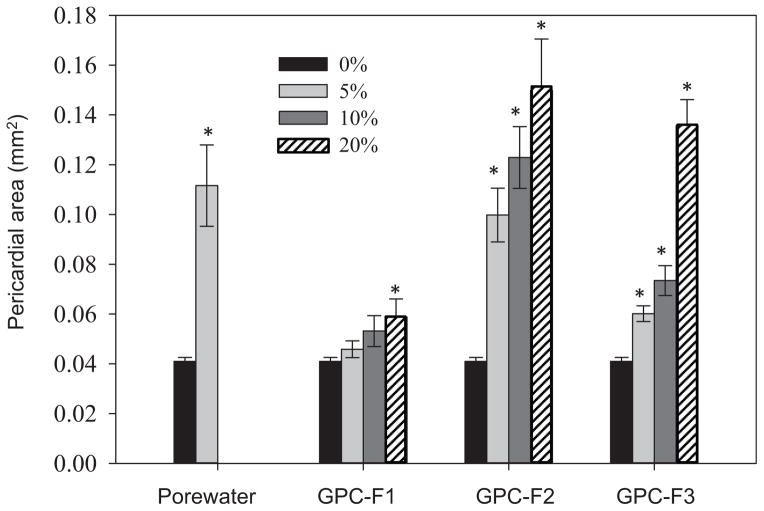
Concentrated porewater extracts were fractionated by GPC into 3 crude fractions (GPC-F1–F3), and the most toxic of these 3 fractions was then further fractionated into 20 NP-HPLC subfractions (F2.1–F2.20). All 3 crude and 20 subfractions were analyzed for PAHs to investigate the separation performance and elution profiles. As shown in Supplemental Data, Figure S3, few chemicals were eluted in the first fraction of the GPC step (GPC-F1). Most PAHs eluted in GPC-F2 and several chemicals, such as carbazole, pyrene, benzo[a]pyrene, benzo[e]pyrene, indeno [1,2,3-cd]pyrene, and perylene, were present either in both GPC-F2 and GPC-F3 or exclusively in GPC-F3. Since low–molecular weight PAHs (e.g., naphthalene) are quite volatile, we also investigated PAH recovery throughout the fractionation procedure using a standard mixture. After the crude GPC fractionation, approximately 50% of the 2-ring and 3-ring PAHs were lost, but the recovery of the higher–molecular weight PAHs (e.g., benzo[a]pyrene, 5 rings) was acceptable (>70%). Therefore, we examined the toxicity of a nominal 5%, 10%, and 20% porewater concentration, which approximately corresponds to a 2.5%, 5%, and 10% concentration in the raw porewater extract when accounting for recovery of the low–molecular weight PAHs. Figure 2 shows the dose–response relationship of the 3 GPC fractions. Only minor acute toxicity was observed for GPC-F1. Fraction GPC-F2 was overtly toxic at the 5% porewater concentration level and lethal at the 2 highest levels tested (10% and 20% porewater). Compared with GPC-F2, GPC-F3 was notably less severely acutely toxic. Full-scan mass spectrometric analysis of GPC-F3 (Supplemental Data, Figure S4) revealed fewer than 30 peaks (signal-to-noise ratio >20). Because of the lower acute toxicity and small number of compounds present, no further fractionation was conducted for this fraction.
Figure 2.
Pericardial area of 96–h postfertilization zebrafish embryos (n = 3, 10 embryos in each vial) dosed with raw porewater extract and 3 gel permeation chromatography (GPC) fractions with equivalent porewater ratios of 5%, 10%, and 20% in the medium. Fish mortality was 100% in the 10% raw porewater extract. Error bar represents standard error. *Significant difference from the dimethyl sulfoxide control.
The most toxic fraction, GPC-F2, was then further fractionated by NP-HPLC. Preliminary experiments evaluated semipreparative reverse-phase HPLC (Nucleosil 100-5 C18) for the separation of porewater extracts (data not shown); however, toxicity was not recoverable after fractionation. Losses were attributed to the high hydrophobicity of the toxic components, consistent with similar effect-directed analysis approaches reported elsewhere. For example, a recent report found that less than 10% of the EROD-inducing potency could be recovered in reverse-phase fractionation of sediment extracts and wastewater effluent [20,28]. Therefore, reverse-phase HPLC fractionation was rejected in favor of NP-HPLC fractionation, which is a more suitable approach for the fractionation of AhR agonists [29].
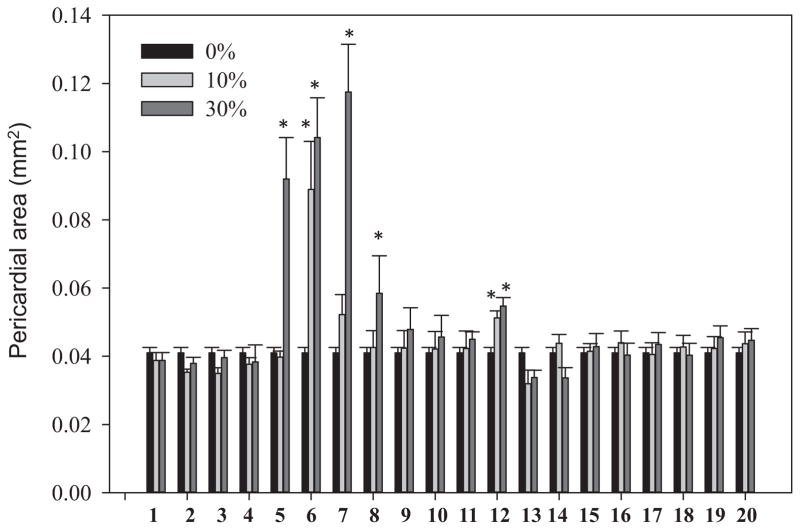
A standard mixture of 36 PAHs (~2 μg/mL for each compound), ranging from 2 rings to 6 rings, was analyzed in parallel with porewater fractions to determine the elution profile of PAHs under the above-described chromatographic conditions. As shown by the UV-visible chromatograms in Supplemental Data, Figure S5, most PAHs eluted during the initial isocratic (100% hexane) regime of the gradient program (retention times, 4–7 min). Based on this observation, we separated the GPC-F2 porewater extract using NP-HPLC with fractions collected every 1 min. The UV-visible absorption profile of the porewater extract (GPC-F2) was found to overlap with the PAH standard mixture, suggesting that PAHs were likely among the most abundant UV-detectable compounds in the extract. Following the NP-HPLC fractionation, the recovery of 3-ring PAHs was close to approximately 30% and the higher–molecular weight PAHs (4 rings) were recovered at approximately 50% relative to the level of PAHs in GPC-F2. Therefore, we adjusted the dosing levels for the NP-HPLC fractions to reflect PAH losses during fractionation. After NP-HPLC fractionation, dosing concentrations of 10% and 30% (porewater equivalent volume) in the final medium were used to conduct toxicity testing for all 20 fractions, which correspond to approximately 5% and 15% for heavier-ring PAHs and 3% and 10% for lower-ring PAHs in GPC fractions, respectively. As shown in Figure 3, the most acutely toxic fraction was F2.6 (i.e., the 6th HPLC fraction of GPC fraction 2), which was followed by F2.7. Severe acute toxicity was observed for F2.6 at an equivalent volume of 10% in the final medium. At this dose, we could recover most of the toxicity observed in the 5% dose of GPC-F2 if 50% recovery was assumed during the NP-HPLC fractionation. This suggests that this fraction might contain the active chemicals that contributed most to the acute toxicity. No obvious overt toxicity was observed for F2.5 at the 10% dose, though obvious toxicity was observed at the 30% dose concentration.
Figure 3.
Pericardial area of 96–h postfertilization zebrafish embryos (n = 3, 10 embryos in each vial) dosed with 20 normal-phase high-performance liquid chromatography fractions from gel permeation chromatography fraction 2 with equivalent porewater ratios of 10% and 30% in the medium. Error bar represents standard error. *Significant difference from the dimethyl sulfoxide control.
Chemical composition of the toxic fractions
The chemical compositions of the 4 most toxic fractions (i.e., F2.6, GPC-F3, F2.7, and F2.5) were investigated using GC/EI–MS in full scan mode as described. Supplemental Data, Figure S4, and Table 1 present a chromatogram and list of identified chemicals, respectively. Based on the NIST-05 library and approximately 36 available PAH standards, most of the major peaks within those 4 extracts were identified either with an authentic standard or based on a library search with a match factor >80%. The concentration of the identified compounds (when authentic standards were available) is also included in Table 1. Most of the major peaks in the toxic fractions were identified as PAHs. The most toxic fraction (F2.6) contained several known AhR agonists (e.g., pyrene, 1,2-benzofluorene, and 1,2-benzanthracene) and CYP1A inhibitors (e.g., dibenzothiophene and fluoranthene). The compounds identified in the less toxic F2.5 were primarily 3-ring PAHs (e.g., phenanthrene and fluorene). Another priority PAH, benzo[k]fluoranthene, eluted in F2.7, which might explain the toxicity observed in that fraction because the cardiac toxicity of benzo[k]fluoranthene was reported in our previous studies [11,16]. The second most toxic fraction (GPC-F3) contained several other carcinogenic AhR agonists (e.g., benzo[a]pyrene and indeno[1,2,3-cd] pyrene) and 1 known CYP1A inhibitor, carbazole. Fractions F2.6 and GPC-F3 contained both an AhR agonist and a CYP1A inhibitor, which suggested that chemical interactions might play an important role in the observed toxicity. Fluoranthene (F2.6 and F2.7) and some PAH heterocycles such as carbazole (GPC-F3) and dibenzothiophene (F2.6) are known CYP1A inhibitors [30]. Previous studies indicate that embryos coexposed to AhR agonistic PAHs and CYP1A inhibitors are deformed with increased severity and frequency compared with embryos dosed with the AhR agonists alone [18,19]. The toxicity of most of the identified chemicals has been well established in simple dosing studies. However, the present study is one of only a few investigations that identified teratogenic compounds in highly complex field-collected samples.
Table 1.
Compounds identified in toxic fractions GPC-F3, F2.5, F2.6, and F2.7 (confirmed with standards or with a match factor of more than 80%)
| Peak no. | Compound identified | CAS number | Identified by | AhR agonist | CYP1A inhibitor | Concentration (ng/mL, if available) of identified chemicals in fractiona
|
|||
|---|---|---|---|---|---|---|---|---|---|
| GPC-F3 | F2.5 | F2.6 | F2.7 | ||||||
| 1 | Carbazole | 86-74-8 | AS | Yes [28] | 550 | ||||
| 2 | 2-hydroxyfluorene | 2443-58-5 | SM | Yes | |||||
| 3 | Pyrene | 129-00-0 | AS | Yes [25] | 597 | 167 | |||
| 4 | Cyclopenta[cd]pyrene | 27208-37-3 | SM | Yes | |||||
| 5 | Benzo[e]pyrene | 192-97-2 | AS | Yes [25] | 170 | 14 | |||
| 6 | Benzo[a]pyrene | 50-32-8 | AS | Yes [25] | 197 | ||||
| 7 | Perylene | 198-55-0 | AS | 63 | |||||
| 8 | Indeno[1,2,3-cd]pyrene | 193-39-5 | AS | Yes [25] | 19 | ||||
| 9 | Benzo[ghi]perylene | 191-24-2 | AS | 81 | |||||
| 10 | Acenaphthene | 83-32-9 | AS | 59 | |||||
| 11 | Dibenzofuran | 132-64-9 | AS | 52 | |||||
| 12 | Fluorene | 86-73-7 | AS | 123 | |||||
| 13 | Phenanthrene | 85-01-8 | AS | 259 | 445 | ||||
| 14 | Anthracene | 120-12-7 | AS | 78 | 33 | ||||
| 15 | Phenanthrene, 2-methyl- | 2531-84-2 | AS | 65 | |||||
| 16 | Phenanthrene, 1-methyl- | 832-69-9 | AS | 66 | |||||
| 17 | Naphthalene, 1-phenyl- | 605-02-7 | SM | Yes | |||||
| 18 | Dibenzothiophene | 132-65-0 | AS | Yes [28] | 22 | ||||
| 19 | Fluoranthene | 206-44-0 | AS | Yes [16] | 516 | 72 | |||
| 20 | Benzo[b]naphtha[2,3-d]furan | 243-42-5 | SM | Yes | |||||
| 21 | Benzo[b]naphtha[1,2-d]furan | 205-39-0 | SM | Yes | |||||
| 22 | 1,2-benzofluorene | 238-84-6 | AS | Yes [25] | 66 | ||||
| 23 | 3,4-benzofluorene | 205-12-9 | AS | 67 | |||||
| 24 | Benzo[ghi]fluoranthene | 203-12-3 | SM | Yes | |||||
| 25 | 1,2-benzanthracene | 56-55-3 | AS | Yes [25] | 102 | ||||
| 26 | Chrysene | 218-01-9 | AS | Yes [25] | 37 | 68 | |||
| 27 | Benzo[k]fluoranthene | 207-08-9 | AS | 42 | |||||
| 28 | Benzo[b]fluoranthene | 205-99-2 | AS | 109 | |||||
| 29 | Benzo[a]fluoranthene | 203-33-8 | AS | 8 | |||||
Values represent the concentration (ng/mL) of compounds for which standards were available. “Yes” means that the chemical was identified but cannot be quantified because of a lack of an authentic standard.
AS = authentic standard (where observed, these compounds were quantified and reported in ng/mL); SM = spectral match (these compounds were identified by match to spectra in the National Institute of Standards and Technology’s 2005 spectral library with a match factor >80%); GPC = gel-permeation chromatography.
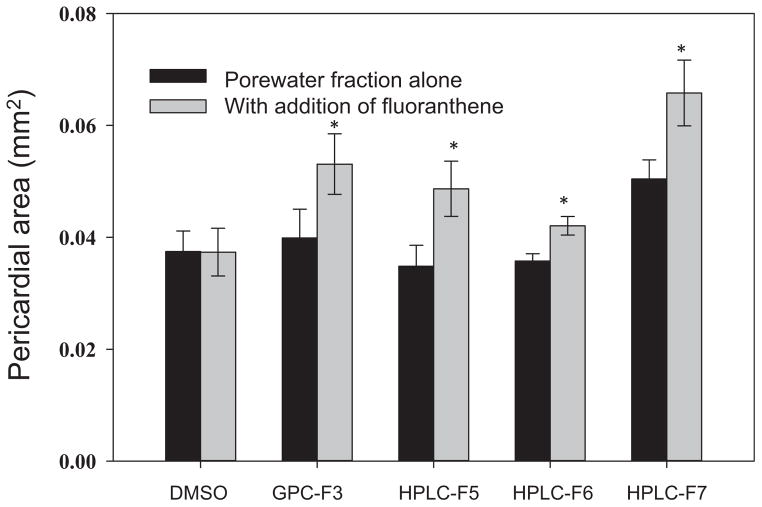
As mentioned, several well-known AhR agonists, as well as CYP1A inhibitors, were identified in different active fractions. Because previous studies have observed synergism between CYP1A inhibitors and AhR agonists, we conducted additional experiments to evaluate changes in the acute toxicity when porewater fractions were spiked with fluoranthene, a CYP1A inhibitor. Porewater fractions that were found to be acutely toxic were dosed with or without fluoranthene (200 ng/mL). In the present study, a lower dosing level (2%) of GPC-F3 and HPLC-F6 was used because of the observed high mortality at 10%. As shown in Figure 4, no acute toxicity was observed in the fluoranthene control. However, a significant increase in toxicity was observed after the addition of fluoranthene in all fractions tested. The highest increase was found in GPC-F3 and F2.7, both of which contained AhR agonists such as benzo[a]pyrene, indeno[1,2,3-cd]pyrene, and benzo[k]fluoranthene. Fraction F2.6 showed only a slight increase in toxicity with fluoranthene amendment, relative to other fractions. This may be because fluoranthene was already present in this fraction (stock concentration, 516 ng/mL) prior to the addition. These findings suggest that the synergistic effect between AhR agonists and a CYP1A inhibitor can occur between multiple types of PAHs, and this effect should be considered in their risk assessment. It should be noted, however, that this finding does not necessarily implicate or exclude the role of AhR-independent developmental cardiotoxicity, which is a key pathway in understanding the observed toxicity caused by other complex PAH mixtures (e.g., weathered oil) [13]. Furthermore, regulation of zebrafish AhR expression through gene knockdown could be used to identify effects of PAHs found in Elizabeth River porewater that act via AhR-independent pathways.
Figure 4.
Pericardial area of 96–h postfertilization zebrafish embryos (n = 3, 10 embryos in each vial) dosed with gel permeation chromatography (GPC) F3, F2.5, F2.6, and F2.7, with equivalent porewater ratios of 2%, 10%, 2%, and 10%, respectively, in medium with and without 200 ng/mL fluoranthene. Error bars represent standard error. A lower dosing level of GPC-F3 and high-performance liquid chromatography (HPLC)-F6 was used because of high mortality at 10%. *Significant difference from the porewater fraction alone. DMSO = dimethyl sulfoxide.
To determine if we could reconstruct the toxicity observed in F2.6, a simulated mixture was prepared containing several available PAHs identified in this fraction (i.e., dibenzothiophene, phenanthrene, fluoranthene, pyrene, 1,2-benzofluorene, 1,2-benzanthracene, and chrysene), and the toxicity was evaluated. Embryos exposed to this simulated mixture showed obvious acute toxicity, which had an average pericardial area approximately 60% of the F2.6 fraction. However, the toxicity of the simulated mixture containing benzo[a]pyrene, benzo[e] pyrene, pyrene, indo[1,2,3-cd]pyrene, and carbazole in GPC-F3 was less than 50% of that observed in this fraction. Furthermore, dosing with a mixture of the most abundant chemicals, phenanthrene and fluoranthene, showed no obvious cardiotoxicity. Therefore, it is likely that some active compounds in those fractions remain unidentified. Also, data on the toxicity of several identified chemicals, such as benzo[b]naphtha[2,3-d] furan, benzo[b]naphtha[1,2-d]furan, and several PAH heterocycles, are not available.
CONCLUSION
In the present study, several chemicals in the porewater from the Elizabeth River Superfund site causing acute toxicity and teratogenesis were tentatively identified using effect-directed analysis. The present study is one of a few investigations that identify probable teratogens in field-collected porewater samples. Our data suggest that the observed acute toxicity in Elizabeth River porewater is the result of high concentrations of PAHs. In particular, fractions containing several known AhR agonists (e.g., pyrene, 1,2-benzofluorene, and 1,2-benzanthracene) and several CYP1A inhibitors (e.g., dibenzothiophene and fluoranthene) exhibited the highest toxicity. These findings support earlier studies, which found a synergetic toxicity when AhR agonists and CYP1A inhibitors were both present [10,18,19]. Although this has been shown in the laboratory with binary mixtures, few studies have demonstrated this synergetic effect in field-collected samples. This interaction between PAHs could complicate the identification of active compounds in complex environmental systems and should therefore be considered in future risk assessments. Furthermore, the present results demonstrate that colloid-bound PAHs, as opposed to PAHs in solution, are primarily responsible for the observed acute toxicity in Elizabeth River porewaters. However, it should be noted that the exhaustive extraction method of the colloids in the present study might overestimate the toxicity. In the future, bioavailable extraction methods and approaches (such as using Tenax beads to extract the porewater) should be conducted.
Supplementary Material
Acknowledgments
This research was supported by a grant from the National Institute of Environmental Health Sciences Superfund Research Program Center (grant P42ES010356-11). Additional thanks are extended to D. Hinton, Duke University, for assistance in microscope use.
Footnotes
All Supplemental Data may be found in the online version of this article.
References
- 1.Brack W, Klamer HJC, da de AML, Barcelo D. Effect-directed analysis of key toxicants in European river basins—A review. Environ Sci Pollut Res. 2007;14:30–38. doi: 10.1065/espr2006.08.329. [DOI] [PubMed] [Google Scholar]
- 2.Brack W, Schirmer K. Effect-directed identification of oxygen and sulfur heterocycles as major polycyclic aromatic cytochrome P4501A-inducers in a contaminated sediment. Environ Sci Technol. 2003;37:3062–3070. doi: 10.1021/es020248j. [DOI] [PubMed] [Google Scholar]
- 3.Clark BW, Cooper EM, Stapleton HM, Di Giulio RT. Compound-and mixture-specific differences in resistance to polycyclic aromatic hydrocarbons and PCB-126 among Fundulus heteroclitus subpopulations throughout the Elizabeth River estuary (Virginia, USA) Environ Sci Technol. 2013;47:10556–10566. doi: 10.1021/es401604b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jung DW, Matson CW, Collins LB, Laban G, Stapleton HM, Bickham JW, Swenberg JA, Di Giulio RT. Genotoxicity in Atlantic killifish (Fundulus heteroclitus) from a PAH-contaminated Superfund site on the Elizabeth River, Virginia. Ecotoxicology. 2011;20:1890–1899. doi: 10.1007/s10646-011-0727-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wassenberg DM, Di Giulio RT. Teratogenesis in Fundulus heteroclitus embryos exposed to a creosote-contaminated sediment extract and CYP1A inhibitors. Mar Environ Res. 2004;58:163–168. doi: 10.1016/j.marenvres.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 6.Wills LP, Matson CW, Landon CD, Di Giulio RT. Characterization of the recalcitrant CYP1 phenotype found in Atlantic killifish (Fundulus heteroclitus) inhabiting a Superfund site on the Elizabeth River, VA. Aquat Toxicol. 2010;99:33–41. doi: 10.1016/j.aquatox.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teraoka H, Dong W, Tsujimoto Y, Iwasa H, Endoh D, Ueno N, Stegeman JJ, Peterson RE, Hiraga T. Induction of cytochrome P450 1A is required for circulation failure and edema by 2,3,7,8-tetrachlorodibenzo-p-dioxin in zebrafish. Biochem Biophys Res Commun. 2003;304:223–228. doi: 10.1016/s0006-291x(03)00576-x. [DOI] [PubMed] [Google Scholar]
- 8.Walker SE, Dickhut RM, Chisholm-Brause C. Polycyclic aromatic hydrocarbons in a highly industrialized urban estuary: Inventories and trends. Environ Toxicol Chem. 2004;23:2655–2664. doi: 10.1897/03-628. [DOI] [PubMed] [Google Scholar]
- 9.Fallahtafti S, Rantanen T, Brown RS, Snieckus V, Hodson PV. Toxicity of hydroxylated alkyl-phenanthrenes to the early life stages of Japanese medaka (Oryzias latipes) Aquat Toxicol. 2012;106:56–64. doi: 10.1016/j.aquatox.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 10.Billiard SM, Timme-Laragy AR, Wassenberg DM, Cockman C, Di Giulio RT. The role of the aryl hydrocarbon receptor pathway in mediating synergistic developmental toxicity of polycyclic aromatic hydrocarbons to zebrafish. Toxicol Sci. 2006;92:526–536. doi: 10.1093/toxsci/kfl011. [DOI] [PubMed] [Google Scholar]
- 11.Clark BW, Matson CW, Jung D, Di Giulio RT. AHR2 mediates cardiac teratogenesis of polycyclic aromatic hydrocarbons and PCB-126 in Atlantic killifish (Fundulus heteroclitus) Aquat Toxicol. 2010;99:232–240. doi: 10.1016/j.aquatox.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garner LVT, Brown DR, Di Giulio RT. Knockdown of AHR1A but not AHR1B exacerbates PAH and PCB-126 toxicity in zebrafish (Danio rerio) embryos. Aquat Toxicol. 2013;142:336–346. doi: 10.1016/j.aquatox.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Incardona JP, Carls MG, Teraoka H, Sloan CA, Collier TK, Scholz NL. Aryl hydrocarbon receptor–independent toxicity of weathered crude oil during fish development. Environ Health Perspect. 2005;113:1755–1762. doi: 10.1289/ehp.8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Incardona JP, Day HL, Collier TK, Scholz NL. Developmental toxicity of 4-ring polycyclic aromatic hydrocarbons in zebrafish is differentially dependent on AH receptor isoforms and hepatic cytochrome P4501A metabolism. Toxicol Appl Pharmacol. 2006;217:308–321. doi: 10.1016/j.taap.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 15.Incardona JP, Linbo TL, Scholz NL. Cardiac toxicity of 5-ring polycyclic aromatic hydrocarbons is differentially dependent on the aryl hydrocarbon receptor 2 isoform during zebrafish development. Toxicol Appl Pharmacol. 2011;257:242–249. doi: 10.1016/j.taap.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 16.Van Tiem LA, Di Giulio RT. AHR2 knockdown prevents PAH-mediated cardiac toxicity and XRE- and ARE-associated gene induction in zebrafish (Danio rerio) Toxicol Appl Pharmacol. 2011;254:280–287. doi: 10.1016/j.taap.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Incardona JP, Collier TK, Scholz NL. Defects in cardiac function precede morphological abnormalities in fish embryos exposed to polycyclic aromatic hydrocarbons. Toxicol Appl Pharmacol. 2004;196:191–205. doi: 10.1016/j.taap.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 18.Billiard SM, Meyer JN, Wassenberg DM, Hodson PV, Di Giulio RT. Nonadditive effects of PAHs on early vertebrate development: Mechanisms and implications for risk assessment. Toxicol Sci. 2008;105:5–23. doi: 10.1093/toxsci/kfm303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wassenberg DM, Di Giulio RT. Synergistic embryotoxicity of polycyclic aromatic hydrocarbon aryl hydrocarbon receptor agonists with cytochrome P4501A inhibitors in Fundulus heteroclitus. Environ Health Perspect. 2004;112:1658–1664. doi: 10.1289/ehp.7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brack W, Schirmer K, Erdinger L, Hollert H. Effect-directed analysis of mutagens and ethoxyresorufin-O-deethylase inducers in aquatic sediments. Environ Toxicol Chem. 2005;24:2445–2458. doi: 10.1897/05-078r.1. [DOI] [PubMed] [Google Scholar]
- 21.Brack W, Schirmer K, Kind T, Schrader S, Schuurmann G. Effect-directed fractionation and identification of cytochrome P4501A–inducing halogenated aromatic hydrocarbons in a contaminated sediment. Environ Toxicol Chem. 2002;21:2654–2662. [PubMed] [Google Scholar]
- 22.Ankley GT, Schubauerberigan MK. Comparison of techniques for the isolation of sediment pore-water for toxicity testing. Arch Environ Contam Toxicol. 1994;27:507–512. [Google Scholar]
- 23.Dong W, Matsumura F, Kullman SW. TCDD induced pericardial edema and relative COX-2 expression in medaka (Oryzias latipes) embryos. Toxicol Sci. 2010;118:213–223. doi: 10.1093/toxsci/kfq254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stein SE. An integrated method for spectrum extraction and compound identification from gas chromatography/mass spectrometry data. J Am Soc for Mass Spectr. 1999;10:770–781. [Google Scholar]
- 25.US Environmental Protection Agency. Method 8272: Parent and alkyl polycyclic aromatics in sediment pore water by solid-phase microextraction and gas chromatography/mass spectrometry in selected ion monitoring mode. Washington, DC: 2007. [Google Scholar]
- 26.Laor Y, Rebhun M. Complexation–flocculation: A new method to determine binding coefficients of organic contaminants to dissolved humic substances. Environ Sci Technol. 1997;31:3558–3564. [Google Scholar]
- 27.Barron MG, Heintz R, Rice SD. Relative potency of PAHs and heterocycles as aryl hydrocarbon receptor agonists in fish. Mar Environ Res. 2004;58:95–100. doi: 10.1016/j.marenvres.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 28.Grung M, Lichtenthaler R, Ahel M, Tollefsen KE, Langford K, Thomas KV. Effects-directed analysis of organic toxicants in wastewater effluent from Zagreb, Croatia. Chemosphere. 2007;67:108–120. doi: 10.1016/j.chemosphere.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 29.Thomas KV, Balaam J, Barnard N, Dyer R, Jones C, Lavender J, McHugh M. Characterisation of potentially genotoxic compounds in sediments collected from United Kingdom estuaries. Chemosphere. 2002;49:247–258. doi: 10.1016/s0045-6535(02)00316-8. [DOI] [PubMed] [Google Scholar]
- 30.Wassenberg DM, Nerlinger AL, Battle LP, Di Giulio RT. Effects of the polycyclic aromatic hydrocarbon heterocycles, carbazole and dibenzothiophene, on in vivo and in vitro CYP1A activity and polycyclic aromatic hydrocarbon–derived embryonic deformities. Environ Toxicol Chem. 2005;24:2526–2532. doi: 10.1897/04-440r1.1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.